Underwater Chemists: Discussion of Design Solutions in Tintinnid Ciliates
Alexa Bailey, Lou Cubberly, Margaux-Blondin Routhier, Niall Slack-Watkins
Abstract
In this essay we explore tintinnids’ intricate design solutions through the lens of chemical processes and pathways, emphasizing the vital role chemistry plays in their survival. These ciliates employ complex chemical processes that regulate digestion, reproduction, self-protection, and swimming mechanisms. We explore the cell and life cycle of tintinnids, their mechanisms for prey selection, capture, and digestion, and the chemical composition of their lorica. We also delve into tintinnids’ ability for chemoreception which allows them to locate prey and avoid predators, and their ability to retract into their lorica when facing danger. Studying these organisms poses challenges due to their microscopic size, especially in the context of chemical reactions. Many of their exact chemical processes are still unknown. However, the insights we can gain from learning about tintinnids’ chemistry can lead to inspiration for biotechnology, engineering, and chemistry applications.
Introduction
Billions of chemical reactions occur every second in all living cells. These reactions are what keep organisms alive and functioning. Chemical reactions drive even the simplest of actions, including eating or self-protection. The tintinnid is a unicellular, microscopic ciliate, but even in tintinnids, we see chemical design solutions at work. They are seemingly simple organisms, yet the adaptations and survival strategies they exhibit are anything but. Their survival depends on the optimization of these chemical reactions. We will examine these chemical reactions, illustrating how chemical pathways are essential to the tintinnid’s survival and growth.
Tintinnids can be characterized by their lorica, a vase-like shell that protects them from predators. Its strength can be attributed in part to its hexagonal patterns that reinforce the shell at the micro-scale. We now explore the chemical composition that further contributes to its durability.
Tintinnids’ unique swimming patterns are presented by a helical swimming motion. Their swimming is controlled by their cilia that beat back and forth in a coordinated fashion. This solution enables them to avoid predators and search for prey. We delve into the chemical reactions involved in this choreography that allow them to move so efficiently through the water.
Tintinnids employ differential predation to seek out their preferred prey. We examine the chemosensory mechanisms and the chemical processes that facilitate this. Furthermore, the chemistry involved in their digestive and reproductive processes is considered.
There are three main abilities necessary for a species’ continued survival: obtaining food, reproduction, and evading danger. We consider the design solutions rooted in chemistry that govern the entire life cycle of the tintinnid and optimize its survival. We explore the chemical aspects and adaptations of tintinnids that allow them to not only survive, but thrive, in their environment. In examining the use of chemistry in this single-celled organism, we can begin to understand and potentially generalize solutions for diverse challenges, which can inspire innovative applications in various scientific fields.
Digestion
The food selection and general consumption process of tintinnids were described in a previous essay. In this sub-section, the digestion and chemical processes surrounding digestion will be explored further.
Once the prey is selected it is sorted by a process that happens in the anterior ciliary region of the tintinnid. To help capture the prey and make sure that it does not escape, tintinnids produce mucus that imprisons their catch (Blackbourn, 1974). To further trap particles, tintinnids induce a beat reversal of local cilia. Another method used by tintinnids to trap particles is the downstream entrapment by a second row of cilia (Blackbourn, 1974; Wasserman, 2017).
Once the food is restrained, it undergoes phagocytosis individually or in groups (Blackbourn, 1974; Montagnes, 2012). Phagocytosis is a form of endocytosis during which particulates or small organisms are absorbed by the cell (in this case, the tintinnid) (Wasserman, 2017). This process takes place in a defined cytosomal region (Montagnes, 2012).
Once the food matter is in the cell, it is contained in a food vacuole and undergoes intracellular digestion by a variety of digestive enzymes (Blackbourn, 1974; Montagnes, 2012). Digestive enzymes contained in the tintinnid’s food vacuoles are hydrolytic enzymes, that cleave organic macromolecules to enable the absorption of nutrients, usually at reduced pH (Montagnes, 2012). However, tintinnids’ enzymes cannot digest every type of food eaten by the ciliates. The maximum efficiency for the heterotrophic digestive system of tintinnids is an assimilation of 90% of all ingested food (Verity, 1985). Some cell structures such as that of dinoflagellates or diatoms are more resistant to the digestive enzymes than others, leading to them being mostly or completely undigested and needing to be egested (Blackbourn, 1974).
Once all essential nutrients are properly absorbed and digested, all the left-over undigested food will be egested from the cell, at the posterior site, located in the cell plasma membrane (Blackbourn, 1974). This posterior site can also be called the cell anus or the cytopyge (Montagnes, 2012). Most of the time, the egested particles will be expelled inside the lorica, stuck between the cell and the interior lorica wall, and will require removal. This is accomplished by a row of thin lateral cilia that will slowly move the egested particle between the cell and lorica wall until it reaches an exit point (Blackbourn, 1974).
Tintinnids are in constant motion, even during digestion, but that motion does not constitute an important part of their total metabolic energy losses, since they are small (Blackbourn, 1974). The metabolism of tintinnids is a protein-based metabolism, proven by their rapid starvation times. The end products of the metabolic pathways of tintinnids are NH4+ and CO (Verity, 1985). Because of the NH4+ that the ciliates emit from their metabolic activities, they are considered to be NH4+ regenerators (Verity, 1985).
Reproduction and cell cycle
Tintinnids mostly reproduce through asexual reproduction, and less often through sexual reproduction (Blackbourn, 1974).
The type of asexual reproduction observed in tintinnids, and other ciliates is asexual binary fission (Reid & John, 1978). That asexual reproduction in a tintinnid is illustrated in Figure 1. During binary fission, organisms double their size and divide into two cells (Wasserman, 2017). Also, the existing macronucleus is destroyed while a new one is formed by the cell’s micronuclei (Wasserman, 2017).
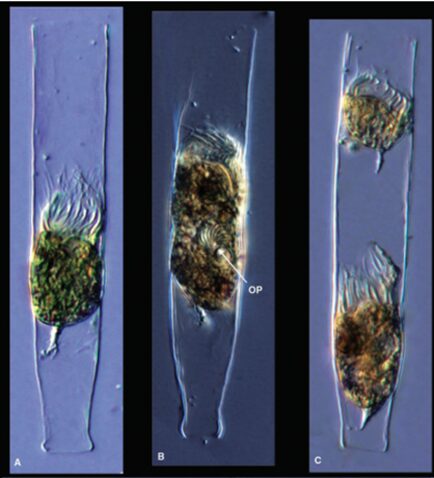
Fig. 1. Binary fission in a tintinnid, where OP is the oral primordium (A corresponds to the feeding stage of the tintinnid, B corresponds to an early stage of binary fission and C corresponds to the end of binary fission) (Dolan, 2012).
Tintinnids need food to grow properly and divide. However, the increase in tintinnid numbers is not related either to the amount of food they ingest or the number of times they engage in sexual encounters (Blackbourn, 1974; Montagnes, 2012). Rather, an increase in tintinnid populations is related to cell divisions, as explained below.
The tintinnid cell takes part in two different cycles. The first one is called the cell cycle while the second one is called the life cycle. Both cycles are represented in Figure 2.
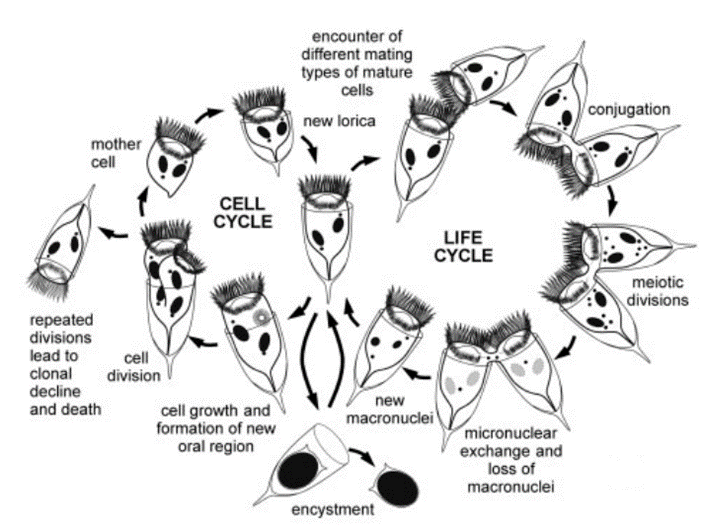
Fig. 2. A generalized tintinnids life cycle and cell cycle (Montagnes, 2012).
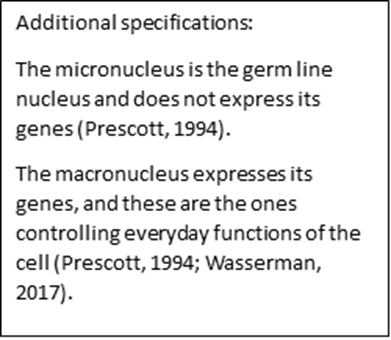
The cell cycle corresponds to the tintinnid’s normal cell activities, excluding conjugation or sexual recombination. These activities include individual cell growth (governed by the macronucleus), mitosis of the micronucleus, amitotic division of the macronucleus, formation of a new oral region, proliferation of somatic cilia, and subsequent cell division (Montagnes, 2012). Cell divisions result in increases in the population of tintinnids. During repeated cell divisions, the amitotic division of the macronucleus can lead to the loss or dilution of some gene fragments essential to the functioning of the cell. It can also lead to an accumulation of handicapping mutations. These two phenomena can create an inability to conjugate and even the death of a tintinnid clone. Furthermore, the viability of asexual clones progressively declines in ciliates (Montagnes, 2012). To counteract this decline, tintinnids use a biological design solution: a form of sexual recombination, or conjugation. This process is however much less common in tintinnids than in other ciliates (Blackbourn, 1974). Sexual recombination or conjugation is what starts the life cycle of tintinnids (Blackbourn, 1974; Montagnes, 2012).
Conjugation is defined as a sexual process in which the two tintinnid cells exchange haploid micronuclei without reproducing (Wasserman, 2017). It is divided into three phases. The first phase is pre-conjugation, during which the two tintinnids search for each other and make contact in the oral region. The second phase consists of the formation of the cytoplasmic connection, or bridge, the meiosis of the micronuclei, and the exchange of gametic nuclei. The third phase is separation (Montagnes, 2012).
During conjugation, the micronuclei of the cell form gametic and haploid nuclei during a process called meiosis, which is a type of cell division consisting of two rounds of cell division and one round of DNA replication (Montagnes, 2012; Wasserman, 2017). One of the nuclei travels to another tintinnid cell to fuse with its stationary haploid nucleus. In other words, the two cells exchange micronuclei through cytoplasmic connections in their oral region, thus going through genetic recombination (Blackbourn, 1974; Montagnes, 2012). Once that genetic recombination is over, the old micronuclei are destroyed and replaced by new ones generated by newly formed micronuclei, like in the asexual binary fission (Montagnes, 2012; Prescott, 1994). Encystment or cyst formation is also an important part of the life of tintinnids, as it corresponds to the resting stage of the tintinnid’s life cycle. (Montagnes, 2012; Reid & John, 1978). It is unclear whether cysts have a reproductive function (Reid & John, 1978). These cysts are formed after the occurrence of conjugation. (Dolan, 2012).
Chemical composition of lorica
The lorica of the tintinnid has yet to be fully chemically characterized. It is hypothesized, however, that the lorica is made of chitin because of its resistance to strong bases; the shell was found to withstand potassium hydroxide at 160 °C for 40 minutes (Dolan, 2013). KOH has a pH of 13.5 so it is remarkable that the lorica could withstand it for so long and not dissolve (Thermo Fisher Scientific, 2009). This experiment suggested a chitinous composition; chitin is a strong polysaccharide due to its ability to H-bond with adjacent molecules. H-bonding is a special dipole-dipole interaction between an electronegative “donor” (denoted by δ– in Figure 3), and a hydrogen (denoted by δ+). It is an electrostatic interaction, not a polar bond which is why it is denoted with a dashed line, not a bond line. Chitin is made up of many oxygen and nitrogen molecules, which are electronegative “donors” to the hydrogens. Examples of H-bonding are highlighted in pink in Figure 3. When submerged in KOH, the base essentially tries to take an H+ from the chitin but fails because of the interactions the hydrogen has with adjacent oxygen and nitrogen atoms; electrostatic interactions essentially “anchor” the H atoms to make a strong polysaccharide (Seenuvasan, 2020).
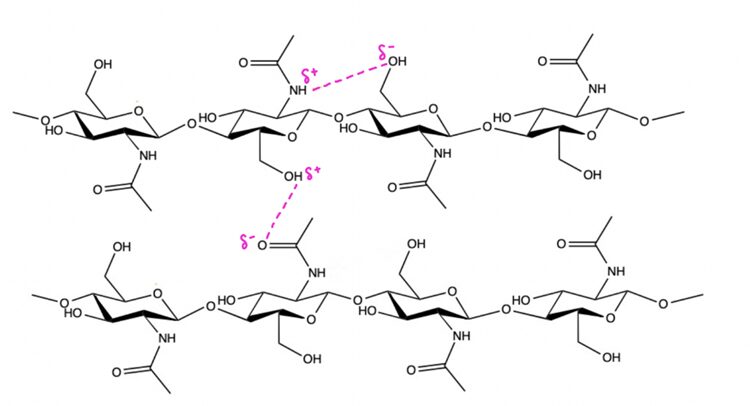
Fig. 3. Diagram of two chitin chains (each made of 2 chitin molecules). Dashed lines in pink represent H-bonding electrostatic interactions.
It can also be hypothesized, though, that the lorica wall may be of proteinaceous nature and composed of glycoproteins. This theory was proposed by Agatha & Simon in 2012. They attempted to characterize the lorica with two methods: enzymatic digestion and high-resolution transmission electron microscopy. The scientists placed the lorica in the chitinase enzyme and it failed to digest the shell, which suggests a non-chitinous composition. The experiment concluded a proteinaceous composition of the lorica shell by submerging the shell in different enzymes and seeing which enzyme did or did not digest it. The high-resolution transmission electron microscopy, which is a method that allows for direct imaging of molecules, revealed that the allegedly proteinaceous layer of the lorica is comparable to the s-layer (proteinaceous surface layer) of bacteria and archaea. This s-layer is made of a 2-D lattice of glycoproteins, an image of which is shown in Figure 4, and a diagram for a representation on the molecular level is shown in Figure 5.
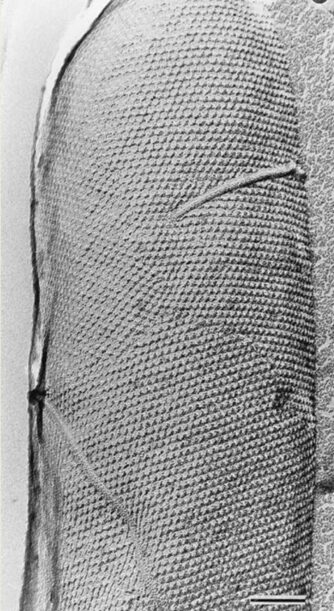
Fig. 4. High-resolution transmission electron microscopy image of S-layer hexagonal lattice of a whole cell. Bar represents 100 nm (Sára, 2000).
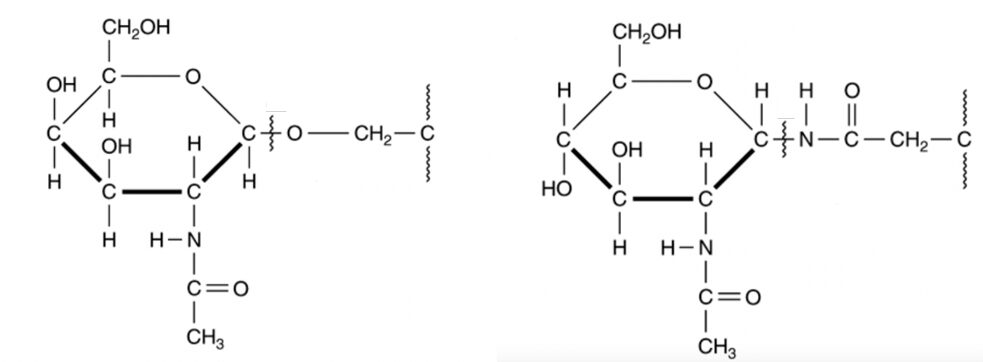
Fig. 5. O-linked (left) and N-linked (right) glycoproteins (Murray, 2017).
Note that the ring component of the glycoprotein is identical to the ring component of the chitin molecule. Therefore, the two hypotheses are not completely contradicting, and much of what was discussed about the structural integrity of the chitin chains applies to the glycoproteins. The difference between the two is the way in which the ring components are bonded to each other. Agatha & Simon (2012) also did an Energy Dispersive X-ray analysis of the lorica which is a way of characterizing the chemical composition of materials by identifying atoms based on the characteristic X-rays they emit when interacting with indecent beam electrons (Figure 6). Nitrogen was found in the Energy Dispersive X-ray analysis of the lorica which may point to N-linkage, however, it is possible that this N found is from the ammonia byproduct of the tintinnid’s chemical reaction for digestion (Verity, 1985). In any case, whether the lorica is composed of N-linked glycoprotein, O-linked glycoprotein, or O-linked chitin, the bottom line is that both Nitrogen and Oxygen can participate in H-bonding which is what gives lorica its strength.
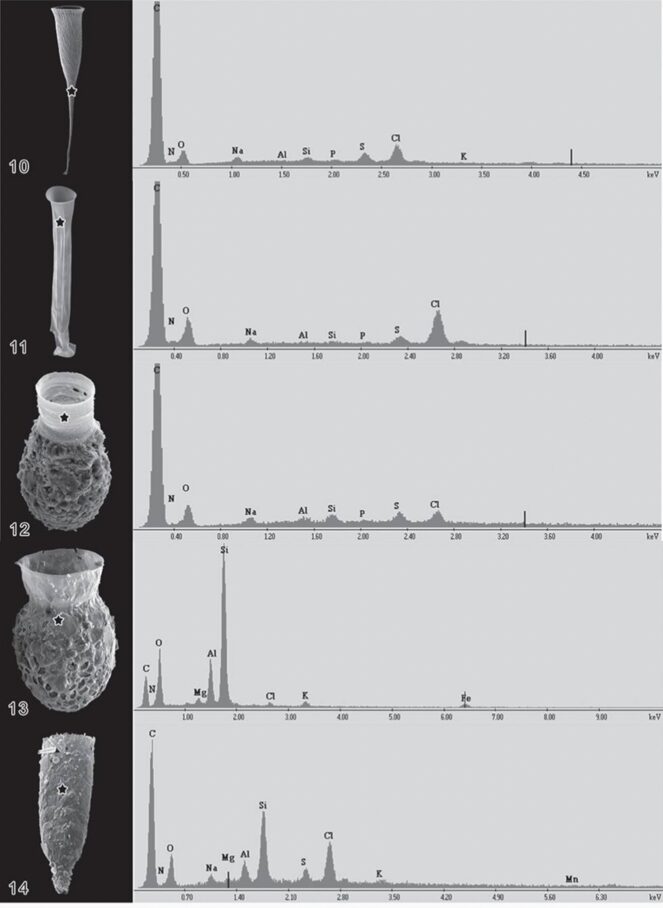
Fig. 6. Energy Dispersive X-Ray analysis of a variety of Lorica, with peaks labelled as corresponding chemical composition (Agatha & Simon, 2012).
Another important factor to consider, aside from structural integrity, is the Lorica’s “stickiness”. Tintinnids attach to agglomerated particles because the particles aid in their mobility to move to a more prey-dense area and they also aid in protection against predators. The larger a tintinnid is, the less vulnerable it is to be preyed upon (Dolan, 2012). What determines how sticky the lorica wall is, and therefore how easily it can attach to other particles, is the lorica wall’s chemical composition. Agatha and Simon (2012) hypothesize that though the lorica is mainly composed of proteins, traces of sodium, potassium, magnesium, and chlorine are also present on the wall of the lorica (Figure 6). These are all elements that are prone to forming ions (Na+, K+, Mg 2+, Cl–). Ion-ion interactions are the strongest intermolecular force, so it is highly beneficial for the lorica to have ions that can participate in these electrostatic interactions. In addition, a variety of anions and cations in the lorica wall allow for a greater variety of potential particles to attach to. For instance, if the lorica was exclusively composed of anions it would only be able to have ion-ion interactions with positively charged particles which limits the possible particles they can attach to. With the lorica being made up of both anions and cations, both negatively and positively charged particles can attach, allowing for more mobility and protection (Agatha & Simon, 2012).
Chemical signaling and ciliate retraction
The ciliate can retract via a contractile stalk into the lorica when faced with danger. This stalk houses a type of fiber called spasmoneme (or myoneme) which contracts when exposed to calcium. It does so by coiling helically as shown in Figure 7. The coiling response is triggered by the protein spasmin in the contractile stalk encountering Ca2+. The velocity at which the coiling occurs depends on the concentration of calcium present; the higher the concentration, the greater the speed of contraction (Misra, 2010). The protein sensors are very sensitive; a Ca2+ concentration of 10-7 M is enough to trigger the contraction. The ciliate then goes back to its original state when calcium is dissociated by the protein spasmin and there is no longer a significant Ca2+ concentration in adjacent waters.
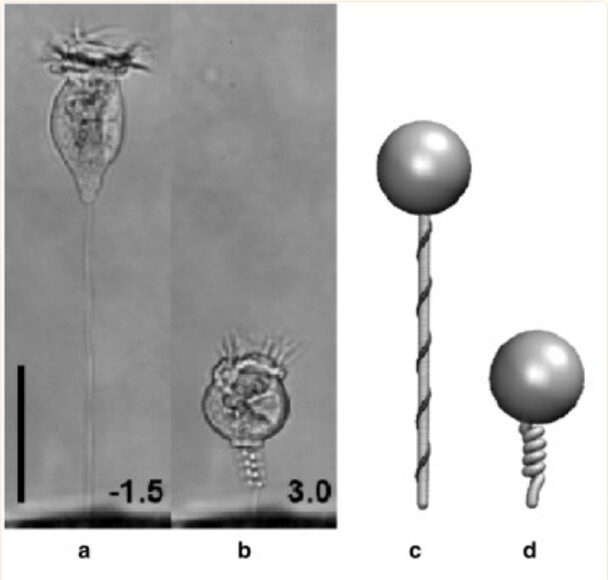
Fig. 7. Image of tintinnid ciliate in extended(a) and contracted(b) state. Represented more simply in a simulation (c, d). Bar represents 50 μm, with a millisecond timescale in (a, b) (Misra, 2010).
What is remarkable about this process is that it is completely ATP-independent and relies on the energy of the chemical interaction between spasmin and calcium, which explains why the rate of contraction depends on the concentration of Ca2+. Another important aspect is that a contraction only occurs when danger is sensed, which is indicated by a certain concentration of Ca2+. Certain types of parasitic protozoa use Ca2+ signaling when they are invading a host cell. This means an increase in Ca2+ concentration in adjacent areas as parasitic protozoa attempt to invade a tintinnid ciliate. It is therefore extremely advantageous that the tintinnid retracts into its shell when it senses an increase in calcium ion concentration; tintinnids have adapted to avoid these parasites (Misra, 2010).
Using the tintinnid species Favella as a case study, we can examine the chemical pathways responsible for various aspects of tintinnids’ survival. These pathways control how tintinnids navigate their environment, locate food, and avoid dangers. Figure 8 below shows an overview of the chemical pathways that will be described throughout the rest of the essay.
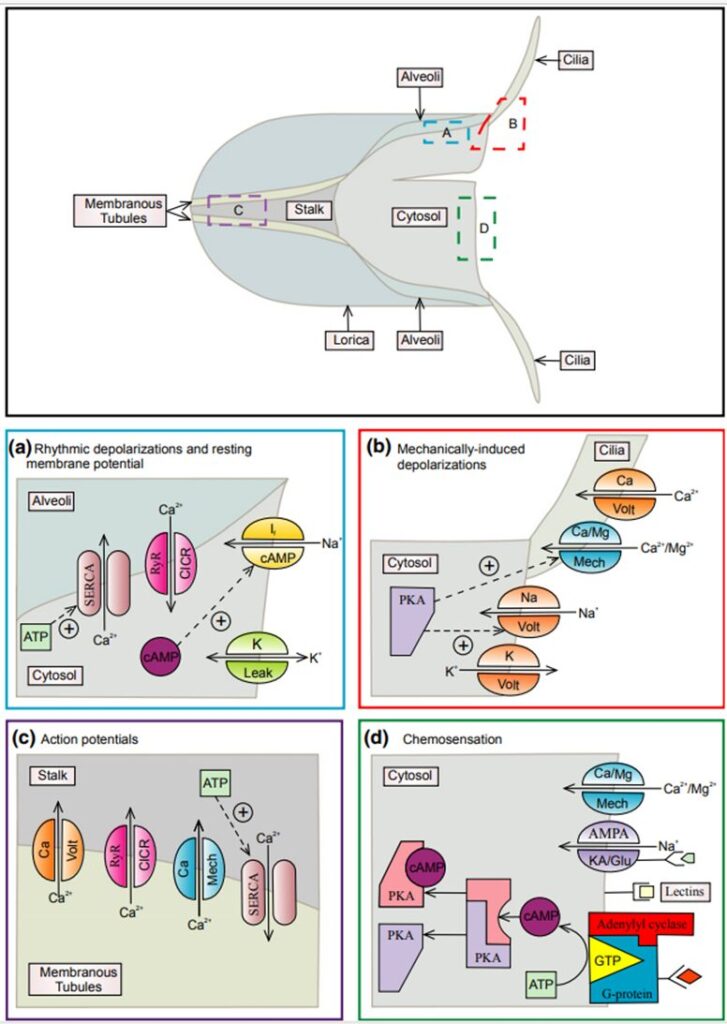
Fig. 8. A working model proposed by (Echevarria et al., 2014) of the sensory mechanisms in Favella. The top of the figure shows the structure of Favella, with colored boxes corresponding to specific sensory mechanisms in the bottom half, illustrating these processes in different regions of the cell. The upper labels of the ion channels identify the ions they are permeable to, and the lower label is the stimuli that activate them. Panel (a) illustrates the chemical pathways involved in regulating the swimming response in tintinnid Favella. Panel (b) depicts the chemical pathways involved in regulating the response to mechanical stimuli in tintinnid Favella. Panel (c) illustrates the chemical pathways involved in regulating the retraction response in tintinnids. Panel (d) depicts some known chemical pathways involved in chemosensation in tintinnid Favella.
Panel (c) of Figure 8 shows an in-depth analysis of the chemical pathways responsible for retraction into the lorica. Membranous tubules surrounding the stalk of Favella store Ca2+ which are pumped in by sarco/endoplasmic reticulum Ca2+-ATPases (SERCA) enzymes (Echevarria et al., 2014). If the cilia or cell membrane comes into physical contact with a powerful stimulus, depolarization occurs, activating voltage-gated calcium channels, located in the membranous tubules. This leads to an increase in Ca2+ levels in the stalk which activates calcium-induced calcium release channels (CICR) called ryanodine receptors (RyR) in the membranous tubules causing a further increase in stalk Ca2+ levels. This creates a positive feedback loop and generates an action potential. Ca2+ then binds with contractile proteins in the stalk resulting in rapid retraction of the cell into the lorica (Echevarria et al., 2014).
Swimming and buoyancy
Tintinnids possess various adaptations that help them regulate their buoyancy and swimming mechanics. Buoyancy is facilitated by inducing a change in the shape of the cell. This is achieved by the specialized contractile organelles known as spasmonemes. The filaments twist to shorten and contract inducing a change in shape and volume of the cell. This change in volume is what aids in buoyancy regulation.
The organization of tintinnid’s specialized structures determines their locomotion, feeding, and ingestion mechanisms. Like many other ciliates, tintinnids’ cilia are fused together in bundles called adoral membranelles that are located around the cell mouth (Echevarria et al., 2014). The axoneme of cilia is connected to a permanent feature, the basal body, which is connected by a complex subcortical system known as kineties (Febvre-Chevalier & Febvre, 1994). This system consists of overlapping microtubules and strands of non-actin filaments that lie beneath the cell membrane. The distribution of this system and the cilia influences the swimming behavior of tintinnids. Depending on the spacing of adjacent cilia in the kineties, the interference of cilia can lead to either a weak delayed force of coordinated metachronal waves or a stronger synchronized movement (Febvre-Chevalier & Febvre, 1994). Swimming mechanisms, and the structures that influence them, are essential to tintinnids’ survival.
Chemical pathways of swimming responses
Tintinnids make use of ions to control hyperpolarization and depolarization of the cell membrane which plays an active role in controlling swimming motions. Hyperpolarization of the cell membrane is defined as the state when the electrical potential across the membrane increases, becoming more negative than the cell’s resting membrane potential. This is caused by an outflow of positive calcium ions from the interior of the cell. Depolarization, on the other hand, is when the electrical potential across the membrane decreases, becoming more positive than the cell’s resting membrane potential. The interior of the cell is now more positive than the exterior. Stimulation initiates a reaction that causes the voltage-dependent calcium channels located at the base of the ciliary membrane to open. This raises the internal concentration of calcium ions above 10-6 M (Febvre-Chevalier & Febvre, 1994).
Two different signal transduction mechanisms control the action of ciliary beating and the beat frequency. Ciliary beating is regulated by ionotropic signal mechanisms and the beat frequency is regulated by metabotropic signal mechanisms.
Ionotropic signals are those controlled by voltage, chemo-sensitive, or mechano-sensitive ion channels. The ion channels, especially calcium ion channels, present in these cilia control various responses to stimuli, in particular, ciliary beating (Echevarria et al., 2014). In order for their cilia to beat, dynein arms must be activated, which are regulated by the intracellular calcium concentration (Febvre-Chevalier & Febvre, 1994).
Metabotropic signaling involves receptor proteins located on the membrane surface, second messengers located in the interior of the cell, and enzymes (Echevarria et al., 2014). In ciliates, these receptor proteins are called G-protein-coupled receptors (GPCR). They transmit signals from the outside to the inside of the cell. When the cell receives a chemical or mechanical signal in the form of external solutes, it binds to a GPCR, activating a G-protein and initiating a cascade of reactions leading to a cellular response (Echevarria et al., 2014). The reactions that follow involve the enzyme adenylyl cyclase which catalyzes the conversion of adenosine triphosphate (ATP) to cyclic adenosine monophosphate (cAMP). This often leads to phosphorylation of target molecules by protein kinase A. These reactions can eventually activate ion channels, demonstrating the link between metabotropic and ionotropic pathways (Echevarria et al., 2014). The beat frequency is regulated by cAMP, cyclic guanosine monophosphate (cGMP), and folic acid (Febvre-Chevalier & Febvre, 1994).
Panel (a) from Figure 8 describes the chemical pathways that are responsible for ciliary movement. It explains the rhythmic depolarizations (RD) and the resting potential of the tintinnid species Favella. The resting membrane potential is primarily determined by the concentration of K+ ions (Echevarria et al., 2014). SERCA is responsible for actively pumping Ca2+ into cellular compartments called alveoli. The location of the alveoli in the tintinnid cell can be seen in the top panel of Figure 8. When alveolar Ca2+ reaches a threshold concentration, CICR and RyR are activated. This leads to the release of Ca2+ into the cytosol from the alveoli, resulting in depolarization of the cytosol. This depolarization activates voltage-gated Na+ channels which results in RDs. RDs open the voltage-dependent K+ channels, which return the cell to its resting membrane potential. The RD loop might also be influenced by cyclic AMP (cAMP) through cyclic nucleotide-sensitive ion channels (Echevarria et al., 2014).
Chemoreception
Chemoreception is a crucial ability for ciliates, such as tintinnids, and their survival as it helps them locate and identify their preferred prey. Chemoreception can be divided into two categories: distance and contact chemoreception, similar to the senses of smell and taste, respectively. Tintinnids are able to sense the presence of prey without making direct contact, effectively “smelling” nearby prey.
Many studies have shown that ciliates will congregate in areas where their preferred prey is detected and actively avoid other organisms that may be harmful. Interestingly, this accumulation does not necessarily mean that the prey is present. An experiment done by (Buskey & Stoecker, 1989) showed that tintinnids accumulated preferentially in pipets that contained only exudates (fluid containing cells and proteins) of prey. This demonstrates that tintinnids’ ability is not in detecting the prey themselves but in detecting the chemical signals of these prey.
Several factors have been found to influence chemosensory responses in ciliates. For example, historical diet influences the prey that tintinnids select. In an experiment conducted by Verity, two identical populations of a tintinnid species were each fed a different prey for several generations: one population was fed the flagellate Isochrysis and the other was fed the diatom Minutocellus polymorphus (Verity, 1988). Both species are of similar size and nutritional content and support the growth of tintinnids. The population that was previously fed Isochrysis, showed a strong preference for Isochrysis over Minutocellus when tested. They accumulated preferentially in areas containing this Isochrysis. And indeed, the converse is true; the population that was acclimated to Minutocellus, also preferentially accumulated around Minutocellus. In other words, their chemosensory response was stronger towards the prey they had been feeding on. The previous diet of tintinnid populations significantly influences their chemosensory behavior when given a choice of prey. It is almost as though tintinnids have “memory”! Their ability to adjust their feeding strategy based on their previous experiences optimizes their foraging efficiency. They capitalize on the most readily available and familiar food sources, allowing them to survive in their environment.
The same study also showed that tintinnid’s chemosensory behavior is influenced by the physiological state of the prey (Verity, 1988). Tintinnids were more attracted to prey that were actively growing (and thus assumed to be more nutritious) than a prey population that was stagnant or declining. The chemical signature of growing prey is still unknown. It was hypothesized that an increase in the carbon-to-nitrogen ratio indicates the growth of prey however, it was found that tintinnids exhibit chemosensory responses even before changes in the ratio are measured (Verity, 1988). Thus, there are two possibilities: tintinnids may be responding to a different chemical, or tintinnids possess extremely precise chemical sensory mechanisms that greatly surpass our current technology. Overall, this process demonstrates that tintinnids can impressively fine-tune their behavior based on the availability and quality of their prey.
While it is clear that tintinnids exhibit chemosensory responses, the role and processes of chemoreception in selective feeding are not well understood (Verity, 1988). The data from the study showed that tintinnids respond to chemical signals in their environment, however exactly how they respond is still unclear. Tintinnids possess receptor systems that enable them to detect changes in the spatial distribution (location) and temporal variation (changes over time) of certain chemicals in their surroundings (Verity, 1988). Tintinnids can detect when the concentration of a particular substance is higher in one direction compared to another and can also if the concentration of a substance is increasing or decreasing over a period. Their receptor systems are sensitive enough to detect minuscule variations in chemical cues.
The response to these changes can be either chemotactic or chemokinetic (Verity, 1988). Chemotactic behavior refers to a directed movement of the cell in response to the chemical gradient. On the other hand, chemokinetic behavior is a random movement of a cell in response to the presence of a chemical. The ciliate may not necessarily move in a specific direction but may exhibit changes in movement patterns or speed. There is still insufficient information to determine whether the ciliate’s responses are chemotactically or chemo-kinetically driven (Verity, 1988). However, some hypothesize that the response is likely kinetic rather than taxic (Echevarria et al., 2014). This means that while the ciliates appear to respond to chemical cues, the exact nature of their responses, in terms of directionality and purpose, is still undetermined. Furthermore, the exact sensory reception and transduction pathways are still unknown (Echevarria et al., 2014).
In response to these chemical signals, tintinnids alter their swimming behavior. When the tintinnid species Favella was exposed to their preferred prey, they showed a decrease in swimming speed, an increase in turning rate, and a decrease in their net displacement (Buskey & Stoecker, 1989). These changes allow Favella to remain in areas with prey abundance. This response is short-lived and lasts for only 30-45 minutes before swimming patterns revert to those present before the addition of any prey. These changes were also observed when Favella was exposed to exudates of prey. When tintinnids sense or encounter prey cells, their ciliary motion reverses, meaning they stop their helical swimming pattern, briefly reverse their swimming, then resume their helical swimming at a lower angle, effectively making a closed circle (Buskey & Stoecker, 1989). The converse is also true. When tintinnids are exposed to potentially toxic chemical signals, their swimming speed increases and turning decreases, allowing tintinnids to swiftly escape the area. Chemoreception thus allows tintinnids to differentiate between food or danger based on the chemical signals they detect and alter their behavior in response. They can remain in areas with high prey concentration as well as avoid and escape areas with toxic or harmful organisms.
Chemical pathways involved in mechano- and chemoreception
Understanding some of the pathways that govern chemoreception and tintinnids’ responses gives us a glimpse of the impressive adaptations crucial to tintinnids’ survival. Searching behaviors in the Favella species of tintinnid are directly controlled by changes in the membrane potential. For example, when prey comes in contact with (contact chemoreception/”taste”) the adoral membranelles, the cell depolarizes resulting in ciliary reversal, backward swimming, and accumulation of Favella in the area of high prey concentration (Echevarria et al., 2014). When the membranelles are stimulated, stretch-activated ion channels open to allow Ca2+ to enter the cell and trigger depolarization and voltage-activated Ca2+ and Na+ channels that lead to the backward-swimming response. To finish the cycle, depolarization activates voltage-dependent K+ channels causing the cell to return to the resting membrane potential, and swimming returns to normal (Echevarria et al., 2014). The chemical pathways following mechanical stimulation are shown in panel (b) of Figure 8. A similar process occurs when tintinnids sense chemical cues in the area through distance chemoreception (“smell”). The mechanism of how these dissolved chemical cues are perceived by tintinnids is not well understood, but it is hypothesized that GPCR pathways are important in chemoreception (Echevarria et al., 2014).
Some genes code for specific processes that are involved in swimming behavior and chemoreception. Studies have found that the genes for adenyl cyclase, an important enzyme in the creation of cAMP and GPCR signal pathways are localized in the cilia (Echevarria et al., 2014). They potentially regulate Ca2+ channels and ciliary movements. Mutating these genes resulted in the inability of the ciliate to respond to chemical signals thus reducing their locomotion and feeding significantly (Echevarria et al., 2014). This shows the importance of signal reception via CPCR and cAMP ion channels in regulating chemoreception, prey location, and swimming behavior.
The final step of the prey capture and selection process likely involves receptor-ligand interactions between the cell surfaces of both predator and prey. As part of this surface recognition system, lectins (carbohydrate-binding proteins) bind to mannose (a sugar) residues found on the surface of prey particles (Echevarria et al., 2014). This then triggers signal cascades that result in the rest of the prey capture and feeding process.
Panel (d) from Figure 8 illustrates the chemosensory chemical pathways. Chemical ligands bind to G-protein receptors, activating the G-protein-adenylyl cyclase complex (Echevarria et al., 2014). Adenylyl cyclase converts ATP to cAMP, which activates protein kinase A (PKA). PKA then opens ion channels when the AMPA/KA receptors are bound by AMPA. The resulting depolarization opens voltage-gated Na+ channels as in (a) (Figure 8. The presence of dissolved AMPA may signal the release of contents from injured neighboring cells, initiating escape responses in Favella (Echevarria et al., 2014) such as increased swimming speed and decreased turns.
Predation
Tintinnids combine the artistry of delicate, often transparent structures with the voracity of predators. Predominantly found in marine waters, they are vital cogs in the machinery of the aquatic food chain. Although small in size, their influence spans the entirety of their ecosystems, highlighting their role as pivotal predators that employ a suite of chemical strategies to secure sustenance. As previously mentioned, the production of mucus, the viscous substance responsible for the capture of prey, is central to tintinnids’ evolutionary resilience and success. This mucus, deployed with remarkable precision, acts as both lure and trap, setting the stage for the tintinnid’s subsequent predatory actions. Prorocentrum cells inside the mucus trap rotate in order to eddy water and prey into the trap, while the viscosity of the outside of the trap sticks to prey not directly oriented into the tintinnid’s mouth. When prey is stuck to the outside, the chance of full entanglement within the trap jumps drastically (Tillman et al. 2023). Once prey is entangled completely, chemical signaling takes center stage. Tintinnids release compounds that alter the behavior of their prey, subduing them and making ingestion easier. Following this, ciliary movements, accentuated by a cascade of chemical reactions, draw the immobilized prey into the tintinnid’s cell, where intracellular digestion begins (Dolan et al. 1991). Here, an array of enzymes break down the prey, converting it into nutrients, a striking example of chemical predation at work.

Fig. 9. Feeding position of D. acuta with prey that has been digested. The arrow indicates the remains of the prey (Ojamäe et al. 2016).
The prey spectrum of tintinnids is varied, encompassing a range of smaller planktonic organisms. Each prey type presents a unique challenge, with chemical interactions tailored to the specific defense mechanisms of the target. Tintinnids exhibit remarkable adaptability, altering their chemical arsenal to neutralize different prey effectively. This versatility ensures their survival and success as predators within the competitive planktonic realm (Dolan et al. 2013). Chemical predation is not an isolated strategy but part of a broader suite of feeding behaviors exhibited by tintinnids. It stands in contrast to the more energy-intensive physical predation strategies employed by larger zooplankton. Through chemical means, tintinnids can capture prey with minimal energy expenditure, an advantageous adaptation in the nutrient-sparse open oceans. However, this method is not completely without its energetic costs, which are factored into the tintinnid’s overall energy budget.
Recent empirical evidence also underscores a critical aspect of tintinnid predation: their precise spatial-temporal predation strategy. This phenomenon reveals tintinnids’ capacity to fine-tune their predatory tactics in response to the dynamic marine environment, ensuring optimal feeding efficiency and survival. Tintinnids exhibit a sophisticated spatial predatory strategy. They actively target regions of high planktonic density, capitalizing on prey abundance. Recent studies from the Department of Marine Science at the University of San Diego confirm that spatial variation accounted for 21% of the variance in species composition. This indicates that the variation of species across different areas is due to environmental factors such as nutrient density (Elliot et al. 2007). Such strategic congregation in nutrient-rich zones enhances their feeding success rate. This spatial precision in predation not only indicates their acute environmental awareness but also reflects an evolutionary response to the heterogeneous distribution of prey in marine ecosystems.
Tintinnids simultaneously align their predation rhythm to match the diurnal cycles of their prey, illuminating why the prime numbered hibernation period of cicadas makes evolutionary sense from a prey standpoint. By intensifying their predatory activities during peak prey activity hours, tintinnids significantly increase their capture rate. This temporal alignment is further refined by seasonal adjustments. Studies from the UCSD Department of Marine Science also indicate that 34% of the variance in species composition was explained by the temporal distribution of species, implying that the types of species found in areas change during different parts of the year (Elliot et al. 2007). These adaptations underscore tintinnids’ role as highly efficient and adaptive predators. Their spatial-temporal precision in predation is a clear manifestation of evolutionary refinement, allowing them to sustain their ecological niche in the fluctuating marine environment.
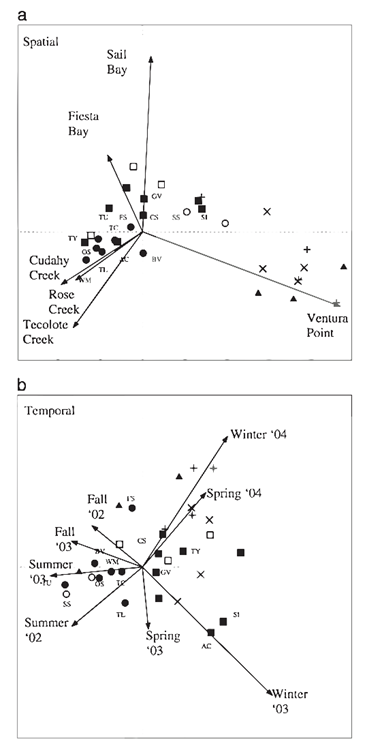
Fig. 10. Spatial and temporal distribution of and relationships among zooplankton species in Mission Bay. Each symbol represents a species (species labels included for common species seen in 25% or more of samples), and symbols correspond with co-occurrence dusters (solid circle = 1A, open circle = 1B, solid square = 2A, open square = 2B, X = 3, + = 4, solid triangle = 5, black point = species not clustered into any group). The vectors represent environmental variables, sampling stations in the case of the spatial ordination, and seasons according to year for the temporal ordination (Elliot et al. 2007).
Role as bioindicators
Tintinnids’ presence and abundance have been observed to correlate strongly with the health and stability of their marine habitats, thus positioning them as valuable bioindicators. Bioindicators are species or groups of species whose function, population, or status can reveal the qualitative status of the environment. In the global context, as well as the specific context of the Indian East Coast, tintinnids provide a window into the health of marine ecosystems, making them crucial for the ecological monitoring of water quality, pollution, and overall ecosystem health (Rakshit et al. 2017).
Historically, the use of living organisms as indicators of environmental change is well-established, and tintinnids have been part of this tradition due to their sensitivity to changes in water quality. Tintinnids are considered exemplary bioindicators because of certain characteristics. They have a rapid turnover rate, meaning their populations can quickly reflect changes in the environment, have large populations which means global comparisons can be made, and are extremely easy to study as they can be collected using traditional plankton nets and studied microscopically (Rakshit et al. 2017). For instance, studies on the migration of tintinnids are currently being used to indicate shifts in the global climate. In a study on Pelagic ciliate communities in the Southern Ocean, a shift of 2 degrees towards the south pole was observed in the monitored populations (Chaofeng, et al. 2023). The shift was a direct result of tintinnids seeking water temperatures more suitable for their needs after the average temperature of the Southern Ocean rose.
Monitoring ecosystem changes through tintinnid populations provides a detailed picture of ecological shifts. These organisms respond primarily to changes in the levels of chlorophyll a (chl a), nitrate, and phosphate which are environmental stressors for most microscopic marine animals (Rakshit et al. 2017). This property of detecting harmful pollutants makes them reliable sentinels not only for themselves but also for entire ecosystem degradation or recovery. For example, a decline in tintinnid diversity has been associated with increased levels of pollutants, while recovery of a population often indicates an improvement in water quality. In a study conducted at the Marine Fishery Research Institute of Zhejiang, the impact of the pollutant soluble reactive silicate (SRSi) was explored. The study found a clear correlation between the abundance of the pollutant and the degradation of species richness in the water. (Meiping, et al. 2015). In the table below, the results of this study are represented using factors total abundance (N), species diversity (H′), species evenness (J′), and richness (D). Total Abundance (N) is the total number of individuals of all species, Species Diversity (H′) measures both the number and relative abundance of species, Species Evenness (J′) assesses how equally different species are represented, and Richness (D) is the count of distinct species in a community
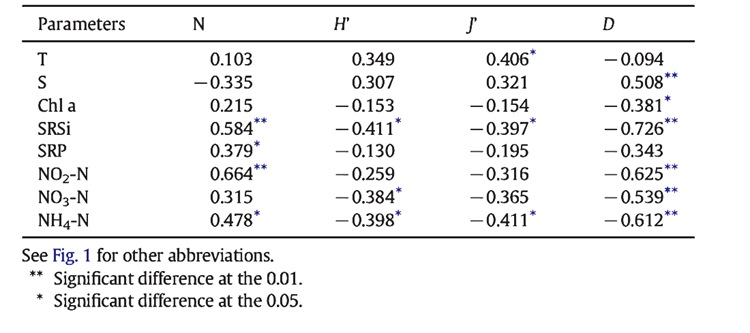
Fig. 11. Correlations between environmental variables and total abundance (N), species diversity (H′), species evenness (J′), and richness (D) of tintinnid community at four sites in Jiaozhou Bay during a 7-year cycle (2006–2012).
The strong correlation within this chart, as well as the sensitivity and self-preservation we have observed in the studies of tintinnids in the South Ocean and on the Indian East Coast, highlights the valuable role that tintinnids play in our understanding of the never-ending shifts of nature and provides us with the context in which to decide the value that these minuscule creatures have for the human race.
Conclusion
Tintinnids are remarkable organisms that employ a wide variety of chemical processes to survive and thrive in the aquatic environment. Reproducing, finding prey, and avoiding danger are among the main challenges they face.
To survive as an organism, tintinnids rely on having effective reproduction mechanisms. Tintinnids reproduce primarily through asexual means i.e. cell division. However, this can lead to the loss of genetic information and genetic mutations that severely impact the functioning of the cell. This presents an interesting dilemma; reproduction is vital, but it simultaneously threatens the survival of the species. Tintinnids overcome this by utilizing a form of sexual reproduction called conjugation. This counteracts the decline in viability of asexual clones and ensures the long-term survival of tintinnids.
The majority of tintinnid’s time and energy is spent on finding and consuming prey. Consequently, they have evolved mechanisms for efficiently locating and consuming prey in their vast marine environment. Tintinnids employ chemoreception to sense the presence of prey even at a distance. Their chemosensory mechanisms are extremely precise, and they are even able to detect the nutritional quality of prey to optimize their foraging efficiency. Furthermore, tintinnids possess a unique sort of “memory” despite not having a brain. Through mechanisms still unknown, tintinnids alter their behavior and feeding patterns based on their previous experiences. The historical diet of tintinnid populations influences their feeding choice, for the current generation and future generations. The observed “memory” in tintinnids suggests a form of learning, where their chemosensory responses are tuned to recognize and prefer the specific chemical signals associated with their historical diet. Thus, they locate prey more efficiently by selecting the most familiar and readily available food sources. When their prey is detected through chemoreception, their swimming behavior is altered to move towards areas with abundant prey. Their chemosensory abilities influence their spatial-temporal tactics that allow them to congregate in regions of high planktonic density. Furthermore, they align their predatory activities with diurnal cycles and adapt to seasonal shifts in prey abundance, all of which are facilitated through “memory” and chemoreception. Once they are surrounded by prey, tintinnids employ a slimy design solution to lure and trap their prey: their mucus. Tintinnids deploy their mucus which sticks to their prey. The cells found within the mucus trap rotate to draw the prey in. The prey then becomes completely entangled and the tintinnid is ready to feast, starting a cascade of chemical reactions. Tintinnids release compounds to immobilize their prey and start the ingestion and digestion process. Within the tintinnid cell, a variety of enzymes break down the captured prey, converting it into nutrients to sustain the tintinnid. Their chemoreception and predation strategies are extremely sophisticated and contribute to the success of tintinnids’ survival in their habitats
Tintinnids evade danger by employing multiple impressive strategies. One notable defense mechanism is their ability to alter swimming behavior in response to chemical cues. Utilizing chemoreception adaptations, tintinnids can adjust their swimming behavior when exposed to areas containing potential threats, harmful organisms, or toxic chemicals. Tintinnids swiftly adjust their swimming patterns, increasing speed and decreasing turning rate, to increase their net displacement. This chemically driven response allows them to navigate away from hazardous zones and actively avoid contact with harmful organisms. Additionally, tintinnids possess a retraction mechanism that protects them when danger is imminent. Tintinnids can rapidly withdraw into their strong, protective lorica, which is chemically engineered to keep them safe. The various chemical processes and the responses they initiate allow tintinnids to evade danger and survive in the vast and dangerous aquatic environment.
Tintinnids’ survival is ultimately governed by the complex and sophisticated chemical reactions that control everything from reproduction to danger evasion. Though tintinnids are a simple organism, their design solutions are not. Not only do these processes help us better understand life, but they also offer intriguing possibilities for applications in engineering. For example, their chemosensory abilities are even more precise than our current technology. This could be an exciting area of research that can help in evaluating water quality and developing new sensors. Additionally, studying their mucus could lead to the development of biological glue alternatives. By zooming in on these microscopic organisms and their design solutions, engineers can draw inspiration and drive innovation across diverse industries.
References
Agatha, S., & Simon, P. (2012). On the Nature of Tintinnid Loricae (Ciliophora: Spirotricha: Tintinnina): a Histochemical, Enzymatic, EDX, and High-resolution TEM Study. Acta protozoologica, 51(1), 1–19.
Blackbourn, D. J. (1974). The feeding biology of tintinnid protozoa and some other inshore microzooplankton [Text, https://open.library.ubc.ca/collections/831/items/1.0053248
Chaofeng, et al. “Pelagic Ciliate (Ciliophora) Communities in the Southern Ocean: Bioindicator to Water Mass, Habitat Suitability Classification and Potential Response to Global Warming.” Progress in Oceanography, vol. 216, 1 Aug. 2023, p. 103081, www.sciencedirect.com/science/article/pii/S0079661123001246, https://doi.org/10.1016/j.pocean.2023.103081. Accessed 4 Nov. 2023.
Coats, D. W., & Bachvaroff, T. R. (2012). Parasites of Tintinnids. In The Biology and Ecology of Tintinnid Ciliates (pp. 145-170).
https://doi.org/https://doi.org/10.1002/9781118358092.ch6
Dolan, J. R., & Coats, D. W. (1991). Preliminary prey digestion in a predacious estuarine ciliate and the use of digestion data to estimate ingestion. Limnology and Oceanography, 36(3), 558–565. https://doi.org/10.4319/lo.1991.36.3.0558
Dolan, J. R., Landry, M. R., & Ritchie, M. E. (2013). The species-rich assemblages of tintinnids (marine planktonic protists) are structured by mouth size. The ISME Journal, 7(6), 1237–1243. https://doi.org/10.1038/ismej.2013.23
Dolan, J. R. (2012). Introduction to Tintinnids. In The Biology and Ecology of Tintinnid Ciliates (pp. 1-16). https://doi.org/https://doi.org/10.1002/9781118358092.ch1
Echevarria, M. L., Wolfe, G. V., Strom, S. L., & Taylor, A. R. (2014). Connecting alveolate cell biology with trophic ecology in the marine plankton using the ciliate Favella as a model. FEMS Microbiology Ecology, 90(1), 18-38. https://doi.org/10.1111/1574-6941.12382
Elliott, David T., and Ronald S. Kaufmann. “Spatial and Temporal Variability of Mesozooplankton and Tintinnid Ciliates in a Seasonally Hypersaline Estuary.” Estuaries and Coasts, vol. 30, no. 3, June 2007, pp. 418–430, https://doi.org/10.1007/bf02819388. Accessed 1 Apr. 2019.
Febvre-Chevalier, C., & Febvre, J. (1994). Buoyancy and swimming in marine planktonic protists. In J. M. V. Rayner, L. Maddock, & Q. Bone (Eds.), The Mechanics and Physiology of Animal Swimming (pp. 13-26). Cambridge University Press. https://doi.org/DOI: 10.1017/CBO9780511983641.003
Feng, M., Lin, S., Zhang, W., Wang, C., Liu, H., Cheung, S., Li, H., Stukel, M. R., Irving, J. P., & Li, N. (2022). Micro-/Meso-Scale distinction and horizontal migration of tintinnid (Ciliophora: tintinnida) assemblages in three regions around the North Pacific Ocean. Frontiers in Marine Science, 9. https://doi.org/10.3389/fmars.2022.863549
Meiping, et al. “Can Tintinnids Be Used for Discriminating Water Quality Status in Marine Ecosystems?” Marine Pollution Bulletin, vol. 101, no. 2, Dec. 2015, pp. 549–555, https://doi.org/10.1016/j.marpolbul.2015.10.059. Accessed 25 Aug. 2022.
Misra, G., Dickinson, R. B., & Ladd, A. J. (2010). Mechanics of Vorticella contraction. Biophysical journal, 98(12), 2923–2932. https://doi.org/10.1016/j.bpj.2010.03.023
Montagnes, D. J. S. (2012). Ecophysiology and Behavior of Tintinnids. In The Biology and Ecology of Tintinnid Ciliates (pp. 85-121). https://doi.org/https://doi.org/10.1002/9781118358092.ch4
Murray, R. (2017, February 17). Glycoproteins. Basic Medical Key. https://basicmedicalkey.com/glycoproteins/ (Accessed 2023-11-18)
Ojamäe, K., Hansen, P. J., & Lips, I. (2016). Mass entrapment and lysis of Mesodinium rubrum cells in mucus threads observed in cultures with Dinophysis. Harmful Algae, 55, 77–84. https://doi.org/10.1016/j.hal.2016.02.001
Prescott, D. M. (1994). The DNA of ciliated protozoa. Microbiological Reviews, 58(2), 233-267. https://doi.org/doi:10.1128/mr.58.2.233-267.1994
Rakshit, D., Sahu, G., Mohanty, A. K., Satpathy, K., Jonathan, M., Murugan, K., & Sarkar, S. K. (2017). Bioindicator role of tintinnid (Protozoa: Ciliophora) for water quality monitoring in Kalpakkam, Tamil Nadu, south east coast of India. Marine Pollution Bulletin, 114(1), 134–143. https://doi.org/10.1016/j.marpolbul.2016.08.058
Rakshit, et al. Bioindicator Role of Tintinnid (Protozoa: Ciliophora) for Water Quality Monitoring in Kalpakkam, Tamil Nadu, South East Coast of India. Vol. 114, no. 1, 1 Jan. 2017, pp. 134–143, https://doi.org/10.1016/j.marpolbul.2016.08.058. Accessed 24 July 2023.
Reid, P. P. C., & John, A. W. G. (1978). Tintinnid cysts. Journal of the Marine Biological Association of the United Kingdom, 58(3), 551-557. https://doi.org/10.1017/S0025315400041205
Sára, M., & Sleytr, U. B. (2000). S-Layer proteins. Journal of bacteriology, 182(4), 859–868. https://doi.org/10.1128/JB.182.4.859-868.2000
Scarpelli, P. H., Pecenin, M. F., & Garcia, C. R. S. (2021). Intracellular Ca2+ Signaling in Protozoan Parasites: An Overview with a Focus on Mitochondria. International journal of molecular sciences, 22(1), 469. https://doi.org/10.3390/ijms22010469
Seenuvasan, M., Sarojini, G., Dineshkumar, M. (2020). Recovery of Chitosan from natural biotic waste. Current Developments in Biotechnology and Bioengineering, 24(1), 115-133. https://doi.org/10.1016/B978-0-444-64321-6.00006-9.
Strom, S. L. (2001). Light-aided digestion, grazing and growth in herbivorous protists. Aquatic Microbial Ecology – AQUAT MICROB ECOL, 23, 253-261. https://doi.org/10.3354/ame023253
Thermo Fisher Scientific, Regulatory Affairs . “SAFETY DATA SHEET POTASSIUM HYDROXIDE.” FisherSci.com, Thermofisher Scientific, 9 Nov. 2009, www.fishersci.com/msdsproxy%3FproductName%3DP2503%26productDescription%3DPOT%2BHYDROXIDE%2BCERT%2BACS%2B3KG%26catNo%3DP2503%26vendorId%3DVN00033897%26storeId%3D10652. Accessed 18 Nov. 2023.
Tillmann, U., Mitra, A., Flynn, K. J., & Larsson, M. E. (2023). Mucus-Trap-Assisted Feeding Is a Common Strategy of the Small Mixoplanktonic Prorocentrum pervagatum and P. cordatum (Prorocentrales, Dinophyceae). Microorganisms, 11(7), 1730. https://doi.org/10.3390/microorganisms11071730
Verity, P. G. (1985). Grazing, respiration, excretion, and growth rates of tintinnids1. Limnology and Oceanography, 30(6), 1268-1282. https://doi.org/https://doi.org/10.4319/lo.1985.30.6.1268
Verity, P. G. (1988). Chemosensory Behavior in Marine Planktonic Ciliates. Bulletin of Marine Science, 43(3), 772-782. https://www.ingentaconnect.com/content/umrsmas/bullmar/1988/00000043/00000003/art00034 (University of Miami – Rosenstiel School of Marine, Atmospheric & Earth Science)
Wasserman, S. A., Minorsky, P. V., Reece, J. B., & Campbell, N. A. (2017). Campbell biology (P. Education, Ed. 11 ed.).
Yang, J., Löder, M. G. J., Jiang, Y., & Wiltshire, K. H. (2019). Are tintinnids picky grazers: Feeding experiments on a mixture of mixotrophic dinoflagellates and implications for red tide dynamics. Marine Pollution Bulletin, 149, 110488. https://doi.org/10.1016/j.marpolbul.2019.110488
Yuan, L. (2022, January 24). Tintinnid ciliates can act as warm eddy bioindicators. phys.org. https://phys.org/news/2022-01-tintinnid-ciliates-eddy-bioindicators.html#:~:text=Tintinnid%20clliates%20are%20sensitive%20to,Sea%20and%20western%20North