Biological Design for Lungs and Gills: Biomolecular and Chemical Engineering
Samuel Bernard, Nabhaan Farooqi, Camille Gagnon, Will Vyse
Abstract
This report describes two of the most important gas exchangers for all living animals: lungs and gills. Throughout the research, cutaneous respiration will also be explained, however, the most efficient oxygen diffusing rates are obtained through lungs and gills, which are our main concern. The purpose of this biological design project is to find out how different species evolved and developed unique respiration systems that allow them to maximize their oxygen intake without spending energy. This report contains information about cellular respiration, the composition of the pulmonary surfactant, as well as the relation between surface tension in the alveoli and surfactant concentration.
Diffusion in The Gill
In gills, the transfer of oxygen between the water and the gills is facilitated by the fish’s gill filaments, also called the lamellae. These lamellae contain capillaries, carrying the fish’s oxygen-deprived blood (Randall and Daxboeck, 1984). As the oxygen-rich water passes over the lamellae, diffusion occurs because of the large disparity in oxygen levels. In the reverse process, carbon dioxide leaves the blood and enters the ocean, diffusing from the CO2 -rich blood into the CO2 -deprived water. The newly oxygenated and CO2 liberated blood then flows back into the fish’s system, while oxygen-deprived and CO2 -rich blood flows into the lamellae, in a continuous system. It is like the blood’s sieve for oxygen! (Randall and Daxboeck, 1984)

Size Matters
The time for the diffusion process to happen is very small, a section of water is in contact with the lamellae for roughly 100 – 300 milliseconds, so a large area of contact is necessary to facilitate large transfers of oxygen and carbon dioxide. It is the surface area of the lamellae that determines the rate of gas transfer between the water and the blood (Randall and Daxboeck, 1984). The lamellae are generally very thin and tall, to allow water to flow between them easily while maintaining a large surface area for capillaries to spread across. In general, larger and more active fish will have larger lamellae, to accommodate their greater need for oxygen (Randall and Daxboeck, 1984).
Controlling the Oxygen
When fish are faced with a scenario where they need to control their oxygen levels, they respond in different ways. One way we can see this is by observing how fish react to hypoxia and hypoxemia: afflictions which result in reduced oxygen levels inside a fish’s body. Different types of fish react differently to these afflictions (Randall and Daxboeck, 1984). Some, called oxygen regulators, react to it by swimming more and swimming faster. This increases the amount of water flowing through their gills and allows for more diffusion to happen in their lamellae (Randall and Daxboeck, 1984). Other fish, called oxygen conformers, instead react by reducing their movement. By reducing movement, they are reducing energy that would otherwise be used in ventilating their gills, thereby preserving themselves until they can recover (Randall and Dexboeck, 1984).

Diffusion in the Lung
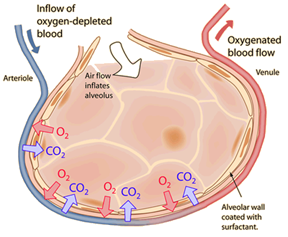
Oxygen enters the blood of mammals by the same process that it does in fish: diffusion from the surroundings. As mammals breathe in, air rushes into the body, through the trachea and into the lungs, filters through small tubes, before reaching small sacs called alveoli (Comroe, 1966). Opposite to them are blood filled capillaries, lacking in O2 and rich in CO2. Oxygen diffuses from its place of high concentration, the alveoli, to low concentration, the blood, while CO2 diffuses in the opposite direction for the same reason (Comroe, 1966).
Much like in gills, the surface area of contact is crucial to fast transmission in large quantities. In humans, this contact area is 70 square meters, over 40 times the surface area of the entire body (Comroe, 1966). The waste CO2 in the alveoli then exits the body when the mammal breathes out, and new oxygen is brought in after breathing back in. Meanwhile, the newly oxygenated blood flows across the body, and acts like an oxygen source for the oxygen-deprived cells elsewhere in the body. Oxygen diffuses from the oxygen-rich blood in the body’s arteries and capillaries, into the body’s billions of cells, which dispense their waste product, carbon dioxide, into the bloodstream (Comroe, 1966). The blood eventually ends up back in the lungs, into those capillaries lining the alveoli, and the cycle continues.
Cellular Respiration
Catabolic Oxidation Reactions
Cells do not directly breakdown high-energy nutrient molecules such as glucose to provide energy for cellular functions. Rather, cells release the energy stored in nutrient molecules through a series of oxidation reactions, in order to synthesize other high-energy molecules, such as adenosine triphosphate (“Cell Energy and Cell Functions” | Scitable by Nature Education, 2014). Oxidation reactions are chemical reactions in which electrons are exchanged from one molecule to another. This results in changes to the composition and energy content of both the donor and acceptor chemicals. Nutrient molecules, such as glucose, are electron donors, which lose energy to electron acceptor molecules during each oxidation reaction. As a complex nutrient molecule is oxidized, it breaks down into simpler separate molecules, releasing energy, which is a process known as catabolism (“Cell Energy and Cell Functions” | Scitable by Nature Education, 2014). In the cells of aerobic organisms, oxygen is used as an oxidizing agent in catabolism, meaning that it is the final acceptor of nutrients’ donated electrons. As a result of catabolic oxidation reactions, a cell is able to use the energy once stored in nutrients to synthesize high-energy storage molecules such as ATP. Cells can directly access the energy stored in ATP to perform countless essential functions and processes (“Cell Energy and Cell Functions” | Scitable by Nature Education, 2014).
The Purpose of ATP Synthesis
If cells already receive high-energy molecules in the form of nutrients like glucose, then what is the purpose of breaking them down just to synthesize different high-energy molecules like ATP? Since nutrient molecules contain more energy than ATP, directly trying to add their energy to chemical reactions in the cell is inefficient, impractical, and unnecessary. It would be comparable to combusting an entire tank of gas in a car at once. For example, in cellular respiration, a single glucose molecule can synthesize up to 38 ATP molecules, so breaking down that glucose molecule all at once for the purpose of adding energy to a normal cellular chemical reaction would be unnecessary. Since so much energy would be released at once, a much larger portion of that energy would be lost as heat, making it inefficient. Alternatively, the gradual dephosphorylation of several ATP molecules slowly releases smaller amounts of energy, which results in less energy lost as heat. Thus, the process of transferring energy from ATP to a chemical reaction is more efficient than transferring energy directly from glucose, which is why cells synthesize ATP and undergo cellular respiration in the first place.
A Map of Aerobic Cellular Respiration
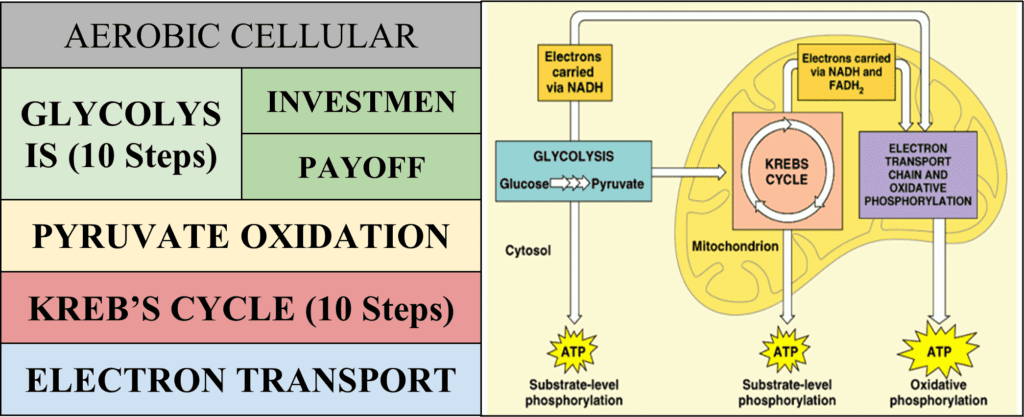
Introduction to Glycolysis
Glycolysis is the first of the four main steps of aerobic cellular respiration and it occurs in the cytoplasm, outside the cell’s mitochondria. The term “glycolysis” itself describes quite briefly what occurs over the course of ten chemical reactions. The prefix “glyco-” means “of relation to glucose,” while the root word “lysis” originates from the Greek verb lyein, “to untie” (Harper, “-lysis”). This is exactly what glycolysis does: it “unties” glucose, breaking it into two separate pyruvate molecules.

When studying the various steps that occur during aerobic respiration, tracking the carbon atoms from the original glucose reactant is crucial to understanding the reaction’s progression. Glucose is a sugar containing six carbon atoms, and throughout aerobic respiration, all six of these carbon atoms are gradually released as carbon dioxide waste; however, this does not happen until the later steps (“Cell Energy and Cell Functions” | Scitable by Nature Education, 2014). In glycolysis, all six of the original carbon atoms remain inside the cell.
Glycolysis is a unique stage in cellular respiration in the fact that it neither requires oxygen gas, nor does it produce any carbon dioxide (“Cell Energy and Cell Functions” | Scitable by Nature Education, 2014). This means that if a person were to stop breathing, their cells would still be able to undergo glycolysis, using it as the only source of ATP for a short period of time, a process known as fermentation. Typically, fermentation only occurs in humans when engaged in strenuous physical activity, since the cells of the skeletal muscles sometimes are not able to get enough oxygen, resulting in only glycolysis being able to occur (“Cell Energy and Cell Functions” | Scitable by Nature Education, 2014). This produces lactic acid as a byproduct, which is the source of the burning sensation that is experienced after extensive physical exercise. Some unicellular organisms, such as yeasts, can live in anaerobic environments by using fermentation as the only mechanism for synthesizing ATP. Glycolysis is a truly ancient mechanism for ATP synthesis that is believed to have developed before eukaryotic life (“Cell Energy and Cell Functions” | Scitable by Nature Education, 2014).
Glycolysis can be broken down into two phases. The first phase, known as the “investment phase” or the “preparatory phase,” consumes energy and uses up ATP. It accounts for the first five steps of glycolysis. The second phase, called the “payoff phase,” releases energy and produces ATP. It accounts for the latter five steps of glycolysis (“Glycolysis: All Steps with Diagram, Enzymes, Products, Energy Yield and Significance” | Laboratory Info’s Editorial Team, 2020).
Glycolysis
Phase I – The Investment
The investment phase consumes energy by dephosphorylating two ATP molecules into ADP molecules, per each glucose molecule being reacted (“Glycolysis: All Steps with Diagram, Enzymes, Products, Energy Yield and Significance” | Laboratory Info’s Editorial Team, 2020). The investment phase phosphorylates glucose, changes it from a six-member ring to a five-member ring, phosphorylates it a second time, cleaves the ring in two, then changes one of the two compounds formed so that it is identical to the other. If the investment phase were to be thought of as a function, the input would be glucose and two ATP, while the output would be two glyceraldehyde 3-phosphate and two ADP (“Glycolysis: All Steps with Diagram, Enzymes, Products, Energy Yield and Significance” | Laboratory Info’s Editorial Team, 2020).

Phase II – The Payoff
The payoff phase phosphorylates four ADP, producing four ATP for each glucose molecule. The specific method of phosphorylating the ADP is called substrate-level phosphorylation. Substrate-level phosphorylation is the synthesis of ATP by the direct transfer of a phosphate group from a substrate to a molecule of ADP (refer to Cavnar, 2013 in Appendix). This type of reaction is usually catalyzed by a kinase enzyme.
In addition, the payoff phase reduces two NAD+ molecules into two NADH molecules. NADH is an important electron transporter, and its role will be discussed in further detail in the section covering the final stage of aerobic respiration, the electron transport chain. The payoff phase transforms the two glyceraldehyde 3-phosphates formed in the first phase into two pyruvate molecules (“Glycolysis: All Steps with Diagram, Enzymes, Products, Energy Yield and Significance” | Laboratory Info’s Editorial Team, 2020). For every investment phase of the reaction that occurs, two of the payoff phases must follow. This is because the initial six-carbon sugar is cleaved into two separate three-carbon molecules in step four, so essentially twice as many reactants enter the payoff phase. This is why all of the components in the following five steps are doubled.

Pyruvate Oxidation
Although it may be the shortest of the stages of cellular respiration, pyruvate oxidation still plays an important role as a key connector that links glycolysis to the rest of cellular respiration. Pyruvate oxidation reduces one NAD+ per pyruvate, so two per initial glucose. In eukaryotes, this step takes place in the mitochondrial matrix, the innermost compartment of the organelle. In prokaryotes, it happens in the cytoplasm, due to a lack of membrane-bound organelles, such as mitochondria (Rye et al., “Oxidation of Pyruvate and the Citric Acid Cycle”).
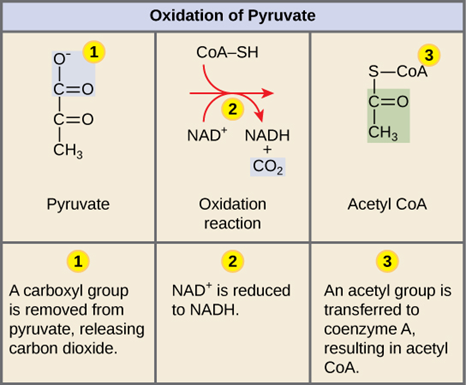
Pyruvate oxidation is the first step in cellular respiration in which carbon atoms from the original glucose begin to be expelled from the cell as CO2 waste. This is because pyruvate oxidation splits pyruvate, a three-carbon molecule, into acetyl CoA, which is a two-carbon molecule bound to Coenzyme A, and the remaining carbon is released as carbon dioxide (“Pyruvate Oxidation” | Biology for Majors I | Lumen Waymaker, n.d.). Acetyl CoA is valuable, since it is an essential reactant for the Krebs Cycle, the next stage in cellular respiration.
Pyruvate oxidation will initially continue to proceed in the absence of oxygen; however, this does not occur for long, since without oxygen to oxidize NADH at the end of cellular respiration, the concentration of NADH would continually build and the concentration of NAD+ would continually decrease, until it is no longer energetically favorable or possible for to reduce NAD+. Once this is the case, pyruvate oxidation would no longer be possible, since, for oxidation to occur, a reduction must also take place (Rye et al., “Oxidation of Pyruvate and the Citric Acid Cycle”).
The net reaction for pyruvate oxidation is shown below in Fig. 17.. Notice how the blue atoms on pyruvate detach and become carbon dioxide. Additionally, the hydrogen atom that bonds with NAD+ to reduce it to NADH is actually not even from pyruvate, but rather it is from the thiol group initially attached to Coenzyme A.

Krebs Cycle
The Krebs cycle, sometimes called the citric acid cycle, takes place in the matrix of mitochondria (Buckley, 2019). Nearly every enzyme in the Krebs cycle is soluble, with the one exception of the enzyme succinate dehydrogenase, which is embedded into the interior membrane of the mitochondrion (Rye et al., “Oxidation of Pyruvate and the Citric Acid Cycle”). Unlike glycolysis, the Krebs cycle is a closed loop: The last part of the pathway regenerates the oxaloacetate used in the first step, which is able to bind to another acetyl CoA. The only component that is used up is acetyl CoA. Essentially, the Krebs cycle involves converting the acetyl CoA molecules produced in pyruvate oxidation into carbon dioxide.
Along with normal electron carriers, such as NADH and FADH2, the Krebs cycle also incorporates another high energy carrier molecule: GTP, guanosine triphosphate. In the conversion of succinyl CoA to succinate, GDP is phosphorylated to produce GTP. Although it is not shown in the diagram below, GTP goes on to react with ADP, phosphorylating it and producing ATP and GDP (Aryal, “Krebs (Citric Acid) Cycle”).

For each acetyl CoA that enters the Krebs cycle, two molecules of carbon dioxide are released, three molecules of NADH are produced via the reduction of NAD+, along with another molecule of FADH2, and one molecule of GTP (Buckley, 2019). For every glucose that is broken down in cellular respiration, two acetyl CoA molecules are produced, which means that the Krebs cycle loops twice per glucose. Using this information, a net equation can be made for the Krebs cycle for the catabolism of a glucose molecule.

The Krebs cycle plays an extraordinarily valuable role in the process of aerobic respiration, since it drives the formation of reduced electron carriers, such as NADH and FADH2 (Buckley, 2019). While these electron carriers may not be particularly useful to the cell as an energy source for most functions, they are still quite important. It is these electron carriers that are used to transport the energy needed to create a large number of ATP molecules in the final stage of aerobic respiration (Buckley, 2019).
Overview of the Electron Transport Chain (ETC)
The electron transport chain is composed of a cluster of proteins that transfer electrons through the inner mitochondrial membrane to form a gradient of protons that drives the creation of ATP (Aryal, “Electron Transport Chain (ETC)”). The process involves multiple redox reactions which rely on protein complexes in order to transfer electrons from a reducing agent donor molecule to an oxidizing agent acceptor molecule. As a result of these reactions, the proton gradient is produced, which enables ATP synthesis.
Electron Transfer Route
At Complex I, NADH molecules are oxidized back to NAD+, transferring their electrons into the complex. This causes Complex I to pump protons into the intermembrane space of the mitochondrion. At Complex II, FADH2 molecules are oxidized to FAD+, transferring their electrons to Complex II. The electrons from Complexes I and II are transferred along to coenzyme Q via a series of redox reactions (BD Editors, 2019).
The electrons are then transferred to cytochrome c, passing through Complex III along the way, causing it to also pump protons into the intermembrane space, using the energy of the electrons being transferred to cytochrome c (BD Editors, 2019).
From there, the electrons are transported to their final acceptor, molecular oxygen. For this to happen, the electrons are transported by cytochrome aa3 complex to Complex IV, which is also the cytochrome oxidase enzyme. Complex IV catalyzes the transfer of two electrons to each atom of oxygen. The oxygens then each bond to a pair of protons, forming water. The energy produced by the transfer of electrons to oxygen is used to allow Complex IV to pump even more protons into the intermembrane space (BD Editors, 2019).

Oxidative Phosphorylation
A majority of the production of ATP is related to the transfer of electrons through the electron transport chain to oxygen. This process is known as oxidative phosphorylation. After the proton pumps have created an electrochemical gradient across the inner mitochondrial membrane, protons flow down the electrochemical gradient through the membrane-bound ATP synthase enzyme. The flow of hydrogen ions through the ATP synthase allows the enzyme to synthesize large quantities of ATP (Aryal, “Electron Transport Chain (ETC)”).
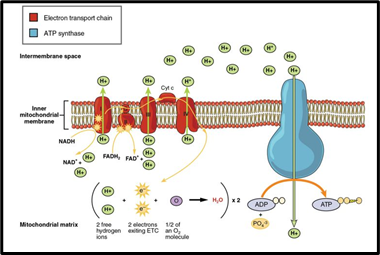
So far, throughout the catabolism of a glucose molecule, there has been a net increase of 10 NADH molecules, 2 FADH2 molecules, and 4 ATP molecules. At the electron transport chain, each NADH has the ability to produce 3 ATP molecules, since electrons from NADH pass by three proton pumps. Electrons from FADH2 only pass by two proton pumps, so each FADH2 results in the production of only 2 ATP by oxidative phosphorylation. This means that a total of 34 ATP molecules are expected to be produced by oxidative phosphorylation due to the electron transport chain. When adding this to the net gain of 4 ATP molecules produced earlier in glycolysis and the Krebs cycle, the total comes out to 38 ATP synthesized per molecule of glucose. This is why aerobic respiration is such an amazing and efficient process. If fermentation were to be used, only the 2 ATP from glycolysis would be gained.
Surface Tension
The drastic change in surface area of the alveoli throughout the respiratory cycle causes such a great force of surface tension in the lungs that airways would simply collapse without something to reduce the surface tension. As the air inside the lungs is moist, the forces exerted by water molecules on the surface of the lung tissue as those molecules pull together due to the strength of the polar bonds have the effect to lower surface area as the tissue is also pulled together. The surface tension of the alveolar air-water interface provides this retractive force opposing lung inflation. Some forms of Laplace’s Law mathematically represented as:
∆P={2T\over r}(1)
illustrates that the difference in pressure between the airspace and the lining (ΔP) depends only on the surface tension (T) and the radius of the alveoli (r) (Prange, 2003). A common representation of this phenomenon would be that the effort required to blow up a balloon is greatest when the diameter of the balloon is least. As the law states, the pressure inside the alveoli is inversely proportional to the radius as long as the surface tension is presumed to change little (Prange, 2003). Fig. 22 shows that, without intervention, a small alveolus shall collapse and inflate its somewhat larger neighbor, resulting in unequal alveolar ventilation. Because all the “grapes” in the model cannot be of exactly the same size, the intervention of a substance with particular properties is needed to minimize and equalize retractive forces, independent of alveolar size (Prange, 2003). Thus, the work of breathing is reduced.

To avoid a difficult and less effective re-inflation of the alveoli during inhalation, inside the alveoli, at the air-liquid interface is located the pulmonary surfactant (Prange, 2003).
Surfactant
What is Surfactant?
First, let us not forget that the alveolar tissue is living tissue and if it is not bathed in fluid it will die. Although it is true that the skin cells covering our body are not bathed in fluid, it is because our outermost epidermal layers are not living tissue. The fluid keeping the alveoli actively functional is surfactant; a lipoprotein molecule that reduces the surface tension to less than 1 mN/m on lung deflation (Veldhuizen and Haagsman, 2000). Research using electron microscopy visualized that this is achieved by forming a surface film that consists of a monolayer which is highly enriched in dipalmitoylphosphatidylcholine and bilayer lipid/protein structures closely attached to it. A more extensive description of composition will be given later in the paper. In addition to a low and stable surface tension, interdependence provided by the fibrous continuum allows the lung to perform efficient gas exchange by maintaining a large alveolar surface area (Knudsen and Ochs, 2018). Furthermore, surfactant is needed not only in the alveolar part of the lung but also in the bronchi through which air is conducted to the alveoli. A lack of surfactant in this area could even lead to closure of the small cylindrical airways (Knudsen and Ochs, 2018).
Cells Producing Surfactant
In the alveolar walls are the type II pneumocytes, also called “surfactant cells”. They are large cells with big nuclei that easily differentiate them from alveolar and capillary endothelial cells. They have a cubic shape and diameter of 9 mm which makes them smaller than the cells covering approximately 95 % of the alveolar surface (Papadakos and Lachmann, 2007). Their most impressive and characteristic morphologic features are the lamellar bodies that can be observed on Fig. 23, the secretory organelles that store surfactant and comprise 25 % of cytoplasm of type II pneumocytes. After synthesis by type II pneumocytes, surfactant is secreted into the alveolar space by exocytosis. The surfactant system is in a continuous state of flux of surfactants due to defects in pulmonary macrophages leads to a disorder known as pulmonary alveolar proteinosis which occurs when surfactant accumulation interferes with gas exchange (Papadakos and Lachmann, 2007).

Surfactant Composition
As previously mentioned, a surfactant is a substance that when added to a liquid, a solid, or a vapor, reduces its surface tension. It is mostly used in detergents as it allows the liquid to spread onto the clothes evenly. Surfactants are known as surface-active agents (Editors of Encyclopedia Britannica, “Surfactant”). These same properties apply to the pulmonary surfactant. All surfactants are basically composed of two different molecular components: lyophilic and lyophobic. Lyophilic means soluble in a fluid, it is usually the polar head group. To better understand what lyophilic is, take a look at hydrophilic molecules or that part of a molecule which means that it is favorable to make interactions with water. On the contrary, lyophobic signifies the part of a molecule that repels fluids, they are the nonpolar tails of the molecule (Bergh, 1999).

Pulmonary Surfactant Composition
Pulmonary surfactant is composed of multiple different lipids and proteins. According to the paper from Intensive Care Med about Pulmonary surfactant, after analyzing lung fluids from a few bronchoalveolar lavage techniques, researchers determined that the surfactant was composed of 80 % phospholipids, eight percent lipids such as cholesterol, and 12 % proteins (Frerking et al., 2001).
Phospholipid Composition
All phospholipids are hydrophobic and hydrophilic. As demonstrated in the figure above, the layer of lipids that form the surfactant shows the air-water interface of the surfactant. This protective layer of surface-active agents allows the surface tension at the air-water interface of the alveoli to decrease by approximately 70 mN/m (Griese, 1998). Counting as more than half of the concentration of phospholipids in the surfactant, the disaturated dipalmitoylphosphatidylcholine’s (DPPC) role is key to reduce surface tension (Alcorn, 2017) (Fig. 24).
The second most abundant phospholipid is the phosphatidylglycerol (PG), it can be replaced by another phospholipid that possesses the same properties, such as evenly spreading the surfactant over the surface of the alveoli. The latter can and will be substituted by another negatively charged phospholipid, the phosphatidylinositol (PI), if the PG’s concentration in the surfactant is too low. It is often the case for smaller air-breathing animals such as cats and chickens. These two phospholipids usually act in pairs, if the PI’s concentration is high, then the PG’s concentration will be lower (Alcorn, 2017). The remaining phospholipids take up around 5 % of the total phospholipid composition. Their roles alternate between the formation of tubular myelin and signals for the surfactant metabolism (Alcorn, 2017).


The Dipalmitoylphosphatidylcholine (DPPC)
The DPPC’s chemical composition makes it the most important phospholipid in the pulmonary surfactant. Having 2 fully saturated fatty acid chains of 16 carbon with no double bonds allows it to be compressed tightly (Fig. 26). In particular, during respiration, the compressed DPPC layer eliminates water molecules that could have been stored in the air-fluid (hypophase) interface reducing tension in the alveoli, preventing it from collapsing (Alcorn, 2017). As mentioned previously, along with all phospholipids that are present in the pulmonary surfactant, the DPPC is amphipathic, which means it has a predisposition to absorb to the air/water interface with its hydrophobic tail and hydrophilic head (Frerking et al., 2001).


Cholesterol
Very little is known about the cholesterol’s purpose in the surfactant as it varies between most species. However, of what we actually know, its properties mainly enable the surfactant’s bilayer to keep a certain fluidity allowing it to spread evenly over the hypophase of the alveoli (Alcorn, 2017). Still, researchers have discovered that cholesterol could also increase the surface tension, which means that a balanced concentration of cholesterol should compose the surfactant to maximize its efficiency (Alcorn, 2017).
Surfactant-Associated Proteins
It is, however, important to know that there are various types of proteins that compose the surfactant. To be able to analyze the content of the lungs, researchers used a technique called bronchoalveolar lavage which empties the alveoli’s fluids (Frerking et al., 2001). This operation usually damages the cells that protect the alveoli allowing contaminants to enter. That explains why a small percentage of the proteins that were located in the surfactant were actually impurities. The rest of the proteins that were analyzed (over 50 %) are named surfactant-associated proteins: SP-A, SP-B, SP-C, and SP-D (Frerking et al., 2001). Each of them is designed to serve a specific purpose. For instance, SP-A and SP-D are hydrophilic, water-soluble. Whereas the SP-C and SP-B are both hydrophobic, which is, as mentioned before, important in order to keep an active interaction between the air and the surfactant (Frerking et al., 2001).
SP-A and SP-D
Both of these proteins play no essential part in the surfactant’s main purpose, tension reduction, instead they assure the lung’s security and the homeostasis of the surfactant. These hydrophilic multimers are able to recognize and “tag” invasive pathogens that enter the alveoli (Alcorn, 2017). They both contain collagen, which is essential for the role they play. SP-A proteins account for 5 % of the pulmonary surfactant’s composition (Fig. 25). Without going into further details about the SP-A’s and SP-B’s structure, it is important to know that these proteins can be divided into 4 domains: N-terminal segment, collagen, neck region, and a carbohydrate recognition domain or CRD (Alcorn, 2017). Once the SP-A completes its division and matures, its final form resembles a bouquet where its carbohydrate recognition domains become the “flowers” that actually bind pathogens (Fig. 27). SP-D proteins represent only 0.5 % of the surfactant. The structural difference between this protein and the SP-A is that its “flowers” are connected through disulfide bridges which allows it to have a “cross like” structure (Griese et al., 2001) (Fig. 27).
Their unique structures allow these proteins to bind to different phospholipids. As being the protein that is in higher concentration, SP-A binds itself to the most abundant phospholipid, the DPPC, absorbing it to the air-water interface, whereas the SP-D binds itself to PI (Griese et al., 2001). The CRD’s that are present in both proteins allow them to recognize microbes. Then, their collagen domains allow them to interact with receptors on certain immune cells allowing them to defend the lungs (Kishore et al., 2006).

SP-B and SP-C
These proteins are less present than the others, only accounting for one percent each of the total surfactant’s composition. Contrary to SP-A and SP-D proteins, they are extremely involved in the surfactant’s role (Griese et al., 2001). Both proteins act directly on the lipid bilayer (Fig. 27). SP-B proteins accelerate and enable the formation of the surfactant’s protective layer of phospholipids over the hypophase of the entire interior of the alveoli (Griese et al., 2001). SP-C proteins, on the other hand, take care of the biophysical side of the surface-active film. These proteins actually stabilize the surfactant’s lipid layer during respiration, as the bilayer often compresses or expands (Griese et al., 2001).
Surfactant Function
Although molecular mechanisms and actions of the film’s components are still the object of some research, studies showed that the three main functions of surfactant (Han and Mallampalli, 2015) are:
(1) Lowering surface tension at the air–liquid interface and thus preventing alveolar collapse at end-expiration
Classified as the biological function, surfactant prevents alveolar collapse at low lung volume, and preserves bronchial patency during normal and forced respiration. As it was first expressed by Neergaad in 1929, surface tension is more important than tissue elastic forces efor the retractive of the lungs at all levels of inflation (Schurch et al., 1992). A summary of how surfactant lower surface tension would be that the phosphate head groups of the surfactant molecules are hydrophilic (i.e., they are attracted to water molecules). As surfactant molecules pull on water molecules, they pull the water molecules apart from other water molecules, causing the attractive forces pulling the water molecules together to be reduced. In other words, the presence of surfactant in the fluid ensures that the alveolar space remains open during the whole respiratory cycle, thus preventing shunts that can result in inadequate oxygenation of the blood (Schurch et al., 1992).
(2) Interacting with and subsequent killing of pathogens or preventing their dissemination
This very important function of the surfactant is classified as the immunological, non-biological function. Constantly exposed to foreign compounds, the lungs need a powerful innate host-defense system (Chroneos et al., 2010). Otherwise, humans would easily contract diseases because of the particles and micro-organisms in the ambient air. Thus, surfactant provides a crucial first line of defense against infection by enhancing the removal of pathogens, modulating the response of inflammatory cells, and optimizing lung biophysical activity (Chroneos et al., 2010). Hydrophilic proteins, which constitute a small portion of surfactant, play a major role in antimicrobial activity. Indeed, the collectins (type of proteins) SP-A and SP-D enhance bacterial and viral clearance. The predominant function of the collectins is their ability to opsonize pathogens, meaning that they facilitate their phagocytosis by cells of the innate immune system, such as macrophages and monocytes (Johansson and Curstedt, 1997). To summarize, those tightly controlled mechanisms exist to preserve surfactant homeostasis and eradicate countless airborne pathogens to keep the lung sterile.
(3) Modulating immune responses
Not only the lung collectins facilitate and activate the immune system but they also act as immunomodulators to inhibit allergen-induced lymphocytes proliferation via multiple mechanisms, especially among children with asthma. Indeed, SP-A and SP-D bind directly to allergens and particles such as pollen grains, house dust mite allergen, etc., inhibiting specific Immunoglobulin E binding to allergens and subsequently decreasing allergen-induced histamine release (Han and Mallampalli, 2015).
The surfactant also has some other important related functions. Indeed, another consequence of low surface tension is that a net fluid flow is directed from the alveolar space into the interstitium (Han and Mallampalli, 2015). Because of their small diameter, with a relatively high surface tension in the alveoli, a thicker fluid may form. Thus, this mechanism to dispose of the non-necessary fluid is of particular importance to keep the alveoli clear of liquid while maintaining a thin fluid film (Han and Mallampalli, 2015). A consequence of a lack of surfactant leads to the accumulation of oedema fluid in the airspace. Furthermore, particulate materials that made their way through the airways are partially removed by surfactant. The physical removal is accomplished by means of the displacement of particles into the hypophase and improvement of mucociliary clearance (Han and Mallampalli, 2015).
Artificial surfactant
A surfactant’s main purpose is to reduce surface tension. Therefore, any substance that possesses a layer composed of both hydrophilic and hydrophobic molecules is considered a surfactant. These properties will allow the component to interact with air-water interfaces. One of the best examples of artificial surfactant is actually soap. Soap has a simple chemical process to remove dirt or grease (“Science on the shelves: Soapy Science” | University of York, 2003). The way it works is the hydrophilic heads of the soap are in the water, but the hydrophobic tails sit with the impurities. As their forces of attraction are too strong, the hydrophobic tails surround the dirt or grease and create a sphere around it (Fig. 28). The surfactant then breaks the dirt and lets water remove it completely from the surface (“Science on the Shelves: Soapy Science” | University of York, 2003).
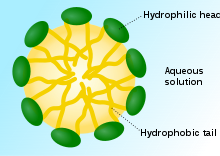
Related Diseases
The crucial functions of surfactant can leave us wondering what consequences an imbalance of pulmonary surfactant will have on a human. There are in fact various diseases that result from problems in the surfactant system. They can be classified in many categories depending on the age of the person, the life habits that may alter the surfactant such as smoking or the specific mechanism that is dysfunctional. For example, the complication can be at functionally active compound level (like in NRDS) or in both the phospholipid and apoprotein fractions (like in pneumonia) (Griese, 1999).
Newborn respiratory distress syndrome (NRDS) usually occurs when the baby’s lungs have not produced enough surfactant (Griese, 1999). Considering that a baby begins producing surfactant sometime between weeks 24 and 28 of pregnancy, if they are born prematurely, then they might not have enough surfactant in their lungs. In fact, babies with NRDS lack surface active material and, consequently, the low surface tension characteristic of pulmonary surfactant is reduced or inexistent (Griese, 1999). This disease results in blue-colored extremities, rapid, shallow breathing and sometimes death. There is however a way to compensate the deficit by administering the missing material or an equivalent substance via the airways. For this treatment, two general types of surfactant that both have their advantages can be used: those processed from animal lungs or human amniotic fluid and those containing only synthetic components (Griese, 1999).
As for pneumonia, it can be caused by a variety of pathogens, including bacteria, viruses and fungi that induce changes in the phospholipid and apoprotein fractions, resulting in changes in the density and surface tension properties of surfactant (Brogden, 1991). In addition, gram-negative bacteria or endotoxin can injure type II epithelial cells causing them to produce abnormal quantities of surfactant, abnormal concentrations of phospholipids in surfactant, and abnormal compositions. The changes in surfactant have a deleterious effect on lung function characterized by significant decreases in total lung capacity, gas diffusing capacity, and a significant increase in mean pulmonary arterial pressure (Brogden, 1991). What is more, decreased concentrations of surfactant or an altered surfactant composition can result in the anatomic changes commonly seen in pneumonia such as pulmonary edema, and atelectasis, shown in Fig. 29 and even hemorrhage (“When Your Child Has Pleural Effusion” | Mount Nittany Health, 2020). For pneumonia, surfactant therapy is really rare.
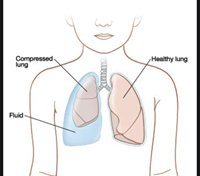

To summarize, many lung problems also come from bad habits or extraordinary situations. Smokers cause the reduction of bronchoalveolar lavage at phospholipid levels which alter the cycling or secretion. All the toxic chemicals in tobacco have effects that result in changes in the dynamics of the surfactant system and, clinically in changes in lung mechanics (Scott, 2004). In other words, surfactant and even the cilia of the airways cannot accomplish to full capacity. In addition, did you know that a near-drowning experience could result in a reduced surfactant function? As an example, a 24-year-old woman developed adult respiratory distress syndrome after near-drowning and the medical tests (i.e., lavage sample) conducted revealed that the surfactant function of this patient was drastically reduced due to a washout effect by aspiration of fresh water (Staudinger et al., 1997).
Surfactant Among Other Mammals
Surfactant’s composition is fairly similar among the vast majority of mammalian species. As mentioned, when describing the NRDS, porcine or bovine surfactant is commonly used as a treatment to compensate the abnormalities in premature babies’ surfactant because these animals have sufficiently the same biophysical properties as they contain the hydrophobic surfactant proteins SP-B and SP-C. However, it can quite differ as surfactant for each animal is tailored to the biology of the animal. Let us take the example of diving mammals who descend the depths of 50-70 meters or greater and fully collapse the gas exchanging portions of their lungs to then re-expand these areas with ascent (Spragg et al., 2004). Experiments on pinnipeds like the sea lion showed that surfactant from several species of diving mammals has a composition that is distinct from that of terrestrial mammals and is uniquely suited to repetitive collapse and expansion of the lung. In contrast to terrestrial mammals, pinnipeds’ surfactant has a higher concentration of phospholipid and relatively more fluidic phosphatidylcholine molecular species to facilitate rapid spreading during alveolar re-expansion (Spragg et al., 2004). To conclude on surfactant distinctions among mammals, its composition is adapted to the differing anatomic and physiological conditions of the respiratory system, both across vertebrate species and during postnatal development. A fair example of those variations regards animals that require a surfactant that is optimized to cope with repetitive collapsing and rapid expansion of fully alveolarized lungs, an event reflected by surface tension that must be repeatedly overcome.

Cutaneous Respiration
Cutaneous respiration allows gas diffusion through pores on the skin. This gas exchanger is rather efficient for fish at their larval stage since their scales are not fully developed yet and their membrane is not at its thickest. However, as they mature, their skin becomes thicker and cutaneous respiration becomes harder, while their gills develop, and the gill surface area expands (Bernard et al., 2020). This also extends to other animals and mammals, for which their skin is usually a lot thicker and impermeable to gasses. As a matter of fact, the skin is the first layer of protection mammals have. Even if warm-blooded animals have more protective skin surfaces, making exchanges from outside to inside the body nearly impossible, the cells that compose the epidermis, the higher layer of the skin, consume a small amount of oxygen from the air (Feder, 1984). That being said, these cells will not serve as a pathway for exterior oxygen to enter the circulatory system. Cutaneous respiration is, therefore, more efficient for terrestrial, semi-aquatic, aquatic amphibians, and larval stage fish. Most amphibians are able to use multiple gas exchangers such as lungs, gills, and their skin; however, 2 out of 3 salamanders are completely lungless (Lewis et al., 2018).

Counter-Current System
Compared to other mammals, larvae and amphibians have a much higher quantity of capillaries that run close to their skin. They do not have any hair, fur, or scales and their epidermis is much thinner. Cutaneous respiration is facilitated because of the countercurrent system (Bosch, 2016). As previously mentioned, gas diffuses towards areas where its concentration is at its lowest. The counter-current system takes advantage of this property, it occurs when blood in the capillaries flows in one direction and air or water flows in the other (Bosch, 2016). As the oxygen-filled blood leaves an area of the capillaries, the concentration of gas lowers allowing oxygen to diffuse easily from the air or water into the bloodstream. This process is mostly present in gills, as oxygen-rich water enters the gills, it diffuses into the capillaries as they have a smaller gradient of concentration of oxygen (Fig. 31). The counter-current system creates an ongoing diffusion gradient allowing oxygen to enter the bloodstream easily. This process is called passive diffusion (Bosch, 2016).
Mucus Skin Composition
A new study published in the Proceedings of the Royal Society B paper, discovered that some salamanders, lungless salamanders or Plethodontidae, express a surfactant-associated protein like that is also found in the human alveoli (Reuell, 2019). The amphibian skin is extremely slimy, they owe this property to the numerous glands that compose their skin. The mucus that is excreted by these glands help keep their skin moist allowing gasses to dissolve and diffuse much easier into the capillaries (“Systems of Gas Exchange” | Boundless Biology | Lumen Candela, n.d.). However, biologists have realized that the mucus that sat on the amphibians’ body (mostly salamanders) was also composed of a copy of the SP-C protein found in the pulmonary surfactant (Reuell, 2019). Researchers are still experimenting on it, though they explain that its properties seem to remain the same even if its location is completely different. SP-C proteins are able to reduce the thickness of the mucus layer over the salamander’s body allowing higher concentrations of gas to diffuse into the bloodstream. This is still an “expression”, they have not yet identified if it was truly present in the skin. It is however present in other components of the salamander’s body (Reuell, 2019) (Fig. 32).
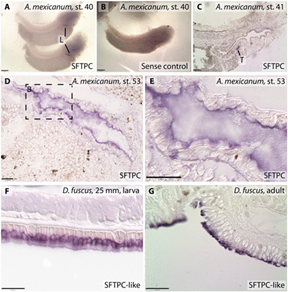
Conclusion
Surfactant is an essential component of lungs and is made of multiple lipids and proteins. The lipids in surfactant have a hydrophilic head and a hydrophobic tail in order to form a film-like layer. The substance’s role is primarily to reduce surface tension and prevent the alveoli from collapsing. Additionally, it plays a crucial role in protecting the body from pathogens entering via the airways. In other words, the surfactant is crucial to the survival of alveoli cells. Although its composition is fairly similar among mammals —the administration of porcine or bovine surfactant to babies with NRDS being a great example — surfactant is tailored to animals’ biological environment. A new study demonstrates that a certain type of surfactant-associated proteins has been identified in most amphibian and salamander skins.
Appendix
“Substrate level vs. Oxidative Phosphorylation.”, 7 February 2013, Peter Cavnar, YouTube, https://www.youtube.com/watch?v=nF3NE40Fmjo.
References
Alcorn, J. L. (2017). Chapter 4 – Pulmonary Surfactant Trafficking and Homeostasis. In V. K. Sidhaye & M. Koval (Eds.), Lung Epithelial Biology in the Pathogenesis of Pulmonary Disease (pp. 59-75). Boston: Academic Press. Retrieved from https://www.sciencedirect.com/science/article/pii/B978012803809300004X
Aryal, S. (2018a, 7 Aug 2018). Electron Transport Chain (ETC)- Components and Steps. Retrieved from https://microbenotes.com/electron-transport-chain-etc-components-and-steps/
Aryal, S. (2018b, 6 Jul 2018). Krebs (Citric Acid) Cycle Steps by Steps Explanation. Retrieved from https://microbiologyinfo.com/krebs-citric-acid-cycle-steps-by-steps-explanation/
Berger, M. (2018). Major Disease Outbreak Strikes California Sea Lions. Retrieved from https://www.smithsonianmag.com/science-nature/major-disease-outbreak-strikes-california-sea-lions-180970755/
Bergh, M. (1999). Allergenic Oxidation Products in Ethoxylated Non-Ionic Surfactants Uppsala University, Sweeden. Retrieved from https://www.medicaljournals.se/acta/download/10.1080/000155599750208040/
Bernard, S., Gagnon, C., Farooqi, N., & Vyse, W. (2020). Biomechanics and Materials Engineering Biological Design for Lungs and Gills. Bioengineering. McGill University Montreal, Canada
Bosch, D. L. (2016). How to breathe without lungs, lissamphibian style. Retrieved from https://allyouneedisbiology.wordpress.com/tag/cutaneous-respiration/
Boutet, E. (2007). Micelle scheme. In (pp. Scheme of a micelle formed by phospholipids in an aqueous solution.). Wikimedia Commons. Retrieved from https://commons.wikimedia.org/wiki/File:Micelle_scheme-en.svg
Britannica, E. o. E. (2020, 11 Feb 2020). Surfactant Retrieved from https://www.britannica.com/science/surfactant
Brogden, K. A. (1991). Changes in pulmonary surfactant during bacterial pneumonia. Antonie van Leeuwenhoek, 59(4), 215-223. doi:10.1007/bf00583673
Buckley, G. (Ed.) (2019). Krebs Cycle. In. Biology Dictionary (online). Retrieved from https://biologydictionary.net/krebs-cycle/
Candela, L. (n.d.). Systems of Gas Exchange. In. Boundless Biology. Retrieved from https://courses.lumenlearning.com/boundless-biology/chapter/systems-of-gas-exchange/
Chandler, D. L. (2013). Explained: Hydrophobic and hydrophilic. In: MIT News. Retrieved from https://news.mit.edu/2013/hydrophobic-and-hydrophilic-explained-0716
Chroneos, Z. C., Sever-Chroneos, Z., & Shepherd, V. L. (2010). Pulmonary surfactant: an immunological perspective. Cell Physiol Biochem, 25(1), 13-26. doi:10.1159/000272047
Comroe, J. H. (1966). THE LUNG. Scientific American, 214(2), 56-71. Retrieved from http://www.jstor.org/stable/24931268
Editors, B. (Ed.) (2019). Electron Transport Chain. In. Biology Dictionary (online). Retrieved from https://biologydictionary.net/electron-transport-chain/
Education, S. b. N. (2014). Cell Energy and Cell Functions. In. nature.com. Retrieved from https://www.nature.com/scitable/topicpage/cell-energy-and-cell-functions-14024533/
FEDER, M. E., & BURGGREN, W. W. (1985). CUTANEOUS GAS EXCHANGE IN VERTEBRATES: DESIGN, PATTERNS, CONTROL AND IMPLICATIONS. Biological Reviews, 60(1), 1-45. doi:https://doi.org/10.1111/j.1469-185X.1985.tb00416.x
Frerking, I., Günther, A., Seeger, W., & Pison, U. (2001). Pulmonary surfactant: functions, abnormalities and therapeutic options. Intensive Care Medicine, 27(11), 1699-1717. doi:10.1007/s00134-001-1121-5
Griese, M. (1999). Pulmonary surfactant in health and human lung diseases: state of the art. European Respiratory Journal, 13(6), 1455-1476. doi:10.1183/09031936.99.13614779
Griese, M., Maderlechner, N., & Bufler, P. (2001). Surfactant proteins D and A in sputum. European Journal of Medical Research, 6(1), 33-38.
Han, S., & Mallampalli, R. K. (2015). The Role of Surfactant in Lung Disease and Host Defense against Pulmonary Infections. Ann Am Thorac Soc, 12(5), 765-774. doi:10.1513/AnnalsATS.201411-507FR
Harper, D. (Ed.) (n.d.). -lysis. In. Online Ethymology Dictionary. Retrieved from https://www.etymonline.com/word/-lysis
Health, M. N. (2020). When Your Child Has Pleural Effusion. Retrieved from https://www.mountnittany.org/wellness-article/pleural-effusion-when-your-child-has
Jackson, A. (2014). Frog. Retrieved from https://animalrespriation.weebly.com/frog.html
Jackson, J. C. (2018). 46 – Respiratory Disorders in the Preterm Infant. In C. A. Gleason & S. E. Juul (Eds.), Avery’s Diseases of the Newborn (Tenth Edition) (pp. 653-667.e652). Philadelphia: Elsevier. Retrieved from https://www.sciencedirect.com/science/article/pii/B9780323401395000462
Johansson, J., & Curstedt, T. (1997). Molecular structures and interactions of pulmonary surfactant components. European Journal of Biochemistry, 244(3), 675-693. doi:10.1111/j.1432-1033.1997.00675.x
Kishore, U., Greenhough, T. J., Waters, P., Shrive, A. K., Ghai, R., Kamran, M. F., . . . Chakraborty, T. (2006). Surfactant proteins SP-A and SP-D: structure, function and receptors. Molecular Immunology, 43(9), 1293-1315. doi:10.1016/j.molimm.2005.08.004
Knudsen, L., & Ochs, M. (2018). The micromechanics of lung alveoli: structure and function of surfactant and tissue components. Histochemistry and Cell Biology, 150(6), 661-676. doi:10.1007/s00418-018-1747-9
Lewis, Z. R., Dorantes, J. A., & Hanken, J. (2018). Expression of a novel surfactant protein gene is associated with sites of extrapulmonary respiration in a lungless salamander. Proceedings. Biological sciences, 285(1888), 20181589. doi:10.1098/rspb.2018.1589
Morita, S. Y., Tsuji, T., & Terada, T. (2020). Protocols for Enzymatic Fluorometric Assays to Quantify Phospholipid Classes. International Journal of Molecular Sciences, 21(3). doi:10.3390/ijms21031032
Moyes, C. D., & Schulte, P. M. (2008). Principles of Animal Physiology: Pearson/Benjamin Cummings. Retrieved from https://books.google.ca/books?id=f-INSQAACAAJ
Nave, R. (n.d.). Laws of Gas Transport: Graham’s Law. Retrieved from http://hyperphysics.phy-astr.gsu.edu/hbase/Kinetic/henry.html
Papadakos, P. J., & Lachmann, B. (2007). Mechanical Ventilation: Clinical Applications and Pathophysiology: Saunders Elsevier. Retrieved from https://books.google.ca/books?id=Fr6Q-xpPSdIC
Policy, S. (2015). Is high fructose corn syrup worse for you than other sugars? Retrieved from https://sciencepolicyivh.wordpress.com/2015/03/21/is-high-fructose-corn-syrup-worse-for-you-than-other-sugars/
Prange, H. D. (2003). LAPLACE’S LAW AND THE ALVEOLUS: A MISCONCEPTION OF ANATOMY AND A MISAPPLICATION OF PHYSICS. Advances in Physiology Education, 27(1), 34-40. doi:10.1152/advan.00024.2002
Randall, D., & Daxboeck, C. (1984). 5 Oxygen and Carbon Dioxide Transfer Across Fish Gills. In W. S. Hoar & D. J. Randall (Eds.), Fish Physiology (Vol. 10, pp. 263-314): Academic Press. Retrieved from https://www.sciencedirect.com/science/article/pii/S1546509808603210
Reuell, P. (2019). Lungless salamanders’ skin expresses protein crucial for lung function. In. The Harvard Gazette. Retrieved from https://news.harvard.edu/gazette/story/2019/01/lungless-salamanders-skin-expresses-protein-crucial-for-lung-function/
Rye, C., Wise, R., Jurukovski, V., DeSaix, J., Choi, J., & Avissar, Y. (2016). Oxidation of Pyruvate and the Citric Acid Cycle. In Biology. Retrieved from https://openstax.org/books/biology/pages/7-3-oxidation-of-pyruvate-and-the-citric-acid-cycle
Schurch, S., Lee, M., & Gehr, P. (1992). Pulmonary surfactant: Surface properties and function of alveolar and airway surfactant. Pure and Applied Chemistry, 64(11), 1745-1750. doi:doi:10.1351/pac199264111745
Scott, J. E. (2004). The pulmonary surfactant: impact of tobacco smoke and related compounds on surfactant and lung development. Tobacco Induced Diseases, 2(1), 3-25. doi:10.1186/1617-9625-2-1-3
Spragg, R. G., Ponganis, P. J., Marsh, J. J., Rau, G. A., & Bernhard, W. (2004). Surfactant from diving aquatic mammals. J Appl Physiol (1985), 96(5), 1626-1632. doi:10.1152/japplphysiol.00898.2003
Staudinger, T., Bankier, A., Strohmaier, W., Weiss, K., Locker, G. J., Knapp, S., . . . Frass, M. (1997). Exogenous surfactant therapy in a patient with adult respiratory distress syndrome after near drowning. Resuscitation, 35(2), 179-182. doi:10.1016/s0300-9572(97)00055-5
Team, L. I. E. (2020, 27 May 2021). Glycolysis: All Steps with Diagram, Enzymes, Products, Energy Yield and Significance. Retrieved from https://laboratoryinfo.com/glycolysis-steps-diagram-energy-yield-and-significance/
Vasconcellos, F. (2007). Dipalmitoylphosphatidylcholine. In Dipalmitoylphosphatidylcholine.svg (Ed.), (pp. Skeletal formula of dipalmitoylphosphatidylcholine (DPPC). Created using ACD/ChemSketch 10.10 and Inkscape.). Wikimedia Commons. Retrieved from https://commons.wikimedia.org/wiki/File:Dipalmitoylphosphatidylcholine.svg
Veldhuizen, E. J. A., & Haagsman, H. P. (2000). Role of pulmonary surfactant components in surface film formation and dynamics. Biochimica et Biophysica Acta (BBA) – Biomembranes, 1467(2), 255-270. doi:10.1016/S0005-2736(00)00256-X
Waymaker, L. (n.d.). Pyruvate Oxidation. In. Biology for Majors I. York, U. o. (2003). Soapy Science. Retrieved from https://www.york.ac.uk/res/sots/activities/soapysci.htm