Healing, Mechanical Loading, and Water-harvesting with Integument Structures and their Biomimetic Applications
Juyoun Bae, Andrei Bocan, Justin Charney, Lan Anh Huynh
Abstract
This report focuses on the structures that enable healing, mechanical loading, and water-harvesting of the integument (i.e., skin, feathers, hair, scales, etc.). By examining the morphologies that allow for these functions, and detailing their interrelation, we develop a basis for understanding how an organism’s design is related to its operation. To conclude, we consider the biomimetic applications for some of the examined structures in engineered materials.
Introduction
Nature’s design of the integument has evolved over millennia, sculpting morphologies that enable remarkable functions in areas like tissue regeneration, mechanical strength, and water-harvesting. These structures are often over- looked in engineering design solutions; though most times, the best design is the one that most closely resembles nature. By examining the integument morphology of several vertebrate, we have distilled integral structures that could inspire innovative solutions to engineering challenges like providing water access to all, improving urban infrastructure, and advancing medicine.
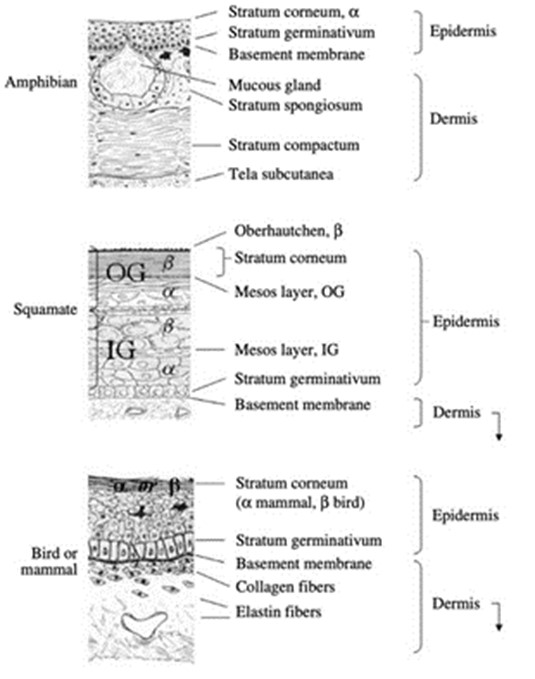
The skin is composed of three layers: the epidermis, dermis, and hypodermis (subcutaneous fat layer). The epidermis is the outward-most layer of the skin and is composed mainly of keratinocytes and dendritic cells (Kolarsick et al., 2011). Its upper layers form a water barrier which prevents excess water loss from the body (Elias and Friend, 1975). This superficial layer also contains Langerhans cells, which take up external antigens passing through the skin, aiding in the skin’s immune barrier function (Matsui and Amagai, 2015). The dermis is composed of various connective tissues, hosts nerve and vascular networks, as well as various types of appendages and cells (Kolarsick et al., 2011). Elastic fiber and collagen networks of the dermis are deeply involved in the skin’s mechanical protection function; the first provides elasticity while the latter provides tensile strength (Joodaki and Panzer, 2018). Additionally, by containing components of the nervous system, it allows for signaling between the environment and host via touch (Kolarsick et al., 2011). The hypodermis is mainly composed of lipids and provides buoyancy as well as energy storage. The skin is also important for thermoregulation, where the dilation/contraction of blood vessels allows for heat conservation or loss (Kolarsick et al., 2011). Finally, if the skin endures a wound, it has an elaborate four-stage healing process.
Healing
As a function of the skin, the process of wound healing is an important physiological property. When damage is done upon this composite material, wound healing according to Guo and DiPietro (2010) occurs through the four main overlapping stages of hemostasis, inflammation, proliferation, and tissue remodeling or resolution. Proper healing ensues if these four stages are done in a specific sequence, for the correct duration of time, and under optimal intensity (Guo and DiPietro, 2010). Elaborated in the study, following an injury, hemostasis is immediately triggered. This results in vascular constriction to reduce the amount of blood loss through the wound opening, with platelet aggregation, degranulation, and fibrin formation following promptly.
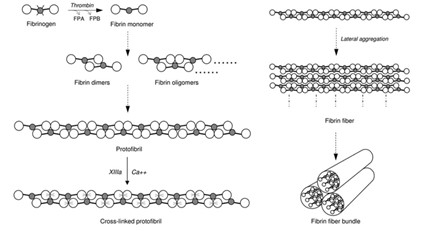
The formation of the fibrin network during coagulation of the hemostasis phase is an important process that helps to reinforce the strength and mechanical rigidity of the platelet clots that form. This clot formation serves to block the wound opening to prevent foreign entities from entering and further blood loss. van Kempen and his team’s 2010 study states that the network formation starts with single fibrinogen molecules being converted to fibrins catalyzed by the enzyme thrombin, then proceeds to polymerizing fibrin polymers to obtain protofibrils — have a double-stranded, half staggered structure (van Kempen et al., 2014); — Finally, protofibrils of a sufficient length will aggregate to form fibers that will connect to create a mature fibrin network (Fig. 1). The 2010 study combined light scattering microscopy and turbidity examinations to identify that the process of fibrin network maturation occurs in two stages. Firstly, the fibers will grow in the longitudinal direction and form branch points, we see this initial formation as a network of thin fibers. Then, the second stage begins with the existing fibers becoming thicker by the addition of protofibrils in the radial direction, constituting the major changes in the network’s mechanical properties (van Kempen et al., 2014).
The architecture and properties of this fibrous network changes in the presence of varying concentrations of erythrocytes (red blood cells [RBCs]), studied by Gersh et al. in 2009. In previous studies of clot structures in vivo, the clots tested were ones done in plasma media with the absence of RBCs; this study aimed at providing analysis of the clot complex in the presences of varying concentrations of RBCs to investigate the changes that occur in terms of the complex’s mechanical properties. The team studied fibrin clot formation in three different RBC conditions: low RBC concentration (2 vol %), intermediate (5 -10 vol %), and high (>or equal 20 vol %), compared to a control with no RBCs at all (Gersh et al., 2009). Through analysis using confocal microscopy, scanning electron microscopy, clot permeability and viscoelastic measurements, the study was able to conclude that heterogeneity in fibrin clot structure was increased in the presence of RBCs. RBCs not only affected the arrangement of the fibers but also their formation (Gersh et al., 2009).
Fig. 2, a scanning electron micrograph taken of the three conditions tested, shows that, as the concentration increases, we see an increase in disruption of the fiber network, creating regions of densely packed fibers and regions with RBCs interlaced in between fibers; this causes irregularities in the network’s overall structure.
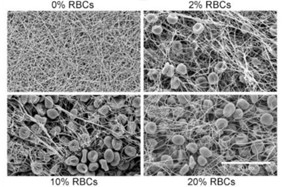
What was further observed by the researchers from these micrographs was that the diameter of the fibers constituting the network also increased in the presence of RBCs allowing the fibers to be more flexible. Therefore, RBCs’ high deformability allows for the formation of a more favorable network formation as heterogeneity is favorable for the fibrin network. If weakness or fault occurs in the network, then it will not be able to propagate throughout the whole structure due to insufficient energy and so will stop after encountering an obstacle within the structure. Therefore, the fibrin network will not fail as a whole. Thus, even if the platelet clot after forming is put under stress, will be supported by a heterogeneous fibrin network that allows it to withstand the impact (to a certain degree) and keep the wound blocked. In addition, the study, “Structural hierarchy governs fibrin gel mechanics” conducted in 2010 further discusses the hierarchical nature of the fibrin network; it explains how the fiber’s 80 % water content contributes to its flexibility and is the cause for its behavior like a simple semiflexible elastic beam when it is in a tightly coupled bundle of protofibrils. In addition, the fibers were found to be “100-fold more flexible to bending than anticipated based on their large diameter” and compared with other biopolymers they essentially “stiffen when stretched” which explains their resilience under deformation (Piechocka et al., 2010), enabling the network to serve its function of reinforcing platelet plugs and adding strength to the scabs covering the wound.
In general, wound healing is a difficult physiological trait to study, especially in living organisms in the wild due to its practicality as well as ethical concerns. Therefore, only the mammalian healing process has been vigorously tested upon as well as a few other vertebrate groups. What is interesting to note, highlighted by Yokoyama and others in their 2018 study, is that most mammals excluding the spiny African mouse and mammal fetuses, respond to injury through wound healing or repair, whilst amphibians deal with the wound through the regeneration of the skin avoiding scarring. Yokoyama’s team (2018) found that amphibian skin may rapidly re-epithelialize after an injury, rapid enough that within 24 hours their dermis-free epidermis was able to recover and completely enclose the wound.
This process seen in mammals may take up to 10 days or more. After the epidermis covers over the amphibian’s wound, dedifferentiation occurs where the underlying subcutaneous myofibrillar proteins, muscles and layers become disorganized around the wound allowing blastema-like cells to accumulate directly under the epidermis (Yokoyama et al., 2018). The study further stated that only after regeneration of the layers and glands around the wound is complete will the subcutaneous organization restore, leaving the skin scar-less. This in comparison to mammals is a very different process as re-epithelization happens in the third phase of healing, only after hemostasis where the wound is blocked, and a structured fibrin network is synthesized.
This process seen in mammals may take up to 10 days or more. After the epidermis covers over the amphibian’s wound, dedifferentiation occurs where the underlying subcutaneous myofibrillar proteins, muscles and layers become disorganized around the wound allowing blastema-like cells to accumulate directly under the epidermis (Yokoyama et al., 2018). The study further stated that only after regeneration of the layers and glands around the wound is complete will the subcutaneous organization restore, leaving the skin scarless. This in comparison to mammals is a very different process as re-epithelization happens in the third phase of healing, only after hemostasis where the wound is blocked, and a structured fibrin network is synthesized.
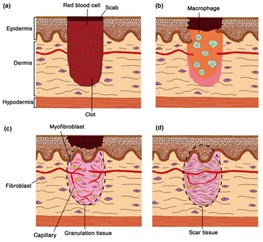
Seen in Fig. 3, the four phases of healing occur in a very specific order and only after the previous step has been initialized. Instead of the immediate formation of the epidermis like seen in amphibians, mammals prioritize blocking the wound opening with platelet aggregates and securing it with the fibrin network before mobilizing macrophages to initiate inflammation and re-epithelization. From Fig. 3b, c we observe that the layers are reformulated in an ordered manner where components responsible for the different stages will enter the wound region to rebuild and fill in the cavity with scar tissues before rebuilding the epidermis layers.
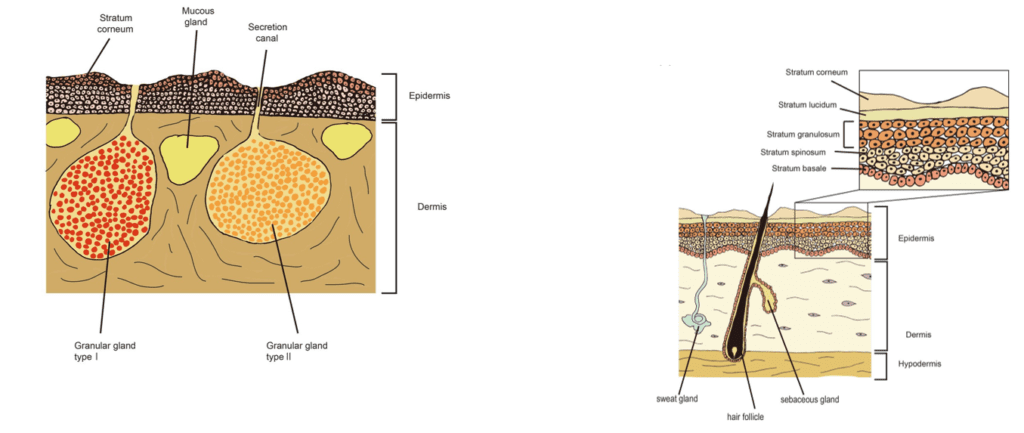
By comparing the anatomy of amphibian and mammal skin in Fig. 4, we observe that the number of components seen in the amphibian skin is a lot fewer than in mammal skin which could explain why amphibians can heal regeneratively by disrupting the organization of its layers. Mammalian skin has up to four different layers (not including the lucidum seen in only thick skin) compared to the two layers seen in the amphibian epidermis. With more layers and components in both the epidermis and dermis, it is much more complex for mammals to disarrange and reorganize the epidermis. From being able to rapidly re-epithelialize the epidermis and to regenerate the skin and its glands and protein components, amphibian skin can heal significantly faster where mammals would take up to weeks or even months to completely heal and their wound region’s tissues would be replaced by scar tissues, unlike amphibians.
Mechanical Loading
The skin plays a major role in protecting the body from mechanical impacts and stresses. From a general perspective, collagen and elastin fibers from the dermis contribute most to the skin’s behavior under stress (Joodaki and Panzer, 2018). The epidermis plays a minor role; attachments between its cells, called desmosomes, give the skin part of its mechanical resistance (Sanders et al., 1995). Human skin does not seem to have an obvious collagen fibril micro-architecture; though collagen fiber bundles often form local “basket weave” arrangements (Graham et al., 2019). Collagen is roughly organized along lines of maximum tension, called Langer’s lines (Fig. 5).
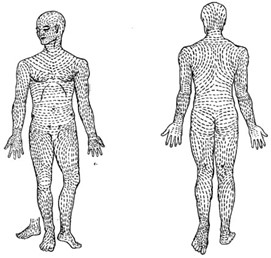
When the skin is stretched in parallel to one of these lines, it cannot extend by much; collagen fibers are pre-maturely aligned in the direction of tension and they rapidly straighten out. If the skin is stretched across a line, it can extend significantly before the fibers align themselves in the direction of tension (Joodaki and Panzer, 2018). Thus, depending on the orientation of mechanical loading, skin responds differently. Elastic fibers are somewhat more structured than collagen; they are arranged in thick horizontal bundles in the deep reticular dermis (Graham et al., 2019).
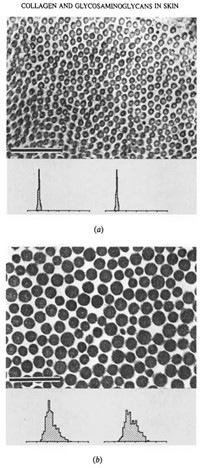
The strength of connective tissues of the skin shows a positive correlation with the mass-average diameter of the constituent fibrils. In other words, the form of the collagen fibril diameter distribution can be directly related to the mechanical properties of the skin (Parry et al., 1978). The size distribution of the collagen fibrils is determined by two factors: first, if the tissue of a skin is designed to have high tensile strength, an increase in the diameter of the collagen fibrils will be proportional to the increase in the potential density of intrafibrillar crosslinks, allowing large collagen fibrils to have greater tensile strength than small fibrils. Secondly, if the tissue is designed to endure compressive stress or to be elastic and hence withstand creep, then a reduction in the diameter of the collagen fibrils will effectively increase the surface area per unit mass of the fibrils, enhancing the probability of interfibrillar crosslinks between the collagen fibrils and the components of the matrix (Parry et al., 1978). Different skins subject to different functional loading have differences in the mass-average diameter of their constituent collagen fibrils. The micrographs and histograms above show the distribution of small diameter fibrils in samples of skin from the lamprey (Fig. 6a) of relatively high compressional stress, and the distribution for protective skin of rats of high tensile strength (Fig. 6b). Likewise, the diameters of the fibrils in the ventral and dorsal abdominal trunk skin in the guinea pig correlate well with the differing mechanical function of the thick, tough dorsal hide and the relatively thin and soft ventral underbelly (Flint et al., 1984).
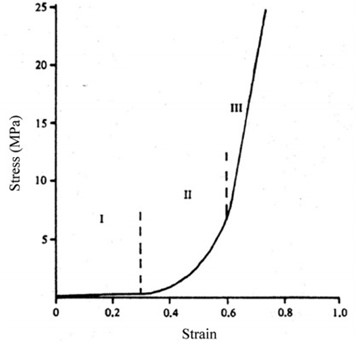
Collagen is relatively stiff, compared to elastin, with a Young’s modulus — the Young modulus of a material is a measure of its stiffness, it is a ratio of stress/strain: stiffer materials have higher moduli, while flexible materials have lower values (French, 1971) — of about 4 GPa versus elastin’s modulus of 300 – 600 kPa. Elastin can sustain many cycles of extension and recoil without any failure (Miranda-Nieves and Chaikof, 2017). This explains the mechanical roles played by both fibers: elastin allows the skin to recoil after deformation and governs the skin’s mechanical responses under small stresses. Collagen gives the skin its strength and supports a major part of the load at high strain levels (Joodaki and Panzer, 2018). When a load is exerted on the skin, it responds in three different phases, depending on the load itself. Collagen fibers, in their resting state, are curled up. When tension is uniaxial, the skin is soft, and the collagen fibers are still slack, the skin’s response is mostly due to elastin; this corresponds to the first phase. In the second phase, the stress on the skin increases, the collagen fibers straighten out and are recruited to carry the load. In the third phase, the system is as it is stiffest , the strain is also at its highest, the collagen fibers are completely straight. Fig. 7 shows how these stages can be modeled, where the curve is linear (phase 3), collagen is the main support structure. As strain gets even higher, collagen fibers will start breaking, resulting in a decrease in stiffness of the tissue (Joodaki and Panzer, 2018).
The skin of the white rhinoceros (Ceratotherium simum) is an interesting illustration of the mechanical protection function of the skin. Rhinos need skin as tough as armor because serious injury can occur during elephant attacks or intraspecific combat between rhino males. Males can deliver blows of significant force with their horns and can deal significant damage to each other. The skin of C. simum is disproportionately thick; back and flank skin is roughly 25 mm thick while belly skin is 15 mm thick, compared to the 7 mm allometric prediction — prediction for the skin thickness of an animal based on its body mass — (Shadwick et al., 1992). C. simum’s skin organization is also different from that of other mammals in many ways. The collagen network in the rhino’s dermis is highly organized, collagen fibers are fairly straight even at rest, and they are heavily cross-linked. The dermis is also fairly devoid of elastic fibers, making the skin less flexible (Shadwick et al., 1992). The epidermis of C. simum contains a high number of desmosomes which, as discussed earlier, enhance its mechanical strength (Plochocki et al., 2017). Fibers in C. simum’s collagen network are densely packed and regularly intertwined, this increased organization yields stronger skin: rhino skin is said to be somewhere between regular mammalian skin and tendons, in which collagen fibers are relatively straight. The increased organization might lead to increased strength by reducing slack in the skin and allowing collagen fibers to bear load faster. Collagen comprises 85 % of the skin’s dry fraction; this is higher than 70 – 80 % seen in the mammalian dermis. The collagen fibers are relatively thick; with diameters of 70µm – 100µm in the dorsum and flanks, and 60µm – 200µm in the belly (Shadwick et al., 1992), compared to 0.07µm-0.1µm in human skin (Meyer et al., 1982). As mentioned earlier, larger collagen fibril diameter and more frequent cross-linking are associated with higher tensile strength.
In comparison, the skin of fishes manifests a different architecture designed mainly for flexibility, since marine animals are involved in continuous body movement for swimming (Hebrank, 1980). An example of marine animal skin worth noting is that of eel (Anguilla rostrata), which interestingly is relatively strong, owing to its relatively large collagen fiber diameter, and a relatively large number of collagen fibers. When the radial section of eel skin is cut parallel to one set of fibers, one can find over seventeen fiber layers (Fig. 8), meaning the animal’s skin affords some tensile resistance .
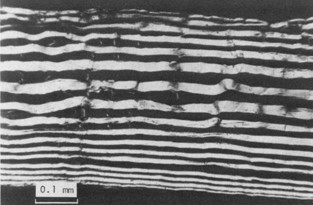
Like the skin in other fish, these collagen fibers are arranged in alternating layers and form left and right helices along the long axis of the fish, which allows flexibility in their locomotion while still being strong. The convex side of the bent body of A. rostrata stretches in the longitudinal direction and compresses in the circumferential direction. The reverse occurs on the opposite, or concave, side. In this way, the fish can undergo a range of movements without changing its body form or volume which allows for shape changes without the skin kinking or wrinkling (Hebrank, 1980).
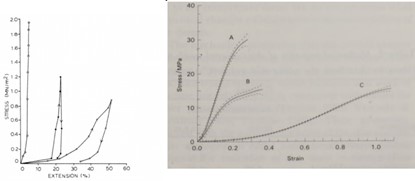
C. simum skin exhibits high stiffness and low extensibility in its operation; the stress-strain curve of the rhino (Fig. 9) has a very short toe region, suggesting the presence of little slack in the skin, less than in human skin. The Young’s modulus for C. simum skin averaged 237 MPa, other animals such as the rabbit or the cat will have lower Young’s moduli; their skin is more extensible and less strong (Shadwick et al., 1992). The skin of C. simum fails at very high stresses and is highly resistant to fracturing and tear propagation. It is also very strong in compression and is much stiffer than in tension. This might be achieved by holding interstitial water tightly within the fiber network, and by generating tension in fibers perpendicular to the compressive force. Interestingly, the force required to tear rhino skin is relatively low; this might be a defensive strategy, allowing the animal to sustain superficial gashes rather than deep punctures which can cause lethal damage (Shadwick et al., 1992). Through its architecture and operation, white rhinoceros skin acts as a very capable dermal armor, which can withstand significant mechanical loads.
A. rostrata skin is somewhat different in its operation; at low strain levels, the eel skin can deform by a large amount. However, as the strain increases, the collagen helices move relative to each other aligned in the direction of the strain, so that the skin no longer extends readily. The fibers are reasonably inextensible, especially within the range of stresses applied to the hoop (circumferential) and longitudinal directions, as seen in Fig. 9a. The stress-strain curves for skin subjected to uniaxial stretching in the hoop and longitudinal directions exhibit the property of anisotropy (Fig. 9a). In both cases, the skin deforms by a relatively large amount while the stress remains low, then the stress-strain curve becomes steeper as the skin no longer deforms as freely under the applied load. In comparison, skin stressed in the longitudinal direction undergoes much greater extensions before it becomes stiffer than skin stressed in the hoop direction (Hebrank, 1980). Importantly, it is not only the level of strain that determines the extent of deformation in A. rostrata. Following the mechanical property of anisotropy — directional dependence, as opposed to isotropy, which means homogeneity in all directions (Irsay et al., 2010), — the flexibility of eel skin also depends heavily on the direction of the stretch. Compared to the rhino skin, in which collagen fibers are mostly straight and heavily cross-linked for its maximum tensile strength, the skin of A. rostrata is composed of collagen fibers in helical arrangement, providing the fish with both flexibility and strength. Moreover, presence of elastin fibers intermingled with fine collagen fibers in the dermis of A. rostrata provides further evidence for higher flexibility in its skin compared to the rhino, which has very few elastic fibers.
Water-harvesting
Water-harvesting is of particular importance to species that live in arid environments with no apparent source of water. Thus, morphological adaptations that increase one or more of the following can be found in over 39 species whose integument enables water-harvesting: surface wettability, spreading area, storage of water, condensation, and gravitational aid (Comanns, 2018). In Moloch horridus (Fig. 10a) and Litoria caerulea (Fig. 10b), this function is characterized by increased surface wettability, along with capillarity, and thermally facilitated condensation respectively. We compared the reptile M. horridus, that resides in the desert covering most of central Australia, and the arboreal tree frog L. caerulea in the tropics of northern Australia because they exhibit both shared and unique methods of water-harvesting despite having different habitats and integument morphologies.

Whereas M. horridus has a hierarchically structured and highly ornamented superficial layer of its epidermis, that is largely impervious to water, L. caerulea lacks significant resistance to cutaneous evaporative loss of water. This results from the amphibian integument lacking an outer layer of cornified cells — cornified cells are the result of keratinocyte differentiation through the terminal process of cornification. Through this process, the cornified envelope develops and provides a highly insoluble barrier to the underlying cutaneous layers (Candi et al., 2005) — unlike mammals, birds, and reptiles (Fig. 11.). Since the stratum corneum of L. caerulea is composed of a thin layer of keratinized cells without significant barrier properties, it is the apical membrane of the stratum granulosum that acts as a permeability barrier across the epithelium. However, since amphibians do not drink orally under most conditions, they also possess a family of water channel proteins called aquaporins that when stimulated allow for rapid movement of water through the apical and basolateral membranes to be transported into capillaries. Related to their highly water-permeable integument morphology, amphibians typically have evaporative water loss (EWL) equivalent to a free water surface of the same shape and size, given similar conditions (Christian and Parry, 1997). With L. caerulea, actual EWL is significantly less than expected — total resistance (s/cm): 11.2 ± 1.6 (Christian and Parry, 1997), — although the morphological mechanism for this is not yet fully understood. Nevertheless, the hypothesis that EWL is restricted by the mucus and lipids secreted from cutaneous mucous glands is considered the most likely explanation.
The morphology discussed so far, regarding L. caerulea‘s high water permeability and self-covering of the body’s surface with a cutaneous secretion of lipids, directly relates to its method of water-harvesting. Unlike other frogs that inhabit similar environments, they remain active during the dry season, exiting from their tree hollows during the night at temperatures as low as 12.5 ºC (Tracy et al., 2011). At this temperature, their performance is below 20 % of peak output, and they are unfit for foraging . Instead, the purpose of this activity is primarily to cool the body before returning to the warm and humid tree hollow. This creates a thermal gradient so that thermally facilitated condensation can occur on the skin and be used to gain up to 0.93 % body mass worth of water (Tracy et al., 2011). To aid with condensation, the mucus secreted is known to have hygroscopic — able to absorb moisture from air — properties that increase surface wettability along with likely reducing EWL. Additionally, the membrane-spanning aquaporins increase water transport by orders of magnitude, especially if there is osmotic flow through several channels, allowing water flux to occur via laminar flow if enough condensation is present on the integument (Lillywhite, 2006). These morphological features of the integument and cutaneous mucus make condensation an appreciable source of water for L. caerulea during the dry season since they operate to both maximize water-harvesting and reduce evaporative water loss.
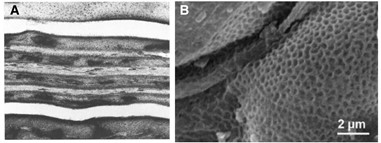
M. horridus have similar needs of reducing EWL and gathering water via condensation; however, these functions result from a more complex integument design and support an alternate primary mechanism for water-harvesting. Reptiles have a distinguishing heavily keratinized epidermis that comprises an outer β-keratin layer, not found in mammals or amphibians, and provides further mechanical strength. Their low EWL can be attributed to an integument feature called the meso layer, which separates the β– and α-keratin layers. Evidence of this comes from ultrastructural studies of the meso layer that show it contains extracellular lipid-rich lamellar sheets, providing a barrier to water flux (Hadley, 1989) (Fig. 12.a). Their integument also comprises a superficial Oberhautchen — a superficial syncytium layer of cornified cells composed of β-keratin with varying microstructures for lepidosauria (Maderson et al., 1998) — with dimple-like ornamentation, sometimes referred to as a “honeycomb” (Fig. 12.b). This microstructure can fully or partially cover the scales and spikes that adorn the body of M. horridus. Similar to L. caerulea, these structures allow for increased surface wettability and condensation, enabling water-harvesting. However, they participate in a more preliminary role of “pre-wetting” to increase the efficiency of the primary method of water-harvesting–cutaneous transport of water.
In a study of three water-harvesting lizards (Moloch horridus, Phrynosoma cornutum, and Phrynocephalus arabicus), researchers dipped a scale from a Phrynosoma cornutum in water to measure the contact angle and quantify the hydrophilicity/surface wettability of the integument. They found that the inwardly facing surface had no Oberhautchen and formed a small meniscus with a contact angle of 60 – 70º. Conversely, the outwardly facing side formed a large meniscus and had a contact angle of less than 10º, rendering it “superhydrophilic” (Comanns et al., “Moisture harvesting and water transport”) (Fig. 13.a, c). Wenzel’s law — cosθwenzel = rcosθcontact where r is a measure of roughness — which accounts for the effect of roughness on contact angle could explain this property for M. horridus, which has a highly irregular surface and roughness of r ≈ 3, but not for the other water-harvesting lizards whose values of r were lower. Additionally, researchers dipped the same scale in water after it had been dried and observed a much smaller meniscus formed by the outer side of the scale (Fig. 13.b). Thus, Cassie’s law — cosθcassie = f1cosθ1 + f2cosθ2 where f1 and f2 are the fractional surface areas of the heterogenous surface and θ1 and θ2 are the corresponding contact angles for the materials — was applied under the assumption that the microstructure of the Oberhautchen can maintain a thin film of water, by a process of pre-wetting, rendering it superhydrophilic. For superphilicity to occur, a minimum required water area of 73 % in the microstructure was calculated. How M. horridus maintains its pre-wet status is still undetermined, though it is hypothesized that the spikes formed from the epidermis act as condensation foci for thermally facilitated condensation when desert temperatures rise in the morning. Support for this comes from measuring an average condensation of 0.2 % body mass for an M. horridus placed in a container at 30 ºC and 95 % relative humidity. This was determined to sufficiently fill the microstructure surface area of an average-sized M. horridus to the 73 % water area needed for superhydrophilicity (Comanns et al., “Cutaneous water collection by a moisture-harvesting lizard”).

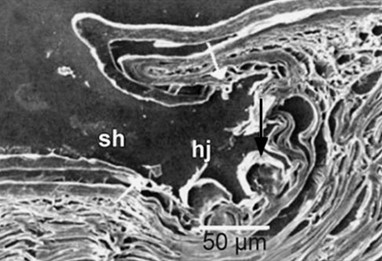
When the integument of M. horridus is pre-wet, the hierarchical structure allows for the rapid uptake and transport of water, leaving the surface dry. Scales of the integument are irregular and hexagonal, formed by the folding of the epidermis into scales that partially overlap (Sherbrooke et al., 2007). The outer β-keratin layer of the epidermis is covered in an Oberhautchen and varies in thickness, with it being thickest at the scale spines and narrowing as it forms a hinge-joint, connecting the scales and allowing for increased flexibility. At the basal region of the hinge joint, it forms a channel with a diameter of 100 – 250 µm and contains several globular protrusions from the α-keratin layer folding on top of itself (Fig. 14.). These globular protrusions form smaller sub-channels between their walls, which allows for an increase of 39 % to the water transport distance since capillary forces are inversely proportional to capillary diameter (Comanns et al., “Adsorption and movement of water by skin of the Australian thorny devil”). The continuous network formed by these hinge joint channels is hexagonal and extends across the surface of the body. However, water movement through these channels is symmetrical and must be transported to the mouth before jaw movements can facilitate drinking. Since M. horridus has only been observed drinking when its capillary system is saturated, with water volume approximately 3.19 % body mass, condensation’s use in pre-wetting is further supported. This leaves rain and wet sand as likely main sources of water since they have a greater potential of filling the capillary system (Comanns et al., “Cutaneous water collection by a moisture-harvesting lizard”).
Biomimetic Applications
The varied structures and mechanisms through which the skin fulfils its functions can inspire many different biomimetic designs. The mature fibrin network used by the skin to aid healing and wound closure can inspire the construction of materials or structures with slender members connected by branch points or joints. By varying the diameter of the individual rods making up the structure we can change the rigidity of the structure. We can also increase rigidity by increasing the density of branch points compared to the volume of the network. Seen when erythrocytes are incorporated into the fibrin network, the structure increases in heterogeneity which is a desirable trait for reinforcement of strength and sturdiness. Heterogeneity will allow the structure to withstand deformation and prevent failure when the system acquires a weakness or fault, a key insightful concept for building structures. The bundles of protofibrils also act as a simple semi-flexible elastic rod when tightly coupled which can inspire the tight coupling of smaller subunits to create beams with semiflexible properties and reduce brittleness as well as increase the strength.
The skin can also be of great inspiration when designing flexible materials designed for protection . A highly resistant and durable fabric could be produced, to be used for heavy-duty clothing, footwear, bags, etc. Such a material can be produced by using a combination of elastin-like and collagen-like fibers. By increasing the diameter and proportion of the collagen-like fibers, the material can be made stiffer. The proportion of elastin-like fibers can also be increased for enhanced elasticity. Linkage of the collagen-like fibers can make the material even stronger. The use of the two fibers allows the fine-tuning of the properties of the materials.
A more novel application would be in the cooling of computer chips: in the United States, data centers consume as much energy and water to cool their systems as the residents of Philadelphia (van Erp et al., 2020). By designing chips with integrated microfluidic cooling channels based on the integument design of water-harvesting lizards, these computer systems can be cooled more efficiently. In current designs of microfluidic cooling systems, a thermal interface material (TIM) is used to transfer heat between the chip and a cooling plate. The problem with a TIM is that it exhibits thermal resistance, decreasing efficiency. To combat this, another design called embedded liquid cooling does not use a TIM and instead brings the coolant in contact with the chips by using a high-powered pump to pump coolant through parallel microchannels on the back of the chip (Wei, 2020). If instead, a three-dimensional structure with channels like those found in the integument of M. horridus and asymmetric microstructures like in Phrynosoma cornutum (enabling directional water transport) were integrated into the back of chips, it could eliminate the need for a TIM and powered coolant pump.
Conclusion
In conclusion, looking at the skin’s architecture from the perspectives of its function in healing, mechanical protection and water harvesting, this report sheds light onto the unique mechanical properties of individual components of this composite material and explains how they contribute to their respective functions. From an examination of the formation and arrangement of fibers to form a highly elastic, stable and viscous network, we find that the skin can heal wounds by reinforcing scab rigidity and speeding-up wound closure. The skin’s mechanical properties are derived mainly from two of its components: collagen and elastin. The study of human, eel, and rhinoceros skin has shown us that the proportions, architectures, and layouts of each of these components can greatly change how the skin responds to mechanical stress. Without a doubt, there exists a variety of different designs in nature, which might be the subject of future studies. Analysis and comparison of the thorny devil and the arboreal Australian tree frog also provide insight into their distinct structures even though they share the same needs to reduce evaporative water loss, increase condensation, and drink in arid environments. The heavily keratinized epidermis of the thorny devil creates a water-impermeable surface that greatly reduces water flux as opposed to the thinly keratinized cell layers seen in the tree frog. Moreover, the structure of the thorny devil’s globular protrusions and its honeycomb Oberhautchen surface provides a distinctive capillary mechanism that relies on pre-wetting and enables the thorny devil to drink water. With these diverse and incredibly unique architectures of the skin in humans, amphibians, and reptiles, future research can certainly be done to create biomimetic materials that utilize these structural findings and propel the advancements of human-engineered designs.
References
Candi, E., Schmidt, R., & Melino, G. (2005). The cornified envelope: a model of cell death in the skin. Nature Reviews Molecular Cell Biology, 6(4), 328-340. doi:10.1038/nrm1619
Christian, K., & Parry, D. (1997). Reduced Rates of Water Loss and Chemical Properties of Skin Secretions of the Frogs <emph type=”2″>Litoria caerulea</emph> and <emph type=”2″>Cyclorana australis</emph>. Australian Journal of Zoology, 45(1), 13-20. doi:https://doi.org/10.1071/ZO96046
Comanns, P. (2018). Passive water collection with the integument: mechanisms and their biomimetic potential. Journal of Experimental Biology, 221(Pt 10). doi:10.1242/jeb.153130
Comanns, P., Effertz, C., Hischen, F., Staudt, K., Böhme, W., & Baumgartner, W. (2011). Moisture harvesting and water transport through specialized micro-structures on the integument of lizards. Beilstein J Nanotechnol, 2, 204-214. doi:10.3762/bjnano.2.24
Comanns, P., Esser, F. J., Kappel, P. H., Baumgartner, W., Shaw, J., & Withers, P. C. (2017). Adsorption and movement of water by skin of the Australian thorny devil (Agamidae: <i>Moloch horridus</i>). Royal Society Open Science, 4(9), 170591. doi:doi:10.1098/rsos.170591
Comanns, P., Withers, P. C., Esser, F. J., & Baumgartner, W. (2016). Cutaneous water collection by a moisture-harvesting lizard, the thorny devil (Moloch horridus). Journal of Experimental Biology, 219(Pt 21), 3473-3479. doi:10.1242/jeb.148791
Elias, P. M., & Friend, D. S. (1975). The permeability barrier in mammalian epidermis. The Journal of cell biology, 65(1), 180-191. doi:10.1083/jcb.65.1.180
Flint, M. H., Craig, A. S., Reilly, H. C., Gillard, G. C., & Parry, D. A. (1984). Collagen fibril diameters and glycosaminoglycan content of skins–indices of tissue maturity and function. Connective Tissue Research, 13(1), 69-81. doi:10.3109/03008208409152144
French, A. P. (1971). Vibrations and Waves (The M.I.T. Introductory Physics Series).
Gersh, K. C., Nagaswami, C., & Weisel, J. W. (2009). Fibrin network structure and clot mechanical properties are altered by incorporation of erythrocytes. Thrombosis and Haemostasis, 102(6), 1169-1175. doi:10.1160/th09-03-0199
Graham, H. K., Eckersley, A., Ozols, M., Mellody, K. T., & Sherratt, M. J. (2019). Human Skin: Composition, Structure and Visualisation Methods. In G. Limbert (Ed.), Skin Biophysics: From Experimental Characterisation to Advanced Modelling (pp. 1-18). Cham: Springer International Publishing.
Guo, S., & DiPietro, L. A. (2010). Factors Affecting Wound Healing. Journal of Dental Research, 89(3), 219-229. doi:10.1177/0022034509359125
Hadley, N. F. (1989). Lipid water barriers in biological systems. Progress in Lipid Research, 28(1), 1-33. doi:10.1016/0163-7827(89)90005-2
Hebrank, M. R. (1980). MECHANICAL PROPERTIES AND LOCOMOTOR FUNCTIONS OF EEL SKIN. The Biological Bulletin, 158(1), 58-68. doi:10.2307/1540758
Irsay, L., Mandl, P., & Balint, P. V. (2010). Chapter 3 – Pitfalls of Gray-Scale Artifacts. In R. J. Wakefield & M. A. D’Agostino (Eds.), Essential Applications of Musculoskeletal Ultrasound in Rheumatology (pp. 29-42). Philadelphia: W.B. Saunders.
Joodaki, H., & Panzer, M. B. (2018). Skin mechanical properties and modeling: A review. Proceedings of the Institution of Mechanical Engineers. Part H: Journal of Engineering in Medicine, 232(4), 323-343. doi:10.1177/0954411918759801
Lillywhite, H. B. (2006). Water relations of tetrapod integument. Journal of Experimental Biology, 209(Pt 2), 202-226. doi:10.1242/jeb.02007
Maderson, P. F. A., Rabinowitz, T., Tandler, B., & Alibardi, L. (1998). Ultrastructural contributions to an understanding of the cellular mechanisms involved in lizard skin shedding with comments on the function and evolution of a unique Lepidosaurian phenomenon. Journal of Morphology, 236(1), 1-24. doi:10.1002/(sici)1097-4687(199804)236:1<1::Aid-jmor1>3.0.Co;2-b
Matsui, T., & Amagai, M. (2015). Dissecting the formation, structure and barrier function of the stratum corneum. International Immunology, 27(6), 269-280. doi:10.1093/intimm/dxv013
Meyer, W., Neurand, K., & Radke, B. (1982). Collagen fibre arrangement in the skin of the pig. Journal of Anatomy, 134(Pt 1), 139-148. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1167944/
Miranda-Nieves, D., & Chaikof, E. L. (2017). Collagen and Elastin Biomaterials for the Fabrication of Engineered Living Tissues. ACS Biomaterials Science & Engineering, 3(5), 694-711. doi:10.1021/acsbiomaterials.6b00250
Parry, D. A., Barnes, G. R., & Craig, A. S. (1978). A comparison of the size distribution of collagen fibrils in connective tissues as a function of age and a possible relation between fibril size distribution and mechanical properties. Proceedings of the Royal Society of London. Series B: Biological Sciences, 203(1152), 305-321. doi:10.1098/rspb.1978.0107
Piechocka, I. K., Bacabac, R. G., Potters, M., Mackintosh, F. C., & Koenderink, G. H. (2010). Structural hierarchy governs fibrin gel mechanics. Biophysical Journal, 98(10), 2281-2289. doi:10.1016/j.bpj.2010.01.040
Plochocki, J. H., Ruiz, S., Rodriguez-Sosa, J. R., & Hall, M. I. (2017). Histological study of white rhinoceros integument. PloS One, 12(4), e0176327-e0176327. doi:10.1371/journal.pone.0176327
Sanders, J. E., Goldstein, B. S., & Leotta, D. F. (1995). Skin response to mechanical stress: adaptation rather than breakdown–a review of the literature. J Rehabil Res Dev, 32(3), 214-226.
Scholz, I., Barnes, W. J. P., Smith, J. M., & Baumgartner, W. (2009). Ultrastructure and physical properties of an adhesive surface, the toe pad epithelium of the tree frog, Litoria caerulea White. Journal of Experimental Biology, 212(2), 155-162. doi:10.1242/jeb.019232
Shadwick, R. E., Russell, A. P., & Lauff, R. F. (1992). The structure and mechanical design of rhinoceros dermal armour. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 337(1282), 419-428. doi:10.1098/rstb.1992.0118
Sherbrooke, W. C., Scardino, A. J., de Nys, R., & Schwarzkopf, L. (2007). Functional morphology of scale hinges used to transport water: convergent drinking adaptations in desert lizards (Moloch horridus and Phrynosoma cornutum). Zoomorphology, 126(2), 89-102. doi:10.1007/s00435-007-0031-7
Sjøland, J. A. (2007). Inflammation and fibrin structure in patients with end-stage renal disease. (PhD). University of Southern Denmark
Tracy, C. R., Laurence, N., & Christian, K. A. (2011). Condensation onto the Skin as a Means for Water Gain by Tree Frogs in Tropical Australia. The American Naturalist, 178(4), 553-558. doi:10.1086/661908
van Erp, R., Soleimanzadeh, R., Nela, L., Kampitsis, G., & Matioli, E. (2020). Co-designing electronics with microfluidics for more sustainable cooling. Nature, 585(7824), 211-216. doi:10.1038/s41586-020-2666-1
van Kempen, T. H. S., Bogaerds, A. C. B., Peters, G. W. M., & van de Vosse, F. N. (2014). A constitutive model for a maturing fibrin network. Biophysical Journal, 107(2), 504-513. doi:10.1016/j.bpj.2014.05.035
Wei, T. (2020). All-in-one design integrates microfluidic cooling into electronic chips. Retrieved from https://www.nature.com/articles/d41586-020-02503-1
Yokoyama, H., Kudo, N., Todate, M., Shimada, Y., Suzuki, M., & Tamura, K. (2018). Skin regeneration of amphibians: A novel model for skin regeneration as adults. Development, Growth & Differentiation, 60(6), 316-325. doi:https://doi.org/10.1111/dgd.12544