The Life Cycle of the Whiskers and Antennae of Animals and Insects
Yen Chuang, Giselle De Leon, Anna Xuyao Shi, Ella (Yaxin) Wang
Abstract
This article explores the various chemical, cellular, and molecular mechanisms relevant to the life cycle of whiskers (barbels) and antennae. The growth of zebrafish barbel is characterized by elongation, vasculature development, and innervation of taste buds. Catfish barbels perform chemical and mechanical sensing; namely, their hyper-sensitive gustatory system enables catfish to identify food sources through amino acid and pH sensing. Zebrafish barbels have regenerative properties and regenerated barbel can regrow after injury. Regenerated barbels are similar to original ones, except the central rod does not regenerate. In insect antennae, the aldehyde oxidase enzyme refreshes the olfactory system by deactivating enzymes involved in the breakdown of the aldehyde sex pheromone. Aldehyde oxidase is crucial for oxidizing plant-derived harmful aldehydes to eliminate hazardous chemicals. Streptomyces bacteria living symbiotically inside beewolf antennae secrete defensive chemicals, such as antifungal compounds. Streptomyces bacteria are integrated into larval cocoons to provide antifungal protection. Antennae of insects and whiskers of mice can also regenerate, including processes like reinnervation to ensure functional regeneration of these appendages. Regeneration requires complex signaling pathways that involve small molecules, hormones, and transcription factors.
Introduction
Animals need to correctly perceive and interpret their environment to survive. Certain environments that animals and insects inhabit make the perception of their surroundings challenging. For instance, harbor seals and catfish both live in murky waters and cannot rely on sight to forage for food. Thus, catfish have evolved barbels sensing amino acids and pH, allowing them to look for food, and seals have evolved wavy whiskers to track prey. Another example is the diamond-back moth, living in environments with harmful chemicals and a plethora of chemical stimuli. These have adopted odorant-degrading enzymes that allow them to correctly interpret their environments by filtering out unnecessary chemical stimuli and degrading harmful chemicals.
In addition, because antennae and whiskers are crucial to the survival of many species, many animals have evolved the ability to regenerate them since the cost of protecting the antennae and whiskers against every possible threat is often too high.
This essay describes how sensory appendages such as barbels and antennae use chemical and cellular mechanisms for survival. The life cycle of fish barbels will be analyzed, from their growth, to their sensing abilities, to their regenerative properties in case of injury. Similarly, the life cycle of insect antennae will be described by analyzing the odorant-degrading aldehyde oxidase enzyme, a symbiotic relationship of Streptomyces bacteria living in beewolf antennae, and antennal regeneration.
Growth of barbels in zebrafish
Barbels are sensory organs located near the mouth of fish such as zebrafish and catfish. The maxillary barbel (Fig. 1) is an elongated integumentary appendage at the base of the zebrafish's face (LeClair & Topczewski, 2010). Studying the stages and mechanism of barbel growth and regeneration can provide insight into bioengineering applications in cellular and tissue engineering. Even if the fish barbels are not equivalent to human tissue, the cell types and mechanisms involved are significantly similar (LeClair & Topczewski, 2010). Mapping out the different phases of barbel growth allows for the design of synthetically grown tissue. For instance, understanding the vascularization of barbels and the development of epidermal tissue can lead to innovations in blood vessel formation in tissue engineering, especially thicker tissue (Goldenberg et al., 2021). Moreover, researching regeneration in animal appendages could lead to developments in regenerative medicine where self-cells are the proponents of healing; this can lead to developments in enhancing skin regeneration after injury or surgery (Tissue Engineering and Regenerative Medicine).
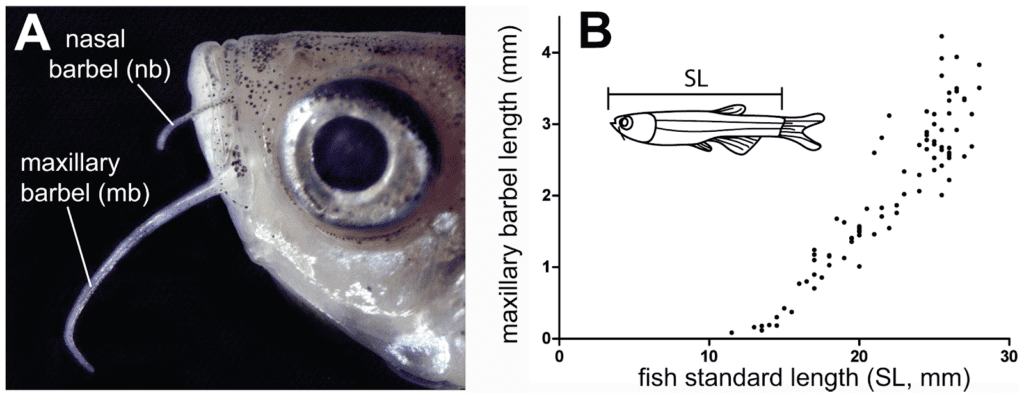
Fig. 1 The maxillary (mb) and nasal barbel (nb) of a zebrafish (Adapted from LeClair & Topczewski, 2010).
Growth of zebrafish barbels
The growth of the zebrafish barbel begins 30-40 days post-fertilization, where the pair of maxillary barbels emerge as unpigmented epithelial buds (LeClair & Topczewski, 2010). As the barbel grows, its distal end shifts from rounded to tapered and becomes more pigmented at the proximal side due to the increase of melanophore cells, chromatophores containing melanin (LeClair & Topczewski, 2010). Angiogenesis, the formation of new blood vessels, occurs near the central rod and innervation begins near the maxillary bud (LeClair & Topczewski, 2010). Barbel elongation is accompanied by thickening and distal lengthening of the central rod, and the extension of vascular, nervous, and dermal structures.
Development of vascular system
As the buds emerge, the proximal plexus, a dense knot of endothelial cells, forms 3-5 sprouts; this structure is the base of the vascular system inside the cheek of the zebrafish (Fig. 2A) (LeClair & Topczewski, 2010). As the barbel grows, the endothelial cells branch into two streams: a large ventral stream for blood transport, and a small dorsal stream presumably for the flow of the lymphatic fluid (LeClair & Topczewski, 2010). When the barbel reaches a length of several hundred microns, the vascular system grows at the distal end due to the formation of single-layered endothelial cells lining blood vessels (Alberts et al., 2002). These endothelial cells migrate using their filopodia (LeClair & Topczewski, 2010), which are minuscule cytoplasmic projections, and release signals that conduct the growth of connective tissue surrounding blood vessels (Alberts et al., 2002). The vascular endothelial growth factor, a protein that regulates the growth of blood vessels, stimulates selective endothelial cells at various steps of blood vessel growth (Alberts et al., 2002). To grow a new capillary vessel, endothelial cells first produce proteases to digest a hole in the basal lamina of the parent vessel; then, the endothelial cells migrate towards a signal emitted by the vascular endothelial growth factor and proliferate to form new vessels (Alberts et al., 2002).
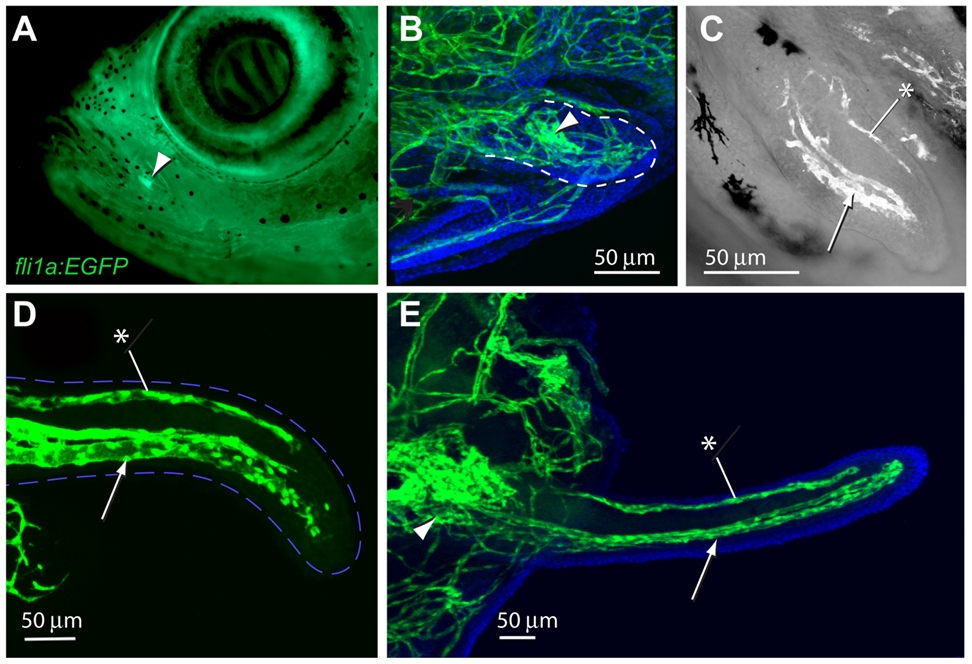
Fig. 2 Development of barbel vasculature. A) The arrow shows the location of the proximal plexus inside the cheek of a juvenile zebrafish. B) Vasculature (green) develops in the barbel bud. Blue staining indicates nuclei. C) The appearance of two streams of endothelial cells. The arrow indicates the larger ventral stream, and the asterisk indicates the smaller dorsal stream. D&E) Further extension of the barbel vasculature. The two streams as seen in C) are visible (LeClair & Topczewski, 2010).
Development of barbel nerves and taste buds
Barbel innervation begins with a small tubulin-positive branch inside the core of the maxillary bud that innervates taste buds in the proximal ventral area (LeClair & Topczewski, 2010). Small sinuous fibers line the barbel's epidermal sheath. As the barbel grows, the nerve trunk thickens and branches into two main nerve tracks: a dorsal track and a ventral track on the back and front of the barbel, respectively (LeClair & Topczewski, 2010). Adult zebrafish possess 1-2 dorsal nerve fascicles (bundle of nerves) and 4-6 ventral fascicles, with more fascicles on the proximal side of the barbel than at the distal ends (LeClair & Topczewski, 2010). LeClair & Topczewski's experiments (2010) using immunohistochemistry, a reaction with fluorescent antibodies to show the location of a particular organ of interest, map out the development of taste buds (Fig. 3). Numerous taste buds appear during the early stages of barbel growth (Fig. 3A). As the barbel extends, onion-shaped cell clusters increase in number along the ventral side of the barbel (Fig. 3B&C) (LeClair & Topczewski, 2010). Some of these onion-shaped clusters of cells contain taste buds, while others are solitary chemosensory cells that detect other chemical stimuli (Fig. 3D), such as acids and other ions (Levanti et al., 2016).
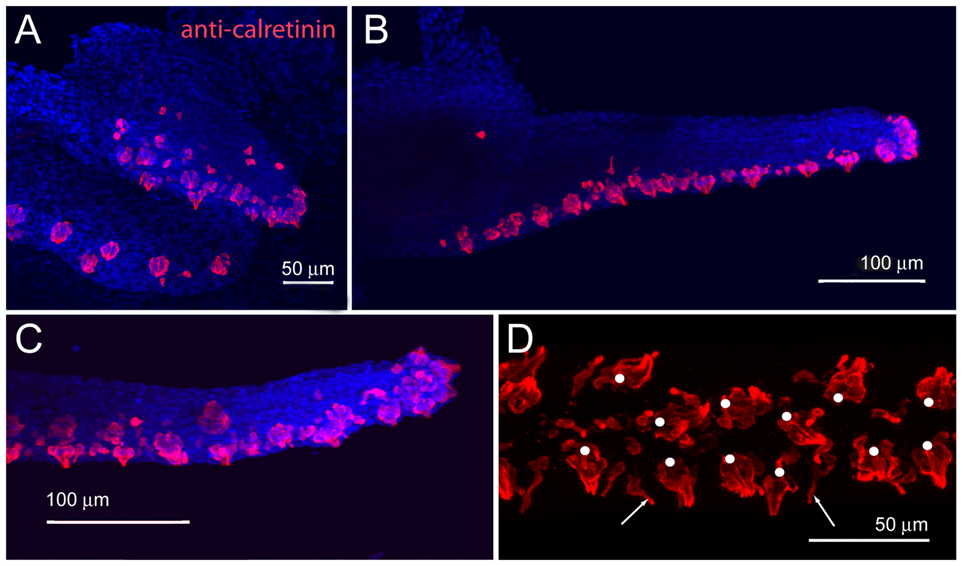
Fig. 3 A) Numerous differentiated taste buds (pink) appear on the early barbel bud. B) As the barbel grows, taste buds form on the ventral and distal sides of the barbel. C) Magnification of the distal tip of the maxillary barbel. D) Ventral view of a mature maxillary barbel. Taste buds are shown by white dots and solitary chemosensory cells are indicated by white arrows (LeClair & Topczewski, 2010).
Life of barbels: catfish look for food with their taste-sensing barbels
Role of gustation
Catfish (Fig. 4) are nocturnal animals that live in dark muddy waters. Their habitat inhibits their sight; thus, they must rely on other senses to navigate and search for food (Gao et al., 2017). They use chemosensory systems such as olfaction and gustation to accomplish these tasks. In most vertebrates, olfaction is considered to be the detection mode that senses volatile stimuli coming from a longer distance while gustation is associated with short-range stimulus recognition (Caprio, 1975). However, with catfish and other fishes, the sense of taste can also react to distant stimuli, rivaling olfaction (Ovalle & Shinn, 1977). Gustation becomes an important tool for food location, especially for species like catfish living in turbid waters. Catfish are often studied to understand gustatory information processing since they have a high concentration of readily accessible taste buds covering their body, notably on the barbels, and use gustation in their environment (Kruger & Cagan, 1976). Of the 2584 species of catfish, the most studied is the Plotosus Japonicus, a species of catfish from Japan, which will be the one investigated in this review (Diogo & Chardon, 2000).

Fig. 4 Sea catfish, Plotosus japonicus (Caprio et al., 2014).
Barbel morphology
Barbels are external organs found near the mouth of catfish, resembling the shape of whiskers. They are distributed in four pairs, namely the lateral mandibular, maxillary, medial mandibular, and nasal barbels. Fig. 5 shows the distribution of the barbels on the catfish's face (Ikenaga & Kiyohara, 2018).
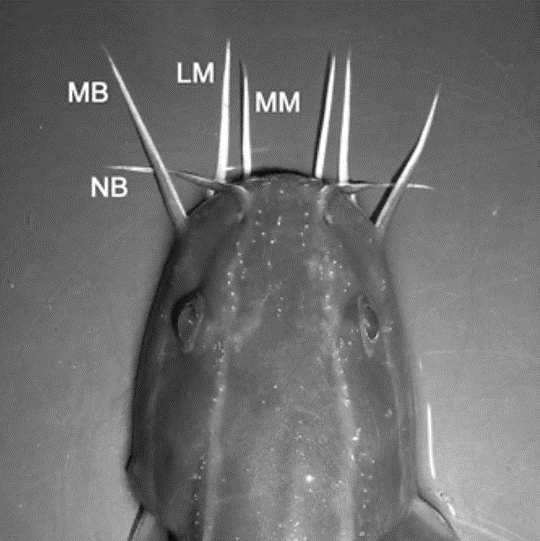
Fig. 5 Enlarged grey-scaled photograph. Dorsal view of the head of P. japonicus showing the distribution of four pairs of barbels. LM lateral mandibular barbel, MB maxillary barbel, MM medial mandibular barbel, NB nasal barbel (Ikenaga & Kiyohara, 2018).
While they differ from the anatomy of whiskers, which are hypersensitive hair follicles, barbels play a similar role in sensing the environment. They are protrusions of the catfish skin performing both mechanical and chemical sensing. They have no intrinsic muscles and therefore cannot move. They rely on tactile stimuli and bending due to external factors for mechanical sensing (Ikenaga & Kiyohara, 2018). The barbels carry the highest concentration of external taste buds on the catfish body, explaining their extreme gustatory sensitivity (Nakamura et al., 2017). The taste buds on these barbels are not distributed evenly along the organ. They are found in a higher density at the barbel's tip and its front edge. On the other hand, the lateral region, also defined as the intermediate region, carries a lower taste bud density (Nakamura et al., 2017). These variations in taste bud density appear to benefit food searching, as regions coming in contact more often with environmental substances coincide with high-density taste bud regions (Nakamura et al., 2017).
Taste bud cell types
Taste buds are composed of three different types of cells. The first type of cells are often referred to as t-cells, light cells, or gustatory cells. These cells have a single large microvillus as the pore center and a complex system of tubules and vesicles that may be involved in connections with nerve fibers. Cells of the second type are called f-cells, dark cells, or supporting cells. They differ from t-cells by having multiple small microvilli extending from their cell pore (Royer & Kinnamon, 1996). Fig. 6 shows a t-cell with multiple microvilli emerging from the pore (Ovalle & Shinn, 1977). Finally, basal cells do not appear on the external surface but are associated with nerve fibers connected to the base of the taste bud (Ovalle & Shinn, 1977).

Fig. 6 Viewed using a scanning electron microscope. Lateral surface view of a single taste bud (TB) showing prominent microvilli emanating from its central pore (above) (Ovalle & Shinn, 1977).
Taste receptors and their properties
Taste receptors are transmembrane receptors expressed in taste buds reacting to chemical stimuli. They relay sensory information through the nerve fibers with which they are associated. Taste buds and taste receptors of the barbels are innervated by the facial nerve (Nakamura et al., 2017). These receptors are responsible for the detection of the five taste sensations, namely bitter, umami, sweet, sour, and salty. The most important one is tasting bitterness, due to its close association with toxic and poisonous substances (Gao et al., 2017).
Nerve impulses are not transmitted by axons small nerve fibers–but are instead made through synaptic connections mediated by chemical neurotransmitters. Each taste receptor is associated with a small hexagonal shaped neuronal network allowing for the discrimination of information in different areas of the barbels (Nakamura et al., 2017). The information is encoded to utilize the spatial distribution of taste buds along the organ. Two main families of taste receptors have been identified on barbels. The first one, the taste receptor of type 1, also known as the T1R family, is divided into three subcategories, namely T1R1, T1R2, and T1R3. T1R3 co-expressed with T1R2 allows for the perception of sweet taste in molecules such as sucrose and D amino acids. T1R3 co-expressed with T1R1 allows for the perception of umami taste in molecules such as L-amino acids and monosodium (Gao et al., 2017). The second family of taste receptors, type 2 or T2R, are responsible for the perception of bitter sensations. It has been determined that the number of taste receptors in this family is much lower than the number of bitter-tasting chemicals found in the catfish environment, indicating that these taste receptors may be used for sensing rather than distinguishing chemicals (Gao et al., 2017).
The binding properties of different chemicals to their taste receptors are also interesting aspects of chemosensory systems, as they affect the efficiency of chemical detection and sensory encoding. Two types of binding usually occur: specific and non-specific binding. Specific binding is the binding of a chemical to the receptor of interest, while non-specific binding is the binding of a chemical to another site. Many factors affect the binding kinetics of chemicals to their TRs (Kruger & Cagan, 1976). Specific binding is time-dependent, indicating that binding increases over a longer time, while non-specific binding remains constant. Binding initially occurs at a faster rate and later decreases, which can be explained by the saturation of taste receptors. Both specific and non-specific binding show a linear relationship with the concentration of taste receptors. In the case that denervation, a loss of nerve supply, occurs, the rate of specific binding decreases while non-specific binding remains constant. This can be explained by the fact that denervation leads to the deterioration of the taste buds, lowering their sensory abilities and efficiency. Finally, temperature changes can cause a decrease in specific binding but will leave non-specific binding unaffected (Kruger & Cagan, 1976). When heated or put under other extreme conditions, the taste receptors lose specificity in binding molecules, which decreases specific binding. Fig. 7 demonstrates the relationships between specific and non-specific binding with time and the effect of temperature on amino acid binding for L-alanine (Kruger & Cagan, 1976).
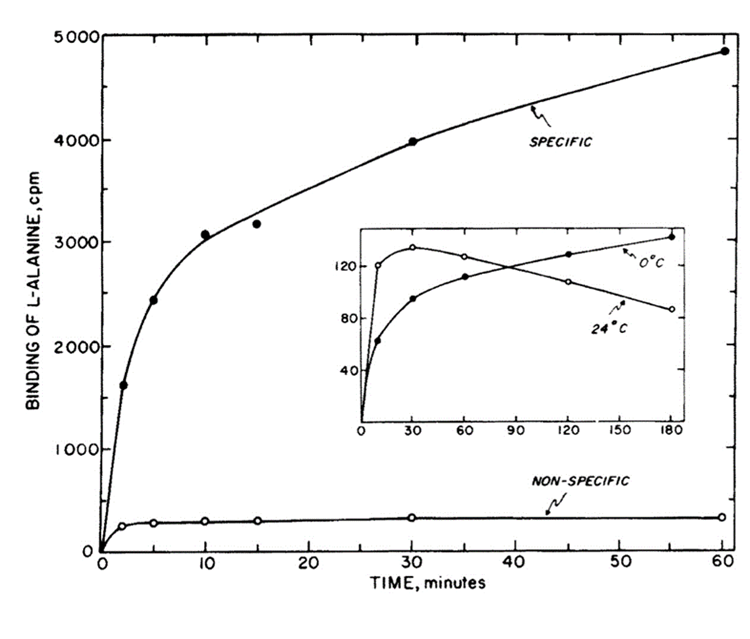
Fig. 7 Binding of L-alanine versus time. Inset shows the binding of L-alanine versus time at two temperatures (Kruger & Cagan, 1976).
Sensitivity to amino acids
Taste buds on the barbels have demonstrated an important sensitivity to amino acids. Amino acid sensitivity is especially important, as it is closely related to food searching. Many catfish prey release amino acids that are found in high concentrations in their tissues, enabling catfish to identify their location. Nerve fibers will react differently in the presence of different amino acids. Table 1 shows the effectiveness of different amino acids as stimuli for catfish barbels (Caprio, 1975).
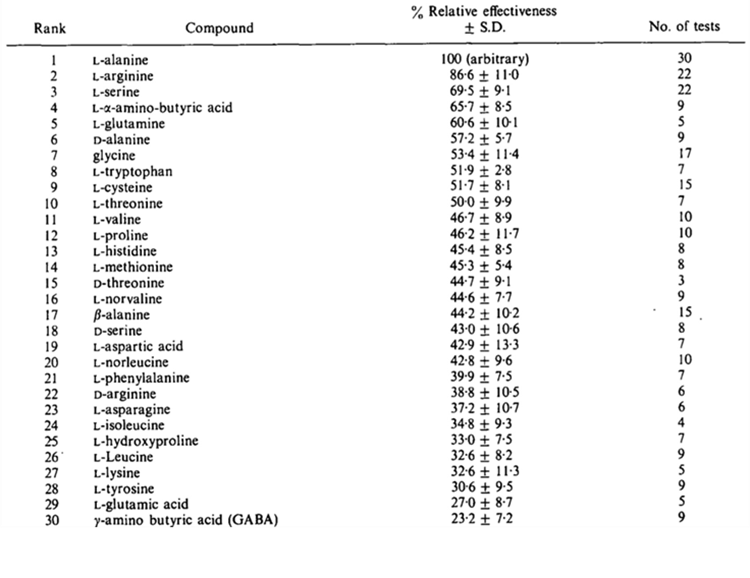
Table 1 Relative stimulatory effectiveness of some amino acids tested on the channel catfish maxillary barbel at 10-4M (Caprio, 1975).
The threshold of amino acid concentration for which taste buds can be stimulated varies for different compounds. Some are only necessary for concentrations as low as 10-10 moles per liter to be sensed by the barbels (Caprio, 1975). Glycine, L-alanine, L-proline, and betaine are among the amino acids detected by the barbels (Nakamura et al., 2017). All these compounds are found in high concentrations in the tissues of marine animals, including catfish prey (Ikenaga & Kiyohara, 2018). Sensitivity to amino acid stimulus is essential in food searching for catfish as it is used as a means to identify the location of their prey. During an experiment, the Plotosus japonicus seemed attracted to a tube releasing betaine in a 10-3M concentration (Ikenaga & Kiyohara, 2018). This behavioral experiment demonstrates an ability to respond to amino acid stimulus as well as a certain attraction to betaine-dense regions, which can be associated with food-searching methods. While the olfactory system of catfish also responds to betaine stimulus, the response is much weaker, indicating that betaine sensing can be attributed to the gustatory system of these fish (Ikenaga & Kiyohara, 2018).
Sensitivity to pH changes
In addition to amino acids, barbels are also sensitive to pH changes in neighboring waters. They can sense pH drops smaller than 0.1 pH unit. This is also useful for food-searching using the barbels' gustatory system since changes in pH are often due to increasing concentrations of H+ ions and carbon dioxide, products of respiration of marine animals, which include the preys of catfish (Nakamura et al., 2017). Decreases in pH are associated with the respiration of prey and allow catfish, through pH sensing, to identify their location. An example of the use of pH sensing for food searching is related to polychaete worms. They are catfish prey living in V-shaped tubes in coastal sediments. During respiration, these worms released H+ and carbon dioxide into the water, allowing catfish to identify their location (Ikenaga & Kiyohara, 2018). A similar behavioral experiment to the one mentioned for amino acid sensing was conducted to demonstrate the pH sensing ability of barbels and its relationship with feeding habits. A U-shaped tube released water with a lower pH, while another released control water with a higher pH. Catfish would not only spend more time in the aquarium with the tube releasing water with a lower pH but would also frequently attempt to bite the tube (Ikenaga & Kiyohara, 2018). This demonstrates a correlation between barbel sensing, water pH, and food searching.
Catfish have been used as templates to study the chemosensory process behind taste buds and gustation. However, the neuronal and chemical basis for this type of sensing is yet to be fully understood. Little is known regarding the processing of chemical information obtained by barbel taste buds. They are some of the most complex and developed organs in terms of chemosensation and should be studied further to open new research areas in this field.
Regeneration of barbels in zebrafish
Maxillary barbels of zebrafish can regenerate, and regenerated barbels can themselves regenerate after proximal amputation (LeClair & Topczewski, 2010). So far, experiments testing the maximum number of times that barbel regeneration can occur have not been found, but research has shown that a regenerated barbel has the capability of regenerating a second time. LeClair & Topczewski (2010) showed that 66% of zebrafish regenerated their barbels after a second surgical cut. However, regeneration is not flawless, as proximal amputation leads to a permanent internal scar and a regenerated barbel shorter and thicker than the original one. The central rod of the barbel does not regrow after amputation: it remains as a stump covered by epidermal sheath cells (Fig. 8), and abnormally organized mesodermal cells extend the barbel (LeClair & Topczewski, 2010). In addition, repeated injuries diminish the likelihood of regeneration, suggesting that physical trauma impacts the barbel's regenerative capabilities. The results of the first regeneration of zebrafish barbel in an experiment by LeClair & Topczewski (2010) are as follows (Fig. 8).
A few hours after amputation, the barbel appears as a stump with blunt ends and an exposed central rod. After 24 hours, an epithelial cap covers the central rod, rounding out the tip. Small blood clots or cloudy necrotic cells lie under the epithelial cap, suggesting that the cap covers the area of platelet clotting. These small dark-colored spots disappear within a few days. After 72 hours, the epithelium cap thickens, and the bulb swells due to the enlargement of the mesodermal layer. Chromatophores, such as melanophores (containing melanin) and xanthophores (containing a yellow carotenoid pigment), differentiate in the regenerated area. Blood flow is significantly decreased in the injured area, with only short capillary sprouts forming in the swollen distal bulb to deliver blood to the regeneration barbel. In addition, calretinin-positive cells are present on the distal epidermis, indicating the presence of nerves. The blastema, a mass of undifferentiated cells forming new tissues, contains GFP-positive cells with filopodia, which aid the active migration of epithelial cells during barbel growth.
After seven days (Fig. 8C), barbel length significantly increases. The vascular system re-establishes, albeit in an abnormally organized fashion: some vessels are thicker than the original, some are replaced by multiple vessels instead of one, and more bends and loops occur than in the original vasculature. The separation between the distal lymphatic system and the vasculature system remains. Taste buds and their nerve supply regrow on the distal and ventral side of the barbel. The ventral taste buds are arranged in two rows, similar to the distribution of the original barbels' taste buds. Both onion-shaped taste buds and solitary chemosensory cells appear in the regenerated barbel.
The regenerated barbel continues its growth for the next 14-28 days (Fig. 8D). The intercellular and vasculature structure of the proximal part of the barbel is more disorganized than those of the distal part (Fig. 8G).
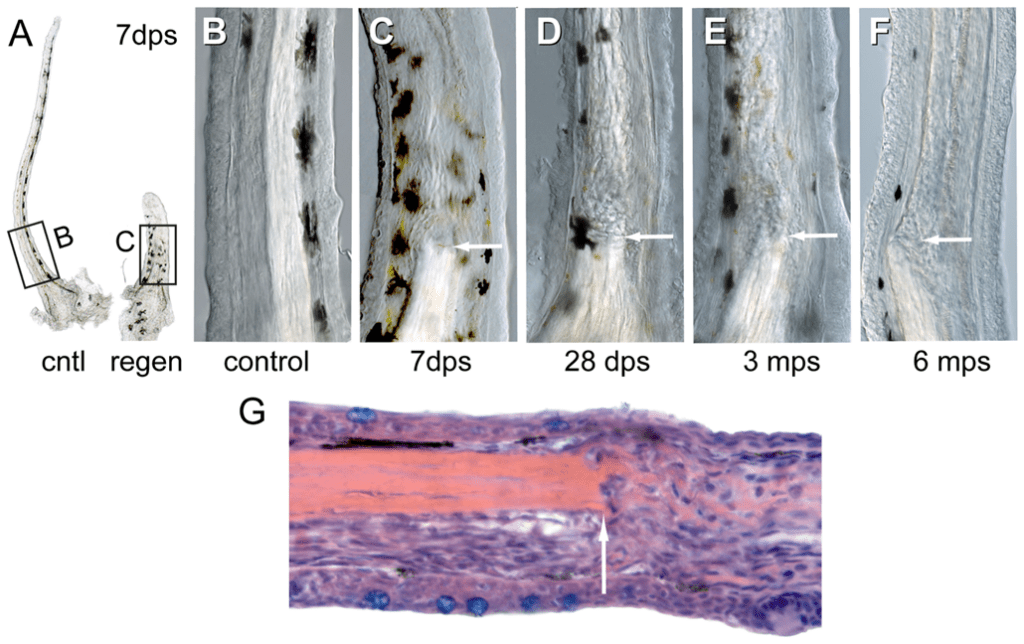
Fig. 8 Stages of regeneration of the barbel (dps: days post-surgery, mps: months post-surgery). A) Comparison of the size and thickness of the control barbel (B) and the regenerated barbel. C, D, E&F) regenerated barbel at different times post-surgery. Notice that the white central rod does not regenerate. The arrow shows the location of the central rod stump. Dark spots are pigmentation due to chromatophores. The pigmentation eventually fades and the barbel becomes translucent. G) Distal (to the right of the figure) to the central rod stump (arrow) are disorganized mesodermal cells that extend the babel (LeClair & Topczewski, 2010).
Barbel cells are pluripotent, meaning they can differentiate into any cell type and renew themselves. However, the exact source of the cells that form the regenerated barbel is yet unknown. Hypothesized cell sources include de-differentiated cells from the injury site, pluripotent cells inside the central rod stump, or cells from other parts of the zebrafish that migrate to the area of injury. Most likely, the cells on the epithelium sheet that cover the injury grow and differentiate, creating the regenerated barbel. Nerve and vascular cells presumably regrow out of surviving cells near the injury site.
Life of antennae: aldehyde oxidase enzyme in diamond-back moth antennae
Insects possess an intricate olfactory system that plays a crucial role in sensing environmental cues to promote specific behaviors, such as aggregation, and locating suitable mates, hosts, and oviposition sites (Robertson, 2019). Insect odorant-binding proteins (OBPs) are believed to be involved in olfactory perception since they solubilize, bind, and integrate an OBP/odorant complex before transporting it through the sensilla lumen to activate the particular receptors on olfactory sensory neurons (ORNs). The odorants must be degraded by various odorant-degrading enzymes (ODEs) to refresh the ORNs (Leal, 2013). Female moths generate sex pheromones intermittently, prompting conspecific male moths to reset their sex pheromone detection system on a millisecond timescale (Ishida & Leal, 2005). Therefore, ODEs are required to convert redundant odorants into inactive molecules so that ORNs can restart detection (He et al., 2014a&b).
The antennae of insects play a crucial role in perceiving and distinguishing a vast array of conventional semiochemicals and host plant-derived scents. It is believed that genes that are firmly connected with the antennae possess olfactory-related tasks related to signal transduction pathways. Multiple mechanisms indicating enzymatic inactivation, such as odorant-degrading enzymes (ODEs), could play a role in the signal termination process. However, only a few ODEs have been found and thoroughly characterized in insects to date due to a lack of research in this field of study (Wang et al., 2021).
What are odorant-degrading enzymes (ODEs) and aldehyde oxidase enzymes (AOXs)?
Based on their substrate specificities, ODEs are categorized into many groups, including carboxylesterases, aldehyde oxidases (AOXs), cytochrome P450 enzymes, glutathione-S-transferases, and UDP-glucuronosyltransferases. ODEs involving ester hydrolysis have been well-documented (He et al., 2014b; He et al., 2015; Younus et al., 2017; He et al., 2020) and identified as key factors in olfactory sensitivity and food-seeking behavior. However, investigations of other forms of ODEs, particularly Aldehyde oxidases (AOXs), remain uncommon, for the in vitro production of the active form of AOXs is highly challenging (Choo et al., 2013).
AOXs are an important class of metabolic enzymes that oxidize a range of aromatic aldehydes; they may also play a vital role in detoxifying and degrading environmental chemical signals. AOXs contribute to the metabolism of xenobiotics and medicines in mammals, plants, and insects (Choo et al., 2013). Few AOXs in insects have been identified as xenobiotic detoxification enzymes. For instance, acetaldehyde is extremely toxic to insects and must be decomposed for their survival (David et al., 1984). Marelja et al. (2014) found four AOX enzymes in D. melanogaster and investigated their role in the acetaldehyde breakdown of an air pollutant. Choo et al. (2013) identified a gene encoding for aldehyde oxidase, AtraAOX2, from the antennae of the navel orange worm, Amyelois transitella. They hypothesized that this enzyme could protect olfactory sensory neurons from xenobiotics and aldehyde-containing pesticides that could enter the sensillum lymph.
Antennae-specific AOXs were initially found in the tobacco hornworm, Manduca sexta (MsexAOXs), expressed exclusively in male antennae and exhibit activity on both the aldehyde sex pheromone, bombykal, and a variety of plant-derived aldehyde odorants (Rybczynski et al., 1989). Researchers have recently identified two forms of AOXs in insects: Drosophila AOX1 in Drosophila melanogaster (Marelja et al., 2014) and AtraAOX2 in the Navel Orangeworm, Amyelois transitella. Using RT-PCR, it was discovered that the AtraAOX2 gene was uniquely expressed in both male and female antennae. In addition, recombinant AtraAOX2 lacked selectivity for the primary component of the sex pheromone Z11Z13–16:Ald, but demonstrated potent oxidation activity for aldehydes produced from plants (Fig. 10). AtraAOX2 may be implicated in the degradation of various aromatic aldehydes and plant volatiles, according to the findings acquired (Choo et al., 2013).
Roles of aldehyde oxidase enzyme in antennae
Researchers identify and characterize a new cDNA encoding a putative aldehyde oxidase, PxylAOX3, from the antennae of the diamondback moth, Plutella xylostella (L.), (DBM) (Fig. 9). The purified recombinant protein exhibited a broad spectrum of substrate zymography activities, oxidizing both sex pheromone molecules and plant-derived aldehydes. The observations indicate that PxylAOX3 may be involved in the breakdown of a wide variety of structurally distinct aldehyde odorants. In addition, PxylAOX3 may protect olfactory neurons by deactivating redundant odorants and xenobiotics, making it a possible target for repellants-development (Wang et al., 2021).

Fig. 9 Diamondback moth adult on a leaf (side view) (Adapted from The Canola Council of Canada).
Aldehyde (Z11-16:Ald) is the major sex pheromone component released by a mature female DBM and is essential to stimulate male mating behavior (Fig. 10) (Wang et al., 2019). Evidence presented in numerous papers demonstrates that aldehyde chemicals are crucial to moth behavior. Understanding the process of aldehyde degradation by AOXs in moth antennae is essential for a full examination of the detoxification/degradation of inactive aldehydes and hazardous xenobiotics (Wang et al., 2021).
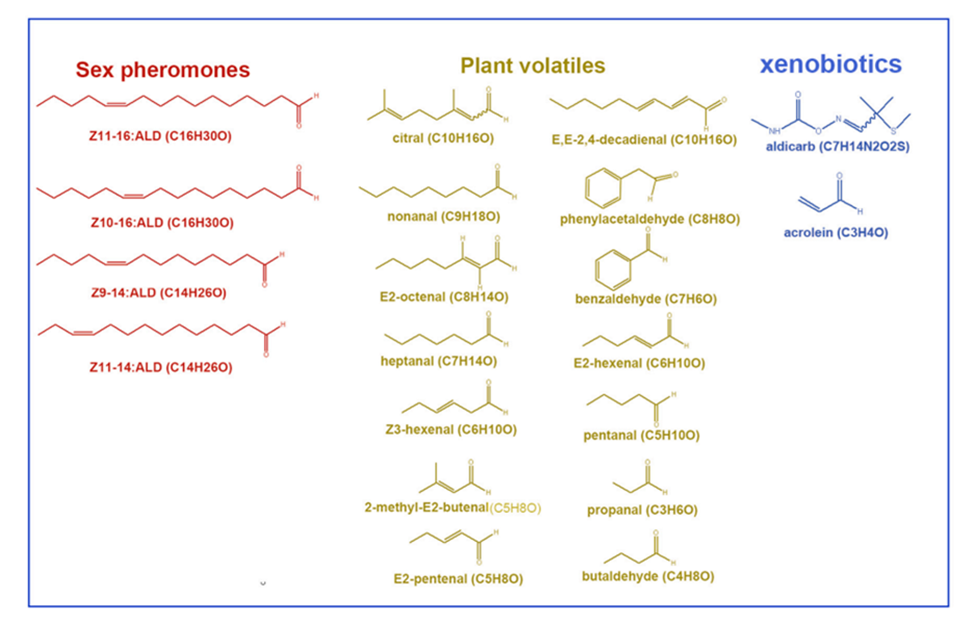
Fig. 10 Chemical structures utilized in the investigation (Wang et al., 2021).
The recombinant enzyme protein was effectively extracted from insect cells infected with baculovirus in order to research the enzyme's function. The properties and functions of the enzyme involved in the inactivation of poisonous plant volatile aldehydes and the detoxification of xenobiotics were also explored (Wang et al., 2021).
Specificities of enzymes for aldehyde volatiles and xenobiotics
PxylAOX3 exhibited an extended degradation zymogram that successfully oxidized plant-derived aldehydes and xenobiotics (at least 24.96% for aldicarb to the control substrate 100% phenylacetaldehyde) (Fig. 11). PxylAOX3 is the most effective in oxidizing phenylacetaldehyde among the studied aldehyde substrates, which have been described as potent insect attractants (Tavares et al., 2018). It is suggested that PxylAOX3 contributes to the decomposition of odorants (Wang et al., 2021).
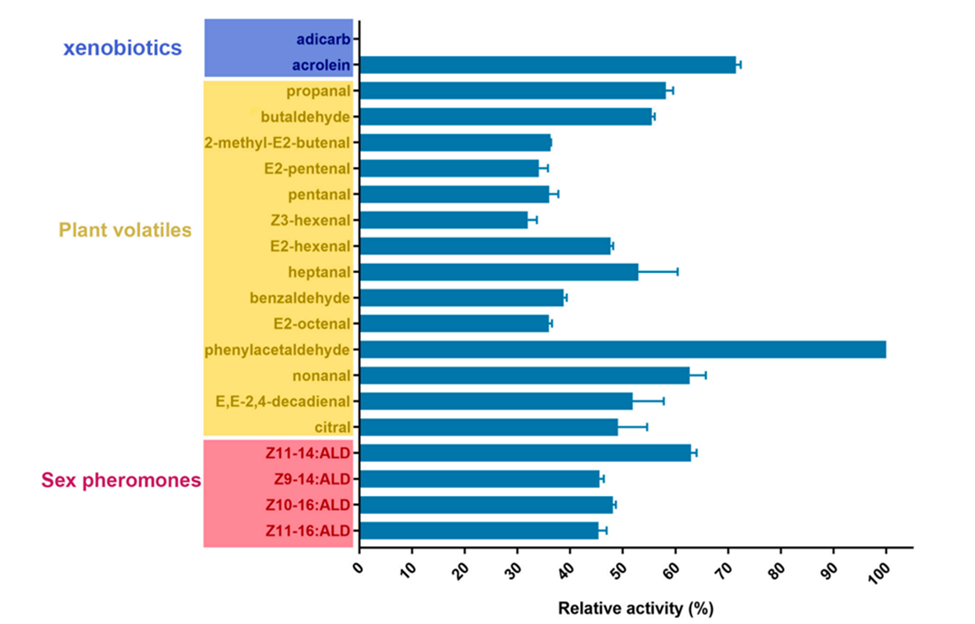
Fig. 11 PxylAOX3's oxidation activity on various aldehyde substrates. MTT reduction was used to determine the relative activity (at 570 nm). The phenylacetaldehyde oxidation activity was adjusted to 100% (means S.D.; n = 3) (Wang et al., 2021).
PxylAOX3, an aldehyde oxidase that is poorly understood, was successfully produced and purified using the Bac-to-Bac baculovirus expression system, a technique allowing for efficient production of recombinant baculovirus for insect cell expression testing. Additional enzymatic-related experiments suggest that PxylAOX3 may work to deactivate enzymes involved in the breakdown of aldehyde sex pheromone components in order to refresh the olfactory system. Moreover, PxylAOX3 is essential for oxidizing plant-derived harmful aldehydes and even aldehyde-containing insecticides to eliminate hazardous chemicals with attached DBM antennae (Wang et al., 2021). Additional research is required to fully comprehend the unique functions and coordination of PxylAOXs in insect antennae.
Life of antennae: symbiotic Streptomyces live inside beewolf antennae
The European beewolves (Philanthus triangulum) are solitary wasps that hunt bees to feed their larvae (Fig. 12). Beewolves dig underground holes in sandy soil to form their brooding nests in which beewolf larvae grow. The brooding nest thus encapsulates a developing larva and the larval cocoon. After hunting and paralyzing the honeybees, the mother wasp embalms the honeybee with a cephalic-gland secretion and places the honeybee in the brood cell. Then, the wasp lays its egg in the brood cell, which hatches into a larva that consumes the paralyzed bee (Takahiro & Takema, 2020).

Fig. 12 A European Beewolf (top) transporting a paralyzed honeybee (bottom) to its brood cell (European Beewolf).
Before the deposition of the egg, the mother wasp secretes a whitish, viscous liquid from its antennae and applies the substance to the ceiling of the brood cell (Kaltenpoth et al., 2005). This liquid is composed of various hydrolytic enzymes, signaling molecules, and antibiotic and antifungal compounds (Gullón & Mellado, 2018). This secretion serves both as a directional cue for the larva and as an antifungal protection for the larva's cocoon (Goettler et al., 2022). The liquid on the brood cell's ceiling indicates the brood cell's exit and the direction of the main burrow. Thus, the larva attaches its cocoon with its head facing the main burrow's direction, allowing the larva to exit its brood cell and emerge out of the soil (Goettler et al., 2022). In addition, the antennal secretion contains antibacterial and antifungal activity due to Streptomyces bacteria. Micrographs of cocoon cross-sections show Streptomyces bacteria integrated into the cocoon structure (Fig. 13) (Kaltenpoth et al., 2005). As the moist environment of the brood cell is conducive to fungus growth on the cocoon, a threat to the beewolf larva, this bacteria is a crucial defense tool. Larvae survival rates drastically decrease when the larva does not receive antennal secretion from its mother (Fig. 14) (Kaltenpoth et al., 2005). In exchange for its antifungal secretions, the Streptomyces bacteria presumably receives nutrients from its host.
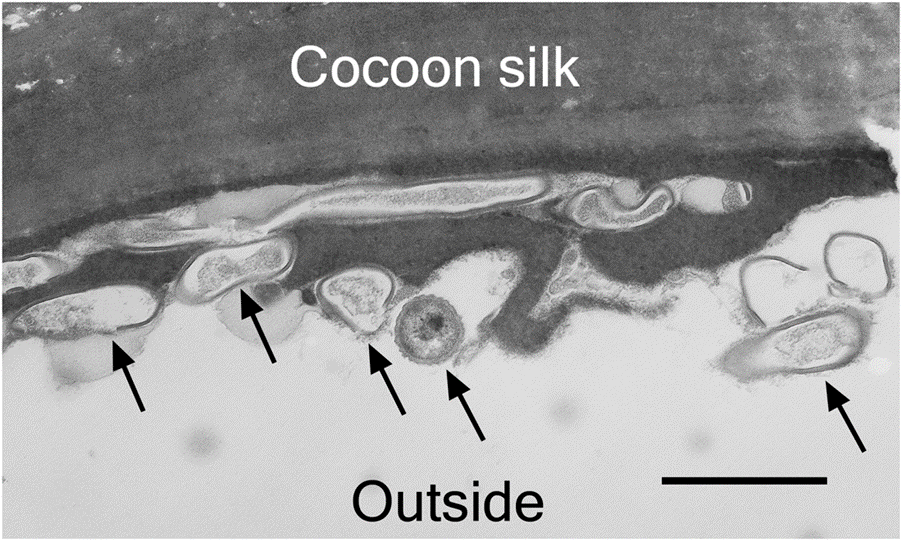
Fig. 13 Transmission electron micrograph of the cross-section of a beewolf larva cocoon (Kaltenpoth et al., 2005). Arrows indicate the Streptomyces bacteria. The scale bar represents 1 μm.
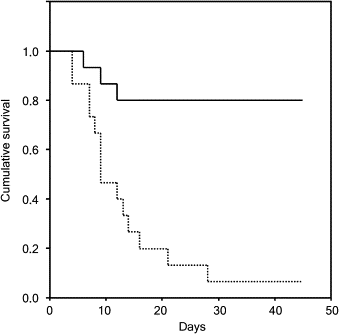
Fig. 14 Graph showing the survival of beewolf larvae: dotted line represents the larvae without antennal secretion, and full line represents the larvae with the antennal secretion (Kaltenpoth et al., 2005).
Morphological analysis of the Streptomyces-beewolf symbiosis
The Streptomyces bacteria live in symbiosis inside the European beewolf's antennal glands, and this mutualism has been found in 39 species of Philanthini (Goettler et al., 2022). Depending on the species, the Streptomyces bacteria inhabit five or six antennomeres (antennal segments). No significant differences exist between the morphology of the five or six antennal glands (Goettler et al., 2022). Figure 15 shows a micro-CT scan of the sixth antennomere (labeled A6) from a female Philanthus ventilabris. The outer cuticle (cu in Fig. 15) is thick and tapers into a thinner cuticle layer inside the antennal reservoir gland. The net-like structure of the cuticle likely allows nutrients from the hemolymph to be delivered to the bacteria. The bacteria (ba in Fig. 15) resides in the reservoir gland (re in Fig. 15), a cavity on the proximal, dorsal side of the antennomere that is half to three-quarters the size of the antennomere diameter. Surrounding the large reservoir gland are smaller class 3 dermal glands (c3 in Fig. 15), which cluster in spherical or cube-shaped acini (sacs of cells) that twist around the reservoir gland (Fig. 15). These class 3 gland cells are composed of a secretory cell connected to the lumen by a canal cell and connected to the reservoir gland by conduction canal cells (Fig. 16). Inside the reservoir, a fibrous structure presumably acts like a door that controls the release of the whitish secretion: when the wasp shakes its head, antennal hemolymph pressure increases, displacing the fibrous structure and allowing the flow of the substance out of its antennae.

Fig. 15 Lateral cross-section of a micro CT scan of the 6th antennomere of Philanthus ventilabris (Goettler et al., 2022). Notice the bacteria (ba) inside the large reservoir gland (re), and the class 3 dermal glands (c3) that surround the reservoir. The cuticle (cu) is thick on the outside and tapers on the inside. The filamentous structure (fl) acts as a plug to contain the secretion inside the reservoir. Other structures include the tracheole (tr), the antennal nerve (an) and the conducting canal (shown by the arrow).
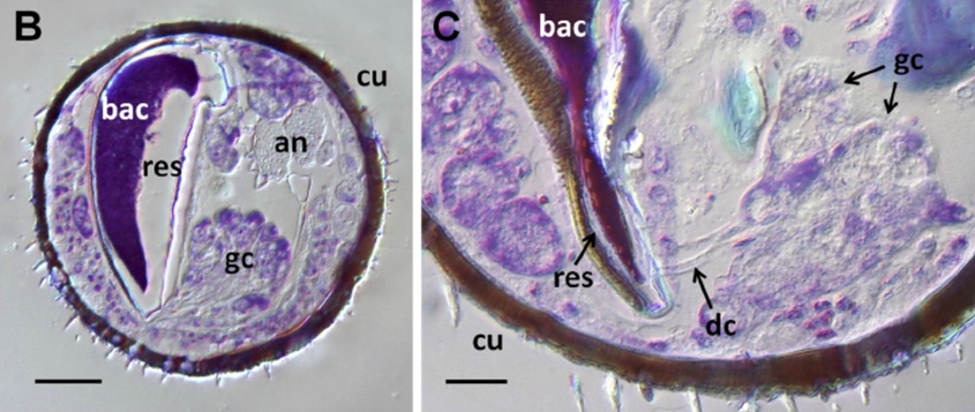
Fig. 16 A medial cross-section of the 6th antennomere of Philanthus triangulum (Adapted from Kaltenpoth et al., 2012). The bacteria (bac) sits inside the reservoir gland (res). The gland cells (gc) are connected to the reservoir gland by conducting canals (dc). The cuticle (cu) envelops the antenna and the antennal nerves (an) run through the antenna. The scale bar represents 25 μm.
Chemical secretions of Streptomyces
The symbiotic bacteria Streptomyces produces extracellular hydrolytic enzymes and secondary metabolites (Gullón & Mellado, 2018). These secondary metabolites have antibacterial properties and immunosuppressive functions. Experiments show that the EMB24 strain is antibacterial towards S. Aureus, E. Coli, and P. Aeruginosa, which are infectious bacteria (Goel et al., 2022). In other studies across 25 Philanthus species, 50 different antibiotics have been found inside their antennae (Goettler et al., 2022). Other examples of antibiotic substances produced by various species of streptomyces include antibiotic alkaloids such as undecylprodigiosin and antifungal compounds such as candicidin and antimycin (Santos-Beneit et al., 2022).
Regeneration of antennae
Antenna regeneration (neuronal)
In the case of insects, odorants reach the hairs of the antennae and diffuse to the olfactory receptor neurons, which, after sending signals to the mushroom body—a structure in the insect brain that deals with short-term memory and odor discrimination (Heisenberg, 1998)—invokes appropriate behavioral patterns (Gomez-Diaz et al., 2018). The neurons play a big part in this process, and regenerating an antenna necessarily involves regenerating the nerves. For most insects' central nervous systems can “reorganize their anatomical connectivity” based on external input, such as the breaking of antennae (Bicker & Stern, 2020). This reinnervation of broken antennae comprises a lot of signaling, which are, in large part, chemical reactions. One such signaling pathway is nitric oxide (NO)/cyclic guanosine monophosphate interaction (cGMP) (Fig. 17). Bicker and Stern (2020) demonstrated that when they crushed the axons in the antennae of Locusta migratoria, the regeneration rate increased when the initial conditions consisted of NO donors and decreased when inhibitors of the NO-cGMP pathway were present (Bicker & Stern, 2020). The NO stimulates the activity of the catalyst, soluble guanylate cyclase, which causes more GTP to be converted into cGMP in neurons (Fig. 18), leading to increased cell motility, which allows faster regeneration of the axon (Ghalayini, 2004). The NO does so by binding to the heme domain of the sGC at the N-terminal, which then activates the cyclase at the C-terminal, which in-turn acts as the catalyst for the conversion from GTP to cGMP (Montfort et al., 2017).
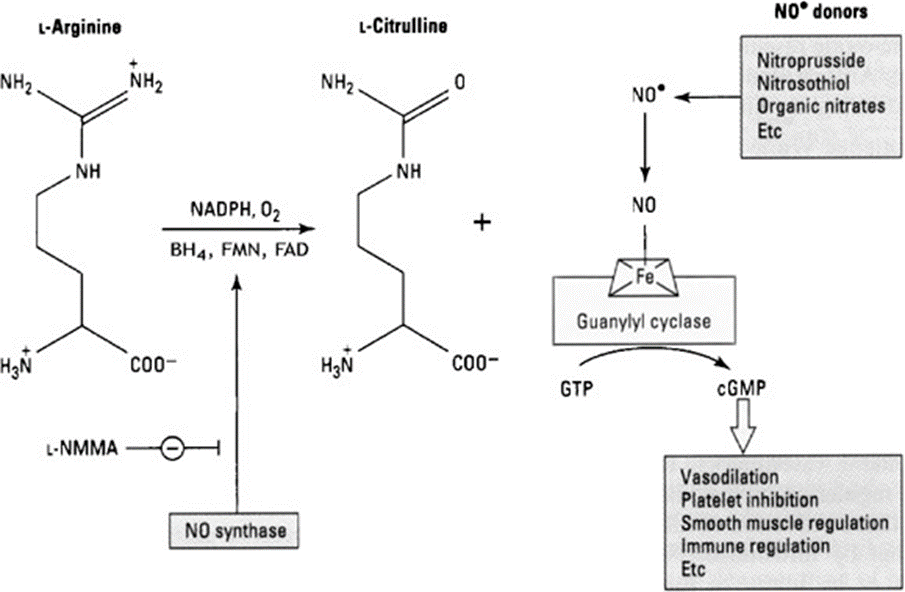
Fig. 17 The NO synthase converts L-Arginine to L-Citrulline and NO, which then catalyzes the GTP to cGMP reaction (Adapted from Ghalayini, 2004).
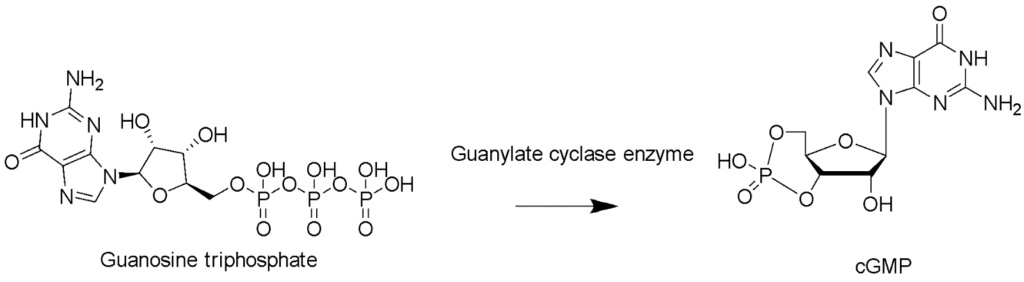
Fig. 18 The reaction from GTP to cGMP. Two of the phosphate groups are removed (Created with ChemDraw software).
Not all signaling is so straightforward. For example, in Hedgehog signaling, which is necessary for the regeneration of larval appendages, there are interactions between many transmembrane proteins like Smoothened, Costal 2, Suppressor of Fused, and Fused, and transcription factors like Cubitus. There is a lot of phosphorylation, which is the process of adding a phosphate group, and cleavages involved in the many interactions. In a study conducted by Villarreal et al. (2015), the silencing of just two things mentioned above, Costal 2 and a Hedgehog signaling receptor Patched, changed the antenna completely. The once smooth antenna became “curved and thick” or “flattened and spherical” (Fig. 19) (Villarreal et al., 2015).

Fig. 19 Antennal anatomy of normal larva compared to those of Costal 2 and Patched removed larva (Adapted from Villarreal et al., 2015): A, C) Normal larva, side view and top view, respectively: B) Patched-silenced larva has curved and thick antennae, compared to A; D) Costal 2-silenced larva has flattened and spherical antennae, compared to C.
Other important signaling pathways known for afferent-regeneration in the antenna include the Pten/Akt pathway, and the CamKII/NOS/PKG pathway (Fig. 20). Pten is phosphatase and tensin homolog, a gene that suppresses tumors (zu Reckendorf et al., 2022) and makes a phosphatase—an enzyme that catalyzes dephosphorylation—suppresses Akt, which is protein kinase B. When there is a lot of Akt, there is greater dendrite and axon regeneration (Song et al., 2012). Interestingly, in the experiment performed by Song et al. (2012), the CamKII/NOS/PKG pathway seems to have opposite results to the earlier experiment done by Bicker and Stern. CamKII regulates the calcium signaling pathway. Calmodulin is a protein that senses calcium levels and relays that information to other proteins (Dutta & Goodsell, 2003). The NO synthase is dependent on Ca2+ and calmodulin, and in the experiment, it inhibited axonal regeneration. The PKG was also an inhibitor of regeneration: it is activated by cGMP, which is catalyzed by NO (Song et al., 2019).
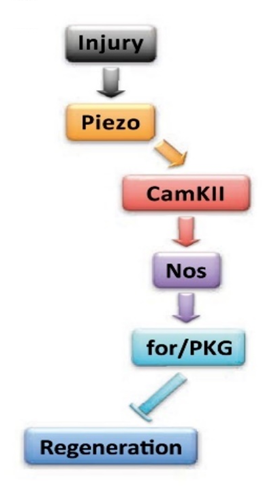
Fig. 20 The CamKII/NOS/PKG pathway (Adapted from Song et al., 2019).
In addition to signaling through molecules like nitric oxide, some hormones help with axon regeneration (Bicker & Stern, 2020). One hormone that does so is 20-hydroxyecdysone (Fig. 21), which controls molting, shedding the outer layer, and metamorphosis, changing form from an immature insect to an adult insect (Nakagawa & Sonobe, 2016). Its chemical formula is C27H44O7 and its IUPAC name is (2S,3R,5R,9R,10R,13R,14S,17S)-2,3,14-trihydroxy-10,13-dimethyl-17-[(2R,3R)-2,3,6-trihydroxy-6-methylheptan-2-yl]-2,3,4,5,9,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-6-one. The fusion between its bottom rings is cis, while the fusion in the other two rings is trans. This hormone has a complicated synthesis process, and has an effect more closely related to mushroom bodies, so its signaling reactions will not be further explained, apart from the fact that it enhances neurite outgrowth (Kraft et al., 1998). The main purpose of this deeper exploration of 20E was to show the complex chemical features involved in signaling molecules and make clear that it is not a simple process that one might suppose from only looking at the starting molecules and final products.
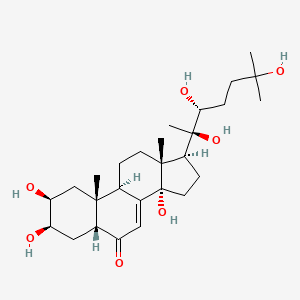
Fig. 21 The chemical structure of 20E (from the PubChem database).
There are also other factors, such as age, that affect the rate of antennal reinnervation. For example, in the example of Locusta migratoria, the antennal nerve regeneration in the fifth instar (Fig. 22) is faster than in adults. This could be because the insect is still growing, so ORN regeneration, synapse connection, and integration of new afferents into existing systems is easier (Bicker & Stern, 2020).
Fig. 22 The fifth instar is the stage of a locust's life before maturing into an adult. There are slight structural differences between the wings, but the main difference is the size (Adapted from Australian Government Department of Agriculture, Fisheries and Forestry).
Antenna regeneration (non-neuronal)
Antenna regeneration does not only involve innervation. For example, gene expression, regulated through transcription factors, can affect regeneration. Transcription factors regulate gene expression by binding to enhancers and silencers to increase the amount of a gene transcribed or decrease it, respectively. It decides where to bind and allows transcription based on the chemical signals it receives. Unfortunately, each transcription factor is different, so one must study each transcription factor's “small molecule binding sites, regulatory mechanisms, and interaction partners” to fully understand its function (Wiedemann et al., 2018). Lee et al. (2013) investigated four transcription factors related to the normal patterning of the antenna of Tribolium castaneum, a flour beetle, and the silencing of one of them, “spineless”, caused a leg-like structure to be formed in place of sensing antennae (Fig. 23) .
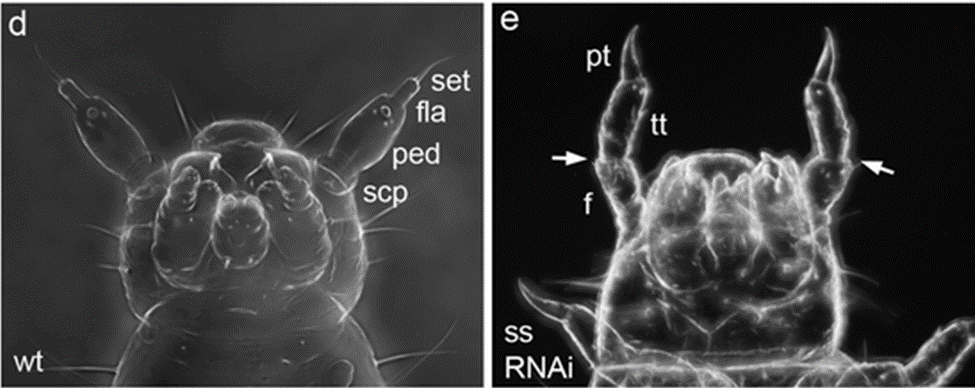
Fig. 23 Comparison of wild-type Tribolium larvae with Spineless-silenced larvae (Adapted from Shippy et al., 2009). A) Wild type Tribolium larva that has normal antennal structure. The antenna contains a basal scape (scp), a pedicel (ped), a flagellar segment (fla), and a terminal seta (set). B) The Spineless-silenced Tribolium that has leg-like structures instead of antennae. This structure was identified as leg-like by its pretarsus (pt), tibiotarsus (tt), and femur (f).
Regeneration of an antenna, no matter how sophisticated, is not foolproof, and insects can have antennae in wrong places if even one thing goes wrong. In Drosophila, some changes to the epidermal growth factor receptor (EGFR) and Notch signaling can sometimes cause antennae to grow on, or in place of, eyes. EGFR is a transmembrane receptor tyrosine kinase that controls cell fate and stops programmed cell death (Kumar & Moses, 2001). Notch is also a transmembrane receptor that sends signals to the nucleus, and helps determine the boundary of the insect eye, in addition to helping determine cell fate (Kumar & Moses, 2001). Figure 24 illustrates the possible complexity of these signaling systems, and how if one small component, like EGFR, or Notch, does not function as they are supposed to, the final eye specification will be faulty, and an antenna will be formed in the wrong place (Kumar, 2001).
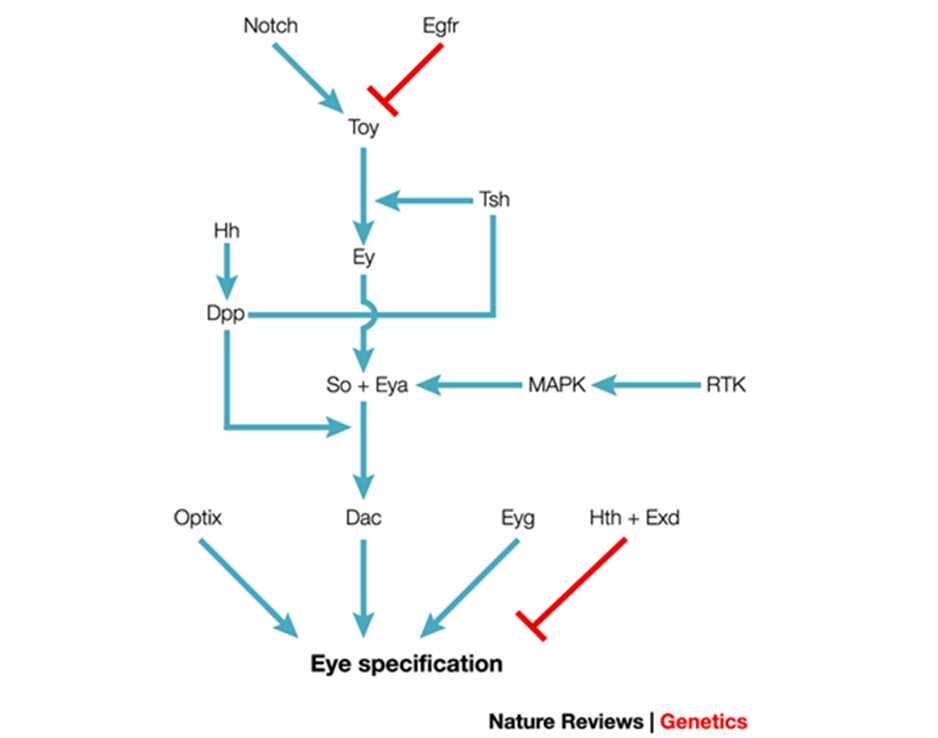
Fig. 24 The complex signaling involved in making an eye in an insect (Adapted from Kumar, 2001). If either the Notch or EGFR does not function as they are supposed to, an antenna can form where the eye is supposed to be.
Regeneration of whiskers
Though oftentimes very good at regenerating structures and organs, insects are not the only animals that will regenerate sensilla. Mice will also regenerate their whiskers. Since whiskers also serve sensing functions, nerve regeneration is essential to antenna regeneration. Interestingly, the Pten/Akt signaling pathway observed in insects is conserved and used by mice (zu Reckendorf et al., 2022). As expected, the deletion of Pten increased the production of Akt, which improved regeneration. Pten is also involved in another pathway though; it can dephosphorylate CREB, a transcription factor that regulates neuronal excitability and survival, and inactivate it. Thus, the inhibition of Pten allows more CREB to be phosphorylated and enhances “circuit formation, and regeneration after CNS injury” (zu Reckendorf et al., 2022). As Pten is a tumor-suppressing gene, its deletion can cause tumors, despite allowing faster axon-regeneration.
Similar to antenna-regeneration, there is more to whisker-regeneration than repairing nerves. Whisker follicles in rats are very good at regenerating due to Wnt signaling, involving Wnt proteins, and β-catenin, as well as the downstream molecules produced by the above, lymphoid enhancer-binding factor 1 and transcription factor 3 (Lin et al, 2015). This pathway is linked with the proliferation of stem cells. The expression of each of the above may be a good indicator of importance in whisker regeneration—as well as the importance of different molecules at different times—and the expressions are shown below. From the figure, it seems both Wnt10a, and Wnt10b proteins, along with their downstream product LEF1 are highly necessary for the entire process of regeneration, while Wnt5a, β-catenin, and TCF3 are important for regeneration on the inside of the whisker (Fig. 25).
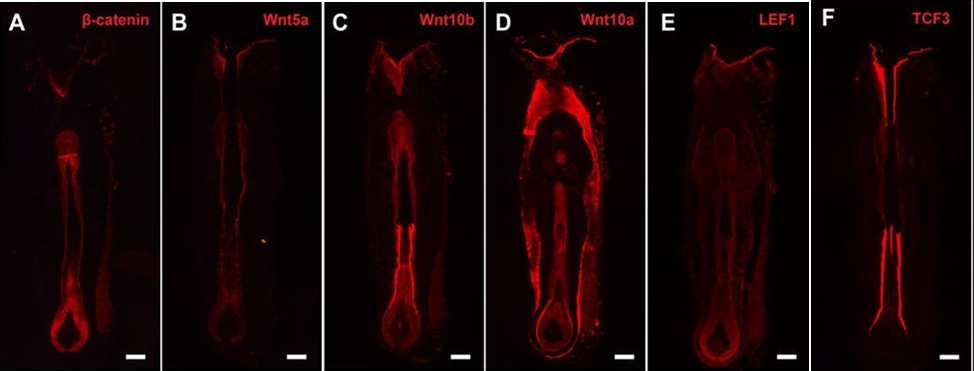
Fig. 25 Different Wnt factors are expressed in different locations of whiskers (Adapted from Lin et al., 2015). The amount of expression of a signaling factor in specific parts of the whisker can serve as an approximation for the importance of that signaling factor in whisker regeneration, especially to that part of the whisker. Additionally, the overall amount of expression of a factor in the whisker can also approximate the importance of that factor to whisker-regeneration in general. The scale bars are 100 µm.
Conclusion and applications
Though very small, antennae and whiskers hold great importance to many species. In cases of their regeneration, complex chemical interactions between many molecules and pathways are necessary. Often, the specific mechanisms are not yet discovered. As such, this paper was not able to fully explain regeneration in specific species nor delve into details of specific chemical reactions and molecule structures, but instead, offers a general review of how antennae and whiskers can be regenerated.
The study of regeneration, particularly of nerves, in “simpler” systems like those of insects, is very important to obtaining a greater understanding of central nervous systems in vertebrae. In fact, the aforementioned NO-cGMP pathway was studied in the context of the insects' ventral nerve cord, which is analogous to the spinal cord in vertebrates, so the signaling pathway gleaned from the study may be applicable to vertebrates (Bicker & Stern, 2020). Further, studies of nerve regeneration in mice may have even greater applications, as PTEN inhibition can induce CNS axon regeneration after an “optic nerve or spinal cord injury” (zu Reckendorf et al., 2022).
Conclusion
Whiskers and antennae, with their many functions and properties, play an essential role in the survival of insects and animals. The stages of their life cycles can be analyzed from a chemical perspective. First, barbels grow into sensory organs with sensitive tasting abilities through their complex distribution of taste buds that provide information for food searching. Their chemosensory sensitivity can explain barbel sensing in feeding behavior to amino acids and pH changes. Next, barbels' regenerative abilities are also key to the survival of animals such as zebrafish. Although imperfect, this process reforms the vesicular system, taste buds, and nerve endings. This allows zebrafish to benefit from the sensing abilities of their barbels following injuries.
Aldehyde oxidase in insect antennae deactivates enzymes involved in the breakdown of aldehyde sex pheromone, thereby refreshing the insect olfactory system. Aldehyde oxidase is also essential for oxidizing aldehydes derived from plants to eliminate hazardous chemicals for the survival of insects. Streptomyces bacteria live in the antennae of beewolves in a symbiotic relationship. The bacteria protect the beewolf by secreting defensive chemicals and antibacterial compounds while the beewolf offers them a home and nutrients. Insects can regenerate their antennae, and mice can regenerate their whiskers, which are important because both are primary sensory organs. In both cases, complex signaling processes involving small molecules, hormones, and transcription factors bring about this regeneration.
All these properties and functions of whiskers and antennas should be studied further to allow an integration of these concepts into technologies that could be beneficial for humans. For instance, understanding barbel, whisker, and antenna regeneration contributes to improvements in cellular and tissue engineering, and discovering complete chemosensory pathways for gustation allows the development of biomimetic chemosensors.
References
Alberts B, Johnson A, Lewis J, et al. (2002). Molecular Biology of the Cell. 4th edition. New York: Garland Science. https://www.ncbi.nlm.nih.gov/books/NBK26848/
Australian Government Department of Agriculture. About locusts. Fisheries and Forestry. Department of Agriculture, Fisheries and Forestry. https://www.agriculture.gov.au/biosecurity-trade/pests-diseases-weeds/locusts/about/about_locusts#lifecycle-of-a-locust
Bicker G., & Stern, M. (2020). Structural and Functional Plasticity in the Regenerating Olfactory System of the Migratory Locust. Frontiers in Physiology, 11. https://doi.org/10.3389/fphys.2020.608661
Caprio, J. (1975). High sensitivity of catfish taste receptors and amino acids. Comparative Biochemistry and Physiology — Part A: Physiology, 52(1), 247-251. doi:10.1016/S0300-9629(75)80160-5
Caprio, J., Shimohara, M., Marui, T., Harada, S., & Kiyohara, S. (2014). Marine teleost locates live prey through pH sensing. Science, 344(6188), 1154-1156. https://doi-org.proxy3.library.mcgill.ca/10.1126/science.1252697
Choo, Y.-M., Pelletier, J., Atungulu, E., & Leal, W. S. (2013). Identification and Characterization of an Antennae-Specific Aldehyde Oxidase from the Navel Orangeworm. PLoS ONE, 8(6), e67794. https://doi.org/10.1371/journal.pone.0067794
David, J. R., Daly, K., & Van Herrewege, J. (1984). Acetaldehyde utilization and toxicity in Drosophila adults lacking alcohol dehydrogenase or aldehyde oxidase. Biochemical Genetics, 22(11-12), 1015–1029. https://doi.org/10.1007/BF00499628
Diamondback moth. (n.d.). The Canola Council of Canada. https://www.canolacouncil.org/canola-encyclopedia/insects/diamondback-moth/
Diogo, R., & Chardon, M. (2000). The structures associated with catfish (Teleostei: siluriformes) Mandibular barbels: origin, anatomy, function, taxonomic distribution, nomenclature and synonym. Netherlands Journal of Zoology, 50(4), 455-478. https://www.researchgate.net/profile/Rui-Diogo-2/publication/233698305_The_Structures_Associated_With_Catfish_Teleostei_Siluriformes_Mandibular_Barbels_Origin_Anatomy_Function_Taxonomic_Distribution_Nomenclature_and_Synonymy/links/5c77aa7292851c695046e2db/The-Structures-Associated-With-Catfish-Teleostei-Siluriformes-Mandibular-Barbels-Origin-Anatomy-Function-Taxonomic-Distribution-Nomenclature-and-Synonymy.pdf?_sg%5B0%5D=started_experiment_milestone&origin=journalDetail
Dutta, S., & Goodsell, D. (2003, August). Molecule of the Month: Calmodulin. PDB-101. https://pdb101.rcsb.org/motm/44
Eickhoff, R., Lorbeer, R. A., Scheiblich, H., Heisterkamp, A., Meyer, H., Stern, M., & Bicker, G. (2012). Scanning Laser Optical Tomography Resolves Structural Plasticity during Regeneration in an Insect Brain. PLOS ONE. https://doi.org/10.1371/journal.pone.0041236
European beewolf. Wikiwand. https://www.wikiwand.com/en/European_beewolf
Gao, S., Liu, S., Yao, J., Zhou, T., Li, N., Li, Q., Dunham, R., & Liu, Z. (2017). Taste receptor and gustatory associated G proteins in channel catfish, ictalurus punctatus. Comparative Biochemistry and Physiology – Part D : Genomics and Proteomics, 21, 1-9. Doi: 10.1016/j.cbd.2016.10.002
Ghalayini, I. F. (2004). Nitric oxide–cyclic GMP pathway with some emphasis on cavernosal contractility. International Journal of Impotence Research, 16, 459-469. https://doi.org/10.1038/sj.ijir.3901256
Goel, N., Singh, R., Sood, S., & Khare, S. K. (2022). Investigation of streptomyces sp. strain EMB24 secondary metabolite profile has unraveled its extraordinary antibacterial potency against drug-resistant bacteria. Marine Biotechnology, doi:10.1007/s10126-022-10168-2
Goettler, W., Kaltenpoth, M., McDonald, S., & Strohm, E. (2022). Comparative Morphology of the Symbiont Cultivation Glands in the Antennae of Female Digger Wasps of the Genus Philanthus (Hymenoptera: Crabronidae). Frontiers in physiology, 13, 815494. https://doi.org/10.3389/fphys.2022.815494
Goldenberg, D., McLaughlin, C., Koduru, S. V., & Ravnic, D. J. (2021). Regenerative Engineering: Current Applications and Future Perspectives. Frontiers in Surgery, vol. 8, 731031. https://doi.org/10.3389/fsurg.2021.731031
Gomez-Diaz, C., Martin, F., Garcia-Fernandez, J. M., & Alcorta E. (2018). The Two Main Olfactory Receptor Families in Drosophila, ORs and IRs: A Comparative Approach. Frontiers in Cellular Neuroscience, 12. https://doi.org/10.3389/fncel.2018.00253
Gullón, S., & Mellado, R. P. (2018). The Cellular Mechanisms that Ensure an Efficient Secretion in Streptomyces. Antibiotics 7(2), 33. https://doi.org/10.3390/antibiotics7020033
Heisenberg, M. (1998). What Do the Mushroom Bodies Do for the Insect Brain? An Introduction. Learning & Memory, 5(1), 1–10. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC311238/
He, P., Li, Z.-Q., Liu, C.-C., Liu, S.-J., & Dong, S.-L. (2014). Two esterases from the genus Spodoptera degrade sex pheromones and plant volatiles. Genome, 57(4), 201–208. https://doi.org/10.1139/gen-2014-0041
He, P., Zhang, J., Li, Z.-Q., Zhang, Y.-N., Yang, K., Dong, S.-L., & He, P. (2014). FUNCTIONAL CHARACTERIZATION OF AN ANTENNAL ESTERASE FROM THE NOCTUID MOTH,Spodoptera exigua. Archives of Insect Biochemistry and Physiology, 86(2), 85–99. https://doi.org/10.1002/arch.21164
He, P., Zhang, Y.-F., Hong, D.-Y., Wang, J., Wang, X.-L., Zuo, L.-H., Tang, X.-F., Xu, W.-M., & He, M. (2017). A reference gene set for sex pheromone biosynthesis and degradation genes from the diamondback moth, Plutella xylostella, based on genome and transcriptome digital gene expression analyses. BMC Genomics, 18(1), 219. https://doi.org/10.1186/s12864-017-3592-y
He, P., Mang, D.-Z., Wang, H., Wang, M.-M., Ma, Y.-F., Wang, J., Chen, G.-L., Zhang, F., & He, M. (2020a). Molecular characterization and functional analysis of a novel candidate of cuticle carboxylesterase in Spodoptera exigua degradating sex pheromones and plant volatile esters. Pesticide Biochemistry and Physiology, 163, 227–234. https://doi.org/10.1016/j.pestbp.2019.11.022
Ikenaga, T., & Kiyohara, S. (2018). Chemosensory systems in the sea catfish, Plotosus japonicus. In: Hirata, H., Lida, A. (eds) Zebrafish, Medaka and Other Small Fishes (pp.295-315). Springer, Singapore. https://doi.org/10.1007/978-981-13-1879-5_16
Ishida, Y., & Leal, W. S. (2005). From The Cover: Rapid inactivation of a moth pheromone. Proceedings of the National Academy of Sciences, 102(39), 14075–14079. https://doi.org/10.1073/pnas.0505340102
Kaltenpoth, M., Göttler, W., Herzner, G., Strohm, E. (2005). Symbiotic bacteria protect wasp larvae from fungal infestation. Current Biology, 15 (5), pp. 475-479. doi: 10.1016/j.cub.2004.12.084
Kaltenpoth, M., Yildirim, E., Gürbüz, M. F., Herzner, G., Strohm, E. (2012). Refining the Roots of the Beewolf-Streptomyces Symbiosis: Antennal Symbionts in the Rare Genus Philanthinus (Hymenoptera, Crabronidae). Applied and Environmental Microbiology, 78. 822-827. 10.1128/AEM.06809-11.
Kraft, R., Levine, R. B., & Restifo, L. L. (1998). The Steroid Hormone 20-Hydroxyecdysone Enhances Neurite Growth of Drosophila Mushroom Body Neurons Isolated during Metamorphosis. Journal of Neuroscience, 18(21), 8886-8899. https://doi.org/10.1523/JNEUROSCI.18-21-08886.1998
Kruger, J. M., & Cagan, R. H. (1976). Biochemical studies of taste sensation. Biding of L [3H] alanine to a sedimentable fraction from catfish barbel. Journal of Biological Chemistry, 251(1), 88-97. https://www-scopus-com.proxy3.library.mcgill.ca/record/display.uri?eid=2-s2.0-0017302562&origin=resultslist&sort=cp-f&src=s&st1=Catfish+taste+buds+barbel&nlo=&nlr=&nls=&sid=94fff7bd928336ccc3dc983b03a56877&sot=b&sdt=cl&cluster=scosubjabbr%2c%22BIOC%22%2ct%2c%22CHEM%22%2ct&sl=40&s=TITLE-ABS-KEY%28Catfish+taste+buds+barbel%29&relpos=3&citeCnt=57&searchTerm=
Kumar, K. P. (2001). Signalling pathways in Drosophila and vertebrate retinal development. Nature Reviews Genetics, 2, 846–857. https://doi.org/10.1038/35098564
Kumar, J. P., & Moses, K. (2001). EGF Receptor and Notch Signaling Act Upstream of Eyeless/Pax6 to Control Eye Specification. Cell, 104(5), 687-697. https://doi.org/10.1016/S0092-8674(01)00265-3
Leal, W. S. (2013). Odorant Reception in Insects: Roles of Receptors, Binding Proteins, and Degrading Enzymes. Annual Review of Entomology, 58(1), 373–391. https://doi.org/10.1146/annurev-ento-120811-153635
LeClair, EE., Topczewski, J. (2010). Development and Regeneration of the Zebrafish Maxillary Barbel: A Novel Study System for Vertebrate Tissue Growth and Repair. PLOS ONE 5(1): e8737. https://doi.org/10.1371/journal.pone.0008737
Lee, A. K., Sze, C. C., Kim, E. R., & Suzuki, Y. (2013). Developmental coupling of larval and adult stages in a complex life cycle: insights from limb regeneration in the flour beetle, Tribolium castaneum. EvoDevo, 4. https://doi.org/10.1186/2041-9139-4-20
Levanti, M., Randazzo, B., Viña E., Montalbano, G., Garcia-Suarez, O., Germanà, A., Vega, J.A., Abbate, F. (2016). Acid-sensing ion channels and transient-receptor potential ion channels in zebrafish taste buds, Annals of Anatomy – Anatomischer Anzeiger, Vol 207. https://doi.org/10.1016/j.aanat.2016.06.006.
Lin, C., Yuan, Y., Chen, X., Li, H., Cai, B., Liu, Y., Zhang, H., Li, Y., & Huang, K. (2015). Expression of Wnt/β-catenin signaling, stem-cell markers and proliferating cell markers in rat whisker hair follicles. Journal of Molecular Histology, 46, 233-240. https://doi.org/10.1007/s10735-015-9616-5
Marelja, Z., Dambowsky, M., Bolis, M., Georgiou, M. L., Garattini, E., Missirlis, F., & Leimkühler, S. (2014). The four aldehyde oxidases of Drosophila melanogaster have different gene expression patterns and enzyme substrate specificities. The Journal of Experimental Biology, 217(12), 2201–2211. https://doi.org/10.1242/jeb.102129
Montfort, W. R., Wales, J. A., & Weichsel, A. (2017). Structure and Activation of Soluble Guanylyl Cyclase, the Nitric Oxide Sensor. Antioxidants & Redox Signaling, 26(3), 107-121. https://10.1089/ars.2016.6693
Nakagawa, Y., & Sonobe, H. (2016). Subchapter 98A – 20-Hydroxyecdysone. In Y. Takei, H. Ando, & K. Tsutsui (Eds.), Handbook of Hormones: Comparative Endocrinology for Basic and Clinical Research (pp. 560-563). Academic Press. https://doi.org/10.1016/B978-0-12-801028-0.00238-5
Nakamura, T., Matsuyama, N., Kirino, M., Kasai, M., Kiyohara, S., & Ikenaga, T. (2017). Distribution, Innervation, and Cellular Organization of Taste Buds in Sea Catfish, Plotosus japonicus. Brain, behaviour and evolution, 89(3), 209-218. https://doi.org/10.1159/000471758
Ovalle, W. K., & Shinn, S. L. (1977). Surface morphology of taste buds in catfish barbels. Cell and tissue research, 178(3), 375-384. https://doi.org/10.1007/bf00218701
Robertson, H. M. (2019). Molecular Evolution of the Major Arthropod Chemoreceptor Gene Families. Annual Review of Entomology, 64(1), 227–242. https://doi.org/10.1146/annurev-ento-020117-043322
Royer, S. M., & Kinnamon, J. C. (1996). Comparison of high-pressure freezing/freeze substitution and chemical fixation of catfish barbel taste buds. Microscopy, Research & Technology, 35(5), 385-412. https://doi.org/10.1002/(SICI)1097-0029(19961201)35:5%3C385::AID-JEMT3%3E3.0.CO;2-K
Rybczynski, R., Reagan, J., & Lerner, M. (1989). A pheromone-degrading aldehyde oxidase in the antennae of the moth Manduca sexta. The Journal of Neuroscience, 9(4), 1341–1353. https://doi.org/10.1523/jneurosci.09-04-01341.1989
Santos-Beneit, F., Ceniceros, A., Nikolaou, A., Salas, J. A., Gutierrez-Merino, J. (2022). Identification of antimicrobial compounds in two streptomyces sp. strains isolated from beehives. Frontiers in Microbiology, 13. doi:10.3389/fmicb.2022.742168
Shippy, T. D., Yeager, S. J., & Denell, R. E. (2009). The Tribolium spineless ortholog specifies both larval and adult antennal identity. Development Genes and Evolution, 219(1), 45–51. https://doi.org/10.1007/s00427-008-0261-9
Song, Y., Ori-McKenney, K. M., Zheng, Y., Han, C., Jan, L. Y., & Jan, Y. N. (2012). Regeneration of Drosophila sensory neuron axons and dendrites is regulated by the Akt pathway involving Pten and microRNA bantam. Genes & Development, 26(14), 1612-1625. https://10.1101/gad.193243.112
Song, Y., Li, D., Farrelly, D., Miles, L., Li, F., Kim, S. E., Lo, T. Y., Wang, F., Li, T., Thompson-Peer, K. L., Gong, J., Murthy, S. E., Coste, B., Yakubovich, N. Patapoutian, A., Xiang, Y., Rompolas, P., Jan, L. Y., & Jan. Y. N. (2019). The Mechanosensitive Ion Channel Piezo Inhibits Axon Regeneration. Neuron, 102(2), 373-389. https://doi.org/10.1016/j.neuron.2019.01.050
Takahiro H. & Takema F. (2020). Relevance of microbial symbiosis to insect behavior. Current Opinion in Insect Science Vol 39. https://doi.org/10.1016/j.cois.2020.03.004.
Tavares, D. da S., Salgado, V. R., Miranda, J. C., Mesquita, P. R. R., Rodrigues, F. de M., Barral-Netto, M., de Andrade, J. B., & Barral, A. (2018). Attraction of phlebotomine sandflies to volatiles from skin odors of individuals residing in an endemic area of tegumentary leishmaniasis. PLOS ONE, 13(9), e0203989. https://doi.org/10.1371/journal.pone.0203989
Tissue Engineering and Regenerative Medicine. National Institute of Biomedical Imaging and Bioengineering. https://www.nibib.nih.gov/science-education/science-topics/tissue-engineering-and-regenerative-medicine
Villarreal, C. M., Darakananda, K., Wang, V. R., Jayaprakash, P. M., & Suzuki, Y. (2015). Hedgehog signaling regulates imaginal cell differentiation in a basally branching holometabolous insect. Developmental Biology, 404(2), 125-135. https://doi.org/10.1016/j.ydbio.2015.05.020
Wang, A., Zhang, K.-X., Gao, Y., Weng, A., Wang, L., Zhang, Y., Zhang, Z., She, D., Ning, J., & Mei, X. (2018). Synthesis and bioactivity studies of sex pheromone analogs of the diamond back moth, Plutella xylostella. Pest Management Science, 75(4), 1045–1055. https://doi.org/10.1002/ps.5214
Wang, M.-M., He, M., Wang, H., Ma, Y.-F., Dewer, Y., Zhang, F., & He, P. (2021). A candidate aldehyde oxidase in the antennae of the diamondback moth, Plutella xylostella (L.), is potentially involved in the degradation of pheromones, plant-derived volatiles and the detoxification of xenobiotics. Pesticide Biochemistry and Physiology, 171, 104726. https://doi.org/10.1016/j.pestbp.2020.104726
Wiedemann, B., Weisner, J., & Rauh, D. (2018). Chemical modulation of transcription factors. MedChemComm, 9(8), 1249-1272. https://10.1039/c8md00273h
Zu Reckendorf, S. M., Moser, D., Blechschmidt, A., Joga, V. N., Sinske, D., Hegler, J., Deininger, S., Catanese, A., Vettorazzi, S., Antoniadis, G., Boeckers, T., & Knöll, B. (2022). Motoneuron-Specific PTEN Deletion in Mice Induces Neuronal Hypertrophy and Also Regeneration after Facial Nerve Injury. The Journal of Neuroscience, 42(12), 2474-2491. https://10.1523/JNEUROSCI.1305-21.2022