An Analysis of Sensors and Systems of Artificial Noses
Curtis Ehlert, Ingi El Shahid, Liv Toft, Mary Wan
Abstract
In the past couple decades, the field of artificial organs has seen immense progress, such as more comfortable prosthetic limbs and artificial livers. However, in recent years, a need for a technology able to detect the presence of molecules that the human nose cannot has emerged, leading to the creation of the electronic nose. In recent years, the technology of the e-nose has been the subject of more research and is slowly being used in different industries. The following essay will examine the parallels between natural and artificial olfaction, the three main components of the electronic nose as well as four families of sensors — electric, gravimetric, bioelectronic, and optical — and some of their respective applications.
The E-Nose: A General Overview
The components of the electronic nose are highly inspired by the steps of mammalian olfaction. There are four working principles of olfaction: acquisition, data processing, comparison, and decision. In mammalian olfaction, acquisition is done by odor receptors, while in artificial olfaction, it is done by sensors array. In mammalian olfaction, data processing is achieved by the brain, while in artificial olfaction, it is achieved by sensing signals. In mammalian olfaction, comparison is executed by the neural system, while artificial olfaction uses the technology of artificial neural networks (ANN). Finally, the results of the comparison of the neural system to decide on a smell is done by pattern recognition in the electronic nose (Hu et al., 2019).
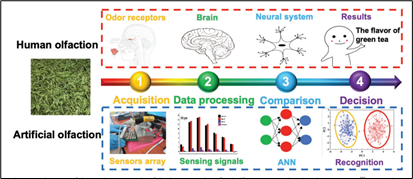
The steps to smell detection mentioned previously can be divided into three main components: the vapor delivery system, the pattern recognition algorithms, and the sensor array (Stitzel et al., 2011).
Vapor Delivery System
The vapor delivery system is responsible for how the sample gets to the sensor array. These systems can either be static or active flow, the latter often being preferred.
Static System
The static system consists of an injection of a few microliters of the sample liquid in a chamber of a volume of a couple of liters, which allows the liquid to evaporate. When the system reaches equilibrium, the sensor starts measuring. This system has certain characteristics necessary for it function properly: the chamber is made of glass to prevent vapor adsorption in its walls and its temperature is maintained constant by placing the whole chamber in a temperature-regulated bath (Nakamoto, 2002).

While there are many active flow systems, diffusion and bubblers are going to be examined in this essay.
Diffusion Method
This method measures the diffusion of vapor from a tube with known dimensions and it works with small concentrations because it is difficult to obtain vapor when the concentration is slightly higher than that of saturation (Nakamoto, 2002).
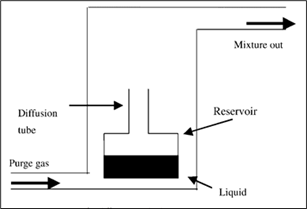
It all starts in the reservoir of sample liquid, where the liquid evaporates to then circulate in the diffusion tube. When it gets out of the diffusion tube, it joins a gas stream at a constant rate. The concentration of the mix is determined by dividing its diffusion rate by the diffusion rate of the flowing gas stream. The diffusion is then determined by the equation:
S={DMPA\over RTL}× ln({P\over (P-p)}) (1)
where S is the diffusion rate out of the tube with the known dimensions (g/mL), M is the relative molecular mass of the vapor (g/mol), P is the pressure at the open end of the tube (atm), A is the area of the tube (cm2), D is the diffusion coefficient (cm2/s), R, the molar constant (mL.Atm/mol.K), T, the temperature (K), L, the length of the tube (cm) and p, the partial pressure of the sample vapor (atm). There is an alternative technique that calculates the diffusion rate in terms of the mass change in the liquid reservoir using a balance (Nakamoto, 2002).
Bubbler Method
A bubbler is a bottle containing the sample liquid. A gas like air is released in the liquid in the bottle to generate vapor, to then take it away to the sensor. Compared to the method described above, bubblers produce vapor more easily. However, it is not as reliable as diffusion. It is possible for tiny liquid particles to accompany the vapor to the sensors because of heavy bubbling (Nakamoto, 2002).

Pattern Recognition Algorithms
The second component of the electronic nose are the pattern recognition algorithms. Their role is to interpret the data collected by the sensor. There are different types of algorithms, some parametric and others nonparametric (Stitzel et al., 2011). With parametric methods, also known as statistical methods, there is an underlying assumption that the sensor’s responses follow the concept of normal distribution that can be described by a probability density function. With nonparametric methods, there are no assumptions about the data, which means it can be used more generally, but with less precision and accuracy (Stitzel et al., 2011). The basis of the algorithms can also differ. Some go through supervised learning, where there is a training stage in which the sensor is exposed to a known set of inputs to create a database of descriptors that define the output classifications for reference. Then, during its identification stage, the unknown response is compared to the database and the output is a response based on the level of similarity with a previously learned output that was recorded in the database. An algorithm with unsupervised learning is not exposed to a known set of inputs; it must experimentally find classes that can define the input responses (Stitzel et al., 2011).
Sensor Array
Finally, in between the vapor delivery system and the pattern recognition algorithms, there is the most important component: the sensor array. It acts as a transducer, converting vapor into a change of signal, which becomes the input of the algorithms (Stitzel et al., 2011). The following subsections examine the principle of the categories of gravimetric, electrical, bioelectronic and optical sensors, as well as more precise types of sensors under each category.
Gravimetric Sensors
While there are several gravimetric sensors, this essay will look at the most common one: the quartz crystal microbalance (QCM). Like indicated in the name, this sensor is made of single crystal quartz, because of its piezoelectric properties (its ability to transform a mechanical stress into electricity), that is coated with a sorbent layer (Stitzel et al., 2011). Some examples of layers are surface-attached molecules or thin-polymer film. The choice of layer is important since it dictates what type of vapor the sensor reacts to. At the opposite sides of the quartz, there are gold electrodes to which is applied an alternative current, generating a resonant wave in the quartz. When a vapor enters the sorbent layer, its mass increases, which will proportionally change the frequency of the resonant wave. This type of sensor produces data that describes change in frequency, which is defined by the Sauerbrey equation (Stitzel et al., 2011):
Δf={-2f_0^2 m_f\over (A\sqrt{p_q μ_q} )}(2)
where f0 is the resonance frequency, mf, the mass of the sorbent film, A, the coated area, pq, the quartz density, and µq, the shear modulus. This sensor is so precise that it can detect mass changes on the order of 1 ng. It is important to note however that these devices are sensitive to changes in temperature and humidity (Stitzel et al., 2011).
Electrical Sensors
Many artificial noses take advantage of electrical sensors to discriminate molecules in a variety of applications. In many instances, multiple electrical sensors of the same type are simultaneously employed so that the sensor array may distinguish and detect specific molecules as does the natural olfactory system (Stitzel et al., 2011). As will be seen in the discussion below, electrical sensor-based artificial noses exploit and mimic one of the fundamental functions of the nose — to recognize odorants — but these sensors lack biological features. The following paragraphs will outline how two types of electrical sensors work and examine two implementations of each.
Metal Oxide Semiconductor Sensors
Metal oxide semiconductor (MOS) sensors are one of the most common gas sensors among electrical sensors (Stitzel et al., 2011). Used in the first artificial nose with tin as the metal (Persaud and Dodd, 1982), MOS-based electronic noses have diversified to detect many gaseous molecules. Before highlighting some of the applications, it is important to understand how the sensor can convert the presence of a gas into a change in signal.
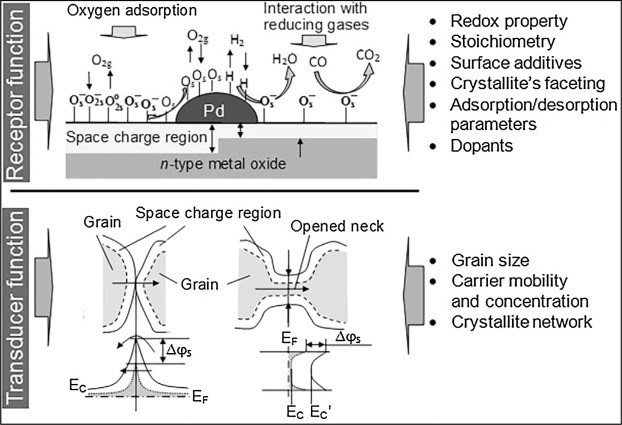
First, the surface of the metal oxide undergoes a change in conductivity in the presence of oxygen (Schierbaum et al., 1991). The film on the surface can be either thick or thin, and many oxide materials — zinc, titanium, tungsten for instance — have been used but the most common is SnO2 (Stitzel et al., 2011). Since SnO2 is an n-type semi-conductor, once it is incorporated into the sensor, it increases the conductivity of the MOS device when exposed to reducible gases such as H2 and CO. The number of charged species that are adsorbed on the semiconductor surface determines the change in conductivity. Essentially, oxygen adsorbs onto the surface (O2 becomes O– by the equation e– + (1/2) O2 → O–) and removes electrons from the SnO2 conduction band as a result. This, in turn, creates a space charge layer — or electron-depleted region — at the grain boundaries, which induces low conductivity (or high resistance) (Schierbaum et al., 1991).
Then, when the target gas molecules — in this case, reducible gasses — come into contact with the sensor, there is a change in the sensor’s electrical resistance due to the adsorption of reducible gases onto the surface currently with adsorbed oxygen molecules (Franke et al., 2006; Kanan et al.,2009; Schierbaum et al., 1991; Yamazoe, 1991). The interaction between the reducible gas and surface oxygen injects electrons back into the conduction band and thus the space charge layer reduces, ultimately leading to increased conductivity (lower resistance) in n-type semi-conductors (Eranna et al., 2004; Schierbaum et al., 1991). P-type semi-conductors, however, will react in the opposite way, with decreased conductivity (increased resistance) upon exposure to a reducing gas (Dey, 2018). Therefore, MOS-based noses detect gases based on the interaction between the target gas and semiconductor surface as well as the changes in conductivity or resistivity generated in the process.
Depending on the application, the properties of MOS sensors, specifically pertaining to the selectivity and stability, can be modified by altering the operating temperature, type of semi-conductor for the oxide films and by adding metal catalysts such as platinum and palladium as dopants (Mädler et al., 2006; Zhang et al., 2010). To enhance the selectivity of the MOS for the target gas molecule, modulating the temperature or using sensor arrays can be done to discriminate between several analytes in the mixture (Bochenkov et al., 2010; Liu et al., 2012). It is also imperative that the sensor is stable, reproducing signals for 2-3 years (Korotcenkov and Cho, 2017); stability can be increased by doping the metal oxide or by calcination for instance (Bochenkov et al., 2010).
There are numerous applications for MOS sensors such as in environmental monitoring, consumer goods, and explosives detection. In this paper, two will be discussed. MOS sensors are often used in environmental pollution detection since these can detect both organic and inorganic toxins with good reproducibility and limited manufacturing costs (Wilson, “Review of Electronic-nose Technologies”). In particular, five on-field calibrated MOS sensors have been utilized together to detect hazardous inorganic chemicals such as CO, NO2, and NOx (De Vito et al., 2009). Multivariate calibration was used as the data-processing method and feature selection algorithms were employed to help select the best array composition to use for each pollutant. Interestingly, the study also found that the calibrations for these multi-sensor devices are not only sensitive to concentration distribution changes of the target gases which appear on a seasonal basis but can also suffer from long term performance degradation. However, the study determined that a training set duration of approximately two weeks can lead to optimal performances in detecting concentrations, sufficient insensitivities to outliers, and to cope with the seasonal changes in the concentration distributions (De Vito et al., 2009).
In addition to pollution monitoring, MOS sensors can be used to identify insects and plant damage. This is paramount for crops, especially to determine their quality at points of transfer and purchase (Evans et al., 2000). Zhang and Wang (2007) successfully used an artificial nose containing 10 MOS chemical sensors to discriminate wheats of different age and with various degrees of insect damage from beetles. Here, headspace sampling, an example of an active flow vapor delivery system, introduced the vapors to the sensor. Following the interaction with the 10 MOS sensors, principal-component analysis (PCA) and linear-discriminant analysis (LDA) for data processing were used to classify both age (or storage time) – based on the amount of volatile compounds such as aldehydes, ketones, and alcohols – and degree of insect damage (Zhang and Wang, 2007).
Conductive Polymer Sensors
Conductive polymer (CP) artificial noses are another popular choice among electrical sensors. CP sensors also detect vapors by changes in conductivity (Lewis, 2004). Intrinsically conductive polymers (ICPs) are commonly based on materials such as pyrrole, aniline, and theophany — all of which are insulating in their neutral state but, through chemical or electrochemical oxidation or reduction, can be made conductive. — Further, the conductivity in ICPs is generated by movement of electrons along the extended pi system in the polymer backbone (Lewis, 2004). Composite conductive polymers (CCPs) are another type of CP artificial noses. These are constructed by blending insulating polymers with conductive material like graphite, metal powders, or carbon black and placed between two noble metal electrodes. Thus, at a baseline resistance, this mixture can conduct current between the two electrodes. Subjected to odorant contact, both intrinsically and composite CPs are assumed to swell, leading to an increase in the distance between the conductive particles (in the case of CCPs) (Lewis, 2004) or disruption of the conjugated backbone (in the case of ICPs). In either case, the result is a change in resistance (Lewis, 2004).
Just like MOS sensors, CP sensors have diverse applications. For this paper, two will be explored. One relevant application is in the detection of pesticides on agricultural and landscape plants using methods that are quick, simple, and reliable. Wilson (“Identification of insecticide residues”) employed a sensor array with 32 ICPs sensors to discriminate eleven insecticides from several different chemical classes. A reference library with the vapor profiles of known insecticides was compiled so that the artificial nose could relate an unknown pesticide to one in the database. Here, an artificial neural network algorithm matched the vapor profile of the insecticide of interest to one already in the library and principal component analysis (PCA) provided details from their vapor profiles regarding the possible chemical relatedness and differences between the insecticides. The sensor array was able to correctly identify 10 out of the 11 insecticide residue types at frequencies ranging from 82 % to 99 % (Wilson, “Identification of insecticide residues”).
CP sensors have the important job of detecting for explosives and nerve agents accurately in a matter of seconds. This is particularly challenging since vapor pressures of these dangerous compounds are in the range of parts per billion by volume at room temperature and since the amount of these vapors are further reduced (by a factor of 103) due to concealment (Kolla, 1997). In fact, CP sensor array composed of polymers doped with conductive carbon black detected nerve agent stimulants dimethylmethylphosphate (DMMP) and diisopropylmethylphosponate (DIMP) (Hopkins and Lewis, 2001). Carbon black CP sensor array detected and discriminated DMMP and DIMP from other test analytes such as water, methanol, benzene, toluene, even when the presented interferents exhibited higher concentrations (Hopkins and Lewis, 2001).
The Bioelectronic Nose and Biosensors
A ‘bioelectronic’ nose is an engineered nose directly based on olfactory receptor cells with surface level odorant recognition elements, that rely on sensors to convert biological signals to electrical ones (Zhang et al., 2018). Bioelectronic noses mimic the animal-olfactory system better than standard e-noses as a result of advances in materials and bioengineering (Wasilewski et al., 2015) as the current science of receptor proteins and odorant molecule binding allows for implementing materials which are much closer to animal systems than standard electronic noses whose odorant sensors are significantly different to the animal olfactory epithelium (Wasilewski et al., 2015). Through focusing on mimicking biological processes, bioelectronic noses are aiming to improve sensor sensitivity, specificity, and speed (Wasilewski et al., 2015). Various kinds of bioelectronic noses can be developed depending on the integrated sensors, the OR type, the immobilization methods, and the desired application fields (Dung et al., 2018).
Traditionally, electronic noses would have sensor arrays to detect ‘volatile olfactory compounds’ (VOCs), via some common sensors such as the quartz crystal microbalance (QCM), bulk acoustic wave (BAW), surface acoustic wave (SAW), field-effect transistor (FET)-type transducers, etc., which would undergo a physical or chemical change that produces signals which are processed digitally upon contact with VOCs (Dung et al., 2018). Despite being sensitive to odorants in a specific way, these sensors often have trouble specifying odorants from others, an issue corrected by bioelectronic olfactory receptor (OR)-based gas sensors, which works towards detecting various odorants with a high level of selectivity and sensitivity (Dung et al., 2018). These novel sensors have a limit of detection at the fM level (in liquid solution) and ppt levels in gas that is similar to the levels of mammals (Kim et al., 2009).
An example of highly specific odorant detection in bioelectronic noses comes from rose scent recognition by nanodisc-based olfactory receptor bioelectronic noses. To target the detection of a rose, Lee et al. used floating electrode (FE)-based carbon nanotube (CNT) field effect transistors (FETs) (which are field effect transistors that use arrays of carbon nanotubes to pass electric current [Tans et al., 1998]) integrated with the olfactory receptor 1A2 (hOR1A2)-embedded NDs – more broadly, hOR1A2NDs (Lee et al., “Mimicking the human smell sensing mechanism”). The receptor hOR1A2 was integrated into the bioelectronic nose as it serves as the receptor for geraniol and citronellol, the two main chemical ingredients of rose scent, and expressed from E. coli samples (Lee et al., “Mimicking the human smell sensing mechanism”). The hOR1A2NDs, incorporated with gold (Au)-based floating electrodes (FEs) on CNT-FETs, could monitor levels of geraniol and citronellol to 1fM and 10fM respectively. To conjure information, the artificial nose was coupled with a semiconductor analyzer, and 9 microlitres of the HEPES buffer (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) was placed on the bioelectronic nose channel surface (Lee et al., “Human-like smelling of a rose scent”). Changes in current were measured when adding the reagents and odorants (Lee et al., “Human-like smelling of a rose scent”).

The importance for being able to detect rose scent and its chemical derivatives comes from its popularity in the fragrance industry, more specifically with perfume. Since the smell of rose is so universally known, it is critical that its ingredients are perfectly balanced, and overdosing on ingredients of rose scent can result in allergic reactions and unpleasant responses (Lee et al., “Human-like smelling of a rose scent”). Because of this, ensuring that the proper ratio and quantity of rose scent ingredients in oil and perfumes is a critical issue, which is and has been studied extensively in pharmaceutical and cosmetic research (Sharma et al., 2015).
More broadly, nanotechnological principles are applicable to bioelectronic nose design as a result of using independent olfactory receptor systems rather than whole cells. As some macromolecular systems require hyper-specific conditions to function, it only makes sense that nanotechnology would be employed to ensure total system stability.

For example, transmembrane proteins need a certain detergent environment to keep their structure and retain function. As a response, Park et al. reported using ultrasensitive flexible graphene-based field-effect transistors (FETs), with the olfactory receptor hOR2AG1, to recognize amyl butyrate (Dung et al., 2018). The minimum detection limit (minimum measured concentration of something with near 100 % confidence) was two whole orders of magnitude larger than previously detected olfactory sensors (Dung et al., 2018).
However, the world of nanotechnological bioelectronic possibilities is vast. One particularly fascinating example of bioelectronic nose nanotechnology is artificial nanovesicles. Human embryonic kidney 293 cells (HEK-293) are injected with a hOR2AG1-expressing construct that results in the production of nanovesicles, which leave the cells: a field effect transistor is incubated with the nanovesicle solution (which retains imitation of the olfactory receptor via retain membrane proteins and cytosolic components) to create a nanovesicle based nose (Dung et al., 2018). These are incredibly effective as a result of odorant and OR binding triggering cellular signal pathways, which leads to charge accumulation, which leads to a transduction of signal with amplified sensitivity; this is contrasted to non-artificial nanovesicle systems which change in the charge of receptors simply comes from odorant-receptor binding (Dung et al., 2018).

Optical Sensors
Some researchers have developed methods for chemical identification and analysis by exploiting the optical properties of certain compounds. Upon interaction with an odorant molecule, characterizable and observable changes occur. Sensors created from such compounds are appropriately called optical sensors. What follows is a more detailed discussion of two specific types of optical sensors, colorimetric sensors which analyze the change in color of the sensor component as a result of ligand binding, and fluorescence sensors which analyze properties of the sensor’s fluorescence upon interaction with an odorant molecule.
Colorimetric Sensors
Colorimetric sensors use color changes in their sensing component upon interaction with an odorant as a means for vapor identification. The colorimetric sensor developed by Rakow and Suslick (2000) exploits the observation that odiferous and toxic compounds often bind readily to metal ions. In fact, it is hypothesized that the naturally occurring olfactory system utilizes a metal ion in its active site. Because of this property of odorants, Rakow and Suslick use metalloporphyrin dyes as a means to characterize vapors. Upon ligand binding, metalloporphyrins exhibit a large spectral shift. In other words, they change color. These metalloporphyrins are engineered porphyrins: 16-atom rings with four nitrogen atoms pointing inwards (Suslick, 2020). The nature of this porphyrin structure makes them perfect for binding to nearly all metal ions (Suslick, 2020). These porphyrins are combined with various metals of different chemical “hardness” and ligand binding affinity, creating metalloporphyrin dyes that exhibit different color changes in response to ligands. Thus, the colorimetric sensor works by analyzing the color difference before and after odorant exposure in an array of these metalloporphyrin dyes. Each odorant molecule has a unique color change signature in the array, thus allowing for both qualitative identification of the vapor as well as quantitative analysis. This can be done not only on a singular molecule type, but also on vapor mixtures. This is because the RGB values of the metalloporphyrin dyes change monotonically with porphyrin concentration and thus a single response to an analyte can be quantified (Rakow and Suslick, 2000).

This colorimetric sensor has many benefits. It can detect a wide range of ligands including alcohols, amines, ethers, phosphines, phosphites, thioethers, thiols, arenes, halocarbons, and ketones and can do so at small concentrations; in some tests, a response was observed for an analyte concentration below 100 parts per billion (Rakow and Suslick, 2000). In addition, unlike other sensors, water vapor does not affect its performance. Because of these properties, this sensor has a wide range of applications including analyte specific detectors such as those for insecticides in the agricultural industry and detection of drugs and neurotoxins in the medical industry (Rakow and Suslick, 2000). A commercialized colorimetric sensor based on these principles was developed by iSense (Stitzel et al., 2011).
Fluorescence Sensors
As suggested by their name, fluorescence sensors utilize a change in the fluorescent properties of its components in response to odorant molecule interactions as a means to identify them (Stitzel et al., 2011). One specific fluorescence sensor developed by White and colleagues will be discussed in further detail. In this sensor, the mechanism that interacts with the odorant molecules is composed of Nile Red dye in polymer matrices in the form of beads arranged in an array.

Nile Red dye was chosen for its slow photobleaching rate and large shift in emission wavelength as a result of changes in the polarity of its environment (Stitzel et al., 2011). In addition, the polymer matrices differ in polarity, hydrophobicity, pore size, elasticity, and swelling tendency. Thus, each bead interacts differently with the same vapor molecule. In addition, the beads are both spatially and temporally distributed, which further changes the way in which each sensor reacts to the vapor. Analyzing and comparing results between sensors allows for a more detailed understanding of the vapor components. The researchers note that vertebrate olfaction properties most likely comes from a system where odorants interact with an array of cross-reactive olfactory sensory cells, rather than specific receptors that are tuned to detect a single molecule. In this way, the bead array sensor mimics this concept (White et al., 1996).
Because optical fibers were used in the sensors, the distal sensing elements can be located several kilometers from the measuring device. This makes this sensor excellent in situations where a remote sensing system is favorable. Specific applications include environmental monitoring and product quality control (White et al., 1996).
Discussion of Nature as an Engineering Inspiration in Relation to Optical Sensors
As discussed briefly above, both colorimetric and fluorescence sensors do draw some inspiration from the naturally occurring olfactory system. However, it is important to note that the fundamental way in which odorant molecules are detected by these sensors differs from that of the natural olfactory system. These rely on and analyze changes in the optical properties of the sensing mechanism as result of interactions with the odorant molecules. It may be unclear why these sensors should be included in a discussion on engineered noses that draw inspiration from the natural mechanism. However, such optical sensors provide an interesting demonstration of when engineering may benefit from diverging from naturally derived solutions. The natural solution to the need to be able to sense vaporous chemicals is a complicated one, and thus mimicry is limited due to the sheer fact that the exact mechanism is still unknown. Even if completely discovered and understood, it may prove to be too complicated, and thus too expensive to reproduce in terms of both time and resources. This is when it is important to identify the issue that chemical detection aims to solve. In animals, the chemical sensor must be dynamical, able to sense a wide range of compounds, able to withstand environmental conditions, and, in some cases, be tunable to specific odorants. However, an engineered chemical sensor does not require all these attributes to be useful. In the optical sensor, researchers aim to recreate the ability to sense a wide range of compounds. Thus, these sensors utilize specific inspirations from the olfactory system of nature (the use of metal ions in the colorimetric sensor and the use of an array of cross-reactive receptors in the fluorescent sensor) that achieves this goal, while opting for simpler substitutions for other components of the device. As with much of bioengineering, the goal is not to exactly recreate the natural olfaction system as this has already been done by evolution, but rather draw inspiration from specific solutions that the mechanism presents that is useful to society. It is this spirit of inspiration that is seen in the optical sensor that still makes it an ode to the olfactory system created by nature.
Conclusion
All the sensors discussed here seek to solve the question of creating an artificial olfactory system. Each draws inspiration from the naturally occurring mechanism. However, the specific method of odorant identification differs as each sensor takes advantage of various physical and chemical properties as a means for detection. Some, such as the bioelectric nose, borrow directly from the natural mechanism, while others such as the optical and electrical sensors, stray away from it. Because of this, each sensor also comes with a unique set of applications. Such applications demonstrate not only how artificial olfaction systems can be incredibly useful to society, but more generally, how inspiring natural solutions can be for designing engineered mechanisms.
References
Brandner, J., Haftek, M., & Niessen, C. (2010). Adherens Junctions, Desmosomes and Tight Junctions in Epidermal Barrier Function. The Open Dermatology Journal, 4, 14-20. doi:10.2174/1874372201004020014
Dey, A. (2018). Semiconductor metal oxide gas sensors: A review. Materials Science and Engineering: B, 229, 206-217. doi:10.1016/j.mseb.2017.12.036
Dubacq, C., Jamet, S., & Trembleau, A. (2009). Evidence for Developmentally Regulated Local Translation of Odorant Receptor mRNAs in the Axons of Olfactory Sensory Neurons. The Journal of Neuroscience, 29(33), 10184-10190. Retrieved from https://www.jneurosci.org/content/jneuro/29/33/10184.full.pdf
Dung, T. T., Oh, Y., Choi, S.-J., Kim, I.-D., Oh, M.-K., & Kim, M. (2018). Applications and Advances in Bioelectronic Noses for Odour Sensing. Sensors (Basel, Switzerland), 18(1), 103. doi:10.3390/s18010103
Eranna, G., Joshi, B. C., Runthala, D. P., & Gupta, R. P. (2004). Oxide Materials for Development of Integrated Gas Sensors—A Comprehensive Review. Critical Reviews in Solid State and Materials Sciences, 29(3-4), 111-188. doi:10.1080/10408430490888977
Eriksson, A., Lindner, P., & Mårtensson, O. (1981). Molecular properties and odour. Effects of substituents on pyridine and pyridine odour. Journal of Theoretical Biology, 90(4), 477-486. doi:10.1016/0022-5193(81)90300-3
Evans, P., Persaud, K. C., McNeish, A. S., Sneath, R. W., Hobson, N., & Magan, N. (2000). Evaluation of a radial basis function neural network for the determination of wheat quality from electronic nose data. Proceedings of the International Symposium on Electronic Noses, 69(3), 348-358. doi:10.1016/S0925-4005(00)00485-8
Franke, M. E., Koplin, T. J., & Simon, U. (2006). Metal and Metal Oxide Nanoparticles in Chemiresistors: Does the Nanoscale Matter? Small, 2(1), 36-50. doi:https://doi.org/10.1002/smll.200500261
Hopkins, A. R., & Lewis, N. S. (2001). Detection and Classification Characteristics of Arrays of Carbon Black/Organic Polymer Composite Chemiresistive Vapor Detectors for the Nerve Agent Simulants Dimethylmethylphosphonate and Diisopropylmethylphosponate. Analytical Chemistry, 73(5), 884-892. doi:10.1021/ac0008439
Hu, W., Wan, L., Jian, Y., Ren, C., Jin, K., Su, X., . . . Wu, W. (2019). Electronic Noses: From Advanced Materials to Sensors Aided with Data Processing. Advanced Materials Technologies, 4(2), 1800488. doi:https://doi.org/10.1002/admt.201800488
Jin, H. J., Lee, S. H., Kim, T. H., Park, J., Song, H. S., Park, T. H., & Hong, S. (2012). Nanovesicle-based bioelectronic nose platform mimicking human olfactory signal transduction. Biosensors and Bioelectronics, 35(1), 335-341. doi:10.1016/j.bios.2012.03.012
Kanan, S. M., El-Kadri, O. M., Abu-Yousef, I. A., & Kanan, M. C. (2009). Semiconducting metal oxide based sensors for selective gas pollutant detection. Sensors (Basel, Switzerland), 9(10), 8158-8196. doi:10.3390/s91008158
Kim, T. H., Lee, S. H., Lee, J., Song, H. S., Oh, E. H., Park, T. H., & Hong, S. (2009). Single-Carbon-Atomic-Resolution Detection of Odorant Molecules using a Human Olfactory Receptor-based Bioelectronic Nose. Advanced Materials, 21(1), 91-94. doi:https://doi.org/10.1002/adma.200801435
Kolla, P. (1997). The Application of Analytical Methods to the Detection of Hidden Explosives and Explosive Devices. Angewandte Chemie International Edition in English, 36(8), 800-811. doi:https://doi.org/10.1002/anie.199708001
Korotcenkov, G., & Cho, B. K. (2017). Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sensors and Actuators B: Chemical, 244, 182-210. doi:10.1016/j.snb.2016.12.117
Lee, M., Yang, H., Kim, D., Yang, M., Park, T. H., & Hong, S. (2018). Human-like smelling of a rose scent using an olfactory receptor nanodisc-based bioelectronic nose. Scientific Reports, 8(1), 13945. doi:10.1038/s41598-018-32155-1
Lee, S. H., Kwon, O. S., Song, H. S., Park, S. J., Sung, J. H., Jang, J., & Park, T. H. (2012). Mimicking the human smell sensing mechanism with an artificial nose platform. Biomaterials, 33(6), 1722-1729. doi:10.1016/j.biomaterials.2011.11.044
Lewis, N. S. (2004). Comparisons between mammalian and artificial olfaction based on arrays of carbon black-polymer composite vapor detectors. Accounts of Chemical Research, 37(9), 663-672. doi:10.1021/ar030120m
Liu, A. H., Zhang, X., Stolovitzky, G. A., Califano, A., & Firestein, S. J. (2003). Motif-based construction of a functional map for mammalian olfactory receptors. Genomics, 81(5), 443-456. doi:10.1016/s0888-7543(03)00022-3
Mädler, L., Roessler, A., Pratsinis, S. E., Sahm, T., Gurlo, A., Barsan, N., & Weimar, U. (2006). Direct formation of highly porous gas-sensing films by in situ thermophoretic deposition of flame-made Pt/SnO2 nanoparticles. Sensors and Actuators B: Chemical, 114(1), 283-295. doi:10.1016/j.snb.2005.05.014
Nakamoto, T. (2002). Odor Handling and Delivery Systems. In Handbook of Machine Olfaction (pp. 55-78). doi: https://doi.org/10.1002/3527601597.ch3
Persaud, K., & Dodd, G. (1982). Analysis of discrimination mechanisms in the mammalian olfactory system using a model nose. Nature, 299(5881), 352-355. doi:10.1038/299352a0
Rakow, N. A., & Suslick, K. S. (2000). A colorimetric sensor array for odour visualization. Nature, 406(6797), 710-713. doi:10.1038/35021028
Schierbaum, K. D., Weimar, U., Göpel, W., & Kowalkowski, R. (1991). Conductance, work function and catalytic activity of SnO2-based gas sensors. Sensors and Actuators B: Chemical, 3(3), 205-214. doi:10.1016/0925-4005(91)80007-7
Sharma, P., Ghosh, A., Tudu, B., Bhuyan, L. P., Tamuly, P., Bhattacharyya, N., . . . Das, U. (2015). A Quartz Crystal Microbalance Sensor for Detection of Geraniol in Black Tea. IEEE Sensors Journal, 15(2), 1178-1185. doi:10.1109/JSEN.2014.2359741
Stitzel, S. E., Aernecke, M. J., & Walt, D. R. (2011). Artificial Noses. Annual Review of Biomedical Engineering, 13(1), 1-25. doi:10.1146/annurev-bioeng-071910-124633
Suslick, K. S. (2020). EXECUTIVE SUMMARY: PORPHYRIN AND METALLOPORPHYRIN CHEMISTRY. Retrieved from https://suslick.scs.illinois.edu/index.html
Tans, S. J., Verschueren, A. R. M., & Dekker, C. (1998). Room-temperature transistor based on a single carbon nanotube. Nature, 393(6680), 49-52. doi:10.1038/29954
Wasilewski, T., Gębicki, J., & Kamysz, W. (2017). Bioelectronic nose: Current status and perspectives. Biosensors and Bioelectronics, 87, 480-494. doi:10.1016/j.bios.2016.08.080
White, J., Kauer, J. S., Dickinson, T. A., & Walt, D. R. (1996). Rapid Analyte Recognition in a Device Based on Optical Sensors and the Olfactory System. Analytical Chemistry, 68(13), 2191-2202. doi:10.1021/ac9511197
Wilson, A. (2013). Identification of insecticide residues with a conducting-polymer electronic nose. Chemical Sensors, 2014, 1-10.
Wilson, A. D. (2012). Review of Electronic-nose Technologies and Algorithms to Detect Hazardous Chemicals in the Environment. First World Conference on Innovation and Computer Sciences (INSODE 2011), 1, 453-463. doi:10.1016/j.protcy.2012.02.101
Yamazoe, N. (1991). New approaches for improving semiconductor gas sensors. Sensors and Actuators B: Chemical, 5(1), 7-19. doi:10.1016/0925-4005(91)80213-4
Zhang, H., & Wang, J. (2007). Detection of age and insect damage incurred by wheat, with an electronic nose. Journal of Stored Products Research, 43(4), 489-495. doi:10.1016/j.jspr.2007.01.004
Zhang, M., Yuan, Z., Song, J., & Zheng, C. (2010). Improvement and mechanism for the fast response of a Pt/TiO2 gas sensor. Sensors and Actuators B: Chemical, 148(1), 87-92. doi:10.1016/j.snb.2010.05.001
Zhang, X., Cheng, J., Wu, L., Mei, Y., Jaffrezic-Renault, N., & Guo, Z. (2018). An overview of an artificial nose system. Talanta, 184, 93-102. doi:10.1016/j.talanta.2018.02.113