The Heart: A Mechanical Perspective
Anne-Sarah Dickman, Lina El Kesti, Junqi Wang, Angela Zhu
Function of the Heart from a Mechanical Perspective
The heart, present in almost all fauna, sometimes more than once in an organism (e.g., octopi possess three hearts) is connected to the rest of the body through a complex circulatory system which allows it to pump blood across the whole body in a coordinated, primarily automated manner, by contracting and relaxing. We can distinguish three types of muscles: skeletal muscles, which are attached to the skeleton and that living species can contract deliberately; smooth muscles, which contract because of visceral, local and hormonal reflexes; and cardiac, the muscles found in the heart, that contract involuntarily in a rhythmical manner as a consequence of automatic membrane potential accrual and discharge, driven by the pace-maker cells (sinoatrial node) (Starkebaum, 2019).
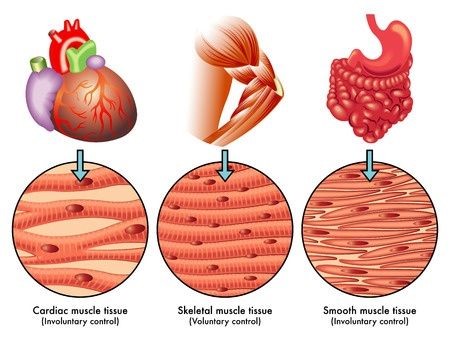
The main function of the heart is to transport blood to the whole body. So, let us look at the function of the heart in more depth, particularly from a mechanical perspective.
The Pump in Engineering
To better understand the role of the heart in a living organism, we can assimilate it to a well-conceived mechanical component used in many domains, notably engineering: the pump. Mechanical pumps are used in our day to day lives, serving many purposes thanks to their versatility (e.g., filtering of pools and aquariums, oil and gas industry, air conditioning and ventilation); pumps also play a major role inside all living organisms. It is important to note that nowadays, biomimicry is being used in to improve mechanical pumps and devise new kinds of chemical and biomechanical pumps (“Pump” | Wikipedia, n.d.).
A pump, in the context of a machine and mechanical tool, is a device that transmits energy to anything that flows through it by ascertaining flow in a particular direction, against the naturally occurring flow direction. By conveying a certain amount of energy, the pump can move liquid and gaseous substances, or fluids more generally speaking, without producing any pressure. The pump follows a predetermined mechanism and consumes energy in order to fulfill its function (“The Basic Working Principle of a Hydraulic (Fluid) Pump & the Concept and Construction” | Bright Hub Engineering, 2009). Therefore, by carrying out some mechanical work, it can propel the fluids to a desired location.
The Heart as a Pump
In the cardiovascular system, the heart fulfills this role by moving blood from an area of low pressure to one of high pressure. Indeed, without it, there would be equilibrium: the pressure will equalize throughout the system and there will be no blood circulation (Downey and Heusch, “CHAPTER 4 – Control of Cardiac Output”). This discrepancy in pressure between veins and arteries is due to their differing capacitance which is defined as the ratio of volume to pressure (Downey and Heusch, “CHAPTER 4 – Control of Cardiac Output”). Indeed, when blood is added to veins, their volume changes significantly while the pressure does not. On the other hand, when blood is added to arteries, the volume changes minimally while the pressure changes considerably (Downey and Heusch, “CHAPTER 4 – Control of Cardiac Output”). This is illustrated in the figure below with the width representing volume and height as pressure.
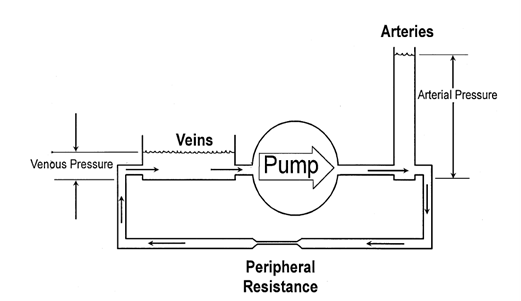
As shown, blood must go from a place of low pressure to that of high pressure which would not be possible without a pump. Pumping blood into a low capacitance vessel which has high pressure results in blood displacement as the vessel does not expand to accommodate the additional blood. Furthermore, because of the disparity in capacitance, an increase in arterial pressure will only result in a minimal increase in venous pressure, thereby maintaining the pressure difference (Downey and Heusch, “CHAPTER 4 – Control of Cardiac Output”).
Moreover, since the system is a closed loop, the total amount of blood will not change. What varies is the distribution of blood in the veins and arteries. This characteristic allows the heart to be self-regulating (Downey and Heusch, “CHAPTER 4 – Control of Cardiac Output”). Indeed, if cardiac output increases too much, venous pressure will decrease since most of the blood would be in the arteries. This will in turn decrease cardiac output thus re-establishing balance since, as will be mentioned later, “venous filling pressure [is] a primary determinant of cardiac output” due to the high compliance of the relaxed heart muscles (Downey and Heusch; “CHAPTER 4 -Control of Cardiac Output”, “CHAPTER 1 – Sequence of Cardiac Activation” p. 12).
Function of the Heart: Transport
The function of the heart is transportation. This organ assures the reliable transmission of blood across the whole body and oversees blood flow, making sure that it flows in a single direction (unidirectional flow of blood).
The heart twists during embryological development and has evolved due to the thickening of the proto-blood vessel. We can see a clear contrast between the prototypic vertebrate cardiovascular system and heart, and the many different heart structures and phenotypes in invertebrates, which have appeared mostly due to selective pressures (Monahan‐Earley et al., 2013). During embryonic development, the heart twists and matures through a process called cardiac looping. This makes the heart chirally asymmetric. In addition, the formed curvatures and twists have dynamic and potential fluidic advantages (Kilner et al., 2000). In fact, the chiral asymmetry of the heart has been proven to help with minimizing the dissipation of energy and interaction between circulating blood streams (Adam, 2000).
One of the attributes of transport is power; the power and rate at which the heart transports blood across the body. Cardiac output is linked to the size of the animal. The larger and heavier the animal, the slower their heart rate and vice-versa; the smaller the animal, the faster their heart rate. Usually, heart size is proportional to animal size, however, that is not always the case. Let us take the example of a hummingbird. The size of a hummingbird heart is two times larger than that of birds having the same mass. In fact, when we consider birds, hummingbirds have the largest heart in proportion to their body. Anatomically, hummingbirds are very small (2-20 grams) and have little wings. For them to be able to fly and sustain flight for long durations of time, they need aerobic power which is dependent on heart size. A larger heart allows the hummingbird to pump more blood to the muscles it needs to fly (Woodward, 2017). A hummingbird heart beats about 500 times per minute at rest and can go up to 1 300 beats per minute during flight. It seems that hummingbirds have “pushed the possibilities of vertebrate design for aerobic performance close to the absolute limit” (Suarez, 1992). Furthermore, it is important to note that some restrictions in the design of vertebrate cardiovascular systems have led to a benchmark in size: birds and mammals have a minimum mass of 2 grams. Such small animals cannot have larger hearts as this might be disadvantageous and even bodily impossible (Suarez, 1992).
General and Pulmonary Circulation for Animals with Several Chambers
After the process evolution seen among animal hearts, the heart becomes 4-chambered (discussed later in the paper). Even though the heart is one single organ, it functions like two pumps: each one accounting for a pattern of circulation. The left and right ventricles work in a coordinated manner to send oxygen and nutrients to all living beings' muscles, heads and bodies by propelling blood across a complex circulatory circuit. Furthermore, blood flows differently, according to the circuit it is in, and follows two separate trajectories:
- On one hand, the right side of the heart receives deoxygenated blood from the veins and sends it to the lungs, which in turn releases the carbon dioxide we produce and replenishes the blood with oxygen. This first pump then sends oxygen-rich blood back to the heart (Healthwise Staff, “How the Heart Works”). This process is known as the pulmonary circulation.
- On the other hand, the left side recuperates this oxygenated blood from the right side and left ventricle is surrounded with much thicker tissue than the right one which allows it to have strong and fibrous walls, ultimately permitting it to act as a more powerful pump propels it through the blood vessels and arteries. Its function is to distribute the oxygen contained in the blood to the other organs inside the body (Healthwise Staff, “How the Heart Works”). This is called systemic circulation.
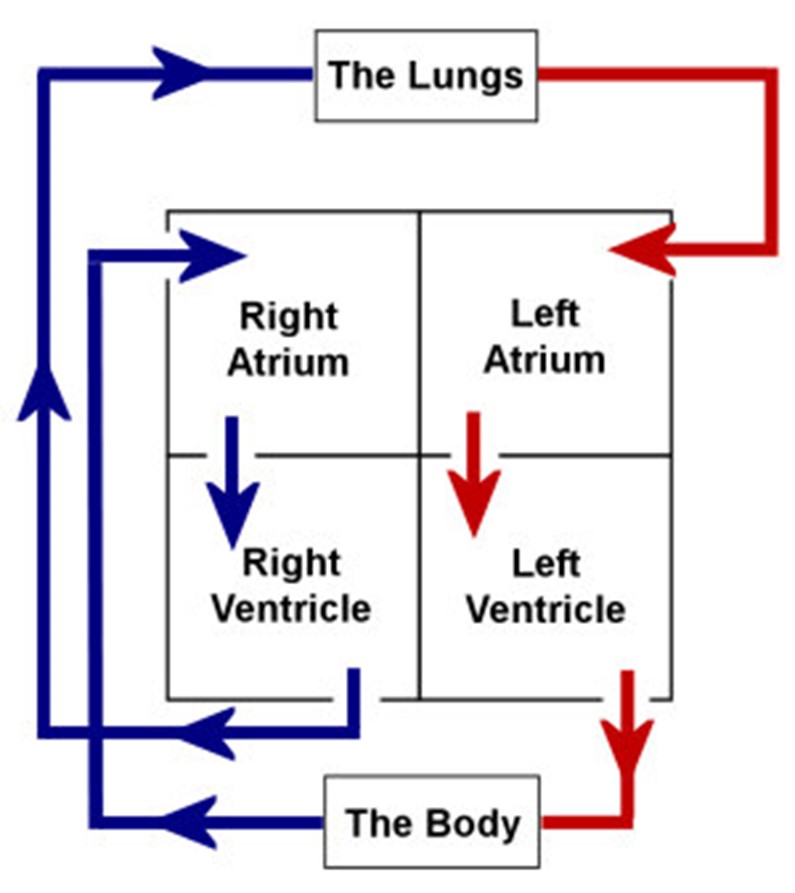
The right and left sides of the heart are both subdivided into two chambers: the upper chamber or atrium and lower chamber or ventricle. These four chambers receive blood that is entering the heart and aid with pumping it to the outside. The right ventricle propels blood to the lungs whereas the left one sends it throughout the whole body. This is due to the nature of both ventricles. In fact, the left ventricle is surrounded with much thicker tissue than the right one which allows it to have strong and fibrous walls, ultimately permitting it to act as a more powerful pump (“Human Body & Mind” | BBC Science & Nature, 2014).
How the Architecture of the Studied Organ is Aligned with the Required Function
The heart is shaped such that veins coming from the upper body enter the heart through the superior vena cava, which points up from the upper right of the heart, and veins coming from the lower body enter through the inferior vena cava, which points down, away from the upper right of the heart. These vessels are positioned in order to facilitate blood flow (“Flow through the heart” | khanacademymedicine | YouTube, 2012).
Compressions and Pressure
The vena cavae carry the blood at a low pressure and passively, therefore there is no need for a valve to control the entrance of the blood in the right atrium. When the heart muscles contract, the blood is pushed into the right ventricle through the tricuspid valve, composed of three flaps. This valve acts as a barrier between the atrium and the ventricle and allows the blood to only flow in the proper direction by preventing backflow (Laurenson, 2011). When the heart contracts a second time, this time in the ventricle, the pressure in the ventricle increases for the blood to pass through the pulmonary valve into the right and left pulmonary arteries that lead to the lungs. The oxygenated blood comes back into the heart from the pulmonary veins to the left atrium. When the left atrium contracts, the blood passes through the mitral valve, also known as the bicuspid valve, composed of two flaps, into the left ventricle. When the ventricle then contracts, the blood pressure rises inside it because as the volume decreases, the pressure increases in incompressible liquids like blood. When the blood pressure in the ventricle exceeds that of the blood in the aorta, the aortic valve is forced open, and the blood is dispersed throughout the body through arteries (Laurenson, 2011).
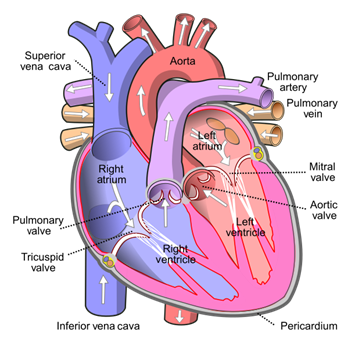
Valves
Valves are a crucial architectural structure that allow the heart to function in order. They are composed of two or three triangular flaps of tissue and are attached to the heart walls by myriad fibers. These flaps, also referred to as leaflets, are supported by chordae tendineae and papillary muscles, which hold the flaps still and prevent them from going backwards. The ring of fibrous tissue around the valve maintains its shape (“Heart Valves” | Cleveland Clinic, 2018). When the pressure behind them is higher than the pressure in front of them, they open. Once opened, the blood can flow upstream, but when the pressure ahead of them is greater, the flaps are pushed backwards and close the opening, preventing a backflow. When a chamber contracts, the pressure within the chamber increases, priming the next valve for maximum stroke volume.
The maximum stroke volume refers to the maximum volume of blood that can be pumped out of the left ventricle after a systolic contraction. There are three factors that can increase the stroke volume: contractility, preload and afterload. Contractility is the force of the contraction created by the myocytes. Preload is the passive ventricular wall stress that takes place at the end of the diastole and before the systole. Afterload englobes all factors that can affect the total tension during systolic contraction. An increase in contractility, an increase in preload or a decrease in afterload can all cause the stroke volume to increase. For example, exercising requires a greater oxygen consumption. The heart reacts by increasing the contractility and thus increasing the stroke volume of blood pumped (Bruss and Raja, 2020).
Cellular Properties
The mechanical properties of the left ventricle, the first chamber to contract, depend on the cellular properties and the extracellular matrix. The tissues of the heart are mostly composed of myocytes, who have passive mechanical properties primarily due to titin, a large, elastic, intracellular protein. Titin connects the z-line and the m-line of the sarcomere, which is the contractile unit of the myofibril. It also provides stiffness in the fiber direction, along the sarcomere, which prevents any damage to the cells due to overextension of the sarcomere. This makes myocardial tissue an anisotropic material since it does not have the same material properties in all directions (Voorhees and Han, 2015).
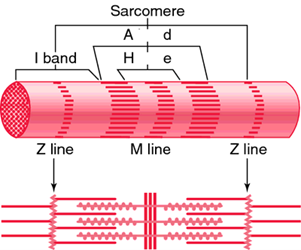
This property is significant since myocytes in the heart tissues do not all align in the same direction. Myofibers are layered with rotating orientation by alternating between longitudinal and circumferential directions. The myocyte sheets are parallel to the heart's surface (Voorhees and Han, 2015). The closer they are to the epicardial surface, the more they approach the circumferential axis from a negative angle. The opposite can be said for the fibers approaching the endocardial surface. They approach the circumferential axis from a positive angle. Within the heart this orientation may vary. The variation of orientation has a direct impact on the mechanical properties of the heart because it provides strength to the tissue in all directions including the longitudinal and the circumferential directions, which face the most stress. This varied orientation also allows for ventricular torsion when the heart contracts and relaxes because the inner surface and the outer surface of the myocardium contract in different directions due to a different timing of the electrical signal between layers (Voorhees and Han, 2015).
When filaments of myosin, a motor protein powered by adenosine triphosphate and hydrolysis, interact with actin, there is an activation of the contractile force. The actin protein is positioned parallel to the myosin along the sarcomere. The head domain of the myosin binds to the actin and changes conformation, which generates tension in the actin filaments. Once this change has occurred, the myosin and the actin detach, and the myosin will reattach to the actin filament in a different area further along. This process happens in a loop as the myosin head makes its way down the actin filament, which contracts the sarcomere. Active contraction causes the myocardial wall to become stiffer. The more actin-myosin cross bridges are formed, the stiffer the tissue will become (Voorhees and Han, 2015).
The above-mentioned action potential causes calcium ions to be released as a response. These ions activate the myofilaments, which starts the contraction process. The stiffness of the myosin also depends on the phosphorylation state of the proteins after their translation. The length of the sarcomere is also subject to change according to the load placed on the ventricle before and after the contractions. All these factors influence the force that can be generated by the sarcomere (Voorhees and Han, 2015).
The Extracellular Matrix
The extracellular matrix of the myocardium greatly affects the mechanical properties of the heart. This matrix is composed of structural proteins including collagen and elastin. It is also composed of non-structural proteins like proteoglycans, proteases and other growth factors (Voorhees and Han, 2015).
Collagen is the most important when it comes to mechanics. This protein is made up of coiled collagen fibers, which are composed of cross-linked collagen protein molecules. Its role is to prevent the myocardium tissue from overstretching and to offer a surface for the myocytes to attach to (Voorhees and Han, 2015). Collagen I is the most important because it is the stiffest kind of collagen, making it the main controller of the myocardial tissue when faced with large deformation. The resistance collagen provides is proportional to the amount of stress it undergoes. As one increases, so does the other. When the collagen fibers are stretched, they tend to become straight. This property explains its non-linear stress profile, which makes it so stiff. Once the fibers are straight, it takes a great amount of force to stretch it (Voorhees and Han, 2015).
Another structural protein that contributes to the mechanical properties of the myocardium is elastin. Elastin has an elastic stress-strain behavior that is needed in healthy myocardium to provide elasticity and resilience to the tissue. It is composed of long, thin fibers composed of an elastin core surrounded by microfibrils, a strand of glycoproteins and cellulose. The microfibril help bind the elastic network, offer integrin binding sites and contribute to the mechanical properties (Voorhees and Han, 2015).
An important non-structural protein to consider is proteoglycan, which impacts the regulation of water in the tissues. This protein contains glycosaminoglycan, which attracts and retains water molecules. This water retention then affects the water in the collagen and elastic neighboring fibers, which in turn can impact the mechanical properties of the myocardium by creating more stress on the tissue and making it stiffer (Voorhees and Han, 2015).
Tension and Cardiomyocytes
Before going through the cardiac cycle, one must first talk about the mechanical properties of cardiomyocytes and their relationship with tension, especially as they display isometric behavior since muscle fiber length remains constant for the better part of the cardiac cycle. Properties of isometric muscle fibers are as follows.
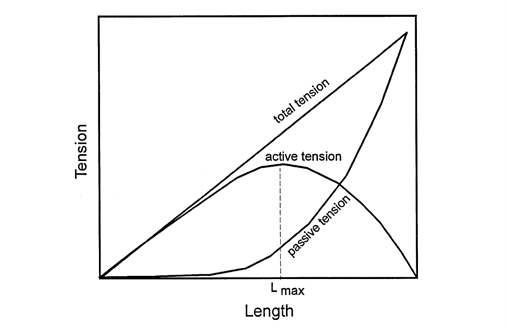
The previous graph depicts tension according to the length curve of three types of tension: active, passive and the total tension which is the sum of the previous two. Active tension is generated by the contractile elements of the cardiomyocytes while passive tension is due to the elastic elements such as elastin (Downey and Heusch, “CHAPTER 1 – Sequence of Cardiac Activation”). As depicted, active tension peaks at Lmax, reaching zero at both extremes. The decreasing tension as length increases is due to the distancing of actin and myosin which, as previously detailed, are responsible for generating force (Katz, 2010). Indeed, when the sarcomere is stretched too much, there will be no overlap between the thick filaments and the thin filaments as the gap between thin filament increases as shown below, and consequently, no tension can be produced (Katz, 2010).
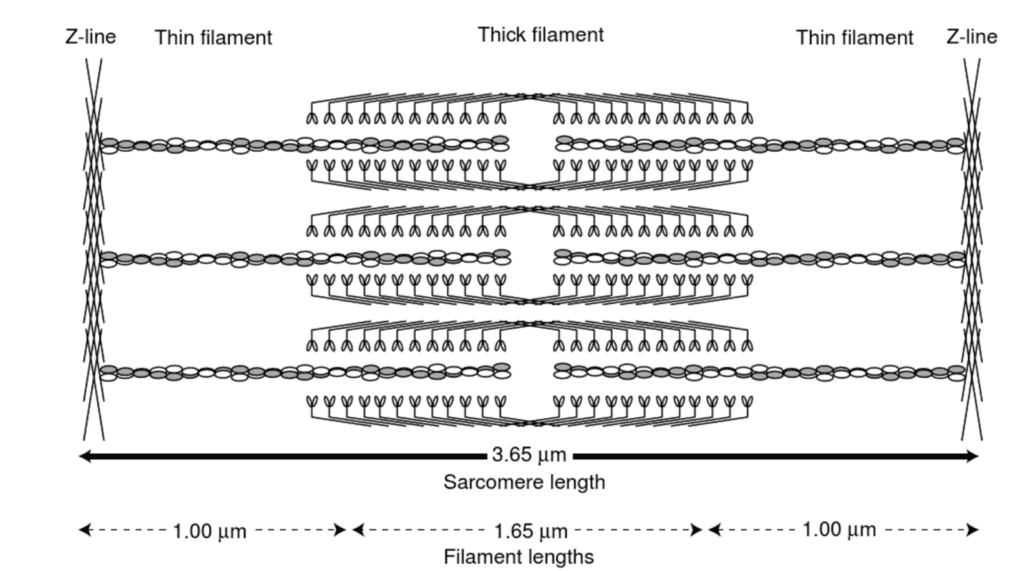
On the other hand, the increasing tension before that point is because past a certain point, the decrease in space between filaments modify the sensitivity of contractile proteins to calcium (Katz, 2010). Thus, the shorter the muscle fiber, the more calcium is needed to produce the same amount of energy (Downey and Heusch, “CHAPTER 1 – Sequence of Cardiac Activation”).
A notable difference between skeletal muscles and cardiac muscles is that the latter is much stiffer at rest than the former whose Lmax is near zero (Katz, 2010). This stiffness which results from the extracellular matrix, collagen and titin prevents sarcomeres from overextending past Lmax (Katz, 2010). This is extremely important as the more stretched the muscle fiber is, the thinner the wall of the heart will be, and, according to Laplace's law, as thickness decreases, stress increases (Katz, 2010). Furthermore, if a dilated ventricle is past Lmax, then as blood flows in from the atrium, tension will shift further along the left of the curve, further decreasing the heart's ability to pump blood as it will require more energy to contract (Katz, 2010).
Pressure and the Cardiac Cycle
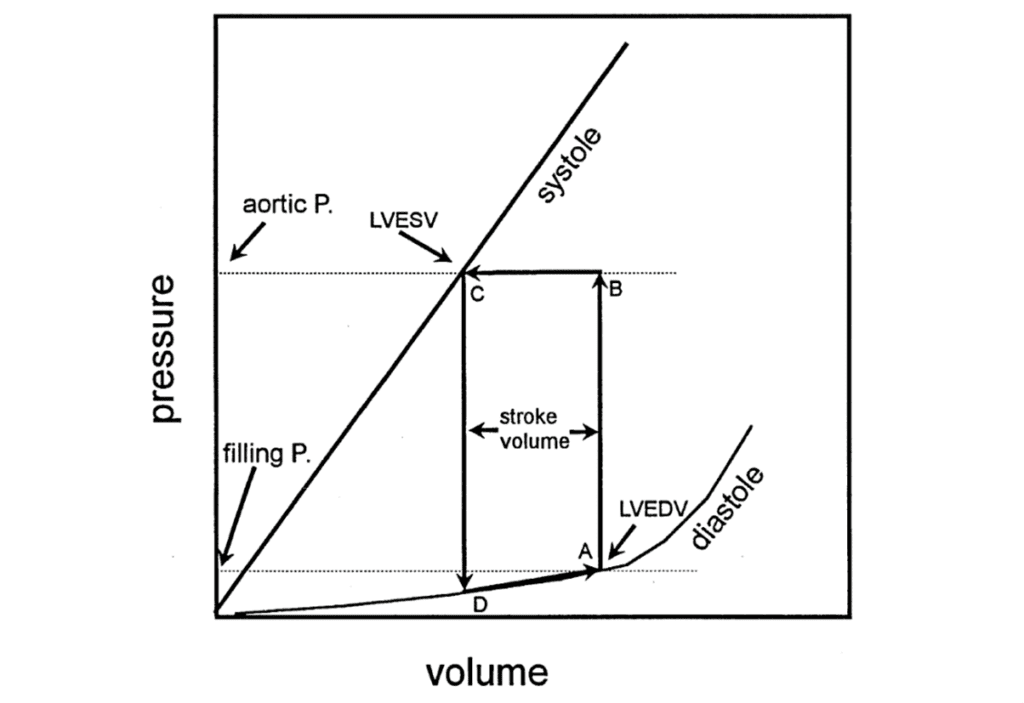
As mentioned previously, venous volume affects cardiac output (Downey and Heusch, “CHAPTER 4 – Control of Cardiac Output”). This is due to the relatively low compliance of the heart when it is relaxed. In other words, a small change in pressure results in a large change in the volume of the ventricle (Downey and Heusch, “CHAPTER 1 – Sequence of Cardiac Activation”). This volume is indicated at point A in Fig. 9 and dictates the resting length of muscle fibers. If point A changes, so will the stroke volume.
As myocytes contract, compliance is lowered (Downey and Heusch, “CHAPTER 1 – Sequence of Cardiac Activation”). As this occurs in an isometric way, the volume will remain the same as compliance decreases causing the pressure within the ventricle to rise to point B (Downey and Heusch, “CHAPTER 1 – Sequence of Cardiac Activation”). This pressure will close the mitral valve, thus preventing blood from entering the left ventricle through the atrium. The pressure will keep increasing until it surpasses that of the aortic vein, allowing it to push open the aortic valve (Downey and Heusch, “CHAPTER 1 – Sequence of Cardiac Activation”). Blood will then be ejected into the arteries.
At point B, the amount of force that could be produced by muscle fibers of that length exceeds the amount needed, and so, the volume of the heart will decrease in an isotonic way until equilibrium is reached at point C. After the action potential is passed, the heart relaxes in an isometric way and pressure decreases, closing the aortic valve followed by the opening of the mitral valve at point D thus ending the cycle (Downey and Heusch, “CHAPTER 1 – Sequence of Cardiac Activation”).
How the Operation of the Studied Organ is Aligned with the Required Function
An Evolutionary Perspective on the Different Circulation Systems
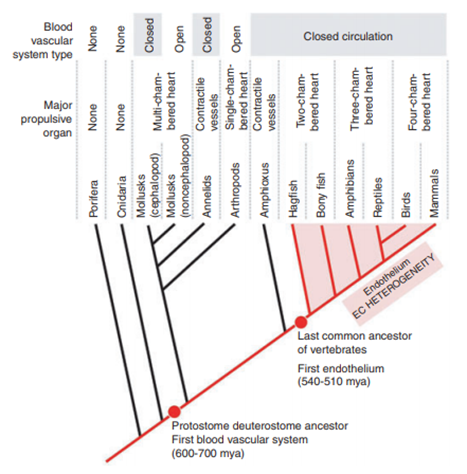
The heart is an important organ that functions as a pump in order to transport blood. Its presence is crucial to the survival of many if not all living species on Earth. The shape of the heart varies between species as a consequence of evolution. In fact, with the appearance of new animals presenting new characteristics (e.g., larger size) and different organs, the need for a stronger heart became more impending. In lower order animals like spiders, snails or annelid worms, it takes the shape of a simple structure displayed in a straight-line. In mollusks, the heart has a more defined architecture with at least one atrium (receiving chamber) and one larger ventricle (pumping chamber): this is when two-chamber hearts surfaced (Editors of Encyclopedia Britannica, “heart”). Vertebrates have multi-chamber hearts. Indeed, when we look at fish, the heart is even more elaborate, showcasing a folded tube containing two chambers. In amphibians, reptiles, birds and mammals, which are animals that all have in common the presence of lungs, the heart exhibits many stages of evolution from a single to a double pump. The latter uses two pumps to transport blood to the lungs as well as the whole organism. In mammals, the heart is settled within the thoracic cavity between the lungs. Amphibians and reptiles have three-chambered hearts (one ventricle and two atria) whereas birds' and mammals' hearts are divided into four chambers, allowing a clear distinction between a general and pulmonary circuit (Editors of Encyclopedia Britannica, “heart”).
Some animals like jellyfish, can live without blood and a heart. Jellyfish are made up of 95 % water, making them almost one with the ocean. They have very thin skin, allowing them to take in oxygen through it, through a process called diffusion. Diffusion occurs when there is a movement of molecules from an area with high concentration to another with low concentration. In this case, oxygen enters the jellyfish because its concentration is lower inside it in opposition to its environment. Similarly, carbon dioxide exits the jellyfish because its concentration is higher inside it than outside. The outer layer's thinness makes the skin semipermeable; so, it becomes easy for the cells to absorb oxygen from the surrounding water. Oxygen enters inside the mesoglea through the jellyfish's epidermis. In the mesoglea, the oxygen is absorbed by the cells. Metabolic waste can also be sent back to the ocean the same way, through the outer layer of the skin cells. Therefore, they do not need a heart to transport blood. Diffusion does not work for most animals because their skin is usually much thicker, meaning that oxygen would not be able to traverse the outer layer of skin cells, which would result in necrosis (“The Importance of Oxygen in Animals” | White Spotted Jellyfish, n.d.; “Stunning Jellyfish” | National Geographic | YouTube, 2008; “Oxygenation of Jellyfish” | CLOUD Reassembly RPI, n.d.).

The first living beings on earth appeared about 1.5 billion years ago; they are eukaryotic cells. These living species were very little in size and consisted of clusters of cells visible on the microscopic level. When compared to any other animal we know today, their size can be considered insignificant. Therefore, unicellular and multicellular animals could just perform diffusion and rely on it for supply of oxygen and nutrients as well as dismissal of carbon dioxide, just like the jellyfish. Diffusion is a slow process which functions over very small distances (less than 1 millimeter) (Bettex et al., 2014).
But with evolution, animals started to become larger in size, prompting a need for a change in structural design, hence the emergence of a reliable transportation system that could carry oxygen, nutrients and metabolic waste. When the size of an organism changes, the ratio of surface area to volume does so too, in a disproportionate manner: this causes design constraints (Monahan‐Earley et al., 2013). Indeed, a modification in the surface area to volume ratio can explain the anatomical and physiological changes seen in larger animals. When the length or radius increases by x, this increase x is squared in surface area (x2) and cubed in volume (x3). Eventually, it became mandatory to implement a transport system (heart and circulatory system) that could deliver substances to and from the living being (Beals et al., 2000).
Having the role of transferring energy and wastes throughout the body, the heart's architecture and operation are constantly evolving in the sake of efficiency, and there is obvious evidence on the evolution process.
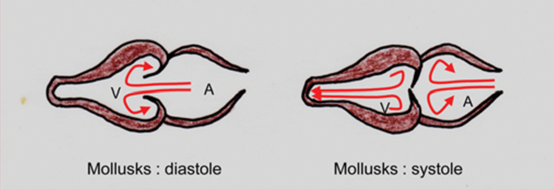
Mollusks, snails, and mussels are the first animals to have a two chambered heart, an atrium and a ventricle. The advantage of possessing an atrium is to increase the ventricular diastole pressure but without increasing the central venous pressure. Many mollusks also own two accessory hearts to push the blood through the gills with a lower pressure, a first appearance of the idea the differentiations of pressure between pulmonary and systemic circulation, which will be discussed later.
The two chambered heart of mollusks, however, form a straight line with the vessels. Such an arrangement largely loses the dynamic energy the blood flow has offered, because once the ventricle is in systole (i.e., contraction phase), the valve between the atrium and the ventricle just suddenly stops the flow of blood (and even backflow). In other words, the kinetic energy is largely lost for the blood in atrium.
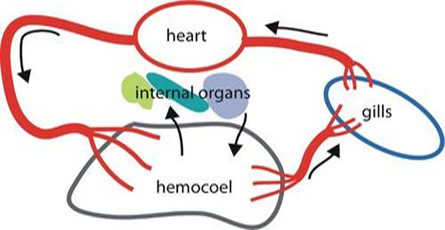
The heart for most fishes, sharks, and rays also consists of two chambers, an atrium and a ventricle. The blood from the body, which is low in oxygen, first enters the sinus venosus, an “accessory chamber” connected to the vessels from the body, and then into the atrium. The cells of the atrium contract and push the blood into the ventricle, where blood pressure is created, and blood is sent to the gill capillary to obtain the oxygen. A unique structure called bulb arteriosus connected to the ventricle reduces the blood pressure so that those fragile capillaries will not be damaged by the overwhelming pressure. Whereas compared to the mollusk's two chambered heart, the two chambers in fishes' heart showed an S-shape in the structure, allowing a curve path of blood in both atrium and ventricle and preserves some kinetic energy which can be reused during the next ventricular diastole, the heart gets more spiraling along the evolution of the vertebrates (Kilner et al., 2000).
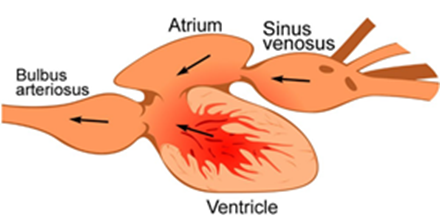
However, such a system may not be able to provide enough energy required by animals with higher metabolic rates such as amphibians, since after being propelled by the ventricle, the blood pressure drops after passing through the gill capillary beds, along with a lower speed of blood circulating through the body. Such a drop will limit the maximum metabolic rate and general energy transferring efficiency. The amphibian heart, however, presents a simple solution — a three chambered heart (Poelmann and Gittenberger-de Groot, 2019).
The frog heart, for example, has two atria and a ventricle. The right atrium receives the deoxygenated blood from the body and transfers it to the ventricle. Afterwards, the ventricle contracts and send the blood to the lung and skin, where frog makes gas exchanges from the outside. What is different from the fish heart is that the oxygenated blood will go back to the heart again though the left atrium and reach the ventricle to maintain a sufficiently high blood pressure in the rest of the body, which allows the amphibian to maintain higher metabolic activity than fishes. The heart of frogs also gives them the flexibility that allows them to behave totally differently between summer and winter, when hibernating occurs, and between on land and diving, where lungs cannot function normally. During diving, the pulmonary artery is vasoconstricted and the flow is diverted to skin, which is also sufficient for the low metabolic activity during a hibernation in a cold swamp (Jensen et al., 2013).

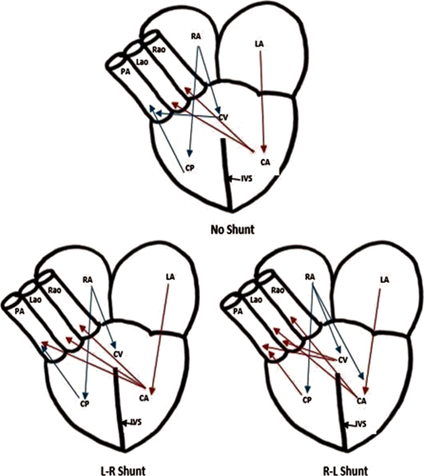
Such a three-chambered heart also brings a problem to the overall efficiency of the energy transferring system: the only ventricle accepting both the oxygenated and deoxygenated blood will mix them together and the body will never receive fully oxygenated blood, which is a potential limit towards the overall efficiency. An effective but incomplete way of preventing mixing of the two types of blood is the presence of a septum in the middle of the ventricle (although still somewhat mixed). This intermediate three-chambered heart is possessed by most of the non-crocodile reptiles like turtles. Interestingly, the ability of partially blood shutting is performed in most of the reptiles (Farmer et al., 2007). Depending on the pulmonary resistance, turtles, for example, could force the oxygenated blood to the pulmonary artery (L-R shoot), or the deoxygenated blood to the aortas (R-L shoot) (refer to Fig. 16). The L-R shut occurs when the reptiles are experiencing a high intensity of actions, so this shut could serve the heart with oxygen in a faster pace. On the contrary, the R-L shut occurs while the reptiles are diving or in low activity to save unneeded energy. The shut cannot be performed by a normal four-chambered hearts owned by birds and mammals, which is explained by a loss of an aorta (Stephenson et al., 2017).
Birds and mammals have evolved a complete four chambered heart to fulfill their high demands of metabolic rates and activity. Birds, for example, have two atria and two ventricles. Amphibian hearts follow a similar procedure, that is, the right atrium receives the deoxygenated blood from the body and sends it to the capillaries at the lung by the right ventricle. The blood with relatively low pressure moves to the left atrium and then the left ventricle where it gets repressured to assure a constantly rapid flow to the rest of the body, where the oxygen and energy are needed. Such an efficient system allows mammals and birds to maintain a constant body temperature even when they are at rest and are thus called endothermic animals (Stephenson et al., 2017).
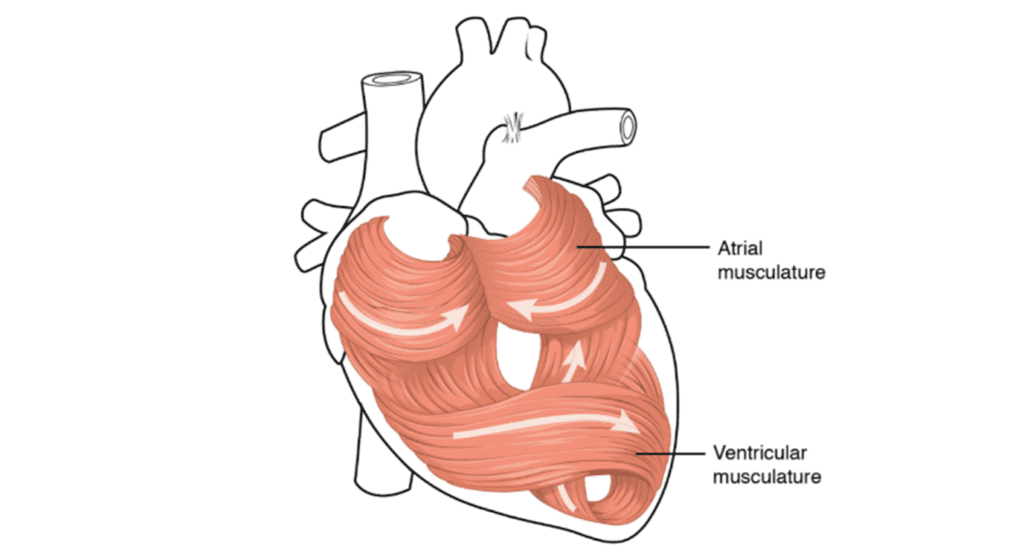
Looking more precisely into the four chambered system, the spiraling course of the heart propels the efficiency even further. As mentioned above, just like in fish, the mammalian heart is not a straight structure, but a helicoidal structure as a product of an embryologic imprinting. There are two simple loops that start at the pulmonary artery and end in the aorta, and this twisting structure prevents the heart contract like a balloon, but more like a mop torsion, which is much powerful as one imagined. Such torsion allows an efficient ejection as well as an active suction in the cardiac filling (Stephenson et al., 2017).
The Rhythmic Beat of Vertebrates
Regardless of efficiency, there is one fantastic property that all these three kinds of heart share: the rhythmic beat. The heart itself contracts and relaxes at a certain frequency without receiving signals from the nervous system, which is accomplished by some autorhythmic cells. What is more important is these cells all contract and relax coordinately, allowing the system to work fluently. This coordination is controlled by the sinoatrial (SA) node in the wall of the right atrium sending electrical signals throughout the whole heart. In fact, the current generated by the SA node is measured, recorded and then made to be what we called electrocardiogram (ECG).
Conclusion
Throughout this essay, we have explored how the heart, a mechanical pump, functions throughout one's circulatory system and how its architecture has evolved across many different species. The heart's main function is to transport blood across all living beings' bodies. It does so in a reliable, automated, and consistent manner. The heart is also responsive and accommodates the rate at which it delivers blood based on environmental stimuli (and other factors), such as weather (e.g., hibernation) or being under attack by a predator. Although the heart is physiologically different from one species to another, it has evolved following the same direction path. The difference in the design of hearts allows different animals to adapt to environments and lifestyle; and the increasing complexity, from the development of multiple chambers to the twisted musculature, has met the demand of higher metabolic rates along the evolution. The materials of its muscular tissue as well as its extracellular matrix form an organ that plays an important role in oxygen dispersion and blood pressure regulation, due to the relationship between tension, volume and pressure during a cardiac cycle as well as the relationship between tension and the length of muscle fibers.
Such a complex organ can be explored from many different angles. Although we have explained how the mechanics of this system functions, we have yet to elaborate on the chemical and electrical aspects of its regulation.
Appendix
“Flow through the heart.”, 10 October 2012, khanacademymedicine, YouTube, https://www.youtube.com/watch?v=7XaftdE_h60.
“Stunning Jellyfish.”, 30 June 2008, National Geographic, YouTube, https://www.youtube.com/watch?v=JoMCTvRkvxo.
References
Adam, D. (2000). Your twisted heart. Nature. doi:10.1038/news000413-9
Beals, M., Gross, L., & Harrel, S. (2000). The surface area to volume ratio. Retrieved from http://www.tiem.utk.edu/~gross/bioed/bealsmodules/area_volume.html
Bettex, D. A., Prêtre, R., & Chassot, P.-G. (2014). Is our heart a well-designed pump? The heart along animal evolution. European Heart Journal, 35(34), 2322-2332. doi:10.1093/eurheartj/ehu222
Betts, J. G., Young, K. A., Wise, J. A., Johnson, E., Poe, B., Kruse, D. H., . . . DeSaix, P. (2013). Cardiac Cycle. In Anatomy and Physiology. Retrieved from https://openstax.org/books/anatomy-and-physiology/pages/19-3-cardiac-cycle
Blair, D. (2017). The Different Types of Muscle Tissue. Retrieved from https://www.ftcollinspersonaltrainer.com/2017/04/different-types-muscle-tissue/
Britannica, E. o. E. (2020, 4 Jun 2021). heart. Retrieved from https://www.britannica.com/science/heart
Bruss, Z. S., & Raja, A. (2020). Physiology, Stroke Volume. In StatPearls [Internet]. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK547686/
Clinic, C. (2018). Heart Valves. Retrieved from https://my.clevelandclinic.org/health/articles/17067-heart-valves
Copstead. (1995). Sarcomere. In. medical-dictionary.thefreedictionary.com. Retrieved from https://medical-dictionary.thefreedictionary.com/sarcomere
Downey, J. M., & Heusch, G. (2001a). CHAPTER 1 – Sequence of Cardiac Activation and Ventricular Mechanics. In N. Sperelakis, Y. Kurachi, A. Terzic, & M. V. Cohen (Eds.), Heart Physiology and Pathophysiology (Fourth Edition) (pp. 3-18). San Diego: Academic Press. Retrieved from https://www.sciencedirect.com/science/article/pii/B9780126569759500031
Downey, J. M., & Heusch, G. (2001b). CHAPTER 4 – Control of Cardiac Output and Its Alterations during Exercise and in Heart Failure. In N. Sperelakis, Y. Kurachi, A. Terzic, & M. V. Cohen (Eds.), Heart Physiology and Pathophysiology (Fourth Edition) (pp. 61-69). San Diego: Academic Press. Retrieved from https://www.sciencedirect.com/science/article/pii/B9780126569759500067
Engineering, B. H. (2009, 17 Mar 2009). The Basic Working Priciple of a Hydaulic (Fluid) Pump & the Concept and Construction. Retrieved from https://www.brighthubengineering.com/fluid-mechanics-hydraulics/29394-the-basic-concept-construction-and-working-principle-of-hydraulic-pumps/
Farmer, C. G. (2011). On the evolution of arterial vascular patterns of tetrapods. Journal of Morphology, 272(11), 1325-1341. doi:10.1002/jmor.10986
Farmer, C. G., Uriona, T. J., Olsen, D. B., Steenblik, M., & Sanders, K. (2008). The right-to-left shunt of crocodilians serves digestion. Physiol Biochem Zool, 81(2), 125-137. doi:10.1086/524150
Jellyfish, W. S. (n.d.). The Importance of Oxygen in Animals. Retrieved from http://whitespottedjellyfishresource.weebly.com/gas-exchange.html
Jensen, B., Wang, T., Christoffels, V. M., & Moorman, A. F. M. (2013). Evolution and development of the building plan of the vertebrate heart. Cardiomyocyte Biology: Cardiac Pathways of Differentiation, Metabolism and Contraction, 1833(4), 783-794. doi:10.1016/j.bbamcr.2012.10.004
Katz, A. M. (2010). Physiology of the Heart. Philadelphia, UNITED STATES: Wolters Kluwer Health. Retrieved from http://ebookcentral.proquest.com/lib/mcgill/detail.action?docID=2031935
Kilner, P. J., Yang, G. Z., Wilkes, A. J., Mohiaddin, R. H., Firmin, D. N., & Yacoub, M. H. (2000). Asymmetric redirection of flow through the heart. Nature, 404(6779), 759-761. doi:10.1038/35008075
Kong, Q. (n.d.). Comparison of Heart Rate and Body Mass in Animals. In: Northern Illinois University. Retrieved from http://www.math.niu.edu/~kong/360/014.pdf
Laurenson, E. (2011). Building a Heart: The Function and Mechanics. In: Yale University. Retrieved from https://teachers.yale.edu/curriculum/viewer/initiative_11.07.04_u
Monahan-Earley, R., Dvorak, A. M., & Aird, W. C. (2013). Evolutionary origins of the blood vascular system and endothelium. Journal of Thrombosis and Haemostasis, 11 Suppl 1(Suppl 1), 46-66. doi:10.1111/jth.12253
Nature, B. S. (2014, 24 Sept 2014). Human Body & Mind. Retrieved from https://www.bbc.co.uk/science/humanbody/body/factfiles/heart/heart.shtml
Pierce, E. (2006). Diagram of the human heart. In D. o. t. h. h. (cropped).svg (Ed.), (Vol. 663 × 651). Wikimedia Commons. Retrieved from https://en.wikipedia.org/wiki/File:Diagram_of_the_human_heart_(cropped).svg
Poelmann, R., & Gittenberger-de Groot, A. C. (2019). Development and Evolution of the Metazoan Heart. Developmental Dynamics, 248. doi:10.1002/dvdy.45
Poelmann, R. E., & Gittenberger-de Groot, A. C. (2019). Development and evolution of the metazoan heart. Developmental Dynamics, 248(8), 634-656. doi:https://doi.org/10.1002/dvdy.45
RPI, C. R. (n.d.). Oxygenation of Jellyfish. Retrieved from http://rpi-cloudreassembly.transvercity.net/2012/11/05/oxygenation-of-jellyfish/
Simões-Costa, M. S., Vasconcelos, M., Sampaio, A. C., Cravo, R. M., Linhares, V. L., Hochgreb, T., . . . Xavier-Neto, J. (2005). The evolutionary origin of cardiac chambers. Developmental Biology, 277(1), 1-15. doi:10.1016/j.ydbio.2004.09.026
Staff, H. (2020, 31 Aug 2020). How the Heart Works. Retrieved from https://www.uofmhealth.org/health-library/tx4097abc
Starkebaum, G. A. (2019, 10 Jan 2019). Types of muscle tissue. Retrieved from https://medlineplus.gov/ency/imagepages/19841.htm
Stephenson, A., Adams, J. W., & Vaccarezza, M. (2017). The vertebrate heart: an evolutionary perspective. Journal of Anatomy, 231(6), 787-797. doi:https://doi.org/10.1111/joa.12687
Suarez, R. K. (1992). Hummingbird flight: sustaining the highest mass-specific metabolic rates among vertebrates. Experientia, 48(6), 565-570. doi:10.1007/bf01920240
TeachPE.com. (n.d.). The Human Heart. Retrieved from https://www.teachpe.com/anatomy-physiology/the-human-heart
Voorhees, A. P., & Han, H. C. (2015). Biomechanics of Cardiac Function. Compr Physiol, 5(4), 1623-1644. doi:10.1002/cphy.c140070
Wikipedia. (n.d.). Pump. In Wikipedia. Woodward, A. (2017). Hummingbirds have massive hearts to power their hovering flight. Retrieved from https://www.newscientist.com/article/2155235-hummingbirds-have-massive-hearts-to-power-their-hovering-flight/