Mathematical Analysis of Amoebas
Hailey Jukes, Adele Omichinski, Nicholas Da-Costa-Bastidas, Gil Qin
Abstract
Despite lacking a conventional nervous system as in more complex organisms, amoebae of various kinds display signs of “intelligence” in their high adaptability to the environment and their problem-solving abilities, which have fascinated scientists for many years. In this essay, we discuss various examples of amoeboid intelligence, such as their maze-solving and network-building capabilities. It has been discovered that amoebae of the species Physarum Polycephalum can exchange information by fusing with other members of their species. This ability inspires the construction of algorithms for solving complex combinatorial optimization problems, which are examined in a later section. In addition, another example of algorithm modeling the motility of an amoeboid cell in the presence of positive and negative stimuli using swarm optimization is presented to further illustrate the rudimentary mental structure of the unicellular amoeba. Finally, amoebae are intriguing not only because of their primitive computational abilities and their ability to effectively respond to changes in their environment, but also because of the precise geometric formations of the crystalline inclusions they contain. Various facets of these inclusions are studied in detail in the final section of the paper.
Introduction
Physarum Polycephalum: background and life cycle
Physarum polycephalum, literally meaning the “multiheaded” slime mold, is a multi-nucleated unicellular protist belonging to the myxomycetes, or acellular slime molds (Dove et al., 1986). Among slime molds, it is classified as a “true” or acellular slime mold due to its distinctive life stage known as the plasmodium (Figure 1). The plasmodium has a syncytial structure, which means it is a single, vast cell that contains millions of diploid nuclei. Even though the plasmodium can grow very large, sometimes covering an area over 900 cm2, they are still considered as a unicellular organism since there are no membranes separating the nuclei (Beekman & Latty, 2015).

Figure 1: Image of a Physarum polycephalum plasmodium growing on a tree bark (Wikimedia Commons).
The migrating plasmodium of an acellular slime mold, such as Physarum polycephalum, showcases a unique behavior. Initially, the cell extends an elongating fan-like sheet at its leading edge. This is followed by the formation of an intricate network of interconnected veins, through which the cytoplasm of the cell flows rhythmically back and forth. By establishing strategic networks of veins that interconnect at various regions, the slime mold can circulate chemical signals as well as acquire nutrients from different areas simultaneously. This organism is also able to efficiently navigate its environment by extending pseudopods (projections of cytoplasm) (Briard et al., 2020).
However, under the combination of nutritional stress and light exposure (light being a stress factor), vegetative growth of the plasmodium is halted (Beekman & Latty, 2015). Instead, the plasmodium forms sexual structures, leading to the formation of haploid spores. These spores will germinate to form microscopic haploid myxamoebae, which feed on bacteria and undergo mitotic cell division. Myxamoebae of different types can fuse to form binucleate cells which will further fuse to yield diploid zygotes. These diploid cells then differentiate into plasmodial cells, restarting the life cycle (Beekman & Latty, 2015).

Figure 2: Life cycle of Physarum polycephalum (Carolina Knowledge Center).
The plasmodium life stage of P. polycephalum distinguishes it from “cellular” slime molds, such as Dictyostelium discoideum. In fact, cellular slime mold exists as individual haploid amoebas during the vegetative state of their life cycle. In nutrient rich environments, these amoebas move independently where they preferably feed on bacteria. However, when faced with nutrient depletion, the individual amoeba aggregate, forming a multicellular “slug”. The slug will then crawl toward light and warmth before further developing into a fruiting body hosting stalk and spore cells (Oeittmeier et al, 2017).
Exploring intelligent behaviors in amoeboid organisms: a case study of Physarum Polycephalum
Although complex behaviors in living organisms are generally associated with the presence of a nervous system, the special case of Physarum polycephalum challenges this belief. Despite lacking a brain and a central nervous system, this slime mold displays a diverse array of sophisticated behaviors that have even been occasionally deemed “intelligent” by scientists (Alim et al., 2017). Examples of the sophisticated behaviors exhibited by P. polycephalum include having the ability to adeptly navigate through mazes by finding the shortest route (Nakagaki et al., 2000), constructing networks with an efficiency rivaling that of human-designed analogues (Tero et al., 2010), solving computationally challenging puzzles in finite time, making nuanced multi-objective foraging decisions, and carefully balancing its nutrient intake (Beekman & Latty, 2015). These design solutions not only ensure the long-term survival and prosperity of the organism but also hold potential for the development of bio-inspired computational algorithms that can solve complex problems (Awad et al., 2023).
Maze solving by Physarum Polycephalum
In recent years, research on Physarum polycephalum has surged in popularity, driven largely by the ground-breaking experiment conducted by Nakagaki and colleagues in 2000. This experiment challenged conventional notions regarding the cognitive abilities of organisms that do not possess a central nervous system. These researchers demonstrated that Physarum polycephalum have the remarkable ability to find the minimum-length solution between two points in a labyrinth (Awad et al., 2023).
To investigate the maze-solving behavior of Physarum polycephalum, the researchers started by extracting a growing tip from a large plasmodium culture (25 x 35 cm). The growing tip was divided into smaller pieces, which were then strategically dispersed throughout a maze constructed of plastic film on an agar surface. The researchers observed that the plasmodial pieces extended and eventually merged to form a single organism that had the ability to efficiently navigate the maze while avoiding the dry film surface (see Figure 3a). Once the slime mold filled the maze, the organism was presented with two sets of two nutrient-containing agar blocks (0.5 x 1 x 2 cm blocks each containing 0.1 mg g-1 of ground oat flakes). Within each set, the two food sources were placed at the start and end points of the maze, thereby creating four potential routes for the slime mold to connect food sources (a1, a2, b1 and b2 in Figure 3a) (Nakagaki et al., 2000).
In the maze, when the plasmodium’s pseudopodia reached a dead end, they initiated a shrinkage process (see Figure 3b). This shrinkage resulted in the formation of a consolidated path – a single thick pseudopodium that spanned the minimum distance between nutrient-containing blocks (see Figure 3c). Although the exact length and position of the pseudopodium varied in each experiment, the a2 path, which is 22% shorter than the a1 path, was consistently favored over the a1 path. In contrast, due to a mere 2% difference in length between the b1 and b2 path, a comparable number of tubes formed through each path (see Figure 3d) (Nakagaki et al., 2000).

Figure 3: a) Organism structure before pathfinding: The image highlights the initial state of the P. polycephalum’s plasmodium inside the maze before the introduction of the food sources. The blue lines represent the shortest routes between the two sets of agar blocks, labelled as α1 (41 ± 1mm), α2 (33 ± 1mm), β1 (44 ± 1mm) and β2 (45 ± 1mm). b) Organism structure during the exploration stage: Following the placement of the agar blocks (AG), the terminal point of the plasmodium recedes, and pseudopodia extend to explore all potential connections within the environment. c) Organism structure four hours after agar block addition: After the exploration period, the plasmodium has identified and strengthened the shortest path. d) Overview of the path selection frequency: A table compiling the frequencies of each pathway selection. “None” signifies instances where no tubes were extended (Nakagaki et al., 2000).
The results from Nakagaki’s experiment demonstrate how the plasmodium can dynamically adapt its shape within the maze, to create a singular, substantial tube that efficiently covers the shortest distance between two food sources (Beekman & Latty, 2015). Nevertheless, a fundamental question remains unanswered: How does this seemingly simple organism execute such a complex task in the absence of a nervous system? It is not so trivial to explain complex behaviors, such as maze solving, in P. polycephalum. However, Alim and colleagues propose an attractive, yet simple hypothesis, which is based on a feedback loop between a signaling molecule and a propagating contraction front (Alim et al., 2017).
The researchers conducted an experiment to observe how P. polycephalum network formation responds to a nutrient stimulus (nutritive liquid). When P. polycephalum forages for food, it constructs a network of tubes with a gel-like outer layer that houses an actin-myosin cytoskeleton which induces periodic contractions. In response to attractants or repellants, the organism produces corresponding changes in contraction amplitude and frequency. In the case of an attractant, the slime mold responds by increasing both the amplitude and the frequency of contractions throughout the organism, whereas in the presence of a repellent, it responds by decreasing the amplitude and frequency of the contractions (Alim et al., 2017).
By utilizing bright-field microscopy, the researchers were able to monitor the network’s behavior pre- and post-stimulation over a period of 2 to 3 hours. Upon exposure to the nutritive liquid, immediate changes in tube contraction were observed (see Figure 4a). The response began with a localized inflation of tubes directly exposed to the food source, followed by a uniform reduction in tube volume elsewhere due to the fixed amount of fluid within the network (Alim et al., 2017).
Analysis of wave patterns revealed that there was an increase in oscillation amplitude at the stimulation site (see Figure 4b and 4c). The change in amplitude was subsequently propagated along the adjacent tubes at an average speed of approximately 13 mm/s. Interestingly, the spread of this change in amplitude was not symmetrical around the stimulation site and the velocity of the amplitude front varied between 1 and 20 mm/s across the different-sized tubes (see Figure 5b). In fact, tubes with larger radii transmitted the change more quickly than tubes with smaller radii (Alim et al., 2017).
This radially asymmetrical increase in contraction amplitude around the stimulus site suggests a basic feedback mechanism. In this feedback mechanism, an initial stimulus, such as a food source, triggers the release of a signaling molecule. This signaling molecule subsequently triggers local wall contractions, leading to an increase in local fluid flow. In turn, the increased fluid flow causes the dispersion of the signaling molecule, creating a downstream signal. The signaling molecule repeatedly triggers local wall contractions and is gradually pushed away from its original source, generating a self-propagating front that travels throughout the entire organism (Alim et al., 2017).


Figure 4: A) Bright-field microscopy images depicting the P. polycephalum’s plasmodial network before (- 3 s; far-left image) and after stimulation with the nutritive liquid (green arrow, added at 0 s.). B) Contraction amplitude recorded at the same time points as in A). The stimulation with the nutritive liquid is represented by a black arrow. The hot colors in the network (yellow, red) represent the amplitude fronts with the larger relative amplitudes, which spread through the tubes with larger radii. C) Dataset of the amplitude fronts and particle speeds extracted from bright-field microscopy. (i) Location of particle speed are indicated by blue arrows and the measured amplitude front in the tube is indicated in purple. (ii) Three-line graphs illustrating the contraction patterns at three different points in the plasmodium, which are progressively farther away from the stimulation site. The doted red line indicates a sudden change in contraction amplitude. (v) Bar graph illustrating the maximal particle speeds (blue) indicated by the blue arrows in (i) and the average front propagation speed (red) along the purple trajectory shown in (i) (Alim et al., 2017).
The researchers also compared the velocity of the amplitude front observed in P. polycephalum to the velocity typically associated with the propagation of action potentials. Action potentials propagate at approximately 2.7 mm/s, which means their propagation is at least 100-fold faster than the signaling molecule-induced propagation in P.polycephalum (see Figure 5a). Therefore, it can be concluded that the networks built by this slime mold are analogous to the system of neurons in mammals, which would represent a later evolutionary achievement. However, the slime mold demonstrates how a living organism can generate sophisticated behaviours using limited biological hardware. This allows the organism to optimize its network of tubes as a design solution to thrive in its environment (Damm, n.d.).

Figure 5: a) Bar graph comparing the observed signal propagation speeds of elastic waves (pink), action potentials (blue) and P. polycephalum (green). b) Correlation graph demonstrating the relationship between a larger tube diameter and an increase in amplitude front. The data for the tube diameter and amplitude front were taken along a network of tubes where the center was stimulated by the nutritive liquid (Alim et al., 2017).
Using Physarum Polycephalum to solve the steiner tree problem
As mentioned above, one of the fascinating characteristics of P. Polycephalum is that it is able to solve computationally intensive problems in logistically acceptable amounts of time. Such problems often fall under the umbrella of combinatorial optimization, a branch of optimization which deals with finding the best solution for a problem out of a finite set of solutions. Examples of problems in combinatorial optimization include the travelling salesman problem, the minimum spanning tree problem, and the Steiner tree problem. These problems, although often formulated abstractly, find many real-world applications in challenges involving optimal network building (Steiner Tree Problem, 2015).
We will be concerned with the Steiner tree problem to showcase P. Polycephalum’s ability to compete with cutting edge computing technologies. This problem deals with connecting a subset of points or “vertices” within a general set of points or “vertices”. In Figure 6 below, the general set of points is all the points, both blue and yellow, and the yellow points are the subset of points we are seeking to connect. These yellow points are known as terminal vertices.

Figure 6: A set of points used in a Steiner tree problem (Steiner Tree Problem, 2015).
Note that the branches connecting the vertices together are each assigned a value. This denotes the weight of that branch, i.e., of the “cost” of connecting the two vertices bridged by that branch. This concept lies at the core of the question defining the Steiner tree problem: what is the minimum weight path or tree connecting all the terminal vertices?
For the set of points above, although it might take a bit of evaluation and trial-and-error, the minimum weight Steiner tree connecting the yellow points (also known as the Steiner minimum tree or SMT) can be found with relative ease, and it is represented in Figure 7.
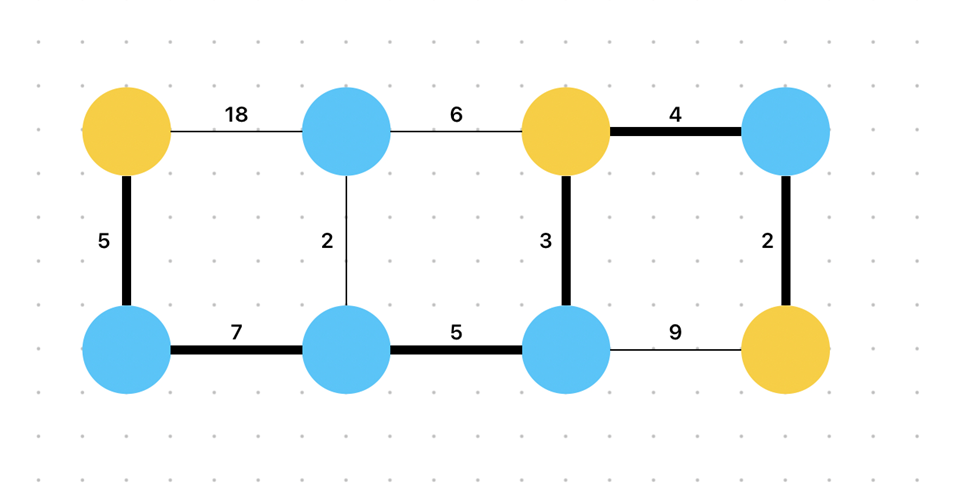
Figure 7: A set of points used in a Steiner tree problem, with a bolded line representing the SMT. This tree has cost 26 (Steiner Tree Problem, 2015).
As one might imagine, the Steiner tree problem becomes much harder as the number of terminal vertices we are seeking to connect increases. In fact, the problem becomes so hard so fast that it is sometimes almost impossible to compute the SMT. Indeed, the Steiner tree problem is an NP-hard problem, meaning it is not solvable in polynomial time (in other words, it is not solvable in any reasonable amount of time, even by a computer). For this reason, people have only developed algorithms that can approximate a solution to the problem within a certain margin of error (Hsu et al., 2022).
The variation of the Steiner problem we will discuss is the Euclidean Steiner tree problem. In this variation, the set of points to be connected is contained on a plane in space, and the weight of each branch corresponds to its length. Thus, the challenge is now to find the shortest path that connects all terminal vertices.
This is where P. Polycephalum comes in. This organism is known for being able to find optimal ways to connect food sources separated on a surface in order to facilitate nutrient transport. This is reminiscent of the Euclidian Steiner tree problem, and indeed researchers have worked to model the amoeba’s behavior to try and find novel solutions to the problem.
One important talent of P. Polycephalum is its ability to fuse with other members of its species. It has been shown that when multiple amoebae fuse together, their individual networks are rearranged in order to optimize the expanded network of food sources now contained in the single bigger amoeba (Hsu et al., 2022). This is fascinating, as P. Polycephalum does not have a nervous system, yet it seems as if upon fusion the individual organisms somehow share the information each one collected on its own and reorganize themselves consequently. The mechanisms underlying this phenomenon are currently being studied but remain unknown.
Several problem-solving algorithms trying to solve the Steiner tree problem have been developed based on Physarum using systems of equations (Hsu). More recently, researchers have been able to build a feature analogous to cell fusion into Physarum-based problem-solving algorithms, despite the details of information transport via cell fusion remaining a mystery. One such model is proposed by Hsu et al.
They make use of several simulated Physarum cell-like agents spread out over an area containing “active zones”, the areas we want to connect. The area is grid-like, meaning everything inside it is modelled using squares, similarly to an image composed of many small pixels. Over every iteration of their algorithm, what they call a one square-sized “bubble” is created in the cytoskeleton of the Physarum models. These bubbles correspond to a sort of hole in the organism, which moves in a certain direction by swapping spaces with the tiles next to it. In order to form a Steiner tree, the researchers set that the bubbles can either appear at an active zone which the cell is already touching, or at a random point in the cytoskeleton. The former option leads to the accumulation of cytoplasm at the active zones as the bubble moves outwards, a desirable effect mirroring the behavior of Physarum in nature, and the latter option allows for a certain degree of randomness in the movement of the model. This randomness is desirable because it allows the model to explore more of the area it is in and thus hopefully find more active zones. However, the amount of randomness in the algorithm is purposefully set to decrease as the different simulated cells fuse and more active zones are discovered, so that the model can switch from a “foraging” mode to a “shrinking” mode, which leads to the shortening of paths and the identification of an SMT. Figure 8 below shows the progression of an iteration of the algorithm.

Figure 8: Progression of the algorithm, from (a) to (i).
In terms of results, after analyzing many iterations of their algorithm across different initial conditions and constraints, the researchers declare that its time complexity competes with the currently most used Steiner tree problem algorithms such as SCIP-Jack (Hsu et al., 2022). Furthermore, it performs just as well on the obstacle-avoidance Euclidean Steiner tree problem, a variation of the problem where certain areas of the plane must be avoided. This is not common, as most algorithms experience much more difficulty with this variation. This is relevant as in many practical applications, there are constraints dictating that certain areas must be avoided, such as mountains or impracticable terrain when designing a railway system for example.
To reiterate, Steiner tree problems can be found in many real-world challenges, such as the design of transportation systems, communication networks, or even electronic chips (Tero et al., 2010). Thus, P. Polycephalum’s ability to solve them could potentially inspire solutions ranging across many disciplines beyond what current technology and computing can yield.
A model of amoeba motility with swarm motion
Swarm intelligence refers to the collective behaviors of individual entities or agents that interact with each other and with the external environment to solve complex problems. Through decentralized decision-making, autonomous agents operate in parallel under in a coordinated manner to achieve a common goal without a centralized controller (Joseph et al., 2023). This characteristic of decentralization in swarm intelligence allows the emergence of global behaviors not necessarily predictable from the properties of individual components, enables optimized performance, and gives rise to high adaptability of the system and effective response to changing conditions in the environment.
Schumann et al. proposed an intricate method to model the motility of a single Amoeba Proteus drawing inspiration from the Particle Swarm Organization (PSO) algorithm proposed by Kennedy and Eberhart and a similar algorithm developed by Reynolds. PSO is an effective algorithm that simulates the complexity of swarm behavior inspired by the social behaviors of flocks of birds and fishes (Kennedy & Eberhart, 1995). It is applied to solve optimization problems and involves a population of agents finding optimal solutions in a search space. Over subsequent iterations, particles adjust their positions – resulting in swarm motion – to converge towards the optimal solution. In Kennedy and Eberhart’s algorithm, two key assumptions are made: (1) each particle of the swarm is aware of its best position found through its individual experience, known as local best, and (2) each particle is aware of the global best, or the best position in the neighborhood discovered by any particle in the entire swarm. The position of particle i, denoted by xi(t), at discrete time step t, is modelled by:
![]()
where the next position is determined by adding a velocity to the current position. The velocity is determined by:
![]()
where c1 and c2 are accelerating coefficients that act as scaling factors to represent the influence of the local best and the global best on how the particle should adjust its velocity. r1 and r2 are random values that introduce stochasticity to the velocity update equation. The regeneration of random values in each iteration ensures diversity in the movement of particles, allows continuous exploration of search space not only limited to historical best positions, improves the chances of finding the optimal solution, and prevents the quick convergence of particles to a suboptimal solution. Therefore, the terms ![]() and
and ![]()
can be viewed as forces acting on the particle to drive its motion to positions that have been historically successful for the individual and the swarm, respectively.
The amoeboid cell is a self-organized system and the reactions of Amoeba Proteus in the presence of external stimuli, whether attractants or repellents, demonstrate self-organization (Schumann et al., 2021). A self-organized process involves the spontaneous emergence of order and structure in a system without external guidance and is a critical characteristic in a system exhibiting swarm intelligence. The amoeba shows diverse responses when encountered with a mixture of attractant and repellents where the behaviors of the plasmodium cannot be explained by a simple stimulus-response model. In addition, the reactions of the amoeba in response to stress and safety conditions showcase the amoeba’s exemplification of a proto-psychic structure, implying that amoeba exhibit characteristics reminiscent of primitive psychic processes.
The deformation of the elastic membrane of the amoeba is caused by active assembly and disassembly of actin filaments in the presence of stimuli. In the algorithm developed by Schumann et. al, they viewed regions of active actin assembly and disassembly in the amoeba as particles or agents as a response to external stimuli. Together, these active zones display swarm-like behavior and operate coordinately to change the behaviors of actin waves across the cell membrane. This means that each active zone is considered as a self-organized agent and they collectively coordinate the response of the organism in the presence of stimuli, resulting in the emergence of a set of diversified reactions of the amoeba when faced with attractive and/or repelling stimuli. Each agent or active zone can be identified by
![]()
where Ai denotes the agent and riA represents the corresponding position of the agent. Similarly, to model amoeba reaction to external stimuli, let
![]()
identify an external signal where Xj is either an attractant Atj or a repellent Rpj and rjX is the radius associated with the external signal that defines its effective range or influence. Additionally, each Xj is associated with an interaction coefficient χj that is a positive value less or equal to one if the stimulus is attractive, and a negative value greater or equal to negative one if the stimulus is repulsive.
A potential function describes the potential energy associated with an agent. The overall potential function can be described by the relation:
![]()
where
![]()
represents the potential energy associated with the spatial relationship between the position of the active zone Ai and the position of the stimulus Xj. In addition, the overall potential of the system is also dependent on χj, which takes into consideration of the attractive or repulsive strength of the stimulus.
The gradient or the rate of change of the potential describes the forces – whether attractive force from attractants or repulsive force from repellents – acting on the agent since force is the negative gradient of potential, given by:
![]()
Therefore, the force on the active zone Ai imposed by stimulus Xj is given by:
![]()
this means that the force FijA is proportional to the gradient of the potential function and the strength of the stimulus. To dissect the formula further, ![]() ,
,
describes how the potential changes with respect to changes in the position of the active zone relative to the external signal. Because the gradient describes the rate of change of the potential, the force associated with the gradient, ![]() ,
,
is directed from regions of higher potential to lower potential, which means that the force is aiming to move the active zone towards the position where the potential function V decreases most rapidly. It is worthwhile to note that the negative sign in the formula accounts for the fact that the force always acts in the opposite direction of the potential gradient. Therefore, given a set of m external signals, identified by ![]() ,
,
the resulting force on each particle can be found by:
![]()
which allows scientists to construct a model for the mechanism of interaction between amoeba and external signals, taking into consideration of the coordinated swarm motion of areas of active actin polymerization and depolymerization across the amoeboid cell.
Through modeling the complex and adaptive swarm-like behaviors of the system of amoeba in response to external stimuli, the improved understanding of collective behavior can be applied to develop more efficient optimization techniques and neurocomputing research. The simulation of amoeba motility effectively showcases its potential to create advanced algorithms with efficient problem-solving capabilities that draw upon the principles of collective intelligence.
Cytoplasmic crystal inclusions of the general amoeba
While specific amoebae use mathematical concepts behaviorally, studies also show evidence of the organisms using them structurally. The amoeboid cytoplasm is a very crowded place, containing various organelles, freely diffusing proteins, and storage vacuoles. It also includes curious inclusions that are not used in metabolism, as energy sources or as sites of energetic transformation (Daniels & Breyer, 1968). Notably, amoebae contain crystalline granules, referred to as amoeboid crystals. It was originally hypothesized that these crystal structures were used as sources of energy during periods when the organism experienced starvation, however studies have shown that energy sources drawn upon by starved amoebae are proteins and lipids.
The cytoplasmic crystals are instead composed of nitrogenous waste products; mainly triuret, also known as carbonyl diurea. They appear to be waste deposits for the organism. In support of this finding, the number of crystals appears to have a positive linear relationship with the age of the amoeba; older amoebae have more cytoplasmic crystals, whereas younger ones show proportionately smaller inclusions (Taylor, 1962). As the amoeba ages, it produces more and more waste, therefore the amount stored in the cytoplasm increases (Luce & Pohl, 1935).
Additionally, the crystals are a distinguishing marker between herbivorous and carnivorous amoebae, as they are noticeably lacking in the herbivorous species (Griffin, 1960). This is likely due to the chemical differences concerning herbivorous and carnivorous digestion material.
Amoeboid crystals appear in the organisms in two specific shapes: the bipyramid and the plate. Plate-like crystals are resemblant of snowflakes, and often have a greater surface area than volume due to their flat nature. They are characterized by their flat nature, as they are significantly wider than they are tall (see Figure 9) and they are often much smaller and less developed than the bipyramidal crystals.

Figure 9: An example of a plate-like crystal, sometimes seen in the amoeboid cytoplasm (Prywer and S. Krukowski, 1998).

Figure 10: A bipyramidal crystal, typically found in the amoeba cytoplasm (Grunbaum et al., 1959).
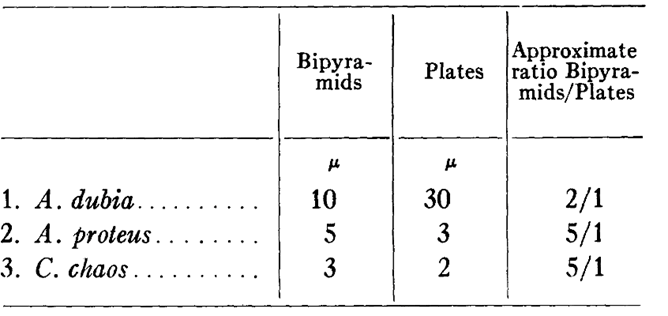
It is hypothesized that the shape that the crystals adopt could depend on the nature of the chemical compound that was metabolized by the amoeba (Griffin, 1960). As shown in Table 1, a preference is shown for bipyramidal crystal structure. This may be due to the greater storage stability of the bipyramidal shape; the crystal lattice is more developed in these structures, and they have a more optimal surface area to volume ratio (see Figure 10). Therefore, they are more storage efficient per unit space in the amoeba and are likely more resistant to degradation. Additionally, it may be due to a tetragonal form of triuret molecules, as seen in Figure 11, preferentially produced as a waste product when it is possible for the amoeba. Thus, it is evident that the preferred structure, specifically the surface area, angles, and 3D shape in general, is an alternative design displayed by the organism.

Figure 11: Diagram of a tetrahedral molecular structure, typically seen in the triuret molecules forming bipyramidal amoeboid crystals (Britannica, n.d.).
Table 1: Relative abundances of crystal inclusion types in amoeboid species A. dubia, A. proteus, and C. chaos (Griffin, 1960).
The characteristics of amoeboid bipyramidal crystalline inclusions are the most prominent and most well studied. The density of these crystals at normal physiological temperatures is 1.74 g/mL, allowing the amoeba to effectively store more nitrogenous waste per unit area than if it were left to freely exist in the cytoplasm (Grunbaum et al., 1959) (Carlström & Møller, 1961). For size reference, they are about 7.00 Å (angstroms) in width, and 13.68 Å in height (Grunbaum et al., 1959).
The angle between the top and side faces of the crystals (see Figure 10) is 27° whereas the larger angle between side faces is 153°. They are about 7.00 Å (angstroms) in width, and 13.68 Å in height on average.
Growth patterns of the crystals appear to occur in a layering fashion; the top and bottom faces of the crystal accumulate more triuret, and the crystal grows lengthwise (Carlström & Møller, 1961).
This allows for uniform growth and works to provide stress or fracture lines in the crystals. If the amoeboid cytoplasm is subject to high stress forces, the crystalline layers can break off uniformly into layers, maintaining their functionality as storage units (Carlström & Møller, 1961). This is another hypothesized explanation for the presence of the thinner, plate-like crystals seen in the organism (Griffin, 1960).
While these studies show hypothetical ways through which amoebae use mathematical optimization to store their nitrogenous waste, studies concerning the amoeboid crystal are still very preliminary (Bernheimer, 1938).
Cubic membrane formation for oxidative damage defense
The amoeba is a eukaryotic organism; therefore, it contains numerous membrane organelles optimized for its survival and proliferation. This includes the cellular powerhouse, the mitochondria. As mitochondria are the site of oxidative phosphorylation to create ATP, they are subjected to high amounts of reactive oxygen species (ROS), and thus oxidative damage. Research on amoeboid mitochondria has revealed an adaptation of their cristae (Deng et al., 1999). Amoebae appear to form cubic mitochondrial cristae, depicted in Figure 12.

Figure 12: Visualization of cubic phospholipid organization present in the inner mitochondrial membrane of amoebae (Landau & Rosenbusch, 1996).
The cubic membrane structures are highly constrained and ordered and cause high condensation of the matrix in these areas (Deng et al., 1999). Their formation is a special design solution to protect against oxidative stress; the cubic structure promotes increased leakage of H2O2 and ROS (Kong et al., 2020). It also reduces the membrane’s sensitivity to oxidants, due to its closely packed and constrained structure.
This information is supported by experiments involving exposure of amoebae to high levels of oxidative stress. Results showed dramatic structural changes occur in the inner mitochondrial membrane under high stress. The membrane adopts cubic morphology almost exclusively, in order to protect itself (Deng et al., 2002).
The downside of this structural morphology is that it appears to be negatively correlated with ATP production. Therefore, amoebae have adapted to utilize it only when necessary (Chong et al., 2018).
Cubic membrane structures are symmetrical, well defined, and three dimensional. Uniquely, they show 3D periodicity, or the reoccurrence of the same 3D structure at intervals (Almsherqi et al., 2006).
Conclusion
As single-celled, eukaryotic organisms, amoebae have adapted to display various mathematical concepts; both within their interactions with their environments, and their methods of carrying out life processes. The “intelligence” of these protists has allowed them to solve computational problems. Firstly, as seen through the maze solving abilities of amoeba species Physarum polycephalum, these organisms can optimize their size and shape to cover the maze distance most efficiently – consistently showing a preference for the shortest path length. They do so using specifically directed reduction and inflation of their morphology, transducing signals in ways that mimic the neurological transmission seen in the brains of higher-order organisms. This design solution allows them to span areas more efficiently and quickly, to get where they need to go, whether that be applied to nutritive purposes or to fulfill other survival requirements. Amoebae also show other problem-solving behaviours, concerning combinatorial optimization. The organism displays the ability to solve Steiner tree problems through its knack for connecting itself to other members of the species and form a farther-reaching network. In the presence of external stimuli, each individual organism continuously optimizes its response over historical iterations. By modeling each organism of amoeba as a swarm of areas of active polymerization and depolymerization across the cell membrane, researchers aim to simulate the emergent proto-psychic structure of the amoeba that provides the organism with high adaptability to environmental changes. Not only do amoeba achieve optimal positions or behaviours through their movement, but also in their internal structures.. Small crystal inclusions within the amoeboid cytoplasm act as deposits of nitrogenous waste. These crystals are also optimized, for example, the volumetric shapes, and densities of the crystals are designed to take up the least amount of space while containing the largest amount of waste. Additionally, the amoeba can change the morphology of its inner mitochondrial membranes to cuboid structures in order to resist oxidative damage. Collectively, amoeba possess integrated mathematical principles into their behaviours, both intracellularly and extracellularly, to optimize their survival and proliferation.
References
Alim, K., Andrew, N., Pringle, A., & Brenner, M. P. (2017). Mechanism of signal propagation in Physarum polycephalum. Proc Natl Acad Sci U S A, 114(20), 5136-5141. https://doi.org/10.1073/pnas.1618114114
Almsherqi , Z. A., Kohlwein , S. D., & Deng , Y. (2006). Cubic membranes: a legend beyond the Flatland* of cell membrane organization. Journal of Cell Biology, 173(6), 839-844. https://doi.org/10.1083/jcb.200603055
Awad, A., Pang, W., Lusseau, D., & Coghill, G. M. (2023). A survey on physarum polycephalum intelligent foraging behaviour and bio-inspired applications. Artificial Intelligence Review, 56(1), 1-26. https://doi.org/10.1007/s10462-021-10112-1
Beekman, M., & Latty, T. (2015). Brainless but Multi-Headed: Decision Making by the Acellular Slime Mould Physarum polycephalum. Journal of Molecular Biology, 427(23), 3734-3743. https://doi.org/https://doi.org/10.1016/j.jmb.2015.07.007
Bernheimer, A. W. (1938). A Comparative Study of the Crystalline Inclusions of Protozoa. Transactions of the American Microscopical Society, 57(4), 336-343. https://doi.org/10.2307/3222488
Briard, L., Goujarde, C., Bousquet, C., & Dussutour, A. (2020). Stress signalling in acellular slime moulds and its detection by conspecifics. Philosophical Transactions of the Royal Society B: Biological Sciences, 375(1802), 20190470. https://doi.org/10.1098/rstb.2019.0470
Carlström, D., & Møller, K. M. (1961). Further observations on the native and recrystallized crystals of the amoeba Amoeba proteus. Experimental Cell Research, 24(2), 393-404. https://doi.org/https://doi.org/10.1016/0014-4827(61)90440-2
Chong, K., Almsherqi, Z. A., Shen, H.-M., & Deng, Y. (2018). Cubic membrane formation supports cell survival of amoeba Chaos under starvation-induced stress. Protoplasma, 255(2), 517-525. https://doi.org/10.1007/s00709-017-1169-x
Damm, P. (n.d.). Thinking While Brainless: Slime Mold Gives Insight Into The Intelligence of Neuron-Less Organisms. NJIT News. Retrieved December 1, 2023, from https://news.njit.edu/thinking-while-brainless-slime-mold-gives-insight-intelligence-neuron-less-organisms
Daniels, E. W., & Breyer, E. P. (1968). Starvation effects on the ultrastructure of amoeba mitochondria. Zeitschrift für Zellforschung und Mikroskopische Anatomie, 91(2), 159-169. https://doi.org/10.1007/BF00364307
Deng, Y., Kohlwein, S. D., & Mannella, C. A. (2002). Fasting induces cyanide-resistant respiration and oxidative stress in the amoeba Chaos carolinensis: implications for the cubic structural transition in mitochondrial membranes. Protoplasma, 219(3), 160-167. https://doi.org/10.1007/s007090200017
Deng, Y., Marko, M., Buttle, K. F., Leith, A., Mieczkowski, M., & Mannella, C. A. (1999). Cubic Membrane Structure in Amoeba (Chaos carolinensis) Mitochondria Determined by Electron Microscopic Tomography. Journal of Structural Biology, 127(3), 231-239. https://doi.org/https://doi.org/10.1006/jsbi.1999.4147
Dove, W. F., Dee, J., Hatano, S., Haugli, F. B., & Karl-Ernst Wohlfarth-Bottermann. (2012). The Molecular Biology of Physarum polycephalum. Springer Science & Business Media.
Frankenstoen. (2011). Українська : Плазмодій слизовика виду Physarum polycephalum. flickr. https://commons.wikimedia.org/wiki/File:Physarum_polycephalum_plasmodium.jpg#/media/File:Physarum_polycephalum_plasmodium.jpg
Griffin, J. L. (1960). The Isolation, Characterization, and Identification of the Crystalline Inclusions of the Large Free-Living Amebae. The Journal of Biophysical and Biochemical Cytology, 7(2), 227-234. http://www.jstor.org/stable/1603546
Grunbaum, B. W., Møller, K. M., & Thomas, R. S. (1959). Cytoplasmic crystals of the amoebae: Amoeba proteus and Chaos chaos. Experimental Cell Research, 18(2), 385-389. https://doi.org/https://doi.org/10.1016/0014-4827(59)90021-7
Hsu, S., Massolo, F. I. S., & Schaposnik, L. P. (2022). A Physarum-inspired approach to the Euclidean Steiner tree problem. Scientific Reports, 12(1), 14536. https://doi.org/10.1038/s41598-022-18316-3
Joseph, A. A., Nambiar, G. S., & Jayapandian, N. (2023, 1-3 June 2023). Swarm Intelligence Decentralized Decision Making In Multi-Agent System. 2023 8th International Conference on Communication and Electronics Systems (ICCES),
Kennedy, J., & Eberhart, R. (1995, 27 Nov.-1 Dec. 1995). Particle swarm optimization. Proceedings of ICNN’95 – International Conference on Neural Networks,
Kong, D., Liu, R., Liu, J., Zhou, Q., Zhang, J., Li, W., Bai, H., & Hai, C. (2020). Cubic Membranes Formation in Synchronized Human Hepatocellular Carcinoma Cells Reveals a Possible Role as a Structural Antioxidant Defense System in Cell Cycle Progression [Original Research]. Frontiers in Cell and Developmental Biology, 8. https://doi.org/10.3389/fcell.2020.617406
Landau, E. M., & Rosenbusch, J. P. (1996). Lipidic cubic phases: A novel concept for the crystallization of membrane proteins. Proceedings of the National Academy of Sciences, 93(25), 14532-14535. https://doi.org/doi:10.1073/pnas.93.25.14532
Luce, R. H., & Pohl, A. W. (1935). NATURE OF CRYSTALS FOUND IN AMOEBA. Science, 82(2138), 595-596. https://doi.org/10.1126/science.82.2138.595
Mineral – Examining Crystal Structures . (n.d.). Britannica. https://www.britannica.com/science/mineral-chemical-compound/Examining-crystal-structures
Nakagaki, T., Yamada, H., & Tóth, Á. (2000). Maze-solving by an amoeboid organism. Nature, 407(6803), 470-470. https://doi.org/10.1038/35035159
Oettmeier, C., Brix, K., & Döbereiner, H.-G. (2017). Physarum polycephalum—a new take on a classic model system. Journal of Physics D: Applied Physics, 50(41), 413001. https://doi.org/10.1088/1361-6463/aa8699
Prywer, J., & Krukowski, S. (1998). GaN Single Crystal Habits and Their Relation to GaN Growth Under High Pressure of Nitrogen. MRS Internet Journal of Nitride Semiconductor Research, 3(1), 47. https://doi.org/10.1557/S1092578300001198
Schumann, A., Bielas, K., & Krol, J. (2021). Logical Duality in Reactions of Amoeba Proteus. https://doi.org/10.5220/0010386102130217
Steiner Tree Problem. (2015, June 16). GeeksforGeeks. https://www.geeksforgeeks.org/steiner-tree/
Taylor, M. (1962). Abnormal Crystals in Amoeba lescherae. Nature, 196(4851), 290-291. https://doi.org/10.1038/196290b0
Tero, A., Takagi, S., Saigusa, T., Ito, K., Bebber, D. P., Fricker, M. D., Yumiki, K., Kobayashi, R., & Nakagaki, T. (2010). Rules for Biologically Inspired Adaptive Network Design. Science, 327(5964), 439–442. https://doi.org/10.1126/science.1177894
Tero, A., Nakagaki, T., Toyabe, K., Yumiki, K., & Kobayashi, R. (2010). A Method Inspired by Physarum for Solving the Steiner Problem. IJUC, 6, 109-123.