Molecular and Histological Structures of Generic Mammalian Hair and Fur
Marc Amin, Lovéni Hanumunthadu, Matthew MacDonald, Thibaud Roy
Introduction
Keratin structures are found throughout the animal world and can be found in diverse forms with varying functions. Hair has evolved to fit the needs of animals in their respective environments and thus has resulted in the range of keratin structures that can be seen in nature, from porcupine quills to polar bear fur. Looking at the molecular level, as well as histological analysis, the properties of keratin structures and their role in animal function become clearer.
Keratin structures in a molecular context allow us to analyze how functions of the hair arise at multiple structures: starting at the elemental level of hair shafts, then onto the primary amino acid level, then onto the secondary 𝛼-helix or β-pleated sheets, then the tertiary infoldings of the aforementioned structures, and ending with quaternary structure protein complexes (Wang et al., 2016). Looking more in-depth at the primary level, keratin distinguishes itself due to extensive intermolecular cysteine cross-linking, particularly disulfide covalent crosslinks, that contribute to important properties such as improved stability, viscoelasticity, and chemical reactivity of keratin (Hinton, 1974; Menefee, 1977). Finally, the epicuticle, composed of the protein matrix and the covalently bound lipid 18-methyleicosanoic acid, contributes to hydrophobicity of hair, important for various functions (Yang et al., 2014).
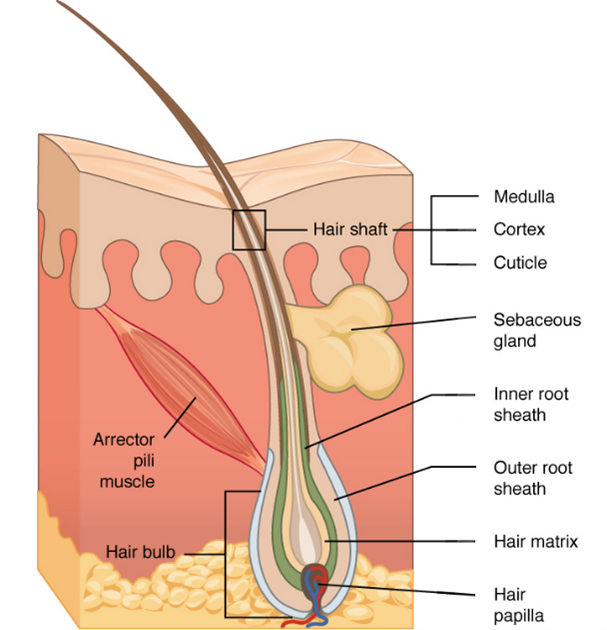
Taking a histological approach, hair can be analyzed and how it contributes to the molecular properties. At the macro level, hair can be broken down into two parts: the hair follicle, which is under the epidermis, and the hair shaft which can be found both in and out of the epidermis (Hoover et al., 2020). Under the hair follicle level, more can be gleaned about properties of the hair such as the role of the sebaceous gland, which provides the necessary sebum for hydrophobicity (Martel et al., 2020). The last histological approach in this paper is the hair growth cycle, which is crucial as it allows for renewal of the hair organ, providing mammals with the ability to protect, dry, and insulate themselves.
Molecular Structure
Keratinous proteins, like all other proteins, are composed of amino acids. Keratin is found in two different forms, namely 𝛼- (alpha) and β- (beta) keratins. Mammalian keratinous structures are most composed of 𝛼-keratin, whereas β-keratin is found in reptilian and avian species (Wang et al., 2016). The pangolin, a mammal who hosts a plethora of other peculiarities, has both 𝛼- and β-keratin structures (Fraser and Parry, 2018). These two forms, while both considered keratinous, vary in molecular structure and hierarchical combination. The hierarchical nature of keratin is easily seen in its use of polypeptides that are continuously joined, folded, and coiled until a final structure emerges (Fig. 1).
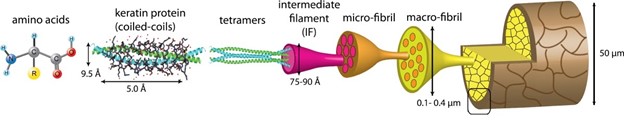
Keratins are composed of a combination of carbon, nitrogen, oxygen, hydrogen, phosphorus, and sulfur (Fig. 2). Amino acids are composed of these elements (Murr, 2015). Though up to 20 amino acids can appear in various keratin proteins, the most commonly occurring in keratin structures are glycine and alanine (Murr, 2015). Another important amino acid in keratin structure is cysteine, which uses the molecular properties of sulfur to form disulfide bridges.
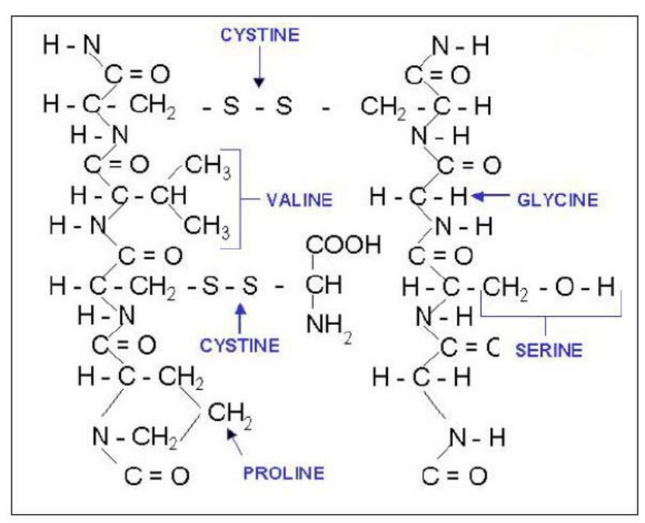
The polypeptides formed in keratin structures are examples of a primary structure characteristic (Wang et al., 2016). Both 𝛼- and β-keratins form polypeptide chains with three main regions. These three regions can be separated into a carbon terminal, a nitrogen terminal, and a central domain (Murr, 2015). The distinction between these domains is important for the bonding properties that will be further analyzed in secondary and tertiary structure. Ultimately, the shape of the keratin structure will be determined by the order in which the amino acids are arranged in the polypeptide.
The interactions between amino acids determine the secondary structure of keratin (Wang et al., 2016). It is in the secondary structure that 𝛼- and β-keratins begin to significantly differ. Alpha-keratin is organized in coils, whereas beta-keratin is organized in pleated sheets. The polypeptide chains in 𝛼-keratin coil to form an alpha-helical coil. The driving force behind this structural feature is hydrogen bonding.
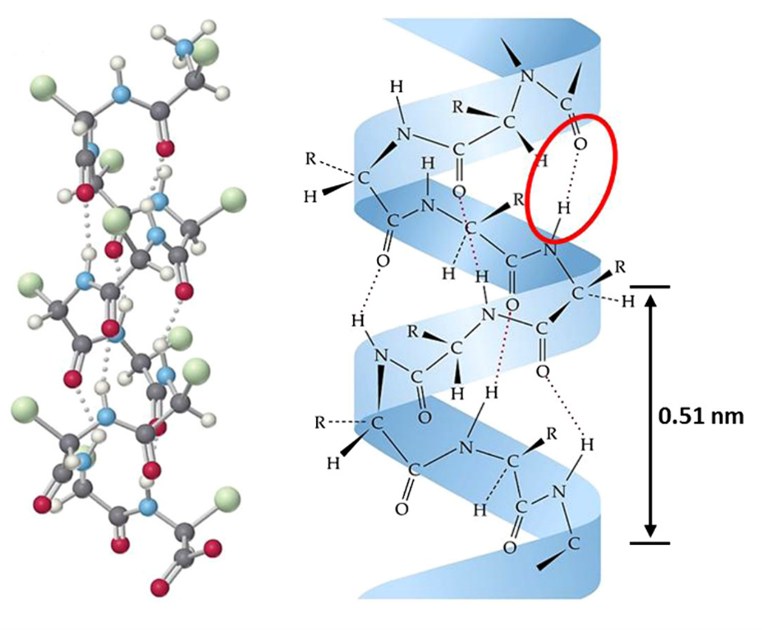
As illustrated in Fig. 3, the carbonyl oxygen of one residue (amino acid) and the amide group of another residue form a hydrogen bond (Wang et al., 2016). In alpha-helical arrangement, there are hydrogen bonds every 4 amino acids. This sequence of hydrogen bonding results in 3.6 residues per full rotation in the coil (Murr, 2015). Maximum stability is achieved by this placement of residues, as it allows for the highest hydrogen bond strength between carbonyl and amide groups. Alpha-helix structures are right-handed, which indicates that if the helix were to be screwed clockwise then it would move away from the observer. Side chains point outwards from the coil in alpha-helical formation, and hydrogen bonds are protected, meaning harder to disrupt, by being closer to the helical axis (Murr, 2015).
Similarly, beta keratins primary structure is determined by hydrogen bonding. The beta-keratin pleated sheet structure arises when two polypeptide chains are arranged in a row either parallel or antiparallel to each other (Fraser and Parry, 2018). In the parallel arrangement, the carbon and nitrogen terminals are on the same ends for both chains. This results in the hydrogen bonding carbonyl and amide groups to form an angled bond between the two chains. In antiparallel arrangement, the carbon and nitrogen terminals are on the opposite ends for the two chains (Fraser and Parry, 2018).
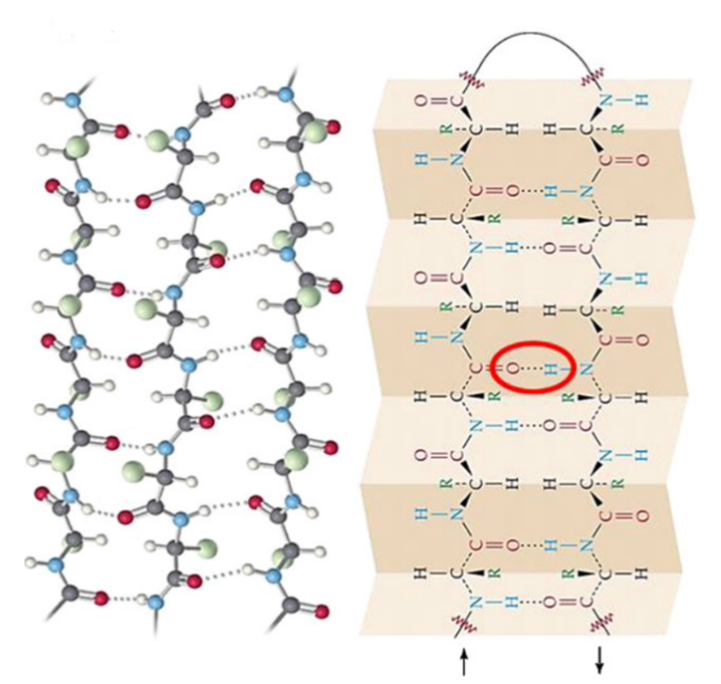
As shown in Fig. 4, this results in the hydrogen bonding groups being able to form a straight, shorter bond. Anti-parallel structure is more stable than parallel, as the bond length is shorter between carbonyl and amide groups in the chain. Sheets can include both parallel and antiparallel sections, however due to the relative instability of parallel structure it is uncommon (Wang et al., 2016). Another part of the secondary structure of β-keratin is the pleated sheet formation. The pleated shape, as shown in Fig. 4, is a result of the tetrahedral geometry of the central atoms. With a bond angle of approximately 109.5°, a slight change in direction of each bond results in the pleated pattern (Wang et al., 2016).
For alpha helices, their smallest unit (aside from amino acids themselves) is the alpha-helical chain. The hierarchical nature of keratin is easily seen in 𝛼-keratin.

As seen in Fig. 5, two 𝛼-helix chains will twist around each other in parallel to form a dimer. Both 𝛼-helix chains are right handed but will form a left-handed coil. Disulfide bonds hold the dimer together (Wang et al., 2016). The dimers will then assemble end to end and side by side to form protofilament. Like dimers, protofilament is stabilized laterally by disulfide bonds. Two protofilaments are again assembled side to side to form protofibril. Four protofibrils will arrange to form a circular or helical intermediate filament (Wang et al., 2016).
Further analysis can be done on tertiary and quaternary structure, but this is better explained in later sections. Cross-linking using disulfide bridges is integral to the structural characteristics of keratin (Fraser and Parry, 2018). It is also important to note that in some keratin molecules there are not only alpha- and beta-keratin, but rather the long strands that switch often (Wang et al., 2016). The alpha or beta structure is determined by the sequence of amino acids in the polypeptide.
Hydrophobicity
When observing the molecular level, much can be learned of the hydrophobic properties of hair. These hydrophobic properties contribute to evolutionary functions such as insulation or protection for the animal. In hair and fur, the hydrophobic properties do not stem from the protein matrix of the hair shaft but rather from a lipid-rich barrier (Wang et al., 2016).
As discussed in later sections, it is the outer layer of the hair shaft, as well as the lipid-rich sebum produced by the sebaceous gland, that contribute to the hydrophobic properties of hair (Makrantonaki et al., 2011). This outer layer is known as the epicuticle and is formed from dead cells, overlapping in layers, which form scales that protect the hair shaft. The cuticle is a hair's outermost layer, made up of 5–10 overlapping keratinized cells, which cover each other like roof tiles and form a protective layer against damage to the hair fiber from environmental and mechanical forces (Makrantonaki et al., 2011).
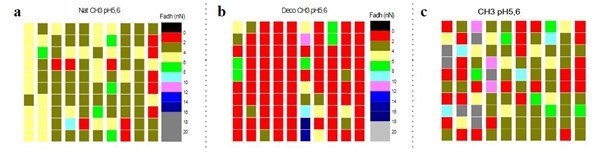
As seen in Fig. 6, the data shows that hair is globally a hydrophobic surface (90 %), but it also contains weak hydrophilic zones localized in the edge of the hair shaft, where proteins are more exposed. This corresponds to a decrease in the amount of bound 18-methyleicosanoic acid (Wang et al., 2016).
The epicuticle consists of approximately three quarters protein and one quarter lipid. As discussed above, the hair shaft is made up of alpha-keratin. Of the amino acids in alpha-keratin, there is a high content of glutamic acid and cystine, both representing roughly 12% of the amino acids in the alpha-keratin complex. These amino acids are rich in disulfide bonds and peptide bonds, as well as COOH and NH2 chemical groups. The protein matrix, in addition to the amino acids, contains 18-methyleicosanoic acid covalently bound to the protein by means of cysteine residues (Wang et al., 2016).
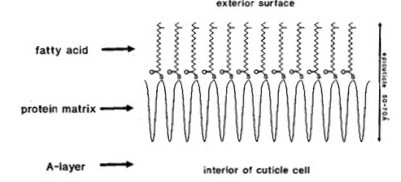
The lipid, 18-methyl eicosanoic acid, covalently attached to the surface confers to the hair hydrophobic character, due to its long CH tail (Yang et al., 2014).
Cross Linking
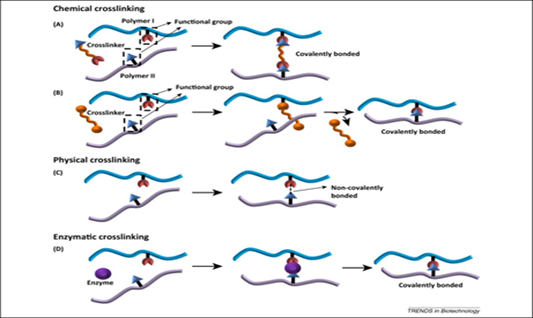
Similarly, to collagen and elastin, keratin is a structural protein that is distinguished by the presence of extensive intermolecular cysteine crosslinking (Menefee, 1977). The structure of keratin is stabilized by chemical — two or more molecules are chemically joined by covalent bonds (“Overview of Crosslinking and Protein Modification” | ThermoFisher Scientific, n.d.), — physical — two or more molecules are physically joined by a hydrogen bond or an ionic interaction. It can be reversed using specific stimuli such as cooling and heating (Oyama, 2014) — and enzymatic — two or more molecules are joined using an enzyme. It can be observed during blood coagulation when red blood cells clot to heal a wound (Levett, 2017) — crosslinks (Reddy et al., 2015) (Fig. 8). Although there are other types of crosslinks, disulfide covalent crosslinks are the most abundant and significant in defining keratin (Hinton, 1974; Menefee, 1977). These high levels of crosslinking are essential for higher mechanical properties, improved stability, viscoelasticity, and chemical reactivity of keratin (Menefee, 1977; Reddy et al., 2015).
Keratin fibers can undergo significant elongation of up to 100 % in water, and still exhibit full recovery, if the extension is done under conditions that minimize chemical crosslink scission (Menefee, 1977). For instance, wool usually has a large extensibility, with a breaking strain of more than 40 % (Yu et al., 2017). This behavior allows for the excellent wrinkle recovery properties of wool fabrics (Menefee, 1977). On the other hand, if the disulfide cross links are chemically treated to allow breakage under strain and then reformation, the fiber will be set into its new arrangement (Asquith and Puri, 1970; Menefee, 1977). The kinetics of setting and long-term relaxation in keratins are governed largely by sulfhydryl-disulfide interchange (Asquith and Puri, 1970; Menefee, 1977). In this process, when strain is applied to a fiber in an appropriate aqueous environment, sulfhydryl groups react with adjacent disulfide bonds, forming new disulfide bridges and sulfhydryl groups, but under less strain (Menefee, 1977). To further stabilize the keratin structure, additional cross links can also be added by heating, radiation. or treatment with formaldehyde or other reagents (Menefee, 1977).
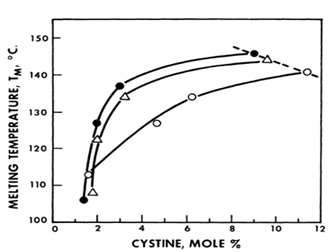
The importance of disulfide crosslinking can also be demonstrated by the heat denaturation of keratin. When subject to increasing temperature, the alpha helical structure of keratin remains intact until it reaches its melting temperature whereby the helical configuration is disrupted. The melting temperature of hair/fur is determined by the amount of cystine present.
As shown in Fig. 9, a low cystine content brings about a low helix disordering temperature while increasing the amount of cystine increases the melting temperature (Menefee, 1977).
The degree of crosslink density can affect the hydrolysis rate of peptide bonds in keratin. Aside from dissolution by crosslink scission, it is possible to hydrolyze peptide linkages completely or partially to obtain a set of keratin oligopeptides or, if hydrolysis proceeds long enough, simple amino acids (Menefee, 1977). While partial acid hydrolysis can be commonly carried out using HCl, hydrolysis can also be done enzymatically, often with more specific peptide cleavage (Menefee, 1977). To have an insight on this effect, a simple model consisting of keratin as a randomly cross-linked network of monodisperse protein chains (each containing U amino acid residues) with random peptide hydrolysis is considered (Menefee, 1977). It is assumed that a fraction Q of the total number of residues is randomly crosslinked, and that peptide bond cleavage is random (Menefee, 1977). Eq. 1:
S = {2PU\over (QU)^2 }(1)
can be approximated where S is the fraction of keratin that becomes soluble, and P is the fraction of peptide bonds (Eq. 2; Menefee, 1977):
P = 1\space –\space e^{-kt}(2)
Substituting P into the Eq. 1, and then differentiating with respect to time t, the initial hydrolysis rate (t = 0) is obtained as Eq. 3 (Menefee, 1977):
{dS \over dt}= {2k\over Q^2 U}(3)
It is hence concluded that the initial hydrolysis rate is inversely proportional to the square of the crosslink density (Menefee, 1977). An increase in crosslinking thus decreases the easiness of hydrolysis of peptide bonds in the molecular structure of keratin.
Histological Structure
Zooming out, away from the molecular structure of hair, we can start exploring its histological structure as well. The histological structure of hair – known as the microscopic anatomy – can be grouped into two main structures: the hair follicle, which lies under the epidermis, and the hair shaft, which one part is in the epidermis and the other part lies in outside the skin (Hoover et al., 2020). The following diagram examines the hair's follicle and shaft cross-sections, labeling the major components.
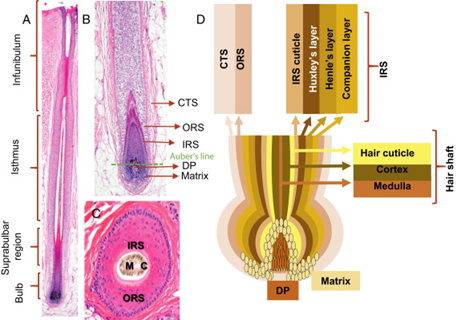
Starting with the primary structure responsible for growth, the hair follicle is divided into two components: the inner and outer root sheaths (Cooper, 2015).
First, the inner root sheath (Fig. 11c) is responsible for binding the growing hair shaft to the follicle, and it consists of four layers (refer to Fig. 10), the most important among them is the cuticle. The cuticle is made from flat cells that enclose the hair shaft while it is in the epidermis, allowing it to come straight out outside the skin (Hoover et al., 2020). Second, the outer root sheath (Fig. 11d), which is an extension of the epidermis, is the second layer of protection and a ready supply of self-renewing stem cells. These stem cells are then differentiated into several useful cell types such as melanin-producing melanocytes and keratin-producing keratinocytes (“Hair” | Biology for Majors II | Lumen Waymaker, n.d.; Hoover et al., 2020). These two cell types are crucial for the pigmentation of the hair and the structural density of the hair, respectively.
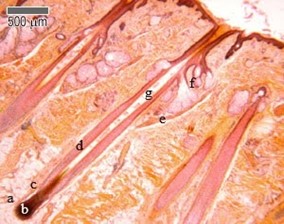
A third follicular structure, mostly engulfed as part of the inner root sheath, is the hair bulb (Fig. 11a), a region of the follicle that produces the hair coming out of the shaft. For most of its life, the hair bulb resides in the dermis and surrounds the dermal papilla (refer to Fig. 11), which consists of nerve fibers and capillaries. Through the papilla, the growing hair can receive nutrients, making it the main contributor to its growth. Growth factors including keratinocyte growth factors, stem cell factors, insulin-like growth factors, and bone morphogenetic protein are all growth factors that the papilla delivers to the hair – more on growth in later sections (Hoover et al., 2020). The last section of the hair bulb is the hair matrix (Fig. 11b), which is a layer of reproducing basal cells that allow the hair shaft (Fig. 11g) to grow (“Hair” | Biology for Majors II | Lumen Waymaker, n.d.).
Two additional structures that are connected to the hair follicle through the hair shaft are the sebaceous glands (Fig. 11f), which produce sebum oils that coat the hair shaft, and the arrector pili muscles (Fig. 11e), which make the hair erect (Hoover et al., 2020). While sebum oils are discussed below, the pili muscles are used to trap air, conserving heat (consult “Comparative Analysis of Biomechanical Properties of Mammalian Fur” by Hanumunthadu et al. (2020) for more information).
Hydrophobicity
The histological makeup of hair determines its hydrophobic properties. These hydrophobic properties provide invaluable function to animals as a way to prevent cracking of hair, provide insulation and warmth, camouflaging, sensory purposes, and prevent injury (Martel et al., 2020).
In the animal kingdom, the difference between oily guard hair and thick underfur is distinct, while for humans, hair is not so differentiated and might be described as a modified combination of the characteristics of guard hairs and fur hairs (Deedrick and Koch, 2011). The central part of the hair follicle is composed of the hair shaft, hair root, hair bulb, hair papilla, and apocrine sweat gland. Attached to this central part of the hair follicle is the sebaceous gland and arrector pili muscle (Martel et al., 2020).
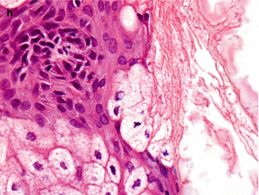
Taking a histological vantage point, as seen in Fig. 13, the sebaceous glands are most relevant to making hair hydrophobic. Sebaceous glands are holocrine glands closely associated with hair follicles and are particularly present on the face and head (Martel et al., 2020). These glands open onto the hair follicles so that, when stimulated by hormones, most notably androgens such as testosterone, sebaceous glands produce and secrete sebum. Sebum is composed of a group of complex oils including triglycerides and fatty acid breakdown products, wax esters, squalene, cholesterol esters, and cholesterol (Martel et al., 2020).
These glands are stimulated to varying degrees depending on temperature (Wang et al., 2016). The secretions emulsify the sweat produced by the eccrine glands and produces a layer of sweat that plays a vital role in delaying dehydration in hot conditions. In colder settings, the sebum becomes more lipid and serves as a coat that can effectively repel moisture (Makrantonaki et al., 2011).
Growth Cycle
As all living things, hair grows, regresses, and eventually dies. Growth is a crucial element for any living organ because it provides adaptation to increasing body sizes, and it allows for cell renewal (Cole, 2010). Each hair follicle in the skin goes through a growth cycle, which is completely asynchronous for each follicle, except for when hair follicles enter the molting stage, more on that later. Asynchronous growth allows each hair on a mammal's body to have a unique growth pattern and cycle (Shitabata et al., 2009). Hence, hair follicle growth is important because it provides constant hair renewal in the epidermis, allowing multiple mammals to insulate, protect, dry, etc. themselves.
The hair growth cycle can be separated into three phases: Anagen, Catagen and Telogen (Fig. 14).
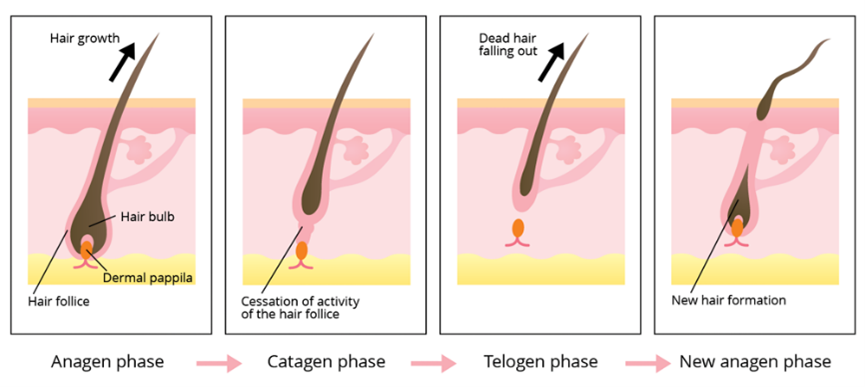
Anagen Phase
The anagen phase, also known as the growth phase, is the first out of the three stages of a hair follicle's life, marking the production of new hair out of stem cells. During this phase, stem cells in the root of the hair follicle divide rapidly, allowing the hair shaft to push up and out the epidermis (“Hair” | Biology for Majors II | Lumen Waymaker, n.d.). This process is possible due to the presence of keratinocytes, which migrate from the root, flatten, and die. These layers of dead cells form keratinized cells, which are composed entirely of keratin (Hoover et al., 2020). This phase is mostly characterized by cell growth and terminal differentiation, which makes hair growth a one-way process. Once the stem cells are differentiated, they remain there with no more reproduction, and, eventually, the build-up of now specialized cells allows the hair shaft to stretch out the epidermis (Peus and Pittelkow, 1996). Depending on the mammalian species, a hair follicle could spend from 2 to 7 years in the anagen phase, which is equivalent to approximately 90 % of its life cycle (Shitabata et al., 2009; “Hair” | Biology for Majors II | Lumen Waymaker, n.d.). The relative length of the anagen phase clearly explains why some species have longer hair follicles compared to others.
Due to hair growth being a cycle, the initiation of the anagen phase is activated by the end of the telogen phase. Hence, the hair follicle may resemble that of its predecessor, but the secondary hair germ adopts a triangular shape and begins extending from there, wrapping itself around the dermal papilla (Hirt and Paus, 2019). In the early stages of anagen, cell transcription begins in the papilla cells and in the secondary hair germ. At this stage of anagen, the papilla delivers growth factors that activate and inhibit certain cells and functions in the hair matrix (Cooper, 2015). Subsequently, the secondary hair germ goes through a phenomenon causing it to thicken and elongate, thus creating a new unpigmented hair matrix. Halfway, the hair matrix thickens to 4 to 5 cell layers, depending on the mammalian species, and encompasses over 60 % of the dermal papilla (Hirt and Paus, 2019). During this stage, the hair follicle creates the inner root sheath and the hair shaft, therefore enabling it to push the hair bulb out into the adipose layer of the skin. The hair shaft reaches maturity once the medulla, cortex, and cuticle are visible. Nearing the end of the stages of anagen, the hair bulb elongates and extends more into the adipose layer, allowing a fraction of the hair shaft to enter the hair canal. During the final stage, the hair shaft can be seen with the naked eye above the skin, where it continues growing for years to come (Hirt and Paus, 2019).
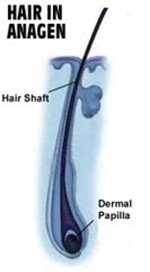
Catagen Phase
The catagen phase, known as the involution phase, marks the transition period between growth and death, when the follicular keratinocytes perform complete apoptosis and their regression starts (Shitabata et al., 2009; “Hair” | Biology for Majors II | Lumen Waymaker, n.d.). This transition period spans about 2-3 weeks and is characterized by a preparation of the hair follicle's temporary death.
The initiation of the catagen phase begins with the detachment of the hair bulb from the dermal papilla. In the early stages of catagen, the hair matrix and the dermal papilla undergo a considerable decrease in size, and in the midst of it, the lower follicle gradually shrinks, retreating an epithelial strand. Hence, the lingering hair matrix partly wraps itself around the dermal papilla, while only having a thickness of one to two cell layers (Hirt and Paus, 2019). The club hair, defined as a bulb detached from the papilla, becomes conspicuous and is located over the adipose layer of the skin. In the final stage of catagen, the hair matrix completely regresses, fundamentally disappearing. Additionally, the dermal papilla becomes compressed and adopts a ball-like shape. The end of catagen is initiated with apoptotic cells being located in the sebaceous gland. All in all, catagen must get rid of old hair shafts and make sure that a new follicle is able to form using the stem cells left behind by apoptosis (Hirt and Paus, 2019).
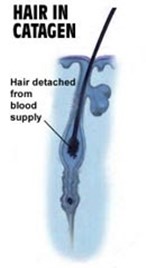
Telogen Phase
The telogen phase, known as resting and shedding phase, is the last stage of the hair follicle's current life when it stops growth and rests. This phase can last from 2 to 4 months, and it is mostly characterized by cell quiescence. During this period, the hair follicle is detached and ready to fall off. (Shitabata et al., 2009; “Hair” | Biology for Majors II | Lumen Waymaker, n.d.)
The telogen stage begins when the hair shaft adopts its mature phase – known as a club hair – and breaks away from the follicle. Although telogen is often perceived as a stage of low activity, even characterised as passive, the hair follicle is exceedingly active (Hirt and Paus, 2019). The dermal papilla rests beneath the secondary hair germ and the bulge, both of which are main epithelial stem cells of the new hair follicle. With telogen and catagen follicles sharing many similar characteristics, clinical examination methods are unable to reliably discern between late catagen and telogen hair follicles (Hirt and Paus, 2019).
Molting Stage
As an additional stage, rarely happening during the hair growth cycle, we have the molting stage. Hair follicle molting can be seen as the final frontier of a hair follicle growth cycle, and it occurs to almost all mammalian species (Ling, 1970). Molting, also known as shedding, occurs as a synchronous stage where all hair follicles of the pelage fall off due to various environmental and hormonal changes. In most mammals, the change of seasons can cause molting, which can be seen in families like the canidae (dogs) and the felidae (cats). Additional factors, like light exposure and nutrition, can also have effects on the occurrence of molting (Ling, 1970).
Conclusion
As observed through analysis, the histological and molecular properties of hair shed light on its function. From specific bond angles to sebaceous glands, every individual member of the system contributes to its overall function. Hair, more particularly 𝛼-keratin, has a hierarchical structure that leads to the formation of a versatile molecule used for varied purposes across many species. Disulfide cross-linking is integral to the structure and function of hair, and through its analysis, properties like extensibility and heat vulnerability can be determined. It is important to note the asynchronous nature of hair growth, and how each follicle follows its own cycle of growth yet contributes to a large cover of hair or fur. Both histological and molecular properties play the role in determining the hydrophobicity of hair; the lipid coating provided by the sebaceous glands is effective in ensuring hydrophobicity of the hair, but the long carbon chain of the epicuticle also provides some hydrophobic character. Further research should be done to recognize any patterns present in hair growth and keratin formation.
References
Asquith, R. S., & Puri, A. K. (1970). The Formation of Mixed Disulfides by the Action of Thioglycollic Acid on Wool—Cystine and its Relationship to Wool Setting. Textile Research Journal, 40(3), 273-280. doi:10.1177/004051757004000311
Bogduk, N. (2005). Clinical Anatomy of the Lumbar Spine and Sacrum: Elsevier/Churchill Livingstone. Retrieved from https://books.google.ca/books?id=UYC_NpoFfAsC
Clinic, M. (n.d.). Phases of hair growth. Retrieved from https://medikemosclinic.com/en/phases-of-hair-growth/
Cole, T. J. (2009). Growth and Organ Development. In G. Goldberg, A. Prentice, A. Prentice, S. Filteau, & K. Simondon (Eds.), Breast-Feeding: Early Influences on Later Health (pp. 1-13). Dordrecht: Springer Netherlands. doi: https://doi.org/10.1007/978-1-4020-8749-3_1
Cooper, G. A. A. (2015). Chapter 1 – Anatomy and Physiology of Hair, and Principles for its Collection. In P. Kintz, A. Salomone, & M. Vincenti (Eds.), Hair Analysis in Clinical and Forensic Toxicology (pp. 1-22). Boston: Academic Press. Retrieved from https://www.sciencedirect.com/science/article/pii/B9780128017005000017
Deedrick, D. W., & Koch, S. L. (2004). Microscopy of Hair Part II: A Practical Guide and Manual for Animal Hairs. Forensic Science Communications, 6(3). Retrieved from https://archives.fbi.gov/archives/about-us/lab/forensic-science-communications/fsc/july2004/research/2004_03_research02.htm
Ebert, G., & Müller, F. H. (1966). Differentialcalorimetrische Untersuchungen über den Einfluß von Cystin- und Thioätherbindungen auf Umlagerungsvorgänge in Keratinfasern. Kolloid-Zeitschrift und Zeitschrift für Polymere, 214(1), 38-45. doi:10.1007/BF01553111
Fraser, R. D. B., & Parry, D. A. D. (2018). Structural Hierarchy of Trichocyte Keratin Intermediate Filaments. In J. E. Plowman, D. P. Harland, & S. Deb-Choudhury (Eds.), The Hair Fibre: Proteins, Structure and Development (pp. 57-70). Singapore: Springer Singapore. doi:https://doi.org/10.1007/978-981-10-8195-8_6
Hanumunthadu, L., MacDonald, M., Amin, M., & Roy, T. (2020). Comparative Analysis of Biomechanical Properties of Mammalian Fur. Bioengineering. McGill University. https://bioengineering.hyperbook.mcgill.ca/comparative-analysis-of-biomechanical-properties-of-mammalian-fur-hair-fur-1/
Hinton, E. H. (1974). A Survey and Critique of the Literature on Crosslinking Agents and Mechanisms as Related to Wool Keratin. Textile Research Journal, 44(4), 233-292. doi:10.1177/004051757404400401
Hirt, P. A., & Paus, R. (2019). Chapter 1 – Healthy Hair (Anatomy, Biology, Morphogenesis, Cycling, and Function). In M. Miteva (Ed.), Alopecia (pp. 1-22): Elsevier. Retrieved from https://www.sciencedirect.com/science/article/pii/B9780323548250000016
Hoover, E., Alhaj, M., & Flores, J. L. (2020). Physiology, Hair. In StatPearls [Internet]. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK499948/
Levett, P. (2017, 15 Jul 2017). Enzymatic crosslinking. Retrieved from http://additivebiomanufacturing.org/blog-enzymatic-crosslinking/
Ling, J. K. (1970). Pelage and Molting in Wild Mammals with Special Reference to Aquatic Forms. The Quarterly Review of Biology, 45(1), 16-54. doi:10.1086/406361
Makrantonaki, E., Ganceviciene, R., & Zouboulis, C. C. (2011). An update on the role of the sebaceous gland in the pathogenesis of acne. Dermato-Endocrinology, 3(1), 41-49. doi:10.4161/derm.3.1.13900
Martel, J. L., Miao, J. H., & Badri, T. (2020). Anatomy, Hair Follicle. In StatPearls [Internet]. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK470321/
Menefee, E. (1977). Physical and chemical consequences of keratin crosslinking, with application to the determination of crosslink density. Adv Exp Med Biol, 86a, 307-327. doi:10.1007/978-1-4684-3282-4_19
Oyama, T. (2021). Cross-Linked Polymer Synthesis. In S. Kobayashi & K. Müllen (Eds.), Encyclopedia of Polymeric Nanomaterials (pp. 1-11). Berlin, Heidelberg: Springer Berlin Heidelberg. doi: https://doi.org/10.1007/978-3-642-36199-9_181-1
Peckham, M., Knibbs, A., & Paxton, S. (n.d.). Hair. Retrieved from https://www.histology.leeds.ac.uk/skin/hair.php
Peus, D., & Pittelkow, M. R. (1996). GROWTH FACTORS IN HAIR ORGAN DEVELOPMENT AND THE HAIR GROWTH CYCLE. Dermatologic Clinics, 14(4), 559-572. doi:10.1016/S0733-8635(05)70384-3
Reddy, N., Reddy, R., & Jiang, Q. (2015). Crosslinking biopolymers for biomedical applications. Trends in Biotechnology, 33(6), 362-369. doi:10.1016/j.tibtech.2015.03.008
Requena, L., & Sangüeza, O. (2017). Embryology, Anatomy, Histology, and Physiology of the Sebaceous Glands. In Cutaneous Adnexal Neoplasms (pp. 755-764). Cham: Springer International Publishing. doi: https://doi.org/10.1007/978-3-319-45704-8_58
Scientific, T. (n.d.). Overview of Crosslinking and Protein Modification. Retrieved from https://www.thermofisher.com/ca/en/home/life-science/protein-biology/protein-biology-learning-center/protein-biology-resource-library/pierce-protein-methods/overview-crosslinking-protein-modification.html
Shitabata, P. K., Tan, B., DeRienzo, D., Fitzgibbon, J., Sung, K., & Yue, C. (2009). Alopecias. In. Dermatopathology Institute in California, Torrance. Retrieved from http://www.dermpathmd.com/Clinical%20Dermatology/Alopecia.pdf
Waymaker, L. (n.d.). Hair. In. courses.lumenlearning.com. Retrieved from https://courses.lumenlearning.com/wm-biology2/chapter/hair/
Yang, F.-C., Zhang, Y., & Rheinstädter, M. C. (2014). The structure of people's hair. PeerJ, 2, e619. doi:10.7717/peerj.619
Yazdan, A. (2014). Hair Growth Cycle and Why it's Important. Retrieved from https://modenahair.com/blog/hair-growth-cycle/
Yu, Y., Yang, W., & André Meyers, M. (2017). Viscoelastic properties of α-keratin fibers in hair. Acta Biomaterialia, 64, 15-28. doi:10.1016/j.actbio.2017.09.012