Copepods Through the Lens of Chemistry
Johanna Turton, Melodi Rousset, Philippe Mercier, Tanjin Sultana
Abstract
Copepods, a resilient group of crustaceans, are adaptable to a variety of environments. This paper explores the captivating world of copepods, focusing on how they are resilient towards cyanobacterial blooms and toxic diatoms, and how they use bioluminescence and pigmentation to increase their fitness levels.
Copepods have evolved symbiotic relationships with bacteria to live among cyanobacterial blooms despite their release dangerous toxins. In addition, some species use aldehyde dehydrogenase to prevent the negative effects caused by diatoms. In terms of their physique, they use bioluminescence for a plethora of reasons such as communicating, avoiding predators, or attracting mates. Lastly, these species can produce a carotenoid known as astaxanthin which gives them a red-orange color that is crucial to their survival.
Introduction
In the vast expanse of aquatic ecosystems, copepods emerge as extraordinary microcrustaceans, playing crucial roles in maintaining ecological balance. When exposed to harsh conditions, these species demonstrate remarkable tenacity. In addition, they harness chemical reactions for unique survival and reproductive strategies.
Copepods live in an environment where there are periodic cyanobacterial blooms. These blooms can release toxins and copepods have evolved the capacity to host a bacterium to produce enzymes that can break the cyanotoxins down within their gut. This symbiotic relationship allows the bacterium a place to live throughout winter while enabling the copepod to avoid dangerous chemicals.
Copepods’ diets as well could be a source of danger, in terms of toxic diatoms. These species can release oxylipins which are dangerous for the development of copepod offspring. Certain species, especially North Sea copepods have evolved to increase their use of the enzyme aldehyde dehydrogenase to detoxify oxylipins through oxidation.
Copepods’ appearance allows them to respond to environmental cues, locate food sources, communicate, and even mate with others. This is done through their impressive chemoreceptive ability within their aesthetascs, which are chemosensory sensilla that facilitate navigation through watery realms. Copepods also utilize bioluminescence in the dark and deep sea for the same purposes.
As if their chemoreception and bioluminescence were not captivating enough, copepods also feature a vibrant array of pigmentation, influenced by the carotenoid astaxanthin. This pigment contributes to their red-orange structure that may vary between copepod species subjected to different environmental conditions. Astaxanthin is accumulated based on the need of reactive oxygen species (ROS) protection, temperature, predator pressures and to increase fitness levels among offspring.
How copepods survive cyanobacterial blooms
In recent years, scientists have started to become concerned by cyanobacteria blooms which, inter alia, release toxins in water and cause lake eutrophication, thus greatly hurting biodiversity (Zhang et al., 2022). A cyanobacteria bloom occurs when these organisms see their environment suddenly become optimal for their survival and reproduction, e.g., when the water reaches a certain warmness and concentration of key nutrients. From that point on, the cyanobacteria’s reproduction-death ratio increases so much that their population explodes, causing great upheavals in their environment (Zhang et al., 2022).
With climate change and the increase in aquatic environments’ pollution via fertilizer runoff, it thus makes sense for such blooms’ frequency of occurrence to increase and for them to become more and more problematic as time passes, and thus, for scientists to search solutions to them (Zhang et al., 2022). One process for finding these involves looking at nature’s own solution to this problem. In fact, cyanobacteria blooms have been happening for so long that many organisms cohabiting with cyanobacteria have evolved ways to resist the toxins they release such as to use them as a source of food. Copepods are one of these species, and the following sections will explore how they manage to eat so many cyanobacteria during blooms without suffering from the resulting massive intake of cyanobacterial toxins.
Toxin degrading bacteria
One of the ways copepods manage to overcome the toxicity of the compounds released by cyanobacteria is by hosting in their gut some bacteria capable of degrading these toxins (Gorokhova et al., 2021). The main one is called Sphingomonas. Four of this bacterium’s genes have been found to encode the information necessary to the production of three enzymes which work together in series to degrade the main cyanobacterial toxin into substances that are less harmful to copepods (Bourne et al., 2001). The degradation process underwent by this toxin when subject to those three enzymes is called an “enzymatic degradation pathway” and is shown in Figure 1.
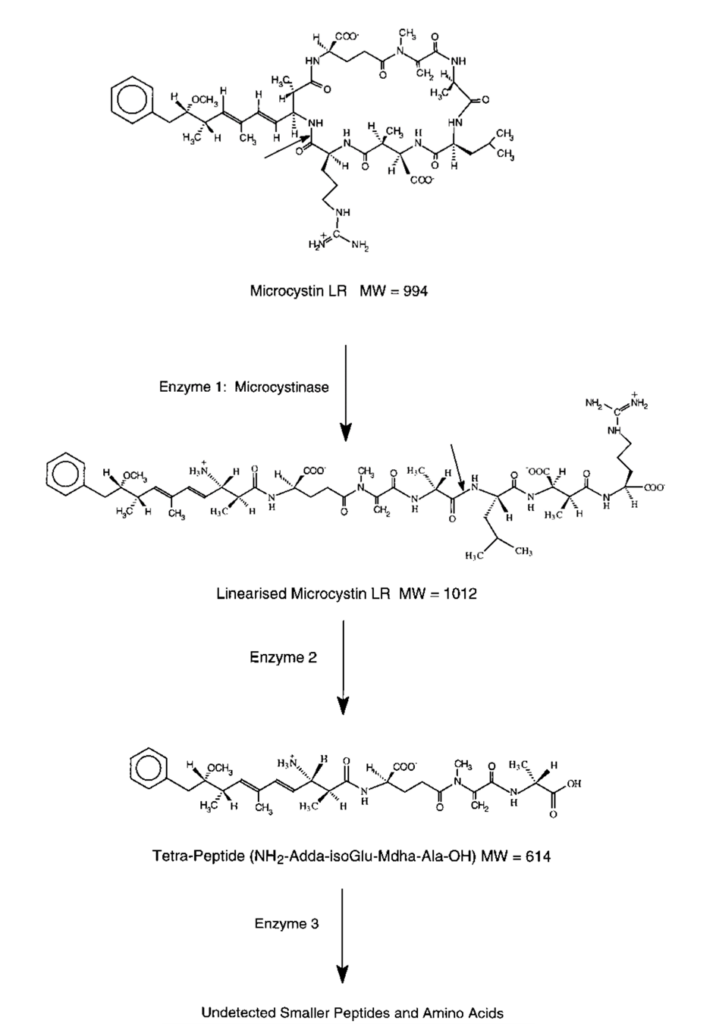
Fig. 1. Enzymatic degradation pathway of the main toxin from cyanobacteria. (Bourne et al., 1996).
To understand how this pathway manages to transform the toxic molecule into less toxic ones, it is first important to know what makes the initial molecule toxic in the first place.
In fact, molecules called “phosphomonoesters” naturally occur in copepods cells. There, enzymes called “phosphatases” act as very powerful catalysts for the hydrolysis reaction shown in Figure 2, which prevents accumulation of phosphomonoesters in the cells by rapidly transforming these molecules into other, harmless compounds (Lad et al., 2003).
| Hydrolysis: Hydrolysis is the process by which a molecule is split in two using a water molecule such that if the given molecule is made of a part A and a part B, the following reaction occurs (Ladisch et al., 1983): AB + H2O → AH + BOH |
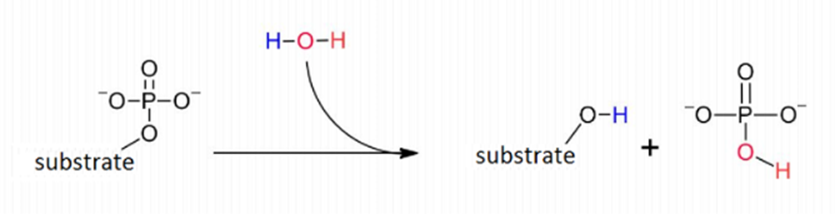
Figure 2: Hydrolysis reaction – called dephosphorylation – catalysed by phosphatase which prevent accumulation of phosphomonoesters in cells by breaking them down. (Lovinne, M., 2023).
When microcystin – one of the toxins secreted by cyanobacteria – finds itself in the presence of this “phosphatase” enzyme, it interacts with it via its N-methyl-dehydroalanine moiety (Mdha) – see Figure 3 (Rzeszotarska et al., 2002). The terminal carbon of microcystin’s Mdha – the one bonded to the nitrogen atom and three hydrogens – binds with a sulphur atom present on the binding site of the phosphatase (Xing et al., 2006). This binding site is where the phosphatase enzyme connects with the phosphomonoesters in the process of transforming the latter into harmless products, process shown earlier in Figure 2. Hence, when the microcystin is interacting with a phosphatase enzyme, the latter cannot transform phosphomonoesters – since it cannot interact with them, for microcystin is in the way. This process is called “steric inhibition”, and it leads to the accumulation of phosphomonoesters in the cells. These molecules can give their phosphate groups to those making the intermediate filaments via a reaction called phosphorylation, which is catalysed by kinase enzymes. Phosphomonoester accumulation in the cells’ cytoplasm when microcystin is ingested by copepods thus leads to the hyperphosphorylation of their cytokeratin, which causes it to lose its structural stability, leading to the collapse of the cytoskeleton in the crustaceans’ cells. This then causes the puncture of these cells and the destruction of the tissues they make – which is why microcystin is said to be toxic to copepods (Bourne et al., 2001).
**Note: When not inhibited by microcystin, the fact that phosphatase dephosphorylates the phosphomonoesters makes the latter unusable by the kinase enzymes as substrates for the phosphorylation of the cytokeratin, which is why it was said earlier that the dephosphorylation of the phosphomonoesters transforms them into harmless compounds.
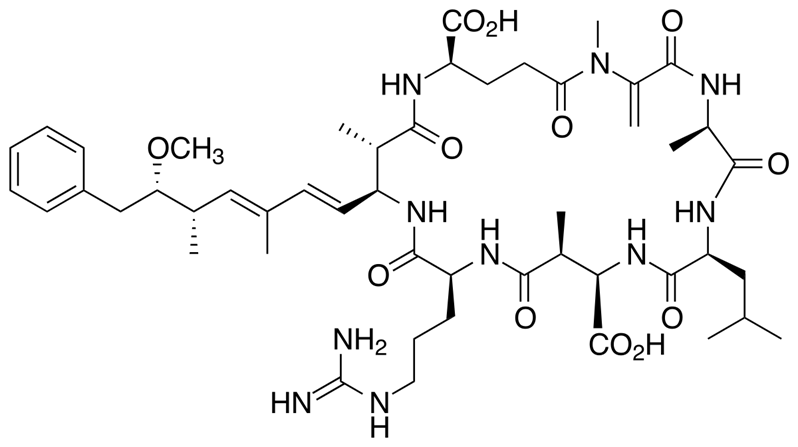
Figure 3: Location of the microcystin’s N-methyl-dehydroalanine moiety (Mdha) in red, and the latter’s terminal carbon in blue, which causes it to be toxic to copepods by allowing it to interact with phosphatases (Rzeszotarska et al., 2002).
It is important to understand that phosphatases are incredibly complex in shape and, more importantly, humongous. Protein phosphatase 1, for example, has a molecular weight of around 38 000 Daltons – with a Dalton being defined as 1/12 of the mass of a carbon 12 atom (Aggen et al., 2000). Also, it can be observed in Figure 3 that microcystin has many hydrogen-bond donors and receivers – many N-H bonds – and so does the phosphatase. Hence, when both get close to one another, a very complex network of H-bonds and other van der Waals forces is formed, which is described in more details in Dr. Xing’s study, “Structure of protein phosphatase 2A Core Enzyme Bound to Toxins” (Xing et al., 2006). The fact that the previously mentioned carbon-sulphur covalent bond – which inhibits the phosphatase – is able to form between the two molecules means that this network of intermolecular forces makes the microcystin dock the phosphatase with the perfect orientation and strength to allow for the carbon and the sulphur to get close enough to form this bond. Now, since the network of forces is so complex, that means that this docking is very restricted – the more constraints there are on a system, the more controlled are its outcomes. Hence, that means that very few, small changes can occur to the microcystin’s shape and constitution before it can no longer dock correctly to the phosphatase binding site and form that carbon-sulfur bond – before it can no longer inhibit the phosphatase. This is how Sphingomonas‘s enzymes manage to make the microcystin non-toxic. Contrarily to what is expected, they do not interact with the Mdha of the toxin, which is directly responsible for the toxic interaction: instead, the first enzyme in the degradation pathway cuts the microcystin via catalyzation of hydrolysis reaction such as to linearize it – see Figure 4 (Bourne et al., 2001).
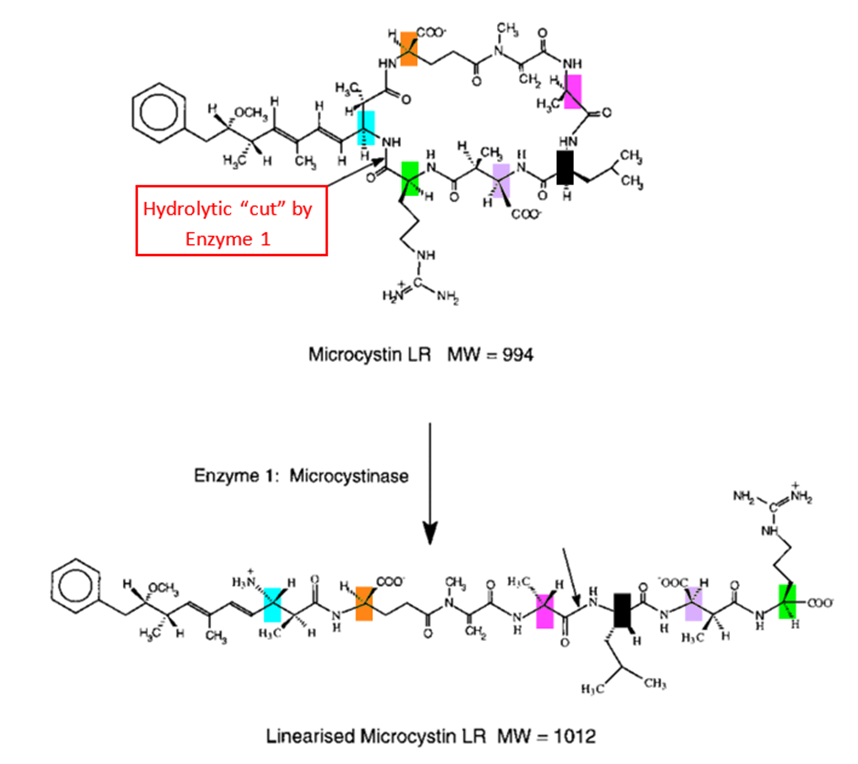
Fig. 4. Enzymatic degradation pathway of the main toxin from cyanobacteria. (Bourne et al., 1996).
This drastic change in shape – occurring at the first stage of the degradation pathway – makes the molecule already negligibly toxic, since it is no longer able to interact with the binding site via an H-bond network in the way that would allow the sulphur-carbon bond to form. The next two enzymes of the pathway perform similar cuts via hydrolysis, still not touching the Mdha, yet making the molecule less and less toxic – thus allowing the copepods hosting the bacteria producing that enzyme to feed on cyanobacteria without significant harm (Bourne et al., 2001).
Symbiotic relationship: Copepods hosting toxin degrading bacteria for mutual benefit
It is important to note that the toxin-degrading bacterium – Sphingomonas – is present outside of the copepod and in the cyanobacteria blooms during summer: they are its main source of food, thus where it proliferates (Gorokhova et al., 2021). However, when winter comes, this bacterium cannot survive if directly exposed to the weather – since it dies under 5°C (Ryan et al., 2016), and is thus not found roaming freely in the water. Copepods, however, can live at much colder temperatures (Hansen et al., 2010), yet they cannot survive alone in cyanobacterial blooms due to their toxicity. The relationship between copepods and Sphingomonas is thus a symbiotic one. It is interesting to note that the bacteria’s presence in copepods’ gut is not innate. In fact, some copepods never acquire them, which is why they do not all survive the blooms. One way they can acquire these symbiotic bacteria is by the ingestion of fecal pellets of other copepods or organism hosting those bacteria, which allows the latter to settle in copepods gut and save the copepods during the next cyanobacterial bloom it will encounter (Gorokhova et al., 2021).
In short, copepods have adjusted to cyanobacteria blooms by evolving a symbiotic relationship with bacteria capable of generating enzymes which can break down cyanobacteria’s toxins.
How copepods survive feeding on toxic diatoms
Diatoms are unicellular organisms that are a type of photosynthesizing algae. Through photosynthesis, they produce 20-30% of the air we breathe and are water dwelling organisms (Spaulding et al. 2021). Diatoms are a very common food source from zooplankton to aquatic insects to whales. They produce long-chain fatty acid making them very rich in energy for their predators.
However, many diatom species produce toxins that negatively impact these predators, including copepods. The toxin, known as oxylipin, is a signaling molecule that affects the development of copepod offspring. Figure 5 shows the chemical structures of different types of oxylipins. Oxylipins can compromise embryonic and larval development by inhibiting fertilization processes or induce malformations in offspring (Lauritano et al. 2012).

Fig. 5. Chemical structures of different types of oxylipins. (Barbosa et al. 2016).
Oxylipins create these issues for copepods by acting as ligands for specific receptors on copepod cells. By doing so, a signaling cascade is initiated and leads to changes in gene expression and cellular metabolism. A study by Lauritano et al., in 2012 looked at the effects of feeding on diatoms of copepods of different aquatic environments. The Calanus Helgolandicus copepod was studied from the North Sea, the Atlantic Ocean, and the Mediterranean Sea. The study found that each population was affected on different levels and responses to the toxins were also population-specific.
Lauritano used knowledge from other studies of toxin effects on copepods to know what genes were of interest within their study. The copepods genes observed were proteins HSP40 and HSP70 (responsible for response to environmental stress factors), the microsomal cytochrome P450 (involved in oxidative modification for turning chemicals into hydrophilic metabolites), catalase, superoxide dismutase (responsible for detoxification of reactive oxygen species), six-aldehyde dehydrogenase (involved in detoxification of certain oxylipins), and three apoptosis regulating genes. The results of the data obtained were that North Sea copepods reacted most effectively to the toxins, the Mediterranean Sea copepods did the worst, leaving the Atlantic Ocean copepods somewhere between them. The results were dependent on whether any, all, or none of the six-aldehyde dehydrogenase were activated. Activation of these genes were observed at 24 and 48 hours after exposure. The data for each populations’ activation is shown in Fig. 6.
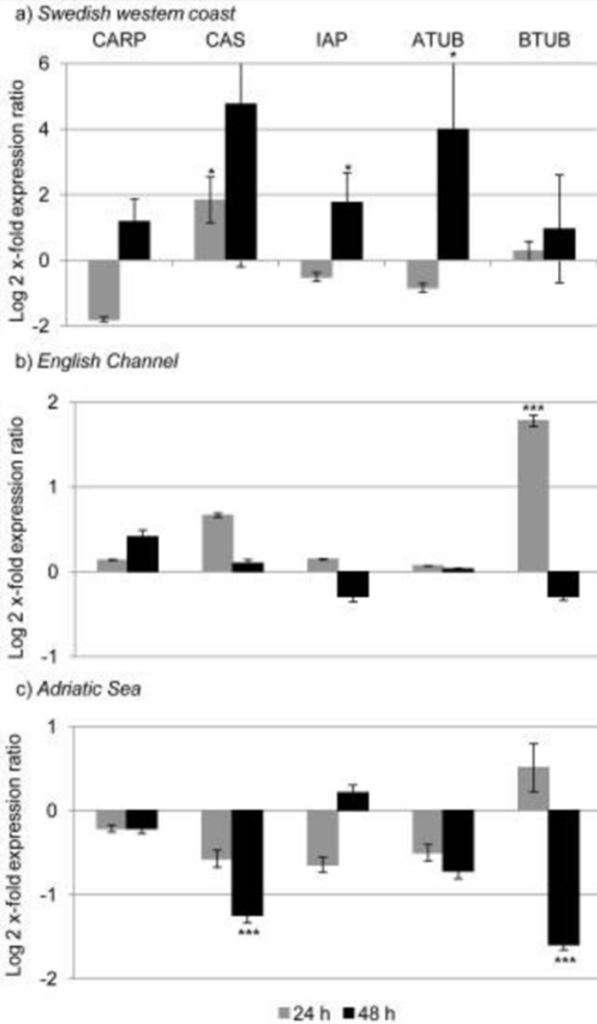
Figure 6 : Aldehyde dehydrogenase activation levels in different populations of copepods (bars in negative being no activation while bars in positive are higher levels of activation) (Lauritano et al. 2012).
The aldehyde dehydrogenase detoxifies the oxylipins through an oxidation reaction to create corresponding carboxylic acids. This converts the toxic oxylipins into compounds that are less harmful to the copepods. This genetic analysis suggests that the Mediterranean Sea copepods have experienced genetic isolation as they have not been able to evolve to activate the genes necessary to fight the toxins. This is why they are most susceptible to the toxic diatom diet. The North Sea and Atlantic Ocean copepods demonstrate the opposite. They have more genetic diversity suggesting they interreact with more copepod species in their respective environment. Another reason is due to weather; diatom blooms are more long-lasting in northern environments. With generations of copepods being exposed to the toxins of the diatoms for longer periods of time, they have more time to evolve to its effects.
Chemoreception
Copepods employ impressive tactics to ensure their survival in arduous conditions. However, copepods have limited visual abilities. How do they perceive their surroundings? Copepods have chemosensory mechanisms to navigate their complex underwater environments. Chemoreception is the ability to detect and respond to chemical signals. These organisms have many sensory adaptations that enable them to detect and respond to chemical cues in their surroundings (Heuschele & Selander, 2014). Chemosensory perception plays a crucial role in copepod survival and reproduction, allowing them to locate food sources, find suitable mates, and avoid predators or unfavorable conditions (Heuschele & Selander, 2014). This section will explore the fascinating world of chemosensory abilities in copepods, shedding light on one of the many ways they use chemistry to thrive in their aquatic habitats.
Anatomy of copepod chemoreception
In copepods, the anatomy of their chemoreception appendages is a marvel of adaptation to their aquatic environment. Chemosensory sensilla, referred to as aesthetascs, are strategically located on both antennules and feeding appendages (Heuschele & Selander, 2014). The aesthetascs are specifically found on the anterior side of the antennules as seen in Figure 7 and possess sensory receptors which transform chemical signals into biological ones (Uttieri et al., 2008). Once these sensory receptors recognize specific chemical stimuli, signal transduction pathways are triggered to relay the information to the central nervous system for signal processing which leads to the initiation of the appropriate behavioral response (Biology-Online, 2023). As shown in Figure 8, these aesthetascs are tubular filaments designed with thin walls and an abundance of tiny pores, a morphology that serves to maximize their contact with the chemical cues in their surroundings (Uttieri et al., 2008). The presence of these sensory organs on the feeding appendages might indicate their role in finding food, which will be explored in a later section. Additionally, some copepods have bimodal sensilla on their cephalic appendages, meaning they possess both mechanoreceptive and chemoreceptive capabilities, enabling them to not only sense their environment but also respond to it effectively (Heuschele & Selander, 2014). The mechanoreception and chemoreception do not work independently but in synergy to fulfill the copepod’s needs. This intricate anatomy highlights the significance of chemoreception copepods and how they interact with their environment.
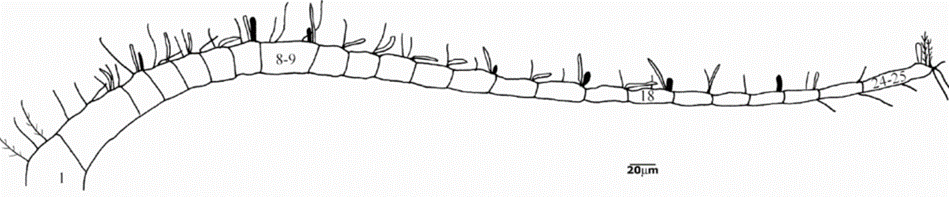
Figure 7: Illustration of copepod antennule with aesthetacs which correspond to the white cylindrical filaments branching off the antennule. (Uttieri et al., 2008).
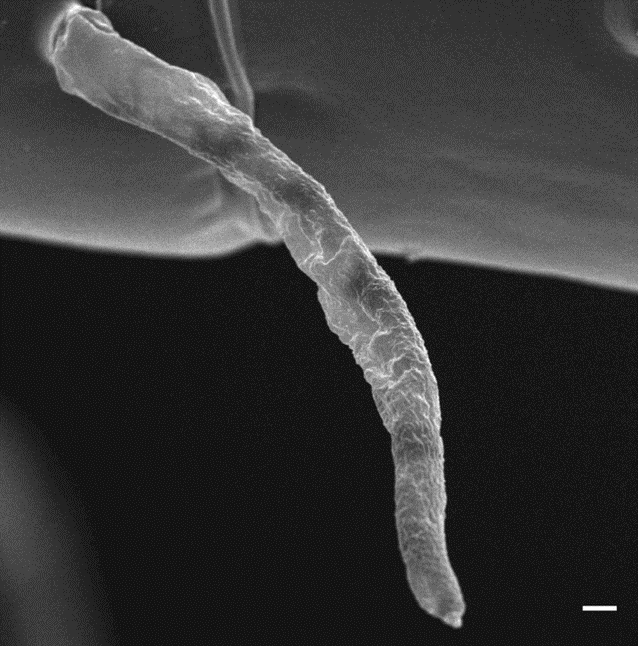
Figure 8: Image of aesthetasc produced by scanning electron microscopy. Scale bar in bottom right corner represents 1 µm. (Uttieri et al., 2008).
Mate-seeking behavior facilitated by chemical signals
Chemoreception plays a pivotal role in the mate-seeking behavior of copepods, particularly in male copepods. These aquatic crustaceans have evolved remarkable chemosensory adaptations to enhance their chances of successful reproduction to ensure the survival of their species over millions of years. The evidence surrounding mating chemoreception is primarily behavioral.
When female copepods emit olfactory signals in the form of pheromones, male copepods exhibit distinct behaviors in response. One of the most striking behaviors is the increase in swimming speed, coupled with a distinctive circular swimming pattern (Heuschele & Selander, 2014). This behavioral shift is triggered by chemoreception and is critical in the pursuit of potential mates.
Upon detecting female pheromones, they modify their swimming trajectory to follow the chemical cues emitted by the potential mates (Seuront, 2013). It is important to note that the chemical cues, in the form of pheromones, can offer some advantages over other sensory cues, such as hydromechanical signals. Chemical cues can be followed for distances of around 200 mm even if the target is immobile (Seuront, 2013). To appreciate this accomplishment, imagine being able to detect a croissant placed on the tip of the Eiffel Tower while being on the ground directly beneath it. This is comparable to a 1 mm copepod sensing chemical signals from 200 times its body length.
| What exactly are pheromones? “A chemical substance that is produced by an animal and serves especially as a stimulus to other individuals of the same species for one or more behavioral responses.” (Merriam-Webster, 2023) |
Male copepods have evolved the ability to evaluate the quality of potential mates based on the pheromones left by females. Chemoreception significantly increases the probability of mate encounters among copepods (Seuront, 2013). In a study conducted with Eurytemora affinis copepods, 85 mating behavioral sequences were orchestrated between adult males and adult ovigerous (carrying eggs) and non-ovigerous females (Seuront, 2013). It was found that in the case of established contact 76.3% of non-ovigerous females were captured in comparison to 0% capture of ovigerous females. Furthermore, while measuring the trail-following behavior of the male copepods, researchers found a significantly higher tracking distance of 19.7 to 147.5 mm for non-ovigerous females while the tracking distance for ovigerous females was between 12.7 and 45.3 mm (Seuront, 2013). This shows that male copepods have a heightened sensitivity to specific pheromones to more efficiently track non-ovigerous females. Therefore, this indicates that male copepods have developed an evolutionary trait to track viable mates for successful reproduction. This preferential behavior proves the role of chemoreception in copepods because they would not be able to distinguish and follow non-ovigerous females if they relied solely on mechanoreception. The precision and efficiency with which male copepods track and locate suitable mates contribute to the overall success of their reproductive efforts.
However, the exact nature of the specific molecules that make up these pheromones in copepods remains a subject of limited research. In an experiment conducted by Snell & Morris at the University of Tampa, male copepods of displayed a response to conspecific (belonging to the same species) females as well other species of copepods. This evidence suggests that these pheromones consist of general metabolic waste products across copepods, not unique to every species of copepods (Snell & Morris, 1993). Overcoming the challenges of deep waters in tracking and locating mates is a testament to the copepods’ remarkable chemoreceptive adaptations, which are essential for their reproductive success in the vast and dynamic aquatic world.
The role of chemoreception in finding food
Chemoreception plays a vital role in the food foraging behavior of copepods, helping them locate and capture prey efficiently. Several key aspects highlight the significance of chemoreception in their feeding strategies. A compelling experiment conducted with two species of harpacticoids, carnivorous copepods, in a Y-maze provides further evidence of chemoreception in their food foraging. On average, approximately 74% of the copepods swam into the branch treated with decaying fish flesh, indicating a modification in trajectory upon detection of chemical cues associated with potential food sources (Fechter et al., 2004). The study concluded that “Both species (tested) and all subgroups chose the treated seawater more often than the control seawater” (Fechter et al., 2004). The formula below presents a simple model that calculates the encounter rate of pelagic copepods with their prey (Heuschele & Selander, 2014).
![]()
![]()
![]()
![]()
![]()
![]()
The encounter rate with prey is directly proportional to the concentration of prey in their environment. To increase the encounter rate of prey, copepods have developed tactics to increase the distance at which they can perceive prey. To optimize this distance R, copepods combine both chemoreception and mechanoreception to detect potential food sources. The integration of these sensory modalities allows them to maximize their detection distance. Stimuli such as amino acids and microalgae trigger an array of responses in copepods such as aggregation in high prey regions, increased swimming speed and increased turning rate (Heuschele & Selander, 2014). In summary, chemoreception is a critical sensory mechanism ensuring the survival and successful feeding of copepods in a highly competitive marine ecosystem.
Protection tactics
Copepods also rely on chemoreception for protection from toxic elements of their environment. They use chemoreception to avoid potential danger and ensure their survival. When exposed to a layer of toxic chemical algal blooms during an experiment, in this case the dinoflagellate Karenia brevis, copepods displayed explicit avoidance behaviors (True et al., 2018). K. brevis produces a neurotoxin called brevetoxin which is responsible for several ecological effects, notably disrupting ecosystems through marine animal mortality (Murray, 2021). After encountering the toxic layer, copepods significantly increase their swimming speed and turn frequency, effectively distancing themselves from the harmful chemicals and limiting contact with the danger (True et al., 2018). The researchers found that “copepods explicitly avoid the K. brevis chemical layer at the individual and population levels and that avoidance strengthens with increasing concentration” (True et al., 2018). When the concentration of the K. brevis exudate reaches a value of 104 cells/mL, the copepods almost completely avoid the toxic layer as seen in Figure 9 (e) compared to 1 cell/mL in Figure 9 (a).
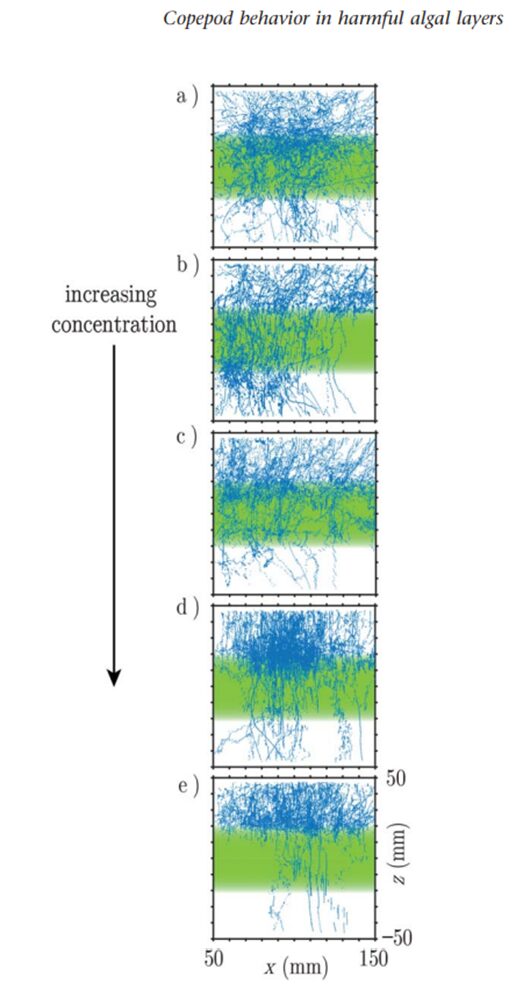
Fig. 9. Plot of copepod swimming trajectories (blue lines) as the concentration of K. brevis exudate in the toxic layer (green rectangle) increases (True et al., 2018).
Additionally, the ability of copepods to avoid toxic prey reaffirms the role of chemoreception in their foraging behavior. They can distinguish between physically similar prey items based on chemical signals, highlighting the specificity and sensitivity of their chemosensory systems.
Bioluminescence
There are many different types of luminescence and can be found all throughout nature, including in certain types of copepods. These families of copepods use bioluminescence which is a branch of chemiluminescence. Chemiluminescence occurs when light is emitted due to a chemical reaction. Bioluminescence uses a chemical reaction to create light as well and this chemical reaction occurs within the body of living organisms (giving it the bio prefix). The reaction that occurs involves the enzyme-catalyzed oxidation of a light emitting molecule and has three essential components: the luciferase enzyme, luciferin substrate, and oxygen. The chemical reaction is shown in Fig. 10.
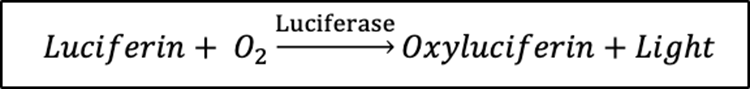
Fig. 10. Chemical reaction of bioluminescence (Baassiri, 2023).
The luciferin substrate attaches to the luciferase enzyme in the presence of oxygen, creating an enzyme-substrate complex. The luciferase enzyme catalyzes the reaction by allowing the oxidation of the luciferin molecule. The energy released when the luciferin is oxidated excites the luciferin molecule and, as it returns to ground state, light is emitted. The products of this reaction are therefore the oxidated luciferin (oxyluciferin) and light. The properties of light emitted, including intensity and frequency, depend on the specific types of substrates and enzymes found in the studied organism. This process is depicted in Fig. 11. Bioluminescence is a very useful light manipulation method for copepods as no incident light is required to emit light, only the presence of oxygen is required. This allows deep sea copepods to emit light and use its properties for their survival.
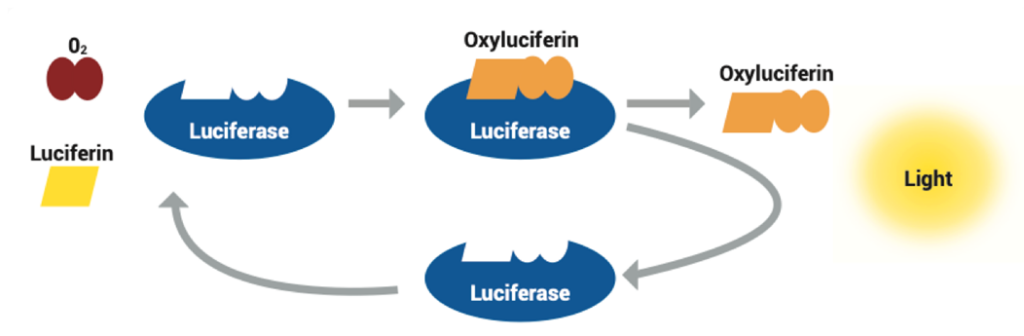
Figure 11: Visual model of bioluminescence reaction (Deep Ocean Education Project, 2022 ).
The copepod family calanoid copepods, uses a substrate known as coelenterazine and the luciferase enzyme in its application of bioluminescence. Coelenterazine acts in the exact same way as the luciferin substrate, the only difference being in the light color emitted. This substrate leads to light emission of 485-488 nm, which is typically a blue-green color. This is logical considering the calanoid copepod’s environment is aqueous. They are found in both oceanic and freshwater habitats, generally they are prevalent in areas where algae blooms occur as they are a food source for these copepods. The calanoid copepod typically uses bioluminescence as camouflage to hide from predators and because they typically are around blue and green color algae, they emit the perfect color of light to blend with their surroundings (Takenaka, 2017). A visual of their bioluminescence is shown in Fig. 12.
Fig. 12. Visual of calanoid copepods(Karin Stubgaard, 2017)
Some families of copepods can emit sudden flashes of light that can potentially confuse their predators. Families such as Metridinid, Lucicutidae, Heterorhabidae, and Augaptilidae, can emit light at 2 flashes per second from glands retracting above where the bioluminescence occurs. One study observed that copepods alternate between retracted glands synchronously while others alternate gland retraction (Herring et al., 1993). They may also keep glands open for longer periods of time to increase frequency of light. These results were found experimentally by stimulating the copepods through exposing them to different frequencies of light. Calanoid copepods can use these variations to their advantage by knowing through evolutionary experience what predators are affected the most by specific methods of light emission. It was also observed in this study that they can use these same methods to attract prey.
Copepods also use bioluminescence as a form of communication. The light they emit can be used as signals to communicate with fellow copepods if there are dangerous predators nearby, to coordinate group behavior, and for mating purposes. They can use light signals to attract mates and to exclaim their readiness for reproduction. (Herring et al.,1993)
Pigmentation within copepods
Amongst the transparent sea, copepods camouflage into their transparent medium (water) to maximize their fitness levels as having color can lead to dangers from predators. Their colors are generally not extensively studied because within labs, the plankton are preserved with certain fixatives such as formaldehyde and this causes the color to leak or be oxidized (Vilgrain et al., 2023).
However, their importance is valuable as the pigment on their bodies can appear in more quantities, or not at all, depending on the usage and the environmental conditions they are exposed to. This is why color on copepods can be known as a form of plastic adaptation, which is such an interesting quality to have (Schneider et al., 2016). The various forms of pigments can be depicted within Fig. 13. This shows that within different regions, the pigment can accumulate differently due to various reasons such as protection from ROS sources, reproductive seasons, predation pressures, food availability and water temperatures and predation pressures.

Fig. 13. Varying pigmentation from different regions within different species of copepods. (Vilgrain et al., 2023).
To first dive into this trait, it is important to note that red-orange pigmentation and blue pigmentation are the two types of shades that are most common among copepods with red being more prevalent (Vilgrain et al., 2023). The blue shade is mostly within neustonic copepods who live on the surface of waters within equatorial regions caused by complexes of proteins and carotenoids that will not be discussed in detail within this paper.
Furthermore, to display these hues, carotenoids, which are lipid-soluble natural pigments that are contained within photosynthetic bacteria, fungi, plants, or algae (Maoka, 2020) have to be consumed as copepods cannot synthesize them de novo (Schneider et al., 2016). Then, through various metabolic pathways, it is converted primarily to astaxanthin or canthaxanthin, carotenoid molecules that are responsible for the red orange tint (Vilgrain et al., 2023).
Carotenoids cause colors to be displayed due to their unique molecular structure. There are two primary groups of carotenoids, and these are carotenes and xanthophylls. These are vital molecules within nature due to the conjugated double-bonds which are found within the polyene chains shown in Figure 14 (Maoka, 2020). These conjugated systems allow light to be absorbed at various wavelengths within the visible region from the range of 400-500 nm (Vilgrain et al., 2023).
| Neuston Definition: “Neuston, group of organisms found on top of or attached to the underside of the surface film of water” (Britannica, T. Editors of Encyclopaedia, 2010). Neustonic is an adjective to describe an organism who is a part of this group. |

Figure 14: a) General Molecular Structure of Carotenoids, b) Common Carotenes and Xanthophylls found in Nature. (Adapted from Maoka, 2020).
The carotenoid we are interested in is a xanthophyll known as: Astaxanthin depicted in Figure 14 which contains end groups that are hydrophilic and nine conjugated double bonds. How it is formed through bioconversion is not clear but there is a proposed mechanism as depicted in Fig. 15. It is also important to note that yellow pigments such as zeaxanthin or lutein are more commonly found within copepods’ various diets over red pigments. For that reason, they are oxidized to convert to astaxanthin through an enzyme known as ketolase (Weaver et al., 2018).
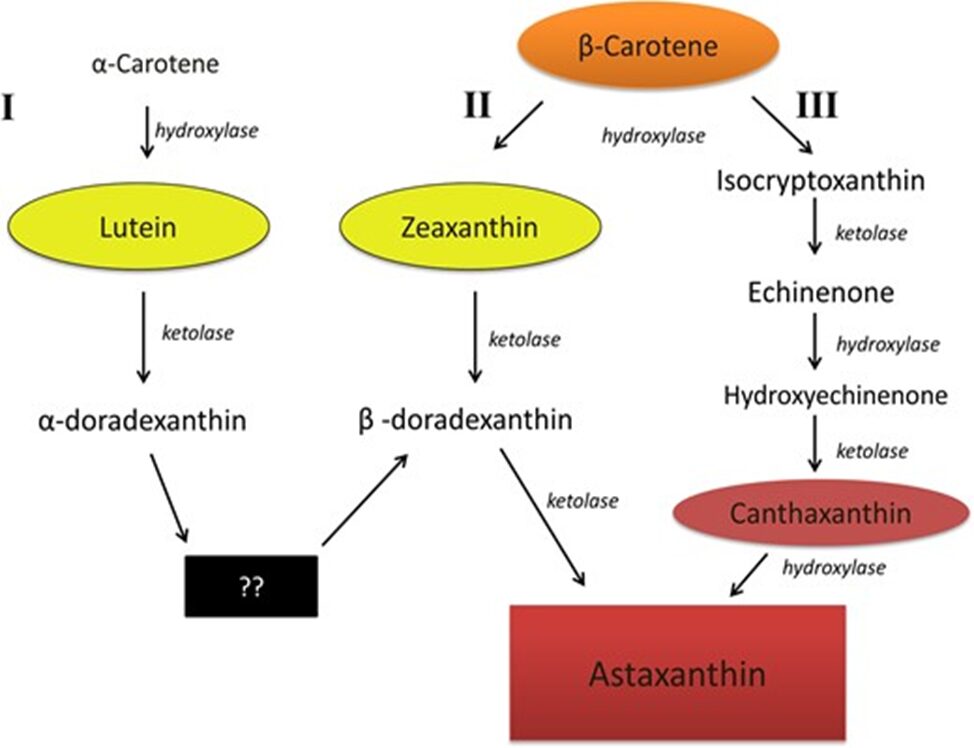
Figure 15:Proposed mechanism on how Astaxanthin is formed within animals. (Weaver et al., 2018).
In addition, studies have also shown that most species rely on echinenone and canthaxanthin as intermediates (Vilgrain et al., 2023) and that β-carotene obtained from phytoplankton is considered to be the main precursor for astaxanthin synthesis (Van Nieuwerburgh et al., 2005).
In the context of copepods, this molecule plays a bigger role than just displaying color tones as it also serves as an antioxidant when it is in free form or when it is esterified with fatty acids or proteins (Schneider et al., 2016). It can prevent oxidative damage since it is an electron acceptor due to its high affinity. It was found that free astaxanthin is the dominant form within copepods (≈ 61%) and the other 39% are astaxanthin esters.
When these species are exposed to harmful molecules known as Reactive Oxygen Species source (ROS), it is found that astaxanthin serves as a form of protection to copepods’ physiques (Schneider et al., 2016) and this process can be depicted by Figure 16. ROS sources are caused by UV radiation or through the respiratory chain and they are known to be the main reason behind cell aging. A single oxygen molecule can produce a singlet oxygen (1O2) and a hydroxyl radical (OH*). This can cause damage to proteins, DNA and especially lipids through peroxidation, which is where the hydroxyl radical will steal electrons from the lipids in cell membranes and create lipid radicals (Vilgrain et al., 2023). The first method is known as singlet oxygen quenching. This is a process where the energy of the excited oxygen molecule gets transferred to the astaxanthin through the carbon chain’s vibration which allows the oxygen to be neutralized. The second method is through free radicals scavenging. This is where the lipid radical is trapped by the molecule and is stabilized thus causing damage to lipids to stop.

Figure 16: How ROS damage is rectified by the molecule of astaxanthin through two methods: Singlet oxygen quenching and Free radicals scavenging. (Vilgrain et al., 2023).
To validate this, experiments have shown that copepods can change their carotenoid content depending on the exposure to UV within highly exposed UV-lakes and that unpigmented individuals cannot tolerate UV-radiation as much as pigmented ones can (Schneider at al., 2016).
To continue, astaxanthin was found to decrease within individuals during reproductive periods which is because the molecules are hypothesized to transfer to the offspring in free form (Schneider at al., 2016). This can explain why younger copepods have a vibrant red along their gut lining and exoskeleton while adults retain a single vibrant red naupliar eyespot (Weaver et al., 2018). It was found as well that more eggs are produced by pigmented individuals which is because copepod nauplii require warmth and astaxanthin helps them stay in hotter regions through photoprotection (Vilgrain et al., 2023).
Even throughout periods where there is low food availability or low temperatures, this carotenoid is significant for its’ role in lipid metabolism within these species (Sheinder et al., 2016). During these times, oxidative stress increases causing damage to storage lipids which are necessary to provide energy. During the winter, lipids are packed together which reduces the movement of ions and molecules across the membrane. For that reason, polyunsaturated fatty acids (Figure 17) play an important role in membrane fluidity as they contain more than two double bonds which leads to kinks that allow openings within the membrane (Membrane Fluidity | BioNinja, n.d.). Thus, astaxanthin increases and incorporates itself within cellular membranes to reduce peroxidation, especially of polyunsaturated fatty acids.
Being pigmented can offer various advantages to copepods but it has its’ tradeoffs since predators are able to detect them better. For that reason, within environments that are fishless, copepods accumulate more pigments. However, as we have come to learn, these creatures are intelligent and able to adapt to their circumstances. For that reason, they have evolved to perform diel vertical migration which is the ability to sink into deeper waters to avoid visual predation. Red is not the color that is best seen by these predators at depth who have evolved to find their prey with green-blue searchlights as water on the surface absorbs wavelength from the 600-750 nm range (Vilgrain et al., 2023).
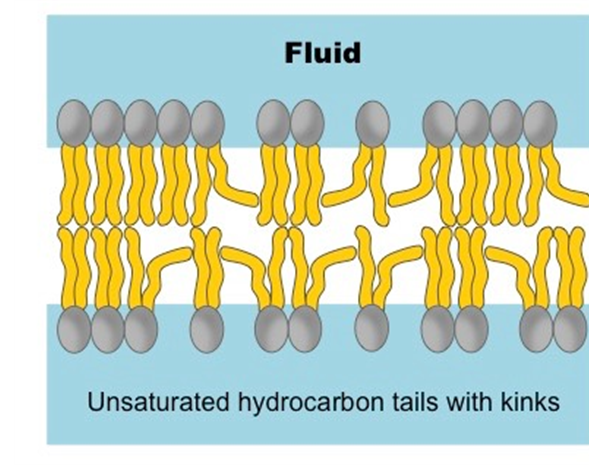
Fig. 17.. Unsaturated hydrocarbon tails allowing kinks. (Membrane Fluidity | BioNinja, n.d.).
Overall, the reasons as to why astaxanthin accumulates can be due to various factors which are mostly all encompassed by the location of where a copepod lives. Figure 18 offers a visual representation of the different components related to pigmented copepods.
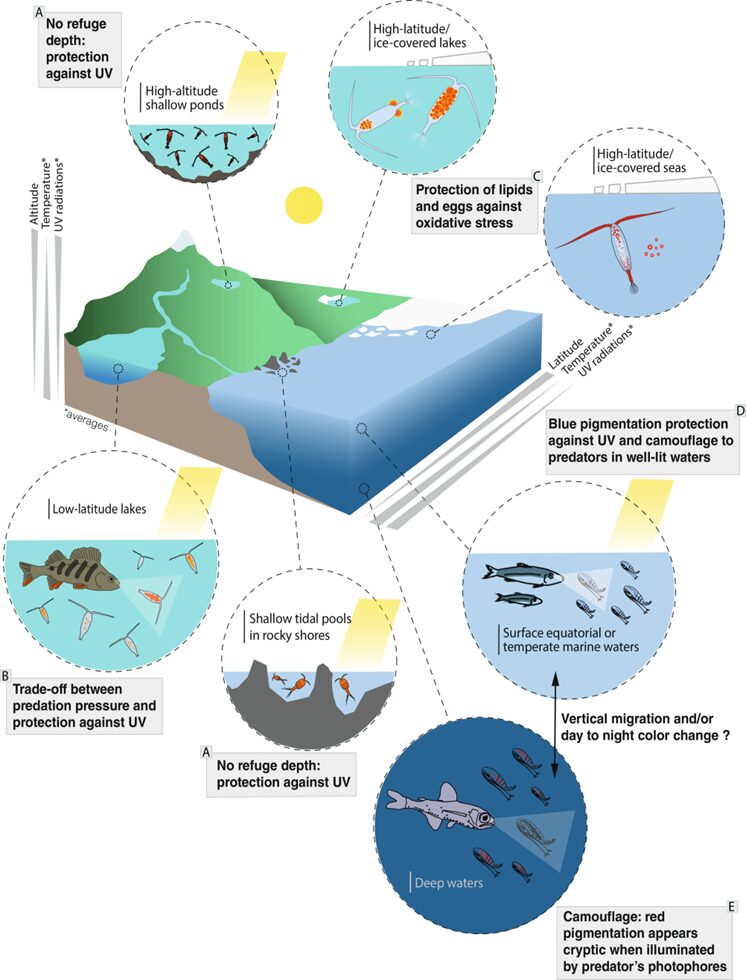
Figure 18: Overview of pigmented copepods subjected to different conditions in different locations (Adapted from Vilgrain et al., 2023).
Conclusion
In summary, copepods emerge as resilient organisms of evolutionary design, wielding a diverse set of chemical mechanisms to conquer challenges in their aquatic world. Their symbiotic relationship with bacteria, capable of breaking down toxins from cyanobacteria blooms, demonstrates an ingenious design solution to environmental adversities. When faced with challenges that their morphology cannot take on, copepods adapt through cooperation with another of nature’s remarkable designs. As copepods face the threat of toxic diatoms, their genetic diversity and evolutionary capabilities allow for progressive learning and adaptation by breaking down oxylipins into less harmful chemicals. The refinement of chemoreception stands out as a key survival strategy, enabling copepods to navigate their surroundings with remarkable precision to evade toxic threats, seek suitable mates, and locate prey with the help of their aesthetascs. Bioluminescence emerges as a unique tool, facilitating communication among copepods and serving as a distraction for potential predators. Furthermore, their carotenoid pigments not only function as camouflage against predators but extend their utility by rectifying damage caused by Reactive Oxygen Species sources.
These intricate chemical solutions highlight the dynamic nature of copepod’s physiology and behavior. Copepods possess a toolbox of evolutionary tactics that manipulate the chemistry within and around them. These tools are brilliant solutions to the new and ongoing problems they must face daily, and these solutions work in conjunction to ensure their survival. Copepods are a reflection of nature’s creativity and innovation through their chemical and behavioral adaptations to help not only themselves but the planet as well.
References
Barbosa, M., Valentão, P., & Andrade, P. B. (2016). Biologically active oxylipins from enzymatic and nonenzymatic routes in macroalgae. Marine drugs, 14(1), 23.
Deep Ocean Education Project. (n.d.). Bioluminescence – NOAA Ocean Exploration. Ocean Exploration. https://oceanexplorer.noaa.gov/edu/materials/bioluminescence-fact-sheet.pdf
Herring, P. J., Latz, M. I., Bannister, N. J., & Widder, E. A. (1993). Bioluminescence of the poecilostomatoid copepod Oncaea conifera. Marine Ecology Progress Series, 94(3), 297-309. http://www.jstor.org/stable/24832715
Lauritano, C., Carotenuto, Y., Miralto, A., Procaccini, G., & Ianora, A. (2012). Copepod population-specific response to a toxic diatom diet. PLoS One, 7(10), e47262. https://doi.org/10.1371/journal.pone.0047262
Maoka, T. (2020). Carotenoids as natural functional pigments. Journal of Natural Medicines, 74(1), 1–16. https://doi.org/10.1007/s11418-019-01364-x
Membrane Fluidity | BioNinja. (n.d.). Retrieved November 9, 2023, from https://ib.bioninja.com.au/standard-level/topic-1-cell-biology/13-membrane-structure/membrane-fluidity.html
Schneider, T., Grosbois, G., Vincent, W. F., & Rautio, M. (2016). Carotenoid accumulation in copepods is related to lipid metabolism and reproduction rather than to UV-protection. Limnology and Oceanography, 61(4), 1201–1213. https://doi.org/10.1002/lno.10283
Van Nieuwerburgh, L., Wänstrand, I., Liu, J., & Snoeijs, P. (2005). Astaxanthin production in marine pelagic copepods grazing on two different phytoplankton diets. Journal of Sea Research, 53(3), 147–160. https://doi.org/10.1016/j.seares.2004.07.003
Vilgrain, L., Maps, F., Basedow, S., Trudnowska, E., Madoui, M.-A., Niehoff, B., & Ayata, S.-D. (2023). Copepods’ true colors: Astaxanthin pigmentation as an indicator of fitness. Ecosphere, 14(6), e4489. https://doi.org/10.1002/ecs2.4489
Weaver, R. J., Cobine, P. A., & Hill, G. E. (2018). On the bioconversion of dietary carotenoids to astaxanthin in the marine copepod, Tigriopus californicus. Journal of Plankton Research, 40(2), 142–150. https://doi.org/10.1093/plankt/fbx072
Aggen, J. B., Nairn, A. C., & Chamberlin, R. (2000). Regulation of protein phosphatase-1. Chemistry & biology, 7(1), R13-R23.
Biology-Online. (2023). Chemoreceptor Definition and Examples – Biology Online Dictionary. https://www.biologyonline.com/dictionary/chemoreceptor#:~:text=(1)%20A%20sensory%20nerve%20cell,to%20the%20central%20nervous%20system.
Bourne, D. G., Jones, G. J., Blakeley, R. L., Jones, A., Negri, A. P., & Riddles, P. (1996). Enzymatic pathway for the bacterial degradation of the cyanobacterial cyclic peptide toxin microcystin LR. Applied and environmental microbiology, 62(11), 4086-4094.
Bourne, D. G., Riddles, P., Jones, G. J., Smith, W., & Blakeley, R. L. (2001). Characterisation of a gene cluster involved in bacterial degradation of the cyanobacterial toxin microcystin LR. Environmental toxicology, 16(6), 523-534.
Fechter, A., Thistle, D., Arlt, G., Suderman, K., & Vopel, K. (2004). Do Harpacticoids (Copepoda) Use Water‐Borne Cues to Aid in Locating Food Parcels? Marine Ecology, 25(3), 217-223. https://doi.org/10.1111/j.1439-0485.2004.00026.x
Gorokhova, E., El-Shehawy, R., Lehtiniemi, M., & Garbaras, A. (2021). How copepods can eat toxins without getting sick: Gut bacteria help zooplankton to feed in cyanobacteria blooms. Frontiers in Microbiology, 11, 589816.
Hansen, B. W., Drillet, G., Kozmer, A., Madsen, K. V., Pedersen, M. F., & Sørensen, T. F. (2010). Temperature effects on copepod egg hatching: does acclimatization matter? Journal of Plankton Research, 32(3), 305-315.
Heuschele, J., & Selander, E. (2014). The chemical ecology of copepods. Journal of Plankton Research, 36, 895-913. https://doi.org/10.1093/plankt/fbu025
Lad, C., Williams, N. H., & Wolfenden, R. (2003). The rate of hydrolysis of phosphomonoester dianions and the exceptional catalytic proficiencies of protein and inositol phosphatases. Proceedings of the National Academy of Sciences, 100(10), 5607-5610.
Ladisch, M., Lin, K., Voloch, M., & Tsao, G. T. (1983). Process considerations in the enzymatic hydrolysis of biomass. Enzyme and Microbial technology, 5(2), 82-102.
Merriam-Webster. (2023). Pheromone Definition & Meaning – Merriam-Webster. https://www.merriam-webster.com/dictionary/pheromone#:~:text=Kids%20Definition-,pheromone,kind%20of%20behavior%20(as%20mating)
Murray, T. F. (2021). Chapter Three – Neurotoxic: Ciguatoxin and brevetoxin—From excitotoxicity to neurotherapeutics. In A. Novelli, M.-T. Fernández-Sánchez, M. Aschner, & L. G. Costa (Eds.), Advances in Neurotoxicology (Vol. 6, pp. 89-104). Academic Press. https://doi.org/https://doi.org/10.1016/bs.ant.2021.03.003
Ryan, M., Butt, A. A., & Adley, C. C. (2016). Sphingomonas paucimobilis. In: Microbes.
Rzeszotarska, B., Siodłak, D., Broda, M., Kozioł, A., & Dybała, I. (2002). Conformational investigation of α, β‐dehydropeptides. X. Molecular and crystal structure of Ac‐ΔAla‐NMe2 compared with those of Ac‐l‐Ala‐NMe2, Ac‐dl‐Ala‐NMe2 and other dimethylamides a: Authors’ affiliations. The Journal of peptide research, 59(2), 79-89.
Seuront, L. (2013). Chemical and hydromechanical components of mate-seeking behaviour in the calanoid copepod Eurytemora affinis. Journal of Plankton Research, 35(4), 724-743. https://doi.org/10.1093/plankt/fbt039
Snell, T. W., & Morris, P. D. (1993). Sexual communication in copepods and rotifers. Hydrobiologia, 255-256(1), 109-116. https://doi.org/10.1007/bf00025828
True, A. C., Webster, D. R., Weissburg, M. J., & Yen, J. (2018). Copepod avoidance of thin chemical layers of harmful algal compounds. Limnology and Oceanography, 63(3), 1041-1055. https://doi.org/10.1002/lno.10752
Uttieri, M., Brown, E. R., Boxshall, G. A., & Mazzocchi, M. G. (2008). Morphology of antennular sensors in <i>Clausocalanus furcatus</i> (Copepoda: Calanoida). Journal of the Marine Biological Association of the United Kingdom, 88(3), 535-541. https://doi.org/10.1017/s0025315408000854
Xing, Y., Xu, Y., Chen, Y., Jeffrey, P. D., Chao, Y., Lin, Z., Li, Z., Strack, S., Stock, J. B., & Shi, Y. (2006). Structure of protein phosphatase 2A core enzyme bound to tumor-inducing toxins. Cell, 127(2), 341-353.
Zhang, W., Liu, J., Xiao, Y., Zhang, Y., Yu, Y., Zheng, Z., Liu, Y., & Li, Q. (2022). The impact of cyanobacteria blooms on the aquatic environment and human health. Toxins, 14(10), 658.