Functions of the Gut through the Perspective of Biological Mechanics
Trevor Hum, Xavier Santerre, Kyle Vamvakas, Mathilde Wagner
Abstract
The main functions of the gut are to digest food, absorb nutrients, and to eliminate waste. These functions vary between species, causing different guts to have different structures. Natural selection has determined the structure of different guts by what the animal consumes, where they live, and how other parts of their body are structured. Gut complexity varies from simple zero-gut organisms, like intestinal parasites, to single-chambered guts observed in carnivores, to complex four-chambered guts observed in ruminants, with intermediate structures in between. In this essay, the gut is evaluated from the perspective of biological mechanics. It will include a portfolio of five examples spanning different gut structures of different organisms, the gut microbiome and its role, and gut plasticity.
Portfolio of Different Guts
Vertebrate mammals are classified by what they eat. From this classification, humans have categorized vertebrate mammal species in three distinct categories: Carnivore, Omnivore and Herbivore. Each category of mammals has a very distinct dietwhich is directly related to their differently evolved gastrointestinal tract. Other interesting digestive tracts can be observed in birds and parasites. These different gut structures have been selected based on the animal’s habitat and environment. Through natural selection, different species have evolved to become generalist or specialist based off where they live and what they consume. A generalist can eat a wider variety of foods, while a specialist is limited to a stricter diet (“Generalist and Specialist Species” | National Geographic, n.d.).
Ruminants
Ruminants are herbivores that consume large amounts of cellulose (i.e., fiber). To facilitate and enable the digestion and absorption of this organic compound, they not only developed a long digestive tract, but also a specialized gut. The latter is comprised of four chambers: the rumen, the reticulum, the omasum, and the abomasum, illustrated in Fig. 1 (General Microbiology at Boundless, n.d.).
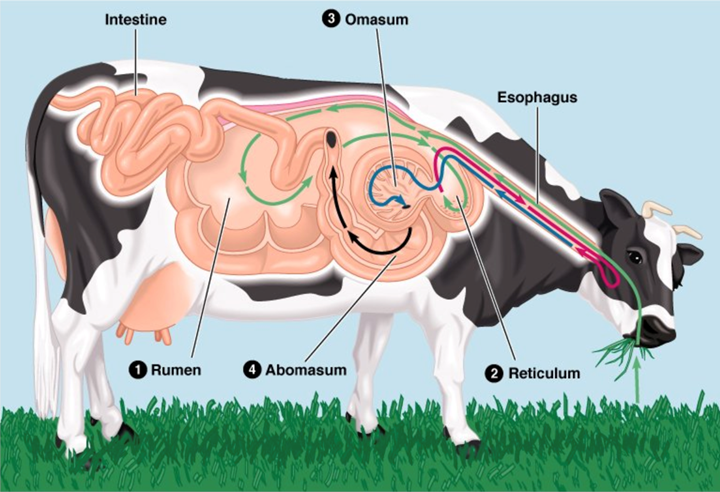
Ruminants are usually considered to be specialists since they only consume plants; however, those that consume a large variety of plants are considered generalists. This plant-based diet has direct consequences on ruminant teeth (Codron and Clauss, 2010). The cow will be the example used to discuss the gut of ruminants.
Ruminant Teeth and Mouth
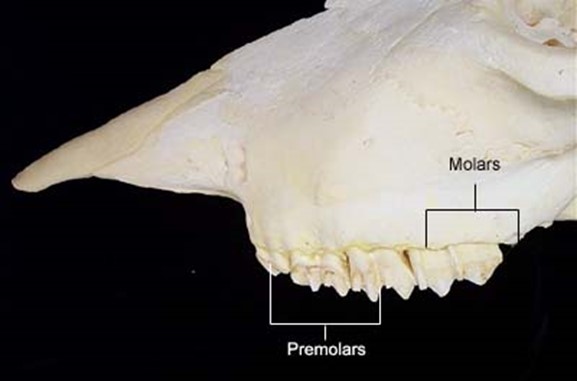
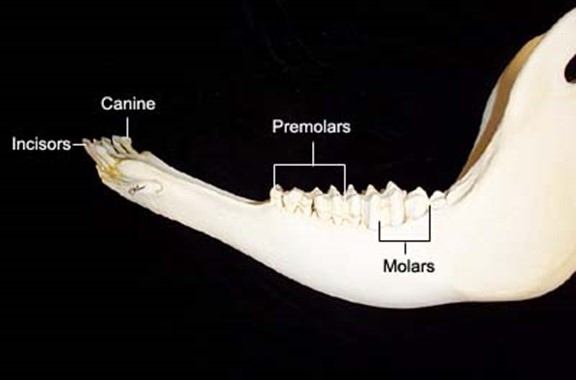
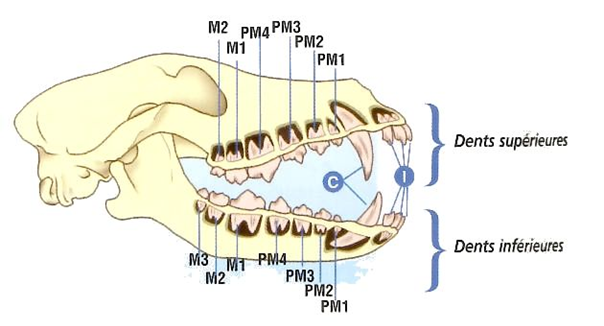
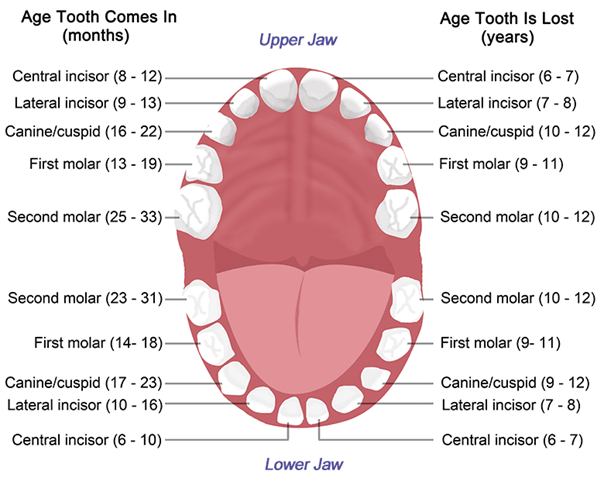
As a consequence of needing to eat large amounts of foliage, most ruminants do not have upper incisor teeth. For example, cows have 8 lower incisors, 12 pre-molars, and 12 molars, as may be seen in Fig. 2. Upper incisors do not allow ruminants to grasp a maximal amount of foliage with each bite, making them less efficient. Hence, through natural selection, they have been eliminated. What is more, ruminants shear the foliage with their lower incisors, while the tongue grasps large portions of foliage (Easley, 2014). Since cellulose is difficult to digest, ruminants have an interesting solution called cud chewing (Care et al., 1979), which will be explained in The Rumen section of this paper.
The Rumen
The rumen is the first of the four chambers of the ruminant gut and it is the largest one. Ingested food arrives to this chamber first, where fermentation takes place. The fermentation of the foliage converts carbohydrates, in this case cellulose, to volatile fatty acids. Hence, through this process, the fiber is being digested into molecules that could be used as energy. There are billions of microbes in the rumen, which enables fermentation, varying from a wide variety of protozoa, bacteria, and fungi (Codron and Clauss, 2010).
In addition to fermenting food, the rumen also stores food. Since the foliage cannot be broken down much by the teeth of ruminants due to the absence of the upper incisors, it is instead swallowed immediately, mostly unchewed. After some time has passed, the foliage will be pushed back up the oesophagus, where it will be chewed by the molar teeth. This process is called cud chewing, and it is repeated several times (Easley, 2014).
The Reticulum
The reticulum is the second chamber of the ruminant stomach. The rumen and reticulum share the same compartment; however, there is a distinction between the two because they have different functions. The reticulum serves as an intermediate compartment between the rumen and the omasum. Essentially, large particles that have not been fully digested are sent back to the rumen from the reticulum to undergo cud chewing once again, whereas particles that have been digested are passed to the omasum (Care et al., 1980). There are two crucial reasons for this: first, if large particles pass, they may lead to potential blockages; and second, returning large particles to be fermented ensures the maximum amount of energy will be absorbed by the ruminant from the digested cellulose (Care et al., 1980). Without large particles being returned, there would be undigested cellulose, and therefore, a loss of energy.
The Omasum
The main function of the omasum is to absorb water and salt by squeezing the digested foliage. The water and salt are absorbed in this compartment because they are needed to fulfil other bodily functions and letting them move to the next compartment would cause them to be lost through excretion (Care et al., 1980). One important structural feature of the omasum is that it is a highly folded structure, having a surface area of over 4 m2. This is crucial because a larger surface area results in more efficient absorption of water and salt (Care et al., 1980).
The Abomasum
The final chamber’s role is to digest proteins. The abomasum is comparable to the monogastric gut observed in omnivores and carnivores because it breaks down protein into amino acids that will later be absorbed in the small intestine (Care et al., 1980). The monogastric gut is discussed in greater detail in the Carnivores section of this report.
Birds
Birds do not have teeth to mechanically break down their food. Though it is still unclear why this is the case, it is apparent that the avian gut had to find a solution to mechanically break down the food a bird ingests. The solution: a unique two-chambered stomach has evolved to allow them to digest un-masticated food (“Digestive Systems” | Boundless Biology | Lumen Candela, n.d.). The chambers that make up this gut are the proventriculus, described as a glandular stomach, and the ventriculus or gizzard, described as a muscular stomach, which can be observed in Fig. 3 (Jacob, n.d.). Different bird species could be classified as either specialists or generalists. It really depends on which food resources are available rather than what their gut is capable of digesting (dos Anjos et al., 2019).
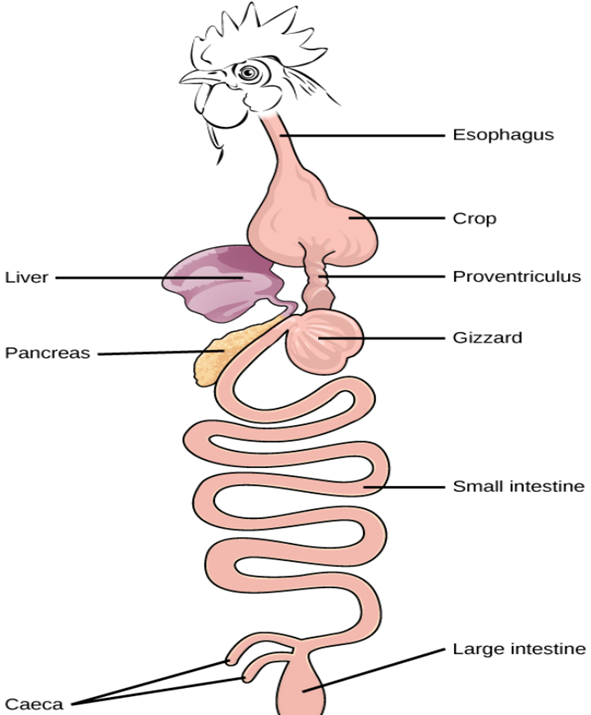
The Proventriculus
After food has been swallowed, it travels down the oesophagus to the first chamber of the avian gut, the proventriculus. In this chamber, digestion of un-masticated food takes place. It starts to get broken down with hydrochloric acid and digestive enzymes so that mechanical digestion in the ventriculus is easier (Jacob, n.d.).
The Ventriculus
In the second chamber of the avian stomach, food is grinded into smaller pieces. This is done with small stones or grit, that has accumulated in the ventriculus from previous meals. If the food is sufficiently digested and broken down, it will move from the ventriculus to the small intestine where the nutrients are absorbed. However, a lot of the time this is not the case, so the food moves back and forth between the proventriculus and the ventriculus until it is sufficiently digested. This ensures the food has been broken down and exposed to an ample number of digestive enzymes for maximal absorption of the nutrients in the small intestine (Jacob, n.d.). After absorption is complete, birds that ingest indigestible components, such as bones, will regurgitate them in the form of a pellet (Westervelt, 2018).
Carnivores
Carnivores have evolved to have a simpler digestive system, but more complex and developed physical features, allowing them to hunt prey efficiently (“Herbivores, Omnivores And Carnivores Explained” | Eden Holistic Pet Foods, n.d.).
The Mouth
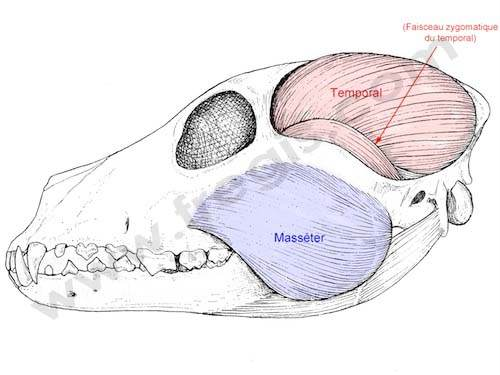
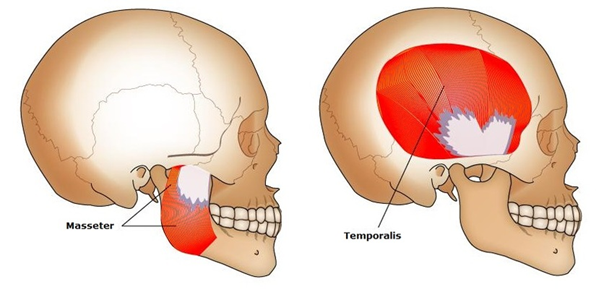
Carnivores usually have big and sharp incisors and canines, with which they can kill their prey with. They often have premolars and molars, which can crush and shred the meat (“Dental and Skull Anatomy of Carnivores, Herbivores, and Omnivores” | Main Street Children’s Dentistry and Orthodontics, n.d.). Their temporalis, which is the jaw muscle that is attached to the bottom jaw, is also larger than their masseter muscles, giving them a significant advantage when hunting and tearing meat apart because it gives them the capacity to close the jaw rapidly and with a lot of force (Dwiggins, 2016).
The Stomach
The carnivore stomach is usually single-chambered. It is much simpler than the ruminant gut because digesting fat and protein is much easier than digesting cellulose. Since carnivores have to chase and hunt their food, it is not always available. Therefore, they have a wide elastic stomach which allows them to ingest large amounts of food. This gut physiology allows them to eat large amounts of food that will supply them with enough energy for the next few days or even weeks (Woodvine and Spencer, 2019).
The Intestine and the Caecum
Carnivores have a smaller digestive tract since meat is easier to digest than plants. They have a shorter small intestine and a shorter large intestine. They also either have a very small caecum or no caecum at all since this organ is mostly useful for digesting plants (Woodvine and Spencer, 2019).
Lions
Lions are probably one of the best examples of carnivores since they reside at the very top of their food chain. They usually possess a total of 12 incisors, 4 canines, 10 premolars and 8 molars for a total of 30 teeth (dental formula: 3.1.3/2.2). Their canines are also spaced in a way that allows them to slip between cervical vertebrae to reach and fracture the spinal cord of their prey and/or opponent. They also possess a tongue that is covered with little spines called papillae, giving them the ability to scrape the meat from the bones more efficiently (“Lion Physiology” | Lion Alert, 2020). They usually have a stomach that is approximately 20 % of their body weight allowing them to store their food for a long period of time (Smith et al., 2006). For reference, the longest time spent between two hunts was registered at 8 days for the Kalahari lions (Eloff, 1984). The small intestine has an average size of 6-7 meters (Smith et al., 2006) while the large intestine is approximately one meter long (Suchodolski, 2011). They also possess a caecum that is about 10 centimeters long which they use to decompose small bones and porcupine quills (Smith et al., 2006). The digestive system of lions, more precisely, felines, is one of the simplest digestive systems because they only need to digest meat (Smith et al., 2006).
Omnivores
Omnivores are generalists; their unspecialized diet results in an unspecialized gut, which prevents them from absorbing all the nutriments in their meals. Due to this, they tend to feed on easier to catch meat and easier to digest plants (Bradford, “Omnivores”).
The Mouth
Omnivores’ set of teeth enable them to shred meat and grind plants. They have all four types of teeth, but all less specialized versions compared to those observed in carnivores or herbivores. Their incisors and canines are sharp, while their premolars and molars are large (“The Teeth of Herbivores, Carnivores and Omnivores” | Miami Center for Cosmetic and Implant Dentistry, n.d.). Even though they cannot shred meat as easily as carnivores, or grind plants like herbivores, their teeth allow them to eat from a wider variety of food, giving them an advantage when confronted by a poor ecosystem (Dwiggins, 2016).
The Intestine and the Caecum
Since omnivores have a generalist gastrointestinal tract, they have a longer small and large intestine than carnivores but shorter ones than herbivores. Despite consuming plants like herbivores, omnivores are not as well equipped to digest them, which results in a less efficient absorption of energy. Omnivores may have a caecum, but not as big as the one in herbivores (Haas, 2018).
Zero-Gut Intestinal Parasites
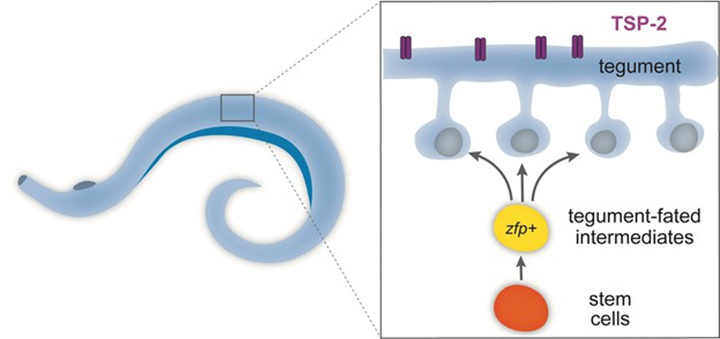
Parasites are extremely abundant throughout different species of animals. According to the World Health Organization, about 130 million people in developing countries live with intestinal parasites. That is roughly 10 % of people in developing countries alone. Without all these hosts, though, parasites would not be able to survive. As a result of residing in a host, some intestinal parasites do not need a digestive system. Intestinal parasites, such as tapeworms (Cestoda), require nutrients to be digested for them, as they cannot digest food themselves (Shao et al., 2019). To make up for not having a digestive tract, these zero-gut parasites have an outer surface called the tegument, which absorbs digested nutrients from the host, and protects them from immune attacks. Intestinal parasites are considered specialists if they are only capable of infecting a single host species, and generalists if they are capable of infecting multiple host species (Walker et al., 2017).
The Tegument: Nutrient Absorbing and Protective Surface
To protect themselves from the host’s immune system, intestinal parasites inhibit complement activation by recruiting complement regulatory proteins of the host onto the tegument (Fig. 11.). In addition to this, parasites will express parasite-encoded proteins to trick the host’s immune to target different components, thus inhibiting an immune attack (Shao et al., 2019).
The Gut Microbiome: A Growing Area of Research
The gut microbiome is a constantly expanding field of study, as it plays such an important, and previously, less well known, role in the body of an individual. Hypotheses and discoveries drawn from observations and experiments of the gut microbiome could lead to many answers. Understanding the diversity, complexity, specificity and partnership of our gut microbiome becomes a major topic, given its direct and crucial link to health.
General information
What Is the Gut Microbiome?
In and on the body of most animals live trillions of microorganisms with which animals maintain and cultivate a relationship and partnership (Cresci and Izzo, 2019). We are surrounded by microbes and our digestive tract is home to most of them. In fact, generally, of all the bacteria that colonize a body, most are found in the gastrointestinal tract (GIT), which extends from the esophagus to the anus. As molecular biologist Joshua Lederberg defines it, the gut microbiome is “the totality of microorganisms, bacteria, viruses, protozoa, and fungi, and their collective genetic material present in the gastrointestinal tract” (Cresci and Izzo, 2019).
Humans and many animals rely on a complex and varying community of bacteria. Different types of microorganisms constitute the gut microbiota: bacteria, non-pathogenic fungi, viruses, and parasites. We classify the different species present in the gut bacteria by genus, by family, phyla, and order (Cresci and Izzo, 2019).
The intestinal microbiome is unique to everyone. While for humans it can represent two to ten times the number of cells in the body and weighs 0.2 kg (Sender et al., 2016), it may also be almost nonexistent in some species (Hammer et al., “Not all animals need a microbiome”).
To analyze and compare the gut microbiota of different individuals, scientists collected fecal samples and then used DNA extraction and 16S rRNA sequencing methods. An experiment, led by several researchers of the Chinese Academy of Medical Sciences, highlights the specificity of the microbiota, varying from one species to the other. They chose five animals to study: the ferret, marmoset, woodchuck, mini pig and tree shrew, as well as humans, and gathered their observations in Fig. 12., comparing the core genera in their gut microbiota (Xiang et al., 2020).
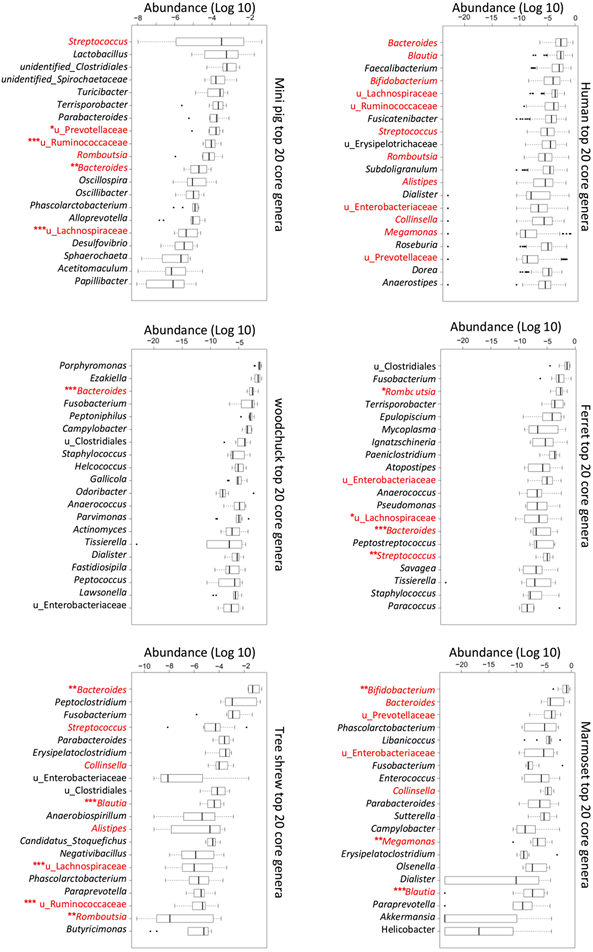
This is only one example of comparison of microbiota, but it shows its unique character. Indeed, the term gut microbiome is “so all-encompassing that the same word lumps very different kinds of microbial associations into a single concept, glossing over fundamental differences across animal species” (Tobin et al., 2019). From a structural point of view, it is also interesting to observe that many organisms carrying few bacteria have a short and simple gut structure. The importance and details about this point will be explained in detail later.
Factors of Influence
The gut microbiome can differ greatly from one species to the another. The composition of the intestinal flora is adapted to the needs of the species in question. It goes through changes, in terms of composition, diversity, and density, due to many different factors, internal as well as external. The intestinal microbiota is very dynamic and may vary under the influence of diet, environment (Goertz et al., 2019), stress, birthing and infant feeding process, lifecycle stages but also pharmaceuticals for humans. Some key factors that the gut microbiome relies on are further elaborated on in the Gut Plasticity section of this paper.
The Significant Role of the Gut Microbiome
Gut microbes can influence their host in many ways. First, the major role of the gut microbiome lays in nutrient absorption. Indeed, the bacteria present in our gut help with the digestion of food that has not been broken down in the stomach or in the small and large intestines.
Our gut bacteria also boost metabolism and balance hormones. Some enzymes, vitamins such as vitamins K (for strong bones) and B (for healthy hair and nails), amino acids, as well as short-chain fatty acids are synthesized by our gut microbiome. In fact, when bacteria digest fiber, they produce short-chain fatty acids that nourish the gut barrier, improve the immune function and help prevent inflammation (Stevens and Hume, 1998). This leads us to the second significant function of the gut microbiome: its role in health and disease. The gut microbiome forms a barrier to pathogens and activates the host immune system. This important function explains why the gut microbiome has been a center of attention recently, as it is related to diseases from metabolic disorders, such as obesity or diabetes, to autoimmune diseases (Davis, 2017).
To understand more about the relationship between the microbiota and host health, many experiments have been carried out. For instance, in an attempt to better understand obesity, an experiment on mice was conducted. Mice were raised in a sterile environment, having no gut microbiome. We know that normal mice have a 40 % higher body fat than germ free mice. When the distal gut microbiota from a normal mouse was transplanted into the laboratory mouse, its body fat increased by 60 % within two weeks without any food consumption increase or modification. The result of the experiment suggests that the gut microbiota influence phenotypic characteristics, associated with obesity (Davis, 2017).
Nevertheless, over the last years, evidence has emerged hinting that the influence of our gut microbiome goes much deeper.
A 2012 study led by Steven Collins of McMaster University demonstrated that the microbiome could affect the behavior of an individual. Mice born without a microbiota were more anxious and spent more time exploring their surroundings for food. Likewise, a transfer of microbiota from aggressive mice to non-aggressive mice caused an increase in their aggressiveness. Researchers are collecting more and more clues that certain mental disorders and neurodegenerative diseases could have originated in the womb (Collins et al., 2012).
Moreover, the depressive character is also linked to our microbiome. We know that serotonin imbalances are involved in this mental disorder. Scientists have found that in humans, approximately 95 % of serotonin is produced by our intestinal microbiota (Gershon and Tack, 2007).
The relationships between complex organisms and their gut microbes are very fascinating. The next section will go through several interesting examples of animals that “ditched” their microbial partners.
Specific Examples of Animals with No Resident Gut Microbiomes
Studying a little more closely animals that depend very little or not at all on their gut microbial partners could teach us a lot about the benefits and costs of living in symbiosis with our gut bacteria.
Bats
Just about every mammal, such as dogs or dolphins, interacts largely with the vast and diverse community of helpful bacteria.
Being the only mammals capable of flight, bats are also an outlier amongst mammals for not relying on their microbiome the same way. There are some bacteria in bat guts, but not as many as in other mammals. Moreover, as animals evolved along with their gut bacteria, closely related host species generally share more similar microbiomes, a pattern called “phylosymbiosis” (Lutz et al., 2019). However, bats seem to buck that trend.
All bats have lightning-fast digestion. Researchers dyed bat food in a colored (magenta) substance, called fuchsin. They observed that their excrement stirred pink about half an hour after ingesting food (Lutz et al., 2019). In fact, bats have extremely shortened guts, to induce a rapid digestion. But why and how would bats digest so quickly?
Scientists suspect that the bats’ unique relationship with gut bacteria is related to the other characteristic separating bats from other mammals: their ability to fly (Lutz et al., 2019). Indeed, bats need to reduce weight for flying, thus they do not want a heavy gut. That is why they do not have a resident microbiome, as a large population of microbes might weigh them down. Therefore, bats have evolved to be more efficient at absorbing nutrients. (Lutz et al., 2019)
Caterpillars
Across many caterpillar species, it was found that their gut microbes are considerably low in abundance (Hammer et al., “Caterpillars lack a resident gut microbiome”). The few bacteria found in their guts are the ones living on the surfaces of leaves. Scientists have dosed caterpillars with antibiotics to see if that disturbs their digestion: it did not affect the caterpillar at all. In fact, these insects even try their best to keep microbes from settling in their digestive tracts. Indeed, they have stomach bases (between ten and twelve pH) that kill bacteria, making them have such an antiseptic inside that it is impossible for microbes to persist in the gut and to contribute to digestion (Hammer et al., “Caterpillars lack a resident gut microbiome”). But why would caterpillars be eager to kill these bacteria, instead of working with them like we, humans, do? Animals weigh the pros and cons! For caterpillars, it may be that the risk of infection is not worth letting microbes settling in their guts.
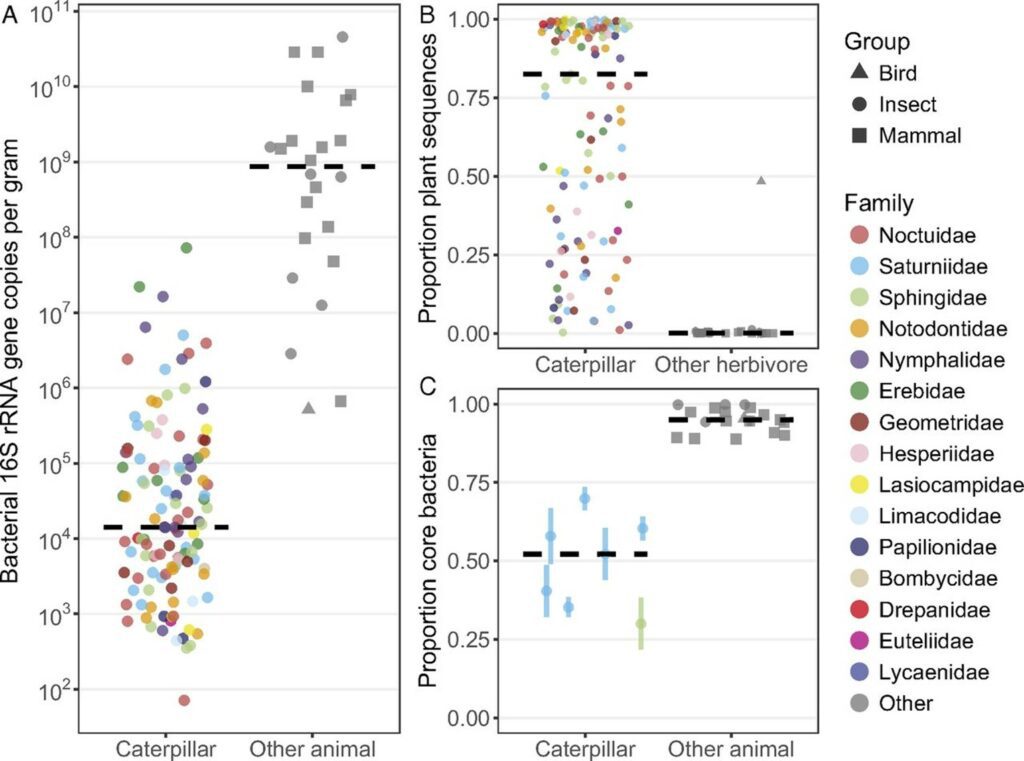
Instead of relying a lot on the gut microbiota to digest food fiber, caterpillars rely on their super basic guts. Resident symbionts are largely absent, and only microbes ingested with food (leaves’ bacteria) are present.
Brine Shrimp
A 2015 study of Montpellier University showed that brine shrimps struggle in lower salinities because their gut microbes do. Without these microbes, these crustaceans would survive in much fresher waters if they have food easy to digest without needing the help of the microbiome (Nougué et al., 2015). Hence, sometimes, animals could benefit from not having certain bacteria.
Stick Insects
A stick insect is an exclusively leaf-feeding insect, which does not need gut microbes to stick around.
Their nutrient source comes from breaking down complex plant molecules, such as the tough fibrous cellulose. But while some animals need the help of their gut microbiota, stick insects, with their long skinny gut structure that has no room for resident microbiomes, do not as they already have the code needed to create enzymes in their genome. The ancestor species acquired the code through horizontal gene transfer, a piece of DNA code that they use every day (Shelomi et al., 2013).
Stick insects are also capable of protecting themselves. When they detect an infectious microbe, they can kill it, as their immune system have an important repertoire of microbe killing molecules (Shelomi et al., 2015).
Gut Plasticity
Around the globe, many different species are put under many different types of stress, whether it is from their surrounding climate, their metabolic needs or even the food that is available to them in their environment. They have thus grown to evolve and adapt their guts to these stresses.
As previously mentioned, the length of the gut can change from species to species depending on their diet; however, this concept is also applicable to individuals within the same species. Furthermore, the gut has been shown to be capable of changing over time, and thus two animals of the same species could have two very different guts, reflecting the conditions they each lived in.
Diet
Two notable studies illustrate the effects of diet on the length of the gut. The first is a study published in 2005 in the journal Evolutionary Ecology and Research. In this research, scientists studied the effects of feeding perch different foods on their gut lengths (Olsson et al., 2007).
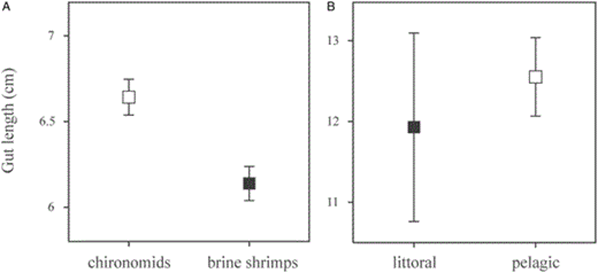
After having individually isolated 192 perch in identical environmental conditions, the fish were fed at the same time, in equal amounts, one of two different foods: frozen adult brine shrimps or chironomids (Olsson and Eklöv, 2005). The experiment assumes that chironomids would be more difficult to digest than shrimps, which would have to be proven as well. Another layer of complexity was added onto the experiment to confirm that the gut length had a direct link to the animals’ ability to digest foods. This was done by switching some of the perch that were initially on a shrimp diet to chironomids, and vice versa. Their body lengths were then measured multiple times after that, as a measurement of their overall wellbeing. After having killed the perch with an overdose of phenoxy-ethanol, each fish was measured in length, and later, so was their gut (Olsson and Eklöv, 2005).
The second study was published in 2007 in the Biological Journal of the Linnean Society (Olsson et al., 2007). The objective was to determine the effects that the available food source would have on the same species of perch in different environments. To do so, researchers simply captured perch from littoral and pelagic habitats from the same lake. Their gut lengths were measured, and part of their dorsal muscle tissue was used for stable isotope analyses with δ15N and δ13C to confirm whether there was even a difference in their diets or not (Olsson et al., 2007).
Before looking at the results, the position regarding the digestibility of shrimp compared to that of the chironomids had to be proven. By inspecting the amount of food left in the digestive tract of the respective fish groups, it was confirmed that the shrimp, with a lower amount of remaining food in the tract, was easier to digest. Scientists also had to confirm that the perch in the littoral and pelagic habitats were feeding on different foods which was, in fact, the case, proven by the stable isotope analysis (Olsson et al., 2007).
The results of the two studies agreed, concluding that diet played a role not only in not only determining, but also changing the length of the gastrointestinal tract of individuals within a species. The gut length of the perch that were on the shrimp diet, which was more easily digestible, was averaged at 6.1 cm, which is considerably shorter than that of the fish-eating chironomids, at 6.6 cm, confirming the hypothesis of plasticity of the gut (Olsson et al., 2007). Furthermore, the length of the guts of the fish from the littoral habitat, averaging 11.9 cm was shorter than that of those in the pelagic zone, with an average of 12.6 cm (Olsson et al., 2007). These results can be seen in Fig. 14.. This implies that the environment in which one lives could influence the length of one’s gut depending on the food that is available in the region and its digestibility (Olsson et al., 2007).
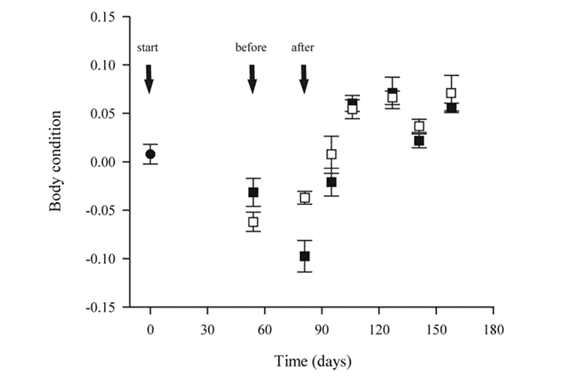
Furthermore, when the perch were forced to change from a harder to digest diet to an easier diet, it was found that their body size grew steadily, since their gut length was already longer and better suited for digesting more difficult food. On the other hand, when the perch were changed from the shrimp diet to the chironomids diet, their body size shrunk for a period of time before rising back up (Olsson et al., 2007). This is visualized in Fig. 15.. This was attributed to the fact that initially having a shorter gut that was adapted to digesting shrimp would make digesting chironomids even harder and would explain the drop in size. Thus, this also proves that the change in gut length is directly tied to the ability of an animal to digest its food properly (Olsson et al., 2007).
High-Energy State
A research, carried out in 2016 and published in the Journal of Experimental Biology, depicts the effects of high energy demands on the gut (Zhang et al., 2016). It examines the effects of high energy states during an animal’s lifetime on the mass of the gut. More specifically, the experiment features hamsters before, during, and after their peak lactation periods (Zhang et al., 2016). The food and litter of the hamsters in the various stages’ masses and energy content, found via Parr 1281 oxygen bomb calorimetry, were used to determine that, as one would expect, the hamsters in peak lactation would require more energy. This was done using the following formulas (Zhang et al., 2016):
Gross \space Energy \space Intake\space ({kJ\over Day})=Dry \space Matter\space Intake\space ({g\over Day}) \sdot Energy \space Content\space of\space Food\space ({kJ\over g})(1)
\small Digestive\space Energy \space Intake\space ({kJ\over Day})=Gross\space Energy\space Intake\space ({kJ\over Day})\\- \\ Dry\space mass\space of\space Feces\space ({g\over Day}) \sdot Energy\space Content\space of\space Feces\space ({kJ\over g})(2)
Digestibility (\%)={Digestive\space Energy\space Intake\space ({kJ\over Day})\over Gross\space Energy\space (kJ/Day)} \sdot100(3)
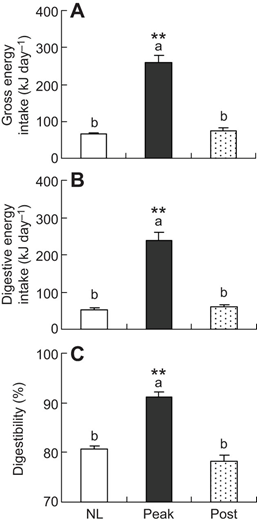
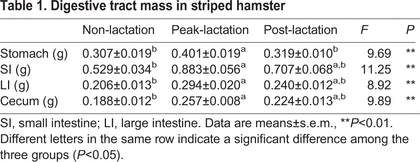
Using the data presented in Fig. 16., it was hypothesized that hamsters in the lactation period, a high energy requiring state, would possess larger guts, which would correlate well with the fact that they exhibit higher digestibility — a consequence of a longer gut. — After having dried and weighed all the parts of the gut, this hypothesis was proven to be true, with every part of the gut from the hamsters at peak lactation weighing considerably more than their counterparts in both before and after lactation, as shown in Table 1 (Zhang et al., 2016). This adaptation is therefore temporary and is to aid in digestion to allow more energy to be absorbed in this high energy state.
Temperature and Climate
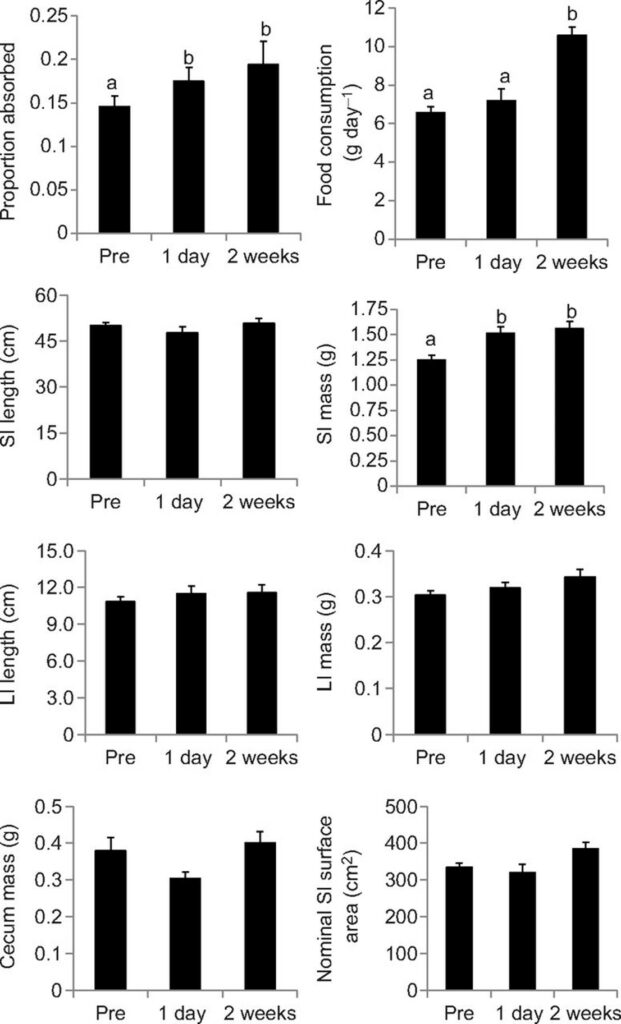
Animals living in colder climates certainly require more energy than those in warmer climates because they need that energy to keep themselves warm. Since that is the case, it was hypothesized that animals exposed to cold must have better digestibility, and thus, a larger gut size (Price et al., 2013). An experiment carried out by Price and colleagues (2013) explores the differences in guts in mice depending on varying cold exposure. As an additional layer to this experiment, researchers wished to see how immediate changes to the gut would compare to long-term changes in the gut.
The mice were placed in three groups. The first group would be maintained in an environment of 20 °C, while the second and third groups were placed in an environment of 4 °C (Price et al., 2013). The second group was euthanized and measured after one day of cold exposure and the third group was measured after two weeks of cold exposure. What the researchers discovered was that, while the length itself did not increase significantly, the mass and surface area had certainly increased due to cold exposure (Fig. 17.). They had also determined that there were very minor changes between the second and third groups of mice, implying that the gut can morph rather quickly to adapt to new environments (Price et al., 2013). What is to note is that, while increasing the mass or length of the gut may lead to better digestibility, having an extremely massive gut would not be optimal in situations where one would not require such high digestibility since it takes a lot of energy as well to maintain and regulate such a large part of the body (Price et al., 2013).
Microbiome Variability
Considering how important the gut microbiome is, animals must have some mechanism to allow it to become more specialized to their environment, notably if they are a migratory species. This dynamic property of the gut microbiome is thus a determining factor of how well an animal can adapt to new environments and conditions. In fact, the diversity in gut microbiome does not take long to become apparent, as the gut microbiota are very different between formula-ed and breastfed babies (Rinninella et al., 2019). Furthermore, once babies begin to eat solid foods, the gut microbiota starts to develop further to break down more complex carbohydrates. Many studies show similar findings, insinuating that the gut microbiota constantly adapts to become increasingly specialized to handle the foods being digested based on the diet of the animal (Derrien et al., 2019).
In a study published in 2020 in the journal Molecular Ecology, the different microbiome diversity of two different factions of fish from various origins were examined, and the fish were later translocated to different environments and re-examined (Webster et al., 2020). As expected, the microbiomes of the fish showed differences initially due to the differing diets available to them in their respective environments, but a large change was detected from their initial microbiome diversity to after the translocation. This suggests, as stated above, that the microbiome updates to become specialized to the food most recently eaten by the fish, and can therefore reflect not only the diet of the animal but also the environment it lived in (Webster et al., 2020).
In a similar paper published in 2018 in the journal Scientific Reports, the microbiomes of swan geese were examined in their breeding areas as opposed to their wintering areas (Wu et al., 2018). This study was done with the intention of proving that despite similar foods being available in both locations, the level of human activity, and thus, pollution could have a significant effect on the microbiome of nearby animals. While their alpha-diversity remained relatively unchanged after migration, the beta-diversity, composition of microbiome and both bacterial and fungal communities were heavily impacted (Wu et al., 2018). This implies that the pollution and garbage that humans create can also threaten the wellbeing of the animals through detrimental influences on their microbiome.
Lastly, temperature can also affect the gut microbiome. Recently, the changing climate has been a hot topic and its disastrous consequences are extremely widespread. One of these consequences is depicted in the microbiome of animals. Experimentally, it has been shown by multiple studies that an increase in temperature can lead to a decrease in alpha-diversity of the microbiome of mice, as well as a decline in the relative abundances of Firmicutes in the guts of rainbow trout, tadpoles, salamanders, common lizards and various tetrapods (Sepulveda and Moeller, 2020). The increase in temperature can also lead to major compositional changes in the communities in the microbiome, leading to potentially unwanted functional changes that could affect the health of the animal (Sepulveda and Moeller, 2020). It has been determined by recent studies that the microbiome’s sensitivity to temperature helps the animal regulate its internal temperature and maintain thermal homeostasis; though the exact mechanism is still somewhat unclear, it has been theorized that it is done through a process involving affecting tight junctions, regulating the transport of heat shock proteins (Sepulveda and Moeller, 2020). Thus, climate change is another way that human pollution could be affecting the microbiome of the animals on Earth.
Conclusion
Through natural selection, a variety of gut structures have evolved depending on the environment of an organism. This essay evaluated gut structure in specialists as well as in generalists with the goal of understanding how each structure maximizes their efficiency of absorbing the nutrients the animal consumes.
Ruminants live in herds and consume plants, but the problem is that cellulose is really tough to digest. To overcome this issue, these animals have developed a long and complex four-chambered gut. Its structure enables ruminants to digest cellulose, making most ruminant species specialists. This feature has also led to ruminants without upper incisors to be favored by natural selection.
Birds have a two-chambered avian gut, which compensates for their lack of teeth. Since their gut does not specialize in the digestion of either cellulose or protein, birds are classified as specialists or generalists depending on what food is available to them.
Carnivores have a single-chambered gut designed to digest meat. As a result, they have a shorter digestive tract, but more incisors and canines than ruminants. Like ruminants, carnivores are also considered specialists.
Omnivores also have a single-chambered gut; however, their digestive tract is longer than that of a carnivore, allowing them to digest meat and plants. This generalized digestive tract is not entirely able to digest cellulose, so a lot of the cellulose they consume is not digested.
Zero-gut intestinal parasites lack a gut altogether. The do not need one since they absorb digested nutrients in their host; however, they had to develop a technique to do so. This is why they have an outer surface: the tegument. In addition to absorbing nutrients, the tegument also serves as a protective surface from the host’s immune system for the parasite.
Furthermore, in light of the high plasticity demonstrated in various experiments, it can be said that one can determine a lot about the climate the animal lived in, the state it was in, and even what type of food it ate just by observing the structure, and composition of the gut and the microbiome within. The gut microbiome, found in the gastrointestinal tract, includes all bacteria as well as viruses, fungi, and protozoa. While more generally, animals, like mammals, depend on their gut microbiota and develop a symbiotic partnership, others do not rely on their gut microbial partners at all or very little, such as bats, the only flying mammal, but also caterpillars, brine shrimps, and stick insects. The gut microbiome is dynamic and varies among species and individuals to suit their needs. Playing a significant role in health and in the immune system, when relied on, the gut microbiome helps in the nutrient absorption, its primary function.
References
Adler, C. E. (2018). Dissecting the schistosome cloak. Elife, 7, e36813. doi:10.7554/eLife.36813
Agarwal, G., Robertson, M. A., Sonar, H., & Paik, J. (2017). Design and Computational Modeling of a Modular, Compliant Robotic Assembly for Human Lumbar Unit and Spinal Cord Assistance. Scientific Reports, 7(1), 14391. doi:10.1038/s41598-017-14220-3
Alert, L. (2020). Lion Physiology. Retrieved from https://lionalert.org/lion-physiology/
Boundless, G. M. a. (n.d.). Vertebrate Digestive Systems. In General Biology (Boundless). Retrieved from https://bio.libretexts.org/@go/page/13843
Bradford, A. (2016). Omnivores: Facts About Flexible Eaters. In. livescience.com. Retrieved from https://www.livescience.com/53483-omnivores.html
Candela, L. (n.d.). Digestive Systems. Retrieved from https://courses.lumenlearning.com/boundless-biology/chapter/digestive-systems/
Canin, P. (2016). La carotte benefiques pour votre chien. In. passioncaninsite.wordpress.com. Retrieved from https://passioncaninsite.wordpress.com/2016/12/11/les-carottes-sont-benefiques-pour-vos-chiens/
Care, A. D., Barlet, J.-P., & Abdel-Hafeez, H. M. (1980). Calcium and phosphate homoeostasis in ruminants and its relationship to the aetiology and prevention of parturient paresis. In Y. Ruckebusch & P. Thivend (Eds.), Digestive Physiology and Metabolism in Ruminants: Proceedings of the 5th International Symposium on Ruminant Physiology, held at Clermont — Ferrand, on 3rd–7th September, 1979 (pp. 429-446). Dordrecht: Springer Netherlands. doi: https://doi.org/10.1007/978-94-011-8067-2_20
Carr, S. (2005). Ruminant digestion in Bos taurus. Retrieved from https://www.mun.ca/biology/scarr/Ruminant_Digestion.html
Codron, D., & Clauss, M. (2010). Rumen physiology constrains diet niche: linking digestive physiology and food selection across wild ruminant species. Canadian Journal of Zoology, 88(11), 1129-1138. doi:10.1139/z10-077
Collins, S. M., Surette, M., & Bercik, P. (2012). The interplay between the intestinal microbiota and the brain. Nature Reviews Microbiology, 10(11), 735-742. doi:10.1038/nrmicro2876
Corporation, M. C. (2010). Mammal Anatomy: An Illustrated Guide: Marshall Cavendish. Retrieved from https://books.google.ca/books?id=mTPI_d9fyLAC
Cresci, G. A. M., & Izzo, K. (2019). Chapter 4 – Gut Microbiome. In M. L. Corrigan, K. Roberts, & E. Steiger (Eds.), Adult Short Bowel Syndrome (pp. 45-54): Academic Press. Retrieved from https://www.sciencedirect.com/science/article/pii/B9780128143308000044
Davis, C. D. (2016). The Gut Microbiome and Its Role in Obesity. Nutrition Today, 51(4), 167-174. doi:10.1097/nt.0000000000000167
Dental, V. (n.d.). Tooth Anatomy Education. Retrieved from https://www.vcdental.com.au/tooth-anatomy-education/
Dentistry, M. C. f. C. a. I. (n.d.). The Teeth of Herbivores, Carnivores and Omnivores. Retrieved from https://www.miamicosmeticdentalcare.com/teeth-herbivores-carnivores-omnivores/
Derrien, M., Alvarez, A.-S., & de Vos, W. M. (2019). The Gut Microbiota in the First Decade of Life. Trends in Microbiology, 27(12), 997-1010. doi:10.1016/j.tim.2019.08.001
dos Anjos, L., Bochio, G. M., Medeiros, H. R., Almeida, B. d. A., Lindsey, B. R. A., Calsavara, L. C., . . . Domingues Torezan, J. M. (2019). Insights on the functional composition of specialist and generalist birds throughout continuous and fragmented forests. Ecology and Evolution, 9(11), 6318-6328. doi:https://doi.org/10.1002/ece3.5204
Dwiggins, J. (2016). The Ins, Outs, and In Betweens of Your Digestive Tract: How Muscle Imbalances in Your Jaw and Neck Affect Digestion – Part One: Chewing. Retrieved from https://www.tuneupfitness.com/blog/the-ins-outs-and-in-betweens-of-your-digestive-tract-how-muscle-imbalances-in-your-jaw-and-neck-affect-digestion-part-one-chewing/
Easley, J. (2014, May 2014). Overview of Dentistry in Large Animals. Retrieved from https://www.merckvetmanual.com/digestive-system/dentistry/overview-of-dentistry-in-large-animals
Eloff, F. (1984). Food ecology of the Kalahari lion Panthera Leo Vernayi. Koedoe : African Protected Area Conservation and Science, 27. doi:10.4102/koedoe.v27i2.584
Foods, E. H. P. (n.d.). Herbivores, Omnivores And Carnivores Explained. Retrieved from https://www.edenpetfoods.com/nutrition/herbivores-omnivores-and-carnivores-explained.html
Geographic, N. (n.d.). Generalist and Specialist Species. In National Geographic Animal Encyclopedia. Retrieved from https://www.nationalgeographic.org/encyclopedia/generalist-and-specialist-species/
Gershon, M. D., & Tack, J. (2007). The Serotonin Signaling System: From Basic Understanding To Drug Development for Functional GI Disorders. Gastroenterology, 132(1), 397-414. doi:10.1053/j.gastro.2006.11.002
Goertz, S., de Menezes, A. B., Birtles, R. J., Fenn, J., Lowe, A. E., MacColl, A. D. C., . . . Taylor, C. H. (2019). Geographical location influences the composition of the gut microbiota in wild house mice (Mus musculus domesticus) at a fine spatial scale. PloS One, 14(9), e0222501. doi:10.1371/journal.pone.0222501
Haas, M. (2018). What Are the Functions of the Cecum? Retrieved from https://sciencing.com/functions-cecum-6809336.html
Hammer, T. J., Janzen, D. H., Hallwachs, W., Jaffe, S. P., & Fierer, N. (2017). Caterpillars lack a resident gut microbiome. Proceedings of the National Academy of Sciences, 114(36), 9641-9646. doi:10.1073/pnas.1707186114
Hammer, T. J., Sanders, J. G., & Fierer, N. (2019). Not all animals need a microbiome. FEMS Microbiology Letters, 366(10). doi:10.1093/femsle/fnz117
Hub, S. L. (2007). Meet some muscles. Retrieved from https://www.sciencelearn.org.nz/resources/1923-meet-some-muscles
Jacob, J. (n.d.). Avian Digestive System. Retrieved from https://poultry.extension.org/articles/poultry-anatomy/avian-digestive-system/
Lutz, H. L., Jackson, E. W., Webala, P. W., Babyesiza, W. S., Peterhans, J. C. K., Demos, T. C., . . . Webster, N. S. (2019). Ecology and Host Identity Outweigh Evolutionary History in Shaping the Bat Microbiome. mSystems, 4(6), e00511-00519. doi:doi:10.1128/mSystems.00511-19
Mark, S. (2017). La dentition du silky. Retrieved from https://majolian.chiens-de-france.com/australian-silky-terrier,de-majolian,rubrique_13226_139158_1_0.html
Nougué, O., Gallet, R., Chevin, L.-M., & Lenormand, T. (2015). Niche Limits of Symbiotic Gut Microbiota Constrain the Salinity Tolerance of Brine Shrimp. The American Naturalist, 186(3), 390-403. doi:10.1086/682370
Olsson, J., & Eklöv, P. (2005). Habitat structure, feeding mode and morphological reversibility: Factors influencing phenotypic plasticity in perch. Evolutionary Ecology and Research, 7.
OLSSON, J., QUEVEDO, M., COLSON, C., & SVANBÄCK, R. (2007). Gut length plasticity in perch: into the bowels of resource polymorphisms. Biological Journal of the Linnean Society, 90(3), 517-523. doi:10.1111/j.1095-8312.2007.00742.x
Orthodontics, M. S. C. s. D. a. (n.d.). Dental and Skull Anatomy of Carnivores, Herbivores, and Omnivores. Retrieved from https://www.mainstreetsmiles.com/dental-and-skull-anatomy-of-carnivores-herbivores-and-omnivores/
Price, E. R., Ruff, L. J., Guerra, A., & Karasov, W. H. (2013). Cold exposure increases intestinal paracellular permeability to nutrients in the mouse. Journal of Experimental Biology, 216(21), 4065-4070. doi:10.1242/jeb.088203
Rinninella, E., Raoul, P., Cintoni, M., Franceschi, F., Miggiano, G. A. D., Gasbarrini, A., & Mele, M. C. (2019). What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms, 7(1), 14. Retrieved from https://www.mdpi.com/2076-2607/7/1/14
Rouge, M. (n.d.). Dental Anatomy of Ruminants. In. vivo.colostate.edu: VIVO Pathophysiology. Retrieved from http://www.vivo.colostate.edu/hbooks/pathphys/digestion/pregastric/cowpage.html
Sender, R., Fuchs, S., & Milo, R. (2016). Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biology, 14(8), e1002533-e1002533. doi:10.1371/journal.pbio.1002533
Sepulveda, J., & Moeller, A. H. (2020). The Effects of Temperature on Animal Gut Microbiomes. Frontiers in Microbiology, 11, 384-384. doi:10.3389/fmicb.2020.00384
Shao, S., Sun, X., Chen, Y., Zhan, B., & Zhu, X. (2019). Complement Evasion: An Effective Strategy That Parasites Utilize to Survive in the Host. Frontiers in Microbiology, 10(532). doi:10.3389/fmicb.2019.00532
Shelomi, M., Lo, W.-S., Kimsey, L. S., & Kuo, C.-H. (2013). Analysis of the gut microbiota of walking sticks (Phasmatodea). BMC Research Notes, 6(1), 368. doi:10.1186/1756-0500-6-368
Smith, Y., de Waal, H. O., & O.B, K. (2006). Aspects of Carcass Digestibility by African Lions ( Panthera leo Linnaeus, 1758) under Captive Conditions. Pakistan Journal of Biological Sciences, 9. doi:10.3923/pjbs.2006.2149.2152
STEVENS, C. E., & HUME, I. D. (1998). Contributions of Microbes in Vertebrate Gastrointestinal Tract to Production and Conservation of Nutrients. Physiological Reviews, 78(2), 393-427. doi:10.1152/physrev.1998.78.2.393
Suchodolski, J. S. (2011). Companion animals symposium: microbes and gastrointestinal health of dogs and cats. Journal of Animal Science, 89(5), 1520-1530. doi:10.2527/jas.2010-3377
Uren Webster, T. M., Rodriguez-Barreto, D., Castaldo, G., Gough, P., Consuegra, S., & Garcia de Leaniz, C. (2020). Environmental plasticity and colonisation history in the Atlantic salmon microbiome: A translocation experiment. Molecular Ecology, 29(5), 886-898. doi:https://doi.org/10.1111/mec.15369
Walker, J. G., Hurford, A., Cable, J., Ellison, A. R., Price, S. J., & Cressler, C. E. (2017). Host allometry influences the evolution of parasite host-generalism: theory and meta-analysis. Philosophical transactions of the Royal Society of London. Series B, Biological sciences, 372(1719), 20160089. doi:10.1098/rstb.2016.0089
Watkins, J. (n.d.). Digestive System of a Lion Retrieved from https://lion-nutrition.weebly.com/digestive-system.html
Westervelt, A. (2018). Owl pellets, or regurgitation, are materials from the bird’s prey. In. myrgv.com. Retrieved from https://myrgv.com/the-monitor/2018/05/18/owl-pellets-or-regurgitation-are-materials-from-the-birds-prey/
Woodvine, A., & Spencer, C. (2019). Wheat Eaters or Meat Eaters. In. viva.org.uk. Retrieved from https://viva.org.uk/materials/wheat-eaters-or-meat-eaters/
Wu, Y., Yang, Y., Cao, L., Yin, H., Xu, M., Wang, Z., . . . Deng, Y. (2018). Habitat environments impacted the gut microbiome of long-distance migratory swan geese but central species conserved. Scientific Reports, 8(1), 13314. doi:10.1038/s41598-018-31731-9
Xiang, Z., Zhu, H., Yang, B., Fan, H., Guo, J., Liu, J., . . . Qin, C. (2020). A glance at the gut microbiota of five experimental animal species through fecal samples. Scientific Reports, 10(1), 16628. doi:10.1038/s41598-020-73985-2 Zhang, J.-Y., Zhao, X.-Y., Wen, J., Tan, S., & Zhao, Z.-J. (2016). Plasticity in gastrointestinal morphology and enzyme activity in lactating striped hamsters (Cricetulus barabensis). Journal of Experimental Biology, 219(9), 1327-1336. doi:10.1242/jeb.138396