Courtship and Mating: the Physics of Sexual Dimorphism and its Evolutionary Function
Liam Fetherstonhaugh, Joseph Grotsky, Chloe Jacquet, Samuel Nidelli
Abstract
Sexual dimorphisms are defined as the differences in size and anatomy between sexes of animals of the same species. These differences arise from reproductive pressures and oftentimes have no survival benefits. The manifestation of such sexual dimorphisms varies significantly based on courting and mating practices. In this essay, we analyze dimorphisms among eight different species and the physics behind such characteristics. From the bright reflective feathers of peafowl to the throat lever of anole lizards, we suggest sexual dimorphisms are supported by physical laws which lend to their efficacy and sexual selection. As engineers, there is much to be learned from these naturally evolved complex and highly effective systems. The sacrificial bonds of the deer antler solve the problem of tough and stiff materials, the calls of the elephant seal and zebra finch teach us about sound production and wave propagation, the peacock and hummingbird help understand optics and light, and the anglerfish and Eurasian blue tit give insight into bioluminescence and ultraviolet spectroscopy. By investigating the courting and mating rituals of these animals, we discovered sophisticated mechanical systems constructed completely by evolution.
Introduction
In nature, animals have an innate drive to pass on their genes. As such, they seek out the strongest and most fit mates with which to produce offspring, so that they will have the best chance of survival. However, competition for reproductive success can be strong. Through millions of years of sexual selection, this has led different species to evolve complex and dramatic characteristics and courtship behaviours to demonstrate their strength and win over potential mates. This evolution often happens differently between males and females of the same species, as the different sexes look for and value different characteristics in a potential mate. This can lead to major differences in appearance between the sexes known as sexual dimorphism.
Sexual Dimorphism of the Red Deer
Red deer are polygynous animals leading to sexual selection amongst males in the species whereas females focus on competitive selection. To produce offspring, females must access the necessary nutrition, making it vital they are successful foragers. Conversely, males must differentiate themselves from other potential mates, making their size and fighting mechanisms vital to finding mates. This dichotomy in selective pressures leads to sexual dimorphisms where male specimens evolve qualities prioritizing reproductive success over survival function. Male deer must, therefore, develop as large as possible in the shortest amount of time. However, a female’s reproductive success depends more on the length of their fertile window, meaning that their focus is on reaching fertility at the youngest age possible. This leads to much smaller young female deer as their energy is expended carrying and developing fetuses, whereas males focus all energy towards growing large and developing antlers (Post et al., 1999).
The courtship process of male red deer involves the dueling of other suitors. A study by Bartoš and Perner (1998) found that sexual success amongst stags is strongly related to their status in the herd. Their study followed 29 stags and 41 courtship episodes. They observed that during the main mating season the dominant stags fathered the majority of offspring. Subordinate stags only engaged in sexual activities in preceding and succeeding time periods. Therefore, it can be concluded that the most reproductively successful males are the dominant or masters (Bartoš & Perner, 1998). The way this dominance is established is dueling (Fig. 1). A later study on the island of Rum in Scotland observed 20 interactions of stags and concluded the hierarchical ranking of stags was directly related to the number of interactions they were involved in (Bartoš & Bubenik, 2011). While dominant stags were involved in almost all duels, intermediate stags were involved in less than half, and subordinate stages less than a fifth.

As shown in figure one, dueling involves the clashing of antlers together to assert dominance. The quality of a stag’s antlers is therefore particularly important to the courtship process. To be successful, the antlers need to be both “stiff and tough,” according to Currey et al. (2009). Manufacturing materials of these conflicting properties has challenged engineers for years. However, somehow deer have naturally solved this through their antlers. Dry bones are stiff but not tough and splinter under pressure. Therefore, Currey expected dried antler to be brittle and break easily when dried. However, even dry the antlers were 2.4 times more tough than wet regular bone. They were capable of withstanding hits of 6 times greater strength than needed to break femur bones (Currey et al., 2009). De Falco et al. (2017) investigated the material properties which give the antlers their strength and toughness. They discovered that deer antlers have 8 layers of hierarchical structures, which work together to make an incredibly strong material (Fig. 2). The most important section was the ‘sacrificial bonds’ (Fig. 2, section III). These bonds break and reform when the material is placed under great stress, which allows for the absorption of energy without damaging the stiff collagen fibrils. The energy therefore dissipates throughout the bone without causing breakage allowing the material to withstand much greater forces even though it is non-deformable. The combination of the staggering inside the lamella and the easily broken interface causes the plastic and elastic fibrils to be deformed at different rates.
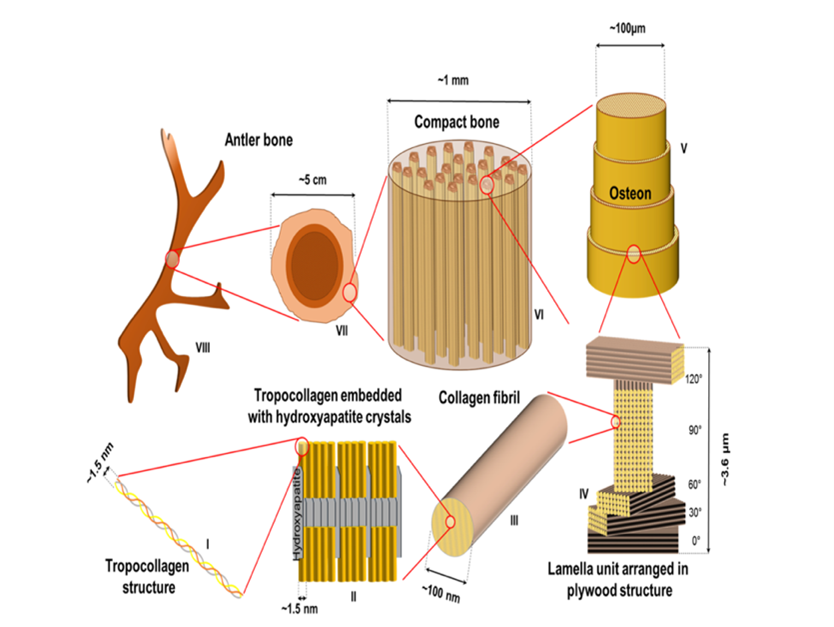
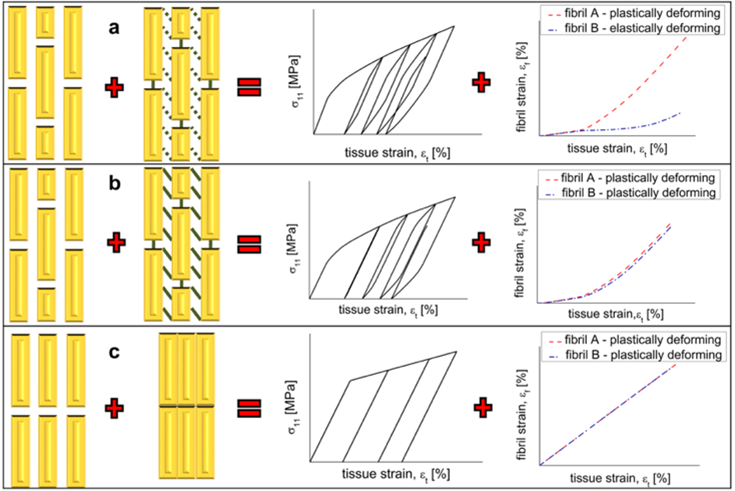
Figure 3 shows different methods of bonding between collagen fibril, and the relationship between percentage strain on each fibril with percentage tissue strain. Situation (a) is what exists in antlers: staggered structures and easily broken bonds. This is what allows less tissue strain on the plastically deforming fibril for the same fibril strain (De Falco et al., 2017).
Sexual Dimorphism of the Elephant Seal
The southern elephant seal (Fig. 4) is a mammal that distinguish themselves by the extreme sexual dimorphism that the male and female undergo. The sexual pattern of the male south elephant seal is very interesting to study about because it is a prime example of a polygynous male. The male elephant seals will mate with multiple females (Sanvito & Galimberti, 2000), which is why the sexual dimorphisms of south elephant seals play such a pivotal role in how the offspring will be born (Sanvito & Galimberti, 2000). The sexual dimorphisms of the male elephant seal are their size compared to the females and their mating call, which is produced by a modified snout, which is unique to the male elephant seals (Sanvito & Galimberti, 2000). Since the sexual pattern of elephant seal colonies is polygynous, it makes it so that males compete to control large groups of females. Their sexual dimorphisms arise from agnostic behaviour since male elephant seals will sometimes engage in violent combat to be able to breed with the most females possible (Mccann et al., 1989).
Often, vocalizations are a main feature in male-male agnostic behaviour, where male-male agnostic behaviour would be settled by vocalizations only (Sanvito et al., 2007). The vocalizations would provide each male with important information on each other’s size and age, giving them a chance to decide if a violent battle is justified. Proboscis expansion is linked with the development of vocalizations (Sanvito et al., 2007). The proboscis (figure 4) is an enlarged and inflatable nasal vestibulum (Frey & Gebler, 2010), which only male elephant seals possess. It is suggested that during vocalization, which is a preliminary step to mating, the proboscis acts as one of many resonators (Frey & Gebler, 2010). This would then alter the frequency after sound has been generated through the laryngeal vocal folds (Frey & Gebler, 2010). The biomechanics of the vocalization production starts with the lungs providing an air stream. This is done to generate energy that will then be transferred to the laryngeal vocal folds, which produce sound by vibrating. The sound travels to the subpharyngeal vocal tract which alters the sound for the first time because of their resonances (Frey & Gebler, 2010). The resonance can be dampened by the walls of the vocal tract if the frequency is too high. The resonances can then be affected by the shape and size of supralarygenal cavities (Frey & Gebler, 2010), caused by tongue positions. Finally, the sound is passed through the proboscis, where the elephant seals act as a supplementary resonator (Frey & Gebler, 2010). A resonator acts when air is pushed into a volume, so that the pressure can be released. The frequency produced through a resonator is in relation to this following formula (Nave, n.d.):
f_{resonance} = \sqrt{ \frac{area \space of \space opening \space port}{volume \space of \space cavity} } * length \space of \space opening \space portThis all means that with a change in the proboscis’ cavity, it could increase or decrease the opening port. This is all with the goal of producing the correct low frequency, of around 55 Hz (Sanvito & Galimberti, 2000), to establish dominance over other males so they can mate successfully.
Sexual Dimorphism of the Anglerfish
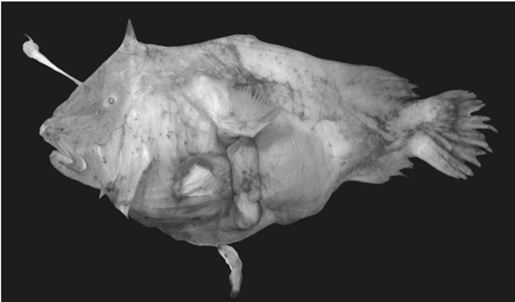
Anglerfish are one of the most intense examples of sexual dimorphism. In extreme cases the female can be more than 60 times as long and about half a million times as heavy as the males (Fig. 5). The mating ritual is parasitic where the males attach to the females and fuse tissues with them. The circulatory systems connect, and the male becomes completely dependent on the female for nourishment. Females have bioluminescent danglers used to lure the males and males have large, advanced eyes to spot a mate. This dangler emits a blue/green light that the males’ eyes are especially susceptible to. Although this bioluminescent dangler makes females more susceptible to predators, it is sexually selected for, as they are essential for reproductive success. Additionally, the bioluminescence facilitates predation allowing the female to receive enough nutrients to support both herself and her male parasite (Pietsch, 2005).
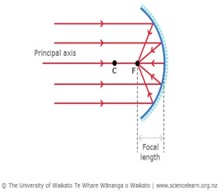
Bioluminescence occurs through the release of energy as light. The enzyme luciferase aids the pigment luciferin in reacting with oxygen to emit light. The female anglerfish produces these enzymes and pigments to attract their mates (Gokhale & Sood, 2012). Additionally, the organisms have a symbiotic relationship with surrounding bioluminescent bacteria. The bacteria are hosted in the organ atop the illicium called the esca (Fig. 6). By shifting the lenses, filters, reflectors, filaments, and appendages inside the esca the angler can adjust its bacterial composition. The bacteria reside inside the esca between the epithelium tissue and a layer of organic crystals. The organic crystals are utilized to amplify the organ’s bioluminescence (Freed et al., 2019). These crystals make up the reflector. The main reflector contains a complex layout of glandular tubules forming a cup shape at the bottom of the esca, as seen in figure 6. It is coated with a thick layer of pigments which attract the light. Once the light is attracted towards the reflector, the complex system of crystals reflects the light back outwards intensifying the luminous properties of the esca. When incident light enters the esca and is reflected by the concave reflector, all the light is concentrated towards the focal point (Fig. 7). This intensifies the concentration of light at this specific point giving the luminous illusion from the organ (“Reflection of Light,” 2020).
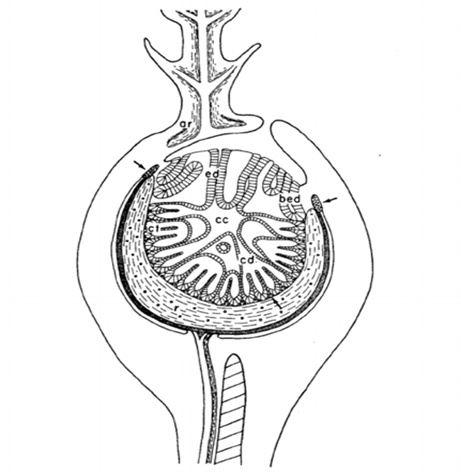
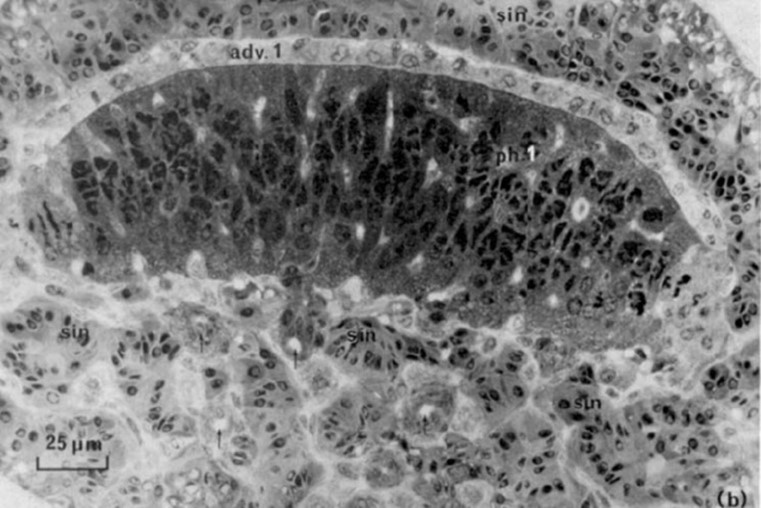
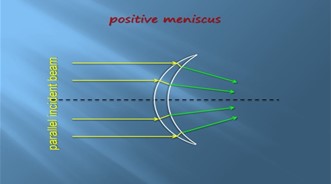
Another thing inside the esca is a lens-shaped glandular core (Fig. 8). This core contains two different layers, the photogenic layer and the adventitious layer. The photogenic layer consists of both large glandular cells, or photocytes, and capillary loops. The glandular cells both have many mitochondria and have organized paracrystalline granules. These structures make up much of the cytoplasm giving the photocytes their photogenic properties (Hansen & Herring, 1977). This lens is a positive meniscus causing light convergence when it passes through the center, again concentrating the light to give the esca its bioluminescent glow (“Reflection of Light,” 2020) (Fig. 9).
Sexual Dimorphism of the Indian Peafowl
The primary sexually dimorphic characteristics of the Indian peafowl (Pavo cristatus) are the peacocks’ colourful and iridescent feathers, as well as their train rattling behaviour that they use to attract peahens. The peacocks’ tail feather display is one of the most well-known and extravagant examples of courtship in the animal kingdom. Their train-rattling involves a multitude of biomechanical processes to impress and attract potential mates most effectively.
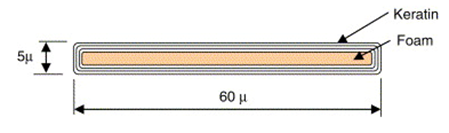
The beauty of the peacocks’ train comes primarily from their lustrous eyespot feathers. These feathers have a complex structure to allow them to be self-supporting when displayed. To remain strong, the peacocks’ tail feathers have a hierarchical structure (Fig. 10). The tiny barbules are supported by the larger barbs, and the barbs are then supported by the stem of the feather. This creates a gradual flow of mass that concentrates the load from the weak barbules down to the strong stem, allowing for high stiffness of the structure (Burgess et al., 2006). All these parts are made up of a thin-film structure, where layers of keratin film coat a low-density core, the medullar layer (Fig. 11.). This helps make the feather very stiff while having a low material requirement (Burgess et al., 2006).
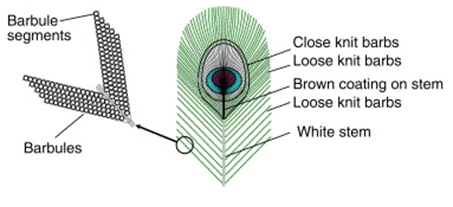
The thin-film structure not only serves to increase the strength/stiffness of the feathers, but also to allow for thin-film interference in the barbules, which produces vibrant colours and lustre. Thin-film interference is an optical phenomenon involving the reflection of light in a very thin layer of material (Burgess et al., 2006). When light reaches the film, some is reflected off the top layer, while some passes through the film. Because the refractive index of the film is different from air, the light that passes through the film slows down before reflecting off the bottom surface. By slowing down the light, some wavelengths shift phase. This phase-shifted light then interferes destructively with the same wavelength light that was reflected off the top surface of the film, causing that colour to be removed from the reflection (Burgess et al., 2006). Other wavelengths do not shift phase, and these then interfere constructively with their counterparts from the top surface, amplifying them. The layers of keratin are 0.4-0.5µm thin, and because visible light has wavelengths of 0.4-0.8µm, when visible light reflects in these layers, it experiences thin-film interference and certain colours are amplified. These colours are called optical colours because they are not produced through pigment (Burgess et al., 2006). Thin-film interference occurs simultaneously in the multiple keratin layers, combining different components of light to create vibrant colours. Different colours can be seen when looking at the feathers from different angles, giving them their iridescence (Burgess et al., 2006). The different colours in the peacocks’ tail feathers are produced through different thicknesses in the layers of keratin and different refractive indices. Each barbule can only be one colour, but because of the hierarchical structure, they are able to be so small that they can act as pixels and produce the eyespot pattern at very high resolution. Small changes in the thickness of the keratin film of adjacent barbules allow for gradual shifts in colour to form the pattern (Burgess et al., 2006).
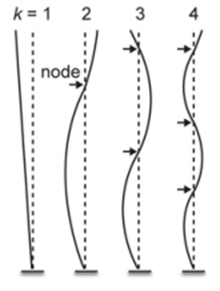
When courting a peahen, peacocks don’t simply display their train/tail feathers, they rattle them. The peacock vibrates their tail laterally (in the plane of the feathers), at or near the resonant frequency of their train feathers, between 22 and 28Hz. This resonance allows for less energy to be required to drive the display (Dakin et al., 2016). The feathers in the peacocks’ train are stiffer in the forward direction, to allow them to remain erect during the display and against the wind, and more compliant in the lateral direction, to allow them to oscillate in this manner (Dakin et al., 2016). Resonance characteristics of feathers can be analyzed by treating them as a type of cantilever because they are only anchored at one end. Based on this, different lengths of tail feathers oscillate at different normal modes (Fig. 12.), the shortest feathers in the first mode and the longest in the third (Dakin et al., 2016). In their train, peacocks also have a large array of eyespot feathers of various lengths, but the resonant frequency of these feathers has been found to be nearly independent of length, allowing for them all to resonate at a single train rattling frequency (Dakin et al., 2016). An important part of the display is that the eyespots on the end of these feathers remain still even as the feathers oscillate. This happens because the centre of the eyespot is located very close to a node – a point on the oscillatory wave where no movement occurs (amplitude is 0). The barbs that make up the eyespots are held together by very small structures, allowing the entire eyespot to act as one entity and remain nearly still at the node (Dakin et al., 2016). These eyespot feathers also have loose barbs that experience a large amount of displacement from oscillation, as they can move freely, creating an iridescent display around the stationary eyespots (Dakin et al., 2016).
Sexual Dimorphism and its Relation to Courtship Dives in the Broad-Tailed Hummingbird
When examining courtship behaviours, hummingbirds are no stranger to dazzling spectacles, with males performing intricate dives to woe potential partners. This type of display is unique and is completed with reference to sexual dimorphisms. Hummingbirds (Trochilidae) can be classified by over 300 species; amongst them, the presence of sexual dimorphisms are widespread (Post et al., 1999). Most notably, hummingbirds are sexually dimorphic in size and exhibit Rensch’s rule: in small species females are larger than males and in large species, males are larger than females (Post et al., 1999). Likewise, vibrant colouration differences are often seen with males dawning bright iridescent crown and gorget feather patches not part of female plumage (Sosa et al., 2019). These exhibits of colour play a key role in the courtship practices of males when they are flaunted in athletic performances.
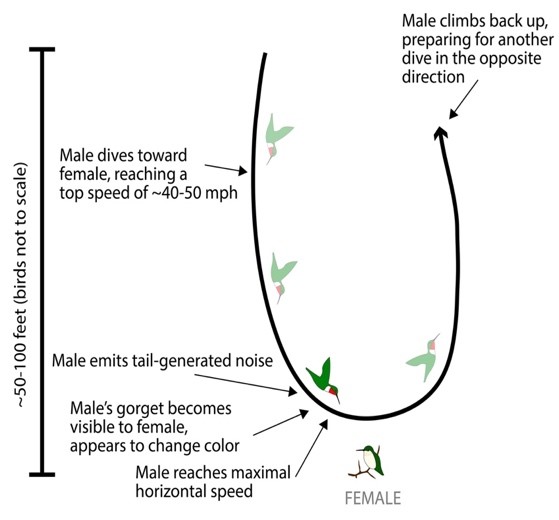
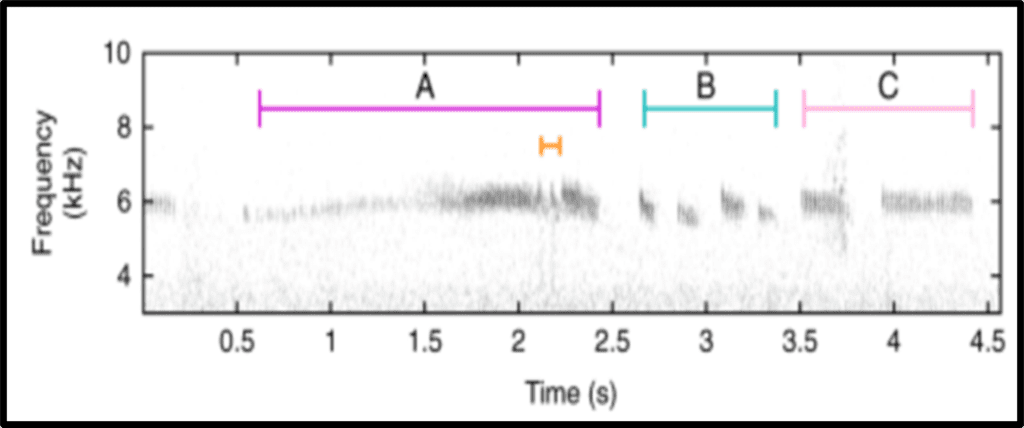
Herein, we investigate the courtship behaviour of the broad-tailed hummingbird (Selasphorus platycerus) (Fig. 13.). Male broad-tailed hummingbirds demonstrate exceptional biomechanics by combining rapid movements, mechanical noises, and visual signals from their iridescent gorgets to execute striking U-shaped courtship dives. The motion consists of the male flying vertically 30m and diving sequentially in U-shapes in front of a female. Near the trough of its flight path, the male produces a distinct ‘buzz’ with its tail feathers while flashing its iridescent (sexually dimorphic) throat patch (Fig. 14.) (Hogan & Stoddard, 2018). This is combined with a non-facultative noise generated by the flapping motion of its wings. What makes this especially unique, is the fact that all these key events occur within a 300-millisecond window (Hogan & Stoddard, 2018). Speed is a crucial wooing factor in the male’s efforts, and so, its tail generated noise and iridescent throat colour contribute to the dynamics of the dive. As the male approaches and quickly passes the female, its tail trill generates an upward and then downward shift in pitch; this is a result of the Doppler effect. The Doppler effect (fo=(v+vo)/(v+vs) ∙ fs) accounts for the change in the male’s sonar frequencies as he passes the female (Fig. 15.). This means that the female perceives a gradually increasing sound, which fades upon the males passing motion; this is the same effect heard from the siren of a passing ambulance. Similarly, due to the geometry of the dive, the male’s red iridescent throat feathers are only visible for a brief period (∿120ms), before being perceived as black (Fig. 16.) (Hogan & Stoddard, 2018). This, in combination with the noises and speed, gives the illusion that the male is travelling at a faster rate. Although comprehensive behavioural data on the female response is currently insufficient, the multimodal signals are suggestive to the male beneficiary. In one notion, the acoustic and visual components of the dives may support each other based on environmental conditions (i.e., sound may be more effective than colour in an overcast climate) (Hogan & Stoddard, 2018). Alternatively (or complementarily), the acoustic and visual elements may signal information about the male’s fitness, quality, or condition (Hogan & Stoddard, 2018). To either measure, females must consider a magnitude of dynamic movements when perceiving the courtship display of the male broad-tailed hummingbird.


Ultraviolet realm of the Eurasian blue tit
Sexual dimorphism takes form in various ways. For mammals, it is not uncommon to observe body size discrepancies, or special ornaments such as antlers or horns. In birds, colourful plumages and hindering long tails are frequent. And, amongst all forms, the commonality can often be related to courtship and mating practices. This holds true for the Eurasian blue tit (Parus caeruleus), a polygynous bird species that demonstrates a unique form of sexual dimorphism (Andersson et al., 1998). Typically, blue tits appear similar amongst the opposite sex; they dawn an azure-blue crown, a yellow underbelly, blue wings and tail, and have a dark blue line passing through their eyes against a white face (Fig. 17.). While the distinct appearance is observed on both males and females, blue tits are sexually dichromatic in ultraviolet light (UV). It is important to note that bird vision extends to the UV-A waveband (320-400 nm), which is broader than the human spectra of visible light (400-700 nm) (Andersson et al., 1998).Coincidently, to the naked eye, humans are unable to discern the UV sexual dichromatism of blue tits. The colour variation amongst male and females is nonetheless influential in the courtship and mating practices of the species (Andersson et al., 1998).

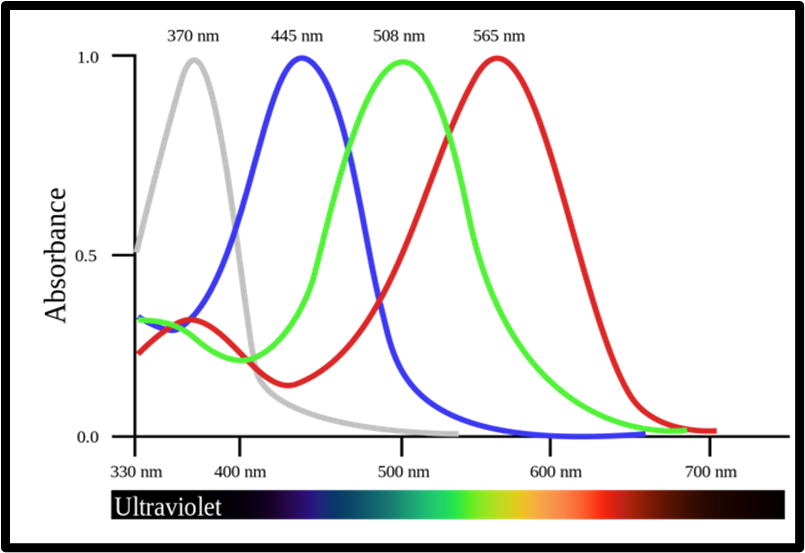
Avian vision and the perception of the UV-A spectra is achieved through a UV cone mechanism not present in humans. In comparison, birds are generally equipped with at least four spectrally unique cone types to our three (Hunt et al., 1998). This permits the observation of an entirely new spectra well engrained in the vision of birds. Since this cone is present in the retina of the blue tit, we must study the 320-700 nm spectral window to explore the social function of its sexual dimorphism.In the UV-A spectra, male blue tits express vivid plumage colouration (Fig. 18.); this is put on display in courtship dances involving horizontal posturing and erected nape feathers. This behaviour supports the role of male plumage as an epigamic ornament that ushers mate choice for females (Andersson et al., 1998; Hunt et al., 1998). In Blue tits are ultraviolet tits, Hunt et al. (1998) suggested that female blue tits tend to favor males of brightest plumage in the UV spectra. While difficult to imagine from a human perspective, the disparity amongst the plumage of males and females is immense (Fig. 19.). This is especially true in the crown and crest areas, where males express much lighter colouration in the 320-400 nm range. Hence, UV vision and receptors take on an integral role in the courtship practices of female blue tits, an important dimension of the sexual dimorphism (Andersson et al., 1998).
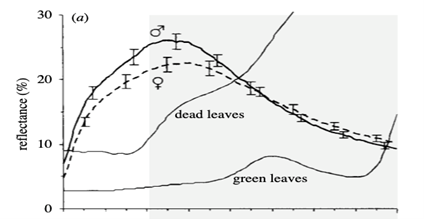
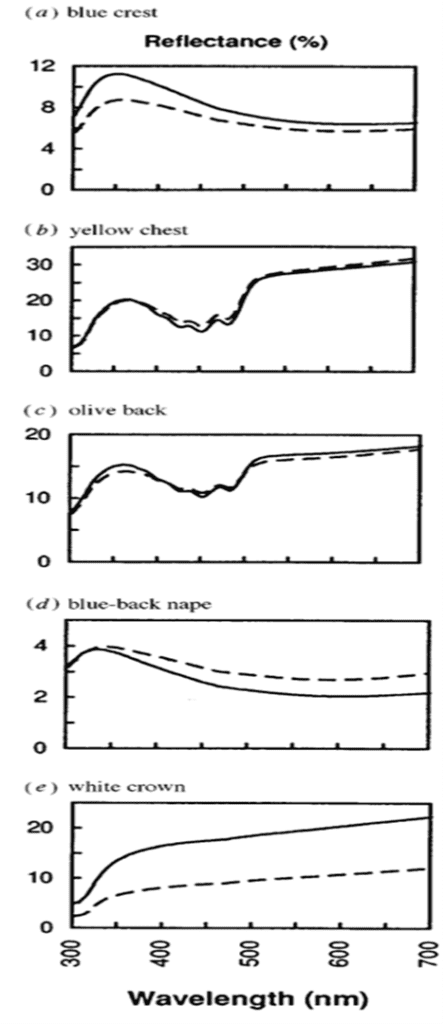
The case of the blue tit presents a conspicuous model for the nature of matting and attraction in UV dimorphism. Using reflectance spectrometry, electromagnetic radiation (Fig. 20) of human invisible wavelengths is studied to discover that blue tits are sexually dichromatic (Andersson et al., 1998; Hunt et al., 1998; Johnsen et al., 2003). This is peculiar from the point of light and optics, and suggests a plethora of undiscovered realms in the animal kingdom. Blue tits, and avian species in general, are said to have tetrachromatic colour vision; this comes through the presence of the fourth cone type (Hunt et al., 1998). Sight is a phenomenon which occurs when light is absorbed through the retina of an eye. Colour, a subset of vision, results from different wavelengths of light being reflected or absorbed by an object; the wavelengths that are reflected make up the colour viewed through sight. Coincidently, tetrachromatic colour vision permits blue tits the ability to absorb reflected light in the UV-A spectra (Fig. 21). This is phenomenally decisive in discerning the sexual dimorphisms of the Eurasian blue tit.
Sexual Dimorphism of the Anoline Lizard
For courtship, male lizards of the genus Anolis primarily use a skin flap on their throat, known as the dewlap, to signal to females that they are a potential mate. In addition to extending the dewlap, males will perform head and body movements in an attempt to attract a mate.
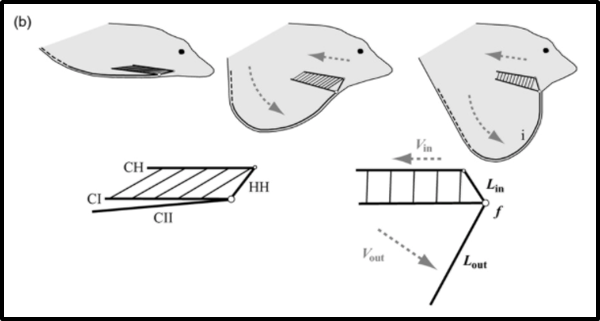
The dewlap of Anolis lizards has an elaborate mechanism that allows for it to be extended, increasing the appearance of the animal’s size to better attract mates, and retracted to an almost invisible state, to avoid predation resulting from their increased visibility. In the throat of the lizard, there is a set of bones called the hyoid apparatus. In dewlap extension, this apparatus acts as a first-order lever (Fig. 22), where the hypohyal (HH) is the power arm, the second ceratobranchials (CII) act as the weight arm, and the role of the fulcrum is performed by the first ceratobranchial (CI) joint. The hyoid starts at a resting position, with the apparatus lying parallel to the lizard’s head. The hypohyal, the power arm, is then pulled by contraction of the ceratohyoideus muscles, rotating from near the nose of the lizard, up and back towards its body. This causes the second ceratobranchials to pivot about the CI joint, and rotate down and outward, away from the lizard’s body, pushing the dewlap out to its extended position (Ord et al., 2013). The CII bone is less mineralized and thus less rigid as it grows further from the CI joint, so when it is extended, the end of CII is bent back as a result of the stress exerted by the taught skin of the dewlap. This creates the dewlap’s distinct curved shape (Ord et al., 2013). The action of the hyoid apparatus as a first-order lever allows for the speed of the ceratohyoideus muscle contraction to be amplified for faster dewlap extension. This is because the length of the CII, the weight arm, is longer than the HH, the power arm. The shorter the HH, the closer the applied force is to the fulcrum, causing a greater speed of extension (Ord et al., 2013). Faster dewlap extension is important for efficient signalling (Ord et al., 2013). This mechanism is consistent across all species in the genus Anolis, but the speed of extension varies due to differences in the ratio of HH length to CII length (Ord et al., 2013).
In addition to helping the lizard appear larger, the Anolis dewlap is also brightly coloured and can appear to glow, aiding in signalling. In its extended position, the dewlap is translucent. The translucence of the dewlap means that while some light is reflected or absorbed by its coloured skin, some passes through it. This creates translucent transmission, where the light that does pass through is diffused (Fleishman et al., 2016). Because of this, when light is coming from the direction opposite the viewer, the diffused light makes the dewlap appear to glow (Fig. 30). By increasing the intensity of light coming from the dewlap, translucent transmission helps the dewlap stand out against distracting colours found in the lizards’ natural habitat. A more visible dewlap means that male anoline lizards can signal to attract females more quickly (Fleishman et al., 2016).

Sexual Dimorphism of the Zebra Finch
The zebra finch (Fig. 24) is a songbird that relies on sexually dimorphic behaviours to mate and reproduce successfully.

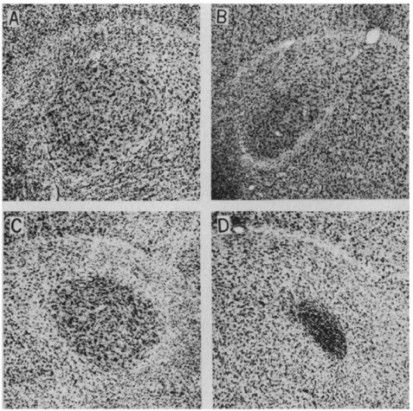
Within zebra finch groups, their calls can change composition depending on their mating status (Gill et al., 2015). The male zebra finch has the role of producing mating calls due to having four vocal control areas in the brain that are larger than those of female zebra finches: (i) area X of the lobus parolfactorius, (ii) the hyperstriatum ventral (HVc), (iii) the robust nucleus of the archistratium (RA), and (iv) the hypoglossal nucleus of the medulla (nXII) (Nottebohm & Arnold, 1976). These size differences will cause a difference in singing behaviour between male and female zebra finches, where the male zebra finches perform birdsongs, and the females do not (Nottebohm & Arnold, 1976). Additionally, male zebra finches have a greater cell packing in the robustus nucleus of the archistratium, which is the primary song output nucleus (Fig. 25) (Nottebohm & Arnold, 1976). Male zebra finches primarily learn songs through auditory reference, and these are known as tutor songs (Nottebohm & Arnold, 1976). Young male zebra finches memorize songs from older males to develop an ‘internal model’ (Aamodt, 1999).
The goal of producing a song is to improve the likelihood of reproduction. It was shown that the most important criteria for female zebra finches when choosing a mate is the song rate (Gill et al., 2015). The reason behind this choice is likely due to the increased “good gene” mechanisms found in a higher song rate (Collins et al., 1994).
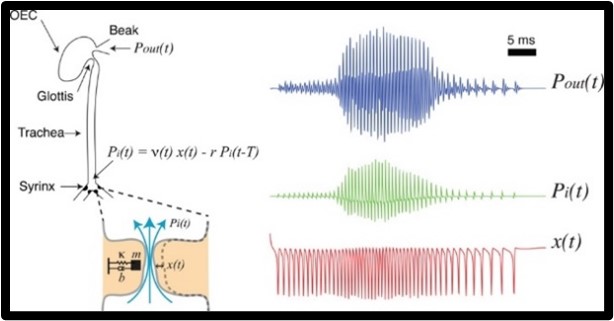
The study of birdsong production has baffled neuroscientists because it involves the integrative study between the nervous system, the peripheral biomechanical system, and the environment (Mindlin, 2013). The avian vocal organ has distinct features that are either produced by specific neural instructions and which are produced from biomechanics (Mindlin, 2013). The production of birdsongs is related to the way humans produce sound with our voices because in both cases, there is a modulation of airflow through vocal folds that oscillate (Mindlin, 2013). The basic mechanism of birdsong production starts when the bird exhales, which establishes airflow through the syrinx, the avian vocal organ (Fig. 26). At the base of the trachea, where the syrinx is located, there are two junctures that contain a pair of labia. At the correct conditions, airflow will induce oscillations in the labia, which produces an acoustic wave. This acoustic wave travels through the trachea and oro-esophageal cavity to change harmonic content and produce a sound signal (Mindlin, 2013). Mindlin (2013) described two important parameters to produce a sound signal: labial tension and air sac pressure. Mindlin (2013) described an excellent way to visualize what is occurring by imagining that the labium is a mass in a spring, where the stiffness equates to the labial tension. The bird controls the labial tension by relaxing or tightening syringeal muscles. These muscles are responsible for the development of frequency modulation since they are attached to the medial ventral cartilage (MVC), which is then a part of the medial labia (Mindlin, 2013). Therefore, its contraction would affect length and tension within the labia since these two are positively correlated. As shown in figure 26, a quicker displacement of the labia would induce a higher internal pressure which then correlates to a higher external pressure, making it so that the song rate is higher (Mindlin, 2013).
All in all, the sexual dimorphism of zebra finches is their birdsong because it is used to attract mates to reproduce successfully, and is only seen in one of the sexes. The birdsong is learnt by tutor songs and is produced through the biomechanics of the avian vocal organ, which dictates the oscillations of the labia.
Conclusion
In this paper, the physics of sexual dimorphisms of eight animals was discussed. These adaptations, which favour courtship and mating, are paramount to their survival because reproduction can be very competitive in nature. The analysis of the physics of the sexual dimorphisms in this article range from the material science of red deer antlers, the vocalization of male elephant seals, the bioluminescence of angler fish, the colourful and iridescent feathers of the peacock, the dives and optical properties of hummingbirds, the UV sexual dichromatism of Eurasian blue tits, the signaling caused by the dewlap of Anoline lizards, to the birdsong production of male zebra finches. Each sexual dimorphism is unique because it must combat different environments and survive the test of evolution. Yet again, these behaviours are a prime example of how nature can be so broad and beautiful. These sexual dimorphisms and other behaviours alike can surely offer humans a deeper understanding of our complex world and inspire us to innovate and improve science.
References
Aamodt, S. (1999). Singing in the brain: song learning in adult zebra finches. Nature Neuroscience, 2(7), 590-590. https://doi.org/10.1038/10139
Andersson, S., Örnborg, J., & Andersson, M. (1998). Ultraviolet sexual dimorphism and assortative mating in blue tits. Proceedings of the Royal Society of London. Series B: Biological Sciences, 265(1395), 445-450. https://doi.org/10.1098/rspb.1998.0315
Bartoš, L., & Bubenik, G. (2011). Relationships between rank-related behaviour, antler cycle timing and antler growth in deer: Behavioural aspects. Animal Production Science, 51, 303-310. https://doi.org/10.1071/AN10195
Bartoš, L., & Perner, V. (1998). Distribution of mating across season and reproductive success according to dominance in male red deer. Folia Zoologica (Czech Republic).
Boyle, L. (2018, April 1). Zebra Finch. Flickr. Retrieved October 4, 2021, from https://www.flickr.com/photos/92384235@N02/40555089585/
Burgess, S. C., King, A., & Hyde, R. (2006). An analysis of optimal structural features in the peacock tail feather. Optics & Laser Technology, 38(4), 329-334. https://doi.org/https://doi.org/10.1016/j.optlastec.2005.06.021
Collins, S. A., Christene, H., & Houtman, A. M. (1994). Female Mate Choice in the Zebra Finch: The Effect of Male Beak Colour and Male Song. Behavioral Ecology and Sociobiology, 35(1), 21-25. http://www.jstor.org/stable/4600971
Currey, J. D., Landete-Castillejos, T., Estevez, J., Ceacero, F., Olguin, A., Garcia, A., & Gallego, L. (2009). The mechanical properties of red deer antler bone when used in fighting. Journal of Experimental Biology, 212(24), 3985-3993. https://doi.org/10.1242/jeb.032292
Dakin, R., McCrossan, O., Hare, J. F., Montgomerie, R., & Amador Kane, S. (2016). Biomechanics of the Peacock’s Display: How Feather Structure and Resonance Influence Multimodal Signaling. PLOS ONE, 11(4), e0152759. https://doi.org/10.1371/journal.pone.0152759
De Falco, P., Barbieri, E., Pugno, N., & Gupta, H. S. (2017). Staggered Fibrils and Damageable Interfaces Lead Concurrently and Independently to Hysteretic Energy Absorption and Inhomogeneous Strain Fields in Cyclically Loaded Antler Bone. ACS Biomater Sci Eng, 3(11), 2779-2787. https://doi.org/10.1021/acsbiomaterials.6b00637
Electromagnetic Radiation. (2019, October 21). National Aeronautics and Space Administration. Retrieved October 4, 2021, from http://www.ces.fau.edu/nasa/module-2/radiation-sun.php
Eurasian Blue Tit. (n.d.). Animalia. Retrieved October 4, 2021, from https://animalia.bio/eurasian-blue-tit
Fleishman, L. J., Ogas, B., Steinberg, D., & Leal, M. (2016). Why do Anolis dewlaps glow? An analysis of a translucent visual signal. Functional Ecology, 30(3), 345-355. https://doi.org/10.1111/1365-2435.12502
Freed, L. L., Easson, C., Baker, L. J., Fenolio, D., Sutton, T. T., Khan, Y., Blackwelder, P., Hendry, T. A., & Lopez, J. V. (2019). Characterization of the microbiome and bioluminescent symbionts across life stages of Ceratioid Anglerfishes of the Gulf of Mexico. FEMS Microbiology Ecology, 95(10). https://doi.org/10.1093/femsec/fiz146
Frey, R., & Gebler, A. (2010). Chapter 10.3 – Mechanisms and evolution of roaring-like vocalization in mammals. In S. M. Brudzynski (Ed.), Handbook of Behavioral Neuroscience (Vol. 19, pp. 439-450). Elsevier. https://doi.org/https://doi.org/10.1016/B978-0-12-374593-4.00040-1
Fuller, L. C. (n.d.). Broad-tailed Hummingbird. The Cornell Lab of Ornithology. Retrieved October 4, 2021, from https://celebrateurbanbirds.org/learn/birds/hummingbird/broad-tailed-hummingbird/
Gill, L. F., Goymann, W., Ter Maat, A., & Gahr, M. (2015). Patterns of call communication between group-housed zebra finches change during the breeding cycle. Elife, 4. https://doi.org/10.7554/elife.07770
Gokhale, T., & Sood, N. (2012). Research of marine isolates in development of biosensors for environmental pollutants. Engineering Reviews, 32, 17-22.
Hansen, K., & Herring, P. J. (1977). Dual bioluminescent systems in the anglerfish genus Linophryne (Pisces: Ceratioidea). Journal of Zoology, 182(1), 103-124.
Heald, T. (2020, October 26). Red Deer (Cervus elaphus) stags fighting during the rutting season. Bushy Park, London, UK. October. Nature Picture Library. Retrieved October 4, 2021 from https://www.naturepl.com/stock-photo/red-deer-(cervus-elaphus)-stags-fighting-during-the-rutting-season-bushy/search/detail-0_01662091.html
Hogan, B. G., & Stoddard, M. C. (2018). Synchronization of speed, sound and iridescent color in a hummingbird aerial courtship dive. Nature Communications, 9(1). https://doi.org/10.1038/s41467-018-07562-7
Hunt, S., Bennett, A. T. D., Cuthill, I. C., & Griffiths, R. (1998). Blue tits are ultraviolet tits. Proceedings of the Royal Society of London. Series B: Biological Sciences, 265(1395), 451-455. https://doi.org/10.1098/rspb.1998.0316
Johnsen, A., Delhey, K., Andersson, S., & Kempenaers, B. (2003). Plumage colour in nestling blue tits: sexual dichromatism, condition dependence and genetic effects. Proceedings of the Royal Society of London. Series B: Biological Sciences, 270(1521), 1263-1270. https://doi.org/10.1098/rspb.2003.2375
McCann, T. S., Fedak, M. A., & Harwood, J. (1989). Parental investment in southern elephant seals, Mirounga leonina. Behavioral Ecology and Sociobiology, 25(2), 81-87. https://doi.org/10.1007/bf00302924
Mindlin, G. B. (2013). The physics of birdsong production. Contemporary Physics, 54(2), 91-96. https://doi.org/10.1080/00107514.2013.810852
Nave, C. R. (n.d.). Cavity Resonance. HyperPhysics. Retrieved October 8, 2021, from http://hyperphysics.phy-astr.gsu.edu/hbase/Waves/cavity.html#c1
Nottebohm, F., & Arnold, A. P. (1976). Sexual Dimorphism in Vocal Control Areas of the Songbird Brain. Science, 194(4261), 211-213. https://doi.org/doi:10.1126/science.959852
Ord, T. J., Collar, D. C., & Sanger, T. J. (2013). The biomechanical basis of evolutionary change in a territorial display. Functional Ecology, 27(5), 1186-1200. https://doi.org/10.1111/1365-2435.12110
Pietsch, T. (2005). Dimorphism, parasitism, and sex revisited: Modes of reproduction among deep-sea ceratioid anglerfishes (Teleostei: Lophiiformes). Ichthyological Research, 52, 207-236. https://doi.org/10.1007/s10228-005-0286-2
Post, E., Langvatn, R., Forchhammer, M. C., & Stenseth, N. C. (1999). Environmental variation shapes sexual dimorphism in red deer. Proc Natl Acad Sci U S A, 96(8), 4467-4471. https://doi.org/10.1073/pnas.96.8.4467
Princeton University. (2018, December 18). Dive-bombing for love: Male hummingbirds dazzle females with a highly synchronized display. Science X. Retrieved October 4, 2021, from https://phys.org/news/2018-12-dive-bombing-male-hummingbirds-dazzle-females.html
Reflection of Light. (2020, August 27). Science Learning Hub. Retrieved October 4, 2021, from https://www.sciencelearn.org.nz/resources/48-reflection-of-light
Sanvito, S., & Galimberti, F. (2000). Bioacoustics of southern elephant seals. I. Acoustic structure of male aggressive vocalisations. Bioacoustics-the International Journal of Animal Sound and Its Recording – BIOACOUSTICS, 10, 259-285. https://doi.org/10.1080/09524622.2000.9753438
Sanvito, S., Galimberti, F., & Miller, E. H. (2007). Vocal signalling of male southern elephant seals is honest but imprecise. Animal Behaviour, 73(2), 287-299. https://doi.org/https://doi.org/10.1016/j.anbehav.2006.08.005
Shyamal, L. (2009, March 21). File:BirdVisualPigmentSensitivity.svg. Wikimedia Commons. Retrieved October 4, 2021, from https://commons.wikimedia.org/wiki/File:BirdVisualPigmentSensitivity.svg
Sosa, J., Parra, J. L., Stavenga, D. G., & Giraldo, M. A. (2020). Sexual dichromatism of the Blue-throated Starfrontlet, Coeligena helianthea, hummingbird plumage. Journal of Ornithology, 161(1), 289-296. https://doi.org/10.1007/s10336-019-01709-z