Mathematical Modeling and Evolution: The Guiding Prong for Shaping Avian Eggs
Leticia Le Goff, Ula Mastej, Jake Pringle, Ryan Romero
Introduction
The avian egg is predominantly perceived as a static structure. In reality, the avian egg is influenced by many internal factors, such as gaseous exchanges and the development of the embryo, as well as external factors such as the nesting environment and the impacts of the human industry on shell thinness as detailed in “Mechanical and Material Design Principles of the Avian Eggshell” (Le Goff et al., 2020). Beyond this, egg shape proves important for distributing impact forces and stress, stabilizing the egg and limiting travel, as well as creating a proper climate for incubation inside the egg both by gas regulation, water and heat exchange. Important measurements, such as length and breadth of the egg, can be used to derive various equations that can accurately model egg shape, thus providing a link between the egg’s architecture and its functions. Being able to understand how factors such as flight efficiency and egg roll capacity can influence the shape of the egg will then broaden our understanding of why eggs acquire specific egg shapes. Finally, analysis of avian species dating as far back as 250 million years ago, to the Mesozoic Era, will provide a variety of case in point studies that indicate a relationship between egg function and shape. Ultimately, these discussions provide the basis explanation for the immense variation in egg physiologies observed across species in the class Aves and outline methods as well as functions of object shaping that we may adapt into engineering practices.
Function
The eggshell is built in such a way that it may protect the bird embryo and later fetus until it is ready to hatch. The function of its shape is to allow it to resist several types of impact. Furthermore, its shape plays a key role in having it remain in or near the nest when disturbed. Egg shape additionally has an important role in the protection of the bird embryo and fetus from environmental and climatic conditions.
Providing Resistance to Stress
During the incubation process, eggshells are exposed to a variety of stresses that can be either gradual or sudden. Protecting the egg from gradual compression — resulting from quasi-static loadings — is a paramount function of the eggshell as during incubation the mother and sometimes father will often sit on the egg. It is also important that the egg be able to withstand sudden forces or cracking resulting from shock loadings such as egg to egg collisions in the nest and predators or parasites pecking in an attempt to break the shell. Thus, the shape of the egg must be that which can withstand these forces optimally, such as an arch from all angles: resulting in the round egg shape. If an egg were to be, for example, a rectangular prism, then it would be strong on the corners but weak on the faces (Kruszelnicki, 2012). This could be mitigated with support structures within, but these would take up space and so interfere with the egg’s role of housing the young bird. Thus, the round shape is ideal.
The most symmetrical shape is the sphere, and so intuitively it would appear that eggs should have this shape to evenly distribute forces when stressed. Yet, in practice, avian eggs rarely are spherical. A common method of quantifying egg shape is the Shape Index (SI):
SI = W /100L
(1)
where W is the width diameter and L is the length diameter of the egg. It has been found that greater rupture forces are required to break eggs with high SI values — most symmetrical or spherical — at various speeds, but most noticeably at lower speeds (Altunas and Sekeroglu, 2007). A study conducted by applying slowly increasing crushing force to an egg found that although shell thickness accounts for about 56 % of variation in crushing strength, the remaining 15 – 35 % of the variation is accounted for by the SI index (Richard and Swanson, 1965). It is thus evident that egg roundness plays a key role in distributing forces along the eggshell upon impact, allowing it to fulfill its function of protecting the egg contents. However, seeing that many other factors contribute to eggshell strength — such as its thickness and composition, — it is reasonable to suggest that the reason that some of egg sphericity is sacrificed in favor of other round shapes is so that the egg shape may serve other functions as well.
Stabilizing the Egg
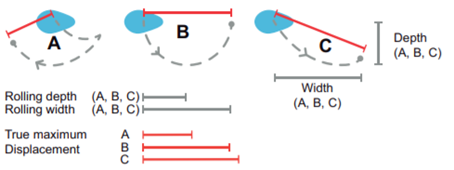
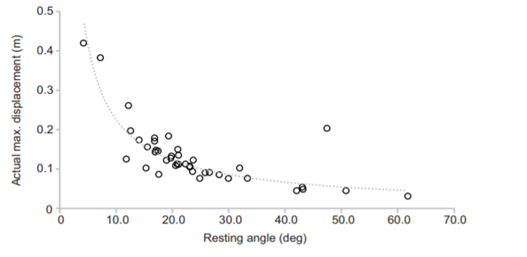
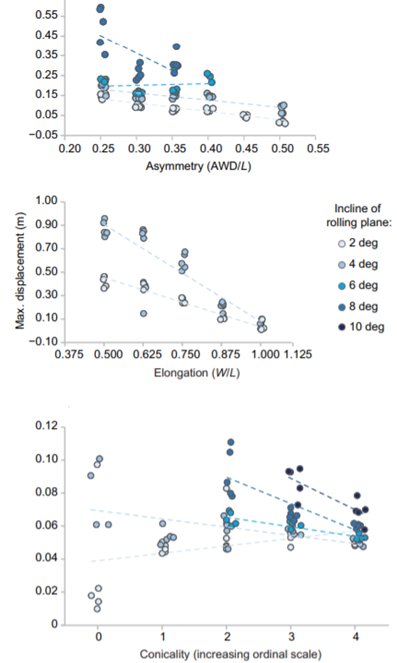
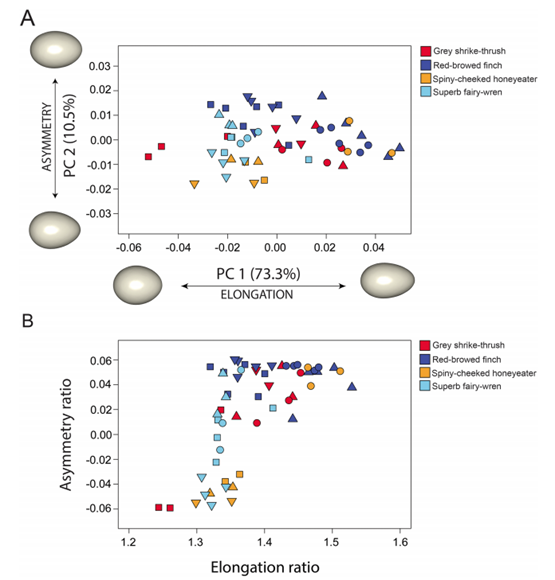
Another key function of eggshell shape is providing stability to the egg on its resting surface. One such hypothesis suggests that pyriform eggs are so shaped to follow an arc as they roll. This is most useful for avian species which set up their nests on cliffs and inclined surfaces, as the eggs will only roll a short distance rather than straight down the cliff. Hays and Hauber (2018) have aimed to quantify this phenomenon by predicting the rolling path — radius, width, depth and overall displacement — of an egg based on its resting angle (Fig. 2 and 3). Their model predicts that the larger the resting angle is, the smaller the radius of the egg’s trajectory will be. It is important to note that this angle is not only dependent on the egg shape but also on the location of the bird embryo and fetus, which tends to attach itself on the pointed end of the egg, further increasing the resting angle (Hays and Hauber, 2018). They additionally measured the relationship of the elongation: width to length ratio (W/L), the asymmetry — apical width to length ratio (AWD/L) — and the conicality (C) of the egg to the displacement. Further on in the paper, this will also show how the resting angle can impact the rolling factor of the egg.
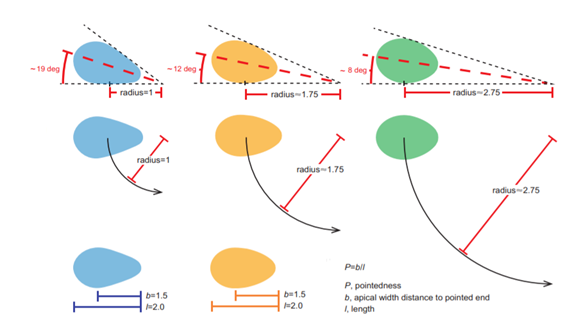
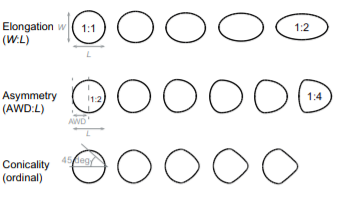
The results of this study have supported the longstanding theory that egg shape influences rolling trajectory and displacement. An inverse relationship occurs with the resting angle to the maximum displacement as postulated (Fig. 5). Furthermore, the maximum displacement decreases linearly with elongation, asymmetry and conicality (Fig. 6). Thus, it can be said that one function of egg shape is to prevent eggs from rolling down inclines in their surroundings (Hays and Hauber, 2018).
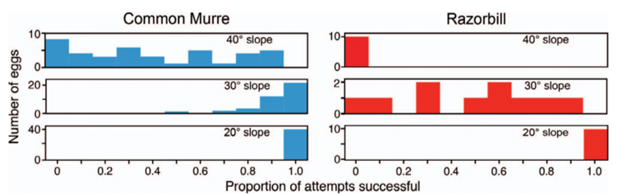
Another reason for more conical egg shapes is that not only do they change rolling trajectory, but they may prevent egg movement in general on sloped surfaces. A comparative study on the common murre (Uria aalge) which lays its pyriform eggs often on slopes and its close relative, and the razorbill (Alca torda), which lays its elliptical eggs on flatter surfaces amongst pebbles, was carried out to determine which eggshell shape is more stable when placed on an inclined plane. As can be seen in Fig. 7, the study concluded that eggs which are more pyriform are indeed more stable than their elliptical relatives (Birkhead et al., “The pyriform egg of the Common Murre”). This phenomenon further highlights the egg shape’s role in protecting eggs from dangerous movements that can occur as a result of the slope of their surroundings.
Dry Environment Adaptations and Protecting Against Climatic Conditions
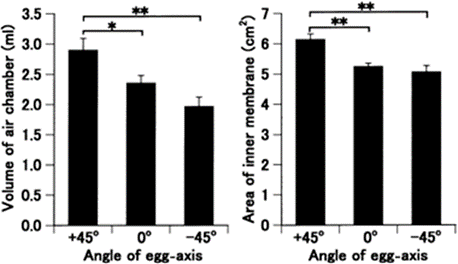
Perhaps a less obvious function of the egg shape is that it serves as an adaptive measure to various climatic conditions. Most notably, the asymmetry of the avian egg can be seen as an adaptation to reproduction on dry land. In a study done on quail eggs, Mao et al. (2007) have found that during the incubation process there is a shift in how much an egg is tilted. They found that both fertilized and unfertilized eggs that gradually tilt their blunt end upwards and conical end down over time and that there is a gradual accumulation of gas in the blunt end of the egg (Fig. 8, 9 and 10).

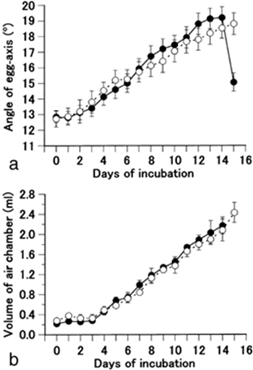
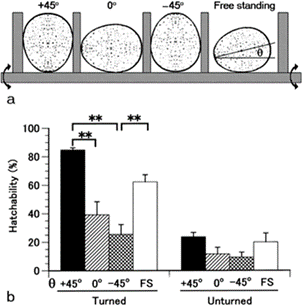
Furthermore, by restricting the eggs to various positions (Fig. 11.), it was found that hatchability is very high for eggs which are blunt end up vertically, similarly for the horizontal and natural positions, but hatchability is very low for eggs with the blunt end down, and the hatchability further improves when the eggs are rotated every two hours (Mao et al., 2007). The positioning of an egg and its contents is thus key to its development.
Since the egg houses all nutrients necessary for life-saving oxygen, it must have a method of obtaining it. Due to the porousness of the egg, gas may diffuse in and out in a dry environment. Yet this diffusion occurs more readily in the blunt end than in the conical end due to the relative porousness of the eggshell structure. For this reason, it is postulated the embryo slides down within the egg and a gas bubble forms up top where oxygen can be stored. This leads to the phenomenon of the egg tilting. The conical shape aids this in occurring as eggs are often placed conical end down in nests and can easily sink into the correct position, allowing for the necessary gas exchange to occur (Mao et al., 2007).
A preference for elongated eggs over spherical eggs can also be seen for climatic reasons. Elongated eggs have a greater surface area to volume ratio. Thus, a spherical egg will lose less water, have less exposure to solar radiation, and gain and lose heat more slowly. Hence, eggs which are in hot or high-altitude climates must have lower shell conductance, so as to avoid extensive water loss, or heat changes. This however can also be mitigated by nest microclimates, which can provide protection from sun and increase humidity near the egg, and so a fine balance must be found between egg shape and nest type (Duursma et al., 2018).
A study conducted in Australia on various passerine birds found several relationships which confirm this. Cavity-nesting species had significantly less elongated eggs than those nesting in the open, thus giving them properties to better retain heat and water. Furthermore, avian eggs were less elongated and dome nests were more common in hot and dry areas (Duursma et al., 2018). Therefore, in conditions that encourage water loss, shaded nests are built to hide the eggs from the sun and so retain water and the eggs are round to better retain heat. Hence, the function of the eggshell can be to mitigate heat and water exchange with regard to the regional climate as well as the microclimate of the nest.
Architecture
In “Mechanical and Material Design Principles of the Avian Eggshell” and “Marvels of Eggshell Pigmentation: Why Eggshells Vary so Greatly in Color and Pattern” (Le Goff et al., 2020), the structure of the avian egg was explored on a macro-level, with specific emphasis on the relationship between shape and pressure distribution, and on a nano-level. With this knowledge of the nanostructure, viewing the egg as a whole and model its shape using various mathematical models is now feasible. By doing this, it is possible to model and, later, predict egg shapes of various species. Additionally, once egg shapes can be accurately modelled and predicted, it is possible to further understand the relationship between shape and function.
Two-dimensional and Three-dimensional Modeling
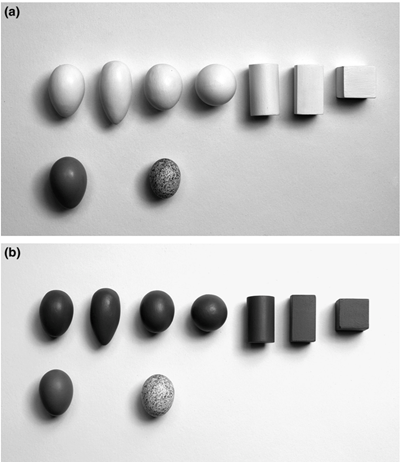
Before going into further detail about shape modeling, it is important to note that egg shape varies greatly between species. Bird eggs can generally be divided into three categories: spherical, elliptical and conical eggs. When studying egg shape, this is done by calculating and comparing ratios of various measurements on the egg – in particular, elongation/ellipticity (E) and asymmetry (A). Elongation is calculated as the ratio between the length (L) and the breadth (B), or maximum diameter, of the egg. Asymmetry uses the ratio between the “distance from the lower vertex of the egg to the point where the polar axis intersects the equatorial axis to egg length” (Attard et al., 2018).
Using this model, one study analyzed nearly 50 000 eggs covering 1 400 species and found that the morphospace representing egg shape is triangular (Fig. 12.). While most eggs have shapes with A ∈ [0.1, 0.2] and E ∈ [0.3, 0.4], asymmetry can range from A = 0.001 up to 0.485 and ellipticity from E = 0.097 to 0.724. Despite this large variation in both elongation and asymmetry, there is a curious lack of eggs that is both low in elongation and high in asymmetry. It is also interesting to note that shapes within orders tend to be widespread (Stoddard et al., 2017) (Fig. 13.).
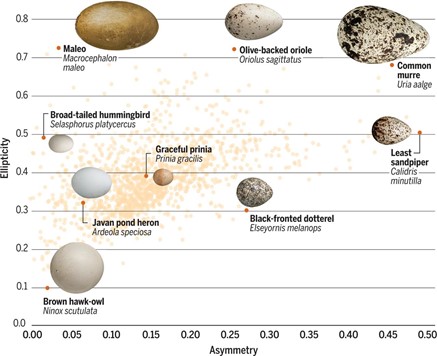
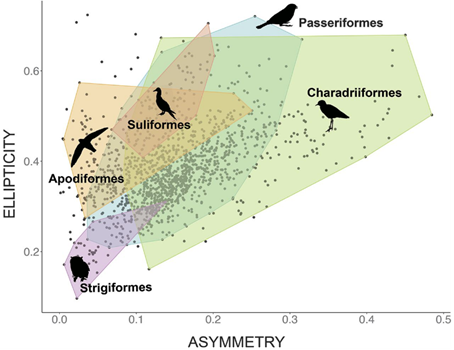
While this two-dimensional modeling can be used to explain egg shape, it does not consider egg curvature: more specifically, it relies on the assumption that radial curvature remains constant and therefore ignores any slight differences in egg curvature. To overcome this, Attard et al. (2018) used computer software to model the 3D surface of the egg.
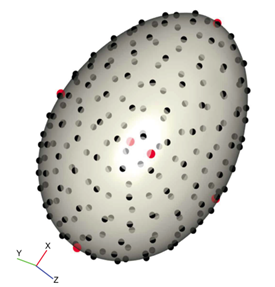
As seen in Fig. 14., the egg was placed so that the x-axis corresponded with the longest side. Red spots were placed at calculated landmarks — marking height, width, depth, and points around the equator — and black spots were equally spaced around the surface (Attard et al., 2018).
Using this, the standard elongation and asymmetry ratios were calculated at the landmark points. The results of using 3D modeling compared to the standard 2D can be seen in Fig. 15.. This demonstrates how elongation is similar in both models. However, there is a difference in asymmetry ratios, which can be explained by the way the axis is defined in each case (Attard et al., 2018). 3D modeling offers the benefit that the principal axis around which asymmetry is calculated is mathematically determined and, consequently, the model can be applied to eggs of any shape. Additionally, mathematical three-dimensional modeling accounts for small changes in egg shape, that might not be caught using traditional 2D modeling.
Mathematical Modeling of Egg Contours
Modeling of Circular and Elliptical Eggs
With this understanding of ellipticity and asymmetry, it is possible to categorize all eggs into four main shapes: circular, elliptical, oval, and pyriform — also known as conical. — To map egg contours, it is easiest to find functions corresponding to spherical and elliptical eggs, as circles and ellipses are already defined by functions. Therefore, the following equation was proposed:
y= ±√(a ^2-x^2 ) f(x)
(2)
where y=±√(a2 – x2) defines either the ellipse or the circle function (Narushin et al., 2020; Barta and Szekely, 1997). On the other hand, f(x) is a polynomial function, usually a cubic function although a quadratic or a linear function may also be used. The basic form of f(x) can be defined as follows:
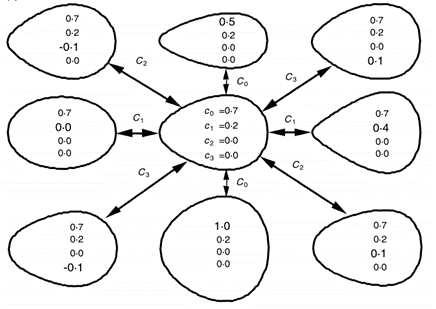
f(x)=c_0 + c_1 x + c_2 x^2+c_3 x^3
(3)
By increasing c0, the egg contour became more circular, while increasing c1 will make the egg more elliptical. Changes in both c2 and c3 have effects on the shape of the ends of the egg (Barta and Szekely, 1997). These findings are summarized in Fig. 16..
Modeling of Ovoid Eggs
While it is somewhat easier to determine functions for the shape of circular and elliptical eggs, oval and pyriform eggs are slightly more complex to estimate. In one instance, it is assumed that the egg is symmetrical along its longitudinal axis and thus only half of the contour is used for calculations (Fig. 17.).
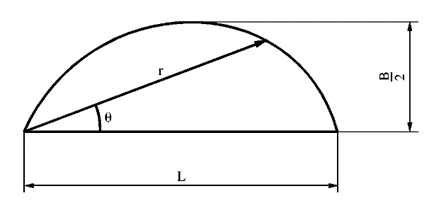
By satisfying the equation r= LcosnƟ, with n=3 being when the curve most closely matched that of an egg, it was possible to derive the following equation:
y= ±1.5396*{B\over L}*√(L^{0.5} x^{1.5}-x^2 )(4)
where B and L represent the breadth and the length, respectively. This function can be used to estimate the contour of oval eggs.
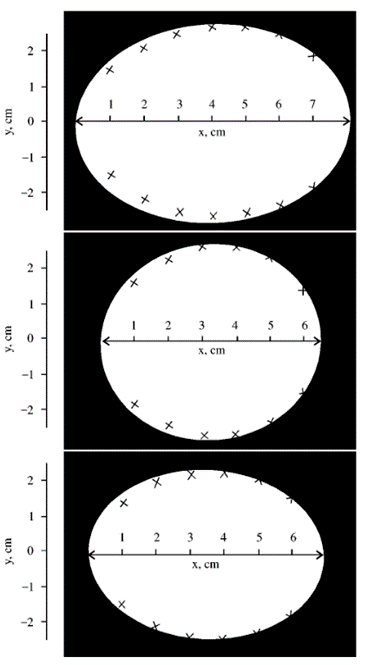
As seen in Fig. 18., measured breadths and lengths were inputted into (1) and the plot of the estimated contour was compared with the actual contour: this is evidence that the derived equation estimates egg shape relatively well (Narushin, “The Avian Egg: Geometrical Description and Calculation of Parameters”).
In a similar experiment, the same author derived a second equation that models the contour of an oval egg. In this model, the starting step remains the same, with r= LcosnƟ . However, instead of defining n=3, n remains a variable in this case. Following the derivation, the contour of the egg was found to be:
y= ±√(L^{2/(n+1)}* x^{2n/(n+1)}-x^2 )(5)
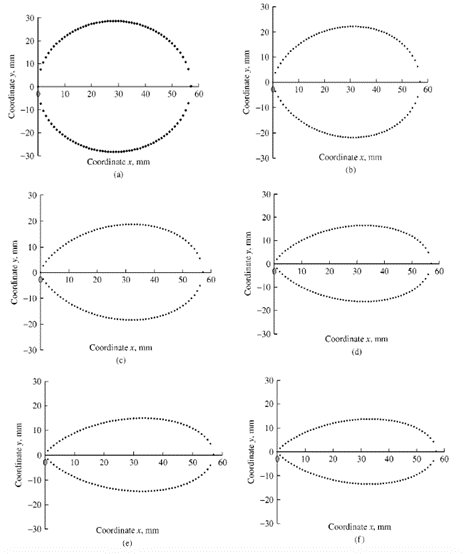
where n=1.057*(L/B)2.372. As a result, modifying the values of L and B will have a direct effect on (n) and, by extension, the shape of the egg (Narushin, “AP – Animal Production Technology: Shape Geometry of the AvianEgg”). Lower (n) values tend to produce more spherical eggs, whereas larger (n) values produce elongated and pointed eggs (Fig. 19.).
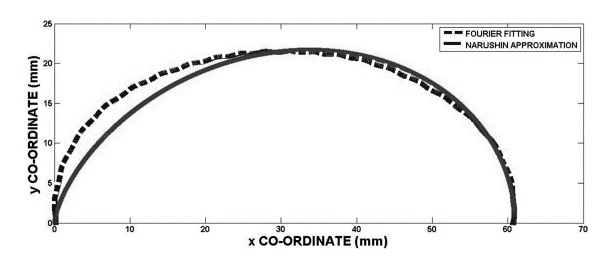
In a separate experiment, 240 eggs were examined and the polar coordinates describing the eggshell shape were plotted by the Fourier series. When the plot produced by the Fourier series was compared with the plot produced by Eq. 5, it was noticed that the contours were reasonably similar throughout most of the surface (Fig. 20). The biggest discrepancies were noted at the egg’s pole (Severa et al., 2013). This points to potential issues with the derived function: the calculated function does not accurately estimate the shape of the egg and more research is required to develop a function that can accurately model any egg shapes.
Construction of a Universal Model
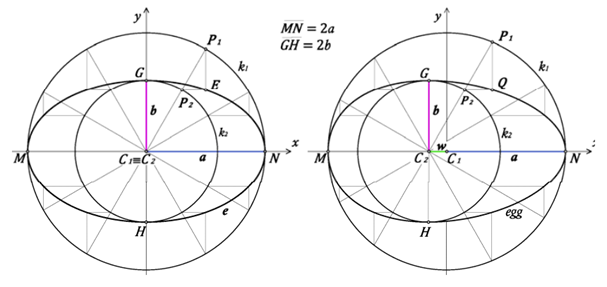
So far, functions used to estimate the contours of circular, elliptical and ovoid eggs have been described. However, pyriform eggs pose a special problem: it is extremely difficult to define a function that can plot a curve with a pointed end. To overcome this, consider Hugelschaffer’s model. Hugelschaffer’s model starts with two concentric circles and shifts the center of the small circle by a parameter away from the center of the first circle (Petrovic and Obradovic, 2010) (Fig. 21).
Using this model, the resulting ellipse can be defined by the Hugelschaffer’s equation:
y= ±{B\over2}*√({(L^2-4x^2)\over(L^2+8wx+4w^2 )})(6)
where L is the length of the egg, B is the breadth and (w) is the parameter defining the shift of the second circle (Narushin et al., 2020). Eq. 6 can be used for circular, elliptical and oval eggs setting L = B will result in a circle, and setting w = 0 will create an ellipse. However, the model breaks down when attempting to estimate the contour of a pyriform egg, since the pointed end cannot be accurately modeled.
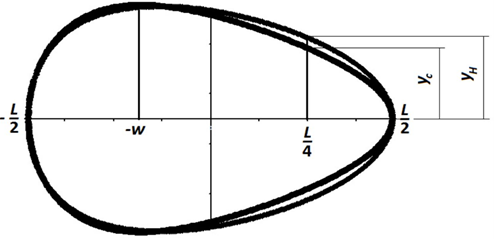
Narushin et al. recalculated the value of (w) so that w = (L-B)/2n, where (n) is a positive number. One of the main problems with Hügelschäffer’s original model is that n≥1, meaning that the only way to obtain pyriform eggs is by setting (n) to be less than 1. Fig. 22 and 23 depict how various values of (n) have an impact on egg shape: only plots using n<1 resulted in pointed ends that are typical of pyriform eggs, but the rest of the contour does not match observed egg contours (Narushin et al., 2020).
As a result, Narushin et al. managed to find an equation that defines the pyriform shape:
y= ±{B\over2}*√({(L^2-4x^2)L\over(2(L-2w)x^2+(L^2+8Lw-4w^2)x+2Lw^2+L^3 )})(7)
which depends on length L, breadth B and distance (w). When plotting both this equation and the Hugelschaffer equation on the same graph, there is a visible region where the two functions diverge, △y (Fig. 24).
Following a series of calculations using △y, a universal formula was determined that can now be applied to any egg:
\small y= ±\frac{B}{2}*√(\frac{(L^2-4x^2)}{(L^2+8wx+4w^2 )})\\*\\(1-\frac{(√(5.5L^2+11Lw+4w^2 ) (√3 BL-2D_{L/4} √(L^2+2wL+4w^2 ))}{(√3 BL(√(5.5L^2+11Lw+4w^2 )-2√(L^2+2wL+4w^2 )) }\\ *\\(1-√(\frac{(L(L^2+8wx+4w^2))}{(2(L-2w)x^2+(L^2+8Lw-4w^2)x+2Lw^2+L^2 w+L^3 )}))(8)
This formula uses the same variables as Eq. 7 with the addition of DL/4, representing the diameter at the point L/4 from the pointed end (Narushin et al., 2020). Finally, the pyriform function and the universal function were both tested with various pyriform eggs and the resulting plots fit reasonably well with actual egg shapes (Fig. 25).
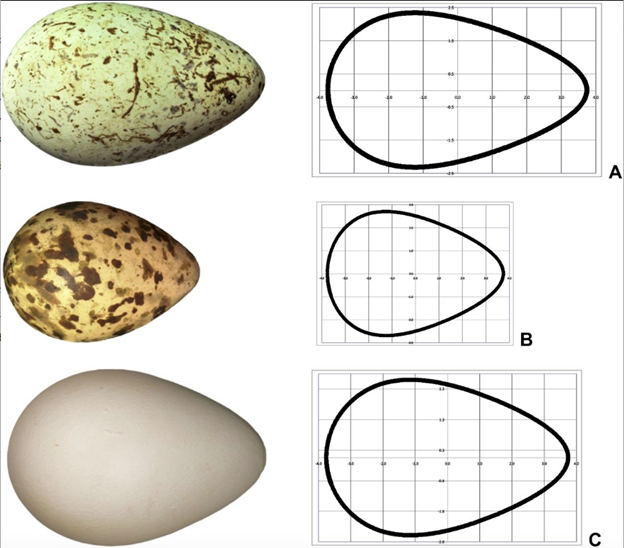
Naturally, the theoretical contours are not a perfect model of actual eggs. However, the curves are reasonably close and manage to consider both the general shape of the egg, as well as the pointed end — something that previous mathematical formulas were unable to do. —This universal formula can therefore be used to model any avian egg — including conical eggs.
Operation
For the egg shape, there exist factors that are transmitted either by the parents or by the breeding habitat that influence the form of the egg in the most interesting ways. Two of these factors are a bird’s specific abilities related to flight and how birds breeding near rocky shore cliffs tend to acquire a very distinguished egg shape. It will also be of importance to note how these factors have been integrated into specific families of birds and why they serve a purpose in promoting an optimum operation and function in the egg.
Influence of Flight Efficiency on Egg Shape
Birds are primarily known to be great flyers, or at least the majority of them and, as was mentioned beforehand, the extent of this ability can influence the egg shape. Most hypotheses seem to consider what it means for a bird to be a strong flyer and how this impacts their body shape. Indeed, to maintain a sleek and streamlined body for an efficient flight, the rest of the bird’s aspect must harmonize with the space available. This would mean having a different shape oviduct compared to birds who do not have a need to possess such body dimensions because of their flight abilities. For example, Hummingbirds (Trochilidae) and Albatrosses (Diomedeidae) are completely different birds, yet both have a very close egg shape (Fig. 26).

The two birds may have evolved similarly shaped eggs because both are high-powered flyers. Naturally, the albatross is one of the best flyers in the animal kingdom and with its wingspan nearly measuring 3.5 m (11 feet), it can travel approximately 800 km (500 miles) in a single day (Chu, 2017). Hummingbirds on the other hand are not far off and could also be considered one of the greatest flyers in the animal kingdom since they have the capacity to fly not only forward, but backwards, sideways and straight up. They can also hover on the same spot for an extensive amount of time and can reach a speed of up to 98 km/h (Yong, 2011).
This leads to establishing a connection between egg shape and Hand-Wing Index (HWI). Birds that are stronger flyers and have a higher HWI tend to lay more asymmetric or elliptical eggs, which maximizes the egg volume without increasing the egg’s width so that the egg fits into the narrower oviduct (Stoddard et al., 2017). The HWI is a characteristic for flight efficiency and dispersal ability in birds. Given by the formula:
Hand Wing Index (HWI)=100×{(WL-SL)\over WL}(9)
where WL is the standard length of the closed wing and SL is the distance from the joint to the tip of the secondary feather (Fig. 27).
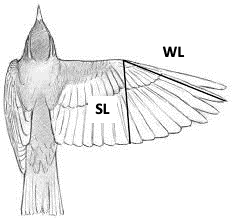
Stronger and more frequent flyers tend to have longer, narrower, and pointed wings, which contribute towards a higher HWI. Contrarily, birds that are weaker and less frequent flyers, for example owls, tend to have shorter, broader and rounded wings, which translates into a low HWI. Noticing the correlation between flight efficiency and egg shape can suggest key adaptations for high-powered flyers, which includes reduced body size, a reduced abdominal cavity and only one functioning ovary which lessens the weight of the body. These factors have considerable effects on the egg shape by constraining the maximum dimensions of an egg to allow accommodation in the reduced oviduct. The changes that can be perceived are an increase in ellipticity and asymmetry of the egg in order to increase the egg’s volume whilst maintaining or reducing the girth of the egg (Stoddard et al., 2017). Moreover, when eggs of flying birds were compared to non-flying birds, it was identified that the egg shape varied greatly (Fig. 28). For instance, Kiwi (Apteryx) birds lay hyper-elliptical eggs with a relatively low asymmetry influence which we find in other flightless birds such as the Ostrich (Struthio camelus) who lay very spherical eggs. This could be explained by the fact that body plans of flightless birds do not require specific dimensions to acquire more efficiency during flight and so eggs can develop more freely inside the oviduct.
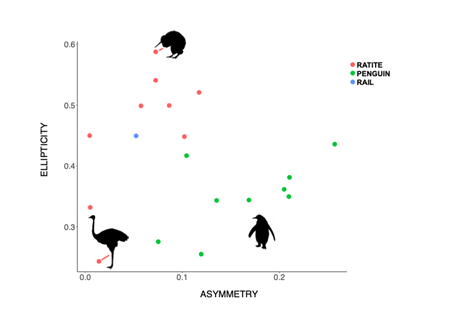
Looking at the graph, egg shapes in flightless birds still differ widely and this can be due to the activities each bird does. For example, looking at the egg shape of a penguin, the graph indicates a more asymmetrical shaped egg but because they are powerful underwater swimmers, the selection for a streamlined body still impacts the asymmetrical aspect of the egg even in the absence of flight.
Influence of Egg Roll and Egg Movement in Pyriform Eggs
As mentioned before, the resting angle plays a key role in understanding the avian egg shape. Taking a look from a different perspective of how the certain factors can alter the egg shape, it will be interesting to see how different environmental habitats include different egg shapes. When presented with eggs from cliff-nesting birds, a pattern can be established between the egg shapes. Indeed these eggs tend to be conical or pyriform eggs. With research focusing on pyriform eggs found in the Alcidae bird family, it was originally thought that this shape was to suppress displacement by having the eggs roll in a tight circle and thus prevent egg loss on the cliffside (Hays and Hauber, 2018). In general, eggs having this conical shape can be understood in the same way. The pointed end typically has a highly conical quality, which is thought to provide a stable point of contact with the resting surface. Furthermore, the pointed end is thought to determine both the angle at which the egg rests and its rolling radius (Hays and Hauber, 2018). More rounded eggs of similar dimensions had much smaller resting angles and a larger radius when the egg was rolled in a circle which thus led to obtaining a larger displacement (recall Fig. 2).
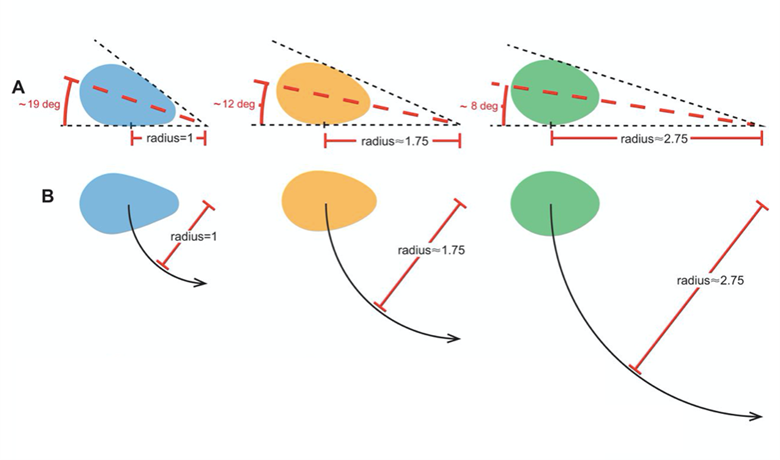
Like mentioned before, a round shape permits a very large radius. Looking at Fig. 2, the radius of a round egg is nearly three times that of a conical egg which rolls in tight circles and is much easier to stay close to the origin of displacement. Hence, conical eggs are much better adapted for birds not having a nest to prevent rolling during incubation (Hays and Hauber, 2018). Previous studies aimed to demonstrate whether the unusual pear shape of the Common Murre (Uria aalge), shown by Fig. 29, can help maximize mechanical strength and decrease the risk of contamination by dirt of the blunt end.

However, research also tried to understand if the shape played a role in preventing egg loss by rolling in a consistent and tight circle, therefore reducing the distance by which an egg is displaced when the incubating bird loses hold of the egg (Preston, 1953). Common Murre are known to breed in difficult and risky environments near the sea with cliffs, rocky shores and slopes (Fig. 30). Furthermore, this species breeds in extremely dense colonies, which makes their eggs vulnerable to mechanical damage from conspecifics, and to contamination by debris such as feces and soil (Birkhead et al., “The point of a Guillemot’s egg”). Thus, having this peculiar shape represents an essential advantage for an ideal functioning and operational egg.

Studies also evaluated if conical eggs would have a different response when put on a slope compared to round eggs. As a matter of fact, it was demonstrated that, when an egg is more pyriform (i.e., more pointed) it tends to demonstrate more stability on sloping surfaces (Birkhead et al., “The pyriform egg of the Common Murre”). Knowing that elongation and pointedness factors also predict the proportion of egg surface in contact with the ground, the stability is most likely due to having a greater surface of contact area, resulting in more friction with the ground (recall Fig. 2). Therefore, having more of a conical shape means the egg has an advantage compared to more rounded shaped eggs when it is on a slope, thus making it an interesting factor when considering the optimum egg shape for specific avian species. It is also important to note that having a greater contact area with a surface means having an easier way of finding the center of gravity and stabilizing the egg so that it does not roll away. Hence, this shape helps the Common Murre egg to be more stable and easier to stabilize on sloping surfaces compared to more rounded or elliptical shaped eggs.
Though there exists many doubts and concerns over such theories regarding the correlation between wingspan and shape or the correlation between pyriform eggs and the surrounding environment of the birds, it remains clear that the egg of the shape depends on how much of an advantage this will represent for the chickling. It will be even more important to understand how the shape of an egg can influence the survival of a species when comparing eggs in the avian domain.
Egg Shape Variation Among Avian Species: A Comparative Discussion
Intra- and inter-specific comparisons of species’ egg shape will now develop and provide case studies of the mentioned avian egg shape properties. From species patterns in the Mesozoic Era to today’s common Troglodytes aedon (house wren) shown in Fig. 31, clutch size, pelvis shape, fitness, nesting, and incubation properties will present the comparison and vulnerabilities across both altricial and precocial bird species.
Clutch Size and Pelvis Shape
Artifacts and fossils of the Mesozoic Era continue to surface and provide insight as to the development of avian egg shape over millions of evolutionary years. That is, to begin species comparison from this period is to begin from what is vastly considered the early onsets of modern-day birds. In examining the Mesozoic Era’s three main periods, it is currently impossible to assign eggs to avian species based on 250-million-year-old remnants. As a result, it is explained in broader terms that both Enantiornithes and Cretaceous ornithuromorphs, predominant avian species of this era, have up to 179 % larger body mass in comparison to egg load mass. Therefore, incubation by contact would ultimately result in egg crushing and so it is inferred that incubation is an evolved trait of avians (Deeming and Mayr, 2018). The operation of clutch size on egg shape is thus only applicable in more modern egg species, as parent-egg contact is required, namely for those of the families Alcidae and Spheniscidae. In such families, incorporation of clutch size increased the accounted variance in the mathematical modeling of egg shape by 19 % to 56 % (Birkhead et al., “The pyriform egg of the Common Murre”). Within Spheniscidae, species Megadyptes antipode (yellow-eyed penguin) indicated increased size, volume, and mass in clutches as the female parent aged, yet minimal deductions to egg shape resulted (Coulson, 1962). This highlights that egg shape is partially attributed to species’ brooding behavior besides disparities in physiology and so there is a lack of succinct interspecific comparison in clutch sizes’ effects on shape.
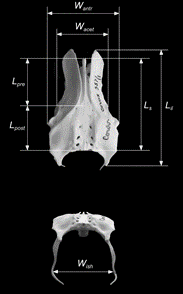
Whilst the discovery of incubation being an evolved trait of avians is significant in the context of evolutionary biology, the methods of arriving at such a conclusion are what is useful in analyzing clutch size and pelvis shape across species for engineering purposes. Maximum breadths of Mesozoic eggs were determined by analyzing species’ pelvic morphologies. According to the pelvic canal, egg breadth must not only fit between the maximum opening – generated by a fused pubic symphysis – but must actually be smaller as to fit through the tissue layers surrounding this cartilage (Deeming and Mayr, 2018). In turn, this affects egg diameter, egg mass, egg size, and ultimately egg shape across Jurassic and Cretaceous avian species. Yet, drawing a focal point to extant avian species indicates that a more ventral pelvic physiology has been evolutionarily adapted and may therefore omit this established pelvis-egg shape relationship (Shatkovska et al., 2018). Existing methods suggest maximum volume through the pelvis opening is to explain some species egg shape, such as Cistothorus palustris (marsh wren) (Underwood and Sealy, 2004). Raptorial birds (Falconiformes, Accipitriformes, and Strigiformes), pigeons (Columbiformes), and diving birds (diving ducks, loons, grebes and cormorants) were consequently examined for their pelvis versus egg shape relationship. Fig. 32 indicates the pelvis dimensions used to then explain that “elongation (smaller Iel) and asymmetry (smaller Ias) of [an] egg [are statistically] well correlated with narrowed pelvis (larger Iil/antr) and elongated post acetabular region (larger Ipost/pre)” (Shatkovska et al., 2018). In context, this finds raptorial birds to have high Iel, Ias, pigeons to have very similar measurements, and diving birds to have larger Iil/antr and Ipost/pre, associating to close to rounded and symmetrical eggs for the former two and more elongated shaped eggs for the latter (Shatkovska et al., 2018). This finally compares and further explains the effect of pelvis on avian egg shape: although inconsistent conclusions can be drawn for early birds of the Mesozoic Era, their inability to incubate provides grounds for explaining today’s common species pelvis to egg shape relationship.
Fitness
Behavioral Selection’s Influence on Egg Shape
Further attention to the family Hirundinidae, Turdus migratorius (American robin), and Dumetella carolinensis (gray catbirds) demonstrates the function of egg shape for survival. Amongst the Hirundines, “a significant negative relationship was found between egg volume and female […] tail fork depth” (Hasegawa1 and Arai, 2017). Forked tails of the Hirundo rustica (barn swallows)species illustrate sexually selected ornamentation, yet for females this proves disadvantageous. As forked tails are deepened, there is an exchange with maternal investment to egg survival. The deepened fork tail requires increased effort of females for culminating maternal resources, thus increasing the species locomotion. This will in turn affect the pelvic physiology selected for in the species, resulting in smaller egg sizes as also found in shorter pelvis post acetabular dimension species Guillemot. Note that this result may have to do with the decreased egg volume to decreased egg shape and size tradeoff as mentioned before for altricial bird species (Shatkovska et al., 2018).
Regardless, the consequence of this necessary gain in mobility for barn swallows is excessive resource expenditure towards locomotive maneuverability, impairing the cumulation of resources for egg size. The then produced smaller egg sizes are advantageous for geometrically arranging the female’s clutch as to ensure efficient incubation (Johnson et al., 2001), a reminder that clutch size has potential effects on egg shape, whilst conclusions on fitness benefits are varied. It is believed that the resulting decrease in egg size may provide grounds for decreased eggshell pigmentation and accordingly increased predatorial concealment, but counter claims suggest that the increased locomotive nature of females with deeply forked tails reduces essential incubation for egg thermoregulation (Hasegawa and Arai, 2017). Such exchange of maternal investment to female tail fork depth elucidates the function of egg shape to species survival, by inferring shape and its aid in chick survival to sexual selection and resulting female physiologies. Similar concepts are investigated for American robins and grey catbirds, finding that egg shape is a statistically significant stakeholder in their egg rejection for brood parasitism. Selection for brown-headed cowbird egg shapes similar to the species’ host clutches, see Fig. 33, demonstrated that survivorship of parasitic eggs has an attributed statistical significance of p < 0.047 (Underwood and Sealy, 2004). Accordingly, egg shapes adaptation to behavioral selection is a deductive argument, ultimately for the purpose of increased species’ fitness.
Nesting and Incubation Sites
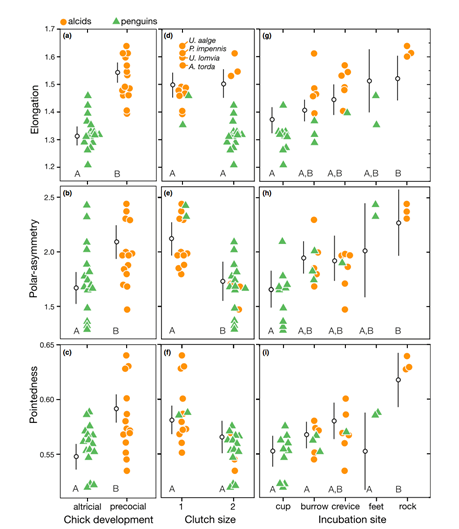
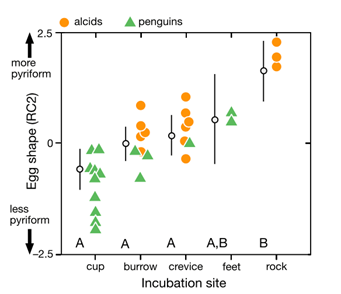
Comparison of species’ nesting and incubation properties provide further examples of varied egg shapes’ for survival benefits. Exploring the family Hirundinidae again, present models indicate that certain species of the genus Hirundo have been evolutionary derived to have open nesting. This pushes for egg shape adaptation towards shells of minimal pigmentation, as for already mentioned concealment measures, created by more spherical and shorter symmetries of the egg (Hasegawa and Arai, 2017). This result of a decreased shell surface area for increased fitness represents the larger picture of egg shape manipulation across the Class Aves based on the selection eggs succumb to during incubation. Common examples include the taxon Alcidae within which species have significantly varied pointedness of egg shape to ensure eggs do not roll off of their respective incubation sites, ranging from more bordered nests to open, flat surfaces. Fig. 34 demonstrates the general trend of egg shape for species within the taxa Alcidae and Spheniscidae — Alcids and penguins respectively. The linear, positive trend of egg pointedness, polar-asymmetry, and elongation as incubation shifts towards more savage sites resembles an egg shaped — nesting relationship developed by adaptation (Birkhead et al., “The pyriform egg of the Common Murre”). Controlling for egg size, a variation between 75-86 % of the shapes of alcids and penguins can be attributed to the incubation site. Demonstrating this in Fig. 35 via 95 % confidence intervals of linear model regression affirms the selection for less spherical egg shapes dependent on survival in a given incubation site (Birkhead et al., “The pyriform egg of the Common Murre”). Specific to the species Branta canadensis moffitt (Canadian geese), intra-clutch variation in egg shape and size ties nesting areas of lesser resources to potentially decreased egg size. However, such species-specific derived nesting arguments remain largely unattainable due to numerous confounding factors, such as endogenous resources of Canadian geese, that may also play an influence on egg shape.
Conclusion
Avian eggs are set apart from those of other oviparous animals, such as reptiles and amphibians, not only by their beautiful colors, but also by their – perhaps sometimes underappreciated — unique variety of shapes. — This fascinating phenomenon of elliptical, oval and pyriform egg shells allows the eggshells to perform a wide variety of functions such as the protection of egg contents from impact forces and stresses, the stabilization of the egg on various surfaces, and the creation of a suitable internal environment. Mathematical modeling can be further used to quantify and describe egg shape in an effort to best understand the effect of an eggshell’s architecture on its operation of the desired functions. The operation of flight, as influenced by egg shape and its efficiency, as well as the interweaving of egg shape with an egg’s rolling capacity can then be understood. Comparison of various avian species from the Mesozoic era to the current age further allows for a detailed understanding of various egg shapes and how they optimize certain functions and operations. The infinite variation of shapes and sizes of avian eggs highlights the intricacies and breadth of engineering in nature, even in something that on the outset appears to be a structurally simple and unassuming eggshell.
References
Altuntaş, E., & Şekeroğlu, A. (2008). Effect of egg shape index on mechanical properties of chicken eggs. Journal of Food Engineering, 85(4), 606-612. doi:10.1016/j.jfoodeng.2007.08.022
Attard, M. R. G., Sherratt, E., McDonald, P., Young, I., Vidal-García, M., & Wroe, S. (2018). A new, three-dimensional geometric morphometric approach to assess egg shape. PeerJ, 6, e5052. doi:10.7717/peerj.5052
Barta, Z., & Szekely, T. (1997). The Optimal Shape of Avian Eggs. Functional Ecology, 11(5), 656-662. Retrieved from http://www.jstor.org/stable/2390408
Birkhead, T. R., Thompson, J. E., Biggins, J. D., & Montgomerie, R. (2019). The evolution of egg shape in birds: selection during the incubation period. Ibis, 161(3), 605-618. doi:https://doi.org/10.1111/ibi.12658
Birkhead, T. R., Thompson, J. E., Jackson, D., & Biggins, J. D. (2017). The point of a Guillemot’s egg. Ibis, 159(2), 255-265. doi:https://doi.org/10.1111/ibi.12458
Birkhead, T. R., Thompson, J. E., & Montgomerie, R. (2018). The pyriform egg of the Common Murre (Uria aalge) is more stable on sloping surfaces. The Auk, 135(4), 1020-1032. doi:10.1642/auk-18-38.1
Charles Deeming, D., & Mayr, G. (2018). Pelvis morphology suggests that early Mesozoic birds were too heavy to contact incubate their eggs. Journal of Evolutionary Biology, 31(5), 701-709. doi:https://doi.org/10.1111/jeb.13256
Chu, J. (2017). Engineers identify key to albatross’ marathon flight. Retrieved from https://news.mit.edu/2017/engineers-identify-key-albatross-marathon-flight-1011
COULSON, J. C. (1963). EGG SIZE AND SHAPE IN THE KITTIWAKE (RISSA TRIDACTYLA) AND THEIR USE IN ESTIMATING AGE COMPOSITION OF POPULATIONS. Proceedings of the Zoological Society of London, 140(2), 211-226. doi:https://doi.org/10.1111/j.1469-7998.1963.tb01861.x
Deeming, D. C. (2018). Effect of composition on shape of bird eggs. Journal of Avian Biology, 49(1), jav-01528. doi:https://doi.org/10.1111/jav.01528
Englert Duursma, D., Gallagher, R. V., Price, J. J., & Griffith, S. C. (2018). Variation in avian egg shape and nest structure is explained by climatic conditions. Scientific Reports, 8(1), 4141. doi:10.1038/s41598-018-22436-0
Fretwell, P. T., Scofield, P., & Phillips, R. A. (2017). Using super-high resolution satellite imagery to census threatened albatrosses. Ibis, 159(3), 481-490. doi:https://doi.org/10.1111/ibi.12482
Hanlon, R. T., & Messenger, J. B. (1996). Feeding and Foraging. In Cephalopod Behaviour (pp. 47): Cambridge University Press.
Hasegawa, M., & Arai, E. (2017). Egg size decreases with increasing female tail fork depth in family Hirundinidae. Evolutionary Ecology, 31(4), 559-569. doi:10.1007/s10682-017-9895-2
Hassan, R., Elnaggar, W., Habib, E.-S., El-Mowafy, A., & El-Sandouby, A. (2017). EVALUATION OF LAL TEST INTERFERENCE IN SOME EGYPTIAN PARENTERAL DRUGS ALONE AND IN COMBINATIONS BY.
Hays, I. R., & Hauber, M. E. (2018). How the egg rolls: a morphological analysis of avian egg shape in the context of displacement dynamics. Journal of Experimental Biology, 221(19). doi:10.1242/jeb.178988
Johnson, L., Leyhe, J., & Werner, C. (2001). The shape of eggs in different-sized clutches of the House Wren (Troglodytes aedon). Canadian Journal of Zoology-revue Canadienne De Zoologie – CAN J ZOOL, 79, 1527-1531. doi:10.1139/cjz-79-8-1527
Kruszelnicki, K. S. (2012). Why are eggs egg-shaped? Retrieved from https://www.abc.net.au/science/articles/2012/05/01/3492086.htm
Le Goff, L., Mastej, U., Pringle, J., & Romero, R. (2020). Mechanical and Material Design Principles of the Avian Eggshel. Bioengineering. McGill University Montreal, Canada.
Leblanc, Y. (1987). Intraclutch variation in egg size of Canada geese. Canadian Journal of Zoology, 65(12), 3044-3047. doi:10.1139/z87-461
Mao, K.-M., Murakami, A., Iwasawa, A., & Yoshizaki, N. (2007). The asymmetry of avian egg-shape: an adaptation for reproduction on dry land. Journal of Anatomy, 210(6), 741-748. doi:https://doi.org/10.1111/j.1469-7580.2007.00737.x
Narushin, V. G. (1997). The Avian Egg: Geometrical Description and Calculation of Parameters. Journal of Agricultural Engineering Research, 68(3), 201-205. doi:10.1006/jaer.1997.0188
Narushin, V. G. (2001). AP—Animal Production Technology: Shape Geometry of the Avian Egg. Journal of Agricultural Engineering Research, 79(4), 441-448. doi:10.1006/jaer.2001.0721
Narushin, V. G., Romanov, M. N., & Griffin, D. K. (2020). A universal formula for avian egg shape. bioRxiv, 2020.2008.2015.252148. doi:10.1101/2020.08.15.252148
Nishiyama, Y. (2010). THE MATHEMATICS OF EGG SHAPE.
Petrovic, M., & Obradovic, M. (2010). The Complement of the Hügelschäffer’s construction of the Egg Curve.
Preston, F. W. (1953). The Shapes of Birds’ Eggs. The Auk, 70(2), 160-182. doi:10.2307/4081145
Richards, J. F., & Swanson, M. H. (1965). The Relationship of Egg Shape to Shell Strength1. Poultry Science, 44(6), 1555-1558. doi:10.3382/ps.0441555
Severa, L., Nedomová, Š., Buchar, J., & Cupera, J. (2013). Novel Approaches in Mathematical Description of Hen Egg Geometry. International Journal of Food Properties, 16(7), 1472-1482. doi:10.1080/10942912.2011.595028
Shatkovska, O. V., Ghazali, M., Mytiai, I. S., & Druz, N. (2018). Size and shape correlation of birds’ pelvis and egg: Impact of developmental mode, habitat, and phylogeny. Journal of Morphology, 279(11), 1590-1602. doi:https://doi.org/10.1002/jmor.20888
Sheard, C., Neate-Clegg, M. H. C., Alioravainen, N., Jones, S. E. I., Vincent, C., MacGregor, H. E. A., . . . Tobias, J. A. (2020). Ecological drivers of global gradients in avian dispersal inferred from wing morphology. Nature Communications, 11(1), 2463. doi:10.1038/s41467-020-16313-6
Spottiswoode, C. N. (2017). The most perfect thing, explained. Science, 356(6344), 1234. doi:10.1126/science.aan2517
Stoddard, M. C., Yong, E. H., Akkaynak, D., Sheard, C., Tobias, J. A., & Mahadevan, L. (2017). Avian egg shape: Form, function, and evolution. Science, 356(6344), 1249-1254. doi:10.1126/science.aaj1945
Underwood, T. J., & Sealy, S. G. (2006). Influence of Shape on Egg Discrimination in American Robins and Gray Catbirds. Ethology, 112(2), 164-173. doi:https://doi.org/10.1111/j.1439-0310.2006.01143.x
Yong, E. (2011). Hummingbird flight has a clever twist. Nature. doi:10.1038/nature.2011.9639