Biomechanics of the Evolutionary Arms Race
Tri Vinh Truong, Steven Xu, Emma Wong, Quinten Bennett
Abstract
The evolutionary race of weaponry may be observed in many natural circumstances. In the context of antagonistic relationships between animals, a fascinating struggle, namely the arms race, is a common occurrence. Species and individuals strive for the advantage over one another in a race for survival and resources by developing specialized traits. General and mechanical properties of the arms race, both asymmetrical and symmetrical, can be observed in competition within and between various animal species, such as rhinoceros beetles, bighorn sheep, bats, and moths. These animal species are several of many that will evolve and develop mechanical features and weapons to withstand evolutionary pressure, stemming from either intraspecies or interspecies competition. Male rhinoceros beetle head horn morphology has evolved to improve performance in sexually driven conflicts, but the length of this exaggerated feature will reach a ceiling as further growth is halted my mechanical limits. Ruminants, such as the bighorn sheep, compete for mates similarly, but on a larger scale. Bats develop complex soundwave detection mechanisms to track moths, to which moths respond with evolving mechanisms to evade the bats. These examples of antagonistic intraspecies and interspecies relationships outline the importance of arms races, and the biomechanical, functional, and evolutionary wonders they ultimately produce.
Arms Race in Nature
Competition, Evolution, and the Arms Race
As a major driving force of the evolutions of animal species, competition forces creatures to adapt rapidly to changes in their surrounding biosphere and to evolve better weaponry and tools to obtain resources that enable the reproduction of species. Failure of such effort usually results in the extinction of animal species by biological causes, not physical causes (Larson, 1996). Besides evolutionary pressure from the environmental changes to biosphere, competition can also occur for the purpose of reproductive attractions. Intraspecies sexual conflicts are often observed for the purpose of mate selections by females due to the prominent benefits of offspring from parents mating with advantageous genes (Parker, 1979). The resulting competition preserves the genetic information of individuals who emerge victorious in either combat or beauty and eliminates individuals who contain flaws that threaten the survival of potential offspring. However, such conflicts can also generate Fisherian ornaments, in which copious amounts of resources are invested into growing traits for the sole purpose of attracting mates (Pomiankowski, 1988). Arms races occurring in nature involve individuals or species improving their characteristics and tools over long stretches of time to overcome the pressure exerted by their rival species or gain advantages over other individuals over limited resources. In an arms race, when one side improves their “weaponry,” their enemies would also progress to become “better enemies” (Dawkins, 1986). While both sides progressively become “better” in this specific competition, the relative advantages gained or lost by one side is diminished by the equal improvement on the opposite side. If an arms race ends with one winner, the results for the imbalanced advantages in the competition would be the extinction of the losing species/individuals. Arms races can be separated into two categories: asymmetrical and symmetrical (Dawkins, 1986).
Asymmetrical Arms Race
The asymmetrical arms race is often observed between species with a predator-prey relationship. Despite both participating in an arms race against the other, the species involved are on distinct levels of the food chain. Accordingly, they will choose different evolutionary paths. Under this condition, predators often choose skills in hunting and tracking, while prey often develop skills in hiding or evading. A typical example is the arms race between bats and moths. Bats use echolocating ultrasonic waves to locate objects and prey; however, under the evolutionary pressure exerted by this weapon, many insect species hunted by bats respond by coevolving specific anti-bat tools. Consider the noctuid moths; they have developed sensory cells that detect soundwaves in the same frequency range as those emitted by bats and may therefore perform evasive flight patterns (Ratcliffe et al., 2011). Remarkably different evolution patterns can be seen within the various predator-prey relationship in nature. Some amphibians, such as newts, become toxic by infusing their skin with tetrodotoxin, a neurotoxin usually found in marine lifeforms, to compensate for their slow movement (Hanifin, 2010). To counterbalance this defense mechanism, one of the predators of newts, the garter snake, increases its tolerance to tetrodotoxin through genetic modifications (Feldman et al., 2010). Asymmetrical arms races involve two distinct species responding to each other through choosing evolutionary directions base on their own position in the food chain and their existing advantages and disadvantages.
Symmetrical Arms Race
Symmetrical arms races have the property that all competitors evolve in the same direction as they try to “out-evolve” each other by developing the same trait or weapon. Consequently, the symmetrical arms race is often found in intraspecies competition, and individuals compete to gain control over limited resources and reproductive benefits. Sexual conflicts and Fisherian runaway are typical examples of this relationship. Exaggerated male ornamentation is often observed within in-fighting over females. The ornamentation directly affects the reproductive ability and success of the male (Fisher, 1958). Examples of Fisherian runaway include the peacock’s tail and colorful patterns on insects. These traits can be seen as a result of cumulation of exaggeration across years of evolution. Besides sexual conflicts, symmetrical arms races are apparent in trees, where individuals compete for resources by height and shape. In a dense forest—due to the need for sunlight in photosynthesis—trees typically grow tall with their crowns extended widely to capture as much light as possible while maintaining the mechanical strength of their trunks (Iwasa et al., 1985). Unlike asymmetrical arms races, symmetrical arms races put emphasis on the individuals’ characteristics and traits, and how they can utilize resources to gain advantages over others who are similar.
Rhinoceros Beetles
Horn Morphology
The head horns of rhinoceros beetles are a typical example of exaggerated morphologies stemming from sexual competition between males to gain reproductive benefits. Male rhinoceros beetles will fight each other for dominance over sap site feeding grounds to have greater access to female rhinoceros beetles that come to feed throughout the day. Typical competition between males of all rhinoceros beetle species involves inserting their horn under the opponent and prying them off the substrate, which can be a tree branch, a narrow shoot, the vertical surface of a tree trunk or a sap site. Under further observation, various horn shapes are seen across species despite their common purpose. A study on the structural variations to diverse fighting styles in sexually selected weapons hypothesized that rhinoceros beetle horns are structurally adapted to meet the functional demands of species-species fighting (McCullough, 2014). These variations are what dictate the finer aspects of rhinoceros beetle fighting styles to minimize strain energies experienced by the horn and maximize on the force transmitted to the opponent. Distinct differences in horn shape are especially apparent in the three species, Trypoxylus Dichotomus, Golofa Porter, and Dynastes Hercules.
The Japanese horned beetle, Trypoxylus Dichotomus, can be easily recognized by its large, pitchfork shaped horn. This distinctly shaped horn is used to pry and twist opponents off the substrate. Comparatively, the horned beetle, Golofa Porter, uses its long and slender head horn for fencing, lifting, and throwing opponents off balance when fighting on narrow branches. Finally, the Hercules beetle, Dynastes Hercules, uses the combination of two horns, the head and thoracic horn, to form a pincer to lift and squeeze opponents. Due to species-specific fighting performances, the direction and magnitude of force experienced on these three horns can differ.
A study conducted by researcher Dr. Erin McCullough and colleagues (2014) investigated the fighting loads experienced by the three beetle species, and consequentially, the structural adaptations they evolved to resist these specialized loads. Using standard finite element analysis, a popular engineering analyses technique applied for the purpose of predicting deformation and failure of complex structures in response to applied force, the mechanical performance of Trypoxylus, Golofa, and Dynastes horns was compared. Vertical, lateral, and rotation force was applied separately to fixed biomechanical models of the three species’ horns, mimicking both species-typical and species-atypical fighting loads (Fig. 1). Two measures of horn performance—strength and stiffness—were assessed. The stress experienced by the biomechanical models was evaluated as horn strength, while total strain energies was evaluated as horn stiffness. After comparison, results confirmed that the horns are more resistant under species-species fighting loads. Once this condition is fulfilled, shape variation may effectively lower the strain energies experienced by the horn, decrease horn deformation, and increase the transmission of force used to dislodge rival males (McCullough, 2014). Fighting performance is therefore an important agent of selection on horn morphology and has evolved to maximize force transmissibility while minimizing horn weight, damage and stress.
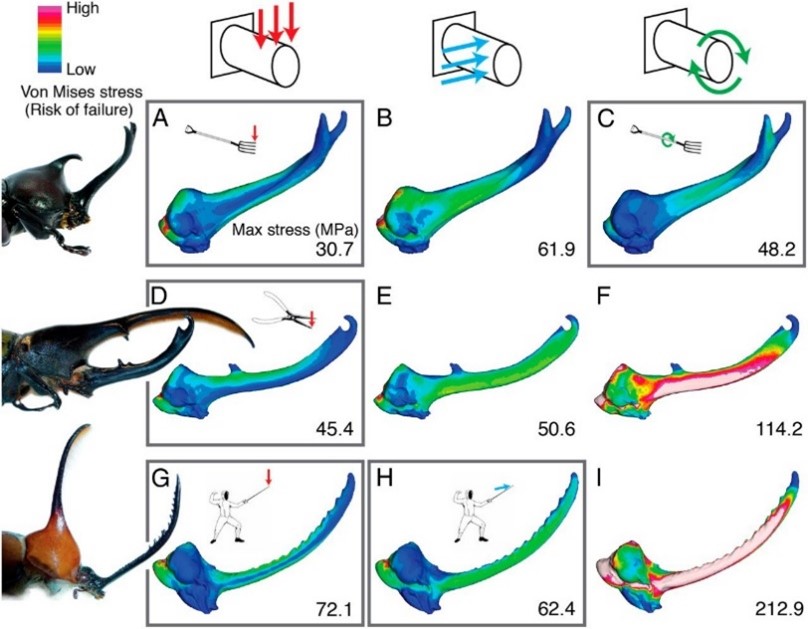
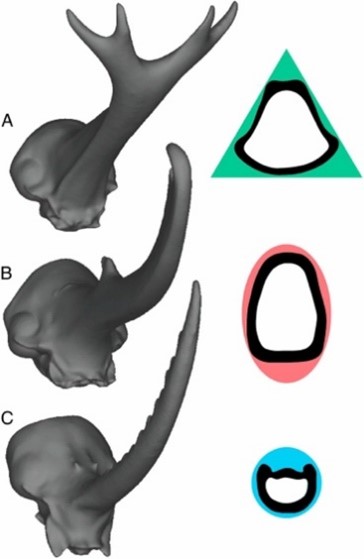
To further assess variations in fighting performance due to horn morphology, cross-sectional shape must be considered. If a beetle’s horn is considered as a beam, its resistance to bending can be directly related to how its mass distributes about a neutral bending axis and the horn’s second moment of area, in accordance to beam theory (McCullough et al., 2014). The Dynastes horn is bent predictably in a single direction during typical application, experiencing only vertical loads, thus it is most resistant to bending when mass is distributed far from its bending axis. To facilitate this resistance, the Dynastes horn adopted an elliptical cross section (Fig. 2B). In comparison, Golofa horns often experience irregular loads from many different directions. They undergo unpredictable bending and therefore have a circular cross-section (Fig. 2C) to distribute the mass equally around all possible bending axes. Lastly, Trypoxylus horns experience vertical and rotational forces. They have adopted a triangular cross-section (Fig. 2A) with a high second moment of area conferring high flexural stiffness, and a high polar moment of area to increase torsional stiffness—both ideal factors for resisting bending and twisting forces. Force application and loading of the three species horns loosely correspond to that of mundane tools: Dynastes with a set of pliers, Golofa with a fencing sword, and Trypoxylus with a pitchfork.
The horns of rhinoceros beetle species have structurally adapted to different fighting performances to maximize strength and stiffness in response to species-typical fighting loads. The study performed by McCullough et al. (2014) has further reinforced the relation between the horn’s shape and function and suggest a history of evolution of the head horn for the purpose of efficacity and gaining advantage in sexually driven competition between male rhinoceros beetles.
Biomechanical Limits of the Head Horn
The size of a male rhinoceros beetle’s head horn is related to success in intra-species competition between males. With a larger horn, it may generate greater prying force and have a range advantage over smaller-horned males when fencing. However, following the trend of all organisms that develop elaborate traits in competition for females, their horn cannot grow to an infinite size and will stop growing once it reaches a certain length. Generally, an organism’s sexual trait will evolve to be increasingly exaggerated until survival costs outweigh reproductive benefits of further trait elaboration (McCullough, 2014). Contrary to this theory, previous studies of the rhinoceros beetle have shown that the growth of their head horn is not limited by cost-benefit equilibrium. For example, a large head horn does not affect the beetle’s maneuverability and speed in flight. What is it that limits further evolution of the head horn? To address this question, a study was conducted on the Japanese rhinoceros beetle, Trypoxylus Dichotomus, at the National Chi Nan University in central Taiwan.
The hypothesis formulated stated that structural failure of the rhinoceros beetle’s oversized horn sets a mechanical limit on the maximum size a horn can grow. When male rhinoceros beetles fight at a sap site (Fig. 3), they must overcome their rival’s grasping force, which is their ability to hold onto the substrate. In the case of Trypoxylus, their forked horns are susceptible to vertical stress when prying their opponent off the substrate. Vigorous fighting can damage and scratch horns, but only in extreme cases where two males of equal size compete will their horns be subject to a maximum load (McCullough, 2014). For research purposes it is necessary that a “safety factor” is established. In the case of Trypoxylus horns, the safety factor is the ratio between force required to break the horn and the maximum force exerted on the horn. To mimic the loading regime experienced by Trypoxylus horns during a typical intraspecies fight, researchers dissected the head from cold-euthanized laboratory-raised males of varying head horn lengths and loaded them into a cantilever system, after which the sample horns were subjected to a vertical force. Imaging software was used to trace the horn’s digital photos and measure the second moment of area after force application, and a mechanical tester measured the maximum force supported by the horn before fracture. The maximum stress experienced within the horn cuticle—where the base of the horn is secured—was then estimated using classic beam theory. The individual horns safety factors were then calculated by the measured maximum breaking force divided by the estimated value of fighting force and compared with horn length (Fig. 4).

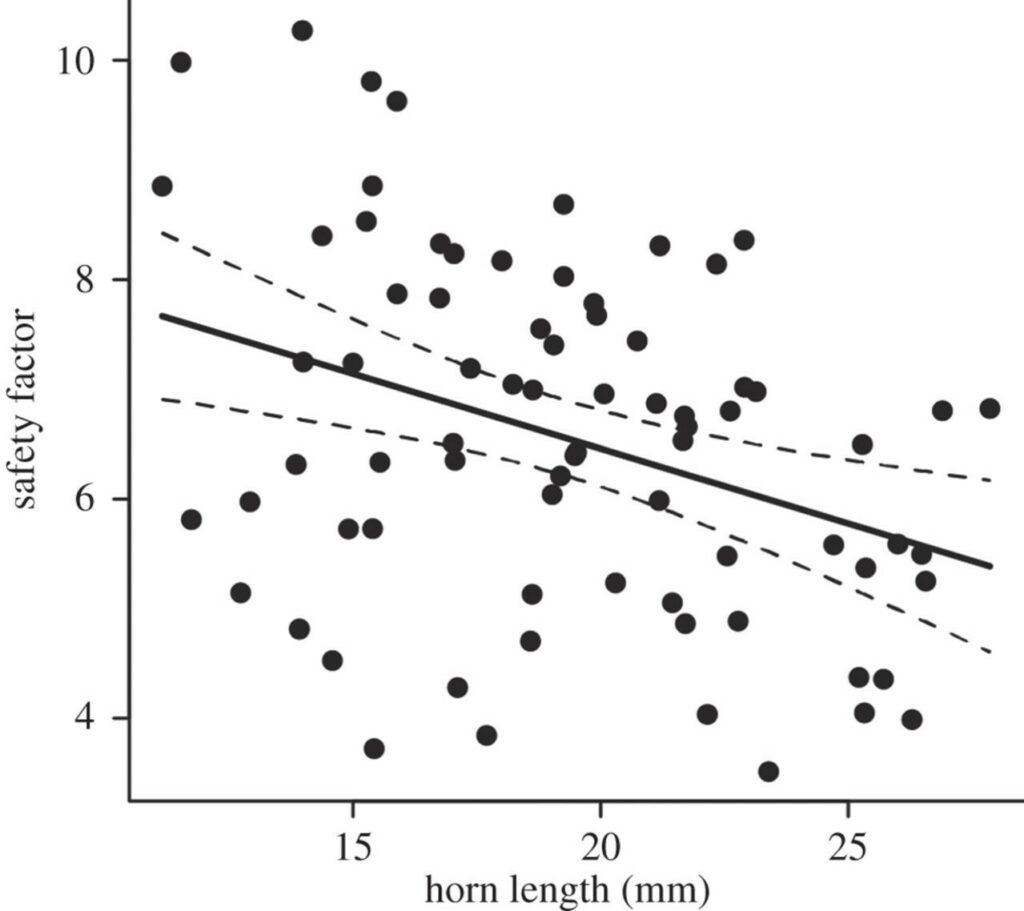
The safety factors calculated in the study ranged from 3.5 to 10.3. The relationship between horn length and safety factor appeared to be negative, suggesting that a longer horn would be more susceptible to fracture and breakage. Conclusively, the results of the study supported the hypothesis that indeed, mechanical limits set an upper bound on horn size as opposed to cost-benefit equilibrium.
The rhinoceros beetle’s horn is adapted to effectively perform in competition based on diverse fighting styles across species. For an effective performance, it is necessary that the horns adopt species-specific cross-sections and shapes, and that an upper limit be set on horn size to minimize risk of breakage.
Bighorn Sheep
Ruminant mammals commonly engage in intraspecific competition to defend their territory or for the opportunity to mate. Typically, males are equipped with cranial appendages, such as ossicles, horns, or antlers, which aid their fighting and defensive abilities. As many of these appendages may have evolved independently of each other (Geist, 1966) and are often sexually dimorphic, their origin is a result of an arms race between competing males. Over time, these arms races have given ruminants the mechanically remarkable organs they sport today.
An example of intraspecific fighting can be observed among bighorn sheep (Ovis canadensis, Fig. 5). Rams will attack each other to gain higher standing in the social and mating hierarchy, with the dominant ram having the best opportunity.

Mechanics of Bighorn Ram Combat
Ideally, rams attack by charging at their opponent, raising themselves onto their hind legs before contact, then slamming their horns down into their target, often meeting their opponents’ (Nat Geo WILD, 2018). This allows the ram not only to control its angle of attack, optimizing defense and offense, but to put their entire weight behind their attack as they slam down, generating a greater force. Rams typically make contact near their base of their horns in the radial direction of the horn.
Actual clashes vary in attack direction, velocity, impact time and deceleration due to numerous factors. Values for the velocity of ramming bighorns between 4.6m/s (Drake, 2015) and 9m/s (Huang et al., 2017) have been reported. Time of impact can occur on the scale of 1×10-3 s (Drake, 2015). Estimated decelerations after impact vary significantly from 30 m/s2 (Kitchener, 1988) to more than 4000m/s2 (Huang et al., 2017). Considering an average case of deceleration of 34m/s2 and sheep mass of 100kg, ram horns must be able to withstand 3400N of force (Kitchener, 1988). Rams engage in several collisions every hour for multiple hours (Drake, 2015). Their horns must be able to withstand these forces with minimal damage until the ram becomes too tired to continue. As horns are permanent, non-regenerating structures, any substantial damage to them negatively impacts the male’s success in reproduction. Applying the arms race theory, rams with more structurally stable horns will win more battles, thus producing more offspring with better horns. After generations of sexual selection, the robust structure and mechanics of the bighorn ram’s horns were developed.
Bighorn Horn Morphology and Physical Characteristics
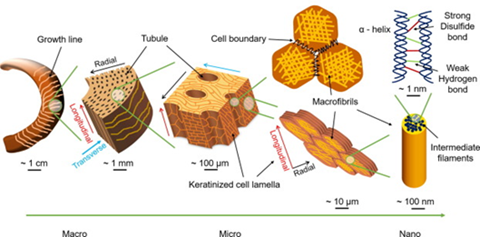
The horns of the ram consist of a bony core covered in a keratin sheath, which will specifically be referred to as the horn for the purpose of this exploration. The horn specifically contains α-helix keratin intermediate filaments with a diameter of 12nm, which are much stiffer than more flexible β-sheet keratin (Drake, 2015). These filaments are embedded in an unstructured protein keratin matrix. Upon closer inspection of the horn, elliptical tubular structures on the scale of micrometers can be identified. These tubules are oriented along the growth direction of the horn. The average major and minor axis lengths of the ellipses are 59µm and 24.6µm along the transverse (normal to growth direction, same as growth lines in Fig. 6) and radial directions respectfully (Huang et al., 2017). The tubules are both surrounded by and part of a matrix of layered cells, meeting the tubules at an angle of approximately 30 degrees. The membranes of the matrix cells are curved, resulting in an interlocking interface due to increased surface area contact between them. This makes the layers of cells in the horn more resistant to delamination. Additionally, the cells are filled with macrofibrils of twisted α-helix keratin intermediate filaments 200nm in diameter. The macrofibrils are oriented randomly inside of the cell but are always parallel to the cell’s surface. This results in the transverse isotropic behavior observed in the horn under certain mechanical stresses (Huang et al., 2019).
Multiple analyses have been conducted using various methods to describe the structure and mechanical properties of the horn. Kitchener (1988) used a modification of the Euler–Bernoulli beam theory to determine the stresses experienced by the horn during impact. Modification of the theory is mandatory due to the curved nature of the horn. The radius of curvature of the trailing edge of the horn is less than a third of the radius of the leading edge, resulting in the neutral axis of the “beam” shifting from the central axis of the horn closer to the rear. This has been shown to change the ratio of maximum compression and tensile stresses experienced in the horn to 1.7. Shearing stresses were also found to be insignificant, as the horns failed in compression long before shearing stresses began to take a toll on the horn (Kitchener, 1988).
It may seem odd to increase this ratio, as the horn and its material are much weaker to compression than tensile or shearing stresses. However, this may show why contact at the base of the horns is ideal during ramming. At this location, the core to horn ratio is highest. As the core is made of mineralized bone, it is much more resistant to compression stresses than the horn. As this makes the bone more susceptible to fracture, the horn can be seen as a tool taking the initial load of the impact, then distributing it to the core underneath (Drake, 2015). Contact near the base also helps with energy dissipation. During impact, once the leading edge of the horn stops, it is compressed by the still-moving trailing edge. This creates a torque at the base of the horns, leading to vibrations travelling along the growth direction of the horn. These vibrations help dissipate some of the energy received from the collision as kinetic energy (Drake, 2015).
Quasi-static and dynamic strain rate experiments have been performed on the horn to determine how it responds to strain in different directions. Under a near constant strain rate under 1 s-1, the horn was weakest to forces applied in the radial direction while similarly more resistant to those in the growth and transverse directions (an example of its transverse isotropic behaviour). However, under dynamic strain rates of up to 4000s-1, more like the sudden impact during a ram clash, the horn’s strength and energy absorption was most pronounced from forces along the radial direction. This is due to not only being the direction most perpendicular to the cell surfaces, with compression increasing keratin density, but also to the collapse of the tubules along their minor axis (Huang et al., 2017). This allows the horn to absorb much more energy without extensive damage to the structure, explaining why rams make contact in the radial direction with respect to their horns.
Adaptions are not only present in the horn. Compared to other ruminants, their first and second cervical vertebrae are shorter and wider relative to body size. This has been shown to improve the animal’s ability to “absorb high compressive forces during impact while also resisting flexion, extension or lateral wrenching” caused by an unideal collision (Vander Lindon & Dumont, 2019, sect. Discussion, para. 4). The seventh vertebra is also comparably longer, allowing for attached muscles to better counteract unwanted flexion (Vander Lindon & Dumont, 2019).
Bats and Moths
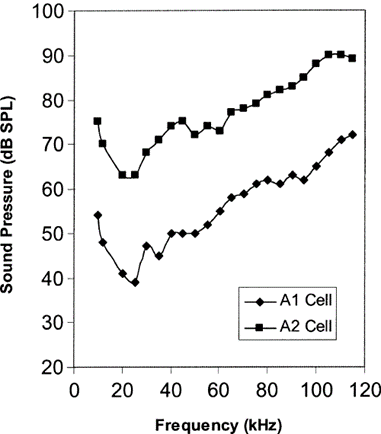
Bats and moths have been engaged in a millennia-spanning evolutionary arms race, with each evolving adaptations to allow them to outdo the other. For example, in order to evade bats more efficiently, at least 5 species of moth have evolved ears, allowing them to detect the ultrasonic echolocation calls bats use to hunt. These ears are specialized in detecting ultrasound at a frequency of about 25kHz, and typically only have two sensory neurons, dubbed A1 and A2, which have different thresholds of activation, A2 cells being roughly 15-20dB less sensitive than A1 cells (Fig. 7) (Waters, 2003). As a result of this, eared moths can estimate the distance at which the bat is making its calls; when the less sensitive A2 cell activates, this means the bat is very close and is about to capture the moth. Thus, the moth can distinguish between a searching bat and an attacking bat, which allows it to evade at the correct moment.
While these cells are effective at detecting ultrasound in the typical frequency range at which bats call, they cannot detect lower or much higher frequencies – a fact that certain bats take advantage of. For example, Horseshoe bats make use of the Doppler shift effect, an effect where a receiver moving towards the source of a sound, or having the source of the sound approach the receiver, will increase the frequency of sound at the receiver’s end. Conversely, the frequency perceived by a receiver will decrease when the receiver and the sound are moving away from each other. Horseshoe bats make use of this effect by emitting calls at low frequencies that are barely audible to them and moths. As the bat flies forwards, the echo frequency of their call is increased due to Doppler shift and is further modulated depending on whether the moth is moving towards or away from the bat. As a result, Horseshoe bat calls are silent towards moths and the bat, but its echo is not, allowing Horseshoe bats to locate and catch moths without alerting them. Additionally, the frequency modulation caused by the moth’s movement provides additional information to the bat, further increasing the chances of catching their prey. (Waters, 2003).
On the other hand, rather than changing the frequency of their calls to one that is inaudible to moths, Barbastelle bats have evolved low-amplitude echolocation calls. As a result, Barbastelle calls go undetected by moths even at closer ranges, since the A1 and A2 cells do not register sufficient ultrasound intensity to trigger. Additionally, as the Barbastelle bat approaches, they further decrease the amplitude of their calls, keeping them undetected even as they approach their prey. Throughout a Barbastelle bat attack, bat calls never reached more than 10dB at the receiver’s end, 10dB being the threshold at which an eared moth will perceive a bat’s ultrasound (Lewanzik & Goerlitz, 2018). The Barbastelle bat’s strategy comes at a cost; however, low amplitude calls view smaller areas and cannot detect moths from far away. As a workaround, Barbastelle bats instead emit two calls at once; one from the nostrils, pointed upwards, and one from the mouth, pointed downwards. Upward calls are used for detecting prey, while downward calls are used for determining distance above the ground. As a result of this adaptation, Barbastelle bats can search a larger volume of space than they could have otherwise (Lewanzik & Goerlitz, 2018).
While ears might be sufficient in increasing the survival rate of moths, some have developed additional defenses against bats. The tiger moth, for example, exhibits a peculiar defense mechanism that takes the form of ultrasonic clicking which can effectively jam bat’s echolocation signals. This clicking is typically emitted using a two tymbal organ located on their abdomen, which click alternatively, allowing for faster clicking. The ultrasonic clicks of tiger moths have a multitude of useful effects: for example, bats who have never heard the clicking before are startled by the sound and actively avoid the moths. However, bats usually habituate themselves to the sound, and begin attacking moths despite the clicking. Even so, these bats fail to catch the moths anyway; by emitting these ultrasonic clicks in a specific time window after hearing a bat’s ultrasonic call, tiger moths can disrupt the ultrasound emitted by bats to sense the location of their prey. In doing so, bats find it much harder to execute their capture maneuvers, allowing the moth precious time to escape the bat. A study by Corcoran et al. (2011) found that bats failed to capture these moths about 70% of the time, showing the success of this defense mechanism.
Other species of moth have also developed ultrasonic clicking, albeit for a different purpose. For example, Yponomeuta moths are deaf and have instead developed toxic chemistry that makes them distasteful to bats. However, bats have poor eyesight; how can these moths communicate that they do not taste good? As a solution, these moths have also developed ultrasonic clicking, much like tiger moths, but adapted to a completely different purpose: advertising their distastefulness. Since these clicks are not used to jam bat sonar, they are constantly emitted using elastic cymbals situated on their wings. By using the force of their wingbeats, these moths constantly produce sound using these elastic cymbals, producing two bursts of clicks every time they flap their wings (O’Reilly et al., 2021).
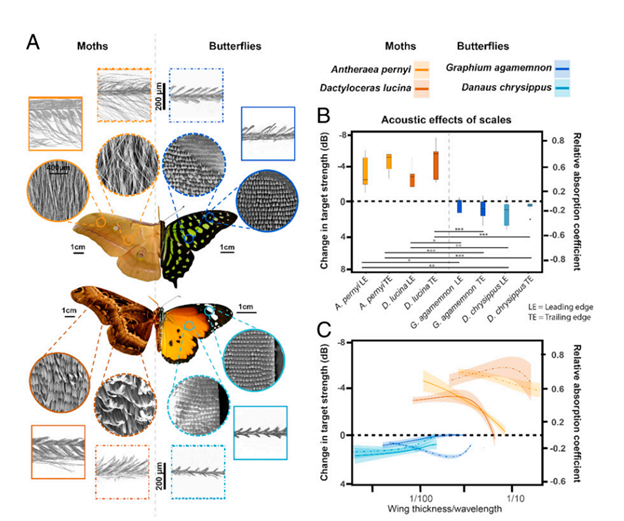
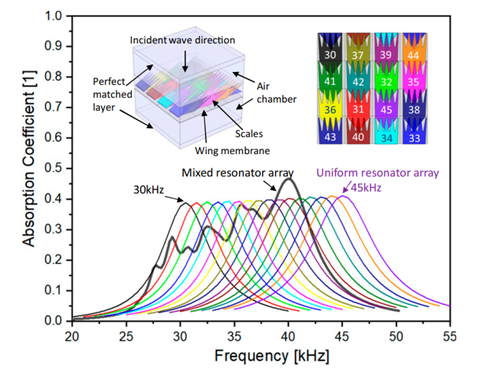
Finally, moths possess yet another adaptation to evade predation by bats: ultrasound absorbing wings. Moths possess wings capable of absorbing up to 72% of ultrasound emissions, effectively camouflaging them against the ultrasound radar of bats (Neil et al., 2020). These wings are made up of an array of scales placed upon a flexible membrane (Fig. 8A) that each function as resonant absorbers. Resonant absorbers are made up of a solid plate with a tight air space behind it and become extremely efficient at absorbing a specific frequency of sound when it matches the resonance frequency of the plate. This property allows resonant absorbers to be powerful sound dampeners without requiring thick material. This advantage is extremely important for moths, as they can easily incorporate sound dampening into their wings without sacrificing flight ability. However, the main drawback of resonant absorbers is that they are only effective at specific frequency ranges. Moths, on the other hand, must defend themselves against a large variety of bats, who use a variety of frequencies for sensing and detection of prey. As a result, while individual moth scales function as resonant absorbers, the moth wing is capable of dampening sound from a broad range of ultrasound frequencies by using an array of differently tuned resonant absorbers (Fig. 9). Each scale is tuned to a different frequency by varying parameters such as length, width, stalk stiffness, and angle of insertion into the wing membrane. This arrangement is even able to achieve greater sound absorption than the sum of its parts because of the way the differently tuned scales interact with each other. Sound absorption technologies will be able to benefit greatly from a similar approach (Neil et al., 2020).
Conclusion
The concept of the evolutional arms race provides a lens through which the impressive abilities and structures present in nature can be explained. The symmetrical arms race between species of rhinoceros beetles has resulted in their specialized horns. Each species’ horn has adapted over generations to best resist the loads that occur due to their distinct fighting styles. However, while longer horns would give the beetles an advantage, their growth will reach an upper bound due to a mechanical limit. Bighorn sheep also engage in a symmetrical arms race concerning combat for the chances of mating. The ram’s horns can withstand large forces in impacts a fraction of a second long by dissipating loads to the sheath to the horn core, as well as dissipate energy through oscillations and microtubule compression in the keratin sheath. In the asymmetrical arms race between bats and moths, the two animals continually evolve to stay ahead of the other. Moths develop ears or methods to jam the ultrasounds generated by bats to search for them, while bats adapt by altering the frequency and amplitude of their calls to remain undetected to the moths. As explored through multiple examples of both intraspecific and interspecific competition, animals require exemplifying peak mechanical fitness within their environment to stand the best chance at continuing their genetic lineage.
References
Corcoran, A. J., Barber, J. R., Hristov, N. I., & Conner, W. E. (2011). How do tiger moths jam bat sonar? J Exp Biol, 214(Pt 14), 2416-2425. https://doi.org/10.1242/jeb.054783
Dawkins, R. (1986). The blind watchmaker. Norton & Company, Inc.
Drake, A. M. (2015). Dynamic Structural Analysis of Ramming in Bighorn Sheep [Master’s thesis, Colorado State University]. mountainscholar.org.
Feldman, C. R., Brodie, E. D., Brodie, E. D., & Pfrender, M. E. (2010). Genetic architecture of a feeding adaptation: garter snake (Thamnophis) resistance to tetrodotoxin bearing prey. Proceedings of the Royal Society B: Biological Sciences, 277(1698), 3317-3325. https://doi.org/doi:10.1098/rspb.2010.0748
Fisher, R. (1958). The Genetical Theory of Natural Selection (2nd ed.). The Clarendon Press.
Geist, V. (1966). The Evolution of Horn-Like Organs. Behaviour, 27(3/4), 175-214. http://www.jstor.org/stable/4533157
Hanifin, C. T. (2010). The Chemical and Evolutionary Ecology of Tetrodotoxin (TTX) Toxicity in Terrestrial Vertebrates. Marine Drugs, 8(3), 577-593. https://www.mdpi.com/1660-3397/8/3/577
Huang, W., Zaheri, A., Jung, J. Y., Espinosa, H. D., & McKittrick, J. (2017). Hierarchical structure and compressive deformation mechanisms of bighorn sheep (Ovis canadensis) horn. Acta Biomater, 64, 1-14. https://doi.org/10.1016/j.actbio.2017.09.043
Iwasa, Y., Cohen, D., & Leon, J. A. (1985). Tree height and crown shape, as results of competitive games. Journal of Theoretical Biology, 112(2), 279-297. https://doi.org/https://doi.org/10.1016/S0022-5193(85)80288-5
Jwanamaker. (2013, June 29). File:New Mexico Bighorn Sheep.JPG. Wikimedia Commons. Retrieved October 6, 2021, from https://commons.wikimedia.org/wiki/File:New_Mexico_Bighorn_Sheep.JPG
Kitchener, A. (1988). An analysis of the forces of fighting of the blackbuck (Antilope cervicapra) and the bighorn sheep (Ovis canadensis) and the mechanical design of the horn of bovids. Journal of Zoology, 214(1), 1-20. https://doi.org/https://doi.org/10.1111/j.1469-7998.1988.tb04983.x
Larson, A. (1996). Evolutionary Transitions: Tempo and Mode in Evolution. Science, 271(5252), 1075-1075. https://doi.org/doi:10.1126/science.271.5252.1075
Lewanzik, D., & Goerlitz, H. R. (2018). Continued source level reduction during attack in the low-amplitude bat Barbastella barbastellus prevents moth evasive flight. Functional Ecology, 32(5), 1251-1261. https://doi.org/https://doi.org/10.1111/1365-2435.13073
McCullough, E. L. (2014). Mechanical limits to maximum weapon size in a giant rhinoceros beetle. Proceedings of the Royal Society B: Biological Sciences, 281(1786), 20140696. https://doi.org/doi:10.1098/rspb.2014.0696
McCullough, E. L., Tobalske, B. W., & Emlen, D. J. (2014). Structural adaptations to diverse fighting styles in sexually selected weapons. Proceedings of the National Academy of Sciences, 111(40), 14484-14488. https://doi.org/doi:10.1073/pnas.1409585111
Nat Geo WILD. (2018, January 5). These Rams Go Head to Head – Literally | Animal Fight Night [Video]. Youtube. https://www.youtube.com/watch?v=5NTvMDEyDpg
Neil, T. R., Shen, Z., Robert, D., Drinkwater, B. W., & Holderied, M. W. (2020). Moth wings are acoustic metamaterials. Proceedings of the National Academy of Sciences, 117(49), 31134-31141. https://doi.org/doi:10.1073/pnas.2014531117
O’Reilly, L. J., Harris, B. J., Agassiz, D. J. L., & Holderied, M. W. (2021). Convergent Evolution of Wingbeat-Powered Anti-Bat Ultrasound in the Microlepidoptera. Frontiers in Ecology and Evolution, 9. https://doi.org/10.3389/fevo.2021.648223
Parker, G. A. (1979). Sexual Selection and Sexual Conflict. In M. S. Blum & N. A. Blum (Eds.), Sexual selection and reproductive competition in insects (pp. 123-166). Academic Press. https://doi.org/https://doi.org/10.1016/B978-0-12-108750-0.50010-0
Pomiankowski, A., & Iwasa, Y. (1998). Runaway ornament diversity caused by Fisherian sexual selection. Proceedings of the National Academy of Sciences, 95(9), 5106-5111. https://doi.org/doi:10.1073/pnas.95.9.5106
Ratcliffe, J. M., Fullard, J. H., Arthur, B. J., & Hoy, R. R. (2011). Adaptive auditory risk assessment in the dogbane tiger moth when pursued by bats. Proceedings of the Royal Society B: Biological Sciences, 278(1704), 364-370. https://doi.org/doi:10.1098/rspb.2010.1488
Vander Linden, A., & Dumont, E. R. (2019). Intraspecific male combat behaviour predicts morphology of cervical vertebrae in ruminant mammals. Proceedings of the Royal Society B: Biological Sciences, 286(1915), 20192199. https://doi.org/doi:10.1098/rspb.2019.2199
Waters, D. A. (2003). Bats and moths: what is there left to learn? Physiological Entomology, 28(4), 237-250. https://doi.org/https://doi.org/10.1111/j.1365-3032.2003.00355.x