A Mathematical Representation of Brain Activity During Sleep
Kelliane Beland, Taisei Fu, Andres Gonzalez, Grace Shi
Abstract
While an organism may seem inactive and static while sleeping, its brain displays a host of unique electrical brain activity. When these voltage fluctuations are graphically represented as waves, it illustrates how the brain processes and consolidates information between its neurons. Delta waves, theta waves, and sleep spindles are each examples of a different wave phenomenon which characterize a process. Meanwhile, certain species are able to buck the trend of bihemispheric sleep, and display unihemispheric sleep in which one half of the brain remains awake while the other shows NREM sleep activity. When applying a mathematical analysis to this brain activity, one can also observe a fascinating synchronization between neuronal groups which explain the patterns of oscillation between NREM and REM sleep. Additionally, another type of neuronal oscillation occurs within the circadian rhythm in response to light input from the environment. When this circadian analysis combined with analysis of homeostatic sleep pressures, a two-process mathematical model of sleep can be constructed. Finally, the brain exemplifies information processing when encoding memories during sleep, a process best demonstrated by the memory and learning capabilities of birds. Hence, analyzing the mathematical modeling of sleep activity can help researchers and engineers gain a much clearer perspective on the mysterious phenomenon of sleep, and can improve problem-solving when designing solutions pertaining to sleeping organisms.
Introduction
Sleep, which is practiced almost entirely throughout the animal kingdom, plays an extremely important and necessary role in restoring and maintaining the functions of each organism. It is loosely defined as a periodic state of unconsciousness and suppressed reaction to external stimuli with functions including memory consolidation, rest and development (Nicolau et al., 2000). Many biological aspects of sleep result from the various types of periodic oscillations that regulate animal sleep on both a cellular and organismal level. The use of mathematical models and equations to analyze these cycles and waves provides insight on what occurs during the different types of sleep, dreaming and memory based on the mapping of brain signals, neural activity and sleep cycles.
An Overview of NREM and REM sleep
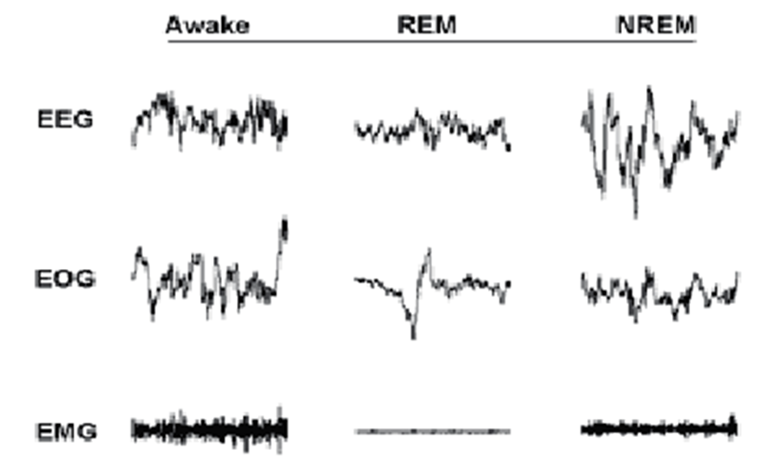
Non-rapid eye movement sleep, or NREM sleep, is the stage of sleep that is characterized by brain activity otherwise unseen in other stages of consciousness such as REM sleep and wakefulness. Indeed, electroencephalography readings (EEG), which measure electrical activity in the animal’s brain, display a predominance of high-amplitude (high-voltage), low frequency cortical waves known as delta (0-4 Hz) waves, as illustrated in Figure 1. Due to the prevalence of these low-frequency oscillations, NREM sleep is thus also known as slow wave sleep (SWS) in animals. The relative prevalence of delta wave activity in the brain has been linked to the depth of sleep: the higher the percentage of delta wave activity, the more stimuli is required to arouse the organism (Zepelin et al., 2005).

During SWS, aside from delta waves, mammals have been observed to exhibit the phenomenon of sleep spindles. As illustrated in Figure 2, Sleep spindles consist of bursts of high-frequency waves ranging from 8 to 16 Hz; thus, they are significantly faster than the predominant delta waves. Each spindle lasts between half a second to six seconds, and occur due to a burst of electrical signals by cells of the reticular thalamic nucleus, a sheet of nerve cells wrapped around the dorsal end of the thalamus (Iotchev & Kubinyi, 2021; Murray Sherman & Guillery, 2001).
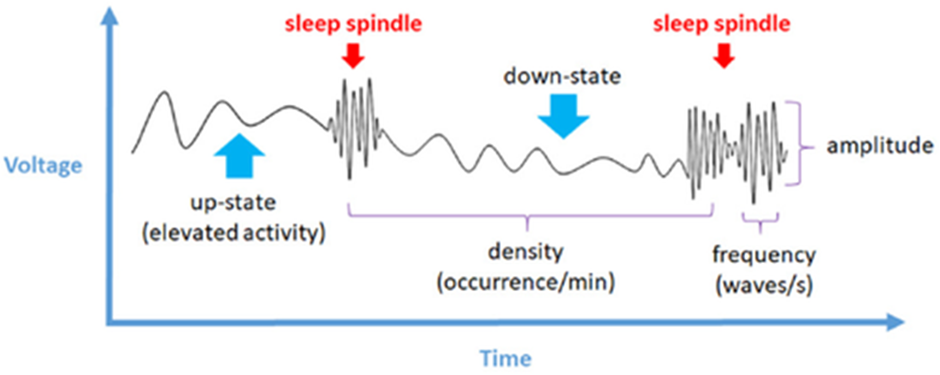
These spindles have been shown to have a causal relationship with memory consolidation in the hippocampus, due to the hierarchical nesting of information: the information is carried by ripples emitted by the hippocampus, which are nested in the troughs of the spindle waves, which are themselves nested into the slower cortical waves. This results in a phenomenon known as phase-locking (Latchoumane et al., 2017). This process enables the transfer of information from short-term memory in the hippocampus to long-term memory in the neocortex (van der Meij et al., 2019). Thus, during sleep, the brain processes and transfers the information across various regions of the brain via the electrical activity
On the other hand, rapid-eye movement sleep, or REM sleep, is a stage of sleep that features significantly different electrical brain activity from NREM sleep. Indeed, REM sleep is characterized by the presence of higher-frequency, lower-amplitude EEG waves in the range of 4 to 10 Hz known as theta waves (Hutchison & Rathore, 2015), as illustrated in Figure 1. As such, REM brain activity actually resembles patterns of activity observed during wakefulness. However, during this period of sleep, the muscles are immobilized, as illustrated by Figure 3, which shows electromyographic (EMG) readings of skeletal muscles. This is caused by the inhibition of motor neurons by neurologic processes that begin in the brainstem (Clauss et al., 2010) Hence, REM sleep is also known as “paradoxical sleep”, due to the perceived contradiction between the wake-like brain activity and the musculoskeletal immobility (Siegel, 2005).
While the norm in the animal kingdom is bihemispheric sleep, which involves both hemispheres exhibiting sleep-like activity, some species can undergo unihemispheric sleep. As the word etymology indicates, the latter comprises sleep in which only one half of the organism’s brain is in a sleeping state, while the other half remains in a state of wakefulness.
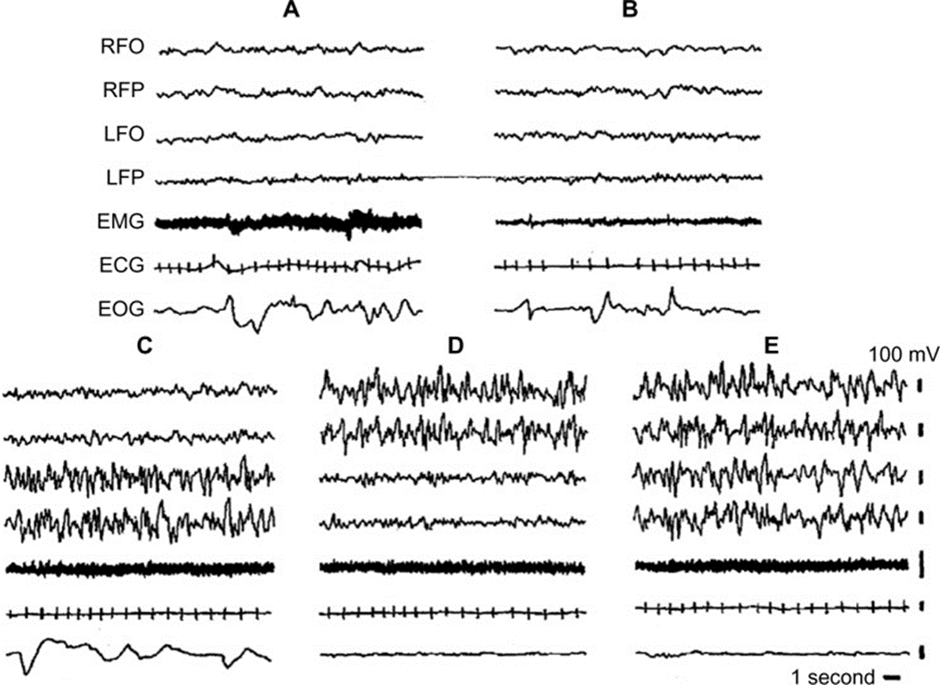
This asymmetry between the hemispheres is best illustrated by electroencephalogram recordings (Mascetti, 2016). As illustrated in Figure 4, during left unihemispheric short wave sleep (C), brain activity measured in the left fronto-occipital cortex and frontoparietal cortex shows the high-amplitude, low-frequency waves that characterize regular bihemispheric NREM sleep (E). On the other hand, the right-side counterparts of the brain displayed low-amplitude, high-frequency waves that match brain activity during wakefulness. It is also observed that during right unihemispheric short wave sleep, the activity in the respective hemispheres is flipped, resulting in an NREM right brain and a wakeful left brain. Thus, the EEG diagrams illustrate the contrast between activity in the two hemispheres of the brain.
This neurological difference is then reflected in behavioural changes which can confer an evolutionary advantage for species that exhibit unihemispheric sleep (Mascetti, 2016). For instance, birds are a well-known example of unihemispheric short-wave sleep (USWS). As such, they have been observed to sleep with one eye open. Thus, given the vulnerability associated with the lack of arousal during sleep, unihemispheric sleep can act as a defense against predation for the animals (Mascetti, 2016).
Additionally, fur seals have been observed to spend about two-thirds of their sleep time in the water in USWS. This results in a motor asymmetry, where the flipper opposite the “wakeful” hemisphere paddles to keep the seal afloat, while the flipper opposite the hemisphere in NREM stays immobile; this setup allows these animals to sleep on the surface of the water while one flipper keeps them afloat, helping the animal adjust to their environment to avoid possible drowning when it is forced to sleep in the water (Siegel, 2005). Thus, the unique nature of unihemispheric short-wave sleep allows certain species to carry out necessary functions to survive while preventing the onset of sleep deprivation.
Kuramoto system to describe the alternation of REM and NREM sleep phases during the night
The sleep cycle alternates between NREM sleep phases, which account for most of the sleep time in regular mammals, and REM sleep phases, a peculiar phase of sleep where numerous face twitches and random rapid-eye movements can be observed. NREM is characterized by a switch from low-amplitude high-frequency state (wakefulness) to a high-amplitude low-frequency state (delta waves), while REM sleep is characterized by the opposite, which means a low-amplitude high frequency state.
It is hypothesised that alternations between REM and NREM sleep phases are orchestrated by different neuronal populations. NREM sleep is maintained by neurons located in the locus coeruleus (LC), while REM sleep is kept by the activity of neurons in the gigantocellular tegmental field (FTG) (Hobson & McCarley 1975). Experimental data by Hobson and McCarley (1975) suggests that interactions between these two cell groups follow a reciprocal interaction model for sleep cycle oscillations. In a nutshell, synchronization emerges from each cell group. Synchronized systems have intricate properties that lead to a much bigger whole than the sum of its parts.
The Kuramoto model accurately describes synchronization, especially when there are coupled oscillators (Acebrón et al., 2005), which can be assumed it is the case. The standard form of the Kuramoto model is presented as:
{(dθ_i) \above{0.5pt} dt}
=ω_i+{K \above{0.5pt} N} \textstyle\sum_{j=1}^N sin(θ_j-θ_i), θ_i ϵ [0,2π], i=1,2…,N, Where θi = θi(t) is the phase oscillator for the oscillator i and ωi is the natural frequency of oscillator i. N is the total number of oscillators and K is the coupling constant.
This model assumes that all oscillators act on equal strength with all other oscillators, and that they are identical in every aspect, except their natural frequencies (Acquistapace et al., 2016). For the scope of modeling synchronization of REM and NREM models, these conditions are met. With N=2, we have two sets of equations, namely:
{dθ \above{0.5pt} dt}=ω_1+ {K \above{0.5pt} 2} sin sin (θ-ϕ) {dϕ \above{0.5pt} dt}=ω_2+ {K \above{0.5pt} 2} sin sin (ϕ-θ) Note that each sin expression is equivalent, but opposite in sign. For simplicity, ϕ = ϕ(t) = θ2 . It follows then that the sum of each expression is simply:
{d(θ+ϕ) \above{0.5pt} dt}= w_1 + ω_2 Which by integration yields:
θ+ϕ = (w_1 + ω_2)t + c
Therefore, this proves that by taking the differences of each phase v = (θ-ϕ), and apply to the second equation we get:
υ=ω_1-ω_2+2Asin sin (θ-ϕ)
Where A = K/2, w1, w2 can be made arbitrarily.
Figure 5 represents the graph of the solution for different values of A, w1, w2. The y axis represents the phase difference v, while the x axis represents time t. A horizontal phase difference would mean synchronization is being kept, if the curve is increasing, there is a desynchronization between phase θ and phase ϕ. If the curve is decreasing while reaching a limit of 0, there is a decay in synchronization (Acquistapace et al., 2016).
Note that the direction of the curve is sensitive to the constant A. Each curved was built based on initial data for the value of θ(0) and ϕ(0).
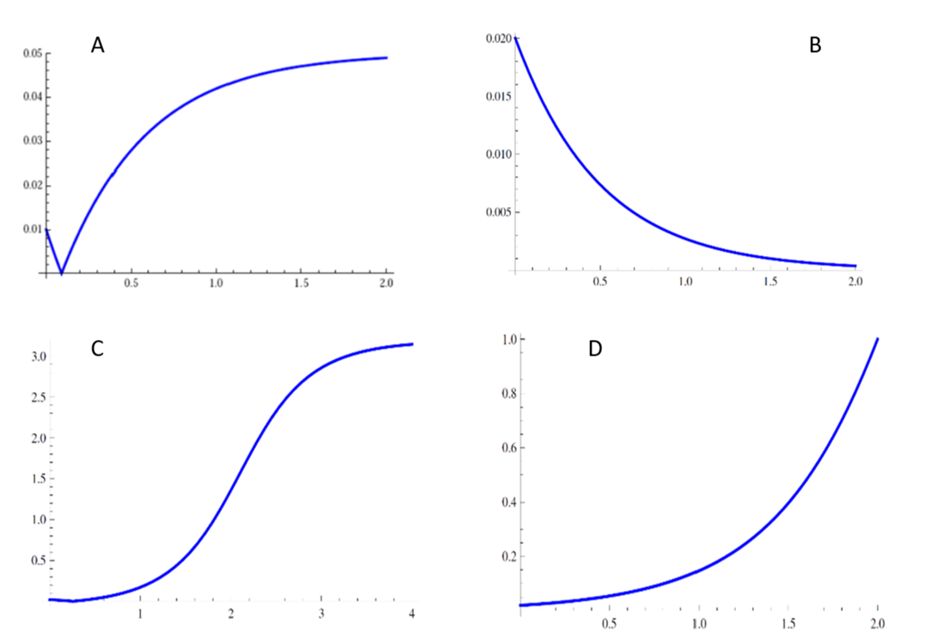
From the graph, it can be seen that θ and ϕ are rarely synchronized (either there is a loss of synchronization from initial conditions or it decays), which reproduces the behaviour of LC (θ) neurons and FTG neurons (ϕ). These results are according to observations, since it is known that REM inducing neurons cease firing during NREM episodes and vice-versa. Therefore, this change in synchronizations from each group lead to transitions between NREM sleep and REM sleep episodes (Acquistapace et al., 2016).
There are many factors that can be taken into consideration to enhance this model (such as the assumption that a group of neurons are a coupled oscillator). Nevertheless, more complicated models require stronger knowledge in mathematics.
This simple equation, with the input of initial conditions, can greatly deepen the understanding of synchronization behaviour between NREM and REM cell groups.
Circadian Rhythm
A shared commonality between nearly all sleeping animals is the periodic biological clock which fuels organismal sleep-wake cycles. Circadian rhythms are characterized by oscillations of roughly 24 hours – when under relatively constant environmental factors – which acts on a cellular, tissue, and organism level (Asgari-Targhi & Klerman, 2019). The oscillatory property of the endogenous circadian system allows for its mathematical modelling, which offers greater insights into this process and its interactions with other biological functions such as cell cycles, sleep/wake cycles and body temperature.
For mammals, the most prominent circadian oscillators are located in the suprachiasmatic nucleus (SNC) of the hypothalamus. It consists of about 20,000 neuronal oscillators that are entrained by ocular light inputs, which then regulate subsequent oscillators in surrounding tissues (Lowrey & Takahashi, 2000). Accordingly, these light inputs, in addition to other external factors such as temperature, can promptly cause phase shifts in the circadian cycle depending on its duration, intensity, and the phase of the circadian cycle itself (Fuentes-Pardo et al., 2002). This alignment of the circadian period with external stimuli is characteristic of the biological pacemaker and is known as entrainment (Nakao et al., 2007).
In terms of mathematical modelling of biological oscillations, there exists three main common categories. This includes self-sustained systems, damped oscillations, and excitable oscillations. For the circadian cycle, it falls under the self-sustained system where the system enters a sustained oscillation that can uphold small perturbations (Asgari-Targhi & Klerman, 2019). A simple ordinary differential equation (ODE) that models a self-sustained system circadian cycle with a stable limit cycle is the van de Pol oscillator of the form dx/dt = f(x,c,u(t)), where f is the rate of change, c is a set of parameters, and u(t) is the external light signal (Asgari-Targhi & Klerman, 2019). Although this simple model reveals the dynamics of circadian cycles, it does not account for the biological noise present at the micro scale such as the cellular coupling in SNC neurons (Hastings & Herzog, 2004). This leads to the more refined stochastic model of the circadian system that takes into consideration the statistical noise. It takes the following form: dX(t,w) = f(t,X(t,w))dt +g(t,X(t,w))dW(t,w), where f corresponds to the deterministic part of the SDE and is called the drift , g is the diffusion coefficient, and dW(t,w) accounts for the noise. (Asgari-Targhi & Klerman, 2019).
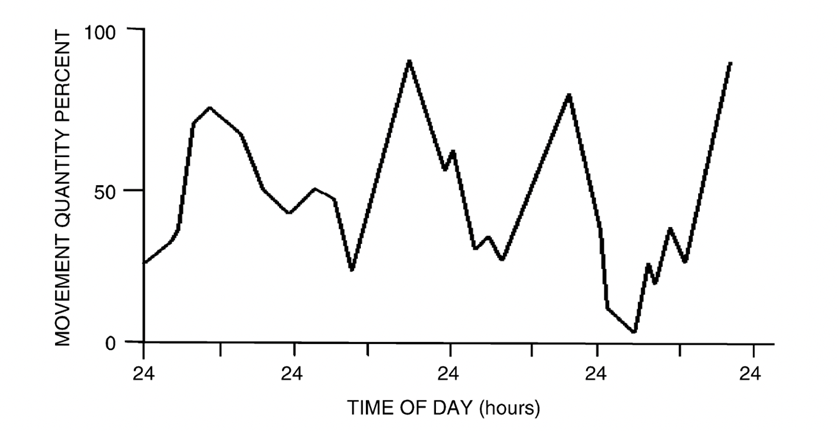
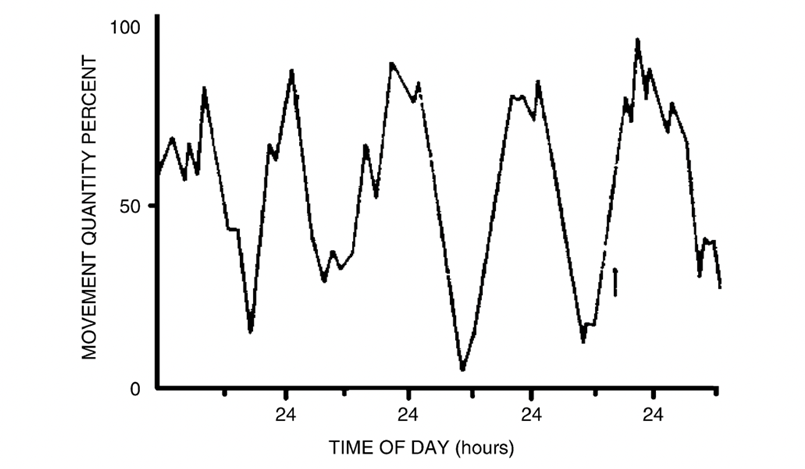
A particularly interesting example of mathematical circadian modelling in animals is the changes of the circadian rhythms in juvenile crayfish as they develop into adulthood. In a study performed by Fuentes-Pardo (2003), they concluded that juvenile crayfish have diurnal behaviour, whereas adult crayfish are nocturnal based on the modelling of their respective circadian rhythms. Observing Figure 6, it shows that the young crayfish have cycle peaks which correlate to daytime light with periods of roughly 24 hours (Fuentes-Pardo et al., 2003). Conversely, Figure 7 shows that adult crayfish have cycles with peaks that correlate to night-time or the lack of light (Fuentes-Pardo et al., 2003). Additionally, the more irregular cycles of the young crayfish prove that the circadian patterns of motor activities are innate biological processes present even in the early stages of animal development that gain stability during adulthood (Fuentes-Pardo et al. 2003). This demonstrates how mathematical modelling can reveal the interesting sleep-wake patterns of certain species in relation to light sensitivity of circadian rhythms.
The two-process model of sleep
The most generalized modelling of sleep-wake cycles is described by the two-process model which describes sleep in terms of circadian and homeostatic processes (Borbely, 1982). This model, as described by Figure 8, contains two circadian boundaries containing the homeostatic cycle of sleep pressure, H(t); therefore, this model determines the polyphasic sleep cycle within the circadian rhythm (Cardon et al., 2018). At the lower threshold, H–(t) = Ho + aC(t), the change from sleep to wake occurs where C(t) = sin(w(t-a)) is the periodic function of the circadian rhythm. Furthermore, the areas of the graph where H(t) is decreasing represents the time during which sleep occurs (Skeldon et al., 2014). Figure 3 illustrates the graph of the two-process model.

Although the commonly used two-process model relates the two foundational regulators of the sleep-wake process, it fails to relate the homeostatic pressure to physiological quantities (Nakoa et al., 2007). Another mathematical model of sleep is the Phillips and Robinson (PR) model which is based on the same processes of the aforementioned model, but instead makes approximations using two timescales, instead of just one (Skeldon et al. 2014). The first scale considers the circadian rhythm, while the second one considers fluctuations on the timescale of physical neuronal potentials to account for higher accuracy (Skeldon et al., 2014).
An interesting analysis using the two-process model of sleep is its application of how external environmental factors affect animal sleep cycles. For example, Skeldon et al. (2014) investigated the sleep patterns of captive animals in comparison to their wild counterparts while considering the opportunity cost of sleep of the wild animals such as activities like foraging for food and avoiding predators. Indeed, it was concluded that their sleep-wake model – a more sophisticated variation of the two-process model – accurately predicted the sleep-wake cycles of wild elephants and sloths based on the differences of opportunity costs between the captive and wild animals along with each species’ circadian rhythm (Skeldon et al., 2014). Together, an understanding of the periodic circadian rhythm combined with mathematical models of sleep cycles can reveal interesting characteristics of animal sleep behaviour.
Dreams
Animals may seem calm and inactive while they sleep, but many phenomena are occurring during this resting period. One of them, dreams, represent an important part of the sleep of animals even if it is not obvious or easy to detect. According to specialists, dreams occur most of the time during rapid eye movements sleep (REM) and non-rapid eye movements sleep. It has been recorded that mammals among other types of animals experience this kind of sleep and therefore could have some dreaming episodes (Manger et al., 2020).
Many areas of the brain are involved in the development of dreams for mammals. The hippocampus could be responsible for the reproduction and somehow deformation of memories in the dreams since its activity increases during REM sleep. The amygdala also shows more activity during REM and NREM sleep (non-rapid-eye movements) (De Gennaro et al., 2011). This region of the brain is known to be the bridge between emotions and memories and could explain how dreams can seem so real, like a dog that starts moving its paws while it sleeps as if it was running (Salzman, 2019). However, dreams are still partly a mystery to scientists, so it is complicated to evaluate with certainty if animals experience dream mentation. Also, there is a possibility that dreams could occur during other stages of sleep for animals that have peculiar sleep patterns. For example, the rock hyrax, a mammal, has three specific stages of sleep: REM, NREM, and SI. SI, somnus innominatus is “a sleep state characterized as a form of low-voltage slow wave sleep” and presents both characteristics of REM and NREM sleep (Manger et al., 2020). Therefore, this animal could experience different types of dreams depending on the amplitude of the waves recorded during each type of sleep, with an additional peculiar type occurring during SI since most mammals do not experience this stage of sleep.
Memory
It is well known that memory is related to sleep and that resting improves the amount of information that is retained. Memories can be defined as information that is stored, encoded, and that can be retrained at any time by the brain (Zlotnik & Vansintjan, 2019). As mentioned before, the memory of an animal lets it recall, sometimes in a deformed way, some significant moments during dreams. There exists also a correlation between the amount of sleep an animal gets and its ability to retain information, that is, to encode the information in its brain. One of the most important parts of the formation of memories is their consolidation. Memories are formed after a stimulus, or a signal given by the brain is sent to let neurons make a connection. This link is initially weak and can eventually break and therefore the memory will be forgotten if it is not consolidated enough. A memory with strong emotions involved will be more retained, and a skill that is practiced a lot will also have more chances to make strong links between the neurons. This consolidation was observed in studies with rats to occur mostly during REM sleep, since it increases a couple of hours after a training session where the animals learned something, like solving a maze, and therefore made new memories. Those studies were however criticized since other research showed that non-REM sleep also increases on certain occasions after learning while REM sleep would either be constant or decrease (Rasch et al., 2013).
There are nevertheless no doubts that memories are strongly dependent on sleep even if the stage in which the consolidation is the most important is not absolutely defined. Some assumptions about the formation of memories propose that the information is first processed and encoded in a “fast learning store”, a role played by the hippocampus, before being stored for long-term in the neocortex. Fast-learning storage is quite effective when an ability is quickly acquired, but the memories are exposed to interference with the new information that is constantly entering the brain. The encoded information will be repeated and recalled during sleep which lets it be strengthened and find a way to the long-term storage and therefore be retained for a longer period. Those new memories will also have to be adapted to the existing ones (Rasch et al., 2013).
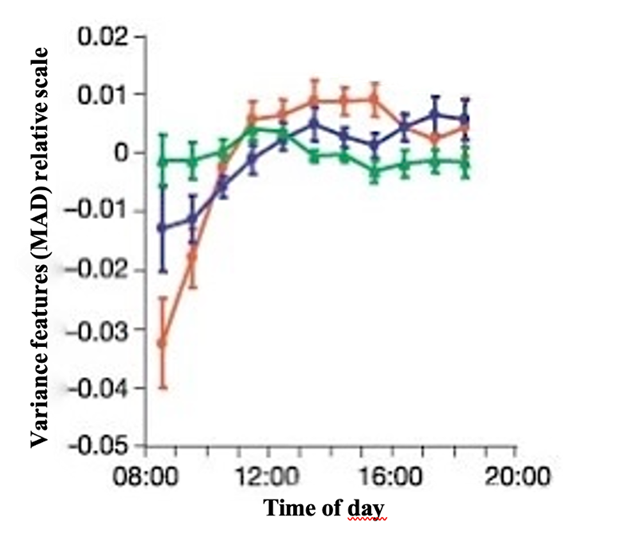
An excellent demonstration of this memory consolidation during sleep is the developmental learning capacities of birds. Some species can learn to reproduce songs and it has been proven that there exists a sleep-associated oscillation of their performance. A study analyzed this correlation by recording song development for a group of 50 zebra finches and analyzing the song activity at a frequency of 44.1 kHz. Many factors like the duration, mean pitch and mean frequency modulation among others helped understand the role of sleep for developmental learning. It was observed that the “vocal changes after 12 h of night-sleep were larger than the overall changes that occurred during 24 h”, leading to the conclusion that the function relating to the vocal changes must be non-monotonic and oscillating on a 24-hour cycle (Derégnaucourt et al., 2005).
Figure 9 shows the variance features between the original songs and the ones performed using their median absolute deviation from the mean (MAD) throughout the day. It is observed that the syllables diversify less in the morning and slowly get more structured after a few hours with or without song training. It represents a relation between developmental learning in birds and therefore the creation of new memories or knowledge and sleep.
Discussion
The sleep experience in ordinary animals is mainly the product of NREM and REM episodes. These episodes exhibit characteristics, such as their wave amplitude and frequency, that can be modeled and expressed in mathematical terms, which enhances the understanding of the phenomena. When REM neurons are active, and information is being consolidated in the hippocampus, the pleasant experience of dreaming might arise. There also exists exotic sleep behaviours, like USWS or SI that differ from the standard ones. Nevertheless, they provide an advantage to the corresponding species. On the other hand, NREM and REM neuronal groups and the two-way process that leads to the circadian cycle can be thought as oscillators that can be described as functions with surprising accuracy, some of these equations arise in other problems in different science fields, such as electricity, or musical synchronization. If different fields that seem unrelated are in fact described by the same model, then it might lead to important discoveries.
Conclusion
By analyzing electrical activity in the brain, two distinct stages of sleep have been identified within mammals: NREM and REM sleep. Each phase is distinguished by the characteristic properties of their respective waves: NREM brain waves are predominantly slower, high-voltage delta waves, while REM brain waves are similar to brain waves during wakefulness in that they are low-amplitude and are characterized by faster theta waves. However, electromyography recordings show that the electrical activity in the musculoskeletal is virtually absent, leading to the muscular immobility during REM sleep that differentiates its electrical activity from wakefulness. Additionally, during NREM sleep, bursts of high-frequency waves known as spindles have been linked to memory consolidation. Moreover, certain species exhibit a fascinating ability to engage in NREM sleep in one brain hemisphere, while maintaining wakeful vigilance in the other half.
Cells responsible for REM and NREM episodes undergo a synchronization phenomenon with its own natural frequency and phase. Simple equations such as the Kuramoto model help explain their behaviour, and why REM neuron activity and NREM neuron activity seems inversely proportional.
The innate periodic nature of certain key biological processes such as the regulation of animal sleep-wake cycles through circadian rhythm allow for the important mathematical modelling and analysis of such processes. Although there exists several mathematical models of animal sleep, there is still much potential for the development of new models with higher accuracy by taking into considerations more external factors or the smaller cellular interactions of circadian activity. On the other hand, analyzing whether they experience dream mentation or not is quite complex. However, the activity of certain areas of the brain of mammals like the hippocampus and the amygdala proves that those animals could dream during REM and NREM sleep stages. Memories are recalled during the night which leads to emotional reactions and consolidation of those memories as well. Consequently, sleep plays an important role in the development of new memories and learning by storing and encoding the information thus allowing the animal to access it.
References
Acebrón, J. A., Bonilla, L. L., Pérez Vicente, C. J., Ritort, F., & Spigler, R. (2005). The Kuramoto model: A simple paradigm for synchronization phenomena. Reviews of Modern Physics, 77(1), 137-185. https://doi.org/10.1103/RevModPhys.77.137
Acquistapace, P., Candeloro, A. P., Georgiev, V., & Manca, M. L. (2016). Mathematical phase model of neural populations intreactino in modulation of rem/nrem sleep. Mathematical Modelling and Analysis, 21(6), 794-810. https://doi.org/10.3846/13926292.2016.1247302
Asgari-Targhi, A., & Klerman, E. B. (2019). Mathematical modeling of circadian rhythms. Wiley Interdiscip Rev Syst Biol Med, 11(2), e1439. https://doi.org/10.1002/wsbm.1439
Borbély, A. A. (1982). A two process model of sleep regulation. Hum neurobiol, 1(3), 195-204.
Cardon, J. H., Eide, E. R., Phillips, K. L., & Showalter, M. H. (2018). Interacting circadian and homeostatic processes with opportunity cost: A mathematical model of sleep with application to two mammalian species. PLOS ONE, 13(12), e0208043. https://doi.org/10.1371/journal.pone.0208043
De Gennaro, L., Cipolli, C., Cherubini, A., Assogna, F., Cacciari, C., Marzano, C., Curcio, G., Ferrara, M., Caltagirone, C., & Spalletta, G. (2011). Amygdala and hippocampus volumetry and diffusivity in relation to dreaming. Hum Brain Mapp, 32(9), 1458-1470. https://doi.org/10.1002/hbm.21120
Derégnaucourt, S., Mitra, P. P., Fehér, O., Pytte, C., & Tchernichovski, O. (2005). How sleep affects the developmental learning of bird song. Nature, 433(7027), 710-716. https://doi.org/10.1038/nature03275
Fuentes-Pardo, B., Guzmán-Gómez, A. M., Lara-Aparicio, M., & López de Medrano, S. (2003). A qualitative model of a motor circadian rhythm. Biosystems, 71(1-2), 61-69. https://doi.org/10.1016/s0303-2647(03)00110-2
Ganesh, B., Sai Venkata, V. P., Shivashanker, M., Sirisha, V., BabuRao, C. H., & Sreekanth, N. (2012). Sleep Paralysis. International Journal of Research in Pharmacy and Chemistry, 2(2). http://www.ijrpc.com/files/21-2123.pdf
Hastings, M. H., & Herzog, E. D. (2004). Clock genes, oscillators, and cellular networks in the suprachiasmatic nuclei. J Biol Rhythms, 19(5), 400-413. https://doi.org/10.1177/0748730404268786
Hobson, J. A., McCarley, R. W., & Wyzinski, P. W. (1975). Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science, 189(4196), 55-58. https://doi.org/10.1126/science.1094539
Hutchison, I. C., & Rathore, S. (2015). The role of REM sleep theta activity in emotional memory [Review]. Frontiers in Psychology, 6. https://doi.org/10.3389/fpsyg.2015.01439
Iotchev, I. B., & Kubinyi, E. (2021). Shared and unique features of mammalian sleep spindles – insights from new and old animal models. Biological Reviews, 96(3), 1021-1034. https://doi.org/https://doi.org/10.1111/brv.12688
Latchoumane, C. V., Ngo, H. V., Born, J., & Shin, H. S. (2017). Thalamic Spindles Promote Memory Formation during Sleep through Triple Phase-Locking of Cortical, Thalamic, and Hippocampal Rhythms. Neuron, 95(2), 424-435.e426. https://doi.org/10.1016/j.neuron.2017.06.025
Lowrey, P. L., & Takahashi, J. S. (2000). Genetics of the mammalian circadian system: Photic entrainment, circadian pacemaker mechanisms, and posttranslational regulation. Annu Rev Genet, 34, 533-562. https://doi.org/10.1146/annurev.genet.34.1.533
Manger, P. R., & Siegel, J. M. (2020). Do all mammals dream? Journal of Comparative Neurology, 528(17), 3198-3204. https://doi.org/https://doi.org/10.1002/cne.24860
Mascetti, G. G. (2016). Unihemispheric sleep and asymmetrical sleep: behavioral, neurophysiological, and functional perspectives. Nature and science of sleep, 8, 221-238. https://doi.org/10.2147/nss.S71970
McCarley, R. W., & Hobson, J. A. (1975). Neuronal excitability modulation over the sleep cycle: a structural and mathematical model. Science, 189(4196), 58-60. https://doi.org/10.1126/science.1135627
Murray Sherman, S., & Guillery, R. W. (2001). Chapter VII – Maps in the Brain. In S. Murray Sherman & R. W. Guillery (Eds.), Exploring the Thalamus (pp. 197-227). Academic Press. https://doi.org/https://doi.org/10.1016/B978-012305460-9/50021-6
Nakao, M., Karashima, A., & Katayama, N. (2007). Mathematical models of regulatory mechanisms of sleep-wake rhythms. Cell Mol Life Sci, 64(10), 1236-1243. https://doi.org/10.1007/s00018-007-6534-z
Nicolau, M. C., Akaârir, M., Gamundí, A., González, J., & Rial, R. V. (2000). Why we sleep: the evolutionary pathway to the mammalian sleep. Prog Neurobiol, 62(4), 379-406. https://doi.org/10.1016/s0301-0082(00)00013-7
Rasch, B., & Born, J. (2013). About sleep’s role in memory. Physiol Rev, 93(2), 681-766. https://doi.org/10.1152/physrev.00032.2012
Salzman, C. D. (2019, February 27). amygdala. Encyclopedia Britannica. Retrieved November 25, 2021, from https://www.britannica.com/science/amygdala
Siegel, J. M. (2005). Clues to the functions of mammalian sleep. Nature, 437(7063), 1264-1271. https://doi.org/10.1038/nature04285
Skeldon, A. C., Dijk, D.-J., & Derks, G. (2014). Mathematical Models for Sleep-Wake Dynamics: Comparison of the Two-Process Model and a Mutual Inhibition Neuronal Model. PLOS ONE, 9(8), e103877. https://doi.org/10.1371/journal.pone.0103877
van der Meij, J., Martinez-Gonzalez, D., Beckers, G. J. L., & Rattenborg, N. C. (2019). Intra-“cortical” activity during avian non-REM and REM sleep: Variant and invariant traits between birds and mammals. Sleep: Journal of Sleep and Sleep Disorders Research, 42(2), 1-13. https://doi.org/10.1093/sleep/zsy230
Zepelin, H., Siegel, J. M., & Tobler, I. (2005). Chapter 8 – Mammalian Sleep. In M. H. Kryger, T. Roth, & W. C. Dement (Eds.), Principles and Practice of Sleep Medicine (Fourth Edition) (pp. 91-100). W.B. Saunders. https://doi.org/https://doi.org/10.1016/B0-72-160797-7/50015-X
Zlotnik, G., & Vansintjan, A. (2019). Memory: An Extended Definition [Perspective]. Frontiers in Psychology, 10. https://doi.org/10.3389/fpsyg.2019.02523