Hormonal Control of Molting
Sidrah Alousi-Jones, Le Chen, Léanne Gauthier, Haley Janvrin
Abstract
Molting is a crucial and intricate process requiring the timely shedding of an animal’s body part, which may include the epidermis, the pelage, or the entire exoskeleton. In accordance with the extraordinary diversity observed within the animal kingdom, molting takes many forms and is characterized by specific processes for each species. However, the precise control of molting is consistent across all species, as the cycles are only initiated at specific moments throughout the animal’s life. The periodicity of the molting cycles is governed by hormonal control; the particular hormones involved in the molting process vary across species. This report examines the involvement of the endocrine system in the regulation of molting for birds, insects, and crustaceans. Various birds shed their old plumage as dictated by thyroid hormones, sex steroid hormones and prolactin. The molting cycle of the tobacco hornworm is initiated by ecdysteroids and culminates with sclerotization, which is triggered by the neurohormone bursicon. Similarly, the wild mud crab sheds its old exoskeleton as controlled by ecdysteroids, molt inhibiting hormones, and the crustacean hyperglycemic hormone. Finally, this report discusses a special case of hormonal control; the East African medicinal plant, Ajuga remota, uses phytoecdysteroids as a chemical mean of protection against invertebrate predators. The plant-derived ecdysteroids disrupt the insect molting cycle, which may lead to the affected insect’s death. The chemical processes associated with the hormonal control of molting allow the timely initiation of the cycles, a key requirement for successful molting.
Introduction
The endocrine system is a web of glands that works hand in hand alongside the nervous system to regulate, initiate and halt biological activities in living organisms. The molting process, which involves the shedding and replacement of an organism’s outer layer to allow for growth, relies heavily on the chemical pathways of the endocrine system to stimulate and regulate the cycle. In birds, the molt is controlled by both thyroid and sex steroid hormones, which prevent the overlap of molting and reproduction. The importance of ecdysteroids in molting is demonstrated by both the tobacco hornworm and the green mud crab, which both stimulate their molt through ecdysteroids. The green mud crab, however, is unique in that it regulates the length and frequency of its molt through inhibiting hormones that counteract the production of ecdysteroids. Although these three species use different hormones to achieve their means, the end goal is the same: a healthy and well controlled molting phase that occurs under ideal conditions. Alternatively, the East African medicinal plant, Ajuga remota, uses molting hormones as a defense. The plant produces phytoecdysteroids, which severely disrupt the insect molting cycle often resulting in death and hence deter insects from eating the plant. Through these examples, it is clear that hormones are an essential aspect of a controlled and successful molt, proving the relevance of chemical processes to an organism’s survival
Hormonal Control of Molting in Birds
Timing of the molting process
Plumage provides birds with many essential functions, and no matter for flight or insulation, quality plumage is crucial for birds to survive (Dawson, 2015). Therefore, molting has always been a key event in life cycle of birds (Dawson, 2015). They undergo molting at least once a year (Dawson, 2015). They replace their feathers “annually in a strictly controlled bilaterally symmetrical sequence” to minimize its negative effects on the functions of plumage (Dawson, 2015). Breeding is another important event for birds. However, since molting and breeding are both energy-demanding and time-consuming activities, these two events usually do not overlap in birds, and molting often occurs after breeding is finished (Dawson, 2015). Many studies investigate the hormonal control of molting in birds, and thyroid hormones, sex steroid hormones and prolactin are shown to be the hormones that regulate molting in birds (Dawson, 2015). This section will discuss the correlation relationship between these three types of hormones and molting in birds.
Thyroid Hormone
“Thyroid hormones are involved in somatic growth and development,” and will therefore be involved in molting since they are required for feather formation (Dawson, 2015). Otsuka et al. (1998) studied the breeding and molting activity of Humboldt penguins within a given year and collected their blood samples throughout the sampling period to measure their circulating hormones. The observed results of plasma concentrations of thyroxine (T4) and triiodothyronine (T3) with respect to sampling period are shown in Fig. 1 and Fig. 2, respectively. As shown in Fig. 1, the concentrations of T4 were low at the start of the year. However, they increased sharply before the molting period, and were at maximum values during molting overall. Moreover, they started to drop after the molting period. As for T3, Fig. 2 shows that the concentrations of T3 did not vary significantly for both sexes throughout the year. Although some variations for males can be seen before molting, they overall did not rise and drop sharply like the concentrations of T4 around the molting period.


Furthermore, the importance of T4 and T3 is studied in Reinert and Wilson’s (1997, as cited in Kuenzel, 2003) experiment. Three groups of thyroidectomized tree sparrows were injected with three different doses (0.1, 1.0, and 10.0 μg) of T4 replacement over 14 days. Similarly, an additional three groups were injected with three comparable doses of T3 replacement. The data in Fig. 3 and Fig. 4 shows that molting only occurred in birds receiving 1.0 and 10.0 μg doses of T4. Particularly, no molting occurred with any doses of T3. The observations from Reinert and Wilson’s (1997, as cited in Kuenzel, 2003), along with the study by Otsuka et al. (1998), show that: thyroid hormones are indeed involved in regulating molting; they are at high concentrations before or during the molting period; among thyroid hormones, T4 plays a more important role than T3.


Sex Steroid Hormone
As with thyroid hormones, it is believed that sex steroid hormones also involve in regulating molting because of the non-overlapping of breeding and molting (Dawson, 2015). The experiment of Otsuka et al. (1998) also measured the plasma concentrations of two sex steroid hormones, testosterone (T) and estradiol (E2), during the sampling period. As shown in Fig. 5, the concentrations of T started to decline 2-3 months before the molting started and they maintained at a low level until the molting was finished. The concentrations were at minimum during molting. After the molting period, the concentrations of T tended to rise. Fig. 6 shows that the concentrations of E2 were low at the start of the sampling period and they also reached minimum during the molting period. However, they started to climb rapidly after the molting period. These observations show that sex steroid hormones are at low concentrations before or during the molting period but will increase after molting have completed.
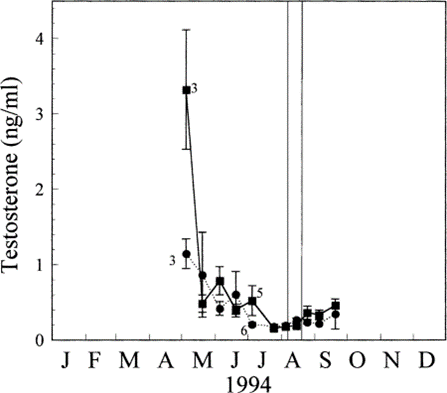

There are also studies show that testosterone administration on birds can impact the molting as inhibitors (Dawson, 2015): testosterone administration prevents a molting that would have normally occurred; when testosterone is removed, molting starts and it progresses faster than usual, trying to compensate the lost time caused by testosterone inhibition; if testosterone is removed after a normal molting, another molting will not occur; implants of testosterone can also stop an ongoing molting, the stopped molting can resume or a new molting can start as soon as the implants are removed. Although a high concentration of testosterone can act to inhibit molting, a low concentration of testosterone or a decrease in concentrations of testosterone cannot act to induce molting (Dawson, 2015).
Prolactin
It is found that prolactin also plays a role in regulating molting in birds (Dawson, 2015). Dawson (2006) conducted a series of experiments regarding molting of starlings and found that in experiments where a molting was successfully induced, the concentrations of prolactin in starlings always increased prior to molting. Nonetheless, the peak of prolactin concentrations and the start of molt usually, if not always, occurred at the same time (Dawson, 2006). Since the time of peak prolactin concentrations mostly coincides with the time of onset of molting, moreover, molting will not start without presence of prolactin, it is hypothesized that high prolactin concentrations play a significant role to initiate a molting (Dawson, 2015). Nonetheless, another hypothesis is made (Dawson, 2015): the high prolactin concentrations, rather than stimulating molting, inhibit molting. The rationale is as follows (Dawson, 2015): since the peak concentrations of prolactin coincide with the onset of molting, it also implies that molting will not start until prolactin concentrations start to decline; molting progresses in a period that concentrations of prolactin are continuously decreasing. Dawson (2015) states that the hypothesis that molting is inhibited by high prolactin concentrations can likely be true because it also corresponds to the mechanism in birds that avoids or minimizes the overlap of breeding and molting activity. Meanwhile, it makes sure that molting can start right after breeding.
Even though there is many evidence showing the involvement of thyroid hormones, sex steroid hormones and prolactin in the process of molting, these relationships are still limited to correlation; causality is unclear (Dawson, 2015). Although molting is extremely important for birds and many studies and research on such topic have been conducted for several decades, the actual mechanism of hormonal control of molting in birds is still unanswerable (Dawson, 2015). Further studies will be needed in order to figure out the actual mechanism.
Hormonal Control of Ecdysis and Sclerotization in Insects
The hormones involved in insect ecdysis
In insects, each step of the ecdysis process is initiated and dictated by a sequence of hormones released throughout the cycle. The molting cycle is initiated by the release of the prothoracicotropic hormone (PTTH) (Riddiford, 2009), a neuropeptide confined in the ventromedial region of the brain, typically within one or two pairs of neurosecretory neurons. The release of the PTTH is triggered by internal or external environmental signals, which vary for different insect species. The distention of the abdomen by a meal, which is relayed to the brain via stretch receptors can constitute a triggering signal (Riddiford, 2009). In Manduca sexta, the tobacco hornworm (Fig. 7), a combination of the size of the moth larva and the time of the day governs the release of the PTTH; the hormone is released during a specific time window at night, called a gate, and only once M. sexta has attained a sufficient size (Riddiford, 2009). Once the PTTH is in circulation, it causes the synthesis of an ecdysteroid, α-ecdysone (E), by stimulating the prothoracic glands (Riddiford, 2009). E is subsequently converted to 20-hydroxyecdysone (20E) within the insect’s tissues, typically the fat body and the Malpighian tubules (Riddiford, 2009). Both Eand 20E are key components in the earlier stages of ecdysis; E is predominantly involved in the early cellular changes associated with the onset of ecdysis, while 20E is involved in the differentiative changes occurring later in the cycle (Riddiford, 2009). More precisely, 20E initiates the deposition of the new cuticle during the intermolt process, and its presence is required to initiate the deposition of the cuticle layer composed of chitin and proteins, a process finalized once 20E recedes form the bloodstream (Riddiford, 2009).

Once the new cuticle is formed and the molting fluid is resorbed, the ecdysis-triggering hormone (ETH) is released into the central nervous system (CNS) and initiates the preecdysis period, characterised by the detachment of the muscles from the old cuticle (Riddiford, 2009). The eclosion hormone (EH) is then released into the blood and within the CNS from a set of neurosecretory neurons following their stimulation by the ETH (Truman, 2005). Similarly, the EH in turn triggers the release of the crustacean cardioactive peptide (CCAP) by activating the network of neurons within which the CCAP is confined (Riddiford, 2009). The CCAP is involved into one of the culminating stages of the molting cycle, triggering the behaviour leading to the shedding of the old cuticle.
Sclerotization in Manduca sexta and the associated hormones
The last stage of the molting cycle consists in the hardening of the new cuticle, which takes place after the expansional period during which the insect reaches its suitable size. This stabilization process, known as sclerotization (Fig. 8) (Hopkins & Kramer, 1992), is initiated in Manduca sexta by the release of bursicon, a neurohormone from the CNS which influences the activities of the epidermal cells (Andersen, 2012). Once initiated, sclerotization involves the crosslinking of the cuticle’s proteins together as well as with chitin via the cross-linking agents N-acetyldopamine (NADA) and N-β-alanyldopamine (NBAD), two dopamine derivatives incorporated into the cuticular matrix (Andersen, 2012).

The sclerotization precursors NADA (Fig. 9, 4) and NBAD (Fig. 9, 5) must first be synthesised from tyrosine (Fig. 9, 1), an amino acid, before the stabilization of the new cuticle can occur (Andersen, 2012). Tyrosine is initially converted to 3,4-dihydroxyphenylalanine (DOPA) (Fig. 9, 2) by hydroxylation, and dopamine (Fig. 9, 3) results from the subsequent decarboxylation of DOPA. NADA and NBAD are then synthesised from dopamine by N-acylation and enzymatically oxidized to form their respective o-quinones (Fig. 10, 6). The resulting o-quinones can readily react with nucleophilic groups to produce catecholic structures with the nucleophile attached to the aromatic ring (Fig. 10, 11). The cuticular nucleophilic groups involved in this reaction are typically the histidine imidazole group as well as free amino groups (Andersen, 2012). The o-quinones can also be isomerized to form p-quinone methides (Fig. 10, 7); in this case, the nucleophiles react with the β-position of the side chain (Fig. 10, 12). The p-quinone methides of NADA can themselves be further isomerized into α,β-dehydro-N-acetyldopamine (dehydro-NADA) (Fig. 10, 8), which can be oxidized to its associated o-quinone (Fig. 10, 9) and p-quinone methide (Fig. 10, 10). The resulting p-quinone methides can react with nucleophiles, typically the phenolic group of tyrosine, or catechols; the latter results in dihydroxyphenyl-dihydrobenzodioxine derivatives (Fig. 10, 13) (Andersen, 2012).


All of the aforementioned reactions between quinones and nucleophiles contribute to the formation of inter-protein cross-links via the interactions of the quinones with the nucleophilic regions of the cuticular proteins (Andersen, 2012). The quinones are also involved in the formation of bonds between the cuticular proteins and chitin (Andersen, 2012). As a result of these increasingly numerous interactions between the cuticular components, a strengthening of the new cuticle of M. sexta is observed up until the end of the sclerotization period is reached; the molting cycle is then completed.
Hormonal Control of Molting in Crustaceans
Molting in Scylla paramamosain
It is no secret that balance is the key to numerous biological processes, and the molting cycle of Scylla paramamosain is no different (Fig. 11.). Similar to insects, the crustacean molting process is controlled by the endocrine system, ensuring that a healthy molt occurs under the correct external conditions such as temperature and substrate concentrations. However, unlike insects which employ a stimulatory control system, crustaceans regulate their molt through inhibitors. The green mud crab, widely sought after in aquaculture for its fast growth rate, is a perfect example of the hormonal inhibition process in action (Hobbs & Lodge, 2010).

Like other crustaceans, the wild mud crab Scylla paramamosain regularly molts by shedding its exoskeleton to allow for new growth. This molting process occurs in four stages: pre-molt, in which the old exoskeleton is partially digested and reabsorbed; ecdysis where the old exoskeleton is shed; post-molt during which the new cuticle expands and hardens; and finally intermolt, where the new exoskeleton is fully formed and Scylla paramamosain is effectively protected (Hosamani & Reddy, 2017). The mud crab’s progression through these stages of molting is primarily regulated by ecdysteroids, molt inhibiting hormones (MIH) and crustacean hyperglycemic hormone (CHH), which are synthesized and secreted by the Y and X organs.
Hormones Involved and Their Effect on Molting Regulation
The mud crab’s Y-organs, located in the maxillary somites, are the primary stimulus for the molting process. Regardless of external conditions, these glands secrete inactive forms of ecdysteroids, specifically ecdysone, 25-deoxyecdyson and 2-dehydroecdysone, which are then hydroxylated by the surrounding tissues into their active forms 20-hydroxyecdysone and ponasaterone A (Chen et al., 2020). Ecdysteroids are a type of hormone responsible for the regulation of the molting process in arthropods as well as for the control of several other important life-events. Increased levels of these ecdysteroids in the hemolymph are what stimulate the premolt processes to begin, namely the reabsorption of the old and synthesis of the new exoskeleton. At the end of the premolt phase, a significant drop in ecdysteroids initiates ecdysis. Ecdysteroid levels then remain low throughout the post-molt and inter-molt phases, allowing calcification of the exoskeleton and growth of the mud crab (Mykles, 2011).
The production of ecdysteroids is controlled by the X-organ sinus gland complex located in the eyestalks. The medulla terminalis X-organs are masses of neurosecretory cells with axons that project distally to form the neurohemal organ that is the sinus gland. In the inter-molt phase, the X-organs release peptide hormones signalling the sinus gland to produce molt inhibiting hormone (MIH). MIH is a class II peptide that binds to receptors on the epidermis of the Y-organs, which prevents the conversion of ketodiol to 25 deoxyecdysone. In this manner, the X-organs interrupt the production of ecdysteroids by keeping the Y-organs in the basal state, thus inhibiting the molt. The concentration of MIH and ecdysteroids are inversely proportional (Fig. 12.) (Chen et al., 2020).
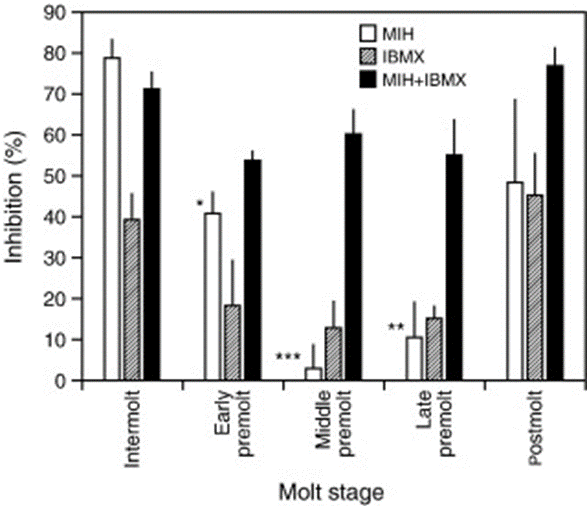
During the pre-moult phase, Scylla paramamosain decreases the production of MIH, causing an increase in hemolymph ecdysteroid levels and thus stimulating the moult. After ecdysis, the mud crab increases the production of MIH and maintains the high hormone levels throughout the inter-molt phase to limit ecdysteroid production. This is how inhibitors regulate the length of the molt cycle (Hosamani & Reddy, 2017).
Since the molt-inhibiting hormones are produced in the eyestalk, many commercial crab farms use the technique of eyestalk ablation in which the eyestalk is removed. This shortens the length of the molting cycle due to the lack of inhibitor hormones, allowing the mud crab to continually molt and thus grow and reach adulthood faster. Upon reintroducing the eyestalk tissue, the molt cycle was lengthened again (Chen et al., 2020).
A Special Case of Hormonal Control of Molting: Phytoecdysteroids
Phytoecdysteroids: A Descriptive Overview
Phytoecdysteroids are plant-derived ecdysteroids and have the similar effect of inducing and regulating insect molting (Das et al., 2021). Plants produce and store such hormones as a means of protection against invertebrate predators. The phytoecdysteroids chemically defend from the threat of plant-eating insects by signalling toxicity to the insect from the bitter taste of their leaves. Most importantly, they disrupt insect hormonal balance and in so doing, the insect molting cycle. This often results in insect death (Das et al., 2021).
The Case of the East African Medicinal Plant and the Fall Army Worm
This phenomenon is illustrated by the interactions between the East African medicinal plant, Ajuga remota (Fig. 13.) and the fall army worm Spodoptera Jrugiperda (Fig. 14.) After the fall army worm ingests the bitter tasting leaves of the Ajuga remota, the timing of its molting cycle will be disrupted due to the phytoecdysteroids contained in the leaves (Kubo et al., 1981). The molting process starts when a soft, new cuticle begins to form beneath the older one. The older shell is then shed as the new cuticle expands due to hydrostatic pressure. After, the new cuticle hardens through a process called sclerotization. The phytoecdysteroids causes the new cuticle to harden in an untimely fashion, before it expands. Therefore, the older cuticle is never shed. This process is repeated until the fall army worm accumulates upwards of three superimposed cuticles (Fig. 15.). The fall army worm becomes unable to feed because the accumulated hard shells obstruct its feeding tract and mouth parts. Fall army worm death occurs when it can no longer feed (Kubo et al., 1981).



Diversity and Distribution of Phytoecdysteroids
Phytoecdysteroids and other ecdysteroid-like compounds have been detected in terrestrial plants, fungi and algae. 5-6% of all terrestrial plants are known to accumulate phytoecdysteroids. It is suggested that most plants contain the genetic material to produce phytoecdysteroids, but that only of a few actually express those genes and accumulate phytoecdysteroids. There is great variation in the amount of phytoecdysteroids that a plant species accumulates. It can range from practically imperceptible to occupying 2-3% of plant dry weight (Dinan et al., 2009). Dry weight is the weight of plant when all water has been removed (Tangahu, 2016). However, even low levels of the hormone can prove to be effective in deterring herbivorous insects. Phytoecdysteroid levels within a single plant vary as well. Levels can vary depending on the plant organ, the time of year, as well as the location in which the plant grows and resides. There is currently no definitive answer to which specific region of plant tissue or of the plant cell contains and stores phytoecdysteroids but it has been hypothesized that it is the vacuole (Dinan et al., 2009).
Hormonal Chemical Structure
Phytoecdysteroids belong to the larger chemical class of steroids. Their carbon skeleton, in other words, the blueprint for phytoecdysteroids, is called cyclopentanoperhydrophenanthrene (Das et al., 2021). A shorter nomenclature for this molecule is sterane. Steranes are comprised of 4 cyclic compounds and at C17, a side chain is attached (Fig. 16.). A gonane is the name for the nucleus of 4 rings in a steroid (Fig. 17.) (Clayton & Kluger, 2021). Phytoecdysteroids possess 27-29 carbon atoms (Fig. 18.) (Das et al., 2021). Looking now to stereochemistry of the phytoecdysteroids, which pertains to the orientation and arrangement of the molecule in space, all phystoecdysteroids share certain defining characteristics (Das et al., 2021). The side chain attached to C17 is a β-chain. In other words, its bond can be represented as a wedge bond. That chain is therefore pointing up from the plane of the gonane (Clayton & Kluger, 2021). Another defining characteristic of phytoecdysteroids, is that the junction of the rings A and B is cis. The hydrogen at C5 is pointing up from the plane of the gonane, it is therefore designated 5β – H (Das et al., 2021). Knowing that C19 is also attached by a wedge bond, this means that the 5β-H and C19 have the same orientation at the junction (Clayton & Kluger, 2021). The other defining characteristic is that at the OH group C14 is attached by a dash bond. This means that it is pointing down. The junction of the C and D rings can therefore be called trans, seeing as C18 has a wedge bond (Fig. 18.) (Das et al., 2021). The trans designation indicates that the orientation of the groups at the junction are opposite (Clayton & Kluger, 2021). Lastly, there is an enone group at ring B (Fig. 19.) (Das et al., 2021). This group is a chromophore, meaning that it is responsible for the color of the molecule (The Editors of Encyclopaedia Britannica, 2011).




Importance of Chemical Structure in Phytoecdysteroids
Though examining the structure of phytoecdysteroids might seem overly detail-focused, understanding the commonalities of the hormonal chemical structure and how they differ from one another help to characterize phytoecdysteroid behaviour. Over 100 different phytoecdysteroids have been identified in terrestrial plants. It is the slight variation in chemical structure which is responsible for this diversity (Das et al., 2021.). The importance of these variations is clear in the following example. Four different phytoecdysteroids (20-hydroxyecdysone, polypodine B, ponasterone A and makisterone A) (Fig. 20) were fed in identical concentrations to the Indian meal worm (Plodia interpunctella) (Fig. 21). When comparing the mortality rates of the four phytoecdysteroids in the Indian meal worm population, it is possible to observe how slight chemical differences in the phytoecdysteroids are responsible for the severity of the effects on the worms. For example, “22 days after the beginning of the experiment, [the mortality rate] was 46.7%, 52.5%, 64%, and 84% for PolB, 20E, PonA and MakA, respectively” (Rharrabe et al., 2010) (Fig. 22).



Conclusion
To summarize, hormones indeed play a crucial role in regulating the molting cycle of several species. In various birds, a correlation was found between hormones and molting: their molting cycle is associated with high levels of thyroid hormones and low levels of sex steroid hormones; peak concentration of prolactin coincides with the start of molting which raises two controversial hypotheses regarding prolactin’s role. In tobacco hornworms, the prothoracicotropic hormone (PTTH), ecdysteroids and bursicon are involved in regulating the molting cycle. In wild mud crabs, active forms of ecdysteroids trigger molting, and molt inhibiting hormones (MIH) inhibit molting by suppressing the production of ecdysteroids. Lastly, phytoecdysteroids, as plant-derived versions of ecdysteroids, can disrupt molting of insects and cause their mortality. Slight variation in phytoecdysteroids’ chemical structures can produce different phytoecdysteroids that cause different mortality rates. The study of these cases regarding hormonal control, from a chemical perspective, shows the significance and indispensability of hormones in molting of many species.
References
Andersen, S. O. (2012). 6 – Cuticular Sclerotization and Tanning. In L. I. Gilbert (Ed.), Insect Molecular Biology and Biochemistry (pp. 167-192). Academic Press. https://doi.org/https://doi.org/10.1016/B978-0-12-384747-8.10006-6
Bauer, S. (2003, May 22). File:Spodoptera frugiperda pupa.jpg. Wikipedia. https://commons.wikimedia.org/wiki/File:Spodoptera_frugiperda_pupa.jpg
Chen, H.-Y., Toullec, J.-Y., & Lee, C.-Y. (2020). The Crustacean Hyperglycemic Hormone Superfamily: Progress Made in the Past Decade [Review]. Frontiers in Endocrinology, 11. https://doi.org/10.3389/fendo.2020.578958
Clayton, R. B., & Kluger, R. H. (2021, April 1). steroid. Encyclopedia Britannica. Retrieved November 4, 2021, from https://www.britannica.com/science/steroid
Das, N., Mishra, S. K., Bishayee, A., Ali, E. S., & Bishayee, A. (2021). The phytochemical, biological, and medicinal attributes of phytoecdysteroids: An updated review. Acta Pharmaceutica Sinica B, 11(7), 1740-1766. https://doi.org/https://doi.org/10.1016/j.apsb.2020.10.012
Dawson, A. (2006). Control of molt in birds: Association with prolactin and gonadal regression in starlings. General and Comparative Endocrinology, 147(3), 314-322. https://doi.org/https://doi.org/10.1016/j.ygcen.2006.02.001
Dawson, A. (2015). Chapter 38 – Avian Molting. In C. G. Scanes (Ed.), Sturkie’s Avian Physiology (Sixth Edition) (pp. 907-917). Academic Press. https://doi.org/https://doi.org/10.1016/B978-0-12-407160-5.00038-5
Descouens, D. (2016, December 21). File:Manduca sexta MHNT CUT 2010 0 104 Caranavi, La Paz Bolivia male dorsal.jpg. Wikipedia. https://en.wikipedia.org/wiki/File:Manduca_sexta_MHNT_CUT_2010_0_104_Caranavi,_La_Paz_Bolivia_male_dorsal.jpg
Dinan, L., Harmatha, J., Volodin, V., & Lafont, R. (2009). Phytoecdysteroids: Diversity, Biosynthesis and Distribution. In G. Smagghe (Ed.), Ecdysone: Structures and Functions (pp. 3-45). Springer Netherlands. https://doi.org/10.1007/978-1-4020-9112-4_1
Fasulo, T. R., & Knox, M. A. (2015, November). Indianmeal moth – Plodia interpunctella (Hubner). Entomology and Nematology Department, University of Florida. Retrieved November 3, 2021, from https://entnemdept.ufl.edu/creatures/urban/stored/indianmeal_moth.HTM
Freshet, A., Daniel, S., & Eyasu, M. (2017). Antihyperglycemic and antihyperlipidemic activities of ethanol extract of Ajuga remota Benth (Harmegusa) leaves in streptozotocin induced diabetic rats. African Journal of Pharmacy and Pharmacology, 11(2), 17-24. https://doi.org/10.5897/ajpp2014.4699
Hobbs, H. H., & Lodge, D. M. (2010). Chapter 22 – Decapoda. In J. H. Thorp & A. P. Covich (Eds.), Ecology and Classification of North American Freshwater Invertebrates (Third Edition) (pp. 901-967). Academic Press. https://doi.org/https://doi.org/10.1016/B978-0-12-374855-3.00022-4
Hopkins, T. L., & Kramer, K. J. (1992). Insect Cuticle Sclerotization. Annual Review of Entomology, 37(1), 273-302. https://doi.org/10.1146/annurev.en.37.010192.001421
Hosamani, N., B, S., & Reddy, R. (2017). Crustacean Molting: Regulation and Effects of Environmental Toxicants. Journal of Marine Science: Research & Development, 07. https://doi.org/10.4172/2155-9910.1000236
Illustrated Glossary of Organic Chemistry. (n.d.). The UCLA Department of Chemistry & Biochemistry. Retrieved November 4, 2021, from http://www.chem.ucla.edu/~harding/IGOC/E/enone.html
Kubo, I., Klocke, J. A., & Asano, S. (1981). Insect Ecdysis Inhibitors from the East African Medicinal Plant Ajuga remota (Labiatae). Agricultural and Biological Chemistry, 45(8), 1925-1927. https://doi.org/10.1271/bbb1961.45.1925
Kuenzel, W. J. (2003). Neurobiology of molt in avian species. Poultry Science, 82(6), 981-991. https://doi.org/https://doi.org/10.1093/ps/82.6.981
Mykles, D. L. (2011). Ecdysteroid metabolism in crustaceans. J Steroid Biochem Mol Biol, 127(3-5), 196-203. https://doi.org/10.1016/j.jsbmb.2010.09.001
NEUROtiker. (2007, June 28). File:Steroid-nomenclature.svg. Wikimedia Commons. https://commons.wikimedia.org/wiki/File:Steroid-nomenclature.svg
Otsuka, R., Aoki, K., Hori, H., & Wada, M. (1998). Changes in Circulating LH, Sex Steroid Hormones, Thyroid Hormones and Corticosterone in Relation to Breeding and Molting in Captive Humboldt Penguins (Spheniscus humboldti) Kept in an Outdoor Open Display. Zoological Science, 15(1), 103-109, 107. https://doi.org/10.2108/zsj.15.103
Rharrabe, K., Sayah, F., & Lafont, R. (2010). Dietary effects of four phytoecdysteroids on growth and development of the Indian meal moth, Plodia interpunctella. Journal of Insect Science, 10(1). https://doi.org/10.1673/031.010.1301
Riddiford, L. M. (2009). Chapter 170 – Molting. In V. H. Resh & R. T. Cardé (Eds.), Encyclopedia of Insects (Second Edition) (pp. 649-654). Academic Press. https://doi.org/https://doi.org/10.1016/B978-0-12-374144-8.00179-X
Shih, H.-T. (2016). Scylla paramamosain. Crab Database. Retrieved November 4, 2021, from https://www.crabdatabase.info/en/crabs/brachyura/eubrachyura/heterotremata/portunoidea/portunidae/scylla/scylla-paramamosain-3181
Tangahu, B. (2016). Growth Rate Measurement of Scirpus Grossus Plant as Preliminary Step to Apply the Plant in Wastewater Treatment Using Reedbed System. Journal of Civil & Environmental Engineering, 05. https://doi.org/10.4172/2165-784X.1000192
The Editors of Encyclopaedia Britannica. (2011, December 6). chromophore. Encyclopedia Britannica. Retrieved November 4, 2021, from https://www.britannica.com/science/chromophore
Truman, J. W. (2005). Hormonal control of insect ecdysis: endocrine cascades for coordinating behavior with physiology. Vitam Horm, 73, 1-30. https://doi.org/10.1016/s0083-6729(05)73001-6