The Colorful Chemistry of Oxygen Carriers in Blood
Arnaud Benchetrite, Ines Hafit, Laura Hebert, Shiyuan Qiao
Abstract
This essay focuses on different types of oxygen carriers in the blood. In “The Well-Kept Secrets of Blood: The Mechanism of a Vital Fluid”, an examination of blood properties was made with a special introduction to physiological aspects of erythrocytes (Benchetrite et al., 2020). Indeed, erythrocytes are a key component of blood manifesting some particularities in the way that they ensure oxygen transportation in organisms. Therefore, this essay is specially dedicated to respiratory proteins in blood. An analysis of the structure and function of hemoglobin for vertebrates and hemocyanin for invertebrates to carry oxygen will be discussed allowing for a better understanding of the dynamics and efficacy of transport.
Introduction
About 2.4 billion years ago, Earth’s atmosphere was not filled with oxygen as it is today and was once anaerobic. Thus, when the oxygen concentration in the atmosphere began to increase, living species had to adapt (Hsia et al., 2013). To them, oxygen was a toxin. The adaptations that took place millions of years ago were plentiful and allowed for life as we know it to develop. One of these such adaptations was the evolution of respiratory proteins which enabled oxygen to be carried through the blood and allowed the organism to create energy.
In Archea, two proteins were discovered explaining the ability of a single cell to bind oxygen. The first one is Aeropyrum pernix and is defined by its capacity to undergo respiration with the use of aerobic metabolism (Wang and Thein, 2018). The second one, Methanosarcina acetivorans, differs from the first protein in its capacity to undergo anaerobic reactions forming methane molecules. Over time, these properties, among others, of prokaryotic organisms were shared with eukaryotes via endosymbiosis, allowing for animals to develop similar abilities (Schäfer, 2004). With these adaptations, oxygen carriers in blood evolved, the most well-known of these proteins being hemoglobin (Burmester, 2015). This protein relies on iron to bind reversibly to oxygen in the blood of vertebrates, such as humans, giving blood a distinct red color. Hemocyanin, although less common than hemoglobin, is another respiratory that relies on copper and is found in mollusks and arthropods, giving their blood a blue hue (Burmester, 2015). Other examples of oxygen carriers exist along with their unique blood coloration, ranging from green to purple and to pink. Some animals have even found ways to survive without any respiratory proteins at all, explaining their clear blood (Ruud, 1965). However, these cases are rarer than the two types of respiratory protein previously mentioned and, for the purpose of this essay, the characteristic properties of hemoglobin and hemocyanin will be focused on.
Respiratory proteins all share a common function. However, they each have their respective traits and detailed evolutionary path (Hsia et al., 2013). This allows for a better understanding of the importance of evolution when considering the many molecular adaptations of oxygen carriers throughout the diverse animal kingdom. Protoglobins evolved into hemoglobins who themselves gave rise to the so-called hemoglobin derived peptides including hemocyanin (Coates and Decker, 2017). Thus, this report will explore the interesting molecular secrets of oxygen carriers in blood.
Hemoglobin
Blood – the life sustaining fluid – travels through cardiovascular systems in various organisms and its main function is to deliver oxygen from the alveoli to different parts of the body and to help transport carbon dioxide back to the lungs. In the case of many species, the ability to transport oxygen throughout an organism is allowed by the respiratory protein that can be found in their red blood cells. Erythrocytes not only have various specialities that allow them to travel throughout the body efficiently, but they also contain hemoglobin. This protein is essential as it can bind to oxygen during oxygen transportation and thus carry it throughout the body to deliver the gas to tissues. Moreover, erythrocytes lack many organelles and in mammals, even lack a nucleus. The cell can thus be described as a sack made up of a plasma membrane that contains mostly hemoglobin. In the case of human adults, hemoglobin has a blood concentration of 13.5–18.0 g dl21 in men and 11.5–6.0 g dl21 in case of women, and each erythrocyte contains approximately 200-300 million molecules of hemoglobin (Thomas and Lumb, 2012). This oxygen carrier plays an important role in sustaining the life of diverse species.
Structure of Hemoglobin
Normal adult hemoglobin has a molecular mass of 64 458 Da (Hsia, 1998) and it is a complex allosteric protein with a defined quaternary structure. Each hemoglobin consists of four polypeptide chains – two α-chains of 141 amino acid residues and two β-chains of 146 amino acid residues (Perutz, 1978) that each bind with a heme group. A heme group is a small molecular complex which consists of an organic portion – containing a porphyrin ring which is a chemical structure formed by four pyrrole rings that provides four nitrogen electron pair donors (Casiday and Frey, 1998) – and an inorganic iron ion in the ferrous state (Fe2+) at the center of the group (Fig. 1). It is the presence of this iron ion that gives blood its red color.

In nature, under normal conditions, iron atoms can exist under two different states that determine the iron-oxygen reaction. The first is ferrous iron (Fe2+) which is the general form of iron that organisms consume. The other state is ferric iron (Fe3+). One general example of ferric iron is iron oxide, or rust. It is a typical example of an iron-oxygen reaction suggesting that such a reaction would typically be irreversible, which is not expected during blood oxygen transportation. To solve this problem, the blood develops a complex porphyrin ring structure such that it ensures the iron-oxygen interaction will be reversible and controlled. The Fe2+ atom forms six bonds in total within the whole structure, where four bonds are with the nitrogen in each of the four pyrrole rings (Fig. 2), the fifth bond is covalent with a nearby histidine residue (Baldwin and Chothia, 1979). This covalent bond is an important ligation site that plays a role in the change of conformation of hemoglobin which will be addressed shortly. The final bond will require the oxygen molecule (Thomas and Lumb, 2012).

Each heme group is considered a single subunit of hemoglobin and in the complete structure, four subunits (heme) are folded and closely jointed, forming a tetramer (Perutz, 1978). Therefore, in the overall structure, each hemoglobin molecule can carry four oxygens which bind reversibly to each heme group.
Structural Change of Hemoglobin During Oxygen -binding
The special structure of hemoglobin gives it efficient binding capacity to oxygen molecules. During transportation, oxygen molecules will bind to each heme group and will be released in the respiring tissues.
To continue, another important characteristic of hemoglobin protein are its allosteric properties. An allosteric protein is a special type of protein with multiple binding-sites where ligands binding to one binding-site can affect another site (Monod et al., 1965). In the case of hemoglobin, the ligand will be the oxygen molecule and the binding of oxygen will trigger a change in the overall conformation of the hemoglobin, resulting in it having a different oxygen affinity. Before the ligand binds with the protein, the entire structure of hemoglobin is held tightly by electrostatic forces in a tense (T) conformation, where only the α-chains can bind with oxygen molecules. This is due to the presence of H-bonding and a salt bridge that prevents the binding of oxygen on the β chains. This T conformation results in the deoxyhemoglobin having a relatively low affinity for oxygen binding (Hsia, 1998).
When a hemoglobin binds to oxygen molecules, it results in a change to both tertiary and quaternary structures of the protein. In the tertiary structure – a single heme group, without the binding of the oxygen, the iron ion is above the heme plane – the surface created by the porphyrin ring which is a flat molecule (Baldwin and Chothia, 1979). When the oxygen binds to the iron ion, it “drags” the iron ion down into the plane, also causing the His F8, which is connected to the iron ion, to be “dragged” down by the same degree (Fig. 3). This change alters the tertiary structural change that breaks the salt bridges which serve to stabilize the low-affinity conformation (Baldwin and Chothia, 1979). The change in the tertiary structure also influences the quaternary structure of hemoglobin.

The four chains that form the protein can be divided in pairs – α1β1dimer and α2β2dimer – these two subunit pairs move as one rigid body. The oxygen-binding process will cause the ionic bond that links the two dimers to break, and a phenomenon will occur where α2β2dimer rotates 15º away from α1β1dimer (Fig. 4). This creates a new distinct conformation of hemoglobin which is called the R (relaxed) state (Traut, 2007). In the R state, oxyhemoglobin is no longer restricted by a salt-bridge or under the influence of steric hindrance, it has an affinity for oxygen 500 times higher than the T conformation of deoxyhemoglobin (Hsia, 1998).

Oxygen Dissociation Curve
Hemoglobin’s property of having different oxygen affinities depending on whether it is in the T or R conformation is extremely important during oxygen transportation. The relationship between the amount of oxygen binding and concentration of hemoglobin is generally expressed as the Hufner’s constant. This constant represents how, in theory, each gram of hemoglobin can bind to 1.39 mL of oxygen. However, due to limiting conditions – such as the presence of abnormal forms of hemoglobin in the blood, this data is often reduced to around 1.31 mL per gram of hemoglobin (McLellan et al., 2004).
The capacity of hemoglobin to carry an oxygen molecule is expressed as the hemoglobin saturation, which is a percentage ratio of the number of bound oxygens over the total number of binding sites available. Hemoglobin saturation change depends on the number of oxygen molecules that it has been exposed to, which is influenced by the partial pressure of oxygen diffusing into the blood stream from the alveoli in the lungs over a concentration gradient. This change in oxygen affinity is due to the hemoglobin’s allosteric property as mentioned previously, and this relationship can be expressed as the oxygen dissociation curve (ODC).
In the general case of adult hemoglobin, the ODC is represented as a sigmoid-shaped curve (Winslow, 2007) (Fig. 5). This curve reveals the important relationship between oxygen partial pressure and percentage saturation of hemoglobin: hemoglobin in the T state has a relatively low affinity to oxygen, where the gradient of the curve is flat at low oxygen pressure. When oxygen molecules start to bind to hemoglobin, changing hemoglobin to the R state, the sigmoid-shaped curve begins to level out.

P50,the oxygen partial pressure, is one important concept from the oxyhemoglobin dissociation curve. This measure expresses the equilibrium point in the curve for which at P50 the oxygen saturation of hemoglobin is exactly 50 %. Under normal conditions –37 ºC, pH 7.40, and carbon dioxide tension of 40 mm Hg, an adult human will have a standard P50 equal to 26.3 mm Hg (Hsia, 1998). This value is very important in measuring changes in blood, it reveals certain abnormalities and factors that can influence the position of the curve – shifting the curve to the right or left.
Factors that Affect Oxygen Affinity
The primary factors that influence the oxygen affinity of hemoglobin other than the change in quaternary structure are temperature, pH (concentration of H+), concentration of CO2, salts, ATP, and 2,3-biphosphoglycerate. In the following section, the focus will be on pH, the involvement of CO2 in oxygen transportation (hemoglobin behaviors when transporting carbon dioxide back to the lungs), and 2,3-biphosphoglycerate (Winslow, 2007).
The level of pH is one of the most important factors that influences the oxygen affinity of hemoglobin. In general, a decrease in pH levels – increase in the amount of hydrogen protons – or an increase in carbon dioxide concentration can lead to a decrease in the oxygen affinity of hemoglobin. This effect is called the Bohr Effect. This special phenomenon is due to different interactions between H+ ions, CO2 molecules, O2 molecules and the binding sites of hemoglobin. When carbon is present in blood, it will be transported under three forms: it could either diffuse as dissolved gas directly in the blood plasma, bind to the α-chain of hemoglobin thus forming carbaminohemoglobin – HbCOO–, a formation of carbamate from deprotonate carbamic acid – (Hsia, 1998), or finally be hydrated – reacts with a proton – and forms HCO3–. The last form of carbon will be released later in lungs under the form of CO2 and H2O. This hydration/dehydration reaction between H+, CO2 and HCO3– is accelerated by a special enzyme present in the red blood cell – carbonic anhydrase (Geers and Gros, 2000). These three forms are present at equilibrium depending on different situations (Fig. 6). Interestingly, the two previously mentioned reactions – formation of carbamate with hemoglobin and the hydration/dehydration reaction of HCO3– – both result in the release of H+ ions into the blood. The released hydrogen ions will bind to specific amino acid residues on hemoglobin chains to stabilize the T conformation of hemoglobin, which results in a decrease in the oxygen affinity (Margaria and Green, 1933).

Moreover, the reverse reaction can also be viewed as the total carbon content in deoxygenated blood being greater than that of oxygenated blood since deoxyhemoglobin has a higher affinity to bind to carbon molecules. This effect is called the Haldane Effect (Klocke, 1973). Both effects summarize the complex relationships between carbon and oxygen affinity binding to hemoglobin (Fig. 7). One interesting example of pH shifting the ODC is when people exercise skeletal muscles. This process will produce large amounts of lactic acid during metabolism, which contribute to a high concentration of H+ ions in blood. This will cause an increase in the concentration of HCO3– and deoxyhemoglobin binding to carbon forming carbamate since these two reactions depend solidly on the presence of H+ (Geers and Gros, 2000). Thus, during intense exercise, hemoglobin in the human body will have a higher capacity of unloading oxygen due to the Bohr Effect.

2,3-Biphosphoglycerate, another very important factor to consider, affects the oxygen binding affinity of hemoglobin. It has been observed that the presence of organic phosphates such as 2,3biphosphoglycerate or adenosine triphosphate (ATP) in blood triggers a change in oxygen binding behavior to hemoglobin and, among all the phosphate groups, 2,3-biphosphoglycerate is the most present in blood – four times greater than ATP – (Bunn and Jandl, 1970; Hsia, 1998). In the general glycolysis process, 1,3-biphosphoglycerate converts directly to 3-phosphoglycerate and produces 1 ATP through phosphoglycerate kinase. However, in special circumstances – for example in red blood cells – 1,3-biphosphoglycerate may convert to 2,3-biphosphoglycerate through a minor pathway, and through such a pathway the glycolysis cycle will not produce any ATP (Fig. 8). Generally, 2,3-biphosphoglycerate has very low concentrations in cells. However, it is frequently present in the case of erythrocytes (Hsia, 1998).

Regarding the influence of 2,3-biphosphoglycerate in blood, Benesch et al. carried out an experiment where the same blood solution was prepared and 2,3-biphosphoglecerate was added to a sample whereas organic phosphates were removed from another (Bunn and Jandl, 1970). The ODC of each solution was compared, and the ODC of the solution with 2,3-biphosphoglycerate added had a shift toward the right – reduced in P50. Other types of organic phosphates had less similar effects. 2,3-biphosphoglycerate has five titratable protons per molecule and it is an effective charge donor in a wild range of pH. This highly charged anion can have electrostatic interactions with the positive charged components of hemoglobin (Bunn and Jandl, 1970). In fact, 2,3-biphosphoglycerate may bind to the charged pocket on the β-chains of hemoglobin (deoxyhemoglobin is preferred) in a 1:1 ratio (Bunn and Jandl, 1970), where it also stabilizes the T conformation of the protein. In addition, the increase in 2,3-biphodphoglycerate concentration in blood also improves the intracellular pH level, which enhance the Bohr Effect (Hsia, 1998). Thus, the complex formed by 2,3-biphosphoglycerate and deoxyhemoglobin effectively reduces the affinity of hemoglobin and is responsible for minor changes in heme-heme reactions (Chanutin et al., 1967). Other factors such as temperature — when body temperature increases, the oxygen affinity decreases facilitating the release of oxygen, — the binding of nitric oxide, and the concentration of salts have an important effect on hemoglobin’s affinity to oxygen. They act as regulators in mammalian blood streams and control the oxygen binding and release process.
Foetal Hemoglobin
Foetal Hemoglobin (HbF) is the protein contained in the red blood cells of a fetus to carry oxygen and has structural and differences compared to adult hemoglobin. This protein plays an important role in oxygen transportation starting during the 12th week of pregnancy until the newborn is approximately 6 months old. The particularity of HbF resides in its composition. This protein consists of two α-chains and two γ-chains. Conversely, adult hemoglobin (HbA) is defined by two β-chains instead of having two γ-chains (Kaufman et al., 2020). The γ-chain is part of a genetic evolution including duplication and regulations of the genes expressed. At the beginning of the pregnancy, a primary form of β-globin is expressed during 5 weeks in the yolk sac of the fetus (Sankaran and Orkin, 2013). This protein, known as the ε-globin but more commonly as the embryonic hemoglobin, makes primitive erythrocytes called erythroids which are the first red blood cells created by the organism. About 3 weeks after the start of ε-globin expression, the liver of the fetus becomes involved in erythropoiesis – creation process of red blood cells. Consequently, stem cells and more precisely progenitor cells, a specific kind of stem cell limited in their division, differentiate to become erythrocytes containing the β-globin under the form of γ-hemoglobin.
The γ-hemoglobin expression is governed by the β-globin gene cluster on chromosome 11. As a result, α-chains and γ-chains are grouped together in a tetramer – association of subunits. Therefore, we could find two α-chains and two γ-chains forming the fetus hemoglobin (Sankaran and Orkin, 2013). The γ-chains are different from the β-chains due to their amino-acid sequence. For example, at position 136, it can be noted that there is a substitution of amino acids between these two proteins (Kaufman et al., 2020). Gamma globin is characterised by a neutral amino acid at this position that could either be an alanine or glycine. This particularity has an impact on the oxygen affinity of HbF.
In red blood cells, hemoglobin is confronted to the 2,3-bisphosphoglycerate regulator molecule able to monitor the hemoglobin affinity to oxygen (Sasaki et al., 1982). 2,3-bisphosphoglycerate is composed of many negatively charged oxygens and would be more likely to associate with β-chains since its amino acids are more positively charged. Due to the nature of opposite charges being attracted to one another, β-globin has less of an opportunity to bind with oxygen. However, γ-globin is favored due to its neutral amino acid establishing a minimal reactivity with this oxygen affinity regulator. This fetus hemoglobin is the result of an adaptation that allows the future newborn to be provided with sufficient oxygen coming from the maternal blood. A study comparing the efficiency of γ-chains and β-globin to carry oxygen considered two parameters: the pressure of oxygen and the saturation of fetal hemoglobin protein. The results show that for the same oxygen pressure, γ-chain oxygen saturation was higher than β-chain oxygen saturation. This demonstrates a better affinity of fetal hemoglobin for oxygen (Kaufman et al., 2020).

This adaptation resulting from a maximization of the oxygen supply to the fetus is due to a physiological aspect. The maternal and fetal circulatory systems are individualized by the placenta in the mother organism and the villous chorion in the fetus organism. However, to ensure the supply of oxygen to the fetus, the future newborn needs an oxygen transfer coming from the maternal blood. In fact, the fetus oxygen supply is made possible through the placenta and the villous chorion linked to an umbilical cord (Wang, 2010)
The placenta represents a gas exchange surface rich in blood vessels. This surface allows for the maternal arteries to bring oxygen to fetal veins and for the maternal veins to take away fetal waste. The particularity in the placenta exchange surface, scientifically named the terminal villi, is that blood flow pressure is considered as being relatively low. Scientifics have been able to measure an approximate blood flow ranging from 600 to 700 mL/min. By knowing this specificity, it can be easier to understand that fetal hemoglobin must ensure an optimal bonding with oxygen if considering this low blood pressure in the placenta and the chorion (Wang, 2010). This capacity of fetal hemoglobin to bond with oxygen at low oxygen pressure is vital for the fetus. If the HbF were not able to carry enough oxygen in a gas exchange surface with a low concentration of O2, the systemic and pulmonary system of the fetus would not be well-supplied, impacting fetal growth. As mentioned previously, the blood flow pressure is low between the maternal uterine artery rich in O2 and the fetal umbilical vein. However, when the blood approaches the lungs via the left atrium, the blood pressure becomes more consequent. Indeed, lungs are not involved in the gas exchange between the fetus and the maternal blood (Wang and Zhao, 2010).
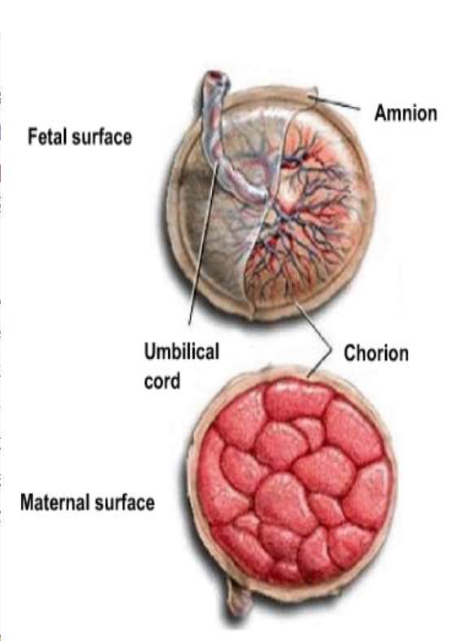
Consequently, the blood circulating next to the lungs is not saturated in oxygen and, as a result, the vessels are much more tolerant towards blood pressure, allowing for the heart to then distribute the oxygenated blood via the aorta in the directions of an organ needing an important supply in O2: the brain. Hence, a physiological adaptation involving a regulation of the fetus circulatory system ensured the supply of the fetus’ organs with oxygen. This is made possible by considering the hemoglobin capacity to carry enough oxygen even when blood flow conditions are not favorable (Vali and Lakshminrusimha, 2017).

To explain the switch between gamma-hemoglobin to beta-hemoglobin, we should study the genes regulatory system in chromosome 11. Scientists have found two gamma-globin genes repressors known under the name of: BCL11A and ZBTB7A. Together, they deactivate the expressions of the genes (HBG) responsible for the synthesis of gamma hemoglobin – part of fetal hemoglobin. By doing so, they more precisely prevent the functionality of a HBG reader, more commonly called a proximal promoter of this specific gene. Thus, a transition occurs and the newborn blood becomes similar to adult blood in terms of hemoglobin. However, even during adulthood there is still a small amount of HbF produced, representing less than 1 % of the total synthesized hemoglobin.

Abnormal Forms of Hemoglobin
Hemoglobin proteins are synthesized in the mitochondria and cytosol of immature red blood cells named reticulocytes (Bunn et al., “Control of hemoglobin function within the red cell”). Basically, the synthesis happens by transcription of globin genes by the mRNA and translation of it to form functioning proteins. Main abnormalities come from mutations – substitution, addition or deletion – modifying the sequence of nucleotides of the gene involved. Still, some abnormalities come from mispairing and crossover during meiosis creating a fusion protein of both gene sequences (Old, 2016). More than 1 000 human hemoglobin variants – abnormal forms – have already been identified (Thom et al., 2013) but we will focus on more common and significant ones. The geographic distribution of common abnormal hemoglobins and related diseases reflects some disparity, mutations are transmitted by heredity.
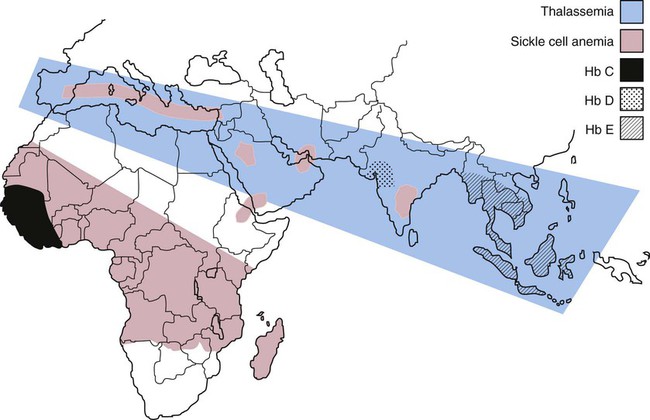
The most common hemoglobin variants are hemoglobin S, hemoglobin C and hemoglobin E (Dominguez et al., 2013). Most of the time, the alleles perpetuating the production of these variants and responsible for significant diseases are recessive, you must have inherited two altered gene copies – from both of your parents – to develop a variant-related disease (“Hemoglobin Abnormalities” | Lab Tests Online, 2017). Therefore, there are three possibilities depending on the number of mutated alleles you have: you can be a heterozygous carrier – having only one altered gene – with mild or no symptoms, a homozygous developing the disease related to the abnormality or a doubly heterozygous if you have two different altered genes responsible for different – symptoms are then related to one or both abnormalities.

Hemoglobin S is the primary hemoglobin in people with sickle cell disease (anemia). It differs from normal hemoglobin (A) by a single amino acid substitution – valine replacing glutamine in the sixth position of the beta-chain of globin – (Davis, n.d.). This little change leads to hemoglobin with abnormal beta-chains that result in erythrocytes deforming into rigid sickle shape that can block small blood vessels causing many symptoms and complications like pain, decreased oxygen delivery or even shortened red blood cells survival. Hemoglobin C is an abnormal hemoglobin that can cause mild symptoms such as the enlargement of the spleen and a minor hemolytic anemia – breakdown of erythrocytes containing hemoglobin not carrying oxygen efficiently. To form those proteins, glutamic acid is replaced by lysine in the sixth position of the globin beta-chain, it produces sickle cell trait but is much benign than the sickle cell disease cause by hemoglobin S seen previously.
Hemoglobin E is another hemoglobin variant that come from a substitution in the 26th position of the globin beta-chain – glutamic acid replaced by lysine. Subjects homozygous for hemoglobin E allele cope with mild symptoms similar to subjects homozygous for hemoglobin C allele such as hemolytic anemia and splenogamy – enlargement of the spleen. In fact, many other hemoglobin variants present the same symptoms with a different severity (e.g., hemoglobin D and hemoglobin J). However, mutations in genes coding for hemoglobin can also involve thalassemia depending on their locus – localization of these genetic changes. Thalassemias are blood disorders characterized by the decrease of hemoglobin production (“Thalassemias” | NHLBI, n.d.). They can coexist with other hemoglobinopathies like hemoglobin S, C or E mentioned earlier.

Hemocyanin
In addition to hemoglobin, there exist other oxygen carriers that allow for different species to send oxygen through their blood. One of these carriers is called hemocyanin and is found in many different species of arthropod and mollusk, including – and certainly not limited to – octopuses, scorpions and horseshoe crabs (Kamerling and Vliegenthart, 1997). Hemocyanin is best known for its copper oxygen binding sites which give the blood, of hemocyanin dependent species, its distinct blue color! Before continuing, it is important to note that arthropod and molluscan hemocyanin evolved independently from one another even if they share a similar method of operation (Burmester, 2001). Both types of hemocyanin have similar binding sites for oxygen, although they have very different structures which often leads to arthropod and molluscan hemocyanin being considered two different proteins (van Holde et al., 2001). This begs the question of why hemocyanin was best suited to these animals instead of the function of a different oxygen carrier such as hemoglobin.
In the subsequent section of this essay hemocyanin will be examined. The structure and function of this protein will be discussed, followed by an analysis of hemocyanin transport. These characteristics of hemocyanin will then be considered when studying hemocyanin in cephalopods in specific – molluscan hemocyanin – and the evolutionary advantages it provides.
Structure of Hemocyanin
Hemocyanin is an oligomeric molecule, meaning it is made up of a small number of repeating units (Klaerner and Padmanabhan, 2016). In mollusks, this molecule is typically made up of ten protein subunits which are each composed of a combination of eight functional units, depending on the species (Markl, 2013). Molluscan hemocyanin molecules are giant and are among the largest proteins studied with each subunit weighing 350-450 kDa (Magnus, 2004). Over time, the functional units of molluscan hemocyanin, named FU-a through FU-h have evolved and diverged from one another, allowing for different species to possess different combinations. For example, cephalopods do not have FU-h whereas gastropods do even though they both belong to phylum Mollusca (Markl, 2013; Kato et al., 2018). The typical quaternary structure of the molluscan protein is a cylindrical decamer (Fig. 16.), collectively made up of ten subunits of seven or eight functional groups. However, hemocyanin can also form multidecamers (Kato et al., 2018). The base structure resembles a tube that may be partially filled by a structure called a collar and has dimensions of 35 nm in diameter and 18 nm in height (Markl, 2013).

In contrast, arthropod hemocyanin is constructed as hexamers or hexamer multiples where six subunit amino acid chains group together, each of which binds one oxygen molecule (van Holde and Miller, 1995). Each arthropod hemocyanin subunit has a mass of approximately 75 kDa (Magnus, 2004). Although the structure of these two hemocyanin molecules are different and vary between species (Fig. 17.), the dicopper binding sites of the two phyla function similarly (Magnus, 2004). The following analysis will focus on oxygen binding and the factors that influence it.

Dynamics and Efficacy of Oxygen Transport
To determine how hemocyanin functions, it is important to further analyze its more minute structure. The active site of molluscan hemocyanin (Fig. 18.) is a type-3 copper-binding site, which consists of a pair of copper atoms each surrounded by three histidine residues (Markl, 2013). It is between these two copper atoms that an oxygen molecule or other ligands – such as carbon monoxide, azide, halides, etc. – can be reversibly bound (Magnus, 2004).

Once this occurs and the copper transitions from Cu (I) to Cu (II), the hemocyanin containing blood turns from a clear color to its characteristic blue shade (Lutz, 2016; Kamerling and Vliegenthart, 1997). The main function of hemocyanin is to act as a respiratory protein which carries oxygen through the blood. Unlike hemoglobin, which is found inside red blood cells, hemocyanin is suspended outside of the blood cells in the hemolymph where it can make up greater than 90 % of the protein constituents (Kamerling and Vliegenthart, 1997). This small change is but one of the many differences between hemocyanin and hemoglobin and may bring up a greater comparison between the two oxygen carriers.
Hemocyanin’s oxygen transport capabilities revolve around its number of copper atom pairs, each of which can bind a single oxygen molecule (Fig. 19.). As seen previously during the analysis of hemoglobin, one hemoglobin molecule can bind four oxygen molecules. For hemocyanin in mollusks, every functional unit has a binding site. For arthropods, each subunit has one oxygen binding site. Therefore, it can be expected that hemoglobin has a greater oxygen affinity than hemocyanin; however, it is not that simple.

Hemocyanin does not operate as individual peptide chains, instead it forms oligomers, the decamer and hexamer multiples previously discussed that form the quaternary structure of molluscan and arthropod hemocyanin respectively. These polypeptides enable hemocyanin to display cooperative binding (Decker et al., 2007). Cooperative binding allows for neighboring binding sites to interact with one another and facilitate or inhibit oxygen binding (Simmons, 2013). In mollusks, this property is moderately low, but in arthropods the cooperative binding noticed in hemocyanin can be very large (Decker et al., 2007). However, cooperative binding requires the presence of certain ions such as Mg+ or Ca+ and protons (Decker et al., 2007). These ions promote the changing of protein conformation and can be considered examples of allosteric regulation (González et al., 2017). As discussed with hemoglobin, the variation in protein conformation is required for efficient oxygen association and dissociation.
Allosteric parameters depend greatly on environmental conditions and can have a large impact on the proper functioning of hemocyanin. It can be understood that different species relying on hemocyanin have adapted to various allosteric affects, along with other factors such as temperature and pH (Hirota et al., 2008) that allow for the optimization of oxygen binding in their specific environment (Decker et al., 2007). With changes in environmental features, a large impact can be had on the operation of oxygen binding in an animals’ blood. What are some environmental features that would promote the use of hemocyanin? In the following section, cephalopod blood will be examined more specifically.
Cephalopod Hemocyanin in Antarctic Octopuses
Cephalopods are a type of mollusk including animals such as octopuses, nautili, squid and cuttlefish. These creatures are found in oceans all around the world in both warm and cold waters rely on the protein hemocyanin to transport oxygen throughout their bodies. Environmental pressures have forced cephalopods to adapt to their surroundings in numerous ways, including how their respiratory protein, hemocyanin, allows for efficient oxygen transport to tissues.
To gain a better understanding of these adaptations it would be helpful to analyze the hemocyanin of a cold-water cephalopod, more specifically, a species of Antarctic octopus called Pareledone charcoti (Fig. 20) which lives in waters between roughly -1.8 – 2 ºC where dissolved oxygen is abundant (Oellermann et al., “Blue blood on ice”). Typically, in cold waters, the metabolism of cephalopods species is lower, which results in less oxygen being required to be delivered to tissues. The abundance of dissolved oxygen and the small metabolic requirements would suggest that oxygenating tissues would not be a large problem for the Antarctic octopuses. Unfortunately, it is not that simple.

It has been observed that as water temperature decreases, hemocyanin develops a higher oxygen affinity (Oellermann et al., “Positive selection in octopus haemocyanin”). This may sound desirable. However, having a higher oxygen affinity means that it will be more difficult for the hemocyanin to release the oxygen to tissues. To combat this issue, evolutionary adaptations have taken place to allow for proper oxygen dissociation (Oellermann et al., “Positive selection in octopus haemocyanin”). Pareledone charcoti has developed the ability to compensate its inability to deliver oxygen to tissues effectively by increasing hemocyanin concentration in its blood (Oellermann et al., “Blue blood on ice”). This feature, along with the abundance of dissolved oxygen in these cold waters, allows for Antarctic octopuses to receive the oxygen needed for cellular respiration. In warmer waters, octopuses had a decrease in concentration of hemocyanin in their blood compared to their Antarctic counterparts and were still capable of reaching their necessary oxygenation levels as the warmer temperatures allowed for better oxygen delivery to tissues (Oellermann et al., “Blue blood on ice”).
Antarctic octopuses are solely one example of temperature influencing the effectiveness of hemocyanin in animals. There is still much we do not know about how rapid temperature fluctuations or other environmental changes could have on the wellbeing of different species (Oellermann et al., “Blue blood on ice”). Many more studies will have to take place to reveal how a changing climate will affect interesting creatures like the Antarctic octopus.
Overview of Hemocyanin Compared to Hemoglobin
The function of hemocyanin and hemoglobin is very similar. The main differences between these two oxygen carriers revolve around their protein structure. At the root of their function to reversibly bind oxygen for transport, hemocyanin and hemoglobin rely on copper and iron respectively. Copper is responsible for the blue blood found in many invertebrates such as cephalopods and arthropods whereas the iron associated with hemoglobin gives blood a bright red color when oxygenated.
The next notable difference between hemocyanin and hemoglobin is that the former is found free flowing in hemolymph as opposed to being directly inside a red blood cell like hemoglobin. Hemocyanin is a much larger protein than hemoglobin, contributing to the reason why it is suspended in hemolymph where it can make up most of the protein constituents and occupy more space (Kamerling and Vliegenthart, 1997). Furthermore, the number of binding sites in hemocyanin and hemoglobin differ. In one hemoglobin protein, there are four oxygen binding sites. In hemocyanin, however – one functional unit in mollusks or one subunit in arthropods – only has one binding site. Hemocyanin instead relies on cooperative binding to improve its oxygen affinity which is possible thanks to the massive quaternary structures formed in multiples of ten subunits – in mollusks – or six subunits – in arthropods – (van Holde and Miller, 1995). It is also important to note that hemocyanin and hemoglobin both flourish under different environmental factors. It can be understood that hemocyanin is more effective at lower temperatures than hemoglobin (“The Difference Between Hemocyanin and Hemoglobin” | University of Manitoba, n.d.). Hemocyanin is also better than hemoglobin at managing the low oxygen levels of an organism’s surroundings. Hemocyanin, however, also has its limits. Hemoglobin is still more efficient at transporting oxygen in oxygen rich environments (Morrison et al., 2013) such as on land or in shallow waters.
Although these two proteins have developed to complete similar functions, they have evolved separately from one another and each bring their own benefits and challenges to consider.
Conclusion
This essay has discussed the various evolutionary advantages associated with different forms of hemoglobin and hemocyanin, two respiratory proteins. Although similar in function, these oxygen carriers have very different structures, allowing them to operate most effectively under specific environmental circumstances.
Hemoglobin, responsible for the red color of blood in many species, including vertebrates is an iron-dependent protein. This oxygen carrier is made up of two α-chains and two β-chain amino acid residues sharing four oxygen binding sites that each bind with a heme group. Hemoglobin can be found inside the red blood cells of vertebrates and operates best in oxygen rich environments. In contrast to adult hemoglobin, fetal hemoglobin has slightly different properties which allow for fetuses to receive adequate oxygen from their mother under low pressure conditions. After several months, the production of fetal hemoglobin stops and the properties of adult hemoglobin take its place after sufficient organismal development. Additionally, examples of abnormal hemoglobin were developed to better appreciate the evolutionary disadvantages and advantages of the adaptations of different carrier protein structures and function.
Hemocyanin is a copper-based oxygen carrier and thus gives blood a blue color. Two forms of this protein can be found in arthropods and mollusks, each of which are very large and share a similar oxygen binding function yet have different structures. Molluscan hemocyanin is massive and is an oligomeric protein made up of multiples of ten amino acid chains called decamers with multiple binding sites per chain. Arthropod hemocyanin on the other hand is made up of hexamer multiples and has one binding site for oxygen in each chain. Both hemocyanins are found free floating in the hemolymph of blood. An example of a more specific hemocyanin from an Antarctic octopus was discussed to address some of the adaptations associated with this oxygen carrier, namely the ability of hemocyanin to change its concentration in the blood depending on the temperature to maximize oxygen delivery to tissues.
This essay has explored some of the many different oxygen carriers in animals throughout the phyla. Since the oxygenation of the atmosphere, Earth has seen many changes in the species that live on it. Blood is not only a vital fluid coursing through the veins of living organisms, but also a key to understanding the evolutionary processes that animals have undergone to adapt to their surroundings. With changing climates, we can now only wonder whether these adaptations will be sufficient in maintaining species survival or if new modes of evolution will be required to sustain life on Earth.
References
Allcock, L. (2016). Antarctic octopuses. Retrieved from http://marinescience.ie/2016/03/18/antarctic-octopuses/
Baldwin, J., & Chothia, C. (1979). Haemoglobin: The structural changes related to ligand binding and its allosteric mechanism. Journal of Molecular Biology, 129(2), 175-220. doi:10.1016/0022-2836(79)90277-8
Benchetrite, A., Qiao, S., Hafit, I., & Hebert, L. (2020). The Well-kept Secrets of Blood: The Mechanism of a Vital Fluid Bioengineering McGill University. Montreal, Canada. https://bioengineering.hyperbook.mcgill.ca/the-well-kept-secrets-of-blood-the-mechanism-of-a-vital-fluid-blood-1/
Bunn, H. F., & Forget, B. G. (1986). Hemoglobin: Molecular, Genetic and Clinical Aspects. Annals of Internal Medicine, 105(5), 820-820. doi:10.7326/0003-4819-105-5-820_2
Bunn, H. F., & Jandl, J. H. (1970). Control of hemoglobin function within the red cell. New England Journal of Medicine, 282(25), 1414-1421. doi:10.1056/nejm197006182822507
Burmester, T. (2001). Molecular evolution of the arthropod hemocyanin superfamily. Mol Biol Evol, 18(2), 184-195. doi:10.1093/oxfordjournals.molbev.a003792
Burmester, T. (2015). Evolution of Respiratory Proteins across the Pancrustacea. Integr Comp Biol, 55(5), 792-801. doi:10.1093/icb/icv079
Casiday, R., & Frey, R. (1998). Hemoglobin and the heme group: Metal complexes in the blood for oxygen transport. In (pp. 13): Washington University
Chanutin, A., & Curnish, R. R. (1967). Effect of organic and inorganic phosphates on the oxygen equilibrium of human erythrocytes. Archives of Biochemistry and Biophysics, 121(1), 96-102. doi:10.1016/0003-9861(67)90013-6
Davis, C. P. (n.d., 29 Mar 2021). Medical Definition of Hemoglobin S. Retrieved from https://www.medicinenet.com/hemoglobin_s/definition.htm
Decker, H., Hellmann, N., Jaenicke, E., Lieb, B., Meissner, U., & Markl, J. (2007). Minireview: Recent progress in hemocyanin research. Integr Comp Biol, 47(4), 631-644. doi:10.1093/icb/icm063
Domínguez, Y., Zurita, C., Calvopiña, D., Villacís, J., & Mora, M. (2013). Prevalence of common hemoglobin variants in an afro-descendent Ecuadorian population. BMC Research Notes, 6(1), 132. doi:10.1186/1756-0500-6-132
Geers, C., & Gros, G. (2000). Carbon dioxide transport and carbonic anhydrase in blood and muscle. Physiological Reviews, 80(2), 681-715. doi:10.1152/physrev.2000.80.2.681
González, A., Nova, E., Del Campo, M., Manubens, A., De Ioannes, A., Ferreira, J., & Becker, M. I. (2017). The oxygen-binding properties of hemocyanin from the mollusk Concholepas concholepas. Biochim Biophys Acta Proteins Proteom, 1865(12), 1746-1757. doi:10.1016/j.bbapap.2017.08.017
Hirota, S., Kawahara, T., Beltramini, M., Di Muro, P., Magliozzo, R. S., Peisach, J., . . . Bubacco, L. (2008). Molecular Basis of the Bohr Effect in Arthropod Hemocyanin*. Journal of Biological Chemistry, 283(46), 31941-31948. doi:10.1074/jbc.M803433200
Hoffbrand, A. V., & Pettit, J. E. (1993). Essential haematology. Retrieved from https://archive.org/details/isbn_9780632019540
Hsia, C. C. W. (1998). Respiratory Function of Hemoglobin. New England Journal of Medicine, 338(4), 239-248. doi:10.1056/nejm199801223380407
Kamerling, J. P., & Vliegenthart, J. F. G. (1997). Chapter 6 – Hemocyanins. In J. Montreuil, J. F. G. Vliegenthart, & H. Schachter (Eds.), New Comprehensive Biochemistry (Vol. 29, pp. 123-142): Elsevier.
Kato, S., Matsui, T., Gatsogiannis, C., & Tanaka, Y. (2018). Molluscan hemocyanin: structure, evolution, and physiology. Biophysical Reviews, 10(2), 191-202. doi:10.1007/s12551-017-0349-4
Kaufman, D. P., Khattar, J., & Lappin, S. L. (2021). Physiology, Fetal Hemoglobin. In StatPearls. Treasure Island (FL): StatPearls Publishing
Klaerner, G., & Padmanabhan, R. (2016). Multi-Step/Step-Wise Polymerization of Well-Defined Oligomers. In Reference Module in Materials Science and Materials Engineering.
Klocke, R. A. (1973). Mechanism and kinetics of the Haldane effect in human erythrocytes. Journal of Applied Physiology, 35(5), 673-681. doi:10.1152/jappl.1973.35.5.673
Lara, M., Monirzad, P., Crawford, L., & Lim, R. (2013). EVOLUTION OF HEMOCYANIN AND HEMOGLOBIN OXYGEN BINDING PROTEINS. In: University of Washington
Lutz, D. (2016). The Many Colors of Blood. In: American Chemical Society. Retrieved from https://www.acs.org/content/dam/acsorg/education/resources/highschool/chemmatters/issues/best-of-chemmatters/sample-lesson-plan-the-many-colors-of-blood.pdf
Magnus, K. A. (2004). Hemocyanins from Arthropods and Molluscs. In Handbook of Metalloproteins.
Manitoba, U. o. (n.d.). The Difference Between Hemocyanin and Hemoglobin. Retrieved from https://sciencecoop.weebly.com/the-difference-between-hemocyanin-and-hemoglobin.html
Markl, J. (2013). Evolution of molluscan hemocyanin structures. Oxygen Binding and Sensing Proteins, 1834(9), 1840-1852. doi:10.1016/j.bbapap.2013.02.020
McLellan, S. A., & Walsh, T. S. (2004). Oxygen delivery and haemoglobin. Continuing Education in Anaesthesia Critical Care & Pain, 4(4), 123-126. doi:10.1093/bjaceaccp/mkh033
Monod, J., Wyman, J., & Changeux, J.-P. (1965). On the nature of allosteric transitions: A plausible model. Journal of Molecular Biology, 12(1), 88-118. doi:10.1016/S0022-2836(65)80285-6
National Heart, L., and Blood Institute (NHLBI). (2020). Sickle Cell Disease. Retrieved from https://www.nhlbi.nih.gov/health-topics/sickle-cell-disease
National Heart, L., and Blood Institute (NHLBI). (n.d.). Thalassemias. Retrieved from https://www.nhlbi.nih.gov/health-topics/thalassemias
Oellermann, M., Lieb, B., Pörtner, H.-O., Semmens, J. M., & Mark, F. C. (2015). Blue blood on ice: modulated blood oxygen transport facilitates cold compensation and eurythermy in an Antarctic octopod. Frontiers in Zoology, 12(1), 6. doi:10.1186/s12983-015-0097-x
Oellermann, M., Strugnell, J. M., Lieb, B., & Mark, F. C. (2015). Positive selection in octopus haemocyanin indicates functional links to temperature adaptation. BMC Evolutionary Biology, 15(1), 133. doi:10.1186/s12862-015-0411-4
Old, J. (2013). Chapter 71 – Hemoglobinopathies and Thalassemias. In D. Rimoin, R. Pyeritz, & B. Korf (Eds.), Emery and Rimoin’s Principles and Practice of Medical Genetics (pp. 1-44). Oxford: Academic Press.
Online, L. T. (2017, 28 Jan 2021). Hemoglobin Abnormalities. Retrieved from https://labtestsonline.org/conditions/hemoglobin-abnormalities
Perutz, M. F. (1978). Hemoglobin structure and respiratory transport. Scientific American, 239(6), 92-125. doi:10.1038/scientificamerican1278-92
PÜNtener, A. G., & Schlesinger, U. (2000). 9 – Natural Dyes. In H. S. Freeman & A. T. Peters (Eds.), Colorants for Non-Textile Applications (pp. 382-455). Amsterdam: Elsevier Science.
Ruud, J. T. (1965). THE ICE FISH. Scientific American, 213(5), 108-115. Retrieved from http://www.jstor.org/stable/24931187
Sankaran, V. G., & Orkin, S. H. (2013). The switch from fetal to adult hemoglobin. Cold Spring Harbor Perspectives in Medicine, 3(1), a011643. doi:10.1101/cshperspect.a011643
Sasaki, R., Ikura, K., Narita, H., Yanagawa, S.-i., & Chiba, H. (1982). 2,3-bisphosphoglycerate in erythroid cells. Trends in Biochemical Sciences, 7(4), 140-142. doi:10.1016/0968-0004(82)90205-5
Schäfer, G. (2004). Chapter 1: Respiratory Chains in Archaea: From Minimal Systems to Supercomplexes. In D. Zannoni (Ed.), Respiration in Archaea and Bacteria: Diversity of Prokaryotic Respiratory Systems (pp. 1-33). Dordrecht: Springer Netherlands.
Simmons, J. (2013). Gas Transport: Cooperative Binding of Oxygen with Hemoglobin. In. study.com
SlideToDoc. (n.d.). DNA Proteins and Ways We Are Different Biological. Retrieved from https://slidetodoc.com/dna-proteins-and-ways-we-are-different-biological/
Thom, C. S., Dickson, C. F., Gell, D. A., & Weiss, M. J. (2013). Hemoglobin variants: biochemical properties and clinical correlates. Cold Spring Harbor Perspectives in Medicine, 3(3), a011858. doi:10.1101/cshperspect.a011858
Thomas, C., & Lumb, A. B. (2012). Physiology of haemoglobin. Continuing Education in Anaesthesia Critical Care & Pain, 12(5), 251-256. doi:10.1093/bjaceaccp/mks025
Traut, T. W. (2007). Allosteric Regulatory Enzymes: Springer US. Retrieved from https://books.google.ca/books?id=CGjT1zxS9NQC
Vali, P., & Lakshminrusimha, S. (2017). The Fetus Can Teach Us: Oxygen and the Pulmonary Vasculature. Children (Basel), 4(8). doi:10.3390/children4080067
van Holde, K. E., & Miller, K. I. (1995). Hemocyanins. In C. B. Anfinsen, F. M. Richards, J. T. Edsall, & D. S. Eisenberg (Eds.), Advances in Protein Chemistry (Vol. 47, pp. 1-81): Academic Press.
van Holde, K. E., Miller, K. I., & Decker, H. (2001). Hemocyanins and Invertebrate Evolution *. Journal of Biological Chemistry, 276(19), 15563-15566. doi:10.1074/jbc.R100010200
Wang, X., & Thein, S. L. (2018). Switching from fetal to adult hemoglobin. Nature Genetics, 50(4), 478-480. doi:10.1038/s41588-018-0094-z
Wang, Y., & Zhao, S. (2010). Integrated Systems Physiology: from Molecules to Function to Disease. In Vascular Biology of the Placenta. San Rafael (CA): Morgan & Claypool Life Sciences
Winslow, R. M. (2007). The role of hemoglobin oxygen affinity in oxygen transport at high altitude. Respiratory Physiology & Neurobiology, 158(2-3), 121-127. doi:10.1016/j.resp.2007.03.011