A Neurochemical Overview of Sleep, its Deprivation and Hibernation
Kelliane Beland, Taisei Fu, Andres Gonzalez, Grace Shi
Abstract
The phenomenon of sleep has been universally observed among animals, and is driven by a plethora of chemical processes that regulate the animal's brain activity. The onset of sleep brings about a number of chemical changes driven by various neurotransmitters. These changes allow the brain to carry out and improve the efficiency of its necessary functions during sleep; notably, the elimination of waste is enhanced, while the microglia responsible for the brain's immune defense and plasticity are improved. By contrast, sleep deprivation can cause a number of negative impacts that affect the organism's memory and learning abilities. Moreover, these sleep-enabling systems are also pliable, and allow for the maintenance of animal health through unique sleeping states such as hibernation. Thus, analyzing the chemistry behind sleep can prove instrumental in understanding a fundamental part of the lives of nearly all animals.
Introduction
Sleep is one of the vital needs of animals and is implicated in different functions of the body and its organs. It is the state in which animals induce upon themselves to recover from the loss of energy resulting from their daily activities. The cells rely a lot on sleep and are strongly affected by this resting period since certain proteins, neurons, and enzymatic receptors among many others base their activity on sleep. Consequently, analyzing sleep from a chemistry perspective helps understand several functions of anatomical subunits of a living organism's body. On the other hand, sleep deprivation can lead to medical issues considering that memory and learning capacities rely on a good amount of sleep. Animals can also use sleep to their advantage, for example, to go through a harsh winter using hibernation. Thus, exploring the mechanisms of sleep can prove beneficial in understanding and engineering for a universal aspect of life.
Neurotransmitters involved in sleep stages in mammals
Mammalian sleep can be subdivided into two types of sleep each occurring multiple times during the night (for diurnal species). In most mammalian species, sleep is initiated as NREM (non-rapid eye movement sleep), then transitions into REM (rapid eye movement) sleep (Datta, 2010).
NREM sleep is characterized by a decrease in body temperature, blood rate, heart rate and respiratory rate. In terms of brain activity, NREM is characterized by a switch from low-amplitude high-frequency state to a high-amplitude low-frequency state (delta waves) (Pace-Schott & Hobson 2002). Higher secretion of sex and growth hormones is typical for this type of sleep. NREM sleep is followed by REM sleep which is characterized by a low-amplitude high-frequency state similar to a wakefulness state (Fig.1). In this form, the individual experiences the characteristic rapid eye movements, facial muscle twitches, large cardio-respiratory rhythm fluctuations, penile erections for the males and clitoral engorgement for the females and muscle atonia (Datta, 2010).
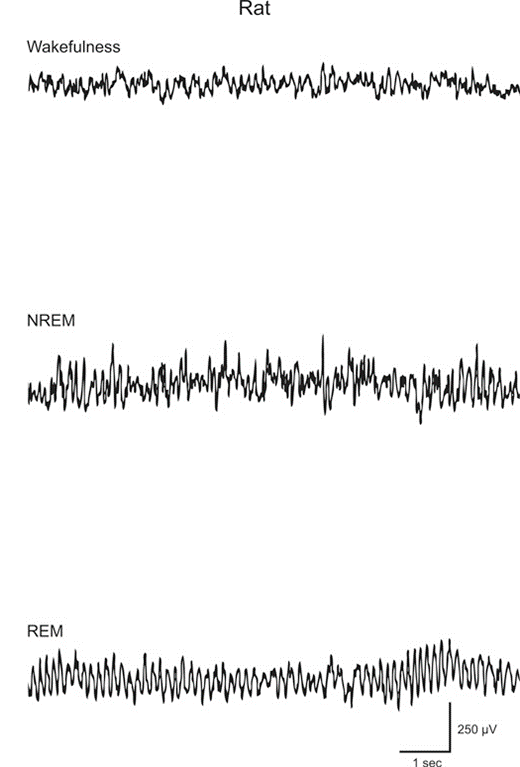
In cats and rats NREM and REM sleep follow a cycle that repeats itself throughout the day, except when the animal is obviously awake. The vast majority of each cycle is spent under NREM sleep (80%), while the remaining is spent in REM sleep (20%) (Datta, 2010). Each type of sleep is regulated by its own neurotransmitters that will be explored in depth in the following pages.
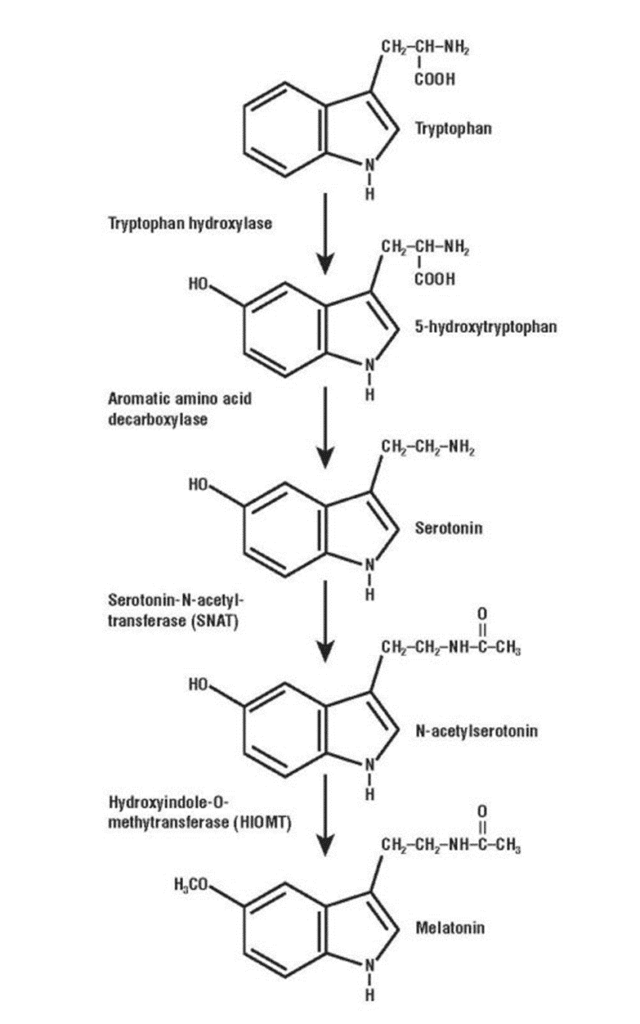
Diurnal species are regulated through the circadian rhythm and melatonin. When sunlight enters the retina, a signal is sent to the suprachiasmatic nucleus whose role is to inhibit the production of melatonin in the pineal gland. When night falls, the cycle is interrupted and melatonin synthesis from tryptophan can start (Fig. 2). This chemical is responsible for the feeling of drowsiness and signals the individual it is time to sleep (Harper, 2015). Furthermore, the accepted theory for the initiation of SWS-1 in most mammal species is a passive-active mechanism guided by the homeostatic regulation of metabolites (Datta, 2010). Metabolites are substances produced during wakefulness and whose concentration increases proportionately to the time awake. Some substances are a subproduct of wake promoting neurons and are harmful if not disposed correctly. GABA and glycine, prostaglandin, cytokines and tumor necrosis factor alpha are the known metabolites (Datta & MacLean, 2007). A high concentration of these substances triggers a strong sleep signal, transitioning the animal body from wakefulness to sleep.
The “loss of consciousness” induced by sleep is the direct product of changes occurring in the thalamic reticular nucleus (Fig. 3). This part of the brain consists of two types of neurons: thalamo-cortical relay neurons, responsible for transferring signals from the brainstem to the cortex, and thalamo reticular neurons, which inhibit the actions of the first type (Gent et al., 2018). As metabolite concentration increases, the efficiency of serotonergic, noradrenergic and cholinergic cells decreases in the brainstem (note all the aforementioned neurons respond to waking associated chemicals serotonin, noradrenaline and acetylcholine). This causes the activity of thalamo-cortical neurons to decrease, but the excitability of thalamo reticular neurons to increase (Datta, 1995). Thalamo reticular neurons further inhibit the action of thalamo-cortical neurons by secreting GABA (γ-aminobutyric acid). GABA is the main neurotransmitter inhibiter in the brain. The means of action of this chemical is by shutting a neuron's potential when received, and therefore removing the possibility for the neuron to communicate (Datta, 2010). As the thalamo-cortical neurons are shutted, the cortex becomes isolated from the outside world. The thalamo reticular neurons are mostly the only cells highly active in the brain during SWS-1, their activity is responsible for the characteristic waves in SWS-1.
SWS-II in mammals is initiated by the preoptic area (POA) in the hypothalamus. Just like the thalamo reticular neurons, but in a much larger scale, this area operates by inhibiting wake-promoting areas of the brain using GABA and galanin (Gaus et al., 2002). The aforementioned area also produces the delta waves seen in the EEG. Growth hormone (GH) has been shown to intensify the activity of this area (Bredow et al., 1996), as a result, the animal body falls in a deep long-lasting sleep.
The mechanisms of REM sleep are far more diverse than the ones responsible for NREM sleep. In fact, many areas of the brain are actively involved in maintaining REM sleep. The system is initiated by a disproportion between aminergic and cholinergic neurotransmitters in the PPT (pedunculopontine tegmental); when awake, both concentrations are proportionate, but when sleep occurs, aminergic substances' concentration drops while cholinergic substances' concentration increases, as a result REM sleep is initiated by cholinergic cells (McGinty & Harper, 1976).
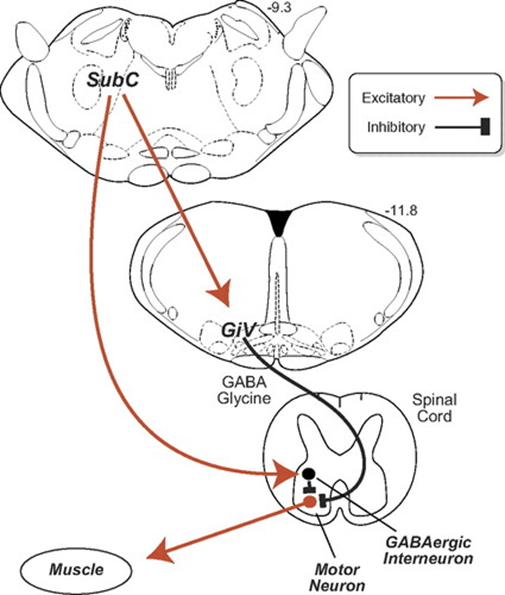
Muscle atonia during REM sleep is induced by changes in the subcoeruleus nucleus (SubC). Current knowledge suggests that the activity of this part of the brain sparks during REM sleep, inducing a control pathway that uses GABA and glycine as main inhibitors (Fig. 4). Glycine hyperpolarizes motoneurons by promoting a strong inhibitory postsynaptic potential, which makes the cell far less likely to reactivate (Chase & Morales, 1990). There is evidence other mechanisms are involved, although there is no consensus among scientists (Brooks & Peever, 2008). Lesions in this control pathway lead to unusual behaviors during sleep such as moving while dreaming.
The three stages of sleep in non-human mammals are the manifestation of complex interactions of different parts of the brain and their neurotransmitters. The most common way for the brain to induce sleep is by inhibiting the potential of wake-related neurons through GABA and glycine. On a chemical level, sleep's role is to clean the body from harmful substances; the mechanisms of this function will be explored in the next chapter.
The Improved Clearance of Waste in the Central Nervous System
As mentioned earlier, metabolites must be eliminated from the brain since neurons are particularly sensitive to toxic waste products. Indeed, certain proteins that build up in the brain as a result of cell metabolism, such as beta-amyloid, tau, and alpha-synuclein, must be removed, and their buildups have been associated with a number of neurodegenerative diseases (Xie et al., 2013). However, unlike the rest of the body, the brain does not possess a lymphatic system to flush out these products. Instead, it uses further metabolism, transport across the blood-brain barrier as well as perivascular clearance (Hladky & Barrand, 2019). The last consists of the flow of cerebrospinal fluid (CSF), combined with the CSF's interactions with interstitial fluid (ISF), to effectively remove unwanted by-products. Channels for the inflow of CSF surround the brain's arteries, while ISF outflows via perivascular channels surrounding cerebral veins. This network of the brain's metabolic waste clearance is known as the glymphatic system.
Changes in this glymphatic system during sleep are indicative of its benefits for various organisms. For instance, the rate of flow in these perivascular networks has been shown to be greatly increased compared to periods of wakefulness. Indeed, in mice, the aforementioned fluid influx into the brain, via the channels surrounding the arteries, increased by approximately 95% during sleep compared to levels during wakeful periods (Xie et al., 2013). This change in flow can be attributed to a reduction in the volume of overall interstitial space during wakefulness. As a matter of fact, interstitial tissue increases in size by 60% during sleep, which decreases the tissue's resistance to fluid flow, which in turn leads to the increase in CSF and ISF flow (Xie et al., 2013). This phenomenon is illustrated in Figure 5, which shows that convection of CSF is greatly inhibited during waking hours when compared to sleep. It has been observed that this increase in volume can be attributed to the neurotransmitters present during arousal (wakefulness). In particular, the inhibition of adrenergic signaling during sleep has been shown to lead to a decrease in cell size, which leads to an increase in overall interstitial volume (Xie et al., 2013). The ensuing greater flow rate improves the efficiency of waste clearance; this is illustrated in Figure 6 by an increased efficiency in clearing the protein beta-amyloid via improved convection. It is noted that the rates of clearance are compared and not merely the rate of elimination, since the latter can vary depending on the concentration of waste, while the former expresses the efficiency of waste elimination. Thus, the expansion of interstitial tissue during sleep leads to a greater flow of CSF and ISF via perivascular channels, which in turn causes a more efficient elimination of potentially harmful proteins that are the products of cell metabolism in the brain.
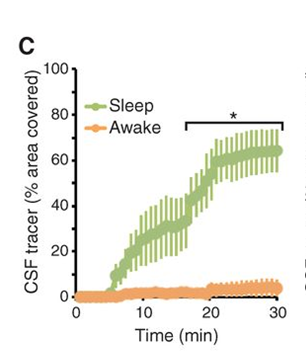
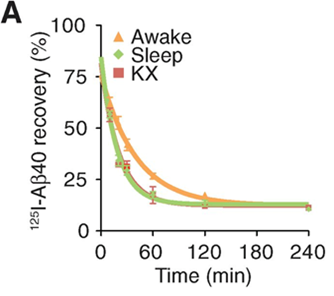
Thus, given that waste clearance has been shown to significantly increase during sleep, it has been proposed as one of the primary functions of sleep for various organisms. Indeed, this change is not limited to mammals, as an augmentation of perivascular clearance has also been observed in invertebrates such as fruit flies during their deep sleep (van Alphen et al., 2021). This provides evolutionary support that improved waste clearance from the brain via perivascular clearance is a fundamental purpose of sleep, since this pattern occurs across the phylogenetic tree, including invertebrates and vertebrates.
Increased activation of the brain's immune system during sleep
Additionally, the mammalian central nervous system's immune defense is more activated during sleep than it is while awake. This is illustrated in the contrast in states of microglial cells during wakefulness and sleep. Microglial cells are a type of glial cell in the brain which serve as the main immune cells that defend the brain and central nervous system. Their mechanisms are mainly used to conduct surveillance and react to traumas and injuries (Stowell et al., 2019). As illustrated in Figure 7, Microglia have β2-adrenergic receptors which are activated by norepinephrine, a neurotransmitter released almost exclusively during wakefulness. This activation results in the shortening of the microglia's arbors. This results in less spatial coverage by these arbors, and thus restricts the microglia's ability to defend the brain and react to injuries, shocks, and other stimuli in the CNS.
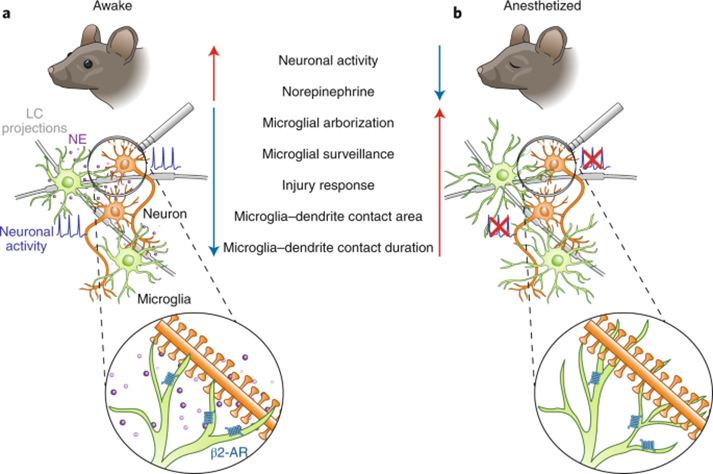
Furthermore, microglia play a large role in maintaining neuroplasticity, which is the brain's ability to change and reorganize networks of neurons. This is accomplished through contact with the neuron's synapses (Stowell et al., 2019). The presence of norepinephrine activating β2-adrenergic receptors inhibits this plasticity by shortening the arbors of the microglia, thereby altering the interactions between microglia and neurons, and changing both the extent and duration of microglia-neuron interactions.
Thus, the increase in the efficacy of waste clearance in the brain helps explain the necessity of sleep, and why it is evolutionarily advantageous for organisms to sleep despite the risks associated with their vulnerability associated with the decreased arousal and immobility that sleep entails. In addition, the absence of norepinephrine released during sleep results in microglia with more complex and longer arbors. This leads to an improved immune response in the CNS, as well as increased neural plasticity.
Sleep deprivation
As mentioned, sleep's main function is to establish homeostasis in metabolites. Furthermore, it allows the regulation of the metabolism and recuperation of energy. It is obvious that a lack of sleep may lead to certain damage and eventually to permanent issues for a living's body. Sleep deprivation has an underestimated impact on the brain and its cognitive functions, the proteins of the body, and even the tolerance of certain specific molecules.
The brain cells that are responsible for memory and cognitive abilities such as learning are called neurons and have a lot of interesting peculiarities that make those cells even more in need of a moment of recuperation. In most if not all animals, this precious phase of recuperation takes place while they sleep. Consequently, the neurons can develop some important dysfunctions or issues that can lead to cognitive deficiency. More precisely, the main area of the brain influenced by this lack of sleep is called the hippocampus which represents the home of memories. The hippocampus is in charge of different kinds of memory such as spatial, recognition, episodic and semantic memory. This specific area of the brain is linking emotions and memories and consolidates them (Looti Bashiyan et al., 2021). Therefore, it is also responsible for the learning capacities of certain animals since repeating a type of movement or behavior re-enforces the memory of this ability and eventually leads to an acquired knowledge. However, those links that make memories are not eternal and can get weaker over time if they are not solicited, that is, if the memory is not brought up or if an ability is not practiced. Sleep deprivation also negatively influences this area of the brain.
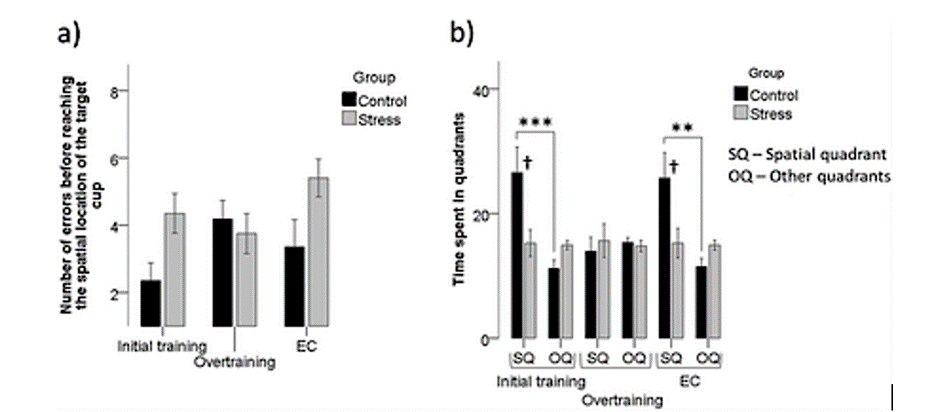
An example of the effect of oxidative stress such as sleep deprivation can be observed in parrots. This species of bird is one of the most intelligent kinds of animals on earth as those avians can learn to talk or do tricks if they are trained, even if they do not naturally behave this way in their original environment. This ability to train makes this species more protected from the oxidative stress that can be caused by sleep deprivation. The more they have trained and therefore the more knowledge they have, the less their memories will be affected. This last statement was demonstrated by a study led by Flore Lormant (2021) and her team in which they trained two groups of birds: one of them had a five-day training corresponding to an initial training while the other had ten additional days for an overtraining. Afterward, they were both exposed to chronic stress that has a similar effect than the one sleep deprivation has on the brain. As seen in the graph (Fig. 8), it was observed that the overtraining protected the birds at a certain point from the stress while the initial impact did not prevent in any considerable way the animals from being affected by the situation. In this study, it was the space memory that caused and proved the relation between learning and memory (Lormant et al., 2021).
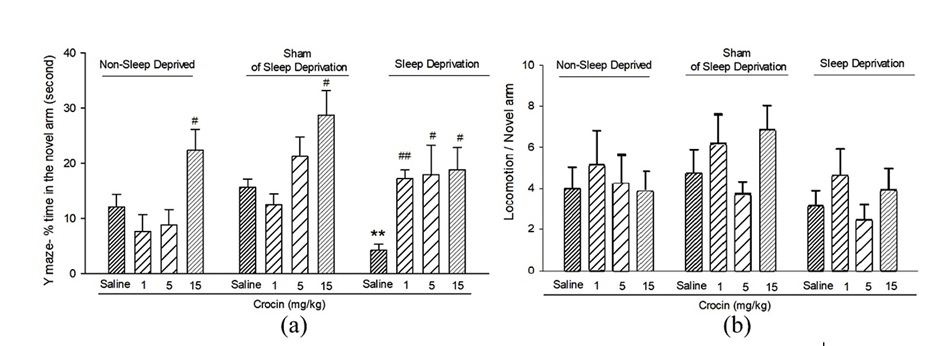
To explain this relationship, the activity of the molecule involved in memory and learning called the brain-derived neurotrophic factor, or BDNF must be analyzed in conditions of sleep deprivation. This protein is expressed mostly in the hippocampal neurons which are the cells of interest in the study of oxidative stress. The activity of BDNF will slow down while aging thus leading to memory deficit or other diseases. It is known that exercise and a great amount of sleep enhance its expression which can help understand why sleep deprivation can cause memory loss (Miranda et al., 2019). The impact of sleep deprivation on the expression of BDNF and therefore on memory deficits was studied through an experimental procedure where rats were put in a state of sleep deprivation and then tested on their ability to escape a maze. This study supports that total sleep deprivation (TSD) decreases the expression of BDNF and the genes in the right and left hemispheres of the hippocampus. Overall, TSD had a considerable effect on the memory of the rats since their regulatory protein was inhibited and their memory could not be consolidated as much as on normal conditions. The study also presented the positive effect of the injection of the molecule called crocin on memory function as can be observed in Figure 9 (Looti Bashiyan et al., 2021).
However, there exists one exception to the rule: elephants sleep approximately 2 hours per day in their natural environment and can even spend days without sleeping with no considerable impact on their activity or behaviors. There exists a relationship between the body mass of an animal and the amount of sleep it typically needs, but it does not apply to elephants. Those mammals and their ability to never experience sleep deprivation are still a mystery, but some differences in their anatomy and their brain were observed and could explain this peculiarity. In the neuronal arousal systems of some African elephants, the presence of a parvocellular cluster of orexinergic neurons and a nuclear subdivision in the periventricular gray matter may be the keys to their short amount of sleep (Gravett et al., 2017). The functions of the orexinergic neurons and their presence in a cluster could explain their sleep patterns since those cells are “implicated in the regulation of blood pressure, neuroendocrine functions, body temperature, the sleep-wake cycle, stimulation of food intake, increased arousal, energy homeostasis and locomotor activity” (Dell et al., 2012). This cluster is observed in most Cetartiodactyla mammals such as the giraffe which present similar sleep behaviors.
Hibernation
While sleep is practiced nearly everywhere throughout the animal kingdom, and allows animals to recuperate and conserve energy, hibernation is a type of specialized sleep evolved by select species which brings the notion of energy conservation via sleep to the next level. Hibernation is described as a survival technique characterized by a decrease in metabolic rates and lowering of body temperature, allowing the species to preserve energy without suffering from the scarcity of food during harsher conditions, such as winter (Kondo & Kondo, 1992). The maintenance of a balanced metabolism under such unique and sustained bodily and environmental circumstances calls for adequately corresponding biochemical changes and processes to ensure a healthy animal upon waking from hibernation.
The Revealing Blood Chemistry of Hibernating Animals
An analysis of the blood chemistry in various hibernating species provides insights as to how levels of specific proteins, blood cells and plasma components vary during hibernation to maintain homeostasis. An investigation into these blood components of both hypothermic hibernating species such as rodents, as well as larger species such as bears, will demonstrate how extreme energy conservation is achieved while simultaneously protecting the health of the animal in these different hibernation methods.
Hibernation in bears is especially interesting due to their large size and maintained high body temperature of around 30°C in which they hibernate (Welinder et al., 2016). For comparison, most hibernating species are much smaller and maintain a body temperature of just over 0°C in hibernation, thus bearing the title of hypothermic hibernators (Kondo & Kondo, 1992). The more apparent physiological changes observed in bear hibernation such as the 6°C drop in body temperature and the decrease in metabolic rate to roughly 25% of the summer rate compliment the animal's less obvious chemical blood changes including the coagulation response and selective suppression or increase of plasma proteins (Welinder et al., 2016). The graphs in Figure 10 give a visual representation of the drastic chemical changes from blood samples in terms of proteins, lipids, and amines in brown bears between the winter (hibernating) and summer (active) months (Welinder et al., 2016).
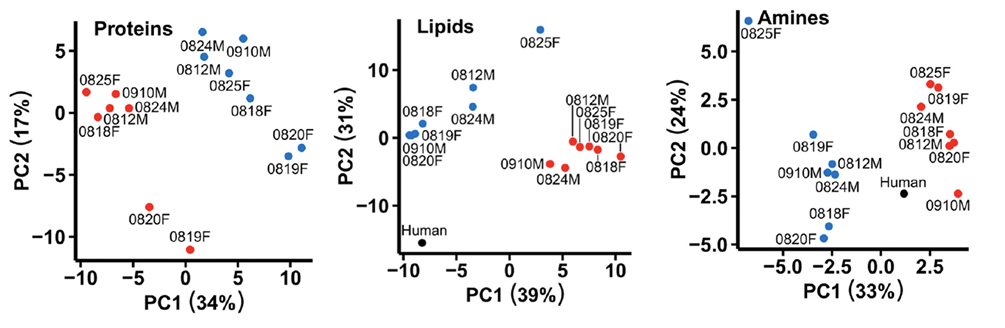
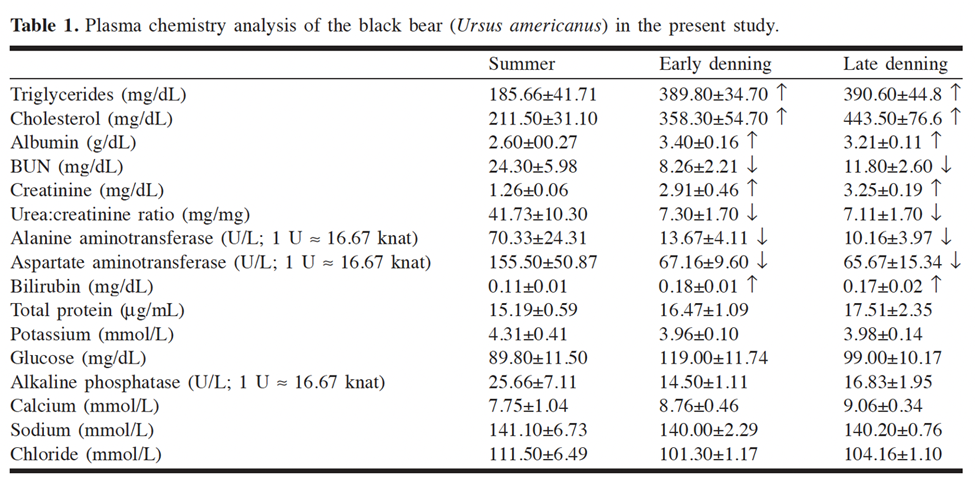
An overarching characteristic with important chemical consequences observed in brown bears is the increase in all plasma protein half-lives largely caused by the 5-7°C drop in body temperature during their extended torpor (Welinder et al., 2016). Observing Arrhenius' equation, chemical reaction rates at room temperature approximately double per 10°C increase in temperature. Therefore, the lowering of body temperature suppresses the reaction rate of protein degradation, in turn, conserving energy for the animal as it requires less synthesis of new proteins (Welinder et al., 2016). Likewise, a study on the blood chemistry of black bears revealed a decrease in the urea to creatine ratio (U:C) during hibernation (Fig. 11), which also corresponds to the saving of plasma proteins (Lohuis et al., 2005). A low U:C ratio indicates decreased protein breakdown since creatine is the product of muscle metabolism, and hibernation involves minimal muscle movement (Lohuis et al., 2005). Another chemical mechanism contributing to the goal of energy conservation through hibernation is the increase of red blood cells (hematocrit) that induce dehydration. Hibernating bears obtain water exclusively via fat metabolism as one mol of triglyceride (C56H110O6) and oxygen yield 55 mols or 1kg of water. This substantial dehydration further maintains plasma protein concentrations thus saving energy from novel protein synthesis (Welinder et al. 2016).
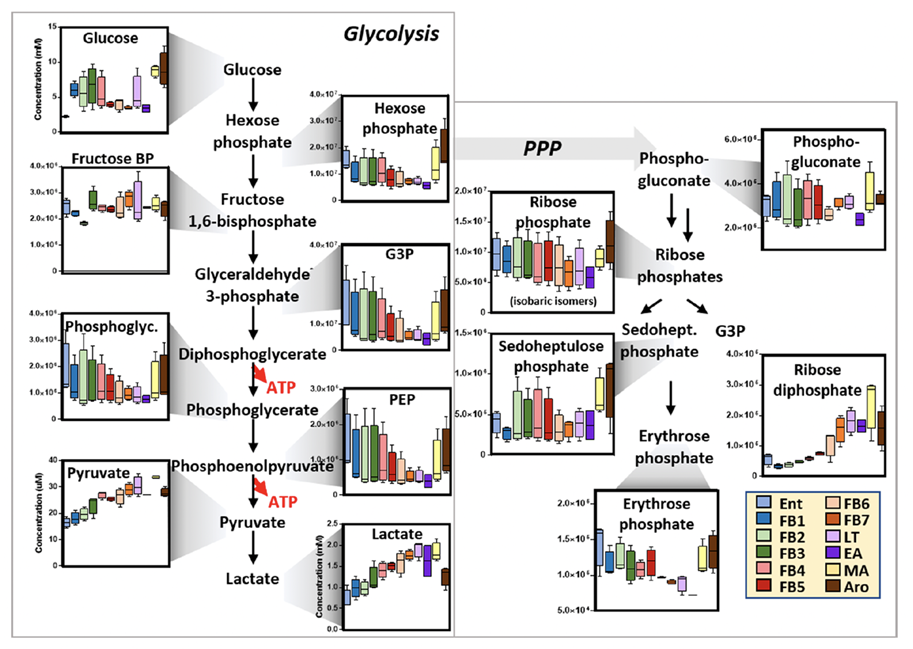
In contrast, the chemistry of hypothermic hibernators differs from that of bears as these species hibernate at near freezing body temperatures, thus requiring biochemical properties to withstand the intense span of body temperatures without jeopardizing the bodily functions and homeostasis. In 70% of winter hibernators, states of torpor and lowered body temperature are interrupted periodically by arousal periods characterized by sudden increases in metabolic rates (Gehrke et al., 2019). These interbout arousals replenish substrates such as lipids and amino acids in the blood consumed by metabolism at low temperature, as well as assist the removal of waste products accumulated in the blood (Gehrke et al., 2019). Significant chemical changes in red blood cells (RBCs) accompany the arousal periods and can be demonstrated by Figure 12, a representation of the changes in glucose metabolism of RBCs in arctic ground squirrels throughout periods of torpor and arousal in their hibernation (Gehrke et al. 2019). Not only is the blood chemistry adapted to this periodic hibernation strategy, but other biochemical compounds also demonstrate adaptive characteristics. Muscle G3PDH (glyceraldehyde-3-phosphate dehydrogenase), an important molecule in the linking carbohydrate and triglyceride metabolism, of the hibernating prairie dog has a less rigid conformation, but a greater temperature stability and is less vulnerable from denaturation when compared to that of a non-hibernating rabbit (de la Roche et al., 2012). Again, this allows the hypothermic hibernator's chemical substrates to maintain functional integrity within a large temperature interval.
Brain Chemistry of Hibernators
Analogous to how hibernating animals have developed elasticity in their blood chemistry functions to account for the extended periods of torpor, their brain chemistry also demonstrates adaptations to satisfy the extreme metabolic shift. The brain is a particularly crucial organ, so it is extremely necessary for the tolerance of hypothermia, hypoxia and hypoglycemia imposed by the decrease of oxygen and glucose cell supply, and the deceleration of blood flow during hibernation (Gordon et al., 2006). The ultimate goal of suppressing protein degradation to conserve energy is a shared commonality between the brain and blood chemistry of hibernating animals.
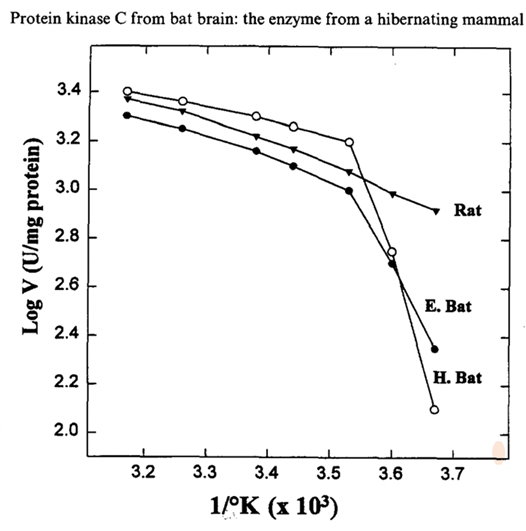
Specific brain proteins vary in function depending on the torpor, periodic arousal and normal states of hibernators. The protein kinase C (PKC) activity levels, abundant in bat brains, showed high variance caused by changes in enzyme properties and body temperature during hibernation, in which it dropped to 63% of the euthermic levels (Mehrani & Storey, 1997). A study compared the PKC of hibernating bats and non-hibernating rats at different temperatures (see Figure 13 for the Arrhenius Plot), where the main differences were that the bat PKC was highly sensitive at lower temperatures and also showed affinity to lipid activators (Mehrani & Storey, 1997). These alterations, exclusive to the bat PKC, align with the need for the protein to sustain affinity to the activators at cold temperatures during hibernation. In terms of the inhibition of neural protein activity, the start of hibernation is characterized by the inhibition of metabolic activities which actually precedes the lowering of body temperature (Gordon et al., 2006). As seen in arctic ground squirrels, entrance into hibernation is marked by an increase in inhibitory neurotransmitters accompanied by a decrease of excitatory transmitters (Gordon et al., 2006). Furthermore, data has shown that isolated hibernator peptides can also regulate the spontaneous biochemical neuronal activity of a non-hibernating rat (Kokoz et al., 1997).
Whether it is changes in the brain or blood chemistry of hibernators, these mechanisms allow the animal to carry out their survival strategy of using sleep to wait out a period of low resource availability, while conserving energy through the general suppression of protein degradation. These adaptive chemical systems in hibernating species allow them to remain healthy throughout conditions such as hypothermia and hypoglycemia which would be detrimental to non-hibernating animals. However, there is potential for the application of these mechanisms to remedy such conditions throughout other non-hibernating organisms, as demonstrated by the aforementioned isolated bat peptides inserted into rats.
Discussion
Sleep is essential for all animals on earth, however, it represents more than just a phase of recuperation for many of them. Understanding the chemical mechanism and the functions of different cells involved in the sleep of those peculiar animals could be the key to certain diseases. Bioengineers can create designs and get inspired by those natural phenomena and possibly find cures and solutions to hypothermia, Alzheimer's, brain injuries, mental disorders, among many others. Overall, the brain is the organ that is, most of the time, mainly implicated and affected by sleep. This state in which animals induce upon themselves is crucial in the regulation of hormones or proteins, the recuperation of the cells, and the maintenance of the metabolism as well. Some exceptions to a regular amount of sleep are impressive and highly interesting under an engineering perspective such as the deprivation and hibernation.
Conclusion
The mechanisms ruling sleep behaviour are profoundly correlated to the concentration of various known and unknown chemicals. The circadian rhythm regulates sleep time through melatonin in diurnal mammals. NREM sleep is induced by a high concentration of metabolites, which in turn activate GABA. GABA itself and glycine are inhibitors which limit the potential of neurons with surprising implications such as the suppression of motor neurons. REM sleep, the final stage, is regulated through a dance between aminergic and cholinergic cells. Also, one of the fundamental functions of sleep is clearance of waste that accumulates as a result of regular metabolic processes in the brain while the animal is awake. During sleep, the inhibition of adrenergic signaling results in higher levels of waste clearance by the convection of CSF and ISF, a phenomenon observed in both mammals and invertebrates. Furthermore, inhibition of norepinephrine during sleep leads to increased microglial size, which leads to improved immune defense and neuroplasticity. On the other hand, sleep deprivation has an important impact on the cognitive abilities of animals, and they must avoid being exposed to this oxidative stress. Their memories and learning capacities are directly linked to the expression of a specific molecule, the BDNF, which has a significantly lower level in the brain when an animal is in the state of sleep deprivation. The opposite, sleeping for a considerably long amount of time also has an interesting mechanism. The intense conditions of hibernation are made possible and regulated by the corresponding chemical flux of the brain and blood, largely involving the lengthening of protein half-lives. These adaptive chemical mechanisms allow hibernators to advantageously conserve energy through a unique sleeping state of suppressed metabolism which would be destructive for non-hibernators.
References
Bredow, S., Taishi, P., Obél, F., Jr., Guha-Thakurta, N., & Krueger, J. M. (1996). Hypothalamic growth hormone-releasing hormone mRNA varies across the day in rats. Neuroreport, 7(15-17), 2501-2505. https://doi.org/10.1097/00001756-199611040-00020
Brooks, P. L., & Peever, J. H. (2008). Glycinergic and GABA(A)-mediated inhibition of somatic motoneurons does not mediate rapid eye movement sleep motor atonia. J Neurosci, 28(14), 3535-3545. https://doi.org/10.1523/jneurosci.5023-07.2008
Brown, R. E., Basheer, R., McKenna, J. T., Strecker, R. E., & McCarley, R. W. (2012). Control of sleep and wakefulness. Physiol Rev, 92(3), 1087-1187. https://doi.org/10.1152/physrev.00032.2011
Chase, M. H., & Morales, F. R. (1990). The atonia and myoclonia of active (REM) sleep. Annu Rev Psychol, 41, 557-584. https://doi.org/10.1146/annurev.ps.41.020190.003013
Datta, S. (1995). Neuronal activity in the peribrachial area: relationship to behavioral state control. Neurosci Biobehav Rev, 19(1), 67-84. https://doi.org/10.1016/0149-7634(94)00043-z
Datta, S. (2010). Cellular and chemical neuroscience of mammalian sleep. Sleep Med, 11(5), 431-440. https://doi.org/10.1016/j.sleep.2010.02.002
Datta, S., & Maclean, R. R. (2007). Neurobiological mechanisms for the regulation of mammalian sleep-wake behavior: reinterpretation of historical evidence and inclusion of contemporary cellular and molecular evidence. Neurosci Biobehav Rev, 31(5), 775-824. https://doi.org/10.1016/j.neubiorev.2007.02.004
de la Roche, M., Tessier, S. N., & Storey, K. B. (2012). Structural and functional properties of glycerol-3-phosphate dehydrogenase from a mammalian hibernator. Protein J, 31(2), 109-119. https://doi.org/10.1007/s10930-011-9376-3
Dell, L. A., Patzke, N., Bhagwandin, A., Bux, F., Fuxe, K., Barber, G., Siegel, J. M., & Manger, P. R. (2012). Organization and number of orexinergic neurons in the hypothalamus of two species of Cetartiodactyla: a comparison of giraffe (Giraffa camelopardalis) and harbour porpoise (Phocoena phocoena). J Chem Neuroanat, 44(2), 98-109. https://doi.org/10.1016/j.jchemneu.2012.06.001
Gaus, S. E., Strecker, R. E., Tate, B. A., Parker, R. A., & Saper, C. B. (2002). Ventrolateral preoptic nucleus contains sleep-active, galaninergic neurons in multiple mammalian species. Barrel Cortex Function, 115(1), 285-294. https://doi.org/10.1016/s0306-4522(02)00308-1
Gehrke, S., Rice, S., Stefanoni, D., Wilkerson, R. B., Nemkov, T., Reisz, J. A., Hansen, K. C., Lucas, A., Cabrales, P., Drew, K., & D'Alessandro, A. (2019). Red Blood Cell Metabolic Responses to Torpor and Arousal in the Hibernator Arctic Ground Squirrel. J Proteome Res, 18(4), 1827-1841. https://doi.org/10.1021/acs.jproteome.9b00018
Gent, T. C., Bassetti, C., & Adamantidis, A. R. (2018). Sleep-wake control and the thalamus. Curr Opin Neurobiol, 52, 188-197. https://doi.org/10.1016/j.conb.2018.08.002
Gordon, R. Y., Ignatiev, D. A., Rogachevskii, V. V., Medvedev, N. I., Kraev, I. V., Patrushev, I. V., Khutsyan, S. S., & Popov, V. I. (2006). Changes of activity of the protein-synthesizing system of brain neurons of the ground squirrel Citellus undulatus during hibernation and hypothermia. Journal of Evolutionary Biochemistry and Physiology, 42(3), 299-307. https://doi.org/10.1134/S0022093006030082
Gravett, N., Bhagwandin, A., Sutcliffe, R., Landen, K., Chase, M. J., Lyamin, O. I., Siegel, J. M., & Manger, P. R. (2017). Inactivity/sleep in two wild free-roaming African elephant matriarchs – Does large body size make elephants the shortest mammalian sleepers? PLOS ONE, 12(3), e0171903. https://doi.org/10.1371/journal.pone.0171903
Harper, K. (2015, January). The Science of Sleep. ACS. https://www.acs.org/content/acs/en/education/resources/highschool/chemmatters/past-issues/archive-2014-2015/the-science-of-sleep.html
Hladky, S. B., & Barrand, M. A. (2019). Metabolite Clearance During Wakefulness and Sleep. In H.-P. Landolt & D.-J. Dijk (Eds.), Sleep-Wake Neurobiology and Pharmacology (pp. 385-423). Springer International Publishing. https://doi.org/10.1007/164_2017_37
Kokoz, Y. M., Zenchenko, K. I., Alekseev, A. E., Korystova, A. F., Lankina, D. A., Ziganshin, R. H., Mikhaleva, II, & Ivanov, V. T. (1997). The effect of some peptides from the hibernating brain on Ca2+ current in cardiac cells and on the activity of septal neurons. FEBS Lett, 411(1), 71-76. https://doi.org/10.1016/s0014-5793(97)00607-8
Kondo, N., & Kondo, J. (1992). Identification of novel blood proteins specific for mammalian hibernation. J Biol Chem, 267(1), 473-478.
Lohuis, T. D., Beck, T. D. I., & Harlow, H. J. (2005). Hibernating black bears have blood chemistry and plasma amino acid profiles that are indicative of long-term adaptive fasting. Canadian Journal of Zoology, 83(9), 1257-1263. https://doi.org/10.1139/z05-120
Looti Bashiyan, M., Nasehi, M., Vaseghi, S., & Khalifeh, S. (2021). Investigating the effect of crocin on memory deficits induced by total sleep deprivation (TSD) with respect to the BDNF, TrkB and ERK levels in the hippocampus of male Wistar rats. J Psychopharmacol, 35(6), 744-754. https://doi.org/10.1177/02698811211000762
Lormant, F., Ferreira, V. H. B., Lemarchand, J., Cornilleau, F., Constantin, P., Parias, C., Bertin, A., Lansade, L., Leterrier, C., Lévy, F., & Calandreau, L. (2021). Training level reveals a dynamic dialogue between stress and memory systems in birds. Behavioural Brain Research, 408. https://doi.org/10.1016/j.bbr.2021.113280
McGinty, D. J., & Harper, R. M. (1976). Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res, 101(3), 569-575. https://doi.org/10.1016/0006-8993(76)90480-7
Mehrani, H., & Storey, K. B. (1997). Protein kinase C from bat brain: the enzyme from a hibernating mammal. Neurochem Int, 31(1), 139-150. https://doi.org/10.1016/s0197-0186(96)00130-1
Mercan, D., & Heneka, M. T. (2019). Norepinephrine as a modulator of microglial dynamics. Nat Neurosci, 22(11), 1745-1746. https://doi.org/10.1038/s41593-019-0526-9
Miranda, M., Morici, J. F., Zanoni, M. B., & Bekinschtein, P. (2019). Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front Cell Neurosci, 13, 363. https://doi.org/10.3389/fncel.2019.00363
Pace-Schott, E. F., & Hobson, J. A. (2002). The Neurobiology of Sleep: Genetics, cellular physiology and subcortical networks. Nature Reviews Neuroscience, 3(8), 591-605. https://doi.org/10.1038/nrn895
Stowell, R. D., Sipe, G. O., Dawes, R. P., Batchelor, H. N., Lordy, K. A., Whitelaw, B. S., Stoessel, M. B., Bidlack, J. M., Brown, E., Sur, M., & Majewska, A. K. (2019). Noradrenergic signaling in the wakeful state inhibits microglial surveillance and synaptic plasticity in the mouse visual cortex. Nat Neurosci, 22(11), 1782-1792. https://doi.org/10.1038/s41593-019-0514-0
van Alphen, B., Semenza, E. R., Yap, M., van Swinderen, B., & Allada, R. (2021). A deep sleep stage in Drosophila with a functional role in waste clearance. Science Advances, 7(4), eabc2999. https://doi.org/doi:10.1126/sciadv.abc2999
Welinder, K. G., Hansen, R., Overgaard, M. T., Brohus, M., Sønderkær, M., von Bergen, M., Rolle-Kampczyk, U., Otto, W., Lindahl, T. L., Arinell, K., Evans, A. L., Swenson, J. E., Revsbech, I. G., & Frøbert, O. (2016). Biochemical Foundations of Health and Energy Conservation in Hibernating Free-ranging Subadult Brown Bear Ursus arctos. J Biol Chem, 291(43), 22509-22523. https://doi.org/10.1074/jbc.M116.742916
Xie, L., Kang, H., Xu, Q., Chen, M. J., Liao, Y., Thiyagarajan, M., O'Donnell, J., Christensen, D. J., Nicholson, C., Iliff, J. J., Takano, T., Deane, R., & Nedergaard, M. (2013). Sleep drives metabolite clearance from the adult brain. Science, 342(6156), 373-377. https://doi.org/10.1126/science.1241224