Nature’s Nanomagnets: A Chemical Perspective on Magnetotactic Bacteria
Alex Gagnon, Catriona Kirk, Jason Li, Sofian Martinais
Abstract
Magnetotactic bacteria (MTB) are complex organisms that have evolved a multitude of internal chemical processes to survive their harsh environments. This paper discusses the details of important biological molecules, metabolic systems, and molecular mechanisms that contribute to the vital functions of MTB. Notably, biomineralization is a chemical, multi-step process that synthesizes magnetosomes to allow MTB to perform magnetotaxis, which perhaps was developed as a result of photoferrotrophy. Moreover, various molecular mechanisms control swimming polarity, while also permitting magneto-aerotaxis and chemotaxis. The genetic makeup of MTB is responsible for regulating all these chemical procedures. Genes clusters are organized such that the crucial process of horizontal gene transfer can occur, which is a major pathway for the evolution of MTB. Finally, the long and complex process of evolution can be investigated using modern technology and research about biogeochemical occurrences, including magnetofossils. Overall, the highly evolved mechanistic chemistry within these organisms provides them with the ability to adapt to and thrive in their aquatic habitat.
Introduction
Recent studies have shown that the canonical definition of a prokaryote is no longer accurate. Prokaryotes used to be described as unicellular organisms which did not contain any membrane-bound organelles (McCausland & Komeili, 2020). However, more and more bacteria with subcellular compartments are being found in the biosphere. Some of them, like magnetotactic bacteria (MTB) can biomineralize organelles with specific magnetotactic functions (McCausland & Komeili, 2020). Gathering information on magnetotactic bacterial species remains a challenge for the scientific community as only a few strains are extensively studied. While it is easy to collect them and separate them for observation, MTB are very fastidious to grow. Their cell-dividing time is very long, and researchers still lack isolation media for their growth (Lefèvre & Bazylinski, 2013). These problems limit the scientific analyses and experiments that can be conducted on these organisms. Growing bacteria in media can be particularly useful to study the compounds responsible for their internal chemistry and compensate for our limited knowledge.
This paper will focus on topics concerning the internal functioning of magnetotactic bacteria. It will begin with a description of the chemical composition of magnetotactic bacteria highlighting the importance of such knowledge in understanding the functioning of this organism. Following that a short description of the origins of magnetotaxis and magnetosome function will be presented. Then, successive internal chemical mechanisms such as biomineralization and magneto-aerotaxis will be analysed as designs to maximize cell survival and functionality. This paper also considers magnetofossils as indicators of magnetosome composition through time. Finally, the organization of gene clusters and their roles in horizontal gene transfer over the course of evolution are investigated.
Chemical composition
Everything on the planet is formed of atoms, no matter if it is an object or a living organism. Atoms binding together form molecules, which eventually leads to life. The elucidation of the chemical formula of living systems provides great insight into the metabolism and reactions taking place inside cells (Naresh et al., 2012). The more complex an organism is the more complex its chemical formula is. Since magnetotactic bacteria are relatively simple bacteria, only five atoms—carbon, hydrogen, oxygen, nitrogen, and iron—are used to represent them chemically (Naresh et al., 2012). Molecules composed of these atoms provide the energy required for the metabolism and growth of the bacteria. Knowledge about the chemical composition allows us to optimize cell growth in media. Studies have found that the chemical formula of Magnetospirillum Gryphiswaldense is CH2.06O0.13N0.28Fe1.74*103 (Naresh et al., 2012). This results from the “mass balance” approach carried inside bioreactors by analysing concentrations of different atoms during the cells’ growth (Naresh et al., 2012). The specific chemical formula for this species of magnetotactic bacteria demonstrates a higher C:O ratio compared to other microbial species. This ratio explains the fastidious nature of the cells in terms of their low-oxygenated specific biotope (Naresh et al., 2012). In other words, they are chemically structured with few oxygen molecules which makes their anaerobic or microaerobic metabolisms favourable (Naresh et al., 2012). Additionally, the study found a strong correlation between the intake of iron and nitrogen. The intake of both elements was measured as consumption rates during cell growth. As can be seen in Figure 1, they follow similar patterns during the initial phase before the magnetosomal synthesis phase labelled S on the Figure (Naresh et al., 2012). Indeed, it has been hypothesized that iron activates the nitrogen transporters in the cell (Naresh et al., 2012). High concentrations of iron act as switches to introduce nitrogen inside the cell. Then, this nitrogen will be used to synthesize essential magnetosome assembly proteins (Naresh et al., 2012). This correspondence between nutrients allows greater control of magnetosome biomineralization, which in turn will give a higher magnetosome yield (Naresh et al., 2012). Altogether, the chemistry behind magnetotactic bacteria reveals the building blocks that these organisms use to proliferate.
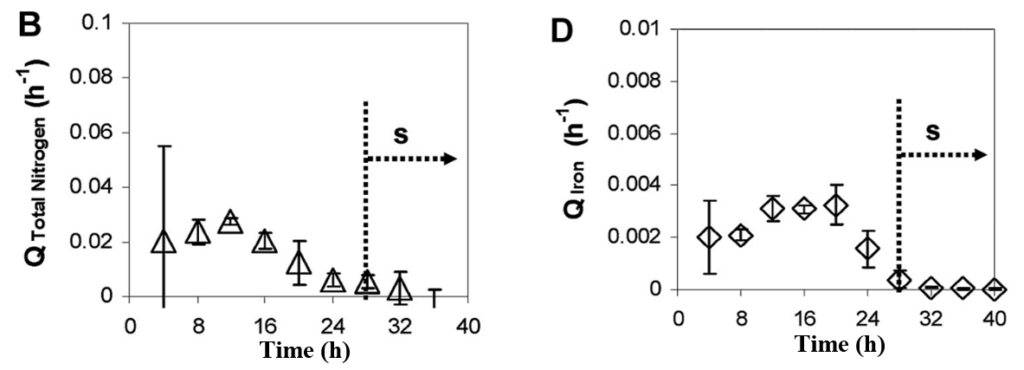
Fig. 1. Specific consumption rate for B) nitrogen, and D) iron during cell growth. The dotted line indicates the end of the initial phase (Naresh et al., 2012).
Origins of magnetosomes and magnetotaxis
Phylogenetic and molecular clock analysis show that magnetosome biomineralization originated in the Archean Eon (Goswami et al., 2022). This leads to a process that is currently observed in magnetotactic bacteria: magnetotaxis. It is believed that this process was driven by the anoxic environment that was present in the Archean and that magnetotaxis evolved to become a crucial feature to allow the bacteria to survive the harsh
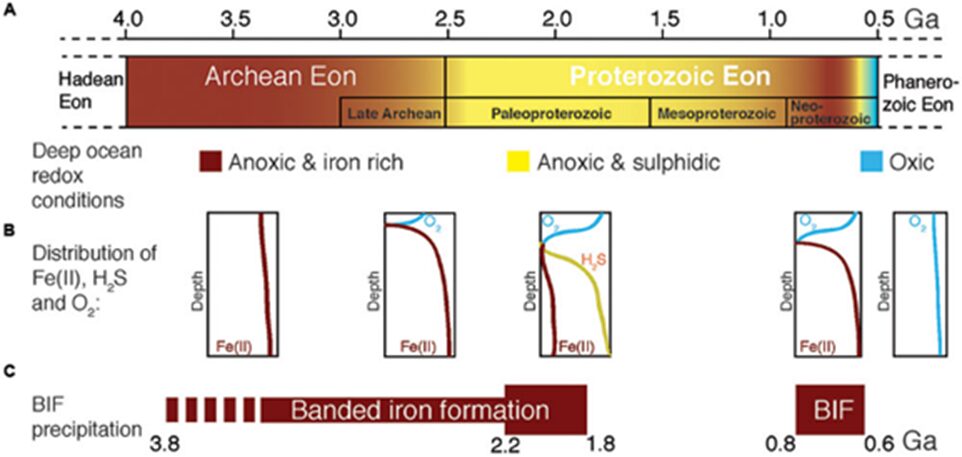
Fig. 2. Geochemical changes of the ocean from the Archean to the Proterozoic era (Goswami et al., 2022).
environment. The changes in geochemical composition in this environment are demonstrated in Figure 2. The initial role of magnetosomal magnetic particles was to mitigate intracellular reactive oxygen species (ROS) toxicity before being adopted for magnetotaxis purposes. Alternatively, photoferrotrophy has been proposed to have caused the development of magnetotaxis (Goswami et al., 2022). This could mean that magnetosome formation is a by-product of Archean iron cycling and that magnetotaxis evolved due to the metabolic accumulation of magnetite, as well as environmental pressures driven by the evolving species of cyanobacteria. Magnetosomes may have been able to provide a protective shield in metal-stressed environments. Photoferrotrophy refers to the process in which inorganic carbon is fixed into organic matter, as a result of electron donations by reduced iron and light energy (Camacho et al., 2017). It is one of the oldest photoautotrophic metabolisms on Earth. It was prevalent in the Archean Ocean due to iron richness and sulphide poorness. However, the ancient ocean underwent major changes in composition. There was a gradual increase in sulphide and oxygen concentrations – oxygen accumulated as a byproduct of early life around 2.3-2.5 billion years ago (Huynh, 2019). This increase was coupled with the occurrence of oxygenic photosynthesis. After all those changes, the ocean became the oxic and anoxic environment that magnetotactic bacteria now experience (Camacho et al., 2017). In these oxic conditions, photoferrotrophs were no longer important primary producers. Hence, their abundance decreased. In essence, photoferrotrophy was a biogeochemically significant process of the ancient Earth that is believed to be a precursor to magnetotactic bacteria’s magnetotaxis.
Magnetosome biomineralization
Magnetosome biomineralization is the pathway that sets apart magnetotactic bacteria from the other types of bacteria found in the biosphere. This reaction is a highly complex process engineered by years of evolution. During this process, bacteria will synthesize either magnetite (Fe3O4) or greigite (Fe3S4) magnetosomes inside their cytoplasm depending on their environment. Usually, greigite-synthesizing bacteria are found in sulphide-rich sediments (Jacob, Jayasri, & Suthindhiran, 2016). There is only little information about the formation of greigite magnetosomes. Most studies are performed on species of Magnetospirillium, namely, Ms. Gryphiswaldense and Ms. Magneticum, because of their ability to grow in culture. These species biomineralize cuboctahedral magnetite magnetosomes. However, genes and proteins that have the same functions as those involved in magnetite synthesis have been identified in the genome of greigite-producing magnetotactic bacteria (Lefèvre & Bazylinski, 2013). These compounds have similar shapes as demonstrated in Figure 3. The only difference in biomineralization between greigite and magnetite magnetosomes is revealed in the number of magnetosomes. Since greigite has a smaller magnetic moment than magnetite, greigite-mineralizing bacteria have to produce larger amounts of magnetosome to get the same efficiency as magnetite-mineralizing bacteria (Bai et al., 2022). Since the process remains the same, this section will describe how the generalized process of magnetosomes would occur in magnetite-producing bacteria.
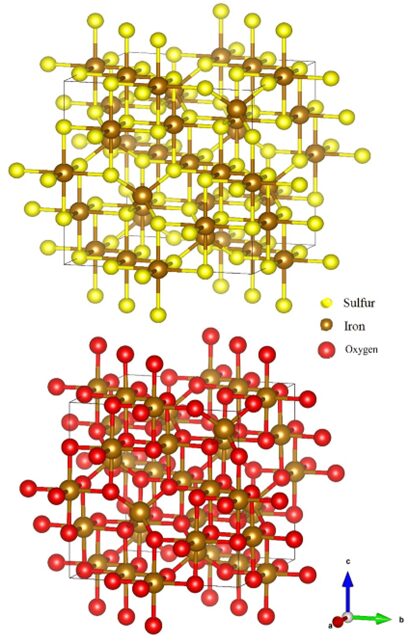
Fig. 3. Illustration of the greigite (top), and magnetite (bottom) atomic structures (Nolan, 2018).
Magnetosome biomineralization is a multi-step process that requires the involvement of approximately 40 proteins with genes located inside the magnetosome genomic island (MGI). This region of DNA, also called the magnetosome island, is about 100 kilobases long (Ying et al., 2022). From this gene cluster, proteins are synthesized to accomplish the four steps of biomineralization. Figure 4 illustrates the name of the proteins associated with the steps of iron uptake, membrane invagination, iron mineralization, and magnetosome chain assembly (Ying et al., 2022).
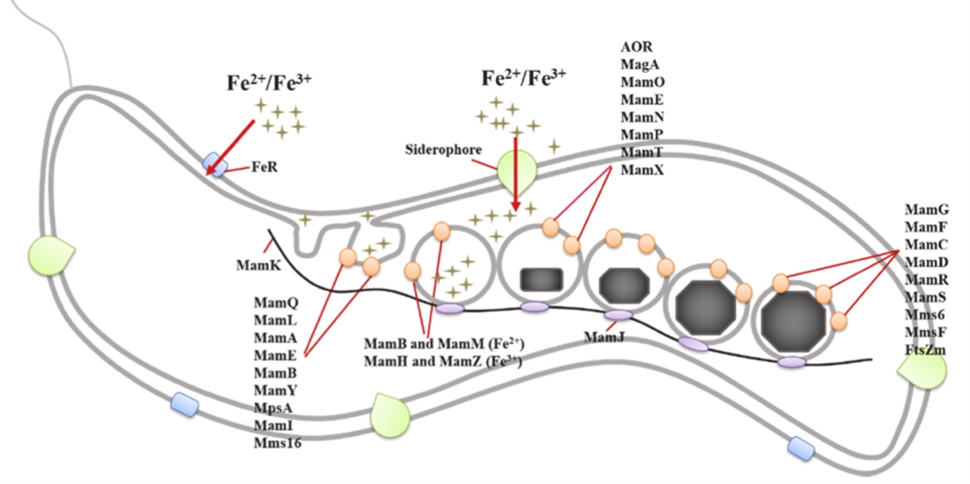
Fig. 4. Model of magnetosome biomineralization in magnetotactic bacteria which consists of the four labelled steps: iron uptake, membrane invagination, iron mineralization, and magnetosome chain assembly (Ying et al., 2022).
Before any magnetosome mineralization can occur, bacteria must absorb iron. Indeed, many enzymes need iron to accomplish their functions which are, often, very important in metabolic pathways. Although all bacteria require iron for proper functioning, the ferric intake observed in magnetotactic bacteria is 100 times larger than the one observed in non-magnetotactic bacteria, and it is strictly controlled by the regulation of genes (Ying et al., 2022). The ferric ions, Fe(II) and Fe(III), must be absorbed from the extracellular matrix and accumulate in the cytoplasm. So far, many mechanisms have been proposed to explain this iron absorption. The first one is the direct transportation of iron inside the cytoplasm done by carrier proteins that are synthesized in magnetotactic bacteria cells in a high iron concentration environment. The second one is the conversion of Fe(III) into Fe(II) by iron reductase to facilitate the absorption (Amor et al., 2018; Ying et al., 2022). The third one is through siderophores. These are light compounds that chelate to ferric ions and solubilize them for uptake (Yan et al., 2012). Another mechanism, very similar to the iron uptake system in yeast, involves a copper dependency (Dubbels et al., 2004). Since biomineralization is essential to magnetotactic bacteria, many proteins help regulate those mechanisms. In the species Magnetospirillium Magneticum, the MagA protein is present in the cytoplasm and membrane and transports ferrous ions through active transportation. Likewise, FeoAB is a transport protein. Studies have also documented that MamB protein contributes to the direct absorption of Fe(II) ions and that MamM increases the stability of MamB. MamA and MamV have similar functions as MamB and MamM. Finally, MamZ and MamH are involved in the direct uptake of Fe(III) from the cytoplasm into the magnetosome vesicles. Ultimately, all these proteins contribute to the first step of the magnetosome’s biomineralization (Ying et al., 2022). Once iron arrives in the cytoplasm it must be transported towards the vesicles. This is where the first two steps get muddled together. Iron uptake occurs continuously inside the cell, but when vesicles are being formed, the iron may be transported from the extracellular matrix to the vesicles with a detour through the cytoplasm or it may go directly to the vesicle through the periplasm (Lefèvre & Bazylinski, 2013).
The second step of magnetosome biomineralization is the invagination of the cytoplasmic membrane. Studies of magnetosomes have revealed that the magnetite crystals are surrounded by a 3-4 nanometers thick membrane that resembles the bacterial cell membrane (Ying et al., 2022). However, the magnetosome membrane acts more like a reactor that provides a redox potential, and a strict pH control on the nanoscale (Ying et al., 2022). This membrane will also limit the space available for magnetosome mineralization. The lipids present in the membrane also play an essential role in the morphology of the magnetosomes and they prevent the aggregation or oxidation of magnetite nanocrystals (Ying et al., 2022). Some compounds, which account for most of this membrane, are phosphatidylethanolamine, phosphatidylglycerol, phosphatidylserine, which are phospholipids and aminophenols (Ying et al., 2022). The current model for the formation of this membrane around the magnetosome crystal is illustrated in Figure 4, where the vesicle forms from the cytoplasmic membrane. This invagination process occurs randomly and rapidly in areas of the cytoplasmic membrane where there are several Mam proteins present. MamB and Mms16 are believed to be responsible for the initiation of the process. MamB, which is situated on the membrane, recruits proteins that are used later on in the process, while Mms16 triggers the infolding (Ying et al., 2022). This pit maintains its structure because of the MamM, MamL, MamI, MamQ, and MamY proteins (Ying et al., 2022). At the end of this step, there may be a permanent invagination in the membrane or a separate vesicle. This remains unclear, as some membrane protein sorting must still occur, but the magnetite precipitation might have begun already (Lefèvre & Bazylinski, 2013). The magnetosome membrane differs from the cytoplasmic membrane from a protein composition standpoint. This can be explained by their different functions in the cell. The proteins that are involved in this key sorting are MamI, MamQ, MamL, MamA, MamU, and MamY (Ying et al., 2022).
Once the vesicles form and the iron penetrates inside the vesicles, the magnetite crystals will start to form. This is the iron mineralization step which can be divided into three phases. Iron enters the magnetosome membrane as phosphate-rich ferrous hydroxides. Then, it undergoes a transient hydrous ferric oxide phase, where the compound is called ferrihydrite. Finally, there is a partial reduction and magnetite is formed inside the vesicle (Baumgartner et al., 2013). These three phases are shown in Figure 5 below. Following this mineralization is a maturation process where many proteins work together to control the pH, the growth, and the redox in the vesicles (Yan et al., 2012). Furthermore, studies have determined the roles of specific Mam and Mms proteins in this step. Mms6 directly impacts the formation and morphology control of the crystals (Ying et al., 2022). MamG, MamF, MamC, and MamD influence the size of the magnetite crystals (Ying et al., 2022). MamE behaves like a switch that triggers the initiation of magnetite biomineralization (Ying et al., 2022). Finally, MamN is a protein that transports protons released from the magnetite formation outside of the vesicle to keep the pH stable (Ying et al., 2022). Furthermore, there has also been observation of discrepancies in the composition of the magnetosomes in different cells dependent on their growth environment. Different transitional metal ions like titanium, cobalt, manganese, and copper can be found in the surface layers of magnetosome crystals. These metals do not incorporate the lattice structure of the crystals, allowing the magnetotactic bacteria to keep its magnetic properties (Lefèvre & Bazylinski, 2013). Navigation of magnetotactic bacteria remains unaffected by these impurities.
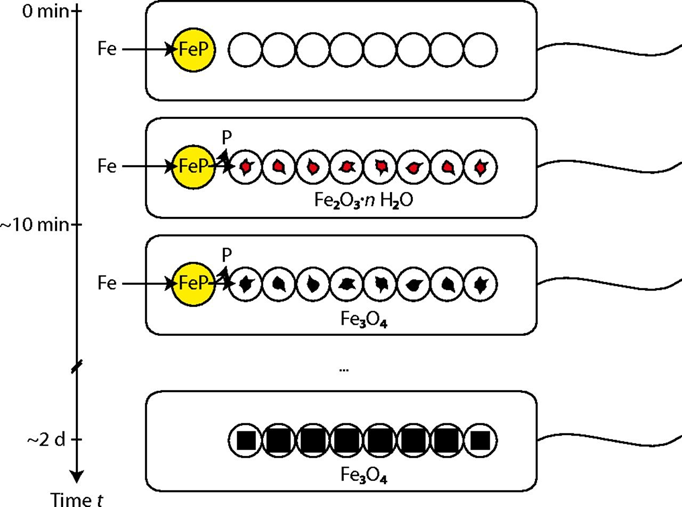
Fig. 5. Illustration of the suggested magnetite biomineralization mechanism in magnetotactic bacteria. Iron (Fe) is stored in a phosphate-rich ferric hydroxide phase (FeP). Fe and P are separated in the transfer process into the magnetosome compartments, leading to the formation of Fe2O3·n H2O (hydrous ferric oxide). Reduction of this phase yields magnetite (Baumgartner et al., 2013).
The last step of magnetosome biomineralization is the chain assembly of these organelles. After multiple magnetosome crystals are formed, their vesicles assemble as a single chain or as multiple chains. The magnetosomes are connected with their magnetic heads to tails to maximize their magnetic dipole moment (Lefèvre & Bazylinski, 2013). However, this structure alone tends to collapse to reduce the monostatic energy of the system. Hence, to fix this issue, the magnetosomes are aligned along a protein filament that stabilizes the structure (Lefèvre & Bazylinski, 2013). This protein, identified in Figure 4, is called MamK. It is part of the large prokaryotic actin-like protein superfamily. MamK protein has an important role in positioning the magnetosome chain, and in completing the vesicle formation (Ying et al., 2022). The presence of ATP prevents the aggregation of MamK. For this step to be completed, another protein is required: MamJ (Ying et al., 2022). This protein displays an acidic polymorphism region. Its main function is to connect the magnetosome vesicles to the MamK filament (Ying et al., 2022). Indeed, experiments have demonstrated that when the mamJ gene is deleted from the bacteria’s genome, the magnetosomes aggregate into a ball, and there is no longer a binding of vesicles to the skeletal actin-like filament (Ying et al., 2022).
The study of biomineralization is still ongoing and many mechanisms involved remain unknown. Additionally, the order of the steps remains debated. The order proposed above is based on the most recent studies, but as can be seen, the range of each step can still be muddled. This is partially explained by the multiple functions of proteins involved. MamA, MamB, MamJ and MamQ‘s influence are not limited to single steps but are important to the process as a whole (Ying et al., 2022). This complicates the study of magnetotactic bacteria’s genome. However, it also simplifies the biomineralization process for the bacteria because it has to synthesise fewer proteins.
Understanding the meaning of MTB polarity
The first study that has shown differences in behaviors of MTB orientation among different magneotactic bacterial species was performed in 1997 by Frankel et al. In this study, two species (Magnetospirillum magnetotacticum and Magnetococcus marinus strain MC-1) were placed in a capillary tube lying in between two magnets which both face their Northern pole to the tube. Initially, stable bands of MTB were observed at the oxic-anoxic region of the capillary tube. Upon reversing one of the poles, the Magnetospirillum magnetotacticum bacteria remained in their place in the tube, while the Magnetococcus marinus population divided into two groups: One going towards the South pole, and the other going towards the North pole (Figure 6). This is an unusual behavior, as MTB going one way would go towards lower oxygen concentrations, but the other subgroup would swim towards higher concentrations of oxygen, which may be detrimental to their growth. These results show that the direction of movement of MTB depends not only on oxygen gradients, but also on the orientation of magnetic fields. This directional dependence on magnetic fields is referred to as MTB polarity, and it is important to acknowledge that the unfavorable behavior of Magnetococcus marinus was obtained under artificial conditions where abrupt field reversals were applied, which is not the case in their natural environment (Frankel et al., 1997).
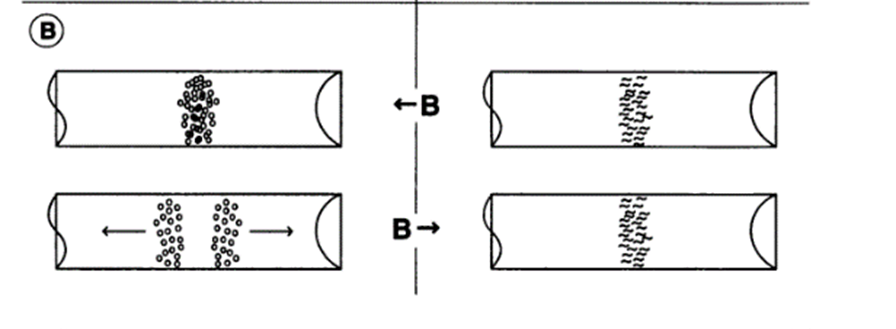
Fig. 6. The effect of a magnetic field reversal on the swimming behaviors of M.magnetotacticum (top) and of MC-1 (bottom) in flat capillaries along an oxygen gradient (increasing to the right). M.magnetotacticum aerotacticbands remain stable, while MC-1 separate into two groups traveling in opposite directions [adapted from Frankel et al].
This experiment is only an example to hint towards an intricate connection between MTB’s use of external magnetic fields as well as oxygen concentrations in their motile response. In fact, there are other types of MTB polarity and it has been shown that polarity can be induced by varying the external magnetic field to which MTB are subjected. For example, predominantly North-seeking MTB collected by Blakemore et al. that were subjected to an external magnetic field a hundred times smaller in strength than that of the Earth’s (Klumpp et al., 2019). This resulted in equal proportions of North- and South- seeking bacteria, showing that MTB polarity can be selected through external magnetic fields (Klumpp et al., 2019). This behavior indicates the existence of a highly adapted chemotactic system that can modify the response of MTB to external fields in addition to conferring the ability of following oxygen gradients. The next sections explore the chemotactic pathways relevant to aerotaxis and discuss the advantages of polar magnetotaxis.
Bacterial chemotaxis
Some of the main molecular features for aerotaxis in MTB are shared across other different types of chemoreception (involving other signals, such as pH, or light intensity) among microorganisms (Wadhams & Armitage, 2004). The histidine-asparate phosphorelay (HAP) system is a common central pathway involved in converting environmental signals into a motile response in bacteria (Wadhams & Armitage, 2004), as shown in Figure 7. The two components that comprise the HAP system are CheA, a histidine kinase that regulates chemoreception, and CheY, which acts as a response regulator (Klumpp et al., 2019). It is important to note that all Che proteins are encoded into the chemotaxis che operons, which all encode the chemotaxis Che proteins, and may vary depending on the species (Popp, Armitage, & Schüler, 2014). When CheA phosphorylates CheY following chemoreception, CheY-P binds to a motor complex to induce some change in flagella movement, which can include motor reversals, or a modulation in flagellum rotational speed (see Figure 7) (Klumpp et al., 2019). After CheY-P’s action on a flagellum, termination of the pathway is mediated by a CheY-P phosphatase to regenerate CheY (these phosphatases can vary depending on the organism) (He & Bauer, 2014). Chemoreception can be mediated by the controlled level of methylation of chemoreceptors, which have a lower sensitivity to signals when they are methylated (Klumpp et al., 2019). This is why these transmembrane chemoreceptors are also known as methyl-accepting chemotaxis proteins (MCPs). Methylation is performed by the methyltransferase CheR, and methyl removal is induced by the CheB protein (Klumpp et al., 2019). This adaptation allows the control of the response over time through down-regulation of that response in the presence of a concentrated signal. In turn, this mechanism makes it possible to increase the sensitivity of chemoreceptors, such that the detection range of chemoreceptors can include low and high concentrations of a signal without the consequence of obtaining an excessive and uncontrolled response (Klumpp et al., 2019). For navigating across spatially varying gradients of substance, bacteria are small enough to require sensing of the corresponding signal and comparison at two separate times (not always the case), which contrasts with the larger eukaryotes that can sense gradients by comparing signal input from two ends of the cell (Klumpp et al., 2019). To effectively measure a concentration gradient, subsequent sensing of the signal is performed from the same chemoreceptor state generated by the previous detection. This creates a reference point from the previous detection relative to which a concentration increase, or decrease, can be sensed (Klumpp et al., 2019).
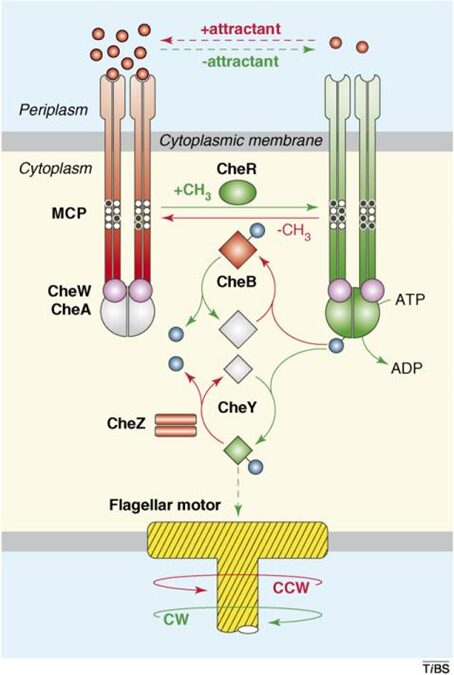
Figure 7. The HAP signaling system. Components in red induce counter-clockwise flagella rotation, while those in blue induce clockwise rotation. Relevant chemotaxis proteins and MCPs are shown (Hazelbauer, Falke, & Parkinson, 2008).
Molecular link between magnetotaxis and aerotaxis
In the case of aerotaxis for microaerophilic MTB, high oxygen concentrations induce a motor response down the gradient, while low concentrations of oxygen promote less movement (Klumpp et al., 2019). Aerotaxis is also a crucial mechanism for maintaining optimal magnetosome formation, which is impeded under higher oxygen concentrations (Popp, Armitage, & Schüler, 2014). It has been shown, by comparing wild type MTB to a mutant strain lacking magnetosome genes, that magnetotaxis provides an advantage in guiding optimal aerotactic responses, but the mechanistic explanations were unclear. It was suggested that biochemical pathways that link magnetic alignment and oxygen sensing allow more efficient aerotaxis. This is where the polarity of MTB becomes important, and there is significant evidence of the existence of biochemical pathways that link aerotaxis to magnetotaxis at the molecular level.
Central role of the cheOp pathway in aerotaxis
cheOp1 operon in Magnetospirillum Gryphiswaldense in aerotactic response (Popp, Armitage, & Schüler, 2014). In fact, mutant M.Gryphyswaldense lacking cheOp1 completely lost its aerotactic response, as supported by the fact that no change was observed when oxygen concentrations were varied from 0% O2 to 2% O2. This was also followed by a lack of direction reversals, where the movement was characterized by long and uninterrupted runs (see Figure 8). More interestingly, it was found that ∆cheOp1 mutants also lacked swimming polarity, and roughly equal numbers of MTB accumulated at both the North and South ends of the assay used. Also, there are other chemotaxis operons in M,Gryphiswaldense (cheOp2, cheOp3, cheOp4) which also encode the putative chemotaxis proteins, and additionally encode for 56 different MCPs and other domain proteins, as seen in Figure 9. In deletion mutants for each of these operons, the growth of magnetosomes was unaffected, and no change in aerotactic behaviour was observed. This shows that cheOp1 controls the major signalling pathway responsible for aerotaxis (Pfeiffer et al., 2020). Having multiple chemotaxis genes that encode for similar Che proteins that differ in the chemotactic signal that is processed, is an important design solution in MTB. In fact, this allows the separation of the roles of chemotactic responses into distinct functional units and allows higher control of these individual responses. This demonstrates that aerotactic signal transduction from cheOp1 can be controlled in a flexible manner, to allow optimized adaptations under different oxygen concentration gradients.
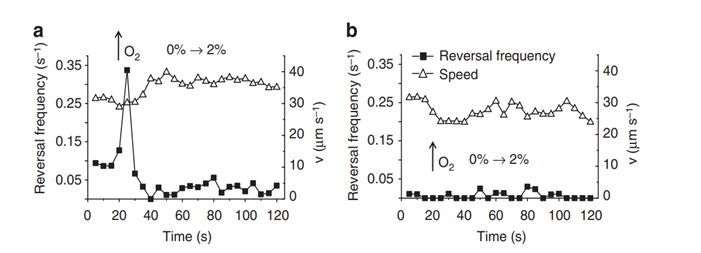
Fig. 8. Reversal frequencies and swimming speeds of non-magnetic (a) and cheOp-lacking (b) M.Gryphiswaldense mutants subjected to an abrupt increase in O2 from 0 to 2% [O2]) at indicated times. Aerotactic response in non-magnetic mutants is conserved, as seen in the reversal rate peak when oxygen concentration was increased, showing efficient aerotactic response. In contrast, no response is observed in cheOp-lacking cells (Popp et al., 2014). Relatively steady and non-zero speeds in (a) and (b) indicate movement of MTB and that reversals do induce a change in movement direction (Popp et al., 2014).
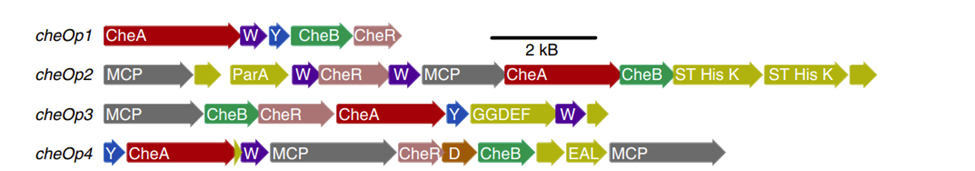
Fig. 9. Organization of the four cheOp operons in M.Gryphiswaldense. as distinct functional sequences. (Aside) Homologues of the same phylogenetic family are illustrated in the same color. Note that W = cheW and Y = cheY. An additional che homologue (i.e. D = cheD) and other domain protein-encoding genes are shown (Pfeiffer et al., 2020).
More recently, the spatiotemporal expression of CheA1 and CheW1 (Che signaling proteins encoded by cheOp1) in M.Gryphiswaldense has been shown to be consistent with their role of controlling aerotactic response by interacting with transmembrane chemoreceptors (Pfeiffer et al., 2020). The role of CheW1, not completely understood previously, is to mediate the interaction of CheA with chemoreceptors, as shown in Figure 7 (Pfeiffer et al., 2020). M.Gryphiswaldense is bipolarly flagellated, allowing its movement to remain axial, which is consistent with it following magnetic field lines. Using 3D-structured microscopy illumination, clusters of fluorescent CheW1-GFP were observed at both poles of the cell, and smaller but growing clusters were observed at the mid-cell, which is important for distributing existing chemotaxis proteins to the two poles of each daughter cell during cell division (see Figure 10). Also, the importance of bipolar localization of the chemotaxis proteins CheA1 and CheW1 was demonstrated. Pfeiffer et al. labelled CheA1 with different fluorophores and observed altered distributions of the CheA1 fluorophore complex. For example, labelled CheA1 with GFP resulted in CheA1-GFP accumulation on one cell pole only and the complete loss of aerotaxis. mCherry-CheA1 also resulted in a 67% decrease in aerotactic efficiency, and asymmetrical distributions of the complex were observed at the cell poles. These drops in aerotactic behaviour supported that symmetric bipolar localization of chemotaxis signalling proteins is important for efficient aerotaxis (Pfeiffer et al., 2020). Even though not all MTB are bipolarly flagellated, these results suggest that the localization of Che proteins close to flagella is an important solution to allow rapid motile responses against changing oxygen gradients. This also allows the maximization of the sensitivity of the signaling system at both cell poles.
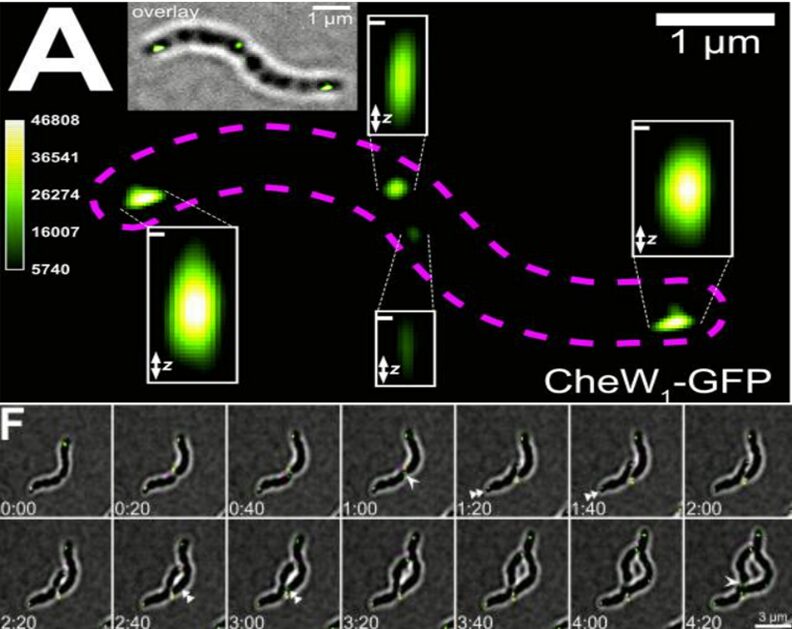
Fig. 10. (A) Green fluorescence of CheW1-GFP localized as clusters at both cell poles and at midcell. Each enlargement corresponds to spotted fluorescence identified by the maximum intensity projection algorithm (which uses pixels with highest intensity in an image) using the z-stack software (indicated by “z” in the Figure). The calibration bar (right) indicates relative intensities represented by each color. 100nm scale bars are shown in each enlargement. (F) Time-lapse 3D-SIM microscopy of CheW1-GFP-expressing cells, showing the cell division process and distribution of CheW1-GFP from the middle in each daughter cell. Indicated times are in hours: minutes [adapted from Pfeiffer et al., 2020].
The final link: aerotaxis coupled with the magnetosome chain
In MTB, magnetosome organelles arrange themselves into linear chains to maximize their magnetic moment, which allows precise magnetic sensing (Katzmann et al., 2013). However, this assembly process requires different magnetosome-specific proteins that position and stabilize the magnetosome assembly, one of which is called MamK (Philippe & Wu, 2010). This actin-like filament serves as a highly dynamic scaffold around the magnetosome chain and is responsible for positioning this chain at mid-cell (Pradel et al., 2006). It has been shown by Pradel et al. that MamK filament extremities localize close to the flagellated cell poles of Magnetospirillum magneticum. Remarkably, from this finding, a direct molecular link between magnetotaxis and aerotaxis could be hypothesized: it was proposed that a magnetic torque applied on magnetosome chains would trigger an interaction between MamK and MCP proteins located at flagellated cell poles, which would guide aerotactic responses and give rise to magneto-aerotaxis (Philippe & Wu, 2010).
To validate this, Phillipe & Wu identified a potential MCP that may be involved in magnetic sensing and tested its interaction with MamK. To identify the most appropriate protein, an important assumption was that this protein should be encoded by genes in the magnetosome island. This led to the identification of Amb0994, an MCP-like protein that was compared with Tsr, a putative MCP protein in Escherichia coli. The fact that the signaling domain of Amb0994 shares 65% sequence identity with that of Tsr was sufficient to demonstrate the potential of Amb0994 to be involved in a signaling mechanism as a chemoreceptor. Moreover, Amb2333 was used as a control to test the compatibility of Amb0994 interactions with MamK, as Amb2333 is not encoded in the magnetosome island (Philippe & Wu, 2010).
The localizations of Amb0994 and Amb2333 were determined to be situated at cell poles and at mid-cell (future cell poles), as observed through fluorescence of the Amb0994-and Amb2333-Venus complexes, as can be seen in Figure 11. This further supported that that Amb0994 and Amb2333 potentially function as chemoreceptors, as these similar localities are characteristic of MCP localities (Philippe & Wu, 2010).
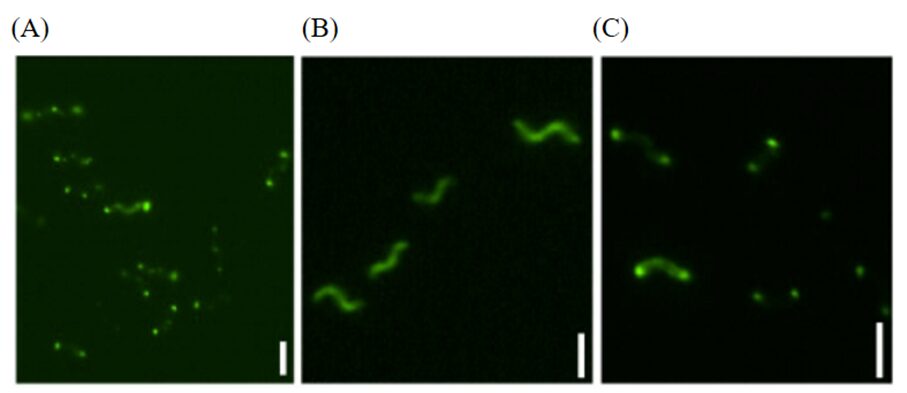
Fig. 11. Fluorescence microscopy of (A) Amb0994-Venus (fluorophore) in polar locations in AMB-1 cells, (B) Venus probe alone, and (C) Amb2333-Venus fluorescence. Scale bars represent 2µm [adapted from Phillippe & Wu, 2010].
To determine whether Amb0994 interacts with MamK filaments, a bimolecular fluorescence complementation (BiFC) assay was used. The BiFC assay is a technique that allows the visualization of protein interactions, by essentially binding to each of the two tested proteins two complementary fragments of a fluorescent probe (Hu, Chinenov, & Kerppola, 2002). This probe becomes visible whenever the two proteins interact to form this fluorescent probe from its individual parts (Hu, Chinenov, & Kerppola, 2002). Using BiFC resulted in fluorescence, showing the existence of an interaction between MamK and Amb0994 (Philippe & Wu, 2010).
Finally, to assess the role of Amb0994 in magnetotaxis, an overproduction interference (OPI) assay was used, i.e. the motility response of MTB with overexpressed levels of Amb0994 to applied magnetic field reversals was compared to that of wild type MTB. This comparison is made on the basis that Amb0994 in excess would interfere with effective magnetotaxis by impeding Amb0994–MamK interaction or by interfering with signal transduction processes that trigger flagellar responses. This OPI was analyzed through a magnetospectrophotometry assay, where MTB were placed inside a cuvette and where their movement was detected at the center of the cuvette by measuring the cuvette’s optical density at that location (see Figure 12). The graphs in Figure 11 B) represent the passage of MTB cells across the center of the cuvette (measured by the absorbance of incident light at that location, indicative of cell density). When a magnetic field was applied, all MTB cells would move mainly North, and when a magnetic field reversal was applied, MTB reversed their direction of movement. An important measurement was the time it took for cells after a magnetic field reversal to return at the center of the cuvette. In unmodified cells, this time was 63.3 ± 8.78 s, while Amb0994-overproducing cells took 108.4 ±10.1s, which is statistically significantly greater than the former. This showed that Amb0994-overexpressing cells had a slower response against the magnetic field reversal (Philippe & Wu, 2010). This serves to demonstrate that Amb0994 is well involved in magnetotaxis. Also, this further confirms the initial hypothesis that magnetic torque causes an interaction between MamK and the Amb0994 chemoreceptor, which might relay a response in the CheA-CheY mechanism to induce a change in flagellar rotation. The MamK-Amb0994 is an elegant design solution that potentially forms the link between magnetotaxis and aerotaxis, and it can explain the complex aerotactic behaviors seen in MTB as discussed in understanding the meaning of MTB polarity.
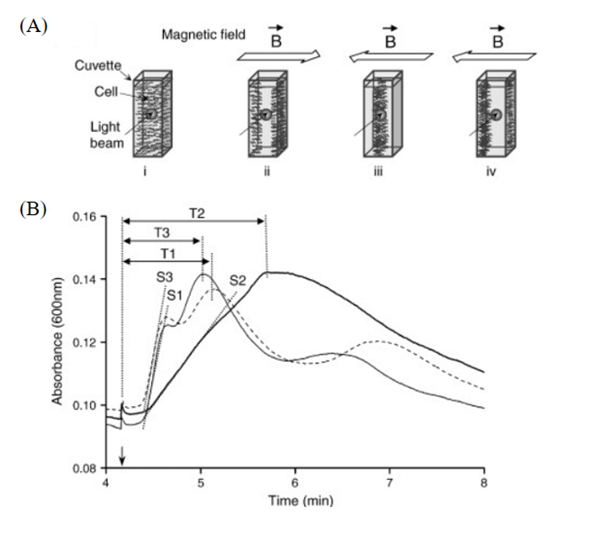
Fig. 12. (A) Working principles of the spectrophotometry assay, with: (i) homogeneous distribution of MTB cells (no magnetic field), (ii) applied magnetic field causing bidirectional movement of MTB cells, and (iv) reversal of the magnetic field generating counter movement, where cell density is measured at the center of the cuvette (iii). (B) Absorbance curves for WT AMB-1 (broken line), Amb-overexpressing AMB-1 (continuous bold line), and Amb0994Δ321–436(lacking the 116 C-terminal residues, which are required for MamK–Amb0994 interaction) are shown, where the arrow indicates the time at which the magnetic field was reversed. S is the slope measured during peak occurrence, and T is the time between magnetic field reversal and peak absorbance [adapted from Phillippe & Wu, 2010].
Magnetofossil revelations
The consistency of magnetosome is incredibly useful in evolutionary analysis. It allows scientist to study magnetotactic bacterial populations over time. These populations are closely related to paleomagnetism (Chang & Kirschvink, 2003). Additionally, this study can be extended to the universe. Magnetite analysis on meteorites from Mars support the existence of extra-terrestrial life. Indeed, magnetite nanocrystals resembling those biomineralized in terrestrial magnetotactic bacteria have been observed on meteorite ALH84001 originating from Mars (Buseck et al., 2001). However, further research must be performed…
The multidisciplinary investigation of microbiology, evolutionary biology, geobiology, biogeochemistry and geochronology are necessary to determine the evolutionary history of magnetotactic bacteria, an aspect that plays a key role in the understanding of the chemistry behind this bacterium. Magnetofossils are magnetosome deposits in sediments and rocks that are preserved after magnetotactic bacteria lyses. They are typically found in freshwater. Recently, they have also been shown to exist in ferromanganese crusts and abyssal manganese nodules (Goswami et al., 2022). These fossils demonstrate the history and the evolution of magnetosome composition. Magnetofossils can trace the origin of magnetotactic bacteria to the Crustaceous, and maybe even the Paleoproterozoic at around ∼1.9 Ga (Goswami et al., 2022). Ever since the formation of the Earth’s magnetic field, there have been many geomagnetic reversals that have impacted the presence of magnetotactic bacteria, but not the composition of their magnetosomes (Chang & Kirschvink, 2003). Geomagnetic reversals are accompanied by transition periods with greatly reduced geomagnetic field intensities (Chang & Kirschvink, 2003). One could think that this would alter magnetosomal composition when bacteria adapt. However, even throughout these transitional periods, Chang & Kirschvink found “abundant single-domain prismatic crystals with hexagonal cross sections” that were deposited in sediment (Chang & Kirschvink, 2003). The specific crystal morphology and composition that are characteristic of biogenic origin remain in the same (Chang & Kirschvink, 2003). Since magnetosomes are not affected by geomagnetic changes, it is believed that the magnetotactic bacterial population decreased due to those changes (Chang & Kirschvink, 2003). This raises an important evolutionary question: why did magnetotactic bacteria not adapt to these changes? While the answer remains debated, one can consider the energy expended in changing the biomineralization process. Additionally, biomineralized magnetite structures form complex lattices that are very stable. Changing the composition and the morphology of magnetosomes would reduce the efficiency and reliability of this crucial organelle (Chang & Kirschvink, 2003).
Various technology can be used to identify magnetofossils. For example, they are identifiable under a transmission electron microscope (TEM) as shown in Figure 13 (Goswami et al., 2022). However, before direct TEM observations, other techniques were used to identify magnetofossils. Low-temperature remanence measurements, first-order reversal curve diagrams and ferromagnetic resonance spectroscopy were all possibilities. Low-temperature isothermal remnant magnetization (IRM) can also be used to distinguish magnetite magnetosome chains (Goswami et al., 2022). The following ratio can be used for this process of identification: δ = (IRM80K-IRM150K)/IRM80K and the δ ratio = δFC/δZFC, where δFC and δZFC are the difference between field cooled (FC) and zero-field cooled (ZFC) IRM curves at 150 K and 80 K, respectively (Goswami et al., 2022). It was concluded that magnetite chains are present when the δ ratio is greater than 2. The ratio is less diagnostic when the magnetosome chains are oxidized. Verwey transition temperature signal is another way magnetofossils can be identified; 100K and 120K are the Verwey transition temperatures of magnetite (Goswami et al., 2022).
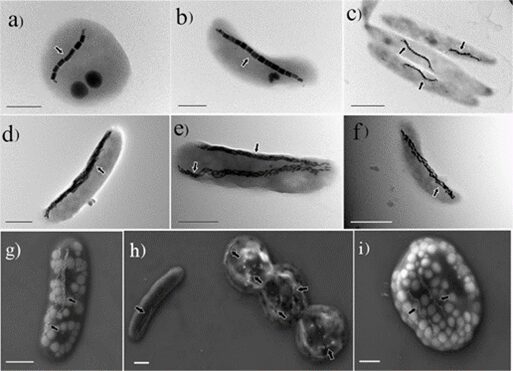
Fig. 13. Electron microscope images of MTB (Goswami et al., 2022).
Genetics of magnetotactic bacteria and horizontal gene transfer
Magnetotactic bacteria genes can be reconstructed due to recently developed metagenomics and single-cell genomics (Goswami et al., 2022). A reconstruction of magnetosomal genes is presented in Figure 14. These studies use environmental samples to reconstruct genomes without cultivation. These genomes can be compared with cultivated bacteria to provide information about the evolution of magnetotaxis. Magnetosome protein phylogeny is very similar to that of organisms at a higher class or phylum level. The evolution of magnetotaxis at lower taxonomic levels involves processes such as horizontal gene transfers, gene duplications and/or gene losses. Horizontal gene transfer occurs when bacteria exchange genes-containing plasmids with other bacteria cells in their environment. The genome sequences of several magnetotactic bacteria are now completed and available to analyze (Lefèvre & Bazylinski, 2013). The functions of several different magnetosome membrane proteins in the biomineralization of the magnetite magnetosome chain have been demonstrated through the development of genetic systems in some magnetotactic bacteria. The genetic determinants for magnetosome synthesis (the Mam and Mms genes) can be found in clusters in the genomes of magnetotactic bacteria. These clusters are near each other and are surrounded by genomic features that indicate that horizontal gene transfer may occur to transmit magnetosome gene islands to different bacteria. MamA and mamB genes belong to the MamAB cluster along with 15 other genes, in Magnetospirillum gryphiswaldense, a genus of magnetotactic bacteria (Lefèvre & Bazylinski, 2013). This segment of DNA is around 16.4 kb in length in collinear order. Also, this cluster is the only one with operon-containing genes that are necessary for magnetosome formation and magnetite biomineralization, while other operon-containing genes are needed for controlling the size and shape of the synthesis of crystals used for magnetic orientation. For example, one of these operons, called the mamGFDC cluster is around 2.1 kb in length and is 15 kb upstream from the mamAB operon. Overall, the magnetosome gene region is around 130 kb in size in Magnetospirillum gryphiswaldense (Bazylinski et al., 2013).
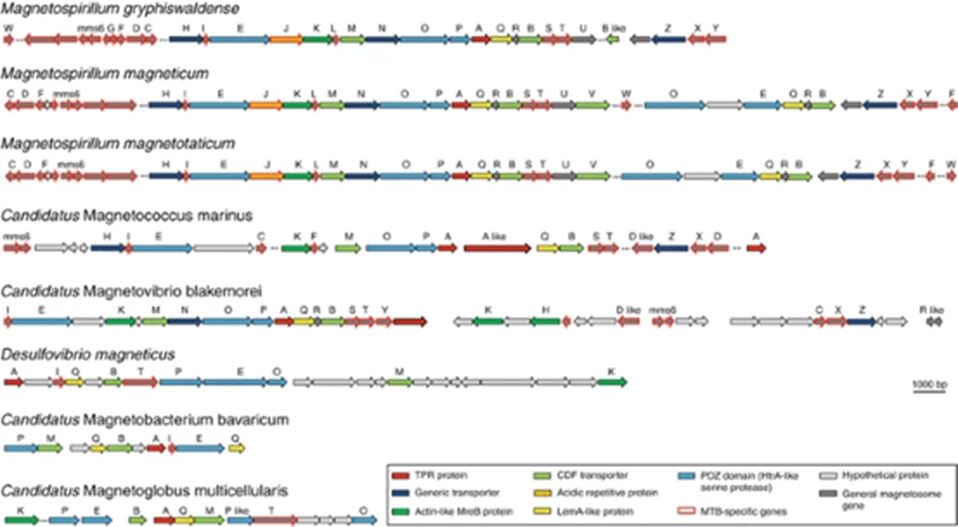
Fig. 14. Organization of magnetosome genes in the magnetosome putative gene islands of various magnetotactic bacteria (Bazylinski et al., 2013).
Gene islands can be given to different bacteria via horizontal gene transfer, which may make them a major pathway for the evolution of magnetotactic bacteria. These gene islands often undergo gene rearrangements, deletions, and duplications – which can frequently cause nonmagnetic mutants of various strains (Lefèvre & Bazylinski, 2013). The presence of tRNA genes is another characteristic of these gene islands that act as insertion sites for integrases (Lefèvre & Bazylinski, 2013). Additionally, the gene island has a different content of cytosine and guanine than the rest of the genome. In some cultivated bacteria, the loss of the gene island resulted in the loss of magnetosome formation, which caused magnetotaxis to no longer occur. This indicates the gene island’s importance to magnetotactic bacteria’s vital functions, like magnetotaxis (Lefèvre & Bazylinski, 2013). Furthermore, the evolution of the mamA protein, which is one of the most protected magnetosome-associated proteins, has been studied in distantly related magnetotactic bacteria by investigating the tertiary structure of the protein. This protein’s folding is very conserved particularly by magnetotactic bacteria of the Nitrospirae phylum and those of the Alphaproteobacteria class. This result supports that horizontal gene transfer of magnetosome genes occurred, since the mamA genes had very similar structural features that must not have evolved as parallel events (Lefèvre & Bazylinski, 2013). In summary, distribution of genomic islands can explain the phylogenetic diversity of magnetotactic bacteria, while gene rearrangements explain why these bacteria may have variations in their genomic islands.
Conclusion
In essence, internal mechanisms and chemistry occurring inside magnetotactic bacterial cells demonstrate remarkable adaptations that lead to the worldwide distribution of this group of bacteria as seen today. As seen previously, magnetotactic bacteria use magnetotaxis to find optimal nutrient concentrations for survival. To be able to perform such a taxis, these cells have developed organelles composed of magnetite or greigite. This composition utilises the magnetic properties of such compounds. Magnetosome biomineralization shows the multifunctionality of different Mam and Mms proteins. This overlap in functions performed minimizes the number of proteins produced. These proteins specifically regulate and work together to allow invagination of the plasma membrane and iron uptake, finally forming vesicles containing magnetic crystals. These magnetic crystals are highly stable, and impurities do not affect their function. Moreover, iron uptake triggers an increase in nitrogen uptake, which was hypothesized to allow synchronous consumption of nutrients while magnetosome formation occurs. This optimizes the conditions in which magnetosome formation takes place. Additionally, magnetosome chain assembly is performed by a specialized actin-like MamK filament. It is used as a scaffold around magnetosomes to maintain optimal linear conformation.
An additional set of design solutions are included in the process of aerotaxis. Aerotaxis permits a quick response from magnetotactic bacteria towards the oxygen concentration. An element that is crucial to its survival. Chemoreceptors can be methylated to down-regulate a response to a signal, which also allows optimizing of the sensitivity of the signalling system over wider ranges of signal concentrations. Moreover, the almost exclusive role of the cheOp pathway in aerotaxis shows the importance of separating the functions of different cheOp operons. Each operon specializes in the signalling pathway for a specific signal. This allows a higher control over individual processes, maximizing the effectiveness of signal transduction systems such as the one responsible for aerotaxis. Remarkably, the molecular mechanism behind magneto-aerotaxis could be clarified: The interaction between MamK and the MCP-like Amb0994 protein, which is triggered by a magnetic torque, likely induces a signaling pathway that relays to the HAP system for signal response. This fascinating mechanism is potentially at the heart of aerotaxis, which might explain the different adapted motility behaviours of MTB in their respective environments.
Although many adaptations often translate to change, magnetofossils reveal that magnetosome composition and structure remain relatively constant through time. This unchanging composition is a result of the highly stable structure that has already been developed. Adaptations of this aspect of the magnetosome would be unfavourable energetically. Finally, the specific locus and clustering of operons involved in magnetosome biomineralization allows horizontal gene transfers to occur more effectively, resulting in a wider distribution of the Mam and Mms proteins crucial in all magnetotactic bacteria. While chemical composition and mechanisms occurring inside cells can be useful to the cells themselves, numerous other applications stem from those mechanisms (Appendix).
Appendix
Bacteria in cancer therapy
For decades, research on bacteria in cancer treatment has been conducted. In fact, bacteria could perform direct oncolysis and stimulate the immune system. By secreting exotoxins in the tumor area, direct oncolysis is mediated. Bacterial infections activate the immune system, which targets not only bacteria but also tumor cells. Furthermore, tumors display irregular and chaotic vasculature which leads to areas with low oxygen concentration and nutrient limitation. Such hypoxic regions are a perfect niche for anaerobic and microaerophilic bacteria such as magnetotactic bacteria to perform selective colonization.
AMB-1 magnetotactic bacteria are promising agents for cancer treatments. They have magnetosomes that are known for their chemical purity and well-defined morphology, which minimizes energy throughput while maximizing magnetic sensing (Fdez-Gubieda et al., 2020). Along with their flagella, their magnetosome chain allows them actively swim, and given their natural attraction towards hypoxic areas, AMB-1 can independently locate tumor areas. External magnetic fields, although not required, could also be used to direct AMB-1, which enables the deeper penetration into the tumor issue, making them appealing for biomedical applications.
Heat generation from an alternating magnetic field (AMF)
AMB-1 are also highly touted for their ability to generate heat from an alternating magnetic field (AMF) for magnetic hyperthermia. Magnetic hyperthermia is the clinical procedure of using magnetic nanoparticles (MNPs) from AMF to increase the temperature of selected tumor regions to result in the changed physiology of cancer cells, which cause their eventual apoptosis (Liu et al., 2020). The appropriate temperature range of the tumor area is 41oC to 46oC, and higher temperatures would lead to a more disruptive and less safe destruction of the cancer cells through thermal ablation (Zhang et al., 2021). In fact, under the induction of the AMF (310kHz), the temperature of AMB-1 suspensions increases significantly within 10 min, and the rising temperature is proportional to the magnetic field strength of the AMF and bacterial concentration (Fig. 1a and b). Infrared thermal imaging also confirms the excellent magnetic heating effect of AMB-1 that can heat up the solution rapidly (Fig. 2). These results confirm that AMB-1 can effectively convert the AMF into heat, showing high potential for magnetic hyperthermia (Chen et al., 2022).
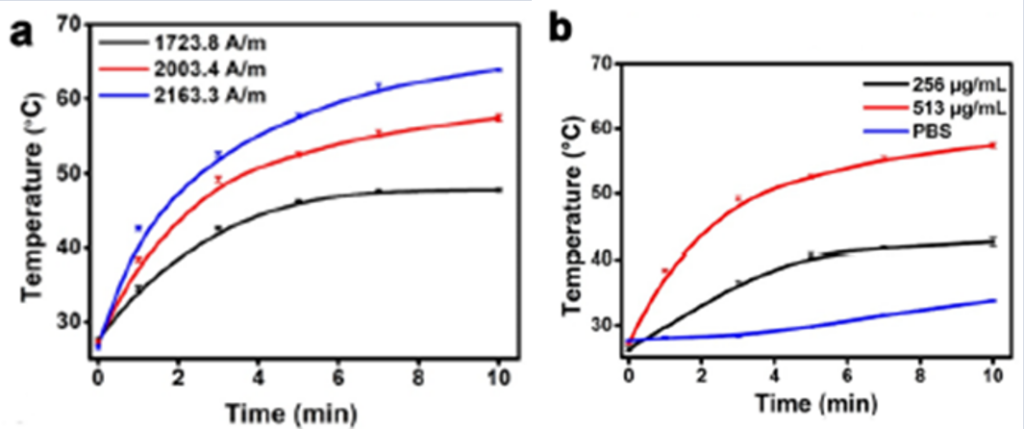
Fig. 1. Temperature elevation curves of AMB-1 suspensions under a 310kHz AMF at (a) different magnetic field strengths (at 513 μg mL−1) and (b) concentrations (at 2003.4 A m−1). (Chen et al., 2022).
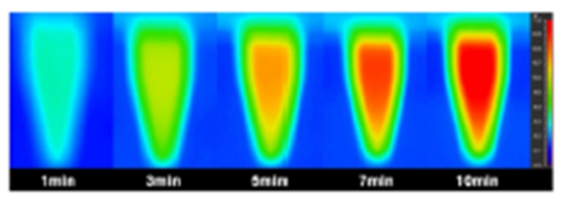
Fig. 2. Infrared thermal images of AMB-1 suspension (513 μg mL−1) under AMF (2163.3 A m−1) at different time points (Chen et al., 2022).
However, AMB-1 remain living bacteria, and therefore, it is best to minimize the dosage given to a patient during cancer treatment. Fortunately, the magnetosome crystals coated by their membranes have high heat efficiency due to well-developed crystallinity and particle uniformity (Muela et al., 2016), strictly regulated by a series of genes (Uebe & Schüler, 2016). The efficiency is parametrized by a magnitude defined as the specific absorption rate (SAR), which refers to the amount of energy absorbed per mass of nanoparticle. It can be determined from the initial slope of the temperature-rising curves with the following equation:
SAR = ms/mn * Cp * DT/Dt
where ms is the mass of the solvent, mnis the mass of the nanoparticles, Cp is the heat capacity of the solvent, and DT/Dt is the initial slope of the temperature rising curve (Zhang et al., 2021). Hergt et al. reported a maximum SAR value of 960 W/g (calculated with magnetosome mass) for magnetosomes with a mean diameter of 30 nm, under 10 kA/m field amplitude and 410 kHz frequency (Hergt et al., 2005). Muela et al. determined a SAR of 2 394 W/g (calculated with magnetosome mass) for magnetosomes of 45 nm exposed to an AMF of 28 kA/m and a frequency of 532 kHz (Muela et al., 2016). In addition, each MTB intact cell with magnetosomes arranged in chains can also be used as a magnetic hyperthermia agent, and the SAR of the intact cells is usually much higher than that of the isolated magnetosomes because of the low magnetostatic interactions (Fdez-Gubieda et al., 2020). Alphandéry et al. obtained the SAR value of 860 W/g (calculated with Fe mass) at 88 mT and 108 kHz for magnetotactic bacteria intact cells using AMB-1 strain (Alphandéry et al., 2011). Gandia et al. directly proved that these cells had higher SAR than the extracted magnetosomes under the same conditions using MSR-1 strain, and then, the intact cells significantly inhibited the proliferation of cancer cells in AMF (Gandia et al., 2019). Therefore, given their high heating efficiency, this ensures lower dosage levels to increase the temperature in tumor areas, making magnetotactic bacteria promising agents for targeted therapy of tumors.
Safety of magnetotactic bacteria
It is important to consider the effects of magnetotactic bacteria for cancer treatment even at low dosages. As previously stated, magnetotactic bacteria have high heating efficiency which ensures minimal usage during therapeutic treatments. Hemolysis is the condition where red blood cells are destroyed so that the contained oxygen-carrying pigment hemoglobin is freed into the surrounding medium. As a result, this can cause vascular dysfunction, injury, and inflammation, putting the patient’s health in a worse state (Rapido, 2017). Fortunately, the generated heat from an alternating magnetic field causes the breakdown and inactivation of AMB-1 bacteria. In fact, scanning electron microscopy (SEM) images confirm that at such temperatures, the cell wall of AMB-1 is damaged, and the cell membrane is ruptured such that the bacteria lose their structural solidity (Chen et al., 2022). After the induction of an alternating magnetic field, AMB-1 inhibits tumor growth, but also prevents its own proliferation. Hemolysis assays also shows that the bacteria do not cause significant hemolysis, with a hemolysis rate below 4.27% (Chen et al., 2022), which suggests the good blood compatibility of AMB-1. Therefore, the loss of bacterial activity after the treatment and low hemolysis rate ensures the biosafety of bacterial cancer therapy.
Nevertheless, much more testing and many more trials are necessary before bacteria can be safely used for cancer treatment. The discovery of magnetotactic bacteria and their advantageous properties has created opportunities for the biomedical world. Their natural attraction to low oxygen and nutrient deficient areas makes them ideal trackers of tumor regions. Their ability to efficiently generate heat and increase the temperature to the desired therapeutic window from an alternating magnetic field leads to the apoptosis of cancer cells and the inhibition of their own growth, ensuring the low widespread damage to organs and tissues.
References
Alphandéry, E., Faure, S., Raison, L., Duguet, E., Howse, P. A., & Bazylinski, D. A. (2011). Heat Production by Bacterial Magnetosomes Exposed to an Oscillating Magnetic Field. The Journal of Physical Chemistry C, 115(1), 18-22. https://doi.org/10.1021/jp104580t
Amor, M., Busigny, V., Louvat, P., Tharaud, M., Gélabert, A., Cartigny, P., Carlut, J., Isambert, A., Durand-Dubief, M., Ona-Nguema, G., Alphandéry, E., Chebbi, I., & Guyot, F. (2018). Iron uptake and magnetite biomineralization in the magnetotactic bacterium Magnetospirillum magneticum strain AMB-1: An iron isotope study. Geochimica et Cosmochimica Acta, 232, 225-243. https://doi.org/https://doi.org/10.1016/j.gca.2018.04.020
Bai, F., Chang, L., Pei, Z., Harrison, R. J., & Winklhofer, M. (2022). Magnetic Biosignatures of Magnetosomal Greigite From Micromagnetic Calculation. Geophysical Research Letters, 49(10), e2022GL098437. https://doi.org/https://doi.org/10.1029/2022GL098437
Baumgartner, J., Morin, G., Menguy, N., Perez Gonzalez, T., Widdrat, M., Cosmidis, J., & Faivre, D. (2013). Magnetotactic bacteria form magnetite from a phosphate-rich ferric hydroxide via nanometric ferric (oxyhydr)oxide intermediates. Proceedings of the National Academy of Sciences, 110(37), 14883-14888. https://doi.org/doi:10.1073/pnas.1307119110
Buseck, P. R., Dunin-Borkowski, R. E., Devouard, B., Frankel, R. B., McCartney, M. R., Midgley, P. A., Pósfai, M., & Weyland, M. (2001). Magnetite morphology and life on Mars. Proceedings of the National Academy of Sciences, 98(24), 13490-13495. https://doi.org/doi:10.1073/pnas.241387898
Camacho, A., Walter, X., Picazo, A., & Zopfi, J. (2017). Photoferrotrophy: Remains of an Ancient Photosynthesis in Modern Environments. Frontiers Microbiology, 8. https://doi.org/https://doi.org/10.3389/fmicb.2017.00323
Chang, S., & Kirschvink, J. (2003). Magnetofossils, the Magnetization of Sediments, and the Evolution of Magnetite Biomineralization. Annu. Rev. Earth Planet. Sci, 17, 169-195. https://doi.org/10.1146/annurev.ea.17.050189.001125
Chen, X., Lai, L., Li, X., Cheng, X., Shan, X., Liu, X., Chen, L., Chen, G., & Huang, G. (2022). Magnetotactic bacteria AMB-1 with active deep tumor penetrability for magnetic hyperthermia of hypoxic tumors [10.1039/D2BM01029A]. Biomaterials Science, 10(22), 6510-6516. https://doi.org/10.1039/D2BM01029A
Dubbels, B. L., DiSpirito, A. A., Morton, J. D., Semrau, J. D., Neto, J. N. E., & Bazylinski, D. A. (2004). Evidence for a copper-dependent iron transport system in the marine, magnetotactic bacterium strain MV-1. Microbiology, 150(9), 2931-2945. https://doi.org/https://doi.org/10.1099/mic.0.27233-0
Fdez-Gubieda, M. L., Alonso, J., García-Prieto, A., García-Arribas, A., Fernández Barquín, L., & Muela, A. (2020). Magnetotactic bacteria for cancer therapy. Journal of Applied Physics, 128(7). https://doi.org/10.1063/5.0018036
Frankel, R. B., Bazylinski, D. A., Johnson, M. S., & Taylor, B. L. (1997). Magneto-aerotaxis in marine coccoid bacteria. Biophys J, 73(2), 994-1000. https://doi.org/10.1016/s0006-3495(97)78132-3
Gandia, D., Gandarias, L., Rodrigo, I., Robles-García, J., Das, R., Garaio, E., García, J., Phan, M. H., Srikanth, H., Orue, I., Alonso, J., Muela, A., & Fdez-Gubieda, M. L. (2019). Unlocking the Potential of Magnetotactic Bacteria as Magnetic Hyperthermia Agents. Small, 15(41), e1902626. https://doi.org/10.1002/smll.201902626
Goswami, P., He, K., Li, J., Pan, Y., Roberts, A. P., & Lin, W. (2022). Magnetotactic bacteria and magnetofossils: ecology, evolution and environmental implications. NPJ Biofilms Microbiomes, 8(1), 43. https://doi.org/10.1038/s41522-022-00304-0
Hazelbauer, G. L., Falke, J. J., & Parkinson, J. S. (2008). Bacterial chemoreceptors: high-performance signaling in networked arrays. Trends Biochem Sci, 33(1), 9-19. https://doi.org/10.1016/j.tibs.2007.09.014
He, K., & Bauer, C. E. (2014). Chemosensory signaling systems that control bacterial survival. Trends Microbiol, 22(7), 389-398. https://doi.org/10.1016/j.tim.2014.04.004
Hergt, R., Hiergeist, R., Zeisberger, M., Schüler, D., Heyen, U., Hilger, I., & Kaiser, W. A. (2005). Magnetic properties of bacterial magnetosomes as potential diagnostic and therapeutic tools. Journal of Magnetism and Magnetic Materials, 293(1), 80-86. https://doi.org/https://doi.org/10.1016/j.jmmm.2005.01.047
Hu, C.-D., Chinenov, Y., & Kerppola, T. K. (2002). Visualization of Interactions among bZIP and Rel Family Proteins in Living Cells Using Bimolecular Fluorescence Complementation. Molecular Cell, 9(4), 789-798. https://doi.org/https://doi.org/10.1016/S1097-2765(02)00496-3
Huynh, M. (2019). Clues to the Early Rise of Oxygen on Earth Found in Sedimentary Rock. NASA Astrobiology Institute. https://astrobiology.nasa.gov/nai/articles/2019/3/5/clues-of-earths-early-rise-of-oxygen/index.html
Jacob, J. J., Jayasri, M. A., & Suthindhiran, K. (2016). Chapter 11 – The chemistry of magnetosomes. In A. M. Grumezescu (Ed.), Surface Chemistry of Nanobiomaterials (pp. 329-358). William Andrew Publishing. https://doi.org/https://doi.org/10.1016/B978-0-323-42861-3.00011-X
Katzmann, E., Eibauer, M., Lin, W., Pan, Y., Plitzko, J. M., & Schüler, D. (2013). Analysis of magnetosome chains in magnetotactic bacteria by magnetic measurements and automated image analysis of electron micrographs. Appl Environ Microbiol, 79(24), 7755-7762. https://doi.org/10.1128/aem.02143-13
Klumpp, S., Lefèvre, C. T., Bennet, M., & Faivre, D. (2019). Swimming with magnets: From biological organisms to synthetic devices. Physics Reports, 789, 1-54. https://doi.org/https://doi.org/10.1016/j.physrep.2018.10.007
Lefèvre, C. T., & Bazylinski, D. A. (2013). Ecology, Diversity, and Evolution of Magnetotactic Bacteria. Microbiology and Molecular Biology Reviews, 77(3), 497-526. https://doi.org/doi:10.1128/mmbr.00021-13
Liu, X., Zhang, Y., Wang, Y., Zhu, W., Li, G., Ma, X., Zhang, Y., Chen, S., Tiwari, S., Shi, K., Zhang, S., Fan, H. M., Zhao, Y. X., & Liang, X. J. Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. (1838-7640 (Electronic)).
McCausland, H. C., & Komeili, A. (2020). Magnetic genes: Studying the genetics of biomineralization in magnetotactic bacteria. PLOS Genetics, 16(2), e1008499. https://doi.org/10.1371/journal.pgen.1008499
Muela, A., Muñoz, D., Martín-Rodríguez, R., Orue, I., Garaio, E., Abad Díaz de Cerio, A., Alonso, J., García, J. Á., & Fdez-Gubieda, M. L. (2016). Optimal Parameters for Hyperthermia Treatment Using Biomineralized Magnetite Nanoparticles: Theoretical and Experimental Approach. The Journal of Physical Chemistry C, 120(42), 24437-24448. https://doi.org/10.1021/acs.jpcc.6b07321
Naresh, M., Das, S., Mishra, P., & Mittal, A. (2012). The chemical formula of a magnetotactic bacterium. Biotechnology and Bioengineering, 109(5), 1205-1216. https://doi.org/https://doi.org/10.1002/bit.24403
Pfeiffer, D., Herz, J., Schmiedel, J., Popp, F., & Schüler, D. (2020). Spatiotemporal Organization of Chemotaxis Pathways in Magnetospirillum gryphiswaldense. Appl Environ Microbiol, 87(1). https://doi.org/10.1128/aem.02229-20
Philippe, N., & Wu, L.-F. (2010). An MCP-Like Protein Interacts with the MamK Cytoskeleton and Is Involved in Magnetotaxis in Magnetospirillum magneticum AMB-1. Journal of Molecular Biology, 400(3), 309-322. https://doi.org/https://doi.org/10.1016/j.jmb.2010.05.011
Popp, F., Armitage, J. P., & Schüler, D. (2014). Polarity of bacterial magnetotaxis is controlled by aerotaxis through a common sensory pathway. Nat Commun, 5, 5398. https://doi.org/10.1038/ncomms6398
Pradel, N., Santini, C.-L., Bernadac, A., Fukumori, Y., & Wu, L.-F. (2006). Biogenesis of actin-like bacterial cytoskeletal filaments destined for positioning prokaryotic magnetic organelles. Proceedings of the National Academy of Sciences, 103(46), 17485-17489. https://doi.org/doi:10.1073/pnas.0603760103
Rapido, F. (2017). The potential adverse effects of haemolysis. Blood Transfus, 15(3), 218-221. https://doi.org/10.2450/2017.0311-16
Uebe, R., & Schüler, D. (2016) Magnetosome biogenesis in magnetotactic bacteria. (1740-1534 (Electronic)).
Wadhams, G. H., & Armitage, J. P. (2004). Making sense of it all: bacterial chemotaxis. Nature Reviews Molecular Cell Biology, 5(12), 1024-1037. https://doi.org/10.1038/nrm1524
Yan, L., Zhang, S., Chen, P., Liu, H., Yin, H., & Li, H. (2012). Magnetotactic bacteria, magnetosomes and their application. Microbiological Research, 167(9), 507-519. https://doi.org/https://doi.org/10.1016/j.micres.2012.04.002
Ying, G., Zhang, G., Yang, J., Hao, Z., Xing, W., Lu, D., Zhang, S., & Yan, L. (2022). Biomineralization and biotechnological applications of bacterial magnetosomes. Colloids and Surfaces B: Biointerfaces, 216, 112556. https://doi.org/https://doi.org/10.1016/j.colsurfb.2022.112556
Zhang, T., Xu, H., Liu, J., Pan, Y., & Cao, C. (2021). Determination of the heating efficiency of magnetotactic bacteria in alternating magnetic field. Journal of Oceanology and Limnology, 39(6), 2116-2126. https://doi.org/10.1007/s00343-021-1071-4