Discussion of Nature’s Design Solutions in Tintinnids: Masters of Microzooplankton Survival
Alexa Bailey, Lou Cubberly, Margaux-Blondin Routhier, Niall Slack-Watkins
Abstract
In this essay, we explore tintinnids’ survival designs within the context of fundamental physics principles. Tintinnids employ diverse mechanisms to outmaneuver predators, locate prey, and safeguard themselves. To avoid predators, tintinnids utilize specific swimming patterns, attach to groups of particles, develop symbiotic relationships with diatoms, and have undergone morphological adaptations as a consequence of natural selection. Furthermore, these adaptations often initiate coevolutionary responses with other species. When hunting for prey, tintinnids employ negative geotaxis, selective predation, and mechanoreception for optimized foraging. Their lorica, primarily composed of chitin, offers protection and helps them move efficiently while drawing on principles of hydrodynamics, electromagnetic forces, and even weight distribution. Tintinnids highlight how nature applies physics to ensure survival. Understanding these strategies could inspire innovation in various fields by creating more efficient, adaptable, and eco-friendly technologies. By harnessing the important physics lessons we learn from tintinnids, we can develop solutions that benefit industries and ecosystems alike.
Introduction
Microzooplankton play a crucial role in any marine or freshwater ecosystem, weaving themselves into the complex web of life below the water‘s surface. Among this diverse group, the tintinnid, a heterotrophic protozoa, stands out as a particularly captivating organism.
This essay explores the ingenious design solutions tintinnids employ, grounded in the principles of physics. It delves into the realms of hydrodynamics, structural integrity, buoyancy control, the intricate dance of differential predation, and evolutionary adaptations. Through these lenses, we unravel the secrets of tintinnids’ success as microzooplankton, shedding light on their evolutionary significance and ecological role.
Tintinnids have a rich and storied history with over 1200 identified species. The first tintinnid was documented in 1776 (Agatha & Strüder-kypke, 2012). Yet, their ancient lineage is not confined to written records; it is etched in the fossilized remnants they left behind. From the Proterozoic period to the present day, tintinnids have imprinted their presence on Earth’s history.
Fossilized tintinnids, when they do occur, offer insights into the past. Typically, these fossils preserve only the lorica, the protective shell that encapsulates the tintinnid’s single-celled ciliate body (Lipps et al., 2012). The diversity of loricae observed in these fossils, ranging from hyaline to hard shells adorned with particles, provides a window into the shapes and compositions of their ancient counterparts (Agatha & Strüder-kypke, 2012). However, the challenge of correctly identifying these ancient organisms persists, as fossil records can only offer limited information. Intriguing sets of fossils believed to be tintinnids emerged from the Proterozoic eon. The first set, dating back 1600 million years, challenges established timelines and hints at the possibility that ciliates originated earlier than previously thought. The second set, dating between 635 and 715 million years ago, presents significant deviations from modern tintinnids, characterized by the presence of silicon within the lorica. This difference casts doubt on their classification as tintinnids. The third set, originating 580 million years ago, bears a closer resemblance to contemporary tintinnids, particularly in lorica shape (Lipps et al., 2012).
Tintinnids form a crucial component of microzooplankton, which is further divided into four groups: ciliates (including tintinnids), dinoflagellates, rotifers, and microcrustacean larvae (McManus & Santoferrara, 2013). However, the tintinnid group stands out as a prominent member of microzooplankton, owing to its distinctive characteristics. Tintinnids can constitute anywhere from 0 to 81% of the total microzooplankton biomass, with a notable prevalence in nearshore environments, where they can comprise 95 to 100% of samples collected in approximately 9% of nearshore microzooplankton communities (McManus & Santoferrara, 2013). This heightened presence underscores their significance in these specific marine habitats.
Tintinnids, distinguished by their single-celled ciliate nature, possess a remarkable feature that sets them apart from other microzooplankton – a protective, armored shell known as the lorica, derived from the Latin word for “armor.” This unique adaptation provides tintinnids with a level of defense unparalleled in the realm of ciliates. The lorica comes in various forms, from transparent, hyaline structures to mineral-infused, agglomerated shells, reminiscent of champagne glasses, vases, or tubes, as can be seen in Figure 1. The opacity of their lorica varies based on their habitat, with coastal tintinnids typically having agglomerated shells, while tintinnids living in the deep sea have hyaline ones. Intriguingly, even within a single tintinnid species, a wide array of lorica shapes can be observed, posing taxonomic challenges until recent advances (Agatha, 2021).
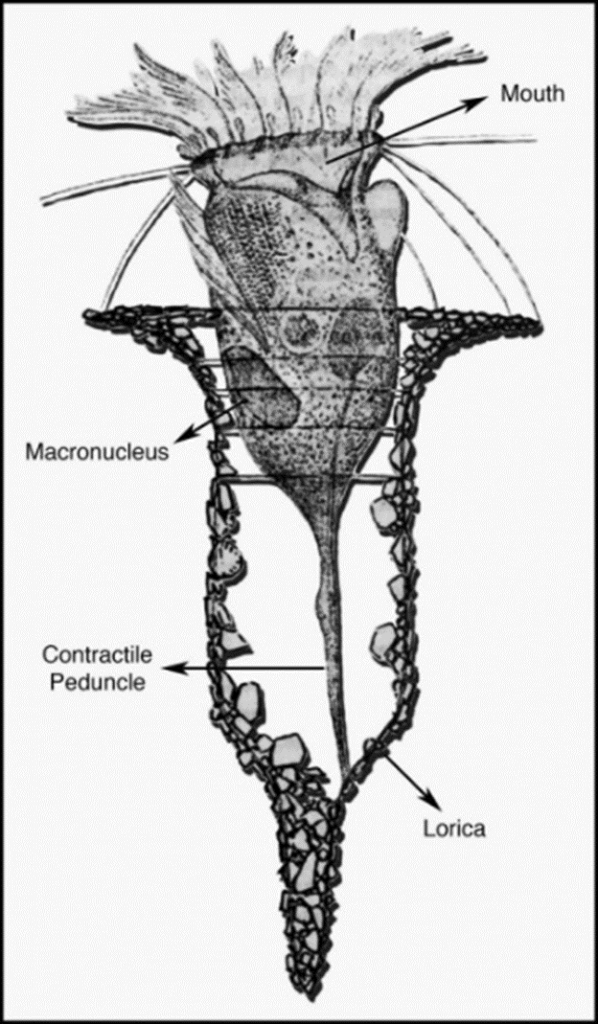
Fig. 1. Diagram of a tintinnid ciliate and its shell (Fauré-Fremiet, 1924).
Structure of tintinnids’ lorica
A defining feature of tintinnids is their loricae. Tintinnids’ loricae are an extremely important part of their biology and survival. They aid in swimming and buoyancy, in prey acquisition, and in protecting tintinnids from predators. The loricae of tintinnids represent a defining and indispensable feature of their biology and survival. The structural integrity of loricae is a product of both material composition and geometric attributes. The hexagonal pattern, found across various tintinnid species, showcases nature’s remarkable adaptation of employing geometric strength to create protective homes for these microorganisms. This section describes the structure of the lorica itself.
Loricae are unique to each species of tintinnid. More specifically, the wall texture of their loricae has been a crucial tool in classifying tintinnids by species (Agatha, 2021). With over 1200 species of tintinnids, this offers an incredibly wide variety of different loricae as can be seen in Figure 2. There are four possible types of loricae that can be identified: the hyaline one with transparent walls, the hard one with particles attached to the walls, the soft one with particles attached to the walls, and the hard one composed of a hyaline collar, and agglomerated bowl (Agatha & Strüder-kypke, 2012). It is important for these creatures that their “homes” be structurally sound. Loricae are composed of chitin, which is a tough, semitransparent material. The intermolecular forces of chitin can be quantified using Coulomb’s law (Equation 1).
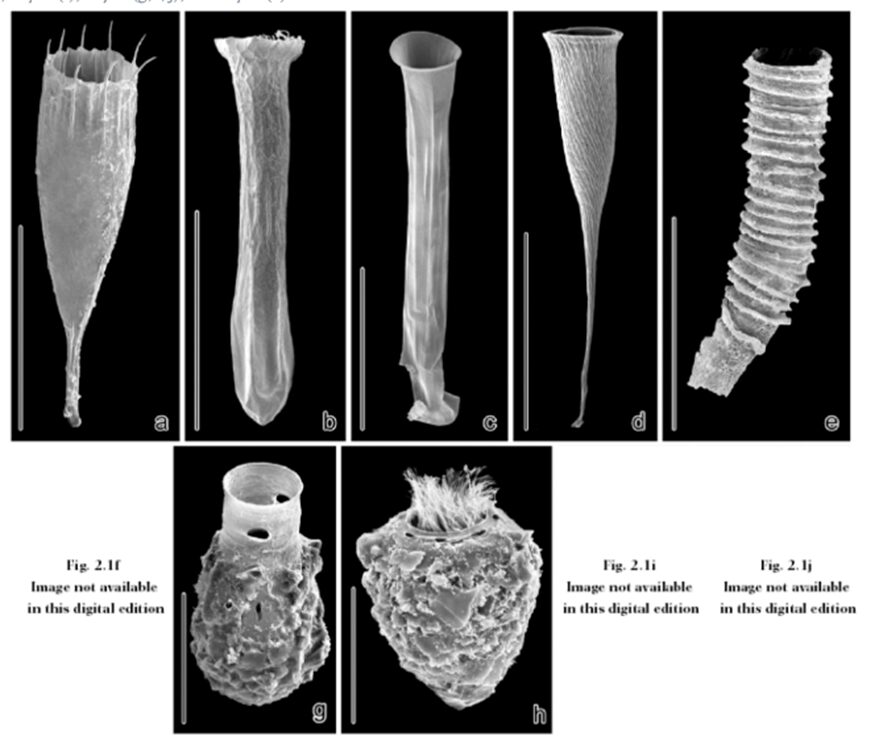
Fig. 2. Scanning electron microscopy images of a variety of hyaline and agglomerated loricae. Scale bars respresent (a, b): 50 μm; (c): 100 μm; (d, e): 150 μm; (g): 50 μm; (h): 30 μm (Agatha, 2008).
Equation 1:
F = (kq_1 q_2)/r^2
Where q1 and q2 represent the charge on each molecule, k is a constant, and r is the distance between the two molecules.
Electromagnetic forces between polymers are part of the reason why the lorica is so strong. However, geometry also plays a role in the strength of the lorica. When a certain species (Eutintinnus angustatus) was analyzed with Fast Fourier Transform (FFT), more was discovered about the structure of their shell. A fast Fourier transform is an algorithm that computes a discrete Fourier transform, which is a mathematical function of frequency. In this case, it is used in the context of spectroscopy; using certain atoms’ unique spectra to learn more about the composition of the shell. Zoomed in at 200-400 nm, a crystal lattice on the surface of the lorica became clear (Figure 3). With increased magnification, a hexagonal symmetry was discovered; the lattice had a periodicity of 23.7 nm (the minimum distance at which the lattice repeats itself) and a hexagonal unit cell size of 27.4 nm (Dolan, 2012). For reference, the cilia of tintinnids range from 20-200 micrometers; this is to say that the units that make up their “homes” are hundreds of times smaller than the actual organism inhabiting it. This tiny hexagon pattern allows for a more structurally sound and protective home for the ciliate; this is because hexagons are made of triangles which are a strong shape due to their ability to distribute weight evenly and their low density.
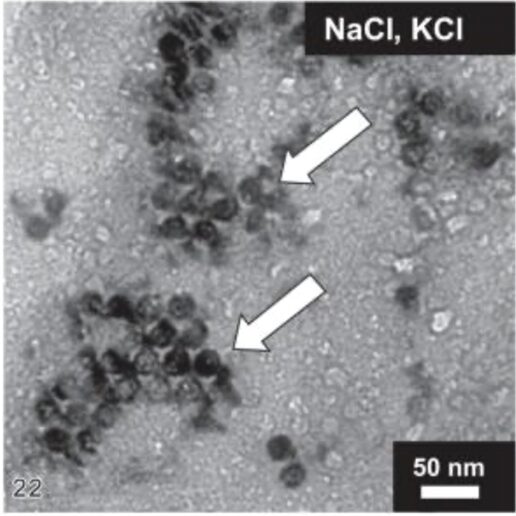
Fig. 3. Transmission electron micrograph of the surface of Eutintinnus angustatus’ lorica (Agatha, 2012).
Most species of tintinnids, not only the Eutintinnus angustatus, have this hexagonal structure in the middle layer of their lorica. Figure 4 shows a cross-sectional view of the Undellopsis‘s shell and a similar hexagonal pattern is clearly identified.
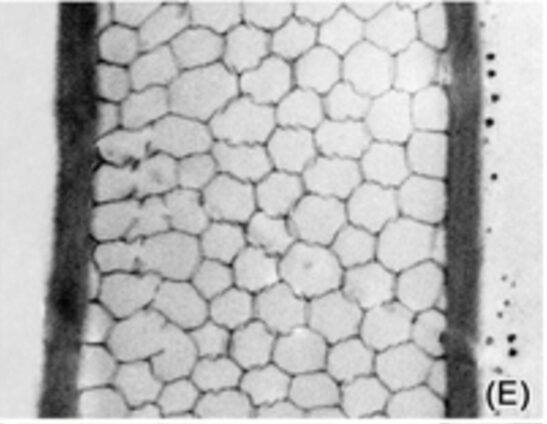
Fig. 4. Tangential view of Undellopsis lorica (Bartel, 2021).
The hexagonal cell lattice (more commonly named “honeycomb”) is not only used in the tintinnid shell, but it is also the most widespread lattice geometry in aerospace and the construction industry (Dizon, 2018). Honeycombs are structurally advantageous because of their high specific modulus and high specific energy absorption. Specific modulus is a material’s property and can be thought of as a stiffness-to-mass ratio. It is represented in Equation 2 as elastic modulus divided by density. Elastic modulus is defined in Equation 3 and can be thought of as the stiffness of a material. Elastic modulus tells us how much a material can resist deformation and specific modulus relates this value to the material’s density. A material with a high specific modulus is therefore one that is stiff and light, which is ideal for a tintinnid because a lighter shell allows for less energy exertion while swimming. Aerospace engineers take advantage of the high specific modulus of honeycomb structures because just like tintinnids in the ocean, aircrafts, satellites, and missiles require a high-strength material that is as light as possible.
Equation 2:
Specific ~modulus = E/ρ
Where E = elastic modulus and ρ = density.
Equation 3:
Elastic ~modulus = stress/strain
Where stress is the force acting upon a material, in units of Pa, and strain is the deformation due to stress (dimensionless).
Specific energy absorption is another crucial factor in considering the structural integrity of a material. It is modelled very simply in Equation 4 in terms of energy absorbed by a certain material and its density. Upon inspection, we can tell that a material with high specific energy absorption will be one with a low density and/or high energy absorption.
Equation 4:
SEA = E/ρ
Where SEA is “specific energy absorption”, E is the energy absorbed by the material in kJ and ρ is the material’s density.
A hexagonal lattice has a high specific energy absorption because it has a low density. The low density also partly contributes to a high specific modulus, though this is also dependent on the hexagon’s ability to withstand stress which we will explore later. What sets a hexagonal lattice apart from other lattices is not only that hexagons are strong, but that they are space efficient, and therefore a material made of a hexagonal lattice will have a low density. With the same perimeter, a hexagon can enclose much more area than a triangle can, as shown in Figure 5. Only three polygons can tesselate; the square, the triangle, and the hexagon. Of these three, the hexagon encloses the most area and is therefore the most advantageous (at least with respect to density). This is precisely why bees employ hexagons into their hives; with minimal beeswax, a larger structure can be built to store the maximum amount of honey. Hexagonal lattices allow a structure composed of minimal materials (and therefore minimal mass) to be more voluminous (and therefore less dense). This low-density feature allows for a high specific modulus and high specific energy absorption for the lorica.
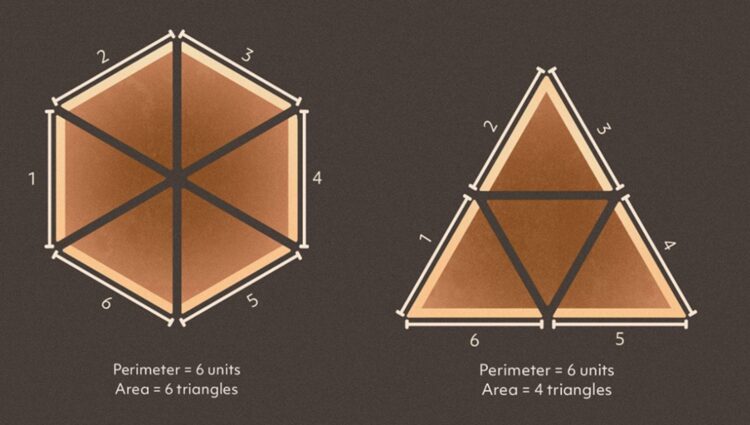
Fig. 5. Comparison of a hexagonal structure versus a triangle one with the same perimeter (Carstens, 2020).
Hexagons are also excellent stress distributors. Stress distribution is more evenly dispersed as the number of struts and nodes increases in a structure (Li, 2022). This supports the notion that a hexagonal lattice is particularly strong because, in one hexagon, there are 6 nodes and 6 struts. This is a lot in comparison to a triangle that has 3 and 3, or a square that has 4 and 4.
Tintinnids need a shell that can protect them from the forces in their environment. They tend to float near the surface of the water, but they still need a shell that can withstand changes in pressure (compressive forces) to go to deeper altitudes. A lattice under compression undergoes elastic deformation, energy absorption, and densification (Wahyudin, 2017). A honeycomb is therefore ideal to protect the ciliate from compressive forces because as mentioned earlier, a hexagonal lattice has a high specific energy modulus (which is directly correlated to a high elastic modulus), a high specific energy absorption, and a low density, meaning it can “afford” to be densified. The honeycomb structure has been used in the tintinnid’s lorica because it is mass efficient while being sturdy enough to protect the ciliate which would otherwise be extremely vulnerable to predators.
Mechanics in tintinnids
This section delves into the physics and adaptations that underlie tintinnids’ buoyancy regulation and locomotion. Buoyancy in tintinnids arises from structural adaptations, while swimming is governed by fluid dynamics. This comprehensive examination aims to shed light on the remarkable capabilities of these microorganisms to thrive in the dynamic marine environment. A fundamental aspect of their survival strategy in their environment involves buoyancy control and swimming mechanics.
Fluid environment
Tintinnids exhibit intriguing swimming mechanics, heavily influenced by fluid dynamics. Their swimming must counteract their sinking tendencies. According to J. Febre, the sinking speed of a tintinnid in seawater can be modelled by the Stokes equation (Equation 5) (Febvre-Chevalier & Febvre, 1994).
Equation 5:
v=(2gr^2 (p_1-p_2 ))/9μ
This equation indicates that the sinking speed (𝑣) is inversely proportional to the viscosity (µ) of the fluid and directly proportional to the difference in densities between the particle and the fluid. When an object moves through a fluid, it encounters two primary forces: viscous forces and an inertial force originating from the acceleration of the fluid that accompanies the object’s motion. The Reynolds number (Re) serves as a vital dimensionless quantity in defining the relative importance of inertial forces and viscous forces within the fluid dynamics surrounding objects. It is defined as the ratio between the inertial forces and viscous forces (Febvre-Chevalier & Febvre, 1994). Its significance becomes particularly evident when examining tintinnids and their interactions with their environment. This parameter defines whether inertial or viscous forces dominate fluid behavior. In instances where Re is greater than one, inertial forces dominate. Conversely, when Re is considerably less than one, viscous drag forces become the primary influence, characterizing the motion of small particles such as protists and tintinnids (Febvre-Chevalier & Febvre, 1994). The Reynolds number is defined as follows:
Equation 6:
Re=ρuL/μ
Where Re = Reynolds number, 𝜌 = density of the fluid, u = flow speed, L = characteristic linear dimension, µ = Dynamic viscosity of the fluid.
In the case of tintinnids, which inhabit a low Reynolds number environment, viscous drag forces dominate due to the absence of significant inertial forces (Febvre-Chevalier & Febvre, 1994; Montagnes, 2012). In this environment, the shape and surface configuration of the tintinnid, such as the lorica, become pivotal factors influencing drag. Fluid behavior significantly differs in a low Reynolds number environment from that observed in higher Reynolds number systems. In such conditions, water behaves as if it were viscous and sticky, similar to if one were swimming through thick syrup. The absence of substantial inertial forces means that when a tintinnid stops propelling itself, it ceases moving almost instantly. Furthermore, the surrounding water effectively “sticks” to the ciliate, forming a thin layer around it. In this low Re environment, tintinnids must actively navigate and continually move this “coat” of water, essential for their food acquisition as they rely on diffusion and active swimming to locate food sources.
Due to the prevalence of viscous forces, tintinnids have evolved to have optimal shapes and surface structures for minimal drag. Unlike organisms swimming in high Reynolds number environments, where shape and surface configuration have a reduced impact on drag, tintinnids must carefully adapt their morphology to minimize resistance. For example, their vase-like lorica may serve several purposes, providing protection, decreasing drag forces, aiding in buoyancy, and aiding in reorientation when sinking occurs.
Buoyancy
Buoyancy control is a defining feature of tintinnid physiology, allowing them to avoid sinking and remain suspended in the water. This ability is crucial for their survival as they must be able to stay in areas with a high density of prey, which are found closer to the surface. It is suggested that this control arises from a combination of structural adaptations, including the presence of capsules or loricae. These protective structures not only shield tintinnids from potential threats but also serve as integral components of their buoyancy control mechanisms, aiding in the ciliate swimming (Dovgal & Gavrilova, 2018).
Furthermore, tintinnids lorica can help them orient themselves in the water and stay near the surface (Dovgal & Gavrilova, 2018). This is achieved through a process called negative geotaxis. Negative geotaxis in tintinnids refers to their inherent tendency to swim or move in an upward direction, against the force of gravity, within their aquatic environment (Winet & Jahn, 1974). Geotaxis is a biological phenomenon referring to the directional movement or orientation of an organism in response to gravity. It is the tendency of organisms to move either toward (positive geotaxis) or away (negative geotaxis) from the gravitational pull of the Earth.
Unlike some other organisms that possess specialized structures or organelles for sensing gravity, most ciliates, including tintinnids, lack such dedicated gravity-sensing mechanisms. Instead, their negative geotactic behavior is believed to be primarily hydrodynamically driven, meaning the way tintinnids interact with fluid dynamics (Montagnes, 2012). When planktonic ciliates like tintinnids find themselves sinking due to gravity, they appear to have the ability to reorient their position so that their anterior ends are facing upward. This reorientation likely helps them counteract the downward pull of gravity, allowing them to effectively swim upward. While the exact mechanisms behind this reorientation are not fully understood, it is a remarkable adaptation that aids in their survival and ecological success. The presence of a lorica may play a role in facilitating this reorientation. The mass of the lorica could potentially act as a stabilizing structure, aiding tintinnids in maintaining the desired orientation with their anterior ends facing upward. Negative geotaxis allows tintinnids to position themselves in the water column to feed on other microorganisms that may be more abundant in the upper layers of the water. Indeed, the advantage of this behavior is reflected in their distribution, as tintinnids tend to be more strongly associated with surface waters as compared to close relatives without loricae (Montagnes, 2012).
Swimming patterns
Research suggests that tintinnid swimming consists of two rotational motions: movement in circles and rotation around their long axis (Montagnes, 2012). This circular and helical motion resembles a spiral trajectory as they move through the water and can be seen in Figure 6. The circular motion in tintinnids is primarily driven by specialized structures called oral ciliature. These are rows or bands of cilia that surround the oral region of the ciliate. These cilia beat in a coordinated, wave-like fashion, creating a flow of water around the ciliate (Figure 7). The beating cilia effectively act like tiny oars, propelling the ciliate forward within the water. By adjusting the angle of these membranelles, tintinnids can enhance their rotational movement, which, in turn, affects their trajectory and direction of swimming. The specific orientation and arrangement of cilia on their body contribute to this helical movement. Interestingly, tintinnids may vary the rotational direction of their helical swimming, even within individual organisms. This variability in swimming direction can be observed as they navigate through their aquatic environment. The ability to change their rotational direction adds an element of adaptability to their swimming behavior, potentially aiding in their response to changing environmental conditions, optimizing their exploration of their surroundings, and avoiding predators (Cicconofri & DeSimone, 2019).
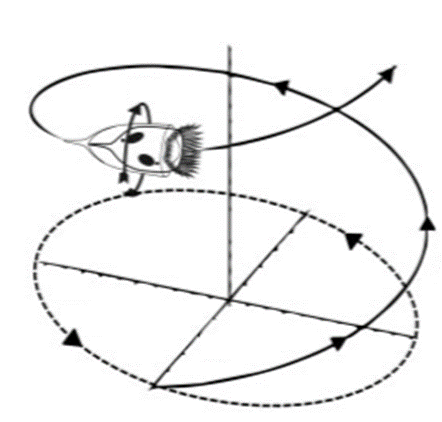
Fig. 6. A generalized helical tintinnid swimming pattern. The characteristic double rotational motion of tintinnids can be seen in this schematic. The helical motion and the rotation along the longitudinal axis of tintinnids are denoted by the arrows (Adapted from Montagnes, 2012).
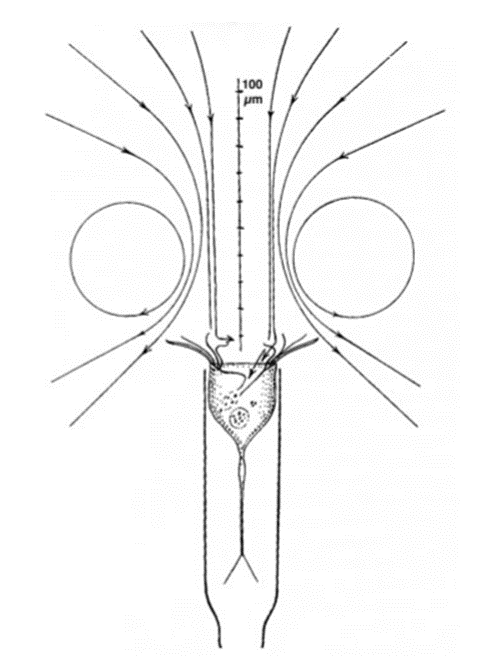
Fig. 7. Water flow generated by Tintinnidium inquilinum (Eutintinnus iniquilinus). This diagram illustrates hydrodynamic patterns generated by the ciliary beating of the Tintinnidium inquilinum species of tintinnid (Montagnes, 2012).
In addition to the circular motion, tintinnids can control the rotation around their longitudinal axis (Figure 6). This means that while they are moving in a circular path, they can also spin or rotate around their own length. This rotation is a deliberate and controlled aspect of their swimming behavior. By varying the degree of rotation, they can fine-tune their swimming path (Montagnes, 2012). Tintinnids may alter the rate of rotation in response to environmental factors. For instance, encountering obstacles or prey may make them adjust their rotation to navigate or capture food more effectively.
Tintinnids’ swimming behavior is not static; instead, they can respond dynamically to various external factors and physical parameters by adjusting their movements and swimming patterns (Montagnes, 2012). Their ability to sense and react to their surroundings is crucial for their survival in the marine environment. Depending on the nature and location of the stimulus, tintinnids may exhibit changes in swimming behavior. Active forward swimming is correlated with posterior stimulation and reverse slow backward swimming occurs following anterior stimulation. Additionally, stimulation at the anterior end induces avoiding reactions, characterized by ciliary reversal and backward movement, followed by reorientation and normal forward swimming. These behaviors are influenced by environmental factors, particularly prey abundance (Montagnes, 2012). Increased swimming rates and changes in reorientation are observed when prey concentrations rise, indicating an adaptive response to local conditions. Tintinnids are also responsive to toxic algae. Exposure to toxic dinoflagellates triggers distinct swimming behaviors, such as increased turns and prolonged periods of backward swimming. This transient behavior allows tintinnids to escape toxic environments effectively.
Tintinnids’ distinctive helical swimming patterns in low Reynolds number conditions offer valuable insights for biomimetic microrobot design. In the realm of engineering, the replication of tintinnids’ helical swimming patterns holds promise for enhancing microrobot locomotion and maneuverability in environments characterized by high viscosity and low fluid flow rates. The helical motion of tintinnids enables efficient propulsion, making it an attractive blueprint for microrobots designed to navigate through viscous fluids, such as bodily fluids or complex fluid systems (Ceylan, 2017). Additionally, tintinnids’ reactivity to toxic environments could be utilized to measure concentrations of chemicals for environmental monitoring. Tintinnids’ swimming behaviors and buoyancy mechanisms, which play a crucial role in their survival by mitigating their inherent tendency to sink and enabling them to remain in regions with abundant prey, also offer intriguing possibilities in the field of bioengineering. Much can be learned from tintinnids’ unique solutions for efficient locomotion and acquisition of prey, essential to their survival.
Interactions with other species
The ecological interactions of tintinnids with their environment and other organisms are multifaceted, with a focus on their prey selection strategies, where they discern among prey types based on prey size and prey movement, influencing their role as both predators and prey in marine ecosystems. Tintinnids engage in mutualistic relationships with diatoms while facing parasitic interactions with other microorganisms. Additionally, tintinnids function as valuable bioindicators, reflecting changes in water quality and environmental conditions, owing to their responsiveness and distinctive characteristics. Tintinnids occupy a pivotal role within microzooplankton communities, influencing ecosystem dynamics and serving as crucial prey for larger organisms.
Prey selection
Regarding prey selection, tintinnids employ a strategy known as “differential predation” (Yang et al., 2019), where they selectively favor specific prey types. These marine microorganisms also exhibit prey rejection and preference patterns (Montagnes, 2012), primarily influenced by their adeptness in handling different prey species (Blackbourn, 1974). Alongside differential predation, tintinnids employ negative selection, largely determined by the ease of digestibility of potential prey species (Blackbourn, 1974). For instance, Favella ehrenbergii, a tintinnid species, exclusively opts for dinoflagellates as prey when presented with a combination of dinoflagellates and non-dinoflagellates (Yang et al., 2019). Tintinnids are not like picky eaters: their food selection mechanism does not depend on the taste of the food itself, but on prey size, search duration, handling time, nutritional content, and assimilation efficiency, with prey size emerging as the most important factor (Yang et al., 2019). Larger prey size augments the encounter probability between tintinnids and their prey, exemplifying why tintinnids predominantly favor larger prey items (Montagnes, 2012). Typically, tintinnids target prey with an average diameter of 8 micrometers, although instances have been documented where they consume prey spanning diameters from 2 to 30 micrometers (Montagnes, 2012).
Encounter probability is intricately linked to the maximum encounter area defined by the tintinnid’s oral region and oral cilia, particularly in sensory tintinnids. The encounter area can be mathematically expressed as:
Equation 7:
Encounter~area = π(r_t+r_p )^2
Where rt represents the tintinnid radius and rp signifies the prey radius (Montagnes, 2012).
Notably, when dealing with larger prey, their size must also be factored into the maximum encounter area equation. Consequently, larger prey contribute to a larger encounter area, subsequently enhancing the likelihood of interaction with tintinnids when compared to smaller prey (Montagnes, 2012). This preference for larger prey is one of the factors guiding tintinnid foraging strategies. Additionally, the speed of the prey and the speed of the ciliate are both significant factors influencing the contact between a tintinnid and its prey. The way the speed of both organisms affects the encounter rate can be expressed in a mathematical expression as follows:
Equation 8:
Encounter rate=(πr^2 P)/3×(u^2+3v^2)/v
where P is the prey concentration, u is the prey speed, v is the predator speed and r is the predator encounter radius (Montagne, 2012).
Another aspect influencing tintinnid prey selection is the mechanical behavior of potential prey, particularly their swimming and movement patterns. To put this in perspective, if the prey moves away from the ciliate in the same way a dog runs away from its owner trying to prevent it not to go in the road, it will be harder for the tintinnid to capture that prey. Tintinnids employ mechanoreception to sense the movements of potential prey, gravitating towards prey exhibiting mechanical properties conducive to a higher encounter probability (Yang et al., 2019). Motility, food quality, and the mechanical characteristics of prey contribute to tintinnids’ discerning prey selection process (Montagnes, 2012).
Following prey selection, tintinnids proceed to capture and consume their chosen prey utilizing a distinct two-step predation strategy. The initial phase, known as “encounter,” encompasses the processing of water and active prey searching, culminating in physical contact between the tintinnid and its prey, primarily facilitated by ciliary structures (Montagnes, 2012). During this phase, tintinnids evaluate and engage potential prey. Subsequently, the second phase, called “processing,” involves prey capture, potential rejection of the captured prey, and the transformation of the prey into a specialized food vacuole within the tintinnid’s cystostomal region. Here, the prey undergoes digestion, with any indigestible portions ultimately expelled through the tintinnid’s cell anus (Montagnes, 2012). Figures 8 and 9 depict the entire sequence of tintinnid feeding processes. While these observable capture mechanisms provide valuable insights, it is proposed that tintinnids may employ unobservable ligands or alternative methods to help in the capture and binding of their prey (Montagnes, 2012). As tintinnids are microscopic organisms, much is still unknown about their intricate feeding processes.
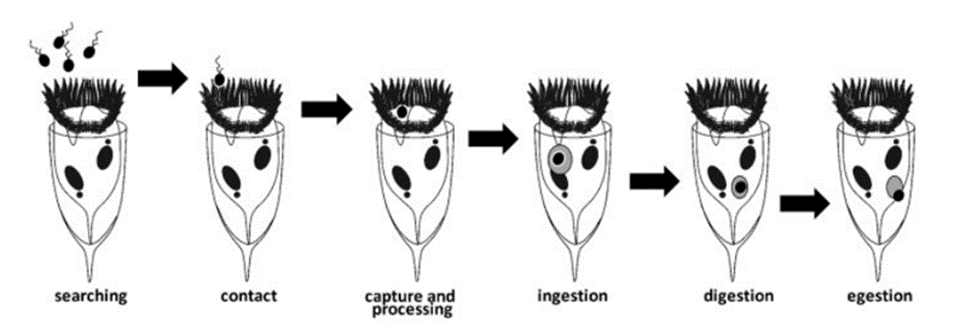
Fig. 8. The feeding steps of tintinnids (Montagnes, 2012).
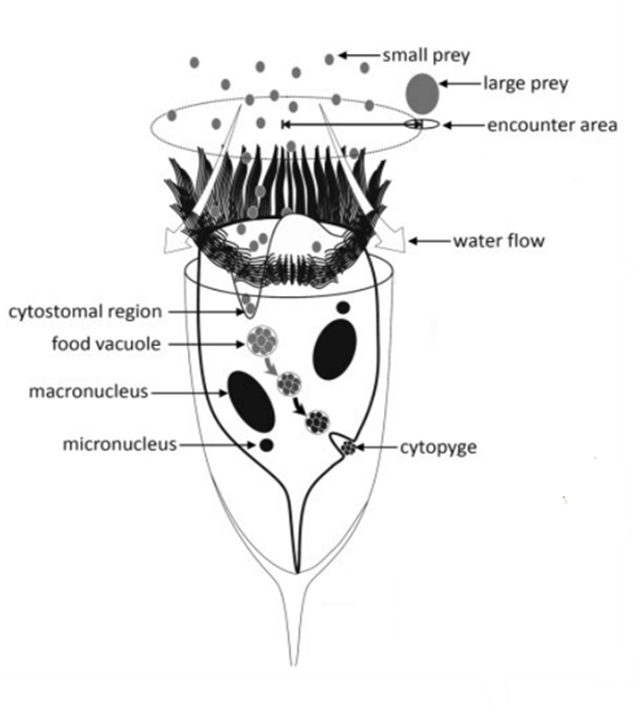
Fig. 9. The generalized tintinnid consumer (Montagnes, 2012).
To determine the duration of tintinnids’ prey consumption, it is analysed as a distinct process from their prey capture and processing mechanism. This separation involves two key steps: first, the search for prey, and second, the handling of prey. The handling phase encompasses all activities related to capturing prey and enclosing it within a food vacuole (Montagnes, 2012). Since tintinnids cannot perform these two steps simultaneously, the total time required to consume a prey is the sum of the time spent searching for prey and the time invested in handling it.
Mutualistic and parasitic interactions
Tintinnids engage with microorganisms not only through selection, capture, and consumption but also through symbiotic and parasitic interactions, giving rise to a spectrum of ecological relationships. These interactions encompass mutualistic associations where different microorganisms derive benefits from their interactions with tintinnids while reciprocating advantages. Conversely, some microorganisms adopt parasitic strategies, imposing harm, or restrictions on tintinnids while exploiting them, ultimately posing a detriment to the ciliates.
One example of a mutualistic interaction involves tintinnids and diatoms, a subgroup of phytoplankton. While tintinnids occasionally prey on diatoms, they predominantly coexist in a symbiotic relationship. In this partnership, diatoms attach themselves to the lorica of tintinnids, leading to modifications in both organisms (see Figure 10 for illustration). The diatom’s attachment preserves its own morphology while modifying the soft body of the tintinnid to enhance its size and protection (Gómez, 2020). This mutualistic bond offers several advantages, including improved buoyancy and motility and access to nutrients for diatoms (Vincent et al., 2018). Indeed, the attachment of the diatom to the tintinnid’s loricae increases the hydrodynamic drag of the ciliate, which leads to the tintinnid going deeper in the water, which helps it to get away from predators. Concerning diatoms, their motility is improved due to the movement of the ciliate they are attached to, allowing them to present horizontal displacements, which are not observed in free-living diatoms. With improved buoyancy and motility, access to nutrients and other resources such as light and food is easier (Gómez, 2020). Tintinnids, in turn, experience enhanced buoyancy, facilitated by the alteration of feeding current dynamics in their favor (Vincent et al., 2018). Their enlarged size affords them increased protection against smaller predators such as copepods (Gómez, 2020). These outcomes underscore the mutual advantages inherent in this symbiotic relationship.
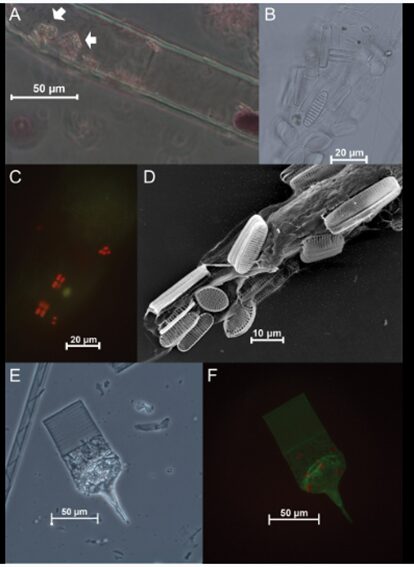
Fig. 10. Illustration of different kinds of tintinnid-diatom associations (A is between live Laackmanniella naviculaefera and live Fragilariopsis spp, B is between empty L. naviculaefera and empty diatom frustules, C is between empty L. naviculaefera and live Fragilariopsis spp, D is between live L. naviculaefera and F. curta with F. rhombica and E and F are between empty Codonellopsis and live Fragilariopsis spp.) (Armbrecht et al., 2017).
Conversely, tintinnids also encounter parasites that can destroy their generative nucleus or release toxins, typically resulting in the demise of the ciliate, though instances of complete recovery have been recorded (Coats & Bachvaroff, 2012). The boundary between tintinnid parasites and predators remains obscure, as both can inflict fatal harm within a similar timeframe (Coats & Bachvaroff, 2012). One example of parasitism towards tintinnids is parasitic dinoflagellates, as depicted in Figure 10. When infected, tintinnid reproduction is significantly hindered. Tintinnid parasites persist across seasons, contributing to common and recurrent declines in tintinnid populations (Coats & Bachvaroff, 2012). Intriguingly, these organisms, though detrimental to tintinnids, play a role in the recycling of materials at the microbial level (Coats & Bachvaroff, 2012).
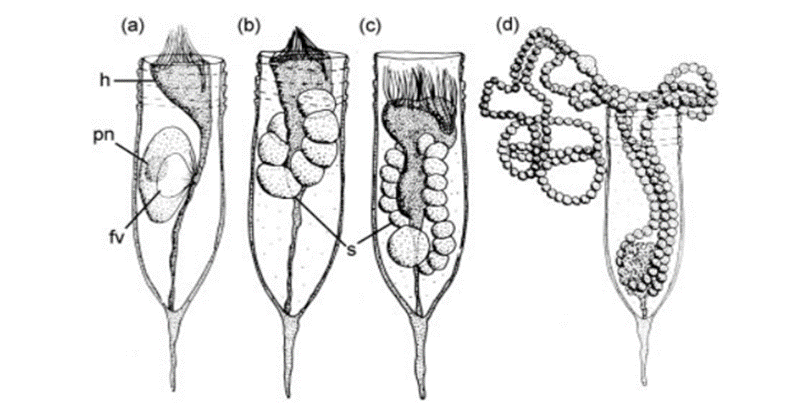
Fig. 11. The infection cycle of a tintinnid by parasitic dinoflagellates (h is the host, fv is the food vacuoles, s are the sporocytes, sporogenic division products preceding spore formation and pn is the parasite nucleus) (Coats & Bachvaroff, 2012).
Their role in the marine environment
Tintinnids establish not only individual relationships with specific organisms but also participate in broader ecological connections within their environment. These microscopic ciliates are integral components of the microzooplankton community (McManus & Santoferrara, 2013), which, in turn, plays a vital role in the marine ecosystem by facilitating the transfer of matter and energy, sustaining the microbial food web, and contributing to the food chain (Li et al., 2016). Tintinnids effectively bridge the gap between nanozooplankton and mesoplankton, functioning as both consumers of nanozooplankton and as prey for mesoplankton.
Tintinnids’ food consumption has a significant impact on ecosystem dynamics, often playing a dominant role in the microzooplankton community (Dolan, 2010). Remarkably, they account for as much as 70% of the overall grazing activity within this community. Tintinnids exhibit rapid responses to fluctuations in phytoplankton abundance, enabling them to effectively mitigate or halt the proliferation of invasive phytoplankton species, including microalgae (McManus & Santoferrara, 2013). This responsiveness positions these marine organisms as potential regulators of ecological balance in their habitats.
Tintinnids also serve as prey for many metazoan zooplankters (Dolan, 2010), like copepods, which again proves their importance in the food chain of the marine ecosystem (Weisse & Montagnes, 2022). Ciliates constitute about 30% of the daily food consumption of copepods (McManus & Santoferrara, 2013), giving a good example of their importance as a prey and food source for the larger organisms.
Organisms that are a part of the microzooplankton are the primary herbivores in the sea and the primary nutrient regenerators of organic nutrients, returning 70% of the food they ingest to be reused by other organisms such as phytoplankton and bacterioplankton (McManus & Santoferrara, 2013).
Furthermore, tintinnids serve as valuable bioindicators, reflecting changes in water quality and environmental contamination. Bioindicators encompass species or communities that embody the abiotic or biotic conditions of an environment, reflecting the consequences of environmental alterations on habitats, communities, or ecosystems, as well as signifying the overall diversity within a given area (Gerhardt, 2002). According to this definition, bioindicators can be classified into three primary categories: environmental indicators, ecological indicators, and biodiversity indicators (Gerhardt, 2002). Tintinnids are environmental indicators. These marine ciliates, renowned for their sensitivity to alterations in their surroundings, particularly excel as bioindicators of water quality and environmental contamination (Rakshit et al., 2017). Their suitability for this role stems from several key attributes. Notably, their short life cycles, delicate pellicles, rapid growth rates, and adaptability to extreme conditions make them exceptionally responsive to environmental alterations (Rakshit et al., 2017). Their distinctive lorica protection, recognizable physical characteristics, and high abundance contribute to their effectiveness as bioindicators (Wang et al., 2021). These qualities render tintinnids essential for assessing water quality in marine environments and monitoring environmental changes (Rakshit et al., 2017).
Tintinnid’s diverse roles illuminate the intricate web of ecological relationships that underpin marine ecosystems. By delving into the world of tintinnids, we gain a deeper understanding of the forces shaping our oceans and the role of microorganisms in maintaining ecological balance.
Evolutionary adaptations
As with every organism on the planet, the tintinnid’s habitat has changed dramatically during the course of Earth’s existence. The harsh nature of these changes means that only those organisms ready to adapt to them will survive. Tintinnids have long been observed to play a significant role in aquatic ecosystems, so it is crucial to study how they have evolved to deal with shifts to the water such as seasonal temperature changes. In a study from the Hooghly River Estuary, a comprehensive view of the seasonal dynamics of these microorganisms has been laid out, shedding light on their community structure, environmental responses, and evolutionary adaptations (Rakshit, 2014).
There are three main factors one must consider when analyzing the patterns of community structure response to seasonal change: species diversity index (H′), species richness (R′), and species evenness (E’). These indices can be calculated with the following formulas;
H′ = −ΣS_i= P_i(ln(P_i));~~~~~E' = H/ln(S);~~~~~R′ = ((S-1))/ln(N)
where Pi = proportion of the total count arising from the ith species, S = total # of species, and N = total # of individuals. (Rakshit, 2014).
A high species diversity index indicates a broad range of species present in similar numbers, a high species richness would correspond to one or more species existing in high volume, and a high species evenness represents a large array of species regardless of quantity. The researchers within this paper note that “an overall species diversity index (H′) was found to be high during post-monsoon and low during pre-monsoon. Species richness (R′) value showed an almost opposite trend with diversity indices. However, species evenness (E′) indicates almost a similar trend throughout the year in all the sites (Rakshit, 2014).” This observation pinpoints a clear cyclical pattern in the population dynamics of tintinnids. During the post-monsoon, the environment supports a diverse population of tintinnids, implying a wealth of different niches being occupied. As the season transitions into pre-monsoon, there is a decline in diversity but a rise in species richness. This indicates that while fewer species may dominate the estuarine ecosystem, those that do thrive are present in large numbers. This seasonal shift suggests that certain species of tintinnids have evolved to capitalize on the resources and conditions prevalent during the pre-monsoon months, outcompeting other species.
As winter approaches, the environmental conditions become harsher, leading to a decline in the overall number of tintinnids. It is during these colder months that the robustness of evolutionary adaptation is tested. Only those species with the necessary genetic traits to endure such conditions can survive, leading to a resetting of the community structure by the time post-monsoon arrives. This observation is supported by the fact that species evenness remains consistent throughout the year, suggesting that the presence of each species within the community does not see drastic changes even though their populations might. Understanding this, we can assume that the population maintains high diversity even under pressure from the dominant species.

Fig. 12. Microphotographs of representative tintinnid species of diverse lorica structures recorded from the Hooghly River Estuary. A: T. karajacensis, B: T. beroidea, C: L. simplex, D: T. parvula, E :Metacylis sp., F :T. Turbo, and G: T. minuta (Jinpeng, 2019).
In another experiment done in Limnology and Oceanography, the tintinnid’s ability to attach to floating aggregates is explored (Jinpeng, 2019). This unique ability of tintinnids to grapple small pieces of detritus is a clear example of an evolutionary adaptation because of said adaptation’s consequences of enhanced tintinnid predation and reduced predation on the respective tintinnid. Attachment to the correct aggregate is crucial for predation because specific aggregates attract certain prey. Aggregates tend to harbor a higher local concentration of nanosized protists, which serve as an important food source for tintinnids. By stationing themselves close to these aggregates, tintinnids are near a constant supply of prey, increasing their feeding efficiency. Furthermore, their attachment provides a significant boost to their feeding mechanics. Tintinnids utilize the flow rate of water through their mouths to feed, drawing in prey with the current they generate. When attached to an aggregate, they experience an enhanced flow rate through their mouths. The research indicates an impressive 50% increase in this flow rate as compared to their free-swimming counterparts (Jinpeng, 2019). This elevated flow rate allows attached tintinnids to capture prey more effectively, thus solidifying their role as superior predators in their environment. The ability to reversibly attach and detach from surfaces, as observed in Eutintinnus Inquilinus, suggests that this adaptation is not a mere chance occurrence. It is a result of evolutionary pressures that favored tintinnids with better feeding efficiencies, leading to the proliferation of those that could attach to aggregates.
While the attachment to aggregates amplifies the tintinnids’ predatory prowess, it also bestows upon them a vital survival tool against their predators. Copepods, especially the calanoid copepods, are primary predators of tintinnids. Intriguingly, copepods are found to feed less efficiently on tintinnids that are attached to aggregates. This reduction in predation efficiency is multifaceted. For one, attached tintinnids present a larger profile, making them more challenging prey for copepods to consume. The act of attachment, especially when tintinnids attach to diatom chains or other structures, increases their drag and consequently their size, making them less appealing or more challenging to predators. Moreover, the swimming behavior of tintinnids also plays a role in their survival. Although it might seem counterintuitive, the research suggests that attached tintinnids exhibit faster swimming speeds compared to free-swimming ones. This enhanced mobility gives them a distinct edge, enabling them to evade predators more effectively.
Future ecological implications of coevolution were highlighted by a study of the morphology of tintinnid loricae by Sorbonne University in France (Dolan 2012). Within this study, an analysis of the data regarding tintinnid ciliates, and their relationship with copepods based on lorica oral diameter (LOD), offers a glimpse into the potential future consequences of coevolution.
The data suggests copepods exhibit a predation preference for tintinnids with larger LODs. Selective feeding implies an imbalance in predation, and over time, this could exert pressure favoring tintinnids with smaller LODs. This evolutionary trajectory could lead to a generational shift towards an overall smaller LOD within the tintinnid population. With the shift towards smaller LODs, one can expect changes in tintinnid feeding behavior. Since a smaller LOD has been correlated with a preference for smaller prey, a shift towards this end might increase the predation pressure on smaller-sized prey items. As these smaller prey items face heightened predation, they might evolve increased defensive mechanisms to improve survival. This would be a classic example of an evolutionary arms race, where predator and prey continually adapt in response to one another.
Fig. 13. “Evolutionary arms race” (Facebook. (n.d.)).
This increased predation on smaller prey could additionally have ripple effects through the ecosystem. As tintinnids with larger LODs become less prevalent, due to copepod predation, predators that typically target these larger LOD tintinnids will be forced to adapt. They will start to seek out smaller LOD tintinnids, introducing a new predation pressure on a previously less targeted population segment. Alternatively, these predators could switch to entirely different prey altogether, removing a crucial predator from the system.
Considering the observations on the coevolutionary dynamics between tintinnids and copepods, the potential for trophic cascades becomes evident. As tintinnids with larger LODs face heightened predation and evolve towards possessing smaller LODs, the ramifications cascade down to smaller prey and up to their larger predators. Such a change could lead to shifts in the abundance, behavior, and adaptations of various marine organisms within this food web. Trophic cascades like these could have drastic consequences on the marine ecosystem, reshuffling species interactions and potentially altering the stability and functionality of marine communities. As ecosystems are interwoven networks of interactions, even subtle shifts in one species can ripple outwards, underscoring the importance of monitoring and understanding these evolutionary battles and their broader ecological implications.
Conclusion
As demonstrated in this paper, Tintinnids are truly exceptional creatures that capitalize on a wide variety of physics concepts in order to survive. To do so, they need to escape predators, find prey, and find shelter. These survival obstacles are not unique; however, their strategies to overcome them are.
To escape predators, tintinnids change the motion of their swimming, latch onto aggregates, form symbiotic relationships with diatoms, and become less appealing to their predators through the course of evolution. Evolution may not be a quick fix, but it is a key reason tintinnids have been able to thrive as a genus. Copepods, their most common predator, prefer tintinnids with a larger lorica oral diameter, so natural selection prefers tintinnids with smaller diameters (Dolan, 2012). This is somewhat of a double-edged sword, though, because this means copepods will coevolve to stay alive, leading to an evolutionary arms race. Latching onto aggregates and the symbiotic relationship with diatoms are similar survival techniques; tintinnids will use either of these for increased buoyancy and to make themselves larger. Both help them not only to be less vulnerable to predators but also aid in catching prey due to the increased mobility that the diatom or aggregate provides (Vincent et al., 2018).
To find prey, tintinnids use negative geotaxis, differential predation, and mechanoreception. The first refers to a unique ability to naturally orient themselves against the force of gravity; swimming toward the surface of the water where there is more prey availability (Winet & Jahn, 1974). Differential predation refers to tintinnids’ preference for large, nutritious, and nearby prey that are easy to ingest. The most important factor to them is prey size because large prey has a higher encounter probability (Montagnes, 2012). Through mechanoreception tintinnids estimate this encounter probability by detecting nearby prey, their size, and their movements.
Furthermore, tintinnid’s swimming patterns are of great interest, especially through the lens of physics as survival design. Tintinnids swim in a helical motion, which is essential to avoid predators and to find prey. This is because they inhabit an environment with a low Reynolds number; meaning the drag forces are more prominent than inertial forces, and the helical path is therefore the most efficient route. The ciliates execute this movement by moving their cilia back and forth at a certain changeable angle to propel themselves in any wanted direction. This simple fact shows us how truly intelligent nature is. Without thinking – because it cannot “think” – it employs this fundamental physics concept in a matter of seconds; far quicker than any physics student could solve a similar problem. This is not because the tintinnids took physics courses but simply because nature has a way of finding solutions to problems “intuitively”. This “intuition” can more formally be called natural selection.
Self-protection is essential to tintinnid’s survival. They achieve this with their lorica, which provides shelter and protection from predators. Tintinnids highly depend on their lorica and naked tintinnids are very rarely found in nature. Their lorica shape allows them to move more efficiently through water, taking advantage of hydrodynamics, and the durability of the shell protects them from predators. The lorica is composed mainly of chitin which is a strong material due to powerful intermolecular forces. In addition to the use of electromagnetic forces between ions, the lorica also uses the principles of even weight distribution in order to be more a structurally sound protector. This is why the middle layer of the lorica is composed of hexagons (Dolan, 2012). These very strong shapes allow the ciliate to stay safe in its home.
Tintinnids’ structure and mechanics offer intriguing possibilities in the realm of engineering. The lorica’s remarkable blend of flexibility, strength, and lightweight characteristics may inspire bioengineers to create sustainable packaging materials that could potentially offer enhanced structural integrity while maintaining a reduced weight profile. Furthermore, tintinnid’s swimming patterns and sensitivity to the marine environment offer interesting applications in micro-robotics and environmental monitoring.
So why should we care about Tintinnids? Though they are a measly single-cell organism that is 100,000 times smaller than we humans, sophisticated physics concepts have aided their survival. By studying the ways tintinnids interact with their environment, we may be able to apply these concepts to the field of engineering. Through natural selection, the tintinnid’s design has adapted to the pressures of its environment and its design has evolved to capitalize on physics to optimize its survival. Due to natural selection, this seemingly simple single-cell organism has optimized its feeding habits, shell composition, life cycle, swimming patterns, buoyancy mechanisms, and countless other adaptations that make it the fascinating creature it is today. Incorporating insights from the study of tintinnid structures and behaviours into engineering practices holds the potential to drive innovation across diverse industries. By gaining a deeper understanding of and emulating nature’s design solutions, engineers can develop technologies that are more efficient, adaptable, and sustainable, thus shaping the future of engineering applications.
References
Agatha, S., Bartel, H. (2021). A comparative ultrastructural study of tintinnid loricae (Alveolata, Ciliophora, Spirotricha) and a hypothesis on their evolution. International society of Protistologists, 69 (2), 10.1111
Agatha, S., & Simon, P. (2012). On the Nature of Tintinnid Loricae (Ciliophora: Spirotricha: Tintinnina): a Histochemical, Enzymatic, EDX, and High-resolution TEM Study. Acta protozoologica, 51(1), 1–19.
Agatha, S., & Strüder-kypke, M. C. (2012). Systematics and Evolution of Tintinnid Ciliates. In The Biology and Ecology of Tintinnid Ciliates (pp. 42-84). https://doi.org/https://doi.org/10.1002/9781118358092.ch3
Armbrecht, L. H., Eriksen, R., Leventer, A., & Armand, L. K. (2017). First observations of living sea-ice diatom agglomeration to tintinnid loricae in East Antarctica. Journal of Plankton Research, 39(5), 795-802. https://doi.org/10.1093/plankt/fbx036
Blackbourn, D. J. (1974). The feeding biology of tintinnid protozoa and some other inshore microzooplankton [Text, https://open.library.ubc.ca/collections/831/items/1.0053248
Bai, Y., Wang, R., Song, W., Li, L., Santoferrara, L. F., & Hu, X. (2020, December 14). Three redescriptions in Tintinnopsis (protista: Ciliophora: Tintinnina) from coastal waters of China, with cytology and phylogenetic analyses based on ribosomal RNA genes – BMC microbiology. BioMed Central. https://bmcmicrobiol.biomedcentral.com/articles/10.1186/s12866-020-02057-2
Carstens, Andy. (2020, November 11). Honeycomb Structure Is Space Efficient and Strong. asknature. https://asknature.org/strategy/honeycomb-structure-is-space-efficient-and-strong/
Ceylan, H., Giltinan, J., Kozielski, K., & Sitti, M. (2017). Mobile microrobots for bioengineering applications. Lab on a Chip, 17(10), 1705-1724.
Cicconofri, G., & DeSimone, A. (2019). Modelling biological and bio-inspired swimming at microscopic scales: Recent results and perspectives. Computers & Fluids, 179, 799–805. https://doi.org/10.1016/j.compfluid.2018.07.020
Coats, D. W., & Bachvaroff, T. R. (2012). Parasites of Tintinnids. In The Biology and Ecology of Tintinnid Ciliates (pp. 145-170). https://doi.org/https://doi.org/10.1002/9781118358092.ch6
Dizon, J. C. (2017). Mechanical characterization of 3D-printed polymers. Additive Manufacturing, 20(1), 44-67. https://doi.org/10.1016/j.addma.2017.12.002
Dolan, J. (2012, February 16). Morphology and ecology in tintinnid ciliates of the marine plankton: Correlates of Lorica dimensions. Acta Protozoologica. https://hal.sorbonne-universite.fr/hal-00670764/
Dolan, J. (2010). Morphology and Ecology in Tintinnid Ciliates of the Marine Plankton: Correlates of Lorica Dimensions. Acta protozoologica, 49, 235-244.
Dolan, J. R.,Montagnes, D., Agatha, S., Coats, D. W., Stoecker, D. (2012). The Biology and Ecology of Tintinnid Ciliates. NHBS Academic & Professional Books
Dovgal, I., & Gavrilova, N. (2018). Diversity and functions of loricae in ciliates (Ciliophora). 3, 13-21. https://doi.org/10.21072/mbj.2018.03.3.02
Facebook. (n.d.). https://www.facebook.com/cellularscribbles/photos/evolutionary-arms-race/1504802626199508/
Febvre-Chevalier, C., & Febvre, J. (1994). Buoyancy and swimming in marine planktonic protists. In J. M. V. Rayner, L. Maddock, & Q. Bone (Eds.), The Mechanics and Physiology of Animal Swimming (pp. 13-26). Cambridge University Press. https://doi.org/DOI: 10.1017/CBO9780511983641.003
Gerhardt, A. (2002). Bioindicator species and their use in biomonitoring. Environmental monitoring, 1, 77-123.
Gómez, F. (2020). Symbioses of Ciliates (Ciliophora) and Diatoms (Bacillariophyceae): Taxonomy and Host-Symbiont Interactions.1, 133-155. https://doi.org/10.3390/oceans1030010
Jinpeng Yang (2019a, August 5). Are Tintinnids picky grazers: Feeding experiments on a mixture of mixotrophic dinoflagellates and implications for Red Tide Dynamics. Marine Pollution Bulletin. https://www.sciencedirect.com/science/article/pii/S0025326X19306265?via%3Dihub
Li, H., Xu, Z., Zhang, W., Wang, S., Zhang, G., & Xiao, T. (2016). Boreal Tintinnid Assemblage in the Northwest Pacific and Its Connection with the Japan Sea in Summer 2014. PLoS One, 11(4), e0153379. https://doi.org/10.1371/journal.pone.0153379
Li, B. (2022). Solid Stress-Distribution-Oriented Design and Topology Optimization of 3D- Printed Heterogeneous Lattice Structures with Light Weight and High Specific Rigidity. Polymers. 14(14), 2807. http://dx.doi.org/10.3390/polym14142807
Lipps, J. H., Stoeck, T., & Dunthorn, M. (2012). Fossil Tintinnids. In The Biology and Ecology of Tintinnid Ciliates (pp. 186-197). https://doi.org/https://doi.org/10.1002/9781118358092.ch8
McManus, G., & Santoferrara, L. (2013). Tintinnids in microzooplankton communities. In: Biology and ecology of tintinnid ciliates: models for marine plankton. In (pp. 198-213).
Montagnes, D. J. S. (2012). Ecophysiology and Behavior of Tintinnids. In The Biology and Ecology of Tintinnid Ciliates (pp. 85-121). https://doi.org/https://doi.org/10.1002/9781118358092.ch4
Norberg, Jon. biodiversity and ecosystem functioning: A complex … – aslo. (n.d.). https://aslopubs.onlinelibrary.wiley.com/doi/epdf/10.4319/lo.2004.49.4_part_2.1269
Rakshit, D., Sahu, G., Mohanty, A. K., Satpathy, K. K., Jonathan, M. P., Murugan, K., & Sarkar, S. K. (2017). Bioindicator role of tintinnid (Protozoa: Ciliophora) for water quality monitoring in Kalpakkam, Tamil Nadu, south east coast of India. Marine Pollution Bulletin, 114(1), 134-143. https://doi.org/https://doi.org/10.1016/j.marpolbul.2016.08.058
Rakshit, D., Biswas, S. N., Sarkar, S. K., Bhattacharya, B. D., Godhantaraman, N., & Satpathy, K. K. (2014, January 9). Seasonal variations in species composition, abundance, biomass and production rate of Tintinnids (ciliata: Protozoa) along the Hooghly (Ganges) river estuary, India: A multivariate approach – environmental monitoring and assessment. SpringerLink. https://link.springer.com/article/10.1007/s10661-013-3601-9
Vincent, F. J., Colin, S., Romac, S., Scalco, E., Bittner, L., Garcia, Y., Lopes, R. M., Dolan, J. R., Zingone, A., de Vargas, C., & Bowler, C. (2018). The epibiotic life of the cosmopolitan diatom Fragilariopsis doliolus on heterotrophic ciliates in the open ocean. The ISME Journal, 12(4), 1094-1108. https://doi.org/10.1038/s41396-017-0029-1
Wahyudim, S. P. (2017). Design and analysis of strut-based lattice structure for vibration isolation. Precision Engineering, 52(1), 494-506. https://doi.org/10.1016/j.precisioneng.2017.09.010
Wang, C., Xu, M., Xuan, J., Li, H., Zheng, S., Zhao, Y., Zhang, W., & Xiao, T. (2021). Impact of the warm eddy on planktonic ciliate, with an emphasis on tintinnids as bioindicator species. Ecological Indicators, 133, 108441. https://doi.org/https://doi.org/10.1016/j.ecolind.2021.108441
Weisse, T., & Montagnes, D. J. S. (2022). Ecology of planktonic ciliates in a changing world: Concepts, methods, and challenges. Journal of Eukaryotic Microbiology, 69(5), e12879. https://doi.org/https://doi.org/10.1111/jeu.12879
Winet, H., & Jahn, T. L. (1974). Geotaxis in Protozoa I. A propulsion—gravity model for tetrahymena (Ciliata). Journal of Theoretical Biology, 46(2), 449-465. https://doi.org/https://doi.org/10.1016/0022-5193(74)90008-3
Yang, J., Löder, M. G. J., Jiang, Y., & Wiltshire, K. H. (2019). Are tintinnids picky grazers: Feeding experiments on a mixture of mixotrophic dinoflagellates and implications for red tide dynamics. Marine Pollution Bulletin, 149, 110488. https://doi.org/https://doi.org/10.1016/j.marpolbul.2019.110488