Masters of Camouflage: Mechanisms of Dynamic Skin Colouration in Cephalopods
Christopher Coluni, John Eric Hamilton, Olivia Lopardo, Olivier Morin
Abstract
Within the animal kingdom, predation pressure has been one of the largest contributing factors towards evolution within species. The presence of predators within communities has forced animals to develop both passive and defensive mechanisms in order to increase fitness. For this research paper, the focus will be directed towards the molluscan class Cephalopoda, as they possess some of the most advanced camouflaging mechanisms in biological history. Being soft bodied, species such as squids, octopus and cuttlefish required non-confrontational methods of survival. Lacking the hard shell of their fellow bivalve species, cephalopods matured into animals with incredible vision and highly advanced nervous systems. The combination of these two elements created a unique camouflaging system tuned to animals capable of night vision, as well as organisms who can interpret ultra-violet and polarised light. In order to mimic one’s environment, cephalopod’s would first need to quantify the light field around them and then find a method of translating this information into a unique pattern. It is truly a miraculous feat of evolution and the fact that these colorful pattern producing species are colorblind makes it all more unbelievable. While there is still lots to be uncovered, this paper focused on some of the few well understood areas, such as the factors involved in the textured camouflage as well as the pigment organs and reflector bodies taking part in dermal coloration.
Introduction
Camouflaging is a very useful feature organisms possess and is helpful in both protecting them from predators and advantageous for hunting. Years of evolution have brought them to develop these special traits so that their species may continue to survive through natural selection. These individuals achieve this using different methods. Some animals permanently copy the colour or texture of their surroundings, a phenomenon called background matching (Boudreau et al., 2012). There are also some individuals in nature that try to resemble other living beings that are toxic to inform their predators that they may be dangerous to them, which is called aposematism (Boudreau et al., 2012). Some living beings can achieve these camouflaging techniques by dynamically changing their own appearance, whether it be their skin colouration, texture, or morphology. A prime example of dynamic skin colouration for camouflage is the molluscan class Cephalopoda, or cephalopods. Their skin is composed of different layers each working together to change the way other individuals see them. It starts with the papillae which is responsible for the textural component of camouflaging. Then, there are the chromatophore, iridophore, leucophore and photophore layers that have the ability to change the perceived colour of the cephalopod’s skin using physical phenomena such as light absorption and refraction.
Change in the Texture of the Skin – Papillae
Camouflage and mimicry are often discussed in the context of colors and complex patterning. However, the concept of texture also plays a fundamental role in masking one’s appearance. Unique to the rest of the animal kingdom, cephalopods have the ability of rapidly modifying the surface of their skin to mimic their environment (Allen et al., 2013). The organs in charge are the dermal papillae, and there are a host of forces responsible for their formation (Allen et al., 2013). These dermal bumps enable cephalopods to camouflage on various irregular substrates such as coral and algae (Gonzalez-Bellido et al., 2018). In the following subsections, the biomechanics responsible for the extension of papilla will be discussed, as well as similarities between this dynamic skin and other known biomechanisms.
The Biomechanics of Papilla Extension and Retraction
As with many living organisms, muscle contractions serve as the basis for all movement. Most of the papillae that take part in adaptive camouflage appear to have three sets of muscles, two tailored towards the extension of the epidermis and one for retraction (Allen et al., 2014). Erector muscle fibers are designed to displace the papilla away from the surface of the skin, while retractor muscles reset their position. (Allen et al., 2014). It is also worth noting that both the expression and retraction mechanisms occur in less than one second (Gonzalez-Bellido et al., 2018).
As previously mentioned, two muscle fibers are likely responsible for the extension of papillae. Circular erectors, which are groups of coaxial muscles, extend the papilla upward by decreasing their circumference (Allen et al., 2013). This radial contraction results in the upward motion of surrounding tissue, which in turn, extends the papilla (Allen et al., 2014). Simultaneously, horizontal erectors are contracting along a level axis to aid both in extension and overall structure (Fig. 1). These horizontal erectors are almost always parallel to the surface of the skin and are responsible for the diversity of shapes that the papillae can create. It is worth noting that this stretched skin is not a risk to rupture as there are folds within the epidermis that contain extra surface area (Fig. 2) (Allen et al., 2013).


Although less understood than the methods of extension, mechanisms of retraction are likely carried out through retractor muscles and stored elastic energy (Fig. 3) (Allen et al., 2013). Retractor muscles appear to be connected to the papillas apex and through contraction, pull the tip of the skin back to level position. Another potential mechanism is that of stored energy (Allen et al., 2013). When both the circular and horizontal erectors contract their muscle fibers, the epidermis surrounding the papila tenses up. When the erector muscles relax, the elastic energy built up in the surrounding tissue is released, allowing for the complete retraction of the papilla (Allen et al., 2013).

Similarities Between Cephalopod Papillae and Muscular Hydrostats
In addition to complex muscle networks, the presence of incompressible fluid provides yet another necessary force mechanism for textured camouflage to occur (Allen et al., 2013). As shown in the above diagrams, papilla can take on a variety of rigid structures (Fig. 3). When the erector muscles contract, interstitial fluid that is found between the muscles is forced into various cavities within the extended papilla (Fig. 4) and creates pressure (Allen et al., 2013). This in turn affects the overall shape of the skin protrusion as volume must be kept constant. Compressions in one dimension will create expansion in others, and this is precisely how papillae form such irregular shapes (Kier & Smith, 1985). With this being said, the small and intricate papillae can be interpreted as small muscular hydrostats (Allen et al., 2013). Larger examples of these anatomical structures are flexible limbs such as squid and octopus tentacles (Kier & Smith, 1985). These structures consist of muscle fibers and rely on the physics of incompressible fluids for movement and rigidity, just like the papillae (Allen et al., 2013).

Potential Catch-Like Biomechanisms in Textured Camouflage
An interesting particularity about cephalopod textured camouflage is that once the dermal papillae is expressed, they can remain upright for long periods of time (Allen et al., 2013). This implies that these organisms may have built in energy preserving systems in order to keep these muscles in contraction. Although the exact method has not been formally discovered, many hypothesise that squids, octopuses and cuttlefish have their own version of “catch” muscles like many other mollusks (Allen et al., 2013).
The term catch-like is often used when discussing bivalve systems such as oysters and mussels. In these systems, the catch mechanisms are used to keep their rigid shells closed without wasting energy (Gonzalez-Bellido et al., 2018). This “catch” state is defined by the ability to maintain a contractile force with very little energy expenditure (Butler & Siegman, 2010). Researchers decided to investigate this possibility as they discovered that papillae expression could be maintained even without continuous input from the central nervous system (Gonzalez-Bellido et al., 2018). This means that the circular and horizontal dermal erector muscles remained in tension even without any neural input. Additionally, the existence of this biomechanism would greatly benefit these species as the extension and retraction of dermal papillae is constantly occurring (Gonzalez-Bellido et al., 2018). Energy expenditure in these organisms is already quite high and being able to express three-dimensional camouflage for long periods of time necessitates some form of an energy moderating system.
Chromatophores
Being able to mimic the colours and textures found within an environment are key aspects to background matching (Boudreau et al., 2012), a type of camouflaging techniques. In the previous section, the mechanics of papillae expression were discussed as well as some unique similarities between textured skin and other known biomechanisms. Moving away from the physics of dermal papillae, cephalopods use pigment and light refracting structures to change the pigmentation of their skin. The following subsections will explain how cephalopods use these structures to create all the colours found within the visible spectrum.
The Structure
Chromatophores are organs in the cephalopod’s skin that contain pigments. They are located at the surface of the epidermis just under the papillae (Fig. 5) and the compartment containing the colour is called the cytoelastic sacculus (Cloney & Brocco, 1983). This pigment sac has the ability to be stretched out in order for light to pass through it. The interaction between oncoming light and the pigment is the physical event that occurs enabling the cephalopods to change colour; otherwise, the chromatophores are barely visible, measuring around 0.1 millimetres (Maëthger et al., 2009) depending on the species.

Chromatophore organs are arranged in subclasses by their colour, and the three main colours are yellow, red, and brown (Messenger, 2001). However, their distribution and amount varies from species to species, but are consistently arranged “yellow over red over brown” (Deravi et al., 2014). As an example, the loliginid squid has these distributed following a pattern of “one brown chromatophore surrounded by a dozen red chromatophores and then interspersed with 20 small yellow ones” (Senft et al., 2021) (Figure 6).

The Function
These are not the only colours these cephalopods can produce. Using a combination of the chromatophores and the underlying leucophores and iridophores, these animals are able to create all the colours of the visible spectrum. They achieve this by choosing specific combinations of chromatophores to expand (Deravi et al., 2014). This will make the light reflect some colours and absorb others so that they can penetrate deeper into the skin and be used by the different coloured chromatophores. The colour of the reflected light is what others see and the absorbed light is modified by the structures below the chromatophores. The hapalochlaena or blue-ringed octopus is able to create rings of a bright blue even though it does not have blue pigmented chromatophores (Fig. 7). This is because it uses tightly packed iridophore plates (Maëthger et al., 2009) that are found in a lower section of its skin. These structures are all placed following specific orientations to create constructive interference of the incoming light which results in the blue colour (Maëthger et al., 2009).

The stretching or contracting is done by a special set of muscles called the radial muscles, attached to the myochromatophoral junctions of the chromatophores (Messenger, 2001) that surround the chromatophore’s pigment sac (Fig. 8). When these muscles contract, the organ is stretched outward in all directions, thus expanding the area of the chromatophore that light can pass through. This will allow the light to go through the sac and create a specific colour, depending on which chromatophore it is, due to the interaction of the light with the pigments.

However, not all the pigment sacs will open at the same time. Since the contracted form is darker and covers less area and stretched-out chromatophores are lighter and cover more area, the cephalopods gain the ability to play with the different possible patterns to best suit their surroundings and needs. They do this at a speed of 100 milliseconds (Senft et al., 2021), which is barely noticeable to the naked eye giving them a useful advantage when escaping attacking predators. Their brain is able to communicate with the radial muscles that are connected to the chromatophores through nerves that go across the muscles themselves (Messenger, 2001). This explains the speed at which they are able to use these muscles.
Contracting the sacculus back to its original state however does not follow the same mechanism. It is said that after being stretched out “part of this energy is stored in the sacculus as if it were composed of highly compliant springs” (Cloney & Brocco, 1983). This means that the muscles that stretch the sac are also providing the energy to contract it. As previously stated, the analogy of the spring or even a rubber band can be thought of in this scenario since the sac does not require another force to return to its original state. It uses a potential energy that was stored from the initial force (Fig. 9).

So far, it has been discussed how incoming light is used by chromatophores to create colours; however, these organs can also alter the perceived colour of incoming light in a different way. The chromatophore can filter the light leaving the iridophores and create colors that are impossible to make with the chromatophore or iridophore alone (Maëthger et al., 2009).
Overall, it can be observed that the chromatophore plays an important role in controlling the patterns and camouflaging abilities of the cephalopod’s skin. Its role could be summarized as filtering the skin of the cephalopod to the incoming light; however, there are other components of the skin that are responsible for creating more specific colours that lie deeper within the dermis.
Iridophores and Leucophores
The colour and pattern changes of the skin of camouflaging cephalopods is achieved by a combination of pigmented chromatophores and light-reflecting cells located beneath these organs, namely iridophores and leucophores. Through their interaction with light, these subjacent structural reflectors produce colours that span the entirety of the visible spectrum, providing cephalopods with dynamic camouflaging abilities (Mäthger et al., 2007). Most cephalopods, apart from some species of squid, contain both iridophores and leucophores in differing proportions and distributions to heighten certain chromatic body patterning components (Hanlon et al., 2018).
The Basics of Light Reflection off Surfaces
Before discussing how these reflective cells help in achieving various skin colourations, it is important to introduce the basics of light reflection. Light travels in a straight line through a medium and when it hits a surface, it bounces, or reflects, off the surface linearly in a different direction (Cloney & Brocco, 1983). However, some surfaces, like spheres, deviate from linear trajectory by localized non-uniformities in the medium for which they pass. In other words, rather than having one straight reflected ray, the light diverges off the surface in multiple directions, a process known as scattering (Videen, 1991). Furthermore, as visible light hits contact surfaces at differing wavelengths, the light gets transmitted and observed as colours on the visible spectrum (Fig. 10). For instance, as the angle of incidence increases, the wavelengths of the reflected light decrease and shift toward the shorter end of the spectrum (Mäthger & Hanlon, 2007).

Iridophores: Structure and Function
Iridophores are colourless cells of variable sizes, made up of stacks of thin plates that reflect light and produce structural colour by thin-film interference (Mäthger & Hanlon, 2007). Thin-film interference is due to the thin platelets and interplatelet spaces within the entire iridophore cell. The thickness of the plate and adjacent spaces between them are precisely and periodically arranged to influence which wavelengths are reflected (Kreit et al., 2013). Iridophores reflect light by thin platelets based on constructive interference. When light strikes the platelets, it becomes partially reflected and partially refracted at the top surface of the platelet. This refracted light then gets partially reflected at the bottom of the platelet and then emerges as a new ray (Fig. 11.). The reflective plates of iridophores are oriented almost parallel to the skin surface, and each has a different refractive index between the reflectin protein and the iridophore platelet (Mäthger et al., 2009). The colour that gets reflected off the iridophore depends on the wavelength of light that hits the cell. For instance, shorter wavelengths will get emitted as blues and greens, and the longer wavelengthswill appear as oranges and reds, in accordance with the visible spectrum as previously discussed.

The combination of the three pigmentary colours of the chromatophores, which are red, yellow, and brown, with these multilayer reflectors enables cephalopods to reflect any wavelength of visible light. For example, the Loligo pealeii, more commonly known as the longfin squid, uses only iridophores to reflect light, and they are capable of reflecting almost any wavelength of the visible spectrum (Mäthger & Hanlon, 2007). Iridophores allow cephalopods colour patterns that chromatophores alone cannot produce, which can be shown through the skin colouration of the longfin squid (Fig. 12.).

The way that the amazing colourations and patterns of cephalopod skin is produced is due to a combination of the light reflection off iridophore cells and the subsequent filtration from chromatophores. Chromatophores have the capacity to expand and contract over subjacent iridophores, thus when light reflects off the iridophore into the chromatophore, a colour is produced that neither chromatophore nor iridophore could produce alone. For instance, Figure 13 demonstrates a study where light was shot through the mantle tissue of the longfin squid, and the way the colours appeared depends on whether the light reflected off the chromatophore alone, the iridophore alone, or if it passed through both (Mäthger & Hanlon, 2007). For example, their results considered the relationship of a yellow chromatophore covering a green iridophore. When the light passed through the chromatophore alone, the peak occurred at 575 nm and appeared yellow. Through the iridophore alone the light emitted was green (peak 530 nm), but when it reflected from the iridophore and passed through the chromatophore, the light peaked at 595 nm, and produced a very bright yellow colour, suggesting the enhancing abilities the iridophores have on the chromatophores (Mäthger & Hanlon, 2007).

Leucophores: Structure and Function
Similar to the actions of iridophores, leucophores are present in cephalopods to reflect white light, but from wavelengths of 300 to 900 nm, producing a white background against which skin patterning is produced to regulate, contract and colour for camouflaging (Hanlon et al., 2018). Leucophores are broad-band diffusers that reflect all ambient wavelengths of light equally well. They have thousands of processes containing globules of proteins with high refractive indices (Cloney & Brocco, 1983). They appear white under direct white light, but have the ability to reflect a specific colour when that colour is shone on them. Not all cephalopods have leucophores, such as the squid, but they are commonly found in both octopus and cuttlefish.
Leucophores are composed of spherical protein assemblages of varying diameters that reflect light by incoherent scattering based upon a randomly ordered system, the process of light scattering as previously mentioned. Like iridophores, the colour that leucophores emit is dependent on whether an expanded chromatophore is present on top of them. Figure 14 presents the interactions of the chromatophores and leucophores on colour emitted on cephalopod skin patterns. When compressed, the red chromatophore does not cover the leucophore, so light is reflected off as white. However, when the red chromatophore is expanded and covers the leucophore, the white light coming off the leucophore gets reflected off as red (Kreit et al., 2013).

In a study of the Sepia officinalis, a species of cuttlefish, researchers found that leucophores were not distributed evenly in the skin of the species, and that they occur in specific areas forming a distinct shape and visual appearance, as shown inFigure 15.

Photophores: Structures and Functions
Bioluminescence and counterillumination are two camouflage techniques that are widely used by crustaceans, fish, and cephalopods to hide in open waters. These techniques utilize organs called photophores to mimic the surrounding ambient lighting conditions. There are two main types of photophores; bacteriogenic photophores, which use bacteria to emit bioluminescent light, and autogenic photophores, which utilise chemical reactions (Sickles, 2020). Although both types differ in their production of light, they each require similar optical structures to aid in characterizing and manipulating the light they produce to adequately camouflage. Photophores modify the emitted light’s properties such as emission angle, refraction, reflection, intensity, and colour through the use of reflectors, scales, and filters (Herring, 2000; Sickles, 2020). Understandably, photophores require distinct combinations of physical structures to adapt their emissions, therefore, their constitution can differ between species and location on an organism (Figure 16).

Reflectors
Used to redirect the light emitted, reflectors can disperse or converge the light shafts. Reflectors within photophores are usually concave, bowl-like structures composed of 70nm thick guanine crystals layered 75 to 125 nm apart under a layer of thin parallel filamentous subunits called tubules (Figure 17) (Cavallaro et al., 2017; Herring, 2000). Additionally, there are some reflectors which contain iridophores and have a hexagonal shape to create an iridescent layer (Paitio et al., 2020). The two major characteristics of reflectors are their composition and their shape. The effect of the curvature resembles the phenomena observed in curved mirrors (Figure 18).


This phenomenon further depends on whether the reflector is specular or diffuse. A specular reflector acts as a smooth surface where light reflects an angle of incidence which is equal to the angle of arrival. Specular reflectors are most likely composed of dioptric materials, such as perfectly aligned hexagonal iridophores, tightly arranged tubules, or guanine crystals which are highly organized in alternating refracting indexes (Copeland, 1991; Paitio et al., 2020). Proper organization of refractive indexes, where the refractive index times the thickness of the layer is a multiple of a quarter wavelength, creates a constructive interference, thus reflecting the light in a homogeneous manner (Figure 19) (Herring, 2000).

Comparatively, diffuse reflectors are composed of granular crystals which are misaligned and unorganized under the thin filamentous subunits that are not tightly packed. Due to the disorder of the surface, the light is reflected in various directions and allows for a greater angle of diffusion. Additionally, the homogeneity of the gleam will be altered by the variation of refractive materials on the uneven surface. Overall, the radius of curvature and the reflector’s structure determines the effect the reflector will have on the light.
Scales
The physical compositions and properties of the scales present within photophores are significant for the perception of the emitted light. The two main types of scales are the lens-scale and the outer accessory-scale. Both scale types act as refractive agents for the light entering and leaving the photophore, additionally accessory-scale acts as protection for the lens-scale. The goal of the accessory-scale’s tougher crystalline guanine structure, other than its protection, is to refract the light towards the desired emission angle (Figure 20) (Cavallaro et al., 2017). Without the accessory-scale, the light from the lens-scale would likely be diffused in straying directions, yet due to guanine’s high refractive index n=1.83, the light’s path is altered and incoming environment light is reflected (Gur et al., 2017). Unlike the reflectors, the accessory-scales are 25 to 95 nm thick and aligned at an oblique angle to the surface of the animal to limit emissions outside the photophore boundaries (Herring, 2000).

The other significant scale type is the lens-scale. The lens-scale lies in the middle of the photophore and acts as a converging lens to refract the light so it concentrates about the axis (Figure 21). There are gelatinous lens-scales and hollowed lens-scales. Both types of lenses are majorly composed of proteins which have high refractive indexes; lens-scales can be composed of aldehyde dehydrogenase, collagen, guanine, or specialized cells (Cavallaro et al., 2017; Dove et al., 1992). In both cases, the material and the shape of the organized layers converge to light by refracting it through a dense medium; such concepts are derived from Snell’s Law (Figure 22). Altogether, the lens-scale and the accessory-scale redirect the light to ensure that the beam is homogenous.


Filters
Two main types of filters are often used in photophores to ensure proper light colouration: absorption filters and interference filters. While these filters’ effects are opposite, both their functions are to process the light emitted to match the ambient lighting. Absorption filters, a type of colour filter, contain pigments that absorb undesirable wavelengths. The absorption filters are composed of pigments similar to chromatophores and can vary in colour. Although there are red or brown filters for contrast purposes, most colour filters are blue with irradiance properties to mimic the overhead water (Sickles, 2020). Since different organisms inhibit selective depths, the colour filters are required to match the extinction coefficient of water (Figure 23; Figure 24) (Denton et al., 1985). Therefore, the light spectra absorbed by the colour filters are very case sensitive: for example, organisms in shallower waters will require pigmented filters that emit high irradiances and absorb longer reddish wavelengths of approximately 650-700nm, whereas the deeper organisms have low irradiances and some reddish emissions (refer to Figure 16) (Denton et al., 1985). Overall, the pigment of the colour filter will depend on whether the photon’s energy in the light will be greater than the electrons in the filter, resulting in the photon transferring its energy to the filter material’s electrons, consequently absorbing the light and emitting the reflected wavelengths between 420 and 470 nm depending on the species (Denton et al., 1985).

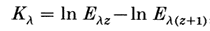
The other type of filter category regroups interference colour filters and light guide structures. While both consist of different materials, they utilize destructive and constructive interference qualities of light through diffraction grating-like structures. The interference filters are composed of stacks of reflector guanine which absorb the nonuniform rays of light and pass them through crystals with high refractive indexes to create phase difference between beams, thus controlling constructive and destructive interference of the rays (Figure 25)(Denton et al., 1985). Generally, filters are used in photophores to limit the unwanted qualities of the light emitted by the other structures.

Conclusively, the combination of a photophore’s components allows it to modify and characterize the light emitted perfectly to accomplish counterillumination camouflage. The reflectors use highly organized refractive material to create a mirror-like reflection, the scales redirect the light to make a homogeneous emission, and the filters are used to selectively purify the colour of the light. The photophore’s subunits are complex and utilize the qualities of the materials as well as the behaviour of light to obtain accurate luminescence. Furthermore, depending on the requirements or the placement of the photophores, some of the subunits may not be present or may have slightly different compositions than the ones analyzed.
Conclusion
Cephalopods have the most diverse and dynamic changeable body patterns, making them masters of camouflage. Through the retraction of dermal papillae, the expansion of chromatophores, the reflecting abilities of iridophore and leucophore cells, as well as light-emitting photophores, cephalopods have the ability to emulate and blend in with surrounding coral, algae, and the underlying seafloor. This mimicry allows cephalopods to protect themselves against hunting predators and also gives them a great advantage for catching prey.
References
Allen, J. J., Bell, G. R., Kuzirian, A. M., & Hanlon, R. T. (2013). Cuttlefish skin papilla morphology suggests a muscular hydrostatic function for rapid changeability. J Morphol, 274(6), 645-656. https://doi.org/10.1002/jmor.20121
Allen, J. J., Bell, G. R., Kuzirian, A. M., Velankar, S. S., & Hanlon, R. T. (2014). Comparative morphology of changeable skin papillae in octopus and cuttlefish. J Morphol, 275(4), 371-390. https://doi.org/10.1002/jmor.20221
Bell, G. R. R., Kuzirian, A. M., Senft, S. L., Mäthger, L. M., Wardill, T. J., & Hanlon, R. T. (2013). Chromatophore radial muscle fibers anchor in flexible squid skin. Invertebrate Biology, 132(2), 120-132. https://doi.org/https://doi.org/10.1111/ivb.12016
Boudreau, D., McDaniel, M., Sprout, E., & Turgeon, A. (2012). Camouflage. National Geographic Society. Retrieved October 16, 2021, from https://www.nationalgeographic.org/encyclopedia/camouflage/
Butler, T. M., & Siegman, M. J. (2010). Mechanism of catch force: tethering of thick and thin filaments by twitchin. J Biomed Biotechnol, 2010, 725207. https://doi.org/10.1155/2010/725207
Cavallaro, M., Battaglia, P., Guerrera, M. C., Abbate, F., Levanti, M. B., Ammendolia, G., Andaloro, F., Germanà, A., & Laurà, R. (2019). Structure and ultrastructure study on photophores of the Madeira lanternfish, Ceratoscopelus maderensis (Lowe, 1839), Pisces: Myctophidae. Acta Zoologica, 100(1), 89-95. https://doi.org/https://doi.org/10.1111/azo.12236
Cloney, R. A., & Brocco, S. L. (2015). Chromatophore Organs, Reflector Cells, Iridocytes and Leucophores in Cephalopods1. American Zoologist, 23(3), 581-592. https://doi.org/10.1093/icb/23.3.581
Copeland, D. E. (1991). Fine Structure of Photophores in Gonostoma elongatum: Detail of a Dual Gland Complex. The Biological Bulletin, 181(1), 144-157. https://doi.org/10.2307/1542497
Costa, F. (2018, October 30). Lanternfishes. Adriaticnature. https://adriaticnature.com/archives/6132
Denton, E. J., Herring, P. J., Widder, E. A., Latz, M. F., & Case, J. F. (1985). The roles of filters in the photophores of oceanic animals and their relation to vision in the oceanic environment. Proceedings of the Royal Society of London. Series B. Biological Sciences, 225(1238), 63-97. https://doi.org/doi:10.1098/rspb.1985.0051
Deravi, L. F., Magyar, A. P., Sheehy, S. P., Bell, G. R. R., Mäthger, L. M., Senft, S. L., Wardill, T. J., Lane, W. S., Kuzirian, A. M., Hanlon, R. T., Hu, E. L., & Parker, K. K. (2014). The structure-function relationships of a natural nanoscale photonic device in cuttlefish chromatophores. Journal of The Royal Society Interface, 11(93), 20130942. https://doi.org/doi:10.1098/rsif.2013.0942
Dove, S., Horwitz, J., & McFall-Ngai, M. (1993). A biochemical characterization of the photophore lenses of the midshipman fish, Porichthys notatus Girard. Journal of Comparative Physiology A, 172(5), 565-572. https://doi.org/10.1007/bf00213679
Gonzalez-Bellido, P. T., Scaros, A. T., Hanlon, R. T., & Wardill, T. J. (2018). Neural Control of Dynamic 3-Dimensional Skin Papillae for Cuttlefish Camouflage. iScience, 1, 24-34. https://doi.org/10.1016/j.isci.2018.01.001
Gur, D., Palmer, B. A., Weiner, S., & Addadi, L. (2017). Light Manipulation by Guanine Crystals in Organisms: Biogenic Scatterers, Mirrors, Multilayer Reflectors and Photonic Crystals. Advanced Functional Materials, 27(6), 1603514. https://doi.org/https://doi.org/10.1002/adfm.201603514
Hanlon, R. T., Mäthger, L. M., Bell, G. R. R., Kuzirian, A. M., & Senft, S. L. (2018). White reflection from cuttlefish skin leucophores. Bioinspir Biomim, 13(3), 035002. https://doi.org/10.1088/1748-3190/aaa3a9
Herring, P. J. (2000). Bioluminescent signals and the role of reflectors. Journal of Optics A: Pure and Applied Optics, 2(6), R29-R38. https://doi.org/10.1088/1464-4258/2/6/202
Kier, W. M., & Smith, K. K. (1985). Tongues, tentacles and trunks: the biomechanics of movement in muscular-hydrostats. Zoological Journal of the Linnean Society, 83(4), 307-324. https://doi.org/https://doi.org/10.1111/j.1096-3642.1985.tb01178.x
Kreit, E., Mäthger, L. M., Hanlon, R. T., Dennis, P. B., Naik, R. R., Forsythe, E., & Heikenfeld, J. (2013). Biological versus electronic adaptive coloration: how can one inform the other? Journal of The Royal Society Interface, 10(78), 20120601. https://doi.org/10.1098/rsif.2012.0601
Ling, S. J., Sanny, J., & Moebs, W. (2016, September 1). Young’s double-slit interference. University Physics Volume 3. Retrieved October 9, 2021, from https://opentextbc.ca/universityphysicsv3openstax/chapter/youngs-double-slit-interference/
Mäthger, L. M., Denton, E. J., Marshall, N. J., & Hanlon, R. T. (2009). Mechanisms and behavioural functions of structural coloration in cephalopods. Journal of The Royal Society Interface, 6(suppl_2), S149-S163. https://doi.org/10.1098/rsif.2008.0366.focus
Mäthger, L. M., & Hanlon, R. T. (2007). Malleable skin coloration in cephalopods: selective reflectance, transmission and absorbance of light by chromatophores and iridophores. Cell and Tissue Research, 329(1), 179-186. https://doi.org/10.1007/s00441-007-0384-8
Messenger, J. B. (2001). Cephalopod chromatophores: neurobiology and natural history. Biological Reviews, 76(4), 473-528. https://doi.org/https://doi.org/10.1017/S1464793101005772
Paitio, J., Yano, D., Muneyama, E., Takei, S., Asada, H., Iwasaka, M., & Oba, Y. (2020). Reflector of the body photophore in lanternfish is mechanistically tuned to project the biochemical emission in photocytes for counterillumination. Biochemical and Biophysical Research Communications, 521(4), 821-826. https://doi.org/https://doi.org/10.1016/j.bbrc.2019.10.197
Roper, C. F.E. and Voss, G. L. (2020, February 13). cephalopod. Encyclopedia Britannica. https://www.britannica.com/animal/cephalopod
Senft, S. L., Kuzirian, A. M., & Hanlon, R. T. (2021). Networks of linked radial muscles could influence dynamic skin patterning of squid chromatophores. Journal of Morphology, 282(8), 1245-1258. https://doi.org/https://doi.org/10.1002/jmor.21379
Sickles, J. E. (2020). Comparative Study of the Effects of Light on Photophore Ultrastructure from Two Families of Deep-Sea Decapod Crustaceans: Oplophoridae and Sergestidae. [Master’s thesis, Nova Southeastern University]. NSUWorks. https://nsuworks.nova.edu/occ_stuetd/524/
Stark, G. (2021, May 7). light. Encyclopedia Britannica. https://www.britannica.com/science/light
The Editors of Encyclopaedia Britannica. (2021a, April 29). Snell’s law. Encyclopedia Britannica. https://www.britannica.com/science/Snells-law
The Editors of Encyclopaedia Britannica. (2021b, May 11). lens. Encyclopedia Britannica. https://www.britannica.com/technology/lens-optics
Videen, G. (1991). Light scattering from a sphere on or near a surface. Journal of the Optical Society of America A, 8(3), 483-489. https://doi.org/10.1364/JOSAA.8.000483
Watson, M., Thurston, E. L., & Nicol, J. A. C. (1978). Reflectors in the light organ of Anomalops (Anomalopidae, Teleostei). Proceedings of the Royal Society of London. Series B. Biological Sciences, 202(1148), 339-351. https://doi.org/doi:10.1098/rspb.1978.0071