Life-Sustaining Processes of the Diatom
Peter Matthews-Crochetiere, Daniel Ibrahim, Wenan Liao, Cedric Mackay
Abstract
The chemical reactions, processes, and mechanisms that occur inside diatoms are key to their ability to survive and dominate the world of microalgae. Through photosynthesis, the diatom can absorb sunlight and carbon dioxide and then convert them into oxygen and glucose with the help of chlorophyll, which is a common chemical reaction seen universally in plants. However, diatoms perform many other chemical reactions apart from the carbon cycle. Other reactions involved urea synthesis and silicon extraction processes. These chemical reactions are full of complexity and harder to understand compared to photosynthesis. Through chemical reactions, the diatom acquires ways to gain nitrogenous compounds without help from animals and synthesize silicon for the formation of their frustules, which allow them to be protected from their predators and some viruses, bacteria, and so on. Due to extracting silicon from silicic acid, diatoms’ frustules are composed almost purely of silica, which increases the hardness of their shells.
Introduction
Diatoms constantly rely on chemical processes to maintain growth and viability. The photosynthetic abilities of diatoms, the biomineralization of silica frustules, and the organism’s urea cycle play a crucial role, not only in a diatom’s survival but also in the larger ecosystem (Znachor et al., 2015). Not only is it fascinating how the chemical reactions of diatoms help to grow the diatom and maintain environmental conditions, but the organism’s physiological response to different types of ecological stress teaches us a lot about how the diatom can withstand and adapt to harsh conditions.
The defining feature of the diatom is its biomineralized silica frustule that creates a rigid cell wall to protect the organism. Without it, the organism would be exposed to the surrounding elements and quickly die off. To perform biosilification, diatoms gather silicon in the form of orthosilicic acid using silicic acid transport proteins. Once collected, the silicon is deposited during mitosis to create new complementary hypotheca using membrane-bound silicon deposition vesicles. At times of silicon depletion, the diatom growth rate decreases due to the inability to create new frustules Znachor et al., 2015). In an interesting beneficial relationship between diatoms and their viruses, silicon nutrient stress causes an increase in viral infection, which lyses some diatoms and consequently allows diatom silicon to remain in the euphotic zone to be used by other diatoms, replenishing the silicon abundance (Kranzler et al., 2019).
In the presence of constantly changing parameters, all phases of a diatom’s life cycle can be affected by ecological stress. The vegetative cell will change the rate of growth and even its chemical composition and sexual reproduction could even be prematurely triggered in cases of nutrient depletion (Podunay et al., 2021; Timmermans et al., 2007; Znachor et al., 2015). Known to be a very resilient organism, the diatom has interesting chemical processes that can be adapted in periods of adversity. In this paper, we will explore in depth the chemical reactions necessary to sustain diatom life and the adaptiveness that allowed it to flourish for millions of years.
For a diatom, there is an adequate light level that will benefit the organism. While under-irradiance causes the diatom to shrink into a resting spore and metabolically shut down, over-irradiance can be equally detrimental. With high concentrations of light, the electron transport chain of a diatom can be interfered with, causing excess reduction of NADP+ from photosystem I which consequently causes the unintentional reduction of O2 (Ruban, 2016). The oxygen radicals can then create peroxides which are toxic to the diatom. Its ingenious way of dealing with over-exposure to light is to produce fewer photosynthetic pigments to decrease the capture of energy, as well as using non-photochemical quenching (NPQ) to dissipate energy in the system (Ding et al., 2023).
The urea cycle of a diatom mainly serves to grow and repair the diatom’s cell. Originally thought to have evolved in multicellular organisms, the discovery of the diatom’s urea cycle gives us insight into the difference between multicellular and unicellular chemical reactions. While multicellular organisms focus on expelling nitrogenous waste, diatoms use the urea cycle to create organic nitrogen compounds to use in cellular processes, such as the exchange of nutrients and photosynthesis. In nitrogen-depleted conditions, the growth rate decreases, and, depending on the species, the diatom can rapidly die off (Cointet et al., 2019; Timmermans et al., 2007). Intriguingly, the cellular content of a diatom will change when experiencing nitrogen stress, decreasing protein concentration, and increasing lipid content.
Diatom growth and death
Life cycle of a diatom
At the start of its life, the diatom zygote, also known as the auxospore, rapidly increases in size (Kooistra et al., 2007). Unlike mature diatoms, the auxospore is not protected by silica frustules. Rather, its cell wall is made up of organic material and silica elements that can accommodate its growing size. Once the auxospore reaches its maximum size depicted in Figure 1, which is specific to each diatom species, it will form the silica frustule. During the diatom’s lifespan, it cannot grow any larger than its original size. The development process from a zygote to a grown cell takes hours to a week to complete, depending on the species and environmental conditions (Chepurnov et al., 2004).
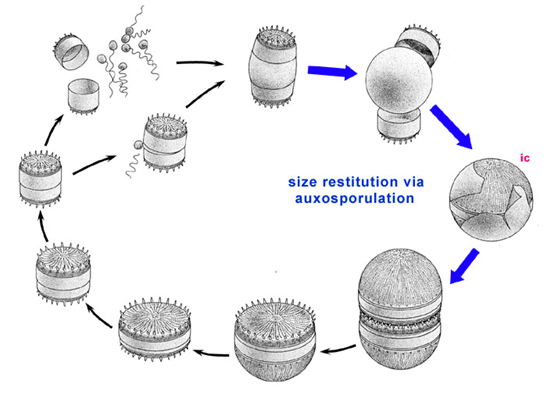
Figure 1: Diatom life cycle. The highlighted region depicts the expansion of the auxospore after sexual reproduction and the formation of the silica frustule to form the initial cell (ic) (Round et al., 1990).
Diatoms participate in both asexual and sexual reproduction (Podunay et al., 2021). During its vegetative stage, individual diatom cells will undergo mitosis. Each cell will take one of the parent cell’s theca (frustule half) and synthesize its own theca during mitosis to create a complete frustule (Chepurnov et al., 2004; Podunay et al., 2021). Due to the nature of the new theca’s creation, it will be smaller than the parent cell’s theca, meaning that one of the daughter cells is slightly smaller than the parent cell. During multiple rounds of mitosis, individual diatom cells will become substantially smaller than the original size of the diatom. Depending on the species, the diatom’s critical size can even be less than half of the original size. The vegetative stage in total lasts anywhere from months to years.
When the diatom reaches its critical size or experiences specific nutrient stresses, it must undergo sexual reproduction (Podunay et al., 2021). Two diatom cells will pair up. One will produce un-flagellated oocytes, while the other will produce flagellated sperm cells that will travel toward the passive gamete (Kooistra et al., 2007). This is very notable in the diatom’s lifecycle, as it showcases the diatom’s ability to create cells with locomotive organelles such as flagella. Once the oocyte fuses with a spermatocyte, the zygote forms. Sexual reproduction in diatoms occurs in a timeframe of a few hours, which is heavily contrasted by the amount of time it spends in the vegetative stage (Chepurnov et al., 2004). As previously discussed, the zygote formed is known as the auxospore, and the life cycle is repeated as it grows into a vegetative cell.
Factors that influence diatom growth and lifespan
The factors that influence cell growth and death are unsurprisingly tied to their metabolic processes as will be discussed in this paper. Factors like irradiance, silicate levels, and nitrogen levels all play a role in cell longevity and viability (Cointet et al., 2019; Timmermans et al., 2007).
Since diatoms are photosynthetic organisms, they rely on light for cell growth (Kooistra et al., 2007). But while light deprivation does show increased cell death in diatoms as seen with the species Thalassiosira Weissflogii, it is hardly substantial compared to other photosynthetic organisms such as the chlorophyte Dunalliela Tertectiola which shows massive cell death with decreased irradiance (Timmermans et al., 2007). This demonstrates the diatom’s ability to withstand long periods of light deprivation and is most likely due to their ability to form into metabolically inactive resting spores which can revive in adequate levels of irradiance (Gross et al., 1948).
On the other hand, nitrogen levels in a diatom’s environment can substantially affect its growth rate (Cointet et al., 2019). Under nitrogen depletion, the photosynthetic mechanism is negatively impacted, and the growth rate decreases. Interestingly, the composition of the diatom is also substantially affected by a decrease in proteins and an increase in lipid content. For example, Thalassiosira Weissflogii, while great at withstanding low light levels, is incredibly sensitive to decreased nitrogen levels, with scientists finding significant cell death in these conditions (Timmermans et al., 2007).
Since Diatoms require silicate to create their frustules, silicate levels are also an important factor in diatom growth and death (Timmermans et al., 2007; Znachor et al., 2015). A decrease in silicate concentrations is accompanied by a decrease in diatom growth rates. Interestingly, there is a difference in the decrease in growth rates when comparing species. For species such as Chaetoceros Calcitrans, the growth rate steadily drops. Meanwhile, with species such as Chaetoceros Brevis and Thalassiosira Antarctica, there is a sudden drop in the growth rate at silicate concentrations of less than 1 µM. Overall, since the consistent creation of new thecae is imperative in diatom asexual reproduction, diatom viability markedly decreases when there is not an adequate concentration of silica in the environment.
Diatom viruses
The impact of diatom viruses on environmental processes has not been thoroughly investigated, and it is still poorly understood (Kranzler et al., 2019). Unlike double-stranded DNA (dsDNA) which is easier to detect and better understood, single-stranded DNA (ssDNA) and single-stranded RNA (ssRNA) that comprises the genome of diatom viruses has not been well researched, making it difficult to identify the effects of these viruses on diatom growth and metabolism.
The population of diatoms is affected differently depending on their growth rate during the beginning of viral infection (Tomaru et al., 2021). In populations that are growing logarithmically, viral inoculations barely affect the growth rate. Meanwhile, populations that are in the stationary phase during infection quickly decrease as cells burst and lyse. After infection, diatom populations are not completely lysed and quickly grow again. Once the population re-establishes, it develops a coexistence with the virus where some diatoms are always infected and contribute to viral proliferation while the rest are not infected.
In a fascinating relationship, diatoms in nutrient-depleted conditions benefit from viral infection and purposely lower their guard to create a more nutrient-rich environment (Kranzler et al., 2019; Tomaru et al., 2021). For example, in silicate-depleted conditions, diatom populations are more likely to become infected. Once infected, elevated ectoprotease activity contributes to increased cell lysis, which decreases the loss of silicate from the euphotic zone to the ocean floor as seen in Figure 2. By keeping silicate in the euphotic zone through cell lysis, the uninfected diatoms have more access to silicate and can continue synthesizing frustules. Bizarrely, diatoms benefit from being infected by the virus and establish an equilibrium in nutrient-depleted environments using increased cell death.
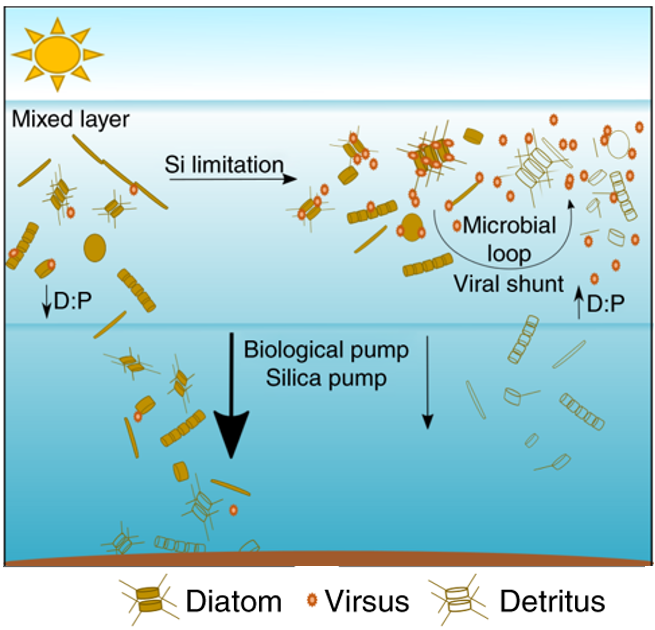
Figure 2: Virus infection and increased cell lysis in a silicate-limited environment weaken the silica pump and increase the supply of regenerative silicate to living diatoms in the euphotic zone (Kranzler et al., 2019).
Diatom silicon biomineralization
The frustule, the characteristic petri dish resembling a rigid cell wall, is the diatom’s number one line of defence. Without it, the diatom would be nothing more than a puddle of cytoplasm and organelles. It is formed by biomineralization during cell reproduction and is formed in a specialized organelle in the diatom: the silicon deposition vesicle. Biomineralization is when biological or organic structures are mineralized, meaning that inorganic minerals are introduced into them. This creates a multi-material matrix, giving that structure specific advantages over comparable structures. This has to do with the idea that structure defines function; the biomineralized structure has some different function, and it is optimized differently, than the similar unmineralized version of it (Estroff, 2008). In the case of the diatom, its frustule is mineralized with beads of silica. This process occurs during cell reproduction, and it is orchestrated by the silicon deposition vesicles, organelles found within the diatom protoplasm (a general term describing the entire cell, except for the cell walls) (Kröger & Poulsen, 2008). The silicon deposition vesicles are membrane-bound organelles and different ones are responsible for the formation of girdle bands and valves, which can be seen in Figure 3 below, and these different vesicles are formed at different times during cell reproduction.
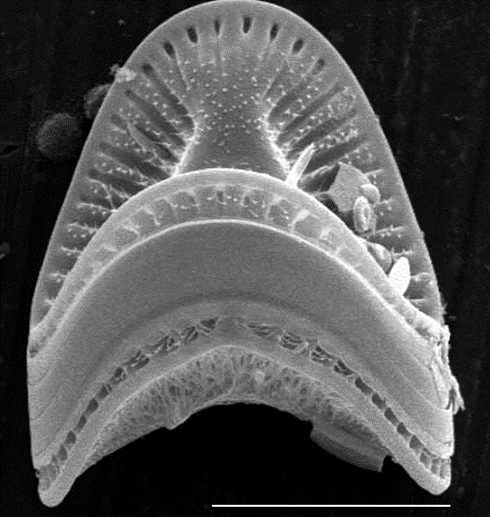
Figure 3: Image of the Campylodiscus diatom, a remarkable saddle shaped diatom, on which the girdle bands and valves can be easily differentiated. The flat section in the middle is the meeting of the girdle bands, and the rest of the frustule, top and bottom, are the valves (Campylodiscus SEM).
Overview of cellular reproduction in diatoms
Diatoms undergo standard mitosis for reproduction, but they then have to undergo additional steps for the formation of new valves and girdle bands, since at the end of standard mitosis, both protoplasts are inside a single set of valves and girdle bands. Cytokinesis is the final step of mitosis, during which the two daughter cells separate entirely from one another, along the cleavage furrow. In Figure 4, the additional steps occur immediately after cytokinesis: silica begins to be biomineralized right away in two separate silicon deposition vesicles right along where the cleavage furrow once was. These silicon deposition vesicles are responsible for the formation of new valves. This deposition continues until the form of the new valve has been achieved. All the while, the membrane of the silicon deposition vesicle is adapting itself to a new form. This membrane is called the silica lemma (Schmid & Schulz, 1979). At this point, exocytosis from the silicon deposition vesicle occurs. Exocytosis is a process during which a cell uses vesicles to move something from inside the cell to outside of it. At this point, the valve-forming silicon deposition vesicles have finished their work. Their fate is not well understood by the diatom community, and we will not delve into this aspect of the silicon deposition cycle. Once this step is complete, two sets of valves are present, one for each protoplast, and the girdle silicon deposition vesicles begin their work. The same steps occur for the valves, and the result is two new diatoms (Kröger & Poulsen, 2008).
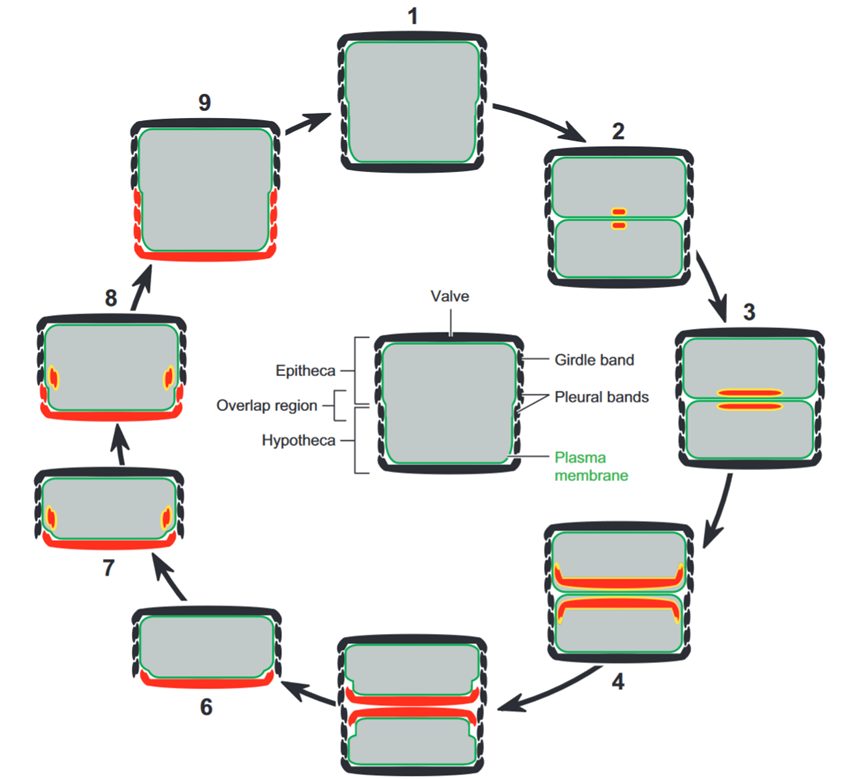
Figure 4: Figure showing the steps that occur after mitosis in the diatom. The grey sections are the protoplasm, the yellow sections are the membranes of the silicon deposition vesicles, and the red sections are the newly deposited silica (Kröger & Poulsen, 2008).
Transport and storage of silicon in the diatom
The scientific diatom community has little to no knowledge of the specific workings of silicon deposition in the cell, but we will here present what is well understood, and some things that are being studied.
Diatoms require silicon for life. Without silicon, they cannot perform cell division, and they eventually die without reproducing. This is of course something to avoid at all costs, so diatoms must have methods of intaking and managing silicon. The main source of silicon for diatoms is orthosilicic acid, Si(OH)4 (Kröger & Poulsen, 2008). This acid is found in the marine environments that diatoms live in, so they are surrounded by a source of silicon. To collect this acid, diatoms have specific proteins, called silicic acid transport proteins. Researchers have proposed two different models of how these proteins transport silicic acid through the cell. Hildebrand et al. propose that the proteins directly bind to the silicic acid and transport it, while Grachev et al. proposed a mechanism in which the protein uses Zn2+ as an intermediary to bind to the silicic acid (Thamatrakoln et al.) (Grachev et al.). These hypotheses are based on analysis of amino acid sequences making up the protein and have not been confirmed through experimental findings. The mechanism through which silicic acid is transported to eventually be deposited as silica, SiO2, is a topic of research and is not known (Kröger & Poulsen, 2008).
However, it is known that diatoms contain pools of soluble silicon, as reservoirs of silicon for the cell. These pools, when placed in experimental or natural conditions, would not be stable, and the result would be uncontrollable precipitation of silicon. Diatoms must therefore have a system to regulate this and control what happens with their silicon. One hypothesis for this is that precipitation inhibitors are present in the diatom’s silicon pools, creating conditions where the pools can form without precipitating, and can then be precipitated as needed by controlling how present this compound is in the pool (Kröger & Poulsen, 2008). Further research is needed on this topic; it is once again part of the intricacies of the diatom which are not well understood.
Diatoms also require specific intracellular conditions for silica deposition to occur properly. One of these conditions is correct pH. The pH of a solution is a measure of its acidity or basicity and is used to quantitatively characterize solutions. The pH is a more friendly way of representing the concentration of free hydrogen ions (H+) or hydroxyl ions (OH–) in a solution, by changing it to a scale that generally goes from 0 to 14. The more acidic, or hydrogen-rich a solution is, the lower its numeric value, with 0 being the lowest. Conversely, basic, or hydroxyl-rich solutions, are closer to 14, with water being the neutral reference at 7. Each increase of 1 on the pH scale is representative of a factor of 10 of the concentration of ions in the solution, i.e. a pH of 3 has 100 times more free hydrogen ions than a pH of 5 (School, 2019). In the silicon deposition vesicle, where the frustules are formed, it is important to avoid degradation and dissolution of the deposited silica. Iler found that a pH around 2 creates conditions in which these negative effects are the least important for synthetic silica gel, and so it is hypothesized that diatoms should have similar conditions in their silicon deposition vesicles (Vrieling et al., 1999). It was also found that pH values around 4 or 5 were not at all conducive to the structural assembly of the silica, but near the pH of 2, even very low concentrations of silica easily assembled into large structures (Vrieling et al., 1999).
To maintain the correct acidity of the silicon deposition vesicle, diatoms feature ion pumps in the silicalemma, the membrane of the silicon deposition vesicle. In their work, Vrieling et al. propose that these pumps are proton pumps, which would allow the diatoms’ system to have control over the acidity of the interior of the silicon deposition vesicle (Vrieling et al., 1999). This is important, as the vesicle may need to change its pH throughout the silicification, as found by Vrieling et al., or react to changes in the environmental pH to prevent interruptions or critical damage to the new frustules, as this would doom the two daughter cells.
The other important factors to consider in the silicon process in the silicon deposition vesicle are the concentration of silica monomers and oligomers (short chains), the temperature, and the concentration of various salts and cations, as these can influence the pH and the interactions of the silica when it is to be polymerized (Vrieling et al., 1999).
Overall, diatoms have a series of highly complex mechanisms, which are not all known to the scientific community. These mechanisms include the intake of orthosilic acid by the diatom, the transport of silica through the cell, the “storage” of silica in pools inside the diatom, the regulation of intracellular conditions, and the deposition of silica inside the silicon deposition vesicle. These processes are all critical, as they allow diatoms to reproduce, and so it is natural that the diatom should have such an intricate system dedicated to this single resource.
Effects of light on diatoms
Diatoms are autotrophic organisms meaning that they rely on light as their main source of energy. As previously discussed in the physics paper, they developed several physical characteristics to control light better and limit their exposure to toxic light frequencies. These diatoms are susceptible to turbulent currents that they live in since they are small, and while they do have some buoyancy control: they still often get dragged down to deeper waters. This is not beneficial to the diatoms since they will have access to less light. Still, the opposite is also dangerous as high concentrations of light will destabilize their functioning by creating radical oxygen species (ROS) (Ding et al., 2023). In these high-light situations, the cell will be under a lot of stress and will alter its biochemical composition to better cope with the changes and avoid these reactive oxygen species at all costs. Light is therefore an abiotic regulatory mechanism that will affect how the cell will behave and will impact its gene expression (since different enzymes will be needed in different quantities to produce different molecules).
Reactive oxygen species
The light-dependent reactions in diatoms are dependent on the absorption of photons by the chlorophyll A present in the light-harvesting complex. When a chlorophyll A molecule is excited after the absorption of a photon, it ultimately has 3 fates (Scheme 1). It can either lead to photochemistry (which is the process by which the energy is transferred to release an electron that will travel through the electron-transport chain), it can dissipate the energy in the form of heat, or it can also reemit the light by fluorescent emission (Buck et al., 2019). The diatom has a high control over these various mechanisms and can influence them depending on their light condition. They will prefer to have a large amount of photochemistry under low-light conditions since this is how they get their energy but under intense light, too much photochemistry will lead to oxidative damage that hurts the organism (Buck et al., 2019). This is because an increase in photochemistry will lead to an over-reduction of the cellular NADP+ which will lead to the production of the O2 superoxide by Mehler’s reaction (Latowski et al., 2011). This superoxide is dangerous to the plant since it forms peroxides and hydroxyl radicals.
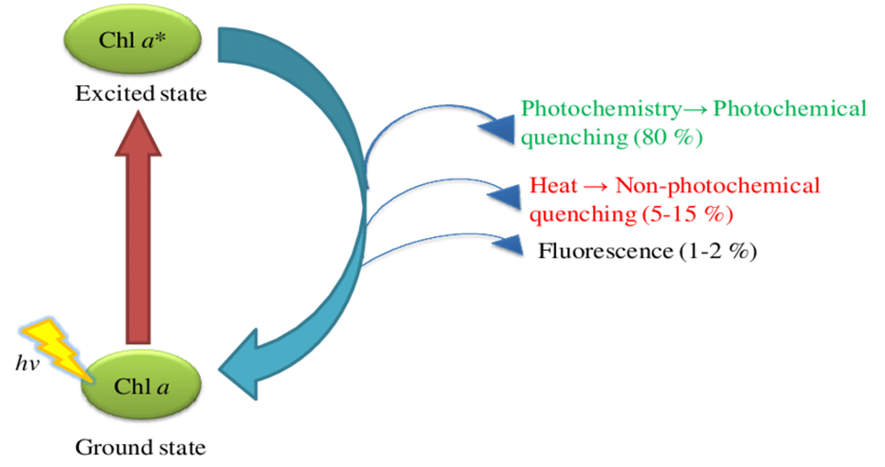
Scheme 1: The possible outcomes after the photon excitation of a chlorophyll A molecule. The percentages represent how often it happens under normal light conditions. In high-light stress, the diatom will increase the non-photochemical quenching (Sardar et al., 2016).
The Mehler reaction was first discovered in 1951 and it is the direct reduction of molecular oxygen (O2) by photosystem 1 (Roberty et al., 2014). This happens when there is no longer enough NADP+ to be reduced at the end electron transport chain in that photosystem. The electrons will then go directly to molecular oxygen and will form the oxygen superoxide ion. This can happen either when there is insufficient utilization of NADPH (so low regeneration of NADP+) or as is the case in the high light environment, there are too many reactions happening at the same time (Polle, 1996). Then, the superoxide ion (which is an example of a reactive oxygen species) will react with the excess protons in the stroma to form hydrogen peroxide (H2O2) and hydroxyl radicals which are toxic to the diatom and to plant cells in general even in moderate quantities. The danger with hydroxyl radicals is that they are highly reactive and so they will attack any available cellular compound to stabilize itself and this will lead to a disturbance in the biochemical functioning of the diatom. The reaction with the protons will also lead to the recovery of the molecular oxygen.
In essence, this whole process can be summarized by the following chemical formula (Mehler, 1951):
O_2+2H^++2e^- → H_2 O_2 → 2•OH
NPQ and diadinoxanthin cycle
Non-photochemical quenching (or NPQ for short) is the name given to a variety of processes whose purpose is to dissipate the excess energy accumulated from absorbed light into heat (Ruban, 2016). They do so by quenching the singlet excited state in the Chlorophyll A molecule before it reaches the electron transport chain and reduces an O2. The NPQ is the main way that the cell deals with and avoids the reactive oxygen species produced in Mehler’s reaction and by other reactions such as the ones involving the reduction of O2 by a metal like iron that produces the ROS (Ruban, 2016). The specific mechanism that we are exploring from the NPQ is the non-enzymatic anti-oxidizing xanthophyll and diadinoxanthin cycle. The xanthophyll cycle refers to the general mode of anti-oxidating action whereas the diadinoxanthin is the specific cycle that diatoms express to defend against ROS (Ruban, 2016).
The diadinoxanthin cycle is composed of 2 molecules that can be changed into 1 another depending on the needs of the cell. These compounds are diadinoxanthin and diatoxanthin (refer to Figure 5) (Kagatani et al., 2022). They are both carotenoids which are compounds that are present in the light-harvesting complex with chlorophyll, and they have 2 functions to perform. First, they can act as photoreceptors and expand the accepted wavelength spectrum of the light-harvesting complex by absorbing the light and producing the singlet excited state that they transfer to the chlorophyll A molecule to continue the process. However, it can also act as a photoprotector by not transferring the energy to chlorophyll A but rather dissipating it as heat. It also can do so by taking the energy from the chlorophyll and dissipating it as heat to avoid photodamage (Kagatani et al., 2022).
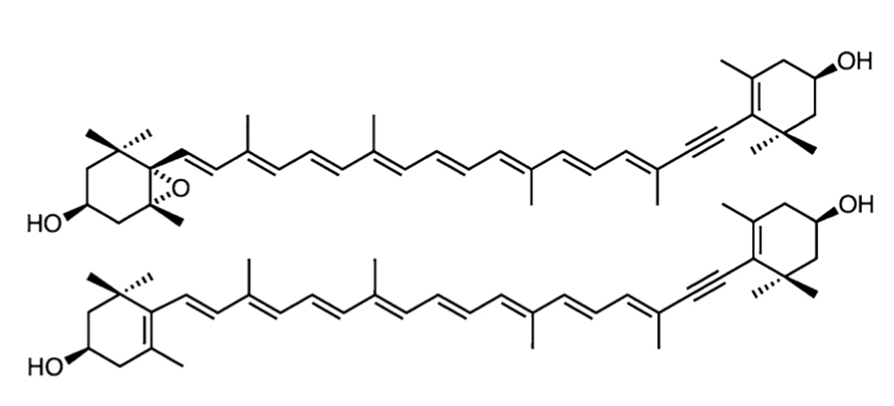
Figure 5: The structures of the carotenoid diadinoxanthin (top) and diatoxanthin (bottom) (Kagatani et al., 2022). Notice that diatoxanthin has a longer conjugated pi system.
The cycle starts when there is an increase in proton concentration (so a lower pH) due to a high quantity of photoreactions (namely from an increased intensity of light) (Consalvey et al., 2005). Diadinoxanthin (Ddx) is transformed into diatoxanthin as light intensity increases since diatoxanthin (Dtx) is worse at transferring energy (due to the larger conjugated pi-system that will be more stable and absorb more energy) and will rather dissipate it as heat (which is what is required in this case). The reaction from Ddx to Dtx and the reverse reaction are both done by enzymes namely the Diadinoxanthin de-epoxidase which will reduce diadinoxanthin by removing the epoxide group on the left and transforming it to a double bond. The reverse of this reaction is the oxidative epoxidation of Dtx by the diatoxanthin epoxidase which will happen to restore the efficiency of the photosystem once the high light stress is removed (Ruban, 2016). So, the diatom has found a clever chemical solution to reduce the efficiency of the light-harvesting complex when it needs to avoid photodamage, but it can also quickly reverse to be able to continue efficient photosynthesis once the stress is gone.
Changes in biochemical composition during high-light stress
During the exposition to high light, researchers noticed that diatoms reproduced considerably less than under a normal (control) environment. In the research conducted by Ding et al., they measured that the volumetric cell number (number of cells per unit of volume) was reduced by 45.5% when the cells of the Phaeodactylum tricornutum diatom were exposed to a higher than optimal light intensity (Ding et al., 2023). The surprising result of this research is that even though cell division was reduced by the stress of the HL (high-light) environment, the biomass was actually slightly higher. This means that even though there is less cell division that happens under stress, the cell is still growing in mass, so you get bigger cells than in the normal environment. The cells that were grown under intense light were however not the same as the ones for the control experiment as they displayed a different macromolecule composition most probably due to the partial shutoff of photosystem. Under this applied light stress, the researchers observed that the cell produced much less of the light-harvesting pigments. So, the diatom produced less Chlorophyll A, Chlorophyll C, fucoxanthin, and beta-carotenoid which are all essential molecules in the light-harvesting complex present inside the chloroplasts. The content of all these molecules was reduced by about 90% (the exact percentage varied for each pigment) which means that the light-dependent reactions in photosynthesis were greatly reduced when the diatom was under stress (Ding et al., 2023). This was also confirmed by analyzing the cytochrome b6 membrane protein which is an indicator for the electron transport between the 2 photosystems. Their activity was also significantly lower when the cell was under a high-light (Ding et al., 2023).
The diatom also had changes to its lipid, carbohydrate, and protein ratios as the cell produced 29.24% less protein but produced 21.33% more carbohydrates and 50.1% more lipids than under the control conditions. The higher lipid content can be attributed to the diatom’s need for polar membrane lipids as they are now forming bigger cells and therefore need more lipids to cope with the increase in volume of the cell. The kind of lipids that form the cell membrane will also change when light changes as the researchers noted that the cell will favor the production of saturated and monounsaturated fatty acids instead of the polyunsaturated ones that are favored under normal conditions (Ding et al., 2023).
Urea cycle
The ancestor of the diatom developed a series of urea metabolism genes similar to mammals so that they do not need to migrate to environments with animals to acquire nitrogen substances. Through the urea metabolism system, the diatom could convert the nitrogenous substance (ammonium salt) that cannot be used up immediately to urea, which would be stored in cells for future use (Prihoda et al.). Urea production begins with the synthesis of carbamoyl phosphate from ammonia and CO2; carbamoyl phosphate and ornithine condensate to citrulline; citrulline and aspartic acid make arginine succinic acid; arginine succinic acid cleave to arginine, and are then hydrolyzed to urea and ornithine, which continue to participate in the cycle of urea synthesis (Prihoda et al., 2012). In diatoms, this cycle serves as a repackaging hub for inorganic carbon and nitrogen, which contributes significantly to the metabolic response of diatoms to episodic nitrogen availability. The urea cycle happens in the mitochondria.
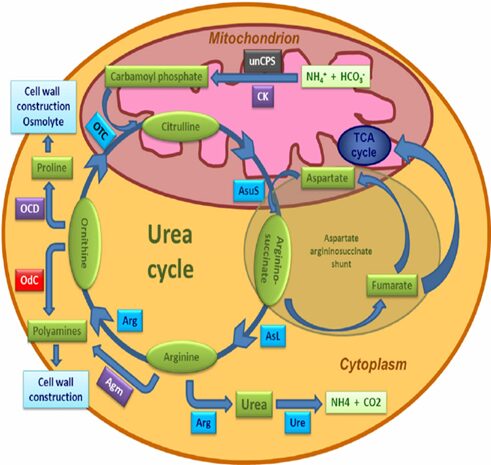
Fig. 6. The urea cycle in diatoms. Scheme showing the principal components of the diatom urea cycle. Genes encoding enzymes of bacterial origin are indicated in purple, metazoan origin in black, and red algal origin in red. Those with uncertain affiliations are shaded in blue. Abbreviations: Agm, agmatinase; Arg, arginase; ASL, argininosuccinate lyase; AsuS, argininosuccinate synthase; CK, carbamate kinase; OCD, ornithine cyclodeaminase; OdC, ornithine decarboxylase; OTC, ornithine transcarbamylase; unCPS, mitochondrial carbamoyl phosphate synthase III; Ure, urease (Prihoda et al., 2012).
Similarity with multicellular organisms
Figure 6 shows the fully functional urea cycle of the diatom (the specific evolution timeline of the urea cycle in diatoms is complex and has not been fully determined). The cycle is surprising, because before this discovery, the cycle was considered to originate from multicellular organisms in adapting to high amino acid diets with life on land. The diatom is the first photosynthetic organism that contains all the genes for the cycle. This discovery of the diatom overturned the theory and reveals that the urea cycle appeared earlier than the first land organisms existed (Armbrust et al., 2004). The first step of the urea cycle is started by a mitochondrially-localized carbamoyl phosphate synthase which is now known as unCPS, it is an enzyme that catalyzes the production of the substrate carbamoyl phosphate for the cycle, it plays an important role in vertebrate metabolic adaptations, which is the survival mechanisms to ensure they have enough energy to perform essential functions (Allen et al., 2011). The analysis of the unCPS confirms their similarities with the multicellular organisms’ enzymes and the scientists found that the enzyme is absent in both red and green algae, which removes the possibility that the gene was acquired from the endosymbiosis of the two algae. The gene had undergone several duplications during its evolutionary history that can explain its neo-functionalization. Other components of the diatom urea cycle closely resemble those found in the multicellular organisms’ cycle from a functional point of view, although the genes do not all appear to be of Ex symbiont origin. In addition, diatom genomes encoded several enzymes thanks to the contribution of horizontal gene transfer from bacteria that potentially expands the functionality of the cycle concerning its animal counterpart. In diatoms, there is a sequence of genes, cyclo deaminase (OCD), which is predicted to convert ornithine into proline, as well as agmatinase (AGM), which has been predicted to link the urea cycle to polyamine biosynthesis (Allen et al., 2006).
Conditions for urea genes expression and the role of the urea cycle
Analysis of the expression of integral and peripheral urea cycle genes illustrates that their expression is coordinately regulated in areas lacking nitrogen, while the gene expression is favored in conditions more suitable for anabolic rather than catabolic metabolism. It is commonly known that in multicellular animals, the urea cycle mainly participates in the breakdown of organic nitrogen compounds, but the above findings seem to tell us that the urea cycle in diatom mainly focuses on the biosynthesis of organic nitrogen compounds. (Krell et al., 2007)
The overall chemical reaction for the urea cycle:
![]()
Allen et al. generated unCPS knockdown mutants in P. tricornutum and they found that in environments lacking nitrogen, the diatom appears to have a delay in their cell recovery compared with the wild-type diatoms. Delayed accumulation of metabolites in the urea cycle reflects that point. Nitrogen is a necessary component for cell repairment in the diatom. As the metabolic role of the cycle in diatoms is to work as a recycling hub, it plays an important role in fixing anaplerotic carbon into organic nitrogen compounds. The urea cycle can provide precursors for biosynthetic proline by converting arginine to ornithine (through the action of OAT that is subsequently converted to GSA) via arginase which is a key urea cycle enzyme. In addition, proline is synthesized in the ribosome and is a precursor of ornithine (product of the urea cycle) and will be then converted into polyamines via ornithine decarboxylase (ODC). Proline is the major osmolyte in diatoms, and proline-rich proteins are common cell wall structural components.
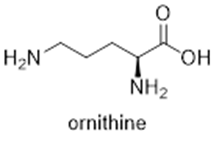
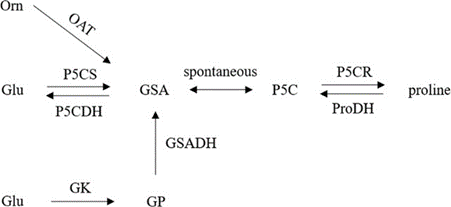
Figure 7: The pathway of proline metabolism. Orn represents ornithine, OAT ornithine aminotransferase, Glu glutamic acid, P5CS delta-1-pyrroline-5-carboxylate synthase, P5CDH delta-1-pyrroline-5-carboxylate dehydrogenase, GK glutamyl kinase, GSA glutamate-1-semialdehyde, GSADH glutamyl semialdehyde dehydrogenase, GP glutamyl phosphoric acid, P5C pyrroline-5-carboxylate, P5CR pyrroline-5-carboxylate reductase, ProDH proline dehydrogenase (by Jin Liu Aug 2020).
The offshoots of arginine and ornithine are precursors for polyamines such as spermidine and putrescine and can be converted into highly modified long-chain polyamines that are used for building diatom-specific siliceous cell walls. Both branches are made feasible through the bacterial genomes captured by the diatom. Thus, the urea cycle of the diatom helps with building organic nitrogen compounds that are particularly critical for building the unique features of diatom cells such as frustule (Allen et al., 2011).
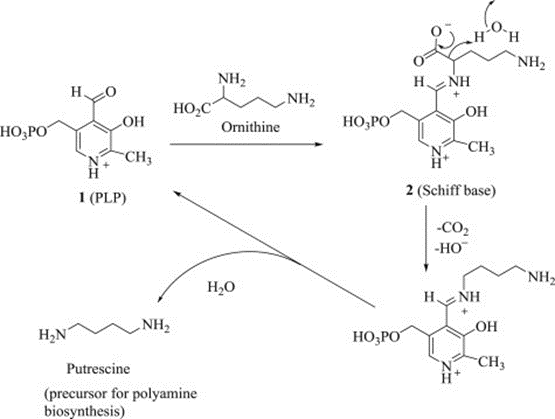
Figure 8: Decarboxylation of l-ornithine leads to the polyamine putrescine, the precursor of sperimidine and sperimine in the polyamine biosynthetic pathway (Satyavan Sharma, Nitya Anand, in Pharmacochemistry Library, 1997).
Relationships among diatoms with urea cycle
It is supposed that the urea cycle is connected to the glutamate synthase and the tricarboxylic acid (TCA) cycle through the aspartate–argininosuccinate shunt. Glutamate synthase works as an enzyme to synthesize glutamate which serves as a substrate for the synthesis of N-acetylglutamate, an essential allosteric activator of carbamoyl phosphate synthetase I, a key regulatory enzyme in the urea cycle. Through the TCA cycle, aspartate is regenerated from fumarate. One of the prime precursors of urea, the bicarbonate ion, is also formed from the CO, which is generated by the TCA cycle which is a tightly regulated and indispensable circle for reallocating carbon in the diatom cell while nutrients cannot satisfy the cells (Kröger and Poulsen, 2008).
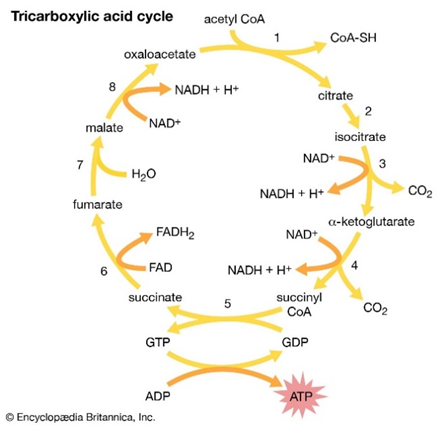
Figure 9: The eight-step tricarboxylic acid cycle (Encyclopedia Britannica, Inc).
The aspartate–argininosuccinate shunt provides an additional point to integrate the urea cycle into central metabolism. Another hypothesis is that the urea cycle may interact with photorespiratory pathways, another is that C4-type photosynthesis may operate in some diatoms by metabolite exchange between chloroplasts and mitochondria. The hypothesis is proposed due to the urea cycle’s position between mitochondria and cytoplasm, it might be an important pathway for the exchange of nutrients. The exchange of nutrients with plastids has not been confirmed yet but according to their closeness to them, such a possibility certainly requires more research (Kroth et al., 2008).
Conclusion
Throughout this paper, we have shown only some of the ways that diatoms were able to exploit the principles of chemistry to improve their survival chances and make them better evolutionary competitors.
First, we saw how the diatom was able to solve the problem it faces during its asexual reproduction. During asexual reproduction, the mature diatom will separate into 2 daughter cells that are about half of the size of the parent. These daughter cells will not be able to grow larger since they are constrained by their frustule. So, if this keeps happening the diatom will get too small and will not be able to survive. The diatom has found a clever solution which is to use sexual reproduction when either the daughter cells get too small, or under environmental pressures.
Then, we explored how the diatom can form its biomineralized silicon frustule. The frustule is a crucial part of diatom anatomy and we have just mentioned how important their optical property is to the well-being of the photosynthetic systems and therefore to the cell. However, silica, with the absence of control mechanisms from the diatom, will precipitate spontaneously and cannot be used to form the frustule. Further, this wastes the precious silica of the diatom. So, the diatoms store their silica in a meta-stable state. This lets the system precipitate the silica on command, and then transport it during reproduction to form 2 new valves in the center of the cell. The current theory is that the diatom contains inhibitory molecules present in the vesicle that prevent spontaneous mineralization. Diatoms have also evolved beautifully to maximize the construction potential of the silica they have by maintaining acidic conditions in the silicon deposition vesicle. To maintain homeostasis, ion pumps are present in the silicon deposition membrane, and they are always adapting to the inner acidity of the membrane.
Next, the diatom faced the problem of varying light intensity in its environment. When there is too little light, the diatom needs to adapt and increase the efficiency of its photosystems to be able to get its energy to live. However, if the light is too intense and the photosystems remain efficient, there will be a production of hydrogen peroxide by Mehler’s reaction which is harmful to the diatom. Nature’s design for this problem is the non-photochemical quenching of the excited chlorophyll A molecules by diadinoxanthin. This solves the problem of excess photochemistry, but nature completes this design with the diadinoxanthin cycle which can quickly change diadinoxanthin to diatoxanthin when the stress of highlight is gone to quickly regain the efficiency.
Finally, another problem that the diatom faces is the lack of available organic nitrogen that it can then use to synthesize various molecules (mainly amino acids). This is where the urea cycle comes in. The urea cycle is a clever design in diatoms that serves to transform inorganic and unusable ammonium and bicarbonate salts into urea. It is then stored in vesicles which will be used if there is a lack of nitrogen and is useful for the cell as it can be used for the synthesis of various amino acids like arginine. The diatom possesses a complex system of enzymes to be able to do so, but it provides a great advantage as cells can tap into freely available nitrogen present in their environment.
References
Allen AE, Dupont CL, Obornik M, et al. 2011. Evolution and metabolic significance of the urea cycle in photosynthetic diatoms. Nature 473, 203–207
Allen AE, Vardi A, Bowler C. 2006. An ecological and evolutionary context for integrated nitrogen metabolism and related signaling pathways in marine diatoms. Current Opinion in Plant Biology 9, 264–273.
Armbrust EV, Berges JA, Bowler C, et al. 2004. The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science 306, 79–86
Buck, J. M., Sherman, J., Bártulos, C. R., Serif, M., Halder, M., Henkel, J., Falciatore, A., Lavaud, J., Gorbunov, M. Y., Kroth, P. G., Falkowski, P. G., & Lepetit, B. (2019). Lhcx proteins provide photoprotection via thermal dissipation of absorbed light in the diatom Phaeodactylum tricornutum. Nature Communications, 10(1), 4167. https://doi.org/10.1038/s41467-019-12043-6
Campylodiscus SEM. Diatoms of North America. Retrieved 11/19/2023 from https://diatoms.org/images/18331
Chepurnov, V. A., Mann, D. G., Sabbe, K., & Vyverman, W. (2004). Experimental Studies on Sexual Reproduction in Diatoms. In International Review of Cytology (Vol. 237, pp. 91-154). Academic Press. https://doi.org/https://doi.org/10.1016/S0074-7696(04)37003-8
Cointet, E., Wielgosz-Collin, G., Bougaran, G., Rabesaotra, V., Gonçalves, O., & Méléder, V. (2019). Effects of light and nitrogen availability on photosynthetic efficiency and fatty acid content of three original benthic diatom strains. PloS one, 14(11), e0224701. https://doi.org/10.1371/journal.pone.0224701
Consalvey, M., Perkins, R. G., Paterson, D. M., & Underwood, G. J. (2005). PAM fluorescence: a beginners guide for benthic diatomists. Diatom Research, 20(1), 1-22.
Ding, W., Ye, Y., Yu, L., Liu, M., & Liu, J. (2023). Physiochemical and molecular responses of the diatom Phaeodactylum tricornutum to illumination transitions. Biotechnology for Biofuels and Bioproducts, 16(1), 1-22.
Estroff, L. A. (2008). Introduction: Biomineralization. Chemical Reviews, 108(11), 4329-4331. https://doi.org/10.1021/cr8004789
Gross, F., Zeuthen, E., & Yonge, M. (1948). The buoyancy of plankton diatoms: a problem of cell physiology. Proceedings of the Royal Society of London. Series B – Biological Sciences, 135(880), 382-389. https://doi.org/doi:10.1098/rspb.1948.0017
Kagatani, K., Nagao, R., Shen, J.-R., Yamano, Y., Takaichi, S., & Akimoto, S. (2022). Excitation relaxation dynamics of carotenoids constituting the diadinoxanthin cycle. Photosynthesis research, 154(1), 13-19.
Kooistra, W. H. C. F., Gersonde, R., Medlin, L. K., & Mann, D. G. (2007). CHAPTER 11 – The Origin and Evolution of the Diatoms: Their Adaptation to a Planktonic Existence. In P. G. Falkowski & A. H. Knoll (Eds.), Evolution of Primary Producers in the Sea (pp. 207-249). Academic Press. https://doi.org/https://doi.org/10.1016/B978-012370518-1/50012-6
Kranzler, C. F., Krause, J. W., Brzezinski, M. A., Edwards, B. R., Biggs, W. P., Maniscalco, M., McCrow, J. P., Van Mooy, B. A. S., Bidle, K. D., Allen, A. E., & Thamatrakoln, K. (2019). Silicon limitation facilitates virus infection and mortality of marine diatoms. Nature Microbiology, 4(11), 1790-1797. https://doi.org/10.1038/s41564-019-0502-x
Kröger, N., & Poulsen, N. (2008). Diatoms—From Cell Wall Biogenesis to Nanotechnology. Annual Review of Genetics, 42(1), 83-107. https://doi.org/10.1146/annurev.genet.41.110306.130109
Latowski, D., Kuczyńska, P., & Strzałka, K. (2011). Xanthophyll cycle–a mechanism protecting plants against oxidative stress. Redox Rep, 16(2), 78-90. https://doi.org/10.1179/174329211×13020951739938
Mehler, A. H. (1951). Studies on reactions of illuminated chloroplasts: I. Mechanism of the reduction of oxygen and other hill reagents. Archives of Biochemistry and Biophysics, 33(1), 65-77. https://doi.org/https://doi.org/10.1016/0003-9861(51)90082-3
Podunay, Y. A., Davidovich, N. A., Davidovich, O. I., Witkowski, A., Gastineau, R., & Solak, C. N. (2021). The Sexual Reproduction and Life Cycle of the Pennate Diatom Entomoneis cf. paludosa (W. Smith) Reimer (Bacillariophyta). Russian Journal of Marine Biology, 47(1), 19-28. https://doi.org/10.1134/S1063074021010089
Polle, A. (1996). Mehler reaction: friend or foe in photosynthesis? Botanica Acta, 109(2), 84-89.
Roberty, S., Bailleul, B., Berne, N., Franck, F., & Cardol, P. (2014). PSI Mehler reaction is the main alternative photosynthetic electron pathway in Symbiodinium sp., symbiotic dinoflagellates of cnidarians. New Phytologist, 204(1), 81-91. https://doi.org/https://doi.org/10.1111/nph.12903
Round, F. E., Crawford, R. M., & Mann, D. G. (1990). The Diatoms : biology & morphology of the genera. Cambridge University Press Cambridge.
Ruban, A. V. (2016). Nonphotochemical Chlorophyll Fluorescence Quenching: Mechanism and Effectiveness in Protecting Plants from Photodamage. Plant Physiol, 170(4), 1903-1916. https://doi.org/10.1104/pp.15.01935
Sardar, A., Srivastava, P., & Grover, S. (2016). Genetically Modified Organism. In (pp. 243-261).
Schmid, A. M. M., & Schulz, D. (1979). Wall morphogenesis in diatoms: Deposition of silica by cytoplasmic vesicles. Protoplasma, 100(3-4), 267-288. https://doi.org/10.1007/bf01279316
School, W. S. (2019). pH and Water. USGS. Retrieved 11/16/2023 from https://www.usgs.gov/special-topics/water-science-school/science/ph-and-water
Timmermans, K. R., Veldhuis, M., & Brussaard, C. P. D. (2007). Cell death in three marine diatom species in response to different irradiance levels, silicate, or iron concentrations. Aquatic Microbial Ecology – AQUAT MICROB ECOL, 46, 253-261. https://doi.org/10.3354/ame046253
Tomaru, Y., Yamaguchi, H., & Miki, T. (2021). Growth Rate-dependent Cell Death of Diatoms due to Viral Infection and Their Subsequent Coexistence in a Semi-continuous Culture System. Microbes Environ, 36(1). https://doi.org/10.1264/jsme2.ME20116
Vrieling, E. G., Gieskes, W. W. C., & Beelen, T. P. M. (1999). SILICON DEPOSITION IN DIATOMS: CONTROL BY THE pH INSIDE THE SILICON DEPOSITION VESICLE. Journal of Phycology, 35(3), 548-559. https://doi.org/10.1046/j.1529-8817.1999.3530548.x
Znachor, P., Rychtecký, P., Nedoma, J., & Visocká, V. (2015). Factors affecting growth and viability of natural diatom populations in the meso-eutrophic Římov Reservoir (Czech Republic). Hydrobiologia, 762(1), 253-265. https://doi.org/10.1007/s10750-015-2417-8