Aquatic Fungi: An Exploration of Aquatic Adaptation and Interaction
Izabela Junqueira Magalhaes, Kristina Kerkelova, Ruizhi Liu, Yasmine Sadr Kaufmann
Abstract
Fungi are eukaryotic microscopic organisms that appear everywhere in the world. The yeast used to make bread and the mushrooms that we eat all belong to Fungi Kingdom. However, there is a big part of fungi, the aquatic fungi, that is not so well-known yet and gains less attention, but still plays an important role in ecosystems. In this paper, aquatic fungi will be introduced through a lens of physics, starting with a definition of aquatic fungi and how they are classified based on their two main habitats. Following that, the history of aquatic fungi and the process of their evolution will be discussed, as well as their diversity in nature and the challenges aquatic fungi are facing now. The structure of aquatic fungi varies a lot but there are still common characteristics among them which will be talked about. Next, the reproduction mechanism of aquatic fungi and their spores‘ movement based on different types will be covered. Finally, we will discuss aquatic fungi and their interaction with other marine organisms, both macroscopic and microscopic, based on their relationship with them.
Introduction
In the complex web of life on our planet, the Fungi Kingdom is still full of unanswered questions. While the spotlight often shines on terrestrial fungi for their roles as decomposers, mycorrhizal associates, and even pathogens, the submerged world of aquatic fungi has remained largely uncharted. These enigmatic organisms, lurking beneath the water‘s surface, have garnered far less attention from researchers. However, their significance in aquatic ecosystems cannot be overstated.
Aquatic fungi inhabit a wide range of aquatic environments, from freshwater streams to the depths of the ocean. Their adaptation to these unique habitats and their intricate interactions with other organisms are the focus of this research paper. Despite their vital contributions to ecosystem dynamics and nutrient cycling, the study of aquatic fungi has been, thus far, a neglected frontier of mycology.
This paper embarks on a journey to shed light on the diverse world of aquatic fungi and presents a literature review of what is known so far about these fascinating beings. We delve into their adaptations to the aquatic environment, unravel their fascinating natural histories, explore the evolutionary trajectories that have led to their diversity, and examine their intricate web of interactions with aquatic life forms. With only a fraction of aquatic fungal species cataloged and an even smaller fraction understood, this research underscores the urgency of studying these organisms. As we strive to comprehend their roles in the aquatic realm, we will unveil the hidden intricacies of ecosystems and the critical functions that aquatic fungi perform, reminding us that there are still vast and unexplored frontiers within the fungal kingdom.
An overview of aquatic fungi
Fungi are a large and phylogenetically diverse group of organisms. They are present in almost all ecosystems on Earth, serving diverse functions, such as decomposition, cycling of organic matter, and renewal of minerals. Due to their various functionalities and broad spatial distribution, there is no question about their key role in ecology.
Fungi are present in many environments, including aquatic ecosystems. More precisely, fungi are present in most, if not all aquatic ecosystems. The presence of fungi in these ecosystems ranges from small lakes to the marine deep sea, from fungi with strictly aquatic life cycles to amphibious fungi, whose life is spent between terrestrial and aquatic phases, or even transient fungi, who live on land but depend on water to reproduce.
A way to classify aquatic fungi is related to their lifecycle and level of dependence on water. There are indwellers, periodic immigrants, and versatile immigrants (Grossart et al., 2019). The first category includes fungi which spend the entirety of their lifecycles in water, in a specific habitat. On the other hand, the immigrant species can migrate from their primary habitat temporarily, when the conditions are not favorable for their development, and then return when they improve, as the periodic immigrants or even be highly adaptable to both aquatic and terrestrial environments, having biphasic or amphibious lifecycles, as versatile immigrants (Gulis, Kuehn, & Suberkropp, 2009).
Water-dwelling, amphibious, and transient fungi
Adaptations seen in aquatic fungi depend on the fungi’s origin and their status as water-dwelling, amphibious, or transient. Water-dwelling fungi (who spend their entire lives in water) and amphibious fungi (who live both in water and on land) can maintain a stable biomass year-round, despite the fluctuations in nutrient availability, and they can sporulate in water. These capabilities make them fully adapted to life in water. Some transient fungi, who spend short periods of time in water, harness aquatic conditions to spread their spores (reproductive structures), while other transient fungi face challenges when in aquatic habitats. Transient fungi that are not adapted to aquatic life may experience a decline in activity shortly after they encounter water, due to the change in oxygen and nutrient availability, as well as the competition for resources with better-adapted organisms. These setbacks may prevent certain transient fungi from sporulating and colonizing new substrates in an aquatic environment (Dix et al., 1995). Whether water-dwelling, amphibious, or transient, all fungi that interact with water at some point during their life are considered aquatic, and their unique relationship with the water should be studied (see Fig. 1).
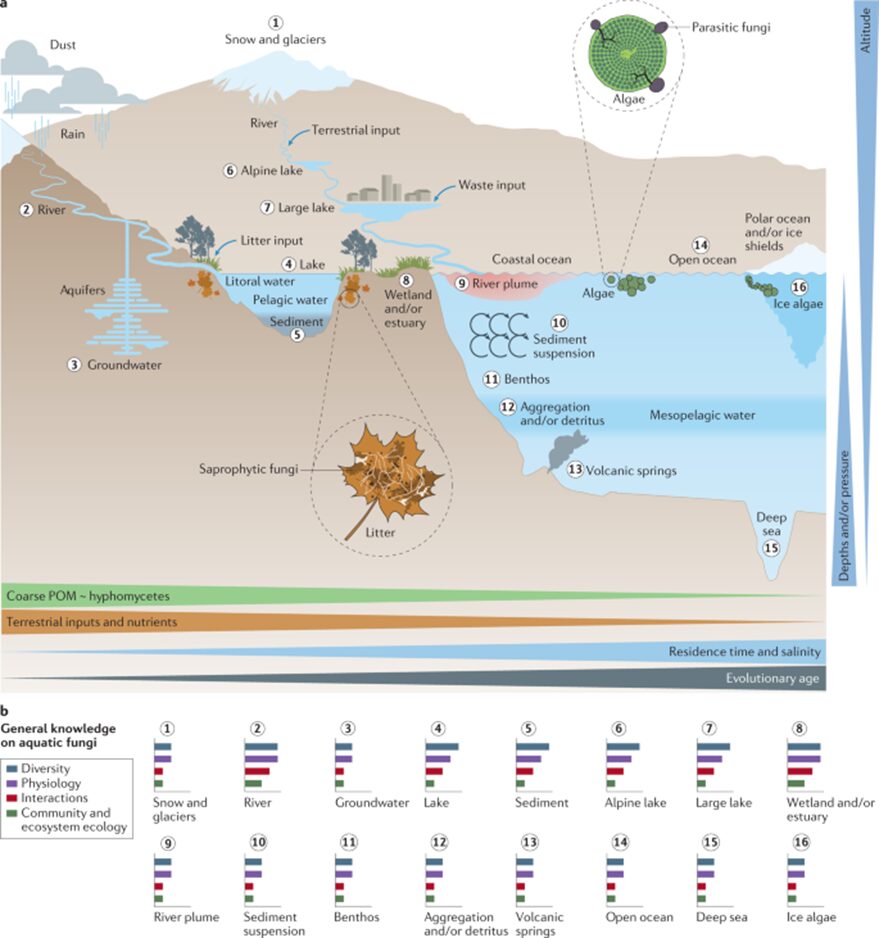
Fig.1: Natural environments for aquatic fungi (Grossart et al., 2019).
Freshwater and marine habitats
The two main categories within aquatic fungi are freshwater fungi and marine fungi. The main difference between the two is the salinity levels of the water in which they are found. Freshwater ecosystems are defined as ones with low to moderate salinity levels, such as lakes, rivers, streams, ponds, and wetlands (Krauss et al., 2011). Furthermore, there are two kinds of freshwater habitats – lentic and lotic. Lentic refers to freshwater habitats lacking continuous water flow. This includes ponds, swamps, lakes, and pools. Low wave action fosters undisturbed fungal growth, but low oxygen levels may impede the growth of certain types of fungi. Lotic habitats refer to bodies of water with a continuous flow of water, such as rivers, streams, creeks, and brooks. Flowing water can help spores distribute widely (Wong et al., 1998). As for the marine aquatic fungi, they inhabit saltwater environments, encompassing the open ocean and deep seas as well as coastal regions and estuaries. Marine habitats offer ideal living conditions for some species of aquatic fungi as their temperatures are relatively stable compared to freshwater environments. Saltwater environments tend to have less fungi than freshwater environments, creating a less competitive environment for marine fungi. There is also a plethora of substrates to choose from. Marine fungi can grow on coral, driftwood, or even other fungi (Richards et al., 2012). Freshwater and marine fungi have unique characteristics that will be explored in this paper.
Although there are species of fungi that can inhabit both, most aquatic fungi are highly adapted to their environment, which in most cases means that they can only be present in one of those categories. A good example of evolution that would differ from marine to freshwater fungi is their mechanisms of osmoregulation: marine fungi have mechanisms to regulate their internal osmotic pressure, allowing them to thrive in a habitat with a specific salinity level.
These classifications help illustrate how fascinating aquatic fungi are, but the specific ecological roles and diversity of these members of the Fungi Kingdom are still largely understudied. Most fungi, especially aquatic fungi, have not yet been characterized, mostly due to the abundance of habitats and the variety of conditions and spaces they occupy. To better understand them in general, it is useful to look back on their evolution.
Evolution and natural history
Fungi belong to the domain Eukarya, and their Kingdom is considered a monophyletic [1] group. Their last common ancestor (LFCA) is believed to have lived about 800 to 900 million years ago, and interestingly in an aquatic habitat, most likely a marine environment (Naranjo-Ortiz & Gabaldón, 2019). Several studies also recommend that fungi, alongside plants, were the first eukaryotes to undergo terrestrialization and colonize the land. This was only possible due to mycorrhizae, a mutualistic ecological association between certain fungi species and the roots of vascular plants that offer nutritional benefits to both species. This theory is based on the belief that this relation was eventually established between fungi who already lived in symbiotic relationships with species of algae and cyanobacteria and early land plants. To better understand the context of this early fungi evolution in natural history, we can illustrate an evolutionary timeline (see Fig. 2).
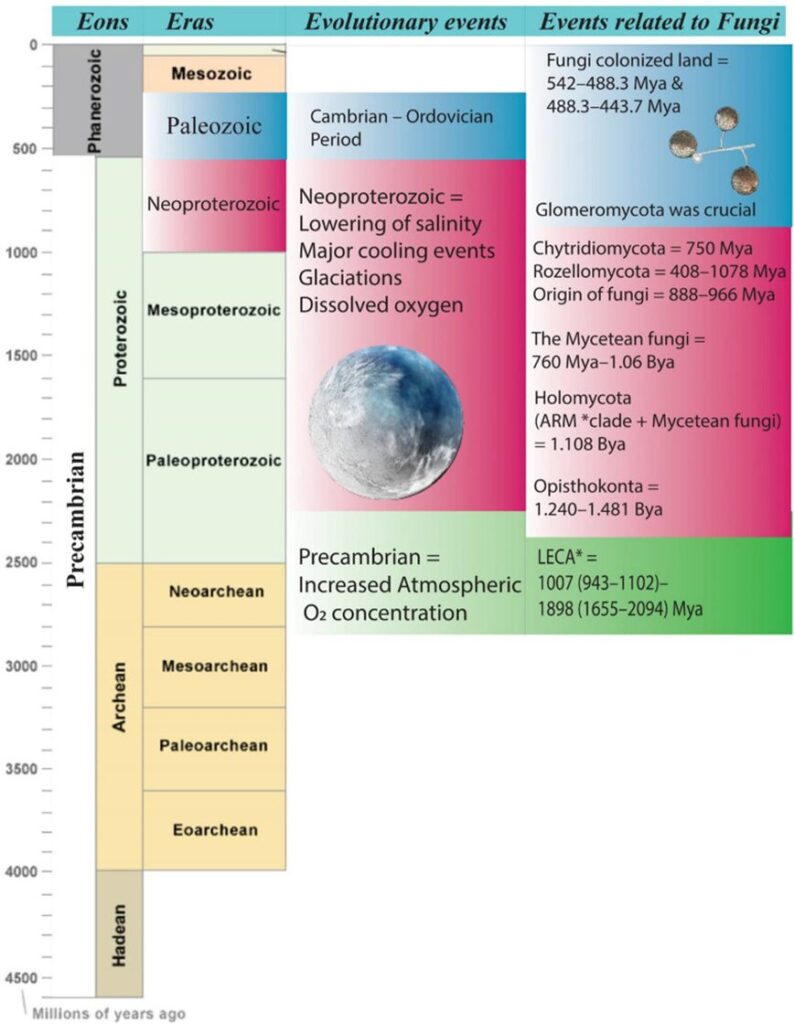
Fig.2: Schematic representation of the evolutionary timeline related to fungi. *ARM, Aphelidomycota, Rozella, Microsporidia; *LECA, last eukaryotic common ancestor (Kumar et al., 2021).
Moving forward in their evolution, their group becomes highly diverse, with a complex branching pattern in their phylogenetic tree. It is important to notice that due to the minimal fossil record of fungi, most of the evolutionary study of their history and of the relationship between the different species is based on biochemical characteristics and recent DNA-sequencing technologies (David Moore, 2023). These same recent genetic analyses are the ones responsible for the considerable changes in the taxonomy of fungi in the last few decades and the discoveries and differentiation of species once considered the same.
In summary, the evolution of aquatic fungi is a key point of study to better understand the fungal kingdom’s evolutionary history in totality. They represent some of the earliest fungal lineages. For example, to get a better idea of how the LFCA could have been, a good place to look is a modern-day chytrid fungus. These are considered the most primitive fungi still in existence and they are primally aquatic, found in both marine and freshwater environments. They are known for their flagellated spores (zoospores) and are considered a basal group in the fungal kingdom (Kumar et al., 2021). This evolutionary aspect of fungi, which still must be extensively studied and understood, contributes to the necessity to join more interdisciplinary efforts in the research of aquatic fungi.
Looking into the current organization of this kingdom, we have seven phyla of those called true fungi, meaning the organisms that can be englobed by the monophyletic clade of the Kingdom. They are Chytridiomycota (discussed briefly already), Blastocladiomycota, Neocallimastigomycota, Microsporidia, Glomeromycota, Ascomycota, and Basidiomycota (the latter two being combined in the subkingdom Dikarya) (David Moore, 2023). Going more specifically into the phylogeny of aquatic fungi, we can look at the following fungal tree of life (see Fig. 3) and appreciate their morphological, phylogenetic, and ecological diversity (Grossart et al., 2019).
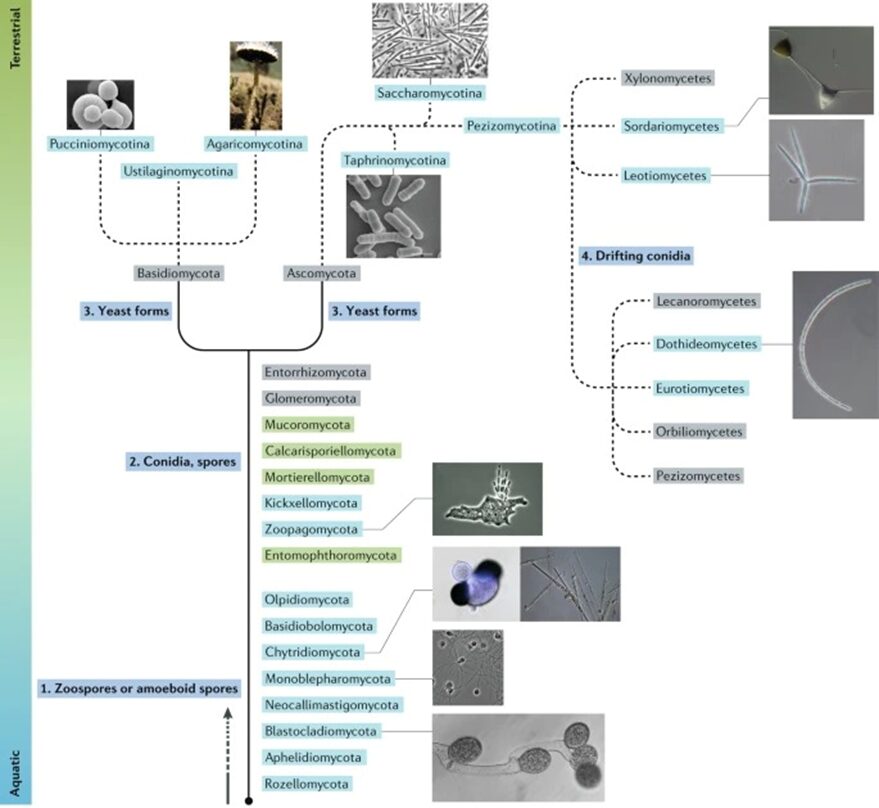
Fig. 3: Fungal tree of life representation highlighting their habitats and main characteristics (Grossart et al., 2019).
In the figure above, the aquatic species of fungi are highlighted in blue. The categories of species highlighted in green belong to phyla that are believed to contain aquatic lineages, while the ones in grey show terrestrial lineages. The solid lines are the basal lineages of the fungal phylogeny, while the dashed lines represent subphylum levels of the tree. As we can see, each fungal lineage has its unique evolutionary path, adaptations, and characteristics.
Aquatic fungi evolution is a marvelous example of convergent evolution. This phenomenon happens when unrelated lineages parallelly evolve to have analogous characteristics due to similar environmental pressures or ecological niches. A good example of this is the evolution of zoospores in different aquatic species (Grossart et al., 2019).
Another point is that fungi have transitioned from marine to terrestrial environments and back multiple times (Kumar et al., 2021). Some species, such as aquatic hyphomycetes, have evolved to go back to aquatic habitats from terrestrial fungi due to environmental pressures. In this case, after territorialization in their evolutionary path, adapting to morphological conditions to go back to aquatic ecosystems and getting attached to submerged substrates was a mechanism to overcome competition for nutrients.
Biodiversity and ecology of fungi
The main ecological part that fungi are responsible for in aquatic environments, similar to terrestrial fungi, is the decomposition of organic matter and recycling of nutrients. Other roles include a diversity of biotic interactions, such as parasitic relations, mutualistic relations, and others. These interactions will be explored in more depth later in the article, but it is important to notice that the still broadly unknown diversity of fungal species also implies unfamiliar interactions still in need of study.
Freshwater ecosystems

Fig. 4: Species and diversity. a–c Colonies of Pseudobactrodesmium spp. on natural substrates. d, e Conidia with sheath of Pseudobactrodesmium spp (Calabon et al., 2023).
The greatest number of aquatic fungi species (examples in Fig. 4) reported so far are in freshwater environments, and the discoveries keep increasing (see Fig. 5). The current number is on the scale of a few thousand species. However, recent molecular data suggests that the number of freshwater fungi species is greatly underestimated in current literature, and it could range from 0.5 to 10 million species (Lepère et al., 2019).
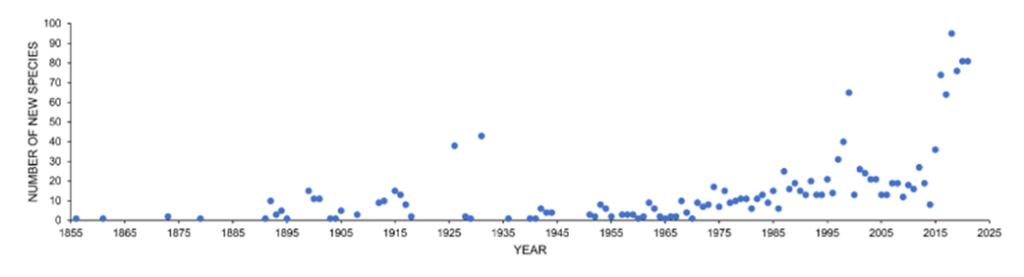
Fig. 5: Number of new species discoveries from freshwater habitats from 1856-2021 (Calabon et al., 2023).
Freshwater fungi, beyond their various functions in their ecosystems and diversity, are also very sensitive to changes. Their biodiversity and activity can be affected by pH and temperature changes in water, nutrient availability, and the other species in their habitat. Furthermore, they are very susceptible to anthropological disturbances, such as the contamination of freshwater habitats with heavy metals and pesticides, nutrient enrichment, nanoparticles, and xenobiotic concentrations (Calabon et al., 2023). These disruptions to natural environments not only impact the fungi population but scale to the dynamic of the ecosystem as a whole.
A great risk within these human-provoked changes in fungi communities comes from water contamination of their habitats with organic fungicides. Pesticides used in agriculture are not tested or regulated having the protection of aquatic fungi in mind (Ittner, Junghans, & Werner, 2018). These cause great impact and risk to the ecosystem once there is contamination of lakes, rivers, and water streams with those biocides.
Marine fungi
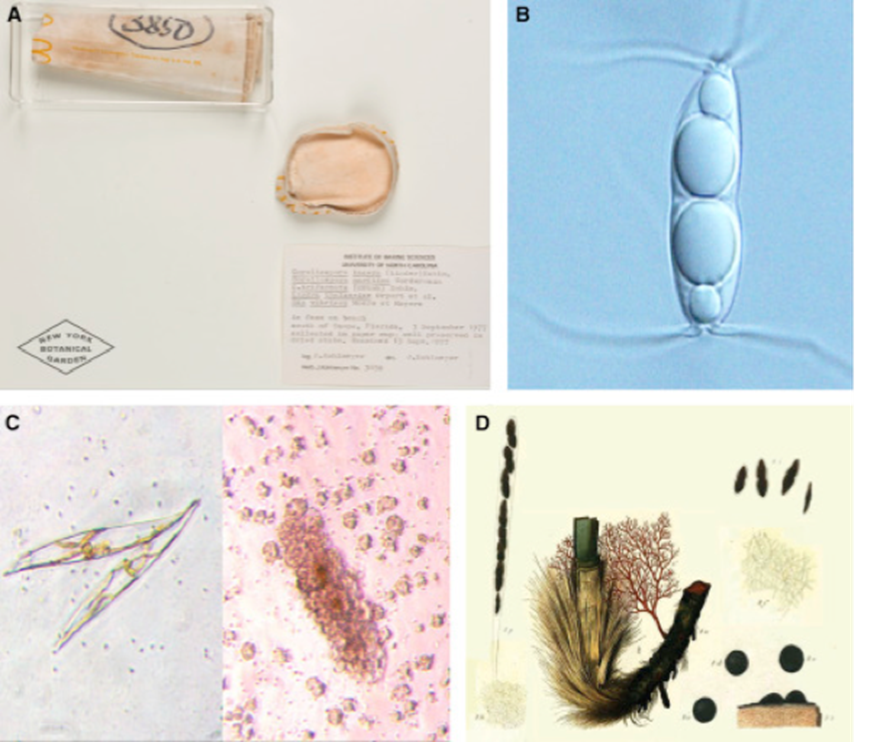
Fig. 6: Marine Fungi Species and Diversity. (A) A collection of five marine fungal species. (B) Ascospore of common Arenariomyces trifurcatus. (C) A diatom, Pleurosigma. (D) A plate depicting Sphaeria posidoniae (Gladfelter, James, & Amend, 2019).
Marine fungi are an even less studied part of aquatic fungi. Their diversity in marine environments is not comprehensively known, particularly when compared to our knowledge of terrestrial and freshwater species. There are only a few hundred marine fungal species identified and reported so far. Most of them are microscopic, as represented in Fig. 6, such as yeasts or swimming zoospore-producing groups. The few macroscopic species known so far are the species that habit coastlines, corals, or even driftwood, such as marine lichens, which are a symbiosis between fungi and algae or bacteria (sometimes even both) (Cunliffe, 2023). Fig. 7 illustrates a few of the spaces and relationships in which aquatic fungi are present.
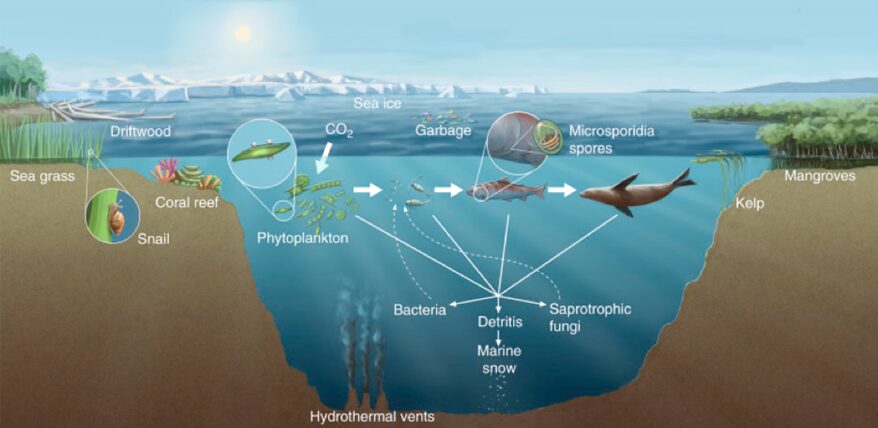
Fig.7: Diversity of marine fungal habitats and the ecological roles of marine fungi (Gladfelter, James, & Amend, 2019).
One of the main challenges that marine aquatic fungi species face currently is the changes to their habitats caused by climate change. Since global warming has an impact on many of the physical properties of marine habitats, besides temperature, such as stratification, acidification, eutrophication, and increased atmospheric carbon dioxide (Kumar et al., 2021), fungi populations undergo changes in their dynamics and physiological processes (Hunter-Cevera, Karl, & Buckley, 2020). These can result in adaptations, migrations, or even extinction.
These are of great concern not only for fungi but also to all the organisms that are in direct or indirect relationships and to the biosphere. Therefore, the study of marine fungi, especially the search for more knowledge about their diversity and the factors that impact their populations, is crucial.
Structure
Mycelium, hyphae, and spores
Aquatic fungi’s structure is largely species-dependent, but they all have a few common characteristics. Most species of aquatic fungi are similar in composition. Fungal tissue is mostly made up of chitin and some (~14 %) nitrogen. Fungi’s reproductive structures, called spores, have higher phosphorus concentrations (Jobard et al., 2010).
Aquatic fungi that grow and live in solid substrates, such as mud, driftwood, or coral, have a large web-like structure called mycelium. Mycelium is made up of many long, tubular branches called hyphae (see Fig.8). Hyphae absorb and transport nutrients to the entire fungal body. Mycelium’s web-like structure is designed to invade substrates and tissues, allowing the fungal body to have greater access to nutrients (Moss, 1986). Large, penetrative, mechanical forces are required in cases of invasion. Fungi that invade plants have an appressorium, a powerful structure that can deliver up to 8 megapascals of pressure to the epidermis of the plant it wants to invade (Howard et al., 1991). It is because of this fascinating skill that fungi are excellent at spreading over most plant substrates they encounter.
Fungi that do not require solid substrates, such as yeasts, reproduce by budding and absorbing nutrients through their cell walls. They do not have hyphae. However, absorbing nutrients through hyphae and cell walls are beneficial tactics, as both structures have a high surface area-to-volume ratio (Moss, 1986). This is one of the many structural characteristics that help aquatic fungi thrive.
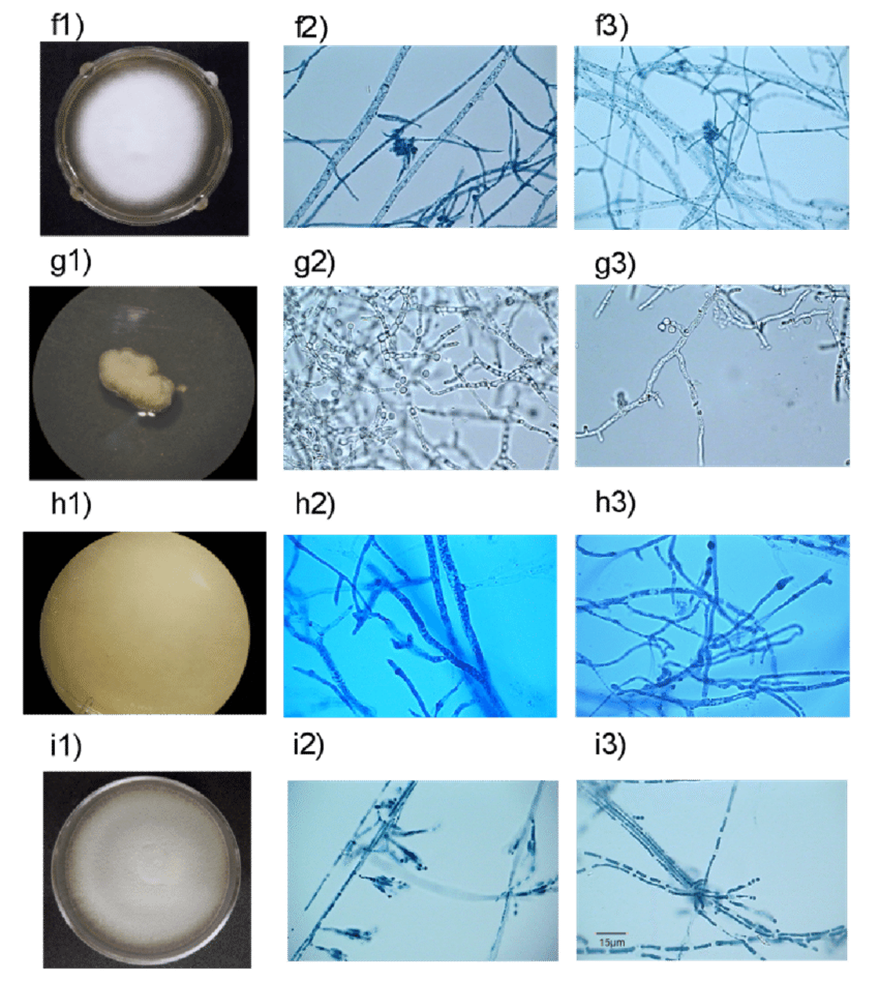
Fig.8: Hyphae of various types of marine fungi (González-Martínez et al., 2017).
Spores come in various shapes and sizes depending on the type of fungi (see Fig. 9). They can be as small as 2 μm or as large as over 200 μm. Spores can be formed sexually or asexually. Asexual spores are formed in a zoosporangium, a sack-like structure on the ends of hyphae that bursts, allowing the spores to break free from the fungal body and spread. Sexual spores are formed in sporangia (see Fig. 10) (Morrow, 2020). Both structures are designed for optimal spreading of spores beyond the existing fungal body.
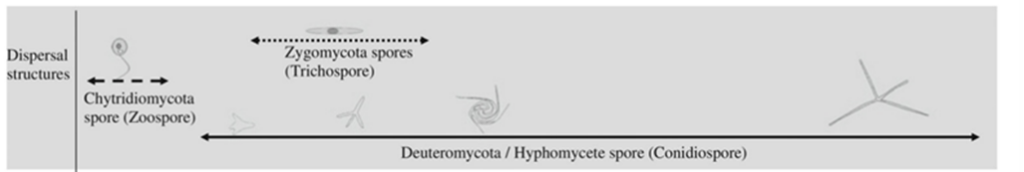

Fig. 9: Dimensions of spores of aquatic fungi (Jobard et al., 2010).
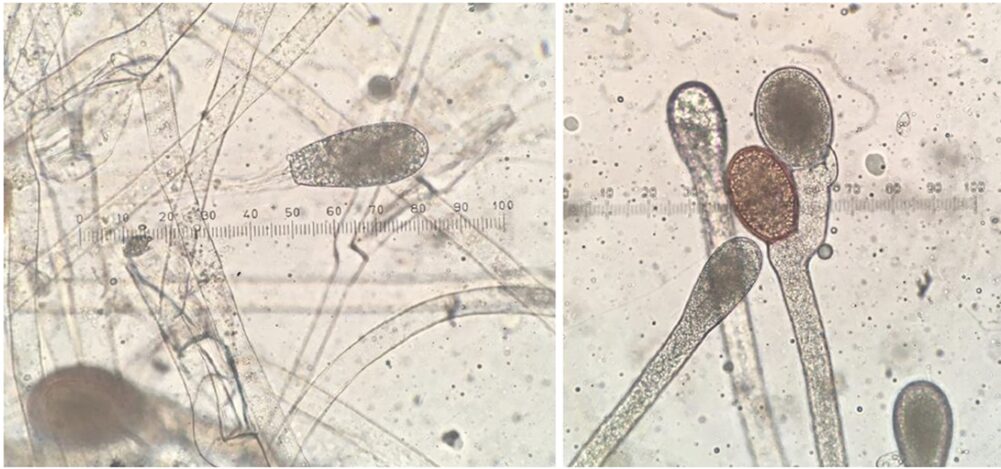
Fig. 10: Asexual zoosporangium at the end of hyphal filament (left). Two resting sporangia, a result of sexual reproduction (right) (Morrow, 2020).
The unique spores of ingoldian aquatic hyphomycetes
Ingoldian aquatic hyphomycetes (AQH) are one of the most studied species of aquatic fungi due to their abundance and ability to be easily recognized. Hyphomycetes’ spores are quite large compared to those of other species, spanning 50-100 μm or sometimes even more. They are mostly seen in one of their two shapes, tetraradiate or sigmoid. Tetradiate hyphomycete spores are star-shaped, with four branched arms, while sigmoid hyphomycete spores are worm-shaped (see Fig. 11.). In contrast to other groups of fungi, hyphomycetes can be identified by their unique spores, which have evolved to live in water (Dix et al., 1995). AQH spores are frequently sourced from bubbles and foam in rivers, which led researchers to find that spores with a tetraradiate shape are easily trapped in the foam. Specifically, the shape of the spores allows them to be trapped on the surface of air bubbles due to lowered surface tension (Selosse et al., 2008). This characteristic may create differences in AQH spore distribution, with more tetradiate spores being able to spread further since they use the foamy, rushing waters to their advantage, while sigmoid spores remain contained in calmer waters (Seena et al., 2022).
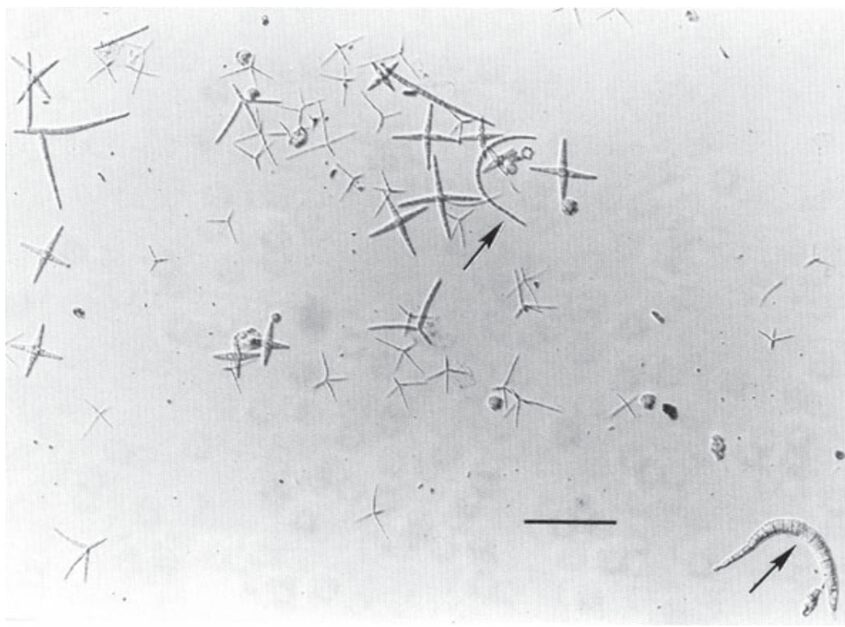
Fig. 11.: Spores of Ingoldian aquatic hyphomycetes collected from foam in River Teigen, England. Most spores in this sample are tetraradiate. Arrows indicate sigmoid spores. Scale bar is 100 μm (Dix et al., 1995).
Common aquatic fungi
Three of the most common species of aquatic fungi include the Ingoldian aquatic hyphomycetes, chytridiales, and ascomycetes.
Ingoldian aquatic hyphomycetes, named after Terence Ingold, the scientist who first classified these organisms as aquatic fungi prefer turbulent, well-aerated, and non-polluted streams. Their usual substrates include leaves, twigs, and even branches fallen into the water from deciduous trees. They spread asexually using their unique spores. Hyphomycetes are of ecological importance since they play a key role in the conditioning and decomposition of plant leaves, making their nutrients available for other aquatic life (Dix et al., 1995).
Chytridiales, commonly known as chytrids, are an aquatic fungus that can be found in marine and freshwater habitats (Richards et al., 2012) Their thalli (plant body) can be eucarpic with only one end forming spores, or monocentric, with only a single spore being produced by one plant body. This simple morphology makes chytridiales very challenging to identify (Dix et al., 1995). They cannot degrade cellulose, which makes wood a poor substrate for them. They prefer living in noncellulosic substrates, such as dead or living insects and pollen grains. Their affinity for live insects makes them parasitic (Wong et al., 1998). They are also known to be decomposers of algae (Dix et al., 1995).
Ascomycetes are common in lotic environments; they prefer woody or leafy substrates and can live in lentic and lotic freshwater environments. Ascomycetes grow long hyphae that are 2-3 μm wide. It is believed that ascomycetes can be terrestrial as well, but ones indigenous to aquatic habitats have sticky appendages that may enable their spores (ascospores) to stick onto substrates in moving water (Dix et al., 1995).
Types of reproduction
Either sexual or asexual reproduction is possible in aquatic fungi (See Fig. 12. for an example). In asexual reproduction, spores form in a structure called a sporangium. Depending on the types of fungi, this could be the conidia, perithecium, or many other structures (Strullu-Derrien, 2016). These spores contain the full DNA of the fungus, making them diploid. These spores are called zoospores. The conidia are made to help the spores disperse effectively. After dispersal, the spores find a place to land and adhere to the substrate. Once attached, if the spore has a tail-like structure known as a flagellum, it pulls it into the cell and a cell wall is formed, creating a cyst. This process is called encysting. The fungus gains access to the substrate through penetration hyphae, originating from the cyst. These hyphae then feed off the substrate and more zoospores are produced and dispersed as the organism multiplies (Sewell et al., 2021). Examples of fungi with flagella are Blastocladiomycota, Chytridiomycota, and Neocallimastigomycota (Money, 2016a).
Within the category of fungal sexual reproduction, there are different mechanisms. First, two compatible haploid hyphae can merge their DNA and then multiply. Second, two meiospores or gametes can merge, and multiply. To increase the likelihood of this, some female gametes release a pheromone to attract the male spores to them (Money, 2016a). This sexual reproduction allows for diversification in the DNA of fungi. Meiosis is performed to produce haploid meiospores. The structure that produces meiospores is called gametangia, as opposed to the non-meiotic sporangia (Money, 2016a).
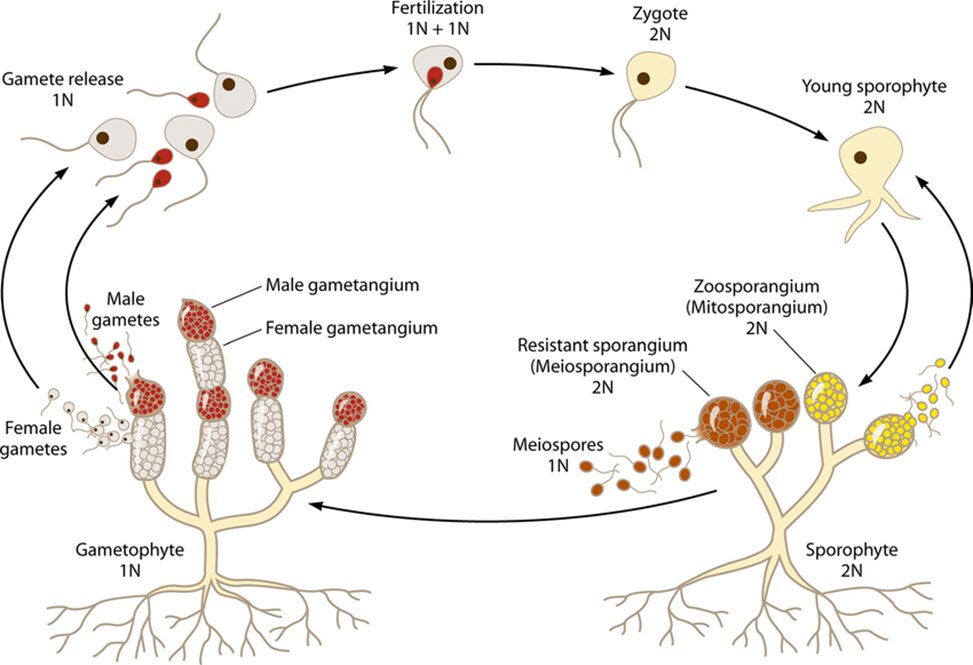
Fig. 12.: A diagram of the Allomyces reproductive cycles (S. C. Lee et al., 2010).
Methods of Spore Dispersal and Movement
Zoospores require energy to travel actively, as they must transit through water before they find an ideal substrate. They acquire this energy by oxidizing lipids and other fuels contained within the spores. This limit of energy supply means that zoospores cannot be active for a very long time. As stated by Money (2016b), “A zoospore swimming at an average speed of 25 μm per second (allowing for frequent stops) for 5 h would travel 0.5 m,” quite a small distance, compared to the sizes of rivers, lakes, and oceans. This indicates that swimming is not the fungi’s main method of dispersal, but merely how it explores the area it has been dispersed in. It is believed that the spore is searching for chemicals and amino acid remnants that indicate there is a potential substrate and food source nearby. This process is called chemotaxis and is described as the migration of a cell towards or away from chemicals that either attract or repulse it (Stock & Baker, 2009).
It has been observed in artificial environments that the zoospores are attracted to dissolved amino acids and sugars. In nature, the spore would also be searching for yet undetermined chemical indicators that signal the presence of an acceptable food source (Money, 2016b). The most likely long-range method of dispersal of fungal spores is by water currents, or through any other movement of water, like a river or, for example, water passing through rocks and soil, carrying spores with it (Golan & Pringle, 2017). This method would require no energy and would leave the zoospore with all its fuel stores once it detects the desired chemicals and starts moving toward them (Money, 2016b).
It is currently unclear whether aquatic fungi release their spores passively or actively, as there is a mix of the two occurring, with a preference for passive release in marine ascomycetes and active ejection in tropical freshwater fungi. It is hypothesized that this occurs due to the seasonal drying of rivers, which affects freshwater fungi. When their primary source of dispersion (water) is gone, they adapt to rely on wind dispersal temporarily. An alternate explanation is that the possibility of flooding causes fungi to end up on the shore and riverbank, creating a necessity for wind dispersal again. In either case, the belief is that freshwater spores can sometimes be dispersed by wind (Golan, 2017 #19).
Ingoldian fungi reproduction
Ingoldian fungi are mostly hyphomycetes and possess many different and unique spore shapes, like crescents, commas, sigmoids, and more (Fig. 13.). Among which is a tetraradiate conidial form, with four branches around a central point of the spore. These spores are created by Ingoldian fungi’s conidiophores and are therefore an asexual method of reproduction that it utilises. It is theorized that these spread out and odd shapes of spores are preferred to the spherical ones because the spores are less heavy but able to cover approximately the same amount of area as spherical spores, with the limbs likely to encounter any organic material the spore might come across. The tetraradiate form contains a lot less cytoplasm and is made up of less matter (Money, 2016b).
Because water is more viscous than air, and spores are generally quite light, aquatic fungi’s spore dispersal is slowed down greatly, in comparison to air dispersion. The spores have a much greater sedimentation rate, meaning that they adhere to a substrate much later than terrestrial airborne spores (Golan & Pringle, 2017).
These Ingoldian spores’ odd shapes could help them disperse through water more easily while also maintaining a larger potential contact area to encounter substrates they could attach to. Studies showed that conidiophores with branches fell through water at the same rate as smaller and denser spores, therefore, the main difference between them is that the branched spores, due to being more spread out, are more likely to come in contact with potential substrates. For example, the tetraradiate spores have a large surface area compared to their volume and weight, and their unique shape with branches going out in all four directions makes it very likely that they will encounter the same substrates a spherical spore would, while weighing less and using less resources to make (Money, 2016b). An oddly shaped Ingoldian spore can be as much as 400 times lighter than a more typical spherical spore of the same diameter. However, it will only have about 30 times less cell wall to produce since the surface area was reduced less compared to the volume. There is a similar development in basidiospores, leading to the theory that this was a useful evolutionary feature since this similarity is believed to be a result of convergent evolution. Spores are believed to have optimized their shape to find a substrate to attach to and feed from more easily. While these spores are asexual and dispersed in water, they do not possess a flagellum to help them move, so they are considered non-motile spores (Money, 2016b).
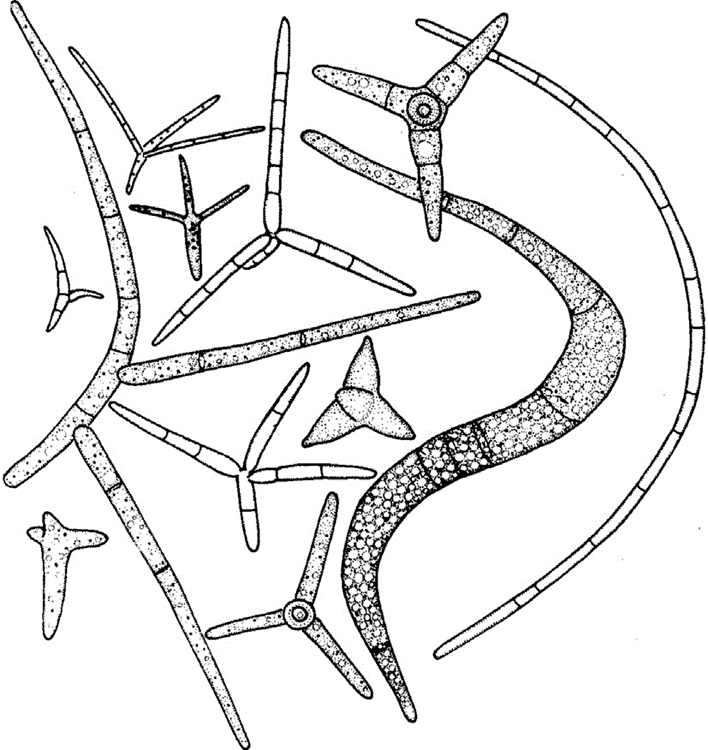
Fig. 13.: Different types of Ingoldian spores produced in freshwater (Money, 2016b).
Non-motile and motile spores
The spores produced by Basidiomycota, Ascomycota, Glomeromycota, and filamentous zygomycetes do not have flagella, unlike other aquatic fungi. It is hypothesized by researchers that there was a single common ancestor for all the non-flagellate fungi groups. (Money, 2016a). Motile Fungi spores are called zoospores. They possess one flagellum which helps them move in water and are produced by Blastocladiomycota, Chytridiomycota, and many more aquatic fungi. They can serve as gametes for the Blastocladiomycota but can also be used for asexual reproduction or to find food for the fungus. Chytrids have only been found to produce asexual spores, but it is hypothesized that, in rare cases, they do produce meiospores. (Strullu-Derrien, 2016). Zoospores tend to have a spherical-like shape with a flagellum (Money, 2016a).
Chytridiomycota spores
Chytridiomycota are both freshwater and marine fungi. In Chytrids, their whip-like flagellum (Fig. 14.) can push the zoospore forward at a maximum speed of 100 micrometers/s, which is equivalent to 40 times the cell’s size per second. A challenge that these zoospores face, since they lack a cell wall, is maintaining cell shape within the water, without lysing, since unimpeded osmosis would lead to turgor pressure, when the fluid within the cell pushes against the walls, causing the spore to burst. Two ways that spores deal with this is by having contractile vacuoles and active ion exchange across the cell membrane to regulate the amount of water in the cell (Money, 2016b). Some spores utilise a contractile vacuole to regulate their osmotic pressure, through a process called osmoregulation. The vacuole collects excess water in the cell and then contracts to expel it through a pore in the membrane. This helps prevent the spore from bursting (Smith et al., 2012). Chytrid zoospores specifically, do not rotate when swimming and move in either straight or circular paths. They use their flagellum to change direction and can act amoeba-like for short periods of time when over surfaces, before returning to swimming. This amoeba-like behaviour includes pseudopodia-like protrusions and movement (Money, 2016b).
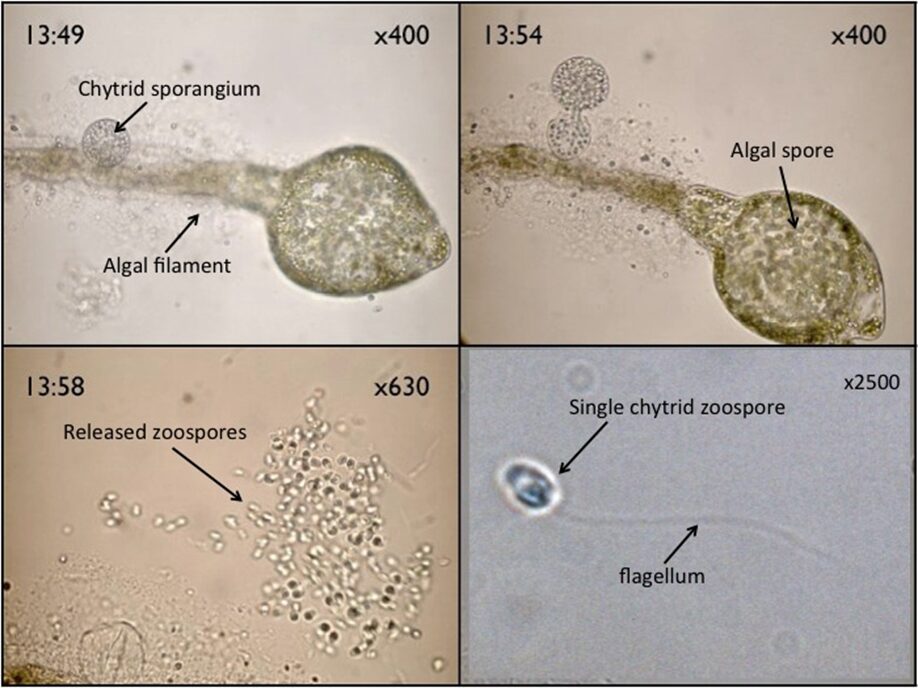
Fig. 14.: Chytrid zoospores, and a sporangium on an algal filament (Walker).
Entomophthorales fungi’s reproductive adaptation
Entomophthorales fungi, which feed off living insects, have an interesting reproductive adaptation. Their sporangium can coat its spores in a sticky substance that facilitates the spore’s adherence to insects, aiding in reproduction. Entomophthorales’s discharge of its spores is an active process, leading to them being called ballistospores, since they are launched by the fungus (Boddy, 2016).
Blastocladiomycota’s reproduction
Allomyces, a type of Blastocladiomycota, are freshwater fungi. They produce separate haploid and diploid fungal colonies, which are difficult to differentiate. A survival characteristic that allomyces possess is the ability to produce different types of spores when nutrients and resources become limited. The hyphae stop extending and produce different types of reproductive structures at their tips (see Fig. 12.). In these circumstances, the diploid colony forms two types of spores, meiospores, and zoospores, with the zoospores each containing a single flagellum to help the spore move in the water. These spores possess a nuclear cap, composed of many membrane-bound ribosomes surrounding the nucleolus of the spore. This structure is unique to Blastocladiomycota. The spores produced when resources are scarce are chemotactic and move towards sources of dissolved amino acids, searching for food and potential sources of sustenance. Once a substrate is located, the zoospores attach themselves to the substrate and form rhizoids that reach into the substrate. The hyphae of the new colony form from where the spore attached itself, reaching into the water (Money, 2016a).
Symbiosis in aquatic fungi
It is defined that marine fungi are those that “form symbiotic relationships with other marine creatures” (Jones et al., 2019). Mutualism, commensalism, and parasitism are three common symbiotic states in nature, all affecting the organism to some extent, from benefiting each other to even leading to the death of both. Marine fungi develop these three relationships with others for survival.
Mutualist relationships
Corals
A special type of marine fungi called endolithic fungi has attracted much attention over the years. They are considered “rock-transforming microorganisms” since they can grow through solid materials (Jones et al., 2019). A common example of endolithic fungi is seen in corals, where studies have shown that the hyphae of the fungi could penetrate layers of carbonate skeletons of corals, while others may pass through a septum (see Fig. 15.), (Kendrick, 1983). It had long been considered that these endolithic fungi penetrators would harm the coral skeleton, making them parasitic to corals. However, a more recent study suggests otherwise. Endolithic fungi have been found to play an important role in nitrogen cycling within corals, as they transform nitrate and nitrite into ammonia, which is then assimilated into essential organic nitrogen used for biosynthesis for corals (see Fig. 16.) (Wegley et al., 2007). Thus, fungi in corals are depicted as being potentially mutualistic, shifting the view on their relationships with marine organisms (Amend, Barshis, & Oliver, 2012). Recent research has also found that coral organisms are able to adapt to different ecological scenarios by changing what microscopic organisms are attached to them (Wegley et al., 2007). “Coral holobiont” is a hypothesized term referring to the coral organism and all the microorganisms that live within the coral, for example, algae, fungi, and bacteria. The coral can switch their partnership with the microorganisms based on what is most beneficial at a particular time to ensure its survival (Wegley et al., 2007). While this mechanism for corals is still very unclear, fungi have been shown to be involved in the beneficial nitrogen cycling in corals.
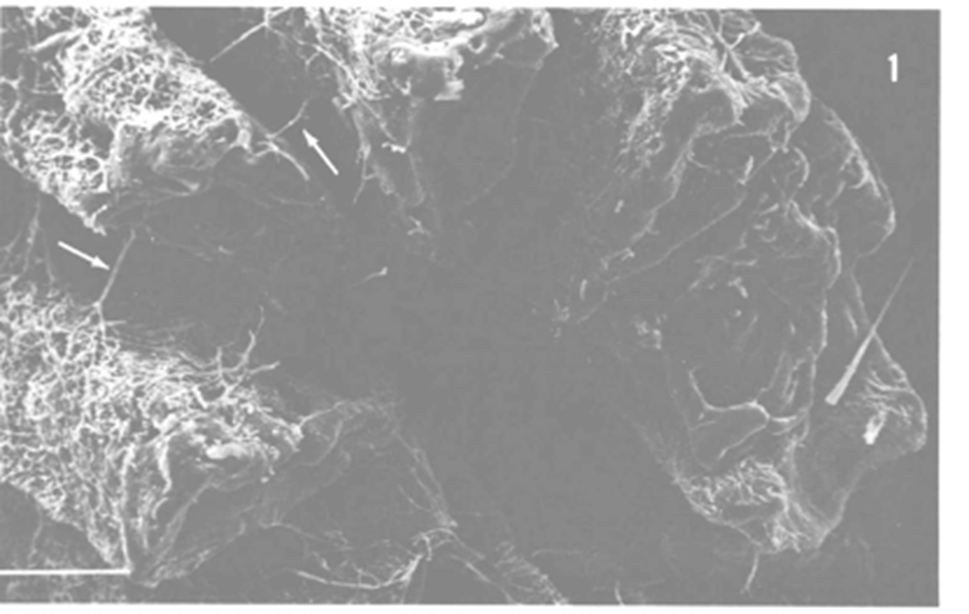
Fig. 15.: Electron micrograph of a fugus-bored corallite. Hyphae can be seen passing through the layers, and arrows indicating passing through a septum (Kendrick, 1983).
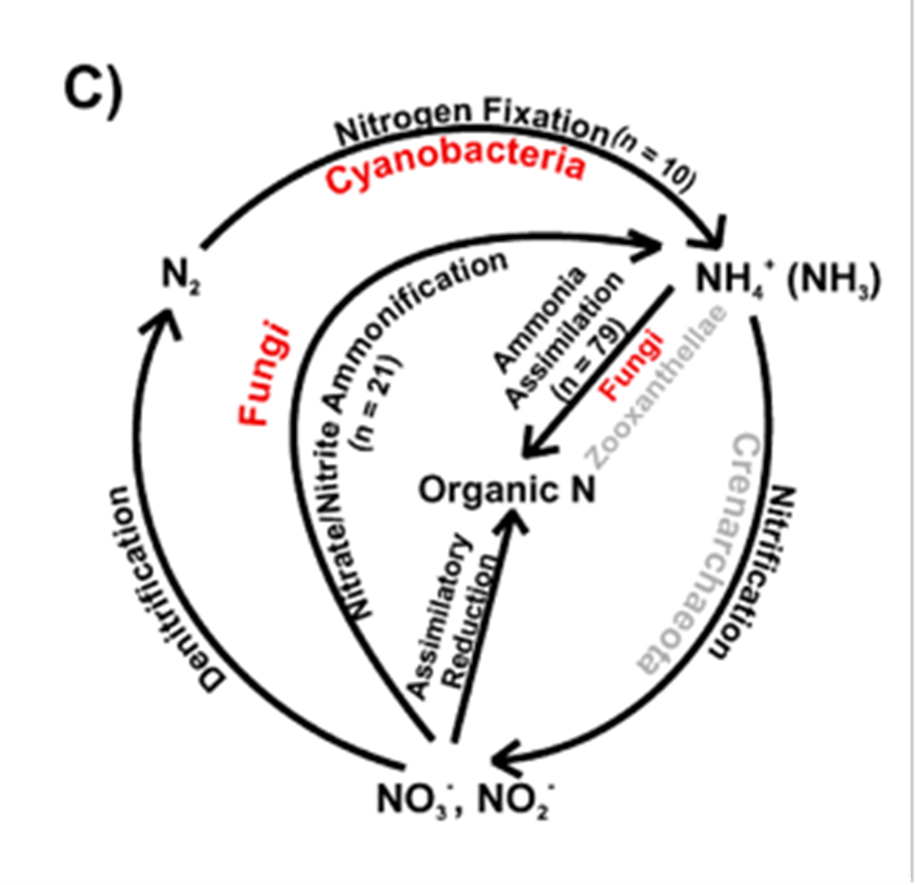
Fig. 16.: Processes of the nitrogen cycle within the coral (Wegley et al., 2007).
Trichomycetes
Trichomycetes, also called “gut fungi”, live in the digestive tracts of freshwater and marine arthropods or larvae, and form an obligate symbiont relationship with the host (Boddy, 2016). All trichomycetes attach to the host’s gut lining, usually the hindgut (Misra, 1998), by a holdfast, whether cellular or noncellular (Benny, 2001), and most of them do not penetrate the membrane of the gut (Boddy, 2016). Based on their species, most of them are categorized as commensal or mutualistic, but a few have been identified as parasitic (Boddy, 2016). As the host’s gut fluids flow past them, they absorb nutrients and sometimes may aid in food digestion (Boddy, 2016). An experiment by Horn has shown that the mosquito larvae growing with the trichomycetes survived better than the mosquito larvae without them under certain same conditions (Misra, 1998), suggesting that fungi are able to provide essential nutrients needed for the larvae to help their survival (Lichtwardt, 2002). Vitamin B and some sterol are provided by the fungi when the larvae are in an environment lacking these necessities (Lichtwardt, 2002). Thus, depending on the situation and the species, their symbiotic relationships may change, from commensal to mutualistic to help the survival of their host (Lichtwardt, 2002).
Parasitic relationships
Chytrid fungi
Marine fungi can also be parasitic and cause catastrophic effects on the biological system. Chytridiomycosis, caused by specific Chytrid fungi Batrachochytrim dendrobatis (Bd) and B. salamandrivorans (Bsal), is a well-known disease for killing frogs (see Fig. 17.). Bsal has been declared to be responsible for the extinction of more than 90 species worldwide, making them “the worst disease to impact biodiversity” (Larissa, 2020). To amphibians, especially frogs, skin is very essential for maintaining homeostasis like gas exchange for their survival (Larissa, 2020). Chytrid fungi appear to kill amphibians by attaching to their permeable skin, leading to the degradation of the skin and interruption of corresponding functions. (Chytrid Fungus, 2022) Specifically, Bd infection causes an electrolyte imbalance in the skin by inhibiting sodium absorption, and thus, disrupts the osmoregulatory function in the skin (Voyles et al., 2009). This change in electrolytes then leads to frog heart contractile dysfunctions and ultimately leads to the disorder of the circulatory system and death (Voyles et al., 2009). The disruption mechanism to the sodium channels still remains unknown, but it could be because of certain toxins that are released by the fungi (Voyles et al., 2009). Bd only invades the keratinized epithelium of amphibians, including the oral disk of tadpoles and the keratinized skin of other animals (Pessier, 2014). Tadpoles do not have keratinized skin, but during metamorphosis, their epidermis is keratinized and chytrid fungi will move from their oral disk to the skin and begin infection (Pessier, 2014). The presence of Bd in the deeper part of the skin or in organs has not been observed so far (Pessier, 2014). These potent fungi can be found mainly on the bird’s feathers, insects, and animals’ feet (Mike, 2023).
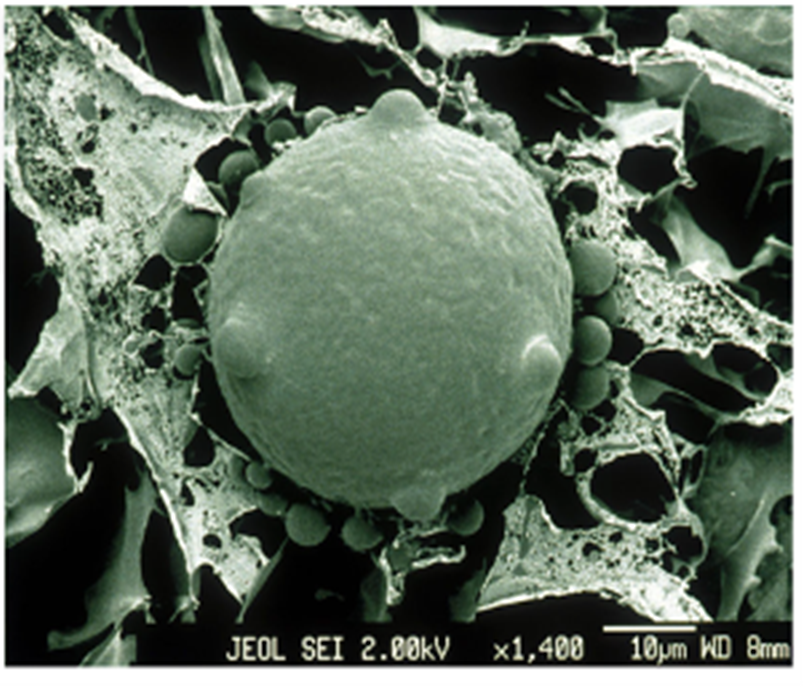
Fig. 17.: An electron micrograph of chytrid fungus (Larissa, 2020).
Mosquito larvae
Another area of parasitic fungi that has also received considerable attention is their attack on mosquito larvae, with the two main attackers being Coelomomyces fromtheChytridiomycetes class and Lagenidium giganteum from the Oomycetes class (Federici, 2009).For instance, they have been known to cause epizootics in Africa and killed more than 95% population of the larval (Federici, 2009). In one case, for Class Oomycetes, the fungi’s zygospore invades a mosquito larva, and once it gets into the hemocoel, it colonizes the larvae’s body by producing massive amounts of mycelium of non-septate hyphae (the partitions on hyphae are called Septa, hyphae without partitions are called aseptate) in about 2 days, making the larva dead (Federici, 2009). Then the hyphae turn septate by the end of their growth, and a motile zoosporangium is formed and exited from the exit tube in each hyphae’s segment end (Federici, 2009). In this asexual cycle, zoospores are constantly produced and looking for a new substrate to continue their survival. For the Chytridiomycetes case, the process is quite similar. By invading the hemocoel of the host, Coelomomyces fungi can release thousands of their uniflagellate gametes by cleaving through the host, which causes their death (Federici, 2009). Then the fungi fuse themselves to be biflagellate to fulfill their life cycle and they will look for the next host (Federici, 2009). Thus, the cycle is completed and continuous for fungi to survive.
Fungi as decomposers
Another big part of aquatic fungi is freshwater fungi, most of them are categorized as decomposers in nature and are said to have evolved from terrestrial ancestors (Wong et al., 1998). Those that decompose wood are called freshwater lingnicolous fungi, and they are usually found on woody debris in water in places like streams and lakes (sometimes in dams as well) (Hyde et al., 2016). Lingnicolous fungi release enzymes to soften the debris, in particular, to decompose the lingnocellulose in plant tissues (Hyde et al., 2016). They grow in the wood’s soft rot S2layer and produce narrow penetration tubes by hyphae to help them stick and grow further (Hyde et al., 2016). An experiment has shown that lignin, the most difficult component in wood to break down is dissolved by freshwater fungi, which proves their role in decomposing woody debris (Bucher et al., 2004). Usually, the debris would lose a significant amount of weight and reduce the debris’ crushing ability (Wong et al., 1998).
Decomposition of coarse particle organic matter
Aquatic fungi, mainly aquatic hyphomycetes (AQH), play a key role in the decomposition of coarse particle organic matter, which is a process that supplies other aquatic life with crucial nutrients. When coarse particle organic matter (CPOM), such as leaves and twigs enters the body of water, AQH colonizes them and uses their enzymes to break down the structural polymers in the plant matter. This breakdown allows bigger aquatic invertebrates called shredders to get nutrients from the CPOM. Without AQH, shredders would not have access to CPOM’s nutrients as they lack the enzymes for CPOM’s breakdown. Fungi also gain nutrients during the breakdown of CPOM, which it uses to spread its spores and increase its biomass. Shredders then continue the breakdown, gaining more nutrients for themselves and releasing further nutrients for other aquatic organisms (Seena et al., 2022).
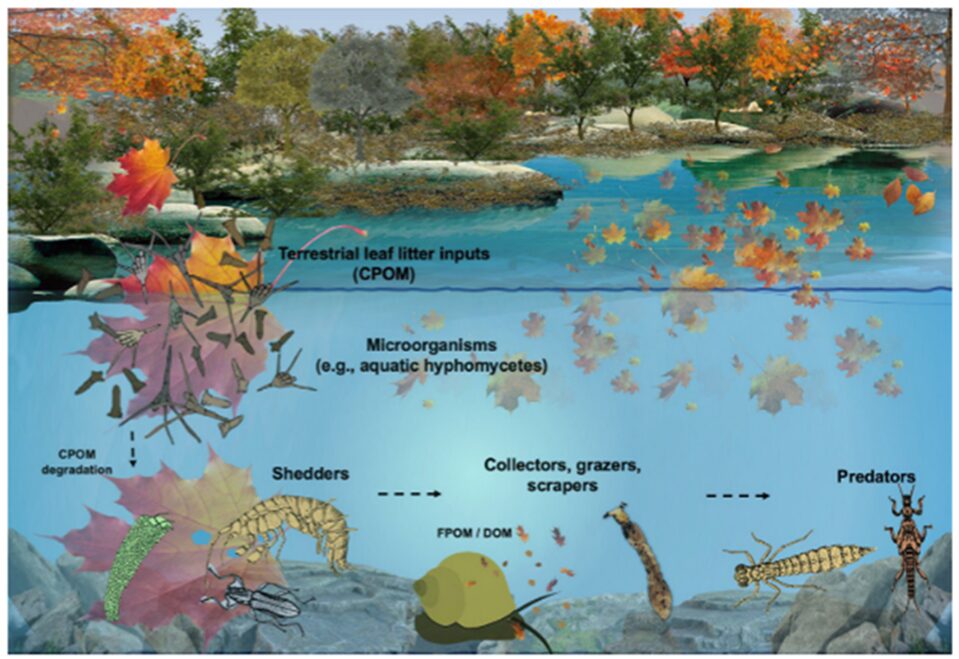
Fig. 18.: Schematic representation of coarse particle organic matter decomposition process in a stream (Seena et al., 2022).
Aquatic fungi and other microorganisms
Besides fungi’s complicated relationship with macroscopic organisms, fungi have also developed diverse relationships with microscopic organisms. It has been shown that there is often resource competition directly between bacteria and fungi. For example, antifungal-producing bacteria have been found on the skin of amphibians, to inhibit the growth of the infectious chytrid fungi as discussed before. Thus, negative interactions are shown between bacteria and fungi in the aquatic environment. Furthermore, negative interactions are also found between fungi and diatoms, and it has been proved their interaction has a negative effect on the production of biofilm. Fungi can also be both predators and prey of heterotrophic protists. For instance, zoosporic fungi have been found to be the food for Heliozoa, a species of eukaryote but at the same time, are parasitic to Heliozoa. Fungi and protists could also share a common host as their niches, like the coral holobiont described before. Fungi can also develop parasitic relationships with other fungi; this relationship is given the name mycoparasitism (Grossart et al., 2019). Therefore, fungi play different roles and have a wide range of interactions with other microorganisms in the aquatic community.
Conclusion
In conclusion, aquatic fungi have many different interactions with their environment, the flora and fauna around them, and even each other. Many of these interactions can be considered as design solutions to conundrums in their environment.
First, they have symbiotic relationships, including mutualistic, communalistic, and parasitic, which ensure that aquatic fungi meet their nutritional needs in all environments. Endolithic fungi’s mutualistic relationship with corals allows both organisms to survive and thrive in the ocean. Trichomycetes, help aquatic larvae gain additional nutrients from their food while feeding from its nutrients too. Parasitic chytrids kill and feast on amphibians. This provides a food source for them and other fungi, by breaking down the complex tissues of dead organisms into something other organisms can consume.
Second, in terms of design solutions in their reproductive methods, the aquatic fungi’s spores have adapted to the movement, pressure, and viscosity of water, the potential lack of resources there could be in an area and have increased the likelihood of their spores attaching to the desired substrate. Specific design solutions aquatic spores can have are a contractile vacuole, which prevents too much water from entering the cell and bursting it; active ion exchange across the cell membrane, to regulate the amount of water entering the cell; spores with a sticky coating, like Entomophthorales, to help them adhere to their substrate better; the ability to alter the types of spores they produce when there are fewer resources, like Allomyces.
Third, to combat the challenges faced in aquatic sporulation, specifically spores not being able to spread in stagnant water, aquatic hyphomycete spores have adapted their shape to stay trapped in the surface of air bubbles in foam, allowing them to travel and spread far. Some spores also have a flagellum, a tail that propels them through water, an advantage for underwater mobility.
Finally, sometimes plant litter is not palatable to invertebrates living in aquatic environments. Aquatic fungi have resolved this issue by breaking down certain polymers in the litter so their co-habitants can also benefit from this source of nutrients. Aquatic fungi use their unique traits to thrive, reproduce, disperse, and decompose, and use their adaptations to solve many of the challenges they are faced with in aquatic environments.
References
Amend, A. S., Barshis, D. J., & Oliver, T. A. (2012). Coral-associated marine fungi form novel lineages and heterogeneous assemblages. ISME J, 6(7), 1291-1301. https://doi.org/10.1038/ismej.2011.193
Benny, G. L. (2001). Zygomycota: Trichomycetes. In D. J. McLaughlin, E. G. McLaughlin, & P. A. Lemke (Eds.), Systematics and Evolution: Part A (pp. 147-160). Springer Berlin Heidelberg. https://doi.org/10.1007/978-3-662-10376-0_7
Boddy, L. (2016). Chapter 9 – Interactions with Humans and Other Animals. In S. C. Watkinson, L. Boddy, & N. P. Money (Eds.), The Fungi (Third Edition) (pp. 293-336). Academic Press. https://doi.org/https://doi.org/10.1016/B978-0-12-382034-1.00009-8
Bryce Kendrick, R., Michael J., John Michaelides, Katherine Bergman,. (1983). Amphibious microborers: bioeroding fungi isolated from live corals. Deep Sea Research Part B. Oceanographic Literature Review, 30(6), 469. https://doi.org/10.1016/0198-0254(83)90258-3
Calabon, M., Hyde, K., Jones, E., Bao, D.-F., Bhunjun, C. S., Phukhamsakda, C., Shen, H.-W., Gentekaki, E., Al Sharie, A., Barros, J., Chandrasiri, S., Hu, D.-M., Hurdeal, V., Rossi, W., Guardia Valle, L., Zhang, H., Figueroa, M., Raja, H., Sahadevan, S., & Balasuriya, A. (2023). Freshwater fungal biology. 14, 195–413. https://doi.org/10.5943/mycosphere/14/1/4
Cunliffe, M. (2023). Who are the marine fungi? Environ Microbiol, 25(1), 131-134. https://doi.org/10.1111/1462-2920.16240
Daugherty, M. Chytrid fungus. Center for Invasive Species Research. (2022, September 28). https://cisr.ucr.edu/invasive-species/chytrid-fungus
David Moore, V. A., Constantine John Alexopoulos. (2023). Fungus. In Encyclopedia Britannica.
Dix, N. J., Webster, J., Dix, N. J., & Webster, J. (1995). Aquatic fungi. Fungal Ecology, 225-283.
Federici, B. A. (2009). Chapter 193 – Pathogens of Insects. In V. H. Resh & R. T. Cardé (Eds.), Encyclopedia of Insects (Second Edition) (pp. 757-765). Academic Press. https://doi.org/https://doi.org/10.1016/B978-0-12-374144-8.00202-2
Gladfelter, A. S., James, T. Y., & Amend, A. S. (2019). Marine fungi. Current Biology, 29(6), R191-R195. https://doi.org/https://doi.org/10.1016/j.cub.2019.02.009
Golan, J. J., & Pringle, A. (2017). Long-Distance Dispersal of Fungi. Microbiology Spectrum, 5(4), 10.1128/microbiolspec.funk-0047-2016. https://doi.org/doi:10.1128/microbiolspec.funk-0047-2016
González-Martínez, S., Soria, I., Ayala, N., & Portillo, A. (2017). Culturable halotolerant fungal isolates from Southern California Gulf sediments. Open Agriculture, 2. https://doi.org/10.1515/opag-2017-0033
Grossart, H.-P., Van den Wyngaert, S., Kagami, M., Wurzbacher, C., Cunliffe, M., & Rojas-Jimenez, K. (2019). Fungi in aquatic ecosystems. Nature Reviews Microbiology, 17(6), 339-354. https://doi.org/10.1038/s41579-019-0175-8
Gulis, V., Kuehn, K. A., & Suberkropp, K. (2009). Fungi. In G. E. Likens (Ed.), Encyclopedia of Inland Waters (pp. 233-243). Academic Press. https://doi.org/10.1016/B978-012370626-3.00129-0
Howard, R. J., Ferrari, M. A., Roach, D. H., & Money, N. P. (1991). Penetration of hard substrates by a fungus employing enormous turgor pressures. Proc Natl Acad Sci U S A, 88(24), 11281-11284. https://doi.org/10.1073/pnas.88.24.11281
Hyde, K. D., Fryar, S., Tian, Q., Bahkali, A. H., & Xu, J. (2016). Lignicolous freshwater fungi along a north–south latitudinal gradient in the Asian/Australian region; can we predict the impact of global warming on biodiversity and function? Fungal Ecology, 19, 190-200. https://doi.org/https://doi.org/10.1016/j.funeco.2015.07.002
Ittner, L. D., Junghans, M., & Werner, I. (2018). Aquatic Fungi: A Disregarded Trophic Level in Ecological Risk Assessment of Organic Fungicides [Review]. Frontiers in Environmental Science, 6. https://doi.org/10.3389/fenvs.2018.00105
Jobard, M., Rasconi, S., & Sime-Ngando, T. (2010). Diversity and functions of microscopic fungi: a missing component in pelagic food webs. Aquatic Sciences, 72(3), 255-268. https://doi.org/10.1007/s00027-010-0133-z
Jones, E. B. G., Pang, K.-L., Abdel-Wahab, M. A., Scholz, B., Hyde, K. D., Boekhout, T., Ebel, R., Rateb, M. E., Henderson, L., Sakayaroj, J., Suetrong, S., Dayarathne, M. C., Kumar, V., Raghukumar, S., Sridhar, K. R., Bahkali, A. H. A., Gleason, F. H., & Norphanphoun, C. (2019). An online resource for marine fungi. Fungal Diversity, 96(1), 347-433. https://doi.org/10.1007/s13225-019-00426-5
Krauss, G.-J., Solé, M., Krauss, G., Schlosser, D., Wesenberg, D., & Bärlocher, F. (2011). Fungi in freshwaters: ecology, physiology, and biochemical potential. FEMS Microbiology Reviews, 35(4), 620-651. https://doi.org/10.1111/j.1574-6976.2011.00266.x
Kumar, V., Sarma, V. V., Thambugala, K. M., Huang, J. J., Li, X. Y., & Hao, G. F. (2021). Ecology and Evolution of Marine Fungi With Their Adaptation to Climate Change. Front Microbiol, 12, 719000. https://doi.org/10.3389/fmicb.2021.719000
Larissa. (2020, December 18). CHYTRID: The frog-killing fungus. Curious. https://www.science.org.au/curious/earth-environment/chytrid-frog-killing-fungus
Lee, S. C., Ni, M., Li, W., Shertz, C., & Heitman, J. (2010). The Evolution of Sex: a Perspective from the Fungal Kingdom. Microbiology and Molecular Biology Reviews, 74(2), 298-340. https://doi.org/doi:10.1128/mmbr.00005-10
Lepère, C., Domaizon, I., Humbert, J. F., Jardillier, L., Hugoni, M., & Debroas, D. (2019). Diversity, spatial distribution and activity of fungi in freshwater ecosystems. PeerJ, 7, e6247. https://doi.org/10.7717/peerj.6247
Lichtwardt, R. W. (2002). Trichomycetes: Fungi in Relationship with Insects and Other Arthropods. In J. Seckbach (Ed.), Symbiosis: Mechanisms and Model Systems (pp. 575-588). Springer Netherlands. https://doi.org/10.1007/0-306-48173-1_36
Medina, E. M., & Buchler, N. E. (2020). Chytrid fungi. Current Biology, 30(10), R516-R520. https://doi.org/10.1016/j.cub.2020.02.076
Mike. (2023, July 12). What is Chytrid fungus? (detection, diagnosis and prevention). Amphibian Life. https://www.amphibianlife.com/chytrid-fungus-guide/
Misra, J. K. (1998). Trichomycetes – Fungi associated with arthropods: Review and world literature [Review]. Symbiosis, 24(2), 179-220. https://www.scopus.com/inward/record.uri?eid=2-s2.0-0031808310&partnerID=40&md5=eb0cdb8fc2c47fcb2f0d8cfa19d0bb37
Morrow, M. (2020, July 16). 3.2: Aquatic Fungi (Chytrids). Biology LibreTexts. https://bio.libretexts.org/Bookshelves/Botany/A_Photographic_Atlas_for_Botany_(Morrow)/03%3A_Fungi_and_Lichens/3.02%3A_Aquatic_Fungi_(Chytrids)
Moss, S. T. (1986). The biology of marine fungi. CUP Archive.
Money, N. P. (2016a). Chapter 1 – Fungal Diversity. In S. C. Watkinson, L. Boddy, & N. P. Money (Eds.), The Fungi (Third Edition) (pp. 1-36). Academic Press. https://doi.org/https://doi.org/10.1016/B978-0-12-382034-1.00001-3
Money, N. P. (2016b). Chapter 3 – Spore Production, Discharge, and Dispersal. In S. C. Watkinson, L. Boddy, & N. P. Money (Eds.), The Fungi (Third Edition) (pp. 67-97). Academic Press. https://doi.org/https://doi.org/10.1016/B978-0-12-382034-1.00003-7
Morrow, M. (2020, July 16). 3.2: Aquatic Fungi (Chytrids). Biology LibreTexts. https://bio.libretexts.org/Bookshelves/Botany/A_Photographic_Atlas_for_Botany_(Morrow)/03%3A_Fungi_and_Lichens/3.02%3A_Aquatic_Fungi_(Chytrids)
Naranjo-Ortiz, M. A., & Gabaldón, T. (2019). Fungal evolution: major ecological adaptations and evolutionary transitions. Biol Rev Camb Philos Soc, 94(4), 1443-1476. https://doi.org/10.1111/brv.12510
Pessier, A. P. (2014). Chapter 22 – Chytridiomycosis. In D. R. Mader & S. J. Divers (Eds.), Current Therapy in Reptile Medicine and Surgery (pp. 255-270). W.B. Saunders. https://doi.org/https://doi.org/10.1016/B978-1-4557-0893-2.00022-3
Richards, T. A., Jones, M. D., Leonard, G., & Bass, D. (2012). Marine fungi: their ecology and molecular diversity. Annual review of marine science, 4, 495-522.
Seena, S., Barros, J., Graça, M. A. S., Bärlocher, F., & Arce-Funck, J. (2022). Chapter 1 – Aquatic hyphomycete spores: What do we know, where do we go from here? In S. A. Bandh & S. Shafi (Eds.), Freshwater Mycology (pp. 1-20). Elsevier. https://doi.org/https://doi.org/10.1016/B978-0-323-91232-7.00016-7
Selosse, M.-A., Vohník, M., & Chauvet, E. (2008). Out of the rivers: are some aquatic hyphomycetes plant endophytes? New Phytologist, 178(1), 3-7. https://doi.org/https://doi.org/10.1111/j.1469-8137.2008.02390.x
Sewell, T. R., Longcore, J., & Fisher, M. C. (2021). Batrachochytrium dendrobatidis. Trends in Parasitology, 37(10), 933-934. https://doi.org/10.1016/j.pt.2021.04.014
Smith, J. J., Wiley, E. A., & Cassidy-Hanley, D. M. (2012). Chapter 16 – Tetrahymena in the Classroom. In K. Collins (Ed.), Methods in Cell Biology (Vol. 109, pp. 411-430). Academic Press. https://doi.org/https://doi.org/10.1016/B978-0-12-385967-9.00016-5
Stock, J. B., & Baker, M. D. (2009). Chemotaxis. In M. Schaechter (Ed.), Encyclopedia of Microbiology (Third Edition) (pp. 71-78). Academic Press. https://doi.org/https://doi.org/10.1016/B978-012373944-5.00068-7
Strullu-Derrien, C. (2016). Fungal Evolution: Aquatic–Terrestrial Transitions. In R. M. Kliman (Ed.), Encyclopedia of Evolutionary Biology (pp. 97-103). Academic Press. https://doi.org/https://doi.org/10.1016/B978-0-12-800049-6.00252-3
Voyles, J., Young, S., Berger, L., Campbell, C., Voyles, W. F., Dinudom, A., Cook, D., Webb, R., Alford, R. A., Skerratt, L. F., & Speare, R. (2009). Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines [Article]. Science, 326(5952), 582-585. https://doi.org/10.1126/science.1176765
Walker, J. (n.d.). The origin and evolution of fungi. The Biology Primer. http://thebiologyprimer.com/the-origin-and-evolution-of-fungi
Wegley, L., Edwards, R., Rodriguez-Brito, B., Liu, H., & Rohwer, F. (2007). Metagenomic analysis of the microbial community associated with the coral Porites astreoides. Environmental Microbiology, 9(11), 2707-2719. https://doi.org/https://doi.org/10.1111/j.1462-2920.2007.01383.x
Wong, M. K. M., Goh, T.-K., Hodgkiss, I. J., Hyde, K. D., Ranghoo, V. M., Tsui, C. K. M., Ho, W.-H., Wong, W. S. W., & Yuen, T.-K. (1998). Role of fungi in freshwater ecosystems. Biodiversity & Conservation, 7(9), 1187-1206. https://doi.org/10.1023/A:1008883716975