Mathematical Marvels of Foraminifera
Emma Bussières, Carolyn Denton, Reno Thompson, Xiao (Rachel) Jiang
Abstract
Foraminifera are a family of marine unicellular eukaryotes whose fossils can be found throughout the world, from the deepest crevices of the ocean to the highest peaks of the Egyptian Pyramids. In this paper, we explore the mathematical models describing the optimization of common adaptations in foraminifera. Beginning with the distribution and density of pores in their calcified tests, we see that foraminifera have found a way to enhance gas exchange with their environment without sacrificing the integrity of their protective shells, namely an increase in pore size and a decrease in pore density. Next, the use of symbiosis as an adaptation to nutrient-deficient environments is explored using a new mathematical model, which accurately predicts the global distribution of symbiont-bearing and symbiont-barren planktonic foraminifera. In a similar manner, the intensity at which calcification occurs in planktonic foraminifera over their life cycle can also be modelled, showing a correlation with environmental pH. Finally, morphogenesis in foraminifera involves discrete, stepwise additions of elements to form an existing morphology. It is through the translation of a foraminifer’s genetic code into specific chamber spaces and shell patterns that mathematical models of shell growth are created. Therein are born important tools in the advancement of our understanding of foraminiferal development, allowing insights into the genetic and environmental factors influencing their morphology.
Introduction
Foraminifera are an abundant unicellular microeukaryote found both in ocean sediment (benthic foraminifera) and floating with the currents (planktonic foraminifera). They are characterized by their test (a calcareous shell, either made up of calcite or aragonite) and their pseudopodia (an extension of their cytoplasm that interacts with the environment, performing functions such as prey capture, test construction, and motility along sediments). Some foraminifera have sharp protrusions on their tests, called spines, which can act as supports for their pseudopodia, as well to increase fluid drag and thus decrease settling velocity. Spines have been shown to increase the prey-encounter rate as well as deter predators from consuming foraminifera – but they also increase the weight of the foraminifera and require extensive construction, which has a metabolic cost.
The presence of pores in the foraminifera tests is a constitutive adaptation that allows for them to exchange nutrients and gases with their environment, but the pores themselves can introduce weakness in the test. Pore size, density, and distribution patterns are determined not just species-specific, but also reflect the environmental conditions and foraminifera’s size, among other things.
Both benthic and planktonic foraminifera have developed symbiotic relationships, which have allowed them to inhabit environmental niches that would normally be inaccessible. For example, symbiosis with photosynthetic organisms enables foraminifera to inhabit both oxygen-depleted and nutrient-poor environments, however this does mean that these foraminifera are forced to find ways to remain within the photic zone – the top 100m of the ocean. Various morphological adaptations enable foraminifera to do so, including the development of spines, and so foraminifera fossils are commonly used to infer past climate conditions – an academic field referred to as paleontology. The explicit relationship between environmental conditions, symbiosis, and spines remains elusive, but the development of mathematical models to describe current foraminifera distribution is an active area of research.
Chambers (refer to Fig 1.cd) are hollow spaces encapsulating the protoplasm and encased by a robust mineral wall (Lipps, 1993). The most basic shells consist of a solitary chamber with a singular opening, which is known as the aperture (Fig. 1cd). On the other hand, more intricate foraminiferal varieties construct their chambers by partitioning them into smaller chamberlets. In those multilocular shells, aged apertures, called foramina (see Fig. 1d), facilitate communication among all chambers formed during the shell’s growth process.

Fig. 1. Views of different foraminiferal morphotypes. Scale bar = 50 um. (a) low helicospiral form; (b) high helicospiral form. (c,d) show external (c) and internal (d) views of the multilocular uniserial species Pseudonosadaria humilis.
The aperture—an opening in the shell—is introduced as fundamental morphologic feature of foraminifera by Tyszka et al., and its coordinates are based on the optimization of the distance between successive openings of chambers (refer to Fig. 1, c-d). In other words, they are based on the minimization of the local communication path (LCP).
Pore function
Marine foraminifera, unicellular eukaryotes living in both the benthic and pelagic realms, play a vital role in marine ecosystems. These organisms have been crucial in paleoceanography, providing insights into past marine conditions through their fossil record (Berggren et al., 1965). In recent years, scientists have become increasingly interested in their unique adaptation strategies, particularly the construction of pore patterns on their test (shell). Pore patterns are not simply random features; they play a critical role in the survival of foraminifera under varying environmental conditions. These pores serve multiple functions, including buoyancy control, gamete expulsion, osmoregulation, and feeding, in addition to facilitating gas exchanges (Bernhard et al., 2010). As a result of climate change and the consequent decrease in ocean water oxygenation, it has never been more crucial to understand these microscopic structures (Diaz & Rosenberg, 2008).
In recent studies, significant strides have been made in understanding foraminifera, particularly through the development of a physical model that correlates their pore patterns with metabolic needs and mechanical constraints, a crucial insight as foraminifera adapt to increasingly low-oxygen environments due to climate change. Benthic foraminifera, for instance, have developed adaptations like nitrate respiration and bacterial symbiosis to cope with low oxygen conditions (Glock et al., 2011). Central to this model is the last formed chamber of the foraminifera, notable for its maximum surface area and minimal thickness, playing a vital role in gas exchange. This focus on the last chamber is critical as it reflects the most recent environmental conditions and foraminiferal responses (Leutenegger & Hansen, 1979). The following model encapsulates key relationships through mathematical equations:
![]()
![]()
![]()
The equations 1-3 relate the pore radius (r) to the pore density (N), porosity (Φ), and mechanical stress at test failure (σ), respectively. These equations, underpinned by principles of fluid dynamics and diffusion, are essential in decoding the adaptive strategies of foraminifera in varying environmental conditions (Richirt et al., 2019). These equations are pivotal in illustrating the foraminifera’s capability to optimize test porosity while maintaining the test’s structural integrity.
The model’s robustness was further validated through a comprehensive empirical data set. The data set’s extensive measurements provided a nuanced view of the morphological variations among foraminiferal species. This data set included measurements of pore density, mean pore radius, and overall test porosity from various specimens. The correlation between these measurements and environmental factors like oxygen concentration affirmed the model’s ecological relevance. The findings were in remarkable agreement with the theoretical predictions, lending credence to the model’s applicability in natural settings. One of the most fascinating aspects revealed by this model is the foraminifera’s balancing act between enhancing gas exchanges and maintaining test solidity. This intricate balance reflects a sophisticated evolutionary response to environmental stresses, ensuring survival in diverse marine habitats. An increase in pore size coupled with a decrease in pore density was observed as a strategy to achieve higher porosity. This observation highlights the complexity of foraminiferal adaptation strategies, blending physiological needs with physical constraints. This pattern aligns with the model’s predictions and highlights a counterintuitive approach: achieving higher porosity not just by increasing the number of pores but by strategically modifying their size and distribution (Richirt et al., 2019a).
Moreover, this research delved into the ecological nuances of different Ammonia phylotypes. These phylotypes, each responding differently to environmental conditions, indicate an intricate web of ecological interactions and niche specializations (Holzmann & Pawlowski, 1997). The study also revealed varied responses to environmental conditions among different phylotypes of the genus Ammonia. This diversity suggests a spectrum of adaptive strategies, shaped by both genetic and environmental factors, to optimize gas exchange in varying dissolved gas concentrations (Jones, 2014). This variability suggests that different phylotypes are adapted to distinct ecological niches, likely influenced by local oxygen concentrations. The model thereby underscores the potential of pore patterns in foraminifera as proxies for past environmental conditions, particularly oxygen and nitrate concentrations. This proxy potential opens new avenues for reconstructing historical marine environments (Katz et al., 2010).
Modeling the global distribution of planktonic foraminifera
Photosynthesizing symbionts provide energy to their foraminifera hosts in nutrient-deficient environments, and foraminifera can optimize the efficiency of their symbiotic relationship by accommodating their symbiont’s metabolic and reproductive needs (Ying et al., 2023). The more that foraminifera invest in the health of their symbionts, the more dependent they become on their symbionts, and thus a spectrum of symbiont-dependency develops. Symbiont-facultative foraminifera are flexible in terms of their dependency on symbionts, while symbiont-obligate foraminifera cannot live without their symbionts. Based on global research observations, symbiont-bearing foraminifera tend to dominate in nutrient-poor environments, such as subtropical gyres[1], while symbiont-barren foraminifera have a high abundance in nutrient rich environments, such as polar regions. Figure 2 shows the global relative abundance of symbiont-barren and symbiont-bearing foraminifera, as modeled by ForamECOGENIE 2 and ForCenS, from 2023 and 2017, respectively.
[1] Subtropical gyres are nutrient poor in the context of planktonic foraminifera, who live suspended in the water; winds push surface waters into the center of gyres and then downwards, taking nutrients away from the sunlit zone (Doddridge et al., 2018). A new model by Doddridge et al. (2018) demonstrates how there is high nutrient concentration and phytoplankton productivity at the edge of the gyres, and lower productivity at the center of the gyres.

Fig. 2. Global Planktonic Foraminifera Distribution. The ForamEcoGENIE 2 model predictions are on the left and are compared to the ForCenS sediment core-top dataset. The ForEcoGENIE 2 model creates 4 groups of foraminifera based on the presence/absence of spines and symbionts. It also takes into account the dominance of symbiont-obligate relationships in spinose foraminifera and symbiont-facultative relationships in non-spinose foraminifera (as opposed to simply symbiont-bearing) (Ying et al., 2023).
Recently, Ying et al. (2023) published an updated model called ForamEcoGENIE2, which describes global planktonic foraminifera distribution based on environmental conditions as well as the absence/presence of both symbiosis and spines. Since their model was able to accurately describe the distribution of symbiont-bearing and symbiont-barren foraminifera, the relevant equations will be described in the following sub-section.
Model equations: symbiosis
The following equations were taken, unchanged[2], from Ying et al. (2018).
![]()
Equation 4 describes the composition of a given foraminifera. For example, QP and QFe describe the amount of phosphorus and iron that have been assimilated, while QChl describes the amount of chlorophyll that has been synthesized (for autotrophy). The variable Bib is the foraminifera’s nutrient biomass (ib = P, Fe, chlorophyll), and BC is its carbon biomass (in any form, e.g. calcite or argonite).
![]()
Equation 5 is used to find the rate of nutrient uptake/chlorophyll synthesis (Qibrate) based on the nutrient quota range (the maximum and minimum quotas: Qibmax,Qibmin) and the current nutrient quota (Qib). For example, according to this equation, when the phosphorous quota (QP) is low, there is a high rate of phosphorus assimilation (QPrate); as the phosphorous quota increases, the rate of phosphorus assimilation decreases. This is relevant for equation 7, which uses the maximum nutrient uptake rate (Vm) and the current nutrient uptake rate (Qibrate).
![]()
Equation 6 describes the volume of a symbiont (VS) as a function of the volume of the foraminifera host (Vh). The ratio of these two volumes is represented by 𝜓; the ForamECOGENIE 2 model assumes that 𝜓 = 1/20, based on photomicrograph observations that symbionts are 1/20th the size of their foraminifera hosts. Note that equation 7 has two variables that are affected by the foraminifera’s size: the maximum nutrient uptake rate (Vm), which is positively correlated to the foraminifera host size (Vh); and the nutrient affinity (![]() ), which is negatively correlated, because of a lower volume-size ratio.
), which is negatively correlated, because of a lower volume-size ratio.
![]()
Equation 7 describes the generic nutrient uptake (![]() ) of a planktonic foraminifer. The subscript “j” denotes the plankton type[3], and the subscript “ir” describes the nutrient (e.g. phosphorus, carbon…). This equation follows a Michaelis-Menten function. Nutrient uptake depends on the efficiency of symbiotic autotrophy[4] (λj,s), the current nutrient uptake rate (
) of a planktonic foraminifer. The subscript “j” denotes the plankton type[3], and the subscript “ir” describes the nutrient (e.g. phosphorus, carbon…). This equation follows a Michaelis-Menten function. Nutrient uptake depends on the efficiency of symbiotic autotrophy[4] (λj,s), the current nutrient uptake rate (![]() ), the temperature (γT), the maximal nutrient uptake rate (
), the temperature (γT), the maximal nutrient uptake rate (![]() ) for that specific nutrient (ir), the nutrient affinity (
) for that specific nutrient (ir), the nutrient affinity (![]() ), and the nutrient resources (
), and the nutrient resources (![]() ). This last parameter refers to the amount of resources available in the environment. As mentioned earlier, subtropical gyres tend to have less nutrients than subpolar regions due to downwelling (Doddrigde et al., 2018). This is why we see different distributions of symbiont-bearing and symbiont-barren types in Figure 1.
). This last parameter refers to the amount of resources available in the environment. As mentioned earlier, subtropical gyres tend to have less nutrients than subpolar regions due to downwelling (Doddrigde et al., 2018). This is why we see different distributions of symbiont-bearing and symbiont-barren types in Figure 1.
[2] Except for changing ![]() to
to ![]()
[3] In this context, “j” denotes that equation 7 is applicable to all types of planktonic foraminifera. If the subscript was “jpred”, then that would denote that equation 7 is only applicable to predatorial planktonic foraminifera
![]()
Equation 8 describes the maximum photosynthesis rate (![]() ) of a foraminifer. This is dependent on the foraminifera’s symbiont size (
) of a foraminifer. This is dependent on the foraminifera’s symbiont size (![]() ) as well as the mixotrophic cost (
) as well as the mixotrophic cost (![]() ). The parameters Pa, Pb, and Pc are dimensionless – their purpose is to improve the accuracy of this equation in describing
). The parameters Pa, Pb, and Pc are dimensionless – their purpose is to improve the accuracy of this equation in describing ![]() .
.
![]()
Equation 9 takes temperature (γj, T), light intensity (γj, I), and the nutrient availability (min[![]() ]) into account to determine the practical photosynthesis rate in a given environment. Like equation 7, this allows us to appreciate the different adaptation strategies seen in different environmental conditions. Comparing different environments, such as the colder yet productive subpolar regions to the warmer yet nutrient poor subtropical gyres, we see that the practical photosynthesis rate increases in subtropical gyres, and it becomes advantageous to perform symbiosis. Equations 10 and 11 describe how the light intensity and nutrient availability are calculated.
]) into account to determine the practical photosynthesis rate in a given environment. Like equation 7, this allows us to appreciate the different adaptation strategies seen in different environmental conditions. Comparing different environments, such as the colder yet productive subpolar regions to the warmer yet nutrient poor subtropical gyres, we see that the practical photosynthesis rate increases in subtropical gyres, and it becomes advantageous to perform symbiosis. Equations 10 and 11 describe how the light intensity and nutrient availability are calculated.
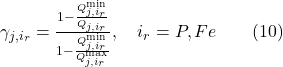
Equation 10 uses the minimum, maximum, and current quotas of a given nutrient (ir=Fe, P) to calculate the nutrient availability. Whichever nutrient is the least available is used in equation 9.
[4] Efficiency of symbiotic autotrophy (λj,s) is a component of the “cost of mixotrophy”. This is the idea that a mixotrophic strategy has decreased efficiency of both autotrophy and heterotrophy. Efficiency of foraminifera heterotrophy would be denoted λj,h
![]()
Equation 11 describes the limiting term of light intensity – this is meant to take into account the fact that two different foraminifera exposed to the same intensity of light will be able to use that light to different degrees. Foraminifera with a greater amount of chlorophyll (Qj,Chl) can more effectively transform light into photosynthesis products. Additionally, photosynthesis efficiency is temperature dependent (in terms of reaction rates), as well as iron dependent (since iron acts as an electron acceptor in the electron transport chain in chloroplasts). In this equation, α is the initial slope of the photosynthesis rate – light intensity curve, which is experimentally determined for a foraminifera type (e.g. spinose symbiont-obligate).
Model strengths and limitations
This model can accurately represent the dominance of symbiont-obligate groups in subtropical gyres and of symbiont-barren groups in subpolar regions (Ying et al., 2023). This gives credibility to the symbiosis-equations described, however, there are some limitations to the model. It assumes that symbiont-bearing foraminifera have constitutive mixotrophy, which is defined as the ability to fully synthesize, repair, and control photosynthetic machinery (Mitra et al., 2016). We know that foraminifera do not have this ability, and thus the model does not account for variability in climate sensitivities of the symbiont and host. This model also does not consider the effect of bleaching at high temperatures (Edgar et al., 2013), or the effect of high CO2 (Barker and Elderfield, 2002). Lastly, this model assumes that symbiosis and spines are independent traits, but there is evidence that symbionts are placed in spines during the day to optimize photosynthesis (LeKieffre et al., 2018).
The main take-away of this model should be an appreciation for the complexity of the environmental challenges that foraminifera face. The evolutionary trade-offs made by their ancestors were based on much more than the parameters discussed here, and this is reflected by their high species diversity. In modeling the current global distribution of foraminifera, researchers can understand how to improve their interpretation of the distribution of fossilized foraminifera samples. Despite its limitations, the ForamEcoGENIE 2 model shows great promise for the future of paleontology.
Mathematical modelling of calcification
One way that foraminifera is affected by its environment is through the process of calcification. Henehan et al. (2017) proposed a model in which the intensity of calcification—the thickness of calcified test material during the period of the organism’s growth—in planktonic foraminifera depends on body size and the pH of the environment.
Calcification: data collection
To observe the calcification effects in a population, Henehan et al. (2017) first collected Globigerinodes ruber of mixed morphotypes taken from the Gulf of Aqaba from a depth lesser than 20 meters. These foraminifera were cultured over their lifespan until gametogenesis with specified conditions such as temperature, light, feeding, and pH. Measurements were periodically taken for the size of the organism under a microscope as well as the pH using electrodes. The size was obtained using the magnification software Orbicule Inc., v2.0 which measured along the major and perpendicular axes of the organism—which can be used to quantify area when multiplied.
![]()
It is important to note that shell mass cannot be measured in pre-culture foraminifera, since the measurement would harm the organism. Therefore, to determine the shell mass of G. ruber pre-culture, the size-weight relationship shown in Equation 12 (Henehan et al., 2017) was devised using a dataset of 205 measurements from different studies of the same species collected under the same conditions. The fit of Equation 12 to the dataset is quantified by an R-squared value of 0.61.
![]()
The calcification intensity, or CI, is defined using the above Equation 13 (Henehan et al., 2017), where Δmass describes the difference in mass from the preculture (Equation 12) to the end of the culture and Δarea describes the difference in area, calculated as the product of major and minor axes—in the same way as Equation 12. The relationship between calcification intensity and size can be estimated using a logarithmic fit as shown below in Figure 3.

Fig. 3. The relationship between CI and size with the dashed logarithmic regression fit y = 503+119.5ln(x). CI is normalized to two chambers being added during the culture, which resulted in an R-squared value of 0.59 to the fit. Adapted from Henehan et al. (2017).
The model
To create a model for CI over its growth, Henehan et al. (2017) collected morphological measurements (using Image J) of 39 G. ruber specimens from a sediment core top sample. Using these foraminifera remains, detailed measurements could be made such as breaking down the test into chambers to measure wall thickness. These many empirical observations were used as constraints in the model: for example, the fraction increase in chamber major axis per chamber addition was determined to be 1.15 and the porosity was determined to be 4.2% (the percentage of the chamber wall that is taken up by pores) (Henehan et al., 2017). Using all these parameters, a MATLAB program was used to determine the addition of calcium carbonate to an ideal G. ruber over its life cycle. Each parameter was allowed to vary within a set range and then the program was run 1 million times—determining which factors specifically linked to changes in CI. Three models were created: Model 1 and Model 2 parametrizes wall thickness as a function of size—Model 1 predicts a variable slope of chamber diameter to wall thickness whereas Model 2 predicts a variable maximum primary wall thickness (the first calcified layer when a new chamber is formed)— and Model 3 parametrizes wall thickness as a function of chamber number.

In each of the models shown in Figure 4, CI increases with body size — which is the same result as the cultured data (Figure 1).
Fig. 4. Model predictions of the relationship between CI and size as blue lines overlayed with relationship between CI and size data from the cultured population as white dots. Graph (a) corresponds to Model 1, (b) corresponds to Model 2, and (c) corresponds to Model 3. Adapted from Henehan et al. (2017).
Predictions relating to the pH
![]()
After observing the models, Henehan et al. (2017) determined that calcification intensity CI depends on two covarying, or jointly-varying, coefficients (“a” and “b”) within an equation that models wall thickness throughout the foraminifera’s growth—shown in Equation 14. This equation, when graphed, is a regression of wall thickness and chamber diameter—as shown in Figure 3 below. Henehan et al. (2017), based on their models, proposed that the shape of the regression controls CI and hypothesized that a change in pH would cause the slope of the regression to also change. This is because Henehan et al. (2017) observed that CI is dependent on pH experimentally in their cultured G. ruber population. In Figure 5 below, it is predicted that lower pH makes the slope of the regression shallower whereas a higher pH results in a steeper regression slope.

Fig. 5. Regressions described by Equation 14 relating wall thickness to chamber diameter. The yellow curve models a higher pH and the blue curve models a lower pH compared to the reference green curve. Adapted from Henehan et al. (2017).
The model regressions proposed in Figure 5 outline a tendency for smaller foraminifera to be less affected by changes in pH compared to larger foraminifera which are more affected; this can be seen with the smaller gaps between regression curves on the left side of Figure 3 and the larger gaps on the right side. The tendency for larger foraminifera to be more affected by changes in pH is explained by the chemical properties of foraminifera’s calcification mechanism. To collect enough dissolved inorganic carbon (DIC) for calcification, foraminifera raise the pH of vacuolized seawater intracellularly to uptake CO2 (Bentov et al., 2009). When seawater pH is low, large foraminifera with large intracellular vacuoles will not be able to raise pH as much, reducing uptake of CO2 and lowering their calcification intensity. On the other hand, smaller foraminifera with large surface area to volume ratios are less dependent on the vacuole pH mechanism (Nehrke et al., 2013) and generally need less DIC to calcify. The same tendencies as predicted by the model have been observed in other studies, such as one conducted by de Moel et al. (2009) which found that large planktonic foraminifera produce thinner walls when exposed to lower pH environments. This implication of this model is that it warns that particularly large foraminifera will be negatively impacted in their ability to perform calcification by the lowering pH of oceans caused by climate change (also known as ocean acidification) (Koch et al., 2013).
The geometry of test formation
In the first theoretical representation of foraminifera’s test growth, presented by Berger (1969), the shell is anchored to what is defined as the center point of the shell. This model, pictured in Figure 4, involves a straightforward sequential rotation of a circle, featuring some overlap and an expansion of the circle’s radius (Ri). It is designed to mimic isometric growth, meaning that all three parameters—the ratio (q-ratio) between successive chamber radii, the angle of advance (a-angle), and the amount of overlap (o-lap)—remain constant throughout the ontogeny (parameters are further defined under Fig.4). It is specifically tailored for planispiral shells consisting of circular chambers.

Fig. 6. Construction of a planispiral foraminiferal shell based on the fixed-reference model by Berger et al.
Other such models typically represent chambers as circles or spheres undergoing rotation and translation along a fixed arbitrary axis (aka reference line). However, these older models cannot simulate some of the more complex patterns found in foraminifera, such as the gradual or sudden changes of growth modes responsible for different chamber arrangements during ontogeny.
Apertures and the moving-reference system
According to Tyszka et al., one way to address these limitations is to shift from a fixed-reference frame to a moving-reference system (see Fig. 8), an approach that is typically grounded in stepwise growth. The first example of that model comes from Webb and Swan (1996), and it is based on three parameters, including the chamber expansion rate. Only one of their parameters, denoted as α, is associated with the fixed coiling axis, serving the purpose of moving chambers along this axis. Parameter β corresponds to the line that moves in conjunction with the created chambers (refer to Fig. 5). While the morphospace generated by this model encompasses a range of forms (including planispiral, trochospiral, biserial, and uncoiled uniserial) it does not significantly expand the spectrum of morphologies when compared to other models. Note that a morphospace is simply a representation of the shape or structure of an organism.

Fig. 7. A moving-reference model. A) shows the spiral view, where β is the angle between the lines connecting the centers of three consecutive chambers. B) shows the peripheral view of a troschospiral foraminifer, where a is the angle between the fixed coiling axis and the line formed by connecting the centers of two consecutive chambers (Webb et al., 1996).
To go beyond the aforementioned models, Tyszka et al. affirm that the moving-reference system should include apertures, for it is essential for locating the new growing chamber. That being said, very little is known about how foraminiferal apertures form, for only tubular foraminifers have open-ended shells, that is shells for which the aperture (shell-opening) is defined by the incremental growth of the shell’s margin (see Fig. 6A,B).
Modeling shell growth: a new approach by Tyszka et al.

Fig. 8. Difference between fixed (A,C) and moving (B,D) reference systems, where z is the axis of coiling. A, B are open-ended shells. Taken from Tyska et al.
The following model integrates two key concepts: the utilization of a moving-reference system and the minimization of the local communication path (LCP). Essentially, this system is based on introducing apertures as reference points for the emplacement of every new chamber, which conforms to the reality of shell growth. In other words, it has the aperture relocating to a new position with each growth step: together with the reference growth axis (RGA), the aperture forms the foundation of the reference system from which a new chamber is constructed. The RFG axis indicates the curling direction during shell growth, and it moves along with the formation of chambers. Note that it has no morphogenetic representation during chamber formation. It also serves as a basis for the calculation of the growth vector, which indicates the growth direction for the new chamber.

Fig. 9. Initial chamber with an arbitrarily defined aperture, denoted r0, with polar coordinated [r0,y0] (Tyszka et al., 2005).
To begin the simulation, the initial chamber, or proloculus (see Fig. 9), must be established. There are then two parts to the procedure: 1) the determination of the positioning of the new chamber (refer to Fig. 10), and 2) calculating the aperture location (refer to Fig. 11.). The latter is done following the local minimization principle, which, as its name implies, relies on the fact that the distance between two successive apertures (that is the apertures of two consecutive chambers) must be as short as possible (Tyszka et al, 2005). It also involves the two following conditions: intuitively, a new aperture cannot be located inside a pre-existing chamber, and the last aperture must be connected to the proloculus by a line of communication that passes through all the apertures (Tyszka et al, 2005).
First and foremost, there are various calculations to consider to be able to position every new chamber (refer to Fig. 10). Note that all of the following equations in this section are taken from Tyska et al, 2005. The radius of the new chamber (ri) is given by the following rule, where GF ≥ 1 and stands for the chamber expansion ratio:
![]()
Next, the direction of the reference growth axis, given by the angle φi, is found from the center (aperture) of the previous chamber (Fig. 10A). Then, referring to Figure 10B, it is from the growth vector vi—with polar coordinated [vi, φi]—that we can determine the center of the new chamber. Indeed, given that two successive chambers overlap, the length of vi cannot exceed the radius of the new chamber, so vi is given by:
![]()
In eq. 16, TF is the chamber translation ratio, which is the overlap, and it is positive and does not exceed 1. Furthermore, to find the second polar coordinate φi, the deviation w.r.t the reference growth axis (Δφ) is determined by Equation 17.
![]()

Fig. 10. Formation of a new chamber. A, Determination of the reference axis (dotted line passing through aperture; ri is given by eq.14). B shows the deviation (Δφ) from the reference axis. C, Emplacement of the new chamber (Tyszka et al., 2005).
Now, onto the steps allowing for the calculation of the new aperture’s location (refer to Fig. 11. for the following). We must first find the vector ri = [ri, γi] since it points to the direction of the new aperture (Fig. 11.A); note that the coordinate ri has been found above (Eq. 15). To do so we define a vector ui which connects two successive apertures and therefore represents the LCP. The latter is defined by equation 18. and we are looking for a γi such that the vector ui has the shortest length.
![]()
The shortest distance lies along a line passing through a point inside the circle and the center of the circle, and this is given by the angle φi (Eq. 17). To find the right value of φi, we examine two values of γi and select the one for whichever the distance is shortest (Fig. 8B).

Fig. 11. Determination of the location of the aperture. A, Calculation for the vector ui. B illustrates the steps to find the shortest distance. The two values of γi are given as follows: ![]() , and
, and ![]() . C, Relocation of the new aperture, outside of the excluded range (Tyszka et al., 2005).
. C, Relocation of the new aperture, outside of the excluded range (Tyszka et al., 2005).
Model review: ontogenetic variability and limitations

According to Tyszka et al., simulations from previous studies used non-random parameters, meaning that they were kept constant through ontogenesis: they therefore resulted in very regular patterns featuring gradually changing chamber shapes (see figure A. G,H). However, such ideal chambers, which follow strict geometric rules, are unrealistic and rarely found in nature. This is whyontogenetic variability was incorporated in the approach, and it was achieved by allowing selected parameters to fluctuate within given ranges*. In other words, different environmental conditions–whether it be nutrition availability, temperature, pH levels, or salinity–are responsible for natural irregularities, and their influence was considered such that the simulations can accurately mimic the reality of morphogenetic processes. Figure 12 shows results from the algorithm when it is fed random parameters, while Figure 13 (A-F) simulates spiral uncoiling after the variation of a single parameter (delta φ), compared to one resulting from the non-random (Fig. 13.G).

Fig. 12. Variability of simulated foraminiferal shells. It is worth noting that all the morphotypes (A–K) resemble 2-dimensional cross-sections of real foraminifers (Tyszka et al, 2005).

Fig. 13. A-E shows spiral morphotypes changing to uniserial pattern with very wide ranges of the Dfi angle. The model simulates such morphologies; they are completely random and nonrecurring. F) Random parameters lead to the creation of uniserial forms. G) Contrastingly, G shows the simulated uniserial arched shell defined by nonrandom parameters (Tyszka et al., 2005).
Overall, the presented model, developed by Tyszka et al., illustrates the geometry behind test growth in foraminifera, as it uses real morphologic characters to follow basic biological processes, which usually act gradually. To imitate reality, some elements of randomness are applied to the selection of parameters.
There are, however, a few important limitations to consider. Firstly, the model is size-independent, while foraminifera are not. Secondly, the spherical shapes used to represent the chambers unfortunately do not come close to reflecting the extent of chamber shapes in nature: the overall shape of the specimen, which by design is dependent on the successive cumulation of chambers. Lastly, the model presented is limited to 2-dimensional cases, and therefore cannot entirely capture the complexity of foraminifera’s morphogenesis.
Globigerina Bulloides: golden ratio and the spiral equation
Planktonic foraminifera grow by the serial accretion of chambers onto their tests, which permits researchers to examine their architecture, and thereby understand the laws of growth governing their shell morphology. A study by Zargogiannis et al. reports the morphological variation in shape and size of the chambers of an adult individual of the species G. Bulloides, for which the shape of the cell remains constant throughout ontogeny. As concluded from the study, this species undergoes isometric growth: the chamber shape remains constant such that the volumes of each successive chamber follow a geometric progression. These spherical chambers used for the analysis are represented in Figure 13 below.

Fig. 13. Position in measurements in individuals of Globigerina bulloides. In a) C1-C4 correspond to the diameters of the chamber, where C1 is the final chamber. The solid lines designate the measured diameters of each chamber. In b) the test is segmented according to its basic geometry, and K1-K4 are the theoretical centers of the chambers. The addition of C1 and C3 forms the segment C13; that of C2 and C4, segment C24 (Zargogiannis et al, 2019).
Zargogiannis et al. illustrate that in G. bulloides, when the chambers of a whorl are projected onto a single plane, they touch the coiling axis, outlining four circles for every two chambers that osculate (that is for every two chambers sharing a tangent line at the point of contact). Note that each chamber is half a whorl. Not only does this geometric similitude make the species ideal for growth models, but that principle happens to be the most plausible explanation for the log-spiral patterns observed in shell coiling. What makes the isometric growth so useful is the characteristics associated to it; the shell’s general geometry allows for the determination of the ratio between the diameters of successive chambers.
The golden ratio is a proportion that operates as a universal constant, and it is found by “dividing a line into two parts so that the longer part divided by the smaller part is also equal to the whole length divided by the longer part” (Zargogiannis et al). Still referring to Figure 12, if C13 is to C1 what C1 is to C3, then we can obtain the following relation, which shows that the golden ratio is met:
![]()
Moreover, from all the measurements of the described diameters collected in Zargoniannis et al.’s study emerges a logarithmic equation of the shell’s evolution. The latter is based on the logarithmic spiral equation by putting the measured lengths on (x,y) points of a Cartesian system, solving the logarithmic spiral equation (eq. X) derives eq. 16 for G. bulloides morphogenesis:
![]()
Ultimately, it becomes evident that the architectural plan for the construction of foraminifera shells (in particular of G. bulloides) heavily features the golden ration.
Conclusion
At first glance, foraminifera appear to be beautiful yet simple creatures – deceptively so, because every spiral of their test, every tip of their spines, every curve of their pores, is the result of millions of years of evolution. Beneath their tests is an even more captivating world of chemistry, often involving a complex network of symbionts.
The use of symbiosis is thought to be a response to oxygen and nutrient depletion in marine environments, but the mathematical model ForamEcoGENIE 2 developled by Ying et al. (2018) shows that a more accurate hypothesis can be achieved by expanding the input parameters, such as nutrient availability, temperature, light, photosynthesis efficiency, symbiont dependency, and more. The model was able to reproduce the abundance of symbiont obligate groups in known nutrient poor regions of the ocean, as well as the abundance of symbiont-barren groups in high productivity environments.
The study of foraminiferal pore functions via mathematical modeling has unveiled key adaptations to environmental challenges. The model effectively correlates pore patterns with metabolic and mechanical needs, highlighting foraminifera’s balance between gas exchange and test integrity. Its empirical validation confirms the model’s accuracy, enhancing our understanding of foraminiferal biology and their ecological strategies. This research is pivotal for future studies in paleoceanography, especially in the context of climate change (Jorissen et al., 2019).
Calcification is one of foraminifera’s greatest assets in the species that undergo the process (calcareous), since calcification results in the formation foraminifera’s characteristic calcium carbonate test. By collecting data relating to calcification from planktonic foraminifera and modelling relationships between this data, researchers can have a greater understanding of the organism. In particular, Henehan et al. (2017) were able to find the way in which foraminifera grows during its lifespan and the way in which environmental pH affects the calcification process.
References
Barker, S. and Elderfield, H. (2002) Foraminiferal Calcification Response to Glacial-Interglacial Changes in Atmospheric CO2, Science, 297, 833–836, https://doi.org/10.1126/science.1072815.
Berger, W. H. (1969). Planktonic foraminifera: basic morphology and ecologic implications. Journal of paleontology, 1369-1383.
Berggren, W. A., Loeblich, A. R., Tappan, H., & Moore, R. C. (1965). Treatise on Invertebrate Paleontology, part C: Protista 2 – sarcodina, chiefly “Thecamoebians” and foraminiferida. Micropaleontology, 11(1), 122. https://doi.org/10.2307/1484826
Bernhard, J. M., Goldstein, S. T., & Bowser, S. S. (2010). An ectobiont‐bearing foraminiferan, bolivina pacifica, that inhabits microxic pore waters: Cell‐biological and Paleoceanographic Insights. Environmental Microbiology, 12(8), 2107–2119. https://doi.org/10.1111/j.1462-2920.2009.02073.x
Berthold, W.-U. (1976). Ultrastructure and function of wall perforations in patellina Corrugata Williamson, foraminiferida. The Journal of Foraminiferal Research, 6(1), 22–29. https://doi.org/10.2113/gsjfr.6.1.22
Bijma, J., Faber, W. W., & Hemleben, C. (1990). Temperature and salinity limits for growth and survival of some planktonic foraminifers in laboratory cultures. The Journal of Foraminiferal Research, 20(2), 95–116. https://doi.org/10.2113/gsjfr.20.2.95
Briguglio, A., Metscher, B., & Hohenegger, J. (2011). Growth rate biometric quantification by X-ray microtomography on larger benthic foraminifera: three-dimensional measurements push nummulitids into the fourth dimension. Turkish Journal of Earth Sciences, 20(6), 683-699.
Diaz, R. J., & Rosenberg, R. (2008). Spreading dead zones and consequences for marine ecosystems. Science, 321(5891), 926–929. https://doi.org/10.1126/science.1156401
Edgar, K. M., Bohaty, S. M., Gibbs, S. J., Sexton, P. F., Norris, R. D., and Wilson, P. A. (2013). Symbiont “bleaching” in planktic foraminifera during the Middle Eocene Climatic Optimum, Geology, 41, 15–18, https://doi.org/10/f4jwbp
Frerichs, W. E., Heiman, M. E., Borgman, L. E., & Be, A. W. (1972). Latitudal variations in planktonic foraminiferal test porosity; part 1, optical studies. The Journal of Foraminiferal Research, 2(1), 6–13. https://doi.org/10.2113/gsjfr.2.1.6
Glock, N., Eisenhauer, A., Milker, Y., Liebetrau, V., Schönfeld, J., Mallon, J., Sommer, S., & Hensen, C. (2011). Environmental influences on the pore density of Bolivina Spissa (Cushman). Journal of Foraminiferal Research. https://pubs.geoscienceworld.org/cushmanfoundation/jfr/article-abstract/41/1/22/77233/ENVIRONMENTAL-INFLUENCES-ON-THE-PORE-DENSITY-OF
Glock, N., Erdem, Z., Wallmann, K., Somes, C. J., Liebetrau, V., Schönfeld, J., Gorb, S., & Eisenhauer, A. (2018). Coupling of oceanic carbon and nitrogen facilitates spatially resolved quantitative reconstruction of nitrate inventories. Nature News. https://www.nature.com/articles/s41467-018-03647-5
Holzmann, M., & Pawlowski, J. (1997). Molecular, morphological and ecological evidence for species recognition in ammonia (foraminifera). The Journal of Foraminiferal Research, 27(4), 311–318. https://doi.org/10.2113/gsjfr.27.4.311
Hottinger, L., & Dreher, D. (1974). Differentiation of protoplasm in Nummulitidae (foraminifera) from Elat, Red Sea. Marine Biology, 25(1), 41–61. https://doi.org/10.1007/bf00395107
Jones, R. W. (2014). Foraminifera and their applications. Cambridge Core. https://www.cambridge.org/core/books/foraminifera-and-their-applications/F8AAB9000F442406778FB15E4B9E194B
Katz, M. E. et al. (2010). Traditional and emerging geochemical proxies in foraminifera. Pubs.geoscienceworld.org. https://pubs.geoscienceworld.org/cushmanfoundation/jfr/article-abstract/40/2/165/77210/TRADITIONAL-AND-EMERGING-GEOCHEMICAL-PROXIES-IN
Łabaj, P., Topa, P., Tyszka, J., & Alda, W. (2003). 2D and 3D Numerical Models of the Growth of Foraminiferal Shells (Vol. 2657). https://doi.org/10.1007/3-540-44860-8_69
LeKieffre, C., Spero, H. J., Russell, A. D., Fehrenbacher, J. S., Geslin, E., and Meibom, A. (2018). Assimilation, translocation, and utilization of carbon between photosynthetic symbiotic dinoflagellates and their planktic foraminifera host, Mar. Biol., 165, 104, https://doi.org/10.1007/s00227-018-3362-7
Leutenegger, S., & Hansen, H. J. (1979). Ultrastructural and radiotracer studies of pore function in Foraminifera. Marine Biology, 54(1), 11–16. https://doi.org/10.1007/bf00387046
Lipps J.H., Fossil Prokaryotes and Protists, Blackwell, Boston, 1993.
Mitra, A., Flynn, K. J., Tillmann, U., Raven, J. A., Caron, D., Stoecker, D. K., Not, F., Hansen, P. J., Hallegraeff, G., Sanders, R., Wilken, S., McManus, G., Johnson, M., Pitta, P., Våge, S., Berge, T., Calbet, A., Thingstad, F., Jeong, H. J., Burkholder, J., Glibert, P. M., Granéli, E., and Lundgren, V. (2016) Defining Planktonic Protist Functional Groups on Mechanisms for Energy and Nutrient Acquisition: Incorporation of Diverse Mixotrophic Strategies, Protist, 167, 106–120, https://doi.org/10/f3p5h2
Richirt, J., Champmartin, S., Schweizer, M., Mouret, A., Petersen, J., Ambari, A., & Jorissen, F. J. (2019a). Scaling laws explain foraminiferal pore patterns. Scientific Reports, 9(1). https://doi.org/10.1038/s41598-019-45617-x
Richirt, J., Champmartin, S., Schweizer, M., Mouret, A., Petersen, J., Ambari, A., & Jorissen, F. J. (2019). Scaling laws explain foraminiferal pore patterns. Scientific Reports, 9(1). https://doi.org/10.1038/s41598-019-45617-x
Ross, B. J., & Hallock, P. (2016). Dormancy in the Foraminifera: A Review. The Journal of Foraminiferal Research, 46(4), 358–368. https://doi.org/10.2113/gsjfr.46.4.358
Tyszka, J., & Topa, P. (2005). A new approach to modeling of foraminiferal shells. Paleobiology, 31(3), 522-537.
Webb, L. P., & Swan, A. R. (1996). Estimation of parameters of foraminiferal test geometry by image analysis. Palaeontology, 39(2), 471-475.
Zarkogiannis, S., Kontakiotis, G., & Antonarakou, A. (2019). Logarithmic expression of Globigerina bulloides shell evolution through the biometric analysis: Paleoceanographic implications for the late Quaternary. IOP Conference Series: Earth and Environmental Science.
Bentov, S., Brownlee, C., & Erez, J. (2009). The role of seawater endocytosis in the biomineralization process in calcareous foraminifera. Proceedings of the National Academy of Sciences, 106(51), 21500-21504.
de Moel, H., Ganssen, G. M., Peeters, F. J. C., Jung, S. J. A., Kroon, D., Brummer, G. J. A., & Zeebe, R. E. (2009). Planktic foraminiferal shell thinning in the Arabian Sea due to anthropogenic ocean acidification? Biogeosciences, 6(9), 1917-1925. https://doi.org/10.5194/bg-6-1917-2009
Henehan, M. J., Evans, D., Shankle, M., Burke, J. E., Foster, G. L., Anagnostou, E., Chalk, T. B., Stewart, J. A., Alt, C. H. S., Durrant, J., & Hull, P. M. (2017). Size-dependent response of foraminiferal calcification to seawater carbonate chemistry. Biogeosciences, 14(13), 3287-3308. https://doi.org/10.5194/bg-14-3287-2017
Koch, M., Bowes, G., Ross, C., & Zhang, X.-H. (2013). Climate change and ocean acidification effects on seagrasses and marine macroalgae. Global Change Biology, 19(1), 103-132. https://doi.org/https://doi.org/10.1111/j.1365-2486.2012.02791.x
Nehrke, G., Keul, N., Langer, G., de Nooijer, L. J., Bijma, J., & Meibom, A. (2013). A new model for biomineralization and trace-element signatures of Foraminifera tests. Biogeosciences, 10(10), 6759-6767. https://doi.org/10.5194/bg-10-6759-2013