An Investigation into the Chemical Properties of Gonium
Andrew D’Argenio, Breanna D’Ettorre, Lucas Elliott, Joseph Gad
Abstract
Gonium is a genus of multicellular green algae with chemical systems of high complexity developed by generations of evolution. Gonium is an autotroph and exhibits a variety of chemical photocycles that control its behavior without a central nervous system. Because Gonium has no cellular differentiation, chemical systems exist separately in each cell, and are controlled by the environmental stimulus individually received. These highly modular chemical principles are essential to the Gonium‘s survivability, as they control reproductive systems, toxin protection, photosynthesis, gene regulation, circadian rhythm, protein function, and more. Simple photocycles create advanced chemical systems, and these advanced systems drive evolution forward. Systems dependent on chemical responses like these can be contextualized as the chemistry dependent evolutionary solutions of Gonium, and in this paper, we will investigate how these chemical systems function and their development through Gonium‘s evolutionary lineage. To understand the chemistry of Gonium, we will begin with the simple photoreceptor processes, expand into the complex chemical systems, and conclude with the chemical differentiation of Gonium‘s evolutionary lineage.
Introduction
The colonial green algae genus known as Gonium stands out as a fascinating element of the microworld. This organism is one of many examples of nature’s diversity and ingenuity in its design. Understanding the chemical processes involved in Gonium‘s lifecycle can further our appreciation of the coloniality and cooperation of green algae as a whole.
Gonium‘s multiple biflagellate cells that are enveloped within a gelatinous matrix vary in number depending on the species. Colonies can contain 4, 8, 16, or 32 cells of the same size organized in a flat plate. These phototrophic organisms rely on phototaxis to orient themselves to perform photosynthesis. The flagellar movement of the multicell organism allows it to orient its eyespot in the direction of the light. It is through the photoreceptor proteins located in the eyespot that Gonium can process light signals and regulate certain cellular responses. Influencing phototaxis, reproduction and other essential biological processes of the organism.
Gonium‘s methods of asexual and sexual reproduction and growth are regulated by many factors besides light. A combination of nutrient availability and light conditions facilitate sexual reproduction, which is induced by the presence of heterothallic strains. Controlling the different aspects of the reproductive cycle ensures the colony can best adapt to the present environmental conditions. These adaptations can ensure the population’s survival by increasing the likelihood of enduring a nutrient-poor environment, increasing the possibility of a successful fusion of gametes, and likely more yet unknown consequences.
It is important to note that Gonium‘s behavioural chemistry is not limited to reproduction. The microorganism faces environmental challenges relating to pollutants like heavy metals, tannins, and reactive dyes. Its sensitivity to heavy metals results in inhibited reproduction. However, the organism’s response to other potentially harmful chemicals demonstrates a magnificent evolutionary adaptive strategy to foreign chemicals. Gonium has an excellent capacity to adapt to toxic pollutants in its environment, in this case by degrading phenolic compounds due to its production of laccase enzymes.
The organism’s genome may provide additional information aside from its own characteristic. Gonium pectorale‘s positioning at the intersection of the unicellular Chlamydomonas reinhardtii and the multicellular Volvox carteri can provide valuable insight into the origin of multicellularity evolution. While the transition from unicellular to multicellular organisms typically involves significant genetic changes, it appears that Gonium‘s genetic makeup bears striking similarity to its unicellular and multicellular counterparts. In essence, the evolution from unicellularity to multicellularity may have occurred in as few as twelve steps, challenging conventional assumptions about genetic complexity.
In the pages that follow, a dissection of Gonium‘s chemical intricacies will be presented, scrutinizing its molecular landscape to uncover the forces governing its biology. Through this exploration, we hope to unveil a deeper appreciation for the chemical marvels within Gonium and draw connections to the grand tapestry of life on our planet.
Photochemical interactions and primary photoreceptors in gonium pectorale
There are three main proteins involved with the reception of light in Gonium. The process of light collection, focusing, and filtering has been detailed significantly in the physics component of this project. To generalize, the eyespot uses a combination of physical devices to focus incident light to a cluster of photoproteins in the center of the eyespot, but also activates photoproteins across a wider area with an interference curtain. These photoreceptors are Channel-Rhodopsins, Phototropins, and Cryptochromes. Together, they process light and produce a chemical response that leads to phototaxis and other behavioral responses.
Channel-rhodopsin activity and photocycle
Channel-Rhodopsins (ChRs) are a specific type of Rhodopsin photoproteins characterized by their activity as light-gated ion channels (Böhm and Kreimer, 2020; Hegemann, 2008). Generally, Rhodopsins, the main function proteins in complexes known as rods, are proteins composed of two significant component parts: the “carotenoid derivative, retinene, […] and colorless protein, opsin” (Hubbard and Kropf, 1958). Rhodopsins are light-activated G-protein signaling molecules, that create a phosphorylation cascade and cyclical GMP (cGMP) production, which generally leads to a cellular response through concentration gradients created from this cGMP activity (Terakita, 2005). Channel-Rhodopsins create their response through opening and closing an ion channel, that facilitates the passage of Ca2+, H+, and Na+ ions. Upon reception of light, ChR channels are opened, H+ and Na+ ions flow in, which causes a change in concentration between the three ions, and a rapid depolarization of the cell membrane is incited (Böhm and Kreimer, 2020). This process can be seen in Figure 1 below, showing the open and closed states of ChRs.
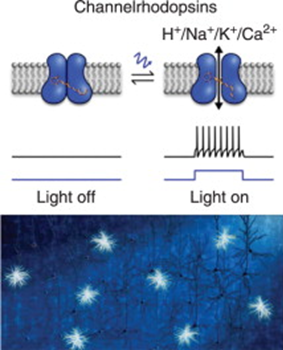
Fig. 1. A simple model of depolarization caused by activation of Channelrhodopsins.
Once activated by light, channelrhodopsin configuration is changed to allow the flow of ions, causing a depolarization signal to be propagated across the cell membrane (Tan et al., 2015).
This rapid change in the concentration gradient of the cell membrane near the eyespot spreads the electric depolarization signal across the membrane and to the flagella. While the exact mechanism of interpretation of the signal by the flagella is unknown, there is an observable “photoshock” response, where the reception of the depolarization signal leads to an immediate increase in the activity of the flagella (Böhm and Kreimer, 2020).
The actual chemical change we see in ChRs can be represented in two photocycles, as seen in figure 2 below. An original ChR without light exists in a dark state, known as D470, before it is excited by light. In this state, ChR is closed to all ion transport. Once excited by light, it will either undergo a conformational change to P480, where one alkene is changed from trans to cis and the nitrile group is changed from anti to syn, or it will be excited to P500, where the same alkene changes conformation but the nitrile group stays anti (Kuhne et al., 2019). This is called the anti-cycle, and P500 will very quickly begin to reconform to P390a and P390b, and then P520. Both P390’s cause ChR to be open for hydrogen ion transport, and P520 causes ChR to open for sodium ion transport. However, these states do not last long, and within thirty milliseconds, the channel will close again. The syn-cycle is the longer, more receptive channel opening response. Once excited to P480, ChR will quickly drop to P500-syn, and then again to P520 syn, which causes opening for hydrogen ion transport. In this case, the syn conformation is very energetically favorable, and will exist for about 250 milliseconds, about eight times as long as anti-cycle, before ChR closes once again.
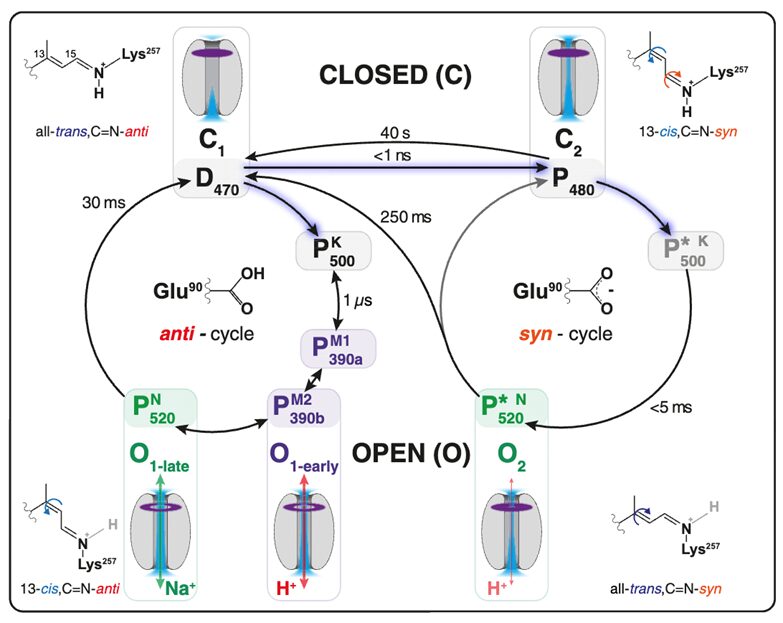
Fig. 2. The Photocycle of Channel-Rhodopsins in Green Algae. This figure shows two possible response cycles to the excitement of electrons by light, a short response cycle (left circle) and extended response cycle (right circle). The significant difference is whether or not the nitrile (C=N) group is in syn or anti conformation with the rest of the alkene chain (Kuhne et al., 2019).
This photocycle is what creates the rapid depolarization that spreads across the cell membrane and delivers a photo-shock to flagella (Böhm and Kreimer, 2020). However, this only considers the light that rhodopsin absorbs, which, in Gonium and other algae of its family, is primarily within the red wavelength range. To consider other wavelengths, we must consider Photoropins and Cryptochromes.
Photropin activity and photocycle
Phototropins absorb light in a wide range of blues (Böhm and Kreimer, 2020). Phototropins (PHOTs) are partially responsible for a wide range of Gonium‘s other interactions, including photosynthesis, eyespot regulation, and the reproductive system (Hegemann, 2008; Huang and Beck, 2003). PHOTs are light activated kinase proteins, which means they have a primary role in the creation and amplification of signals. PHOTs are primary contributors to phosphorylation cascades in Gonium, and as such, they also exhibit a photocycle, but it is one that is a little less complicated than that of ChRs.
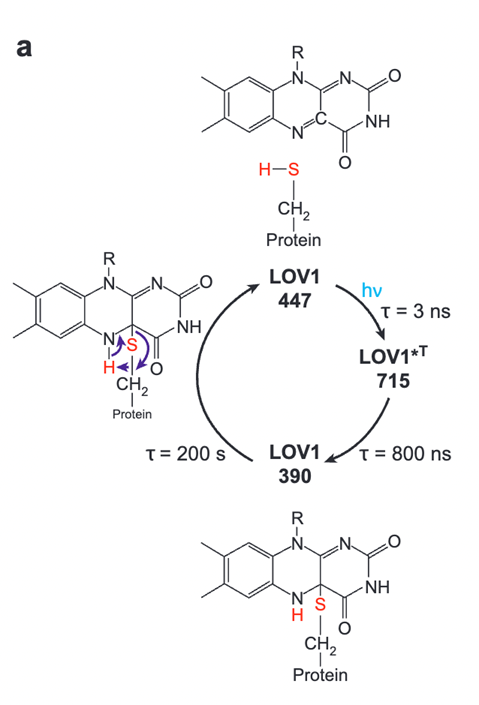
Fig. 3. The Photocycle of Phototropin LOV1 in Green Algal Eyespots. Phototropin has one general cycle in response to excitation from incident light. The cycle has two steps to reach its signalling state. This state is very stable and stays for around 200 seconds before returning to its starting state (Hegemann, 2008).
PHOTs start at a dark state of LOV1-447, until they are excited by light (Hegemann, 2008). Once excited, they enter what is called their signaling state, or LOV1-715. Then, they are quickly oxidized by binding to a protein with a thiol group, entering LOV1-390, a more stable state that they will stay in for more than a minute. In the signaling state, this kinase is active, and contributes to the phosphorylation cascade step of signal transduction, spreading a signal for eyespot control, sexual reproduction, and more. Finally, PHOTs will return and the cellular response will gradually decrease without more light to excite. PHOT activation is shown to have a direct correlation with the regulation of eyespot gene expression, possibly increasing or decreasing the sensitivity of the eyespot with different light conditions to provide a more ideal tool for photosynthesis (Böhm and Kreimer, 2020; Hegemann, 2008). Additionally, PHOT controls the expression of chemotaxis.
Besides phototaxis for orientation to light, Gonium also exhibits chemotaxis in low-light conditions. Essentially, chemotaxis is a method of orientation toward chemical ammonia as an energy source when energy cannot be provided by light in photosynthesis. Because Gonium‘s light energy is mainly provided by the sun, chemotaxis response is generally activated on a circadian rhythm at night, but this is only true for adult vegetative cells. When Gonium cells create gametes and reproduce, chemotactic responses is switched off in gametes, and this response is controlled by PHOTs (Ermilova et al., 2004). PHOTs are mainly associated with gene transcription throughout the reproductive cycle, and controlling the presence of chemotactic response at different reproductive stages is a great example of this role.
Cytochrome activity and photocycle
Finally, there are cryptochromes. Normally, they are strictly absorbent in the blue light range, but the cryptochromes present in Gonium and other algae of the Chlamydomonas genum are absorbent in both red and blue light ranges. This is an evolutionary advantage in cryptochrome absorbance that is similar to cryptochrome proteins found in animals, and likely reduces the energy load of making many proteins by making proteins that can absorb light at broader wavelength spectrums. Cryptochromes (CRYs) exhibit control in many systems, including sexual reproduction, circadian rhythm, photosynthesis, and more (Böhm and Kreimer, 2020; Kottke et al., 2017; Oldemeyer et al., 2016). The CRYs photocycle is visualized in Figure 4.
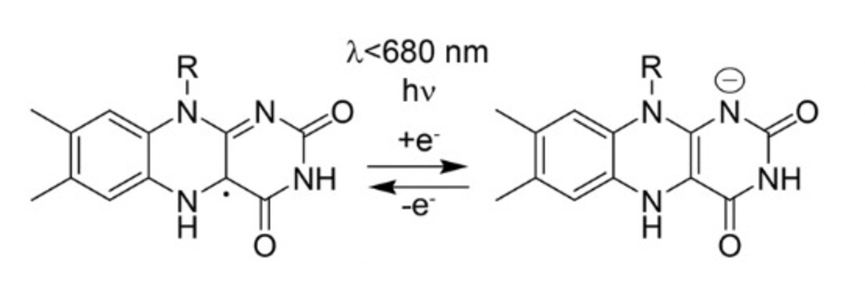
Fig. 4. The Photocycle of Cryptochrome aCRY, present in Gonium sp. aCRY goes through a simple reduction-oxidation cycle. When excited by light, the FAD protein within aCRY is reduced, changing its structure and entering a signalling state. After a while, it is oxidized and returns to its base state. Figure adapted from Oldemeyer et al., 2016.
This cycle is determined by the oxidation and reduction of FAD, a protein many are familiar with due to its role as an energetic carrier in photosynthesis. CRYs are FAD-binding proteins, so when the structure of FAD is changed through reduction or oxidation, CRY function is activated or deactivated. When excited by light, the radical FADH is reduced to FADH-, which is the signalling state of the molecule. This signalling state is directly linked to the gene expression of a variety of genes in chlorophyll, cartenoids, cell cycle regulation, and circadian rhythm, according to Oldemeyer et al., 2016. An example mechanism highlighting how CRYs are involved with gene transcription of proteins involved with circadian rhythm can be seen below in Figure 5:
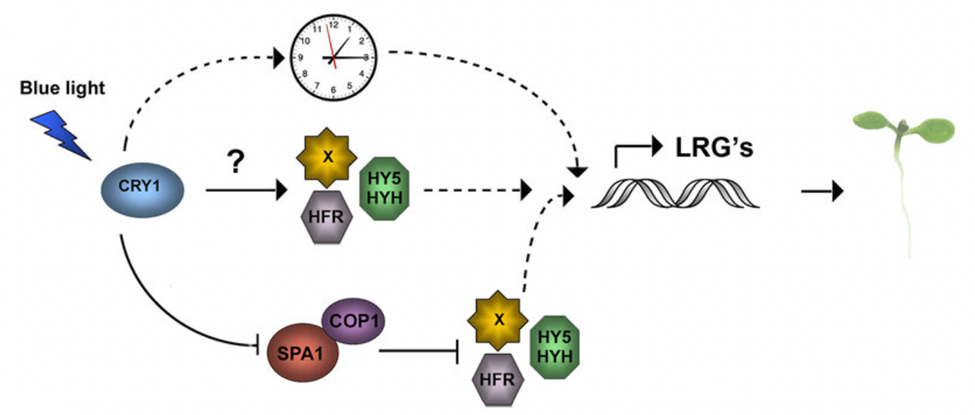
Fig. 5. Cryptochrome signalling pathway in circadian rhythm control of plants. Once excited by light, CRYs exhibit structural changes through their photocycle that lead to the inhibition or activation of certain transcription factors involved with circadian rhythm, which are the various colorful proteins shown. The exact method of action is unknown (Yu et al., 2010).
Within the circadian rhythm of Gonium and other green algae, CRY is notably important in controlling the transcription of genes involved in chemotaxis. During the day, CRY is only present at 1% concentration relative to its highest accumulation (Müller et al., 2017). At night, CRY accumulation is significantly increased, peaking near the end of the night. This is not directly aligned with the activity distribution of chemotaxis in Gonium during a normal circadian cycle, but it is aligned with the highest ammonium uptake of Gonium during a normal circadian cycle, suggesting that CRY is not only associated with chemotaxis but also the direct intake of ammonium (Byrne et al., 1992; Müller et al., 2017; Oldemeyer et al., 2016).
Sexual and growth regulation of gonium
The following section will explore the reproductive processes of Gonium pectorale. Its asexual and sexual modes of reproduction will be briefly explained before focussing on the roles of nutrients and environmental factors that regulate these processes. Nitrogen, acetate, calcium and light play significant roles in sexual reproduction, and while little is known on the specific functions of acetate and light, the roles of nitrogen and calcium can be theorised.
The asexual reproductive process involves four divisions of each colonial cell to form the daughter cells. The initial division takes place in the longitudinal, transversal, or oblique directions within the parent cell in a plane that is nearly perpendicular to the flattened colony. The second division is perpendicular to the first resulting in a four-celled plate containing two diagonal protoplasts slightly attached at the center. The next division, the protoplasts are divided in a direction slightly parallel to the first, forming an eight celled embryo with four near parallel rows of two protoplasts each. The final division takes place perpendicularly to the last division, forming a square 16 celled organism (Nozaki, 1993).
The sexual reproduction of Gonium is only induced when two heterothallic strains are introduced, that is when strains that have their male and female reproductive organs on different undifferentiated cell bodies. Gonium pectorale‘s sexual reproduction, shown in Figure 6, involves two separate processes: gametogenesis and syngamy or fusion (Saito, 1984).
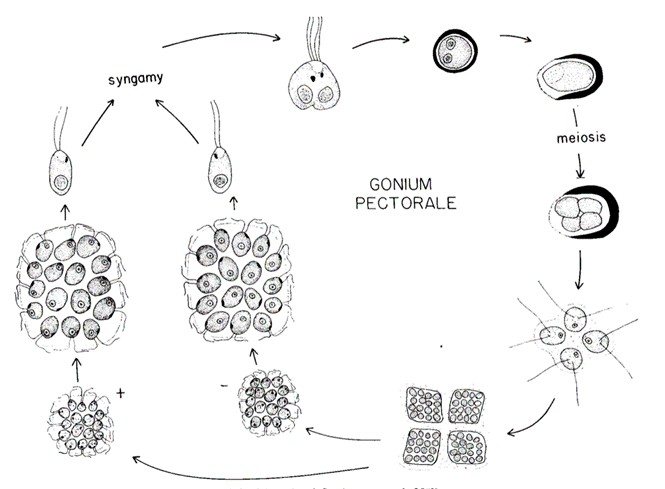
Fig. 6. Lifecycle of Gonium pectorale. The lifecyle of Gonium pectorale in which sexual reproduction is represented. The process beginning with gametogenesis on the left, followed by syngamy, meiosis, the formation of a germ-colony, mitosis and finally cell cleavage (Stein, 1958).
Gametogenesis begins with each cell becoming a gamete and must separate from its colony. Two gametes will then pair up, initially linking with the flagella meeting along their lengths, beginning syngamy. A cytoplasmic bridge between the cells will form between the fusing gametes at the anterior ends of the gametes, eventually enclosing them in a membrane. After the chloroplast and the nuclei fuse, the zygote will lose its flagella, and will either sink to the bottom of their enclosure or float to the surface of the water. This zygospore will develop a cell wall within the primary zygote membrane (Stein, 1958). Once the zygote transfers to fresh media with temperatures ranging from 17-30°C and illumination, germination can begin (Saito, 1984). The germinating zygospore swells, rupturing the cell wall, and its nucleus divides through meiosis. After four nuclei have been formed, cytoplasmic division occurs, ending the meiotic process. The zygospores produce four cells that form a germ-colony, called a gone. The gone will shed the zygospore wall, leaving it as it swims away. The germ-colony will then experience four mitotic divisions resulting in four 16-celled daughter colonies (Stein, 1958). This cleavage process can be seen in Figure 7, where A shows a Gonium pectorale colony not taking part in reproduction and B has the same colony undergoing 4 cell cleavages during mitosis (Lerche & Hallmann, 2009).
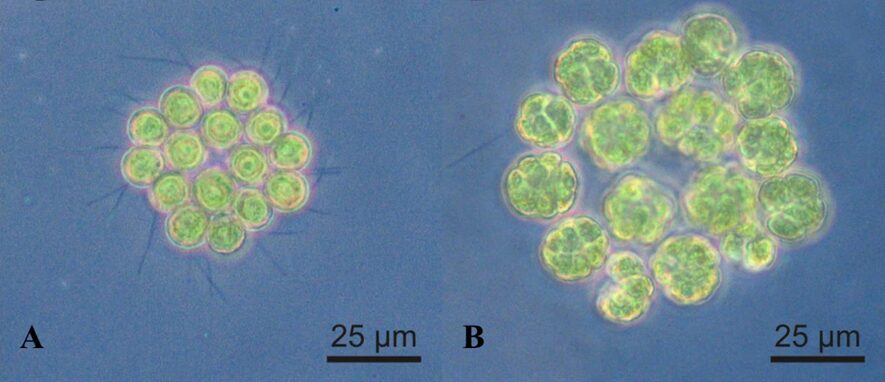
Fig. 7. Gonium pectorale (A) 16 cells prior to reproduction and (B) during mitotic division where each cell is experiencing 4 longitudinal cell cleavages (Lerche & Hallmann, 2009).
The sexual cycle is controlled by the colony’s nutritional and light requirements. These needs either limit or trigger the different processes of Gonium‘s reproductive cycle. Nitrogen, acetate, and calcium are among the nutrients needed for gametogenesis, syngamy, and colony maturation.
Acetate is required for gametogenesis, but it is also a necessary factor for zygote formation and maturation. In acetate-free environments, Gonium populations are unable to grow. The concentration of acetate in the surrounding environment has a liner relationship to the population growth when concentrations are below 300mg%, while concentrations above 500mg% inhibit growth. However, during testing Saito found that pyruvate could be used as a replacement, indicating that exogenous energy sources (from diet or the environment) beside light are also necessary for reproduction.
A nitrogen deficiency is needed to induce gametogenesis with all other nutrients at their optimum levels. In fact, sexual induction is inversely proportional to the nitrogen levels. Colonies that use any inorganic or organic source of nitrogen for vegetative growth would experience gametogenesis if their given source was scarce. This biological design is a protective measure in times of nutritional stress, since once the zygotes are formed, maturation can continue whether or not nitrogen is present in their environment (Saito, 1984). Nitrogen is essential for promoting algal growth and plays an important role the colony’s biochemical composition as it is necessary for protein, lipid and carbohydrate synthesis. An exhaustion in whatever form the element takes (nitrate, nitrite, urea and ammonium) can result in a decrease in growth. However, such a depletion will also result increased lipid production (Yaakob et al., 2021). Given the necessity of nitrogen, the triggering of gametogenesis may prove confusing as the reproduction process will result in more colonies requiring nitrogen for growth. However, this may increase the species chances of survival due to the production of genetically diverse offspring that may be better adapted to a nutrient-poor environment.
Light is also needed to initiate gametogenesis. Nitrogen deficiency is not enough to initiate the process if a light of 1000-4000 lux is not present. The full extent of light’s effect on sexual reproduction is still not clear (Saito, 1984).
Interestingly, calcium is another nutritional requirement for gamete and zygote formation, as well as maturation. The ion interacts with the lipoprotein complexes produced by male flagella, making them stickier (Saito, 1984). This design benefits the fusion of the gametes, likely increasing the strength of the connection between the flagella of the different gametes. This increased adhesion may increase the likelihood of a successful fusion by diminishing the chances of gamete dispersal. The aquatic environment can produce forces that may push the gametes apart, causing the failure of zygote formation. However, the fact that calcium is also needed during zygote maturation, a process that does not involve the flagella, suggests there are different functions needing calcium that is not yet known, potentially relating to the internal metabolism of the cell or zygote (Saito, 1984).
Asexual and sexual reproduction will offer different advantages and disadvantages for a given situation. Gonium‘s ability to perform both betters the organism’s ability to grow its population under a greater variety of conditions. Asexual reproduction’s greater efficiency and genetic uniformity allows for faster reproduction in a stable environment. Yet, in unstable environments, the lack of genetic diversity becomes disadvantageous. Sexual reproduction, while less efficient, provides the population with genetic diversity, allowing for a more adaptable population. The two methods of reproduction have different environmental dependencies as well. Asexual reproduction is dependent on sufficient nitrogen and so asexual growth will be inhibited in nitrogen deficient environments (Yaakob et al., 2021). However, this environment will induce sexual reproduction, still promoting population growth. The two forms of reproduction can be complimentary, in some cases providing the solution to the other’s shortcomings, ensuring the survival of the genus.
Production of depolluting enzymes
Gonium is a genus of freshwater algae, cosmopolitan in brackish waters typically characterized by salinities inferior to 1% (Cahill, 2023; Fabry et al., 1999). These environments are sometimes subject to pollution by toxic compounds, posing a risk not only to organisms like Gonium, but also to their ecosystem.
Amazingly, Gonium has evolved metabolic systems that enable it to participate in the biodegradation of many such compounds. Its versatility in biodegrading toxic pollutants is illustrated by the example that follows.
Production of laccase and biodegradation of toxic pollutants:
Gonium‘s detoxifying capabilities are reflected in its ability degrade phenolic compounds and reactive dyes. Phenolic compounds are molecules whose chemical structure contains one or more hydroxyl functional groups bonded to aromatic hydrocarbon groups (see Figure 8). Such compounds are often highly toxic.
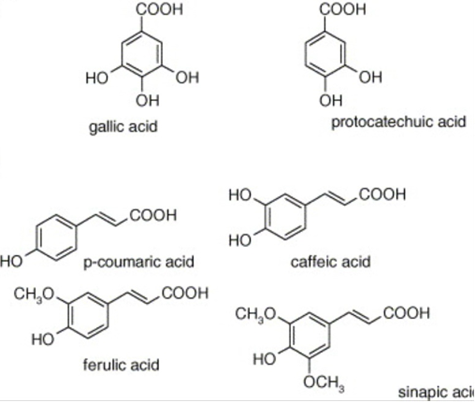
Fig. 8. Examples of phenolic compounds. Each compound contains a hydroxyl (-OH) group attached to a carbon atom that is part of an aromatic ring (Balasundram et al., 2006).
For example, in freshwater environments, tannins, a class of phenolic compound, are leached from decaying vegetation (Bleam, 2017). They are associated with decreases in growth rate, feed intake, and feed efficiency in experimental freshwater animals (Chung et al., 1998). This implies that they are harmful for freshwater ecosystems in high concentrations, as they can negatively impact populations of animals, thereby destabilizing entire food webs and ecosystems.
More dangerous examples of phenolic compounds that pollute freshwater environments are the products of industrial, agricultural, and domestic waste. These include highly toxic compounds present in household disinfectants and antiseptics (e.g. carbolic acid), pesticides such as 2,4-dichlorophenoxyacetic acid and pentachlorophenol, and phenolic azo dyes, which comprise some of the most common toxic dyes produced by the textile industry (Khairul A. M. S. et al., 2021).
The regulation and bioremediation of phenolic compounds in freshwater ecosystems is therefore an important element of ecosystem health and stability. This regulation is ensured in part by green algae such as Gonium as a result of their production of laccases.
Laccases are enzymes that catalyze the oxidation of phenolic substrates. Typically, these phenol oxidases play a role in the synthesis of complex polymers. They perform one-electron oxidations on their substrates. When these substrates are polymers, this oxidation further leads to crosslinking (formation of covalent or ionic bond or bonds) between the oxidated polymer and another polymer. While in Gonium and other green algae the specific synthetic role of laccase is not known, a well-known example of laccase’s role in polymer biosynthesis is found in plants: As seen in Figure 9, laccases oxidate monolignols and promote their oxidative coupling or bonding. This leads to the production of lignin, an essential component of the plants’ cell walls (Solomon et al., 1996).
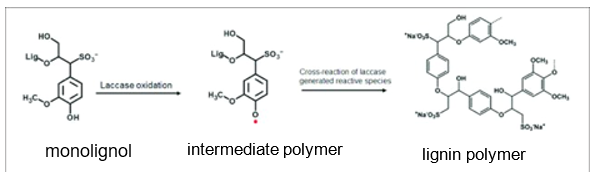
Fig. 9. Oxidation of a monolignol catalyzed by laccase leading to the formation of a lignin polymer. (Mayr et al., 2021).
Laccases are also known to catalyze ring cleavage of aromatic compounds (splitting of bonds within the aromatic rings of these compounds), and namely that of phenolic compounds. This ability allows them to biodegrade phenolic compounds, transforming them into non-toxic molecules. For this reason, the elimination of toxic phenolic reactive dyes is a standard test used to detect the presence of laccases.
Such tests were indeed used by Kılıç et al. and Boduroğlu et al. to support the thesis that Gonium produces laccase. Kiliç et al. showed that, in freshwater-like liquid mediums with a pH of 8 and at concentrations of 0.53 grams of Gonium sp. biomass per liter of liquid, rates of elimination of the dye Reactive Blue 220 reached 96.8%. Boduroğlu et al. were the first to suggest the idea that Gonium sp. produced laccase after showing its potential for eliminating the dyes Reactive Orange 14, Reactive Red 120, Reactive Black 5, and, most of all, Remazol Brilliant Blue R.
Gonium‘s production of laccases is a noteworthy solution developed through evolution as a response to the presence of toxic pollutants in its environment. They catalyze the oxidations that allow the multicellular green algae to sequester toxic compounds (namely phenolic compounds) into non-toxic forms and thereby preserve the integrity of their environment. Even more noteworthy is the possibility that laccases also serve a biosynthetic purpose in Gonium, much like they do in organisms like plants, although more research into the role of laccase in green algae is required to confirm this possibility.
The transition to multicellularity
Multicellular organisms have existed for several hundred million years. Multicellular animals emerged between 600 and 950 million years ago, while multicellular terrestrial plants have existed for roughly 750 million years (Hanschen et al., 2016). Few multicellular organisms still resemble their unicellular ancestors, having undergone a multitude of evolutionary changes to become the organisms they are today over this long period of time. This makes it difficult to understand how unicellular organisms evolved to aggregate together to become one entity. Luckily, Gonium pectorale can provide insight into the origins of multicellularity. Gonium is part of the Volvocales, which only evolved to be multicellular around 200 million years ago, which is much more recent than the evolution of plants and animals to multicellularity (Olson & Nedelcu, 2016). This would suggest that the Volvocales have undergone fewer evolutionary changes than plants and animals. Moreover, in terms of evolution, Gonium pectorale seems to find itself between its volvocine relatives, the unicellular Chlamydomonas reinhardtii and the multicellular Volvox carteri. Unlike Volvox carteri, Gonium‘s cells are undifferentiated, meaning they are all the same and unspecialized, which would suggest that Gonium is a more primitive multicellular organism (Hanschen et al., 2016). The three organisms can be seen in Figure 10.
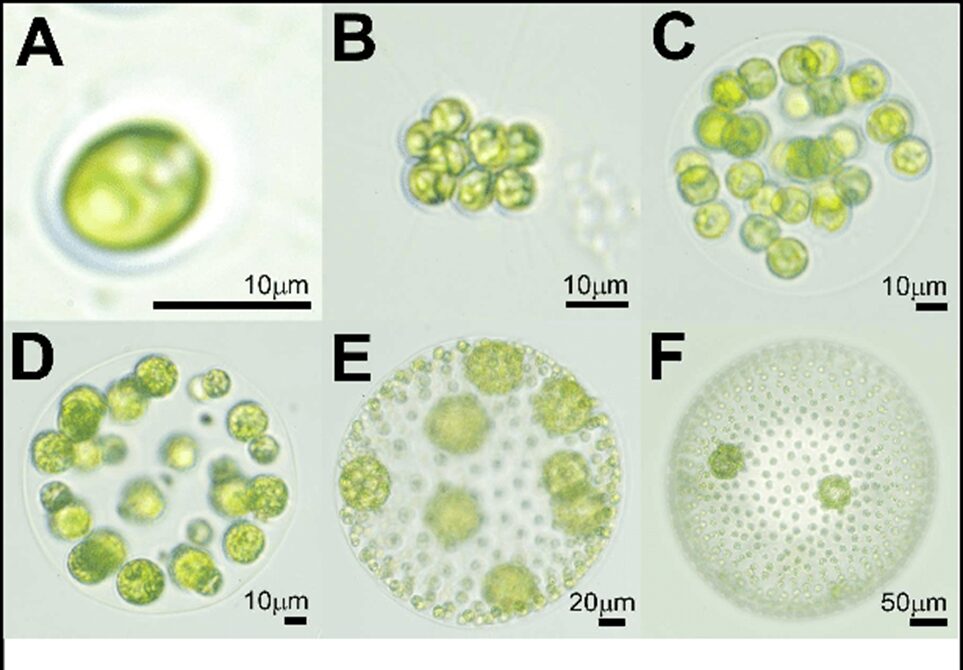
Fig. 10. Images of various Volvocales. A) Chlamydomonas reinhardtii. B) Gonium pectorale. C) Eudorina elegans. D) Pleodorina californica. E) Volvox carteri. F) Volvox aureus. The relevant organisms in this section are Gonium pectorale (B), as well as Chlamydomonas reinhardtii (A) and Volvox carteri (E) (Solari et al., 2016).
An analysis of Gonium‘s genome in comparison to that of Chlamydomonas reinhardtii and Volvox carteri can provide insight into what genetic changes brought about multicellularity. Interestingly, the three algal species are genetically similar and there seems to have been little protein innovation from one species to the next (Hanschen et al., 2016). Moreover, the evolution from unicellularity to multicellularity is thought to have occurred in as few as twelve steps. This begs the question: if genetic changes are not significant factors in the transition to multicellularity, what is responsible for Gonium‘s multicellular nature?
It turns out that the co-option of cell cycle regulation are “the genetic basis for the evolution of multicellularity” (Hanschen et al., 2016). Co-option is “when natural selection finds new uses for existing traits, including genes, organs, and other body structures” (True & Carroll, 2002). Gonium‘s cell cycle, like that of many other eukaryotic organisms, is regulated by its equivalent of the retinoblastoma cell cycle regulatory pathway (Hanschen et al., 2016).
Retinoblastoma is a tumor-suppressing gene (Weinberg, 1995). It produces a protein that is especially important during the first two thirds of G1[1], the first stage of the cell cycle. This protein, called the retinoblastoma protein, or RB1, is found in the cell’s nucleus and regulates E2F[2], which are transcription factors (Fei et al., 2022). When RB1 is hypophosphorylated, it binds with E2F, inhibiting the transcription factors. When RB1 becomes phosphorylated, it unbinds from E2F. E2F is released, which allows the cell to progress to the next phase of the cell cycle. Essentially, unless RB1 is phosphorylated, the cell cycle will not be able to progress to the S phase because the family of E2F transcription factors are inhibited. Thus RB1 serves to suppress the cell cycle (Olson & Nedelcu, 2016). A lack of RB1 in cells typically leads to cancer (Fei et al., 2022).

The phosphorylation of RB1 itself is regulated by cyclin D and CDK4 or CDK6, as well as cyclin E and CDK2. Cyclins are a group of proteins found in every single eukaryotic cell and work with CDK[3] to regulate cell cycles. (Nathan & Richard, 2023). CDK stands for cyclin-dependent kinase. Kinases are a class of enzymes that catalyze reactions involving the transfer of phosphoryl groups (Bruice, 2015). This biochemical pathway can be seen in Figure 11.
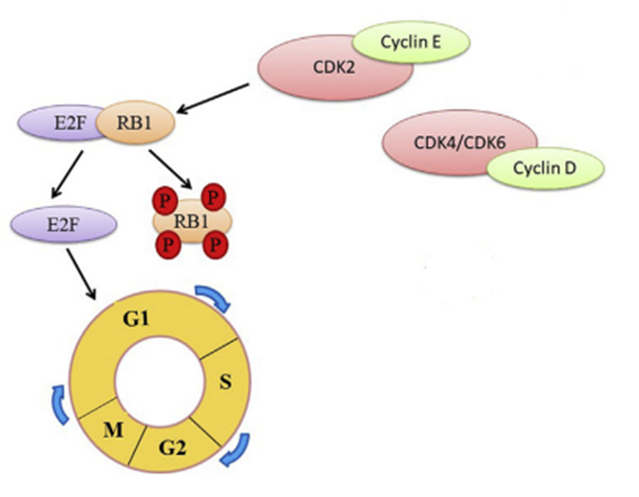
Figure 11. Simplified Diagram of the Retinoblastoma Protein (RB1) Pathway. The retinoblastoma protein is phosphorylated by either CDK2 and Cyclin E, or CDK4, CDK6 and Cyclin D. When RB1 is phosphorylated, E2F proteins are released, which are necessary transcription factors in the cell cycle to progress from the G1 phase to the S phase. (Fei et al., 2022).
[1] G1 is the first stage of the cell cycle, during which cells grow.
[2] E2F refers to transcription factors, which affect DNA synthesis, and are encoded by E2F genes.
[3] CDK stands for cyclin-dependent kinase and CDK2, CDK4, and CDK6 are all types of these kinases.
Both Chlamydomonas and Gonium have the same RB pathways. In addition, both Volvocales undergo multiple fission during their cell cycle (Olson & Nedelcu, 2016). Multiple fission involves the repeated division of cells. Each cell forms two daughter cells after one round of cell division. Since the organisms undergo several rounds of cell division, one cell produces an exponential number of daughter cells. The number of daughter cells produced is equal to 2n, with n being the number of rounds of cell division the cell undergoes. This would mean that a typical 16-cell Gonium colony undergoes four rounds of fission. The RB gene regulates multiple fission and plays a role in determining the number of rounds of cell division. The key difference between the cell cycles of the unicellular and multicellular Volvocales in question is that retinoblastoma protein in Gonium was co-opted to also regulates genes involved in cell to cell adhesion (Olson & Nedelcu, 2016).
In the early stages of G1, Gonium‘s RB1 is phosphorylated in the location that regulates cell adhesion, separate from the area that is involved in cell cycle control (Olson & Nedelcu, 2016). This promotes adhesion. This does not occur in unicellular Chlamydomonas. The mechanism involved in multiple fission is the same for both Gonium and its unicellular counterpart, except for the fact that Gonium produces more daughter cells, seeing that each cell in the colony produces 2n daughter cells. At the end of this mitotic process, Gonium‘s cell adhesion genes that were activated in the early stages of G1 are up-regulated to prevent the groups of daughter cells produced from separating, as they would at the end of mitosis in Chlamydomonas. Thus, these cell adhesion genes that RB was co-opted to regulate are what create multicellular daughter colonies at the end of Gonium‘s multiple fission. This process can be visualized in Figure 12.
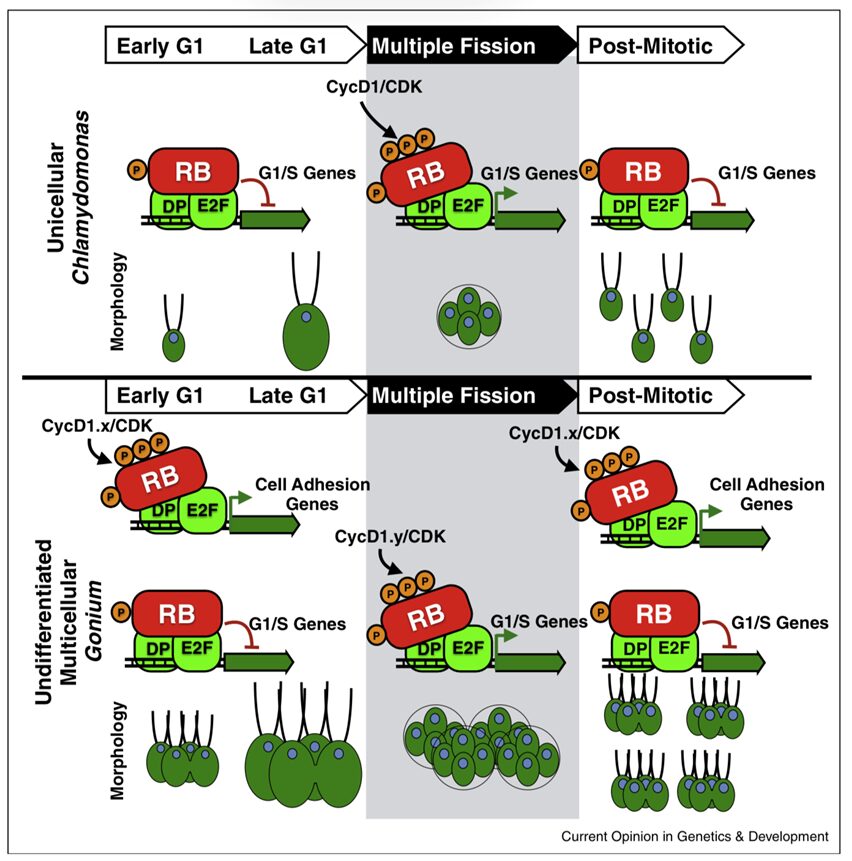
Fig. 12. The cell cycles of unicellular Chlamydomonas and multicellular Gonium. The inhibition and activation of G1/S genes by the transcription factors E2F and DP, which are themselves inhibited and activated by the hypophosphorylation of RB and hyperphosphorylation of RB by cyclin D1 and CDK, respectively, can be seen in both organisms. What differs is the involvement of cell adhesion genes in Gonium’s mitotic cycle, active both pre-mitosis and post-mitosis, creating multicellular offspring. The Chlamydomonas cells lack the cell adhesion genes regulated by RB, which is why the offspring are unicellular at the end of mitosis (Olson & Nedelcu, 2016).
Clearly, the co-option of the retinoblastoma protein is an important factor in the evolution of Gonium‘s multicellularity. In fact, when Chlamydomonas cells are complemented with the RB protein from Gonium, they are capable of exhibiting multicellularity (Olson & Nedelcu, 2016).
By using pre-existing proteins and genes and altering their function to promote cell adhesion, Gonium was able to become a multicellular organism in relatively few steps. Once again, the evolution of Volvocales to become a differentiated multicellular organism like Volvox is said to be a “twelve-step program” (Kulkarni et al., 2022). Seeing that Gonium is undifferentiated and smaller than Volvox, it is safe to assume it’s evolution to multicellularity was done in fewer than twelve steps, thanks to co-option. This raises another question. How does the retinoblastoma protein get co-opted in the first place?
Intrinsically disordered proteins, or IDPs, as well as proteins with intrinsically disordered regions, or IDRs, seem to be involved in co-option (Kulkarni et al., 2022). IDPs and IDRs are “functional proteins that lack rigid 3D structures and exist as highly dynamic conformational ensembles” (Kulkarni et al., 2022). In other words, these proteins are highly plastic or adaptable. This means that the protein’s conformation changes easily. Because of this, IDPs can interact with more substrates than a protein with a rigid structure can. They are also more flexible when it comes to adapting to environmental or cellular changes. When proteins display this kind of flexibility, they are capable of adopting new functions, which is the basis for co-option.
Such is the case for the retinoblastoma protein, which would be considered an intrinsically disordered protein. The protein was able to acquire other functions beyond that of regulating the cell cycle thanks to its disordered nature, which ultimately allowed the cells to adhere and form multicellular colonies. In Gonium pectorale, the RB protein has a 46.69 % disordered level. Interestingly, this number is even higher for the unicellular Chlamydomonas reinhardtii. These values suggest that the RB protein in Volvocales is more disordered in unicellular organisms, which is what allowed Volvocales to evolve into multicellular organisms in the first place. On the other hand, the multicellular Volvocales have less disordered RB proteins, which would be a design solution to increase the stability of the proteins. Decreasing the plasticity of its proteins would be beneficial for a multicellular organism like Gonium since the co-opted proteins would be “locked into developmental pathways, contributing to a new level of order” (Kulkarni et al., 2022), thus maintaining the adaptations it acquired to become a multicellular organism. Being a multicellular organism provides Gonium with many advantages, such as greater locomotive abilities and a greater surface area for photosynthesis, which would explain why the organism would want to stabilize its proteins.
Conclusion
The sixteen-celled Gonium may sound simplistic in comparison to Volvox or other more derived organisms, but exploring the chemical properties present allows us to achieve a better understanding of just how complex it is. As green algae, they exhibit many of the photoreceptor proteins we would expect to see in other photosynthetic organisms, but upon further exploration, we realize they have also evolved photoreceptors similar to animals to reduce the energy cost of making proteins while providing increased functionality. Gonium also exhibits noticeable differences in complexity from its earlier lineage—including an increase in protein stability and relative order from Chlamydomonas. Gonium‘s evolution over centuries has developed advanced chemical processes that function to solve problems in its environments in energy efficient and multifunctional ways.
As Gonium evolved, it faced many selective pressures that progress its evolutionary responses. One example is the conundrum of an energy efficient reproduction system with only the environmental signals of light and ion concentration. Gonium developed not only sexual reproduction but also the relevant proteins and photoreceptors to regulate it. PHOTs and CRYs use the limited stimulus in an ingenious way to regulate sexual selection based on concentration of ions and light absorbance. Environmentally, Gonium also faces the challenge of dealing with toxins and unknown substances. To ensure its own safety, Gonium developed enzymes to be able to process and breakdown harmful phenolic substances and reactive dyes. Another challenge Gonium faced was acting as a multicellular organism efficiently without a central nervous system to control operations. Gonium developed a new function for the disordered protein retinoblastoma to achieve this. By taking a protein that is in involved in the cell cycle and adapting it to regulate cell to cell adhesion, it can ensure multicellularity without spending the energy cost of making more proteins. In fact, Gonium‘s reliance on three main photoreceptors is a design solution itself. In order to photosynthesize, orient, and move efficiently, Gonium needs photoreceptors that can accept light in a wide range of wavelengths and efficiently send signals to Gonium‘s various systems. Gonium‘s evolutionary process opted for using three photoreceptor proteins to limit energy costs, but then expanded the use cases of these proteins to ensure maximum light absorbance. This includes the evolutionary alteration of Cryptochromes in Gonium to include absorbance in a wide spectrum, including reds and even partially some yellows. These design solutions can be more easily visualized and communicated in Figure 13 below.
The chemistry of Gonium tells a wonderful story of evolutionary refinement, using the simplest of means to extreme efficiency to maximize its survival in a harsh world. The tactics crafted by evolution are second to none, and engineers should consider finding inspiration from Nature. For example, the textile industry has already found the ability of dye processing by microalgae to be very useful in the treatment of wastewater (Ferreira et al., 2022). By using the evolutionary devices crafted in organisms like Gonium, we can create a relatively simple—but cost efficient, robust, and high-quality—solution to complex problems.
When someone is tasked with discovering a solution to a problem, they must first ask themselves if this problem has been solved before, and if so, how can they use the working solution to develop a solution that is more advantageous. While many might compare their problem to one of a physicist from the enlightenment era, the greatest engineer in history often goes overlooked. Evolution is the world’s first unshaken design specialist, and as engineers, an idol of reverence that should be everyone’s first step of comparison. Even in Gonium, a microscopic algae that may not seem important in one’s day to day life, the chemical solutions present are something that engineers can build off of for years to come.
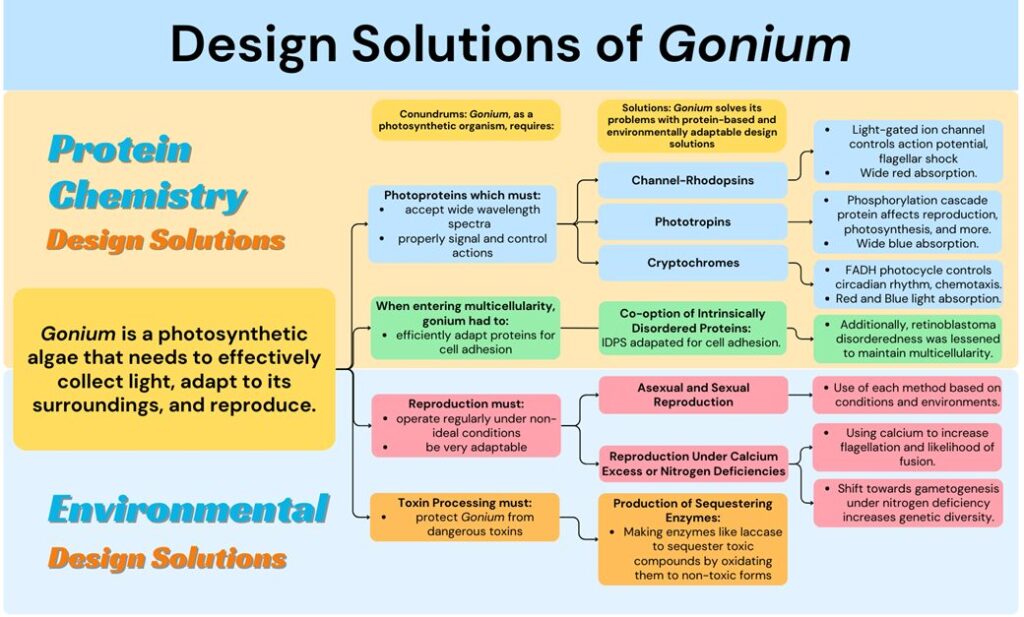
Fig. 13. A flowchart describing notable conondrums faced by Gonium and the protein and environmentally adaptive solutions it has generated through evolution. Protein Chemistry solutions are solutions directly involving specific protein configurations in Gonium and Environmental solutions are design solutions developed by Gonium to increase chances of survival in differing environments. Figure created by our team, 2023.
References
Bleam, W. F. (2017). Soil and Environmental Chemistry. Elsevier, Academic Press. https://doi.org/https://doi.org/10.1016/C2015-0-01022-X
Bruice, P. Y. (2015). Organic Chemistry (8th ed.). Pearson Education Inc.
Byrne, T. E., Wells, M. R., & Johnson, C. H. (1992). Circadian Rhythms of Chemotaxis to Ammonium and of Methylammonium Uptake in iChlamydomonas/i. Plant Physiology, 98(3), 879.
Cahill, N. (2023). Freshwater – Water Education Foundation. Water Education Foundation. Retrieved November 6 from https://www.watereducation.org/aquapedia-background/freshwater
Ehlenbeck, S., Gradmann, D., Braun, F.-J., & Hegemann, P. (2002). Evidence for a Light-Induced H+ Conductance in the Eye of the Green Alga Chlamydomonas reinhardtii. Biophysical Journal, 82(2), 740-751. https://doi.org/10.1016/s0006-3495(02)75436-2
Ermilova, E., Zalutskaya, Z., Huang, K., & Beck, C. (2004). Phototropin plays a crucial role in controlling changes in chemotaxis during the initial phase of the sexual life cycle in Chlamydomonas. Planta, 219(3). https://doi.org/10.1007/s00425-004-1241-6
Fabry, S., Köhler, A., & Coleman, A. ( 1999). Intraspecies Analysis: Comparison of ITS Sequence Data and Gene Intron Sequence Data with Breeding Data for a Worldwide Collection of Gonium pectorale. J Mol Evol, 48, 94–101. https://doi.org/https://doi.org/10.1007/PL00006449
Fei, F., Harada, S., Wei, S., & Siegal, G. P. (2022). Molecular pathology of osteosarcoma. In Bone Sarcomas and Bone Metastases-From Bench to Bedside (pp. 579-590). Elsevier.
Ferreira, J. T., Bortoleti, K. C. D. A., Motta, L. D. S., Gavazza, S., Brasileiro-Vidal, A. C., & Bezerra, R. P. (2022). Microalgae-Based Remediation Approaches in Textile Dye Removal. In (pp. 107-127). Springer Singapore. https://doi.org/10.1007/978-981-19-0526-1_5
Gregory, T. R. (2009). Understanding Natural Selection: Essential Concepts and Common Misconceptions. Evo Edu Outreach, 2, 156–175. https://doi.org/https://doi.org/10.1007/s12052-009-0128-1
Hanschen, E. R., Marriage, T. N., Ferris, P. J., Hamaji, T., Toyoda, A., Fujiyama, A., Neme, R., Noguchi, H., Minakuchi, Y., & Suzuki, M. (2016). The Gonium pectorale genome demonstrates co-option of cell cycle regulation during the evolution of multicellularity. Nature Communications, 7(1), 11370.
Hegemann, P. (2008). Algal Sensory Photoreceptors. Annual Review of Plant Biology, 59(1), 167-189. https://doi.org/10.1146/annurev.arplant.59.032607.092847
Huang, K., & Beck, C. F. (2003). Phototropin is the blue-light receptor that controls multiple steps in the sexual life cycle of the green alga <i>Chlamydomonas</i> <i>reinhardtii</i>. Proceedings of the National Academy of Sciences, 100(10), 6269-6274. https://doi.org/10.1073/pnas.0931459100
Hubbard, R., & Kropf, A. (1958). THE ACTION OF LIGHT ON RHODOPSIN. Proceedings of the National Academy of Sciences, 44(2), 130-139. https://doi.org/10.1073/pnas.44.2.130
Khairul A. M. S., Ahmad F. I., Zulhairun A. K., Mohd S. A., & Asif H. (2021). A review of technologies for the phenolic compounds recovery and phenol removal from wastewater,Process Safety and Environmental Protection, . 151, 257-289. https://doi.org/https://doi.org/10.1016/j.psep.2021.05.015
Kottke, T., Oldemeyer, S., Wenzel, S., Zou, Y., & Mittag, M. (2017). Cryptochrome photoreceptors in green algae: Unexpected versatility of mechanisms and functions. Journal of Plant Physiology, 217, 4-14. https://doi.org/https://doi.org/10.1016/j.jplph.2017.05.021
Kuhne, J., Vierock, J., Tennigkeit, S. A., Dreier, M.-A., Wietek, J., Petersen, D., Gavriljuk, K., El-Mashtoly, S. F., Hegemann, P., & Gerwert, K. (2019). Unifying photocycle model for light adaptation and temporal evolution of cation conductance in channelrhodopsin-2. Proceedings of the National Academy of Sciences, 116(19), 9380-9389. https://doi.org/10.1073/pnas.1818707116
Kulkarni, P., Behal, A., Mohanty, A., Salgia, R., Nedelcu, A. M., & Uversky, V. N. (2022). Co-opting disorder into order: Intrinsically disordered proteins and the early evolution of complex multicellularity. Int J Biol Macromol, 201, 29-36. https://doi.org/10.1016/j.ijbiomac.2021.12.182
Lerche, K., & Hallmann, A. (2009). Stable nuclear transformation of Gonium pectorale. BMC biotechnology, 9(1), 1-21.
Lohmann, D. R., & Gallie, B. L. (n.d.). Retinoblastoma – genereviews® – NCBI bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK1452/
Mayr, S. A., Schwaiger, N., Weber, H. K., Kovač, J., Guebitz, G. M., & Nyanhongo, G. S. (2021). Enzyme Catalyzed Copolymerization of Lignosulfonates for Hydrophobic Coatings. Front Bioeng Biotechnol, 9(14). https://doi.org/10.3389/fbioe.2021.697310
Müller, N., Wenzel, S., Zou, Y., Künzel, S., Sasso, S., Weiß, D., Prager, K., Grossman, A., Kottke, T., & Mittag, M. (2017). A plant cryptochrome controls key features of the chlamydomonas circadian clock and its life cycle [Article]. Plant Physiology, 174(1), 185-201. https://doi.org/10.1104/pp.17.00349
Nathan, H. L., & Richard, T. P. (2023). Cyclins, Cyclin-Dependent Kinases, and Cyclin-Dependent Kinase Inhibitors. In A. B. Ralph, W. H. Gerald, & D. S. Philip (Eds.), Encyclopedia of Cell Biology (Second Edition) (Second Edition ed., pp. 224-234). Academic Press. https://doi.org/https://doi.org/10.1016/B978-0-12-821618-7.00193-0
Nozaki, H. (1993). ASEXUAL AND SEXUAL REPRODUCTION IN GONIUM QUADRATUM (CHLOROPHYTA) WITH A DISCUSSION OF PHYLOGENETIC RELATIONSHIP WITHIN THE GONIACEAE 1. Journal of phycology, 29(3), 369-376.
Oldemeyer, S., Franz, S., Wenzel, S., Essen, L.-O., Mittag, M., & Kottke, T. (2016). Essential Role of an Unusually Long-lived Tyrosyl Radical in the Response to Red Light of the Animal-like Cryptochrome aCRY. Journal of Biological Chemistry, 291(27), 14062-14071. https://doi.org/10.1074/jbc.m116.726976
Olson, B. J., & Nedelcu, A. M. (2016). Co-option during the evolution of multicellular and developmental complexity in the volvocine green algae. Current Opinion in Genetics & Development, 39, 107-115.
Saito, S. (1984). Growth and differentiation of Gonium. 北海道大學理學部海藻研究所歐文報告, 7(2), 195-261. https://eprints.lib.hokudai.ac.jp/dspace/bitstream/2115/48100/1/7%282%29_195-261.pdf
Solari, C. A., Galzenati, V. J., & McGill, B. J. (2016). Exploring the Relationship between Abundance and Temperature with a Chemostat Model. bioRxiv, 054940.
Solomon, E. I., Sundaram, U. M., & Machonkin, T. E. (1996). Multicopper Oxidases and Oxygenases. Chemical Reviews, 96(7), 2563–2606. https://doi.org/10.1021/cr950046o
Stein, J. R. (1958). A morphologic and genetic study of Gonium pectorale. American Journal of Botany, 45(9), 664-672.
Tan, N. G. A., Wu, W., & Seifalian, A. M. (2015). 10 – Optogenetics: lights, camera, action! A ray of light, a shadow unmasked. In M. R. Hamblin & P. Avci (Eds.), Applications of Nanoscience in Photomedicine (pp. 185-203). Chandos Publishing. https://doi.org/https://doi.org/10.1533/9781908818782.185
Terakita, A. (2005). Genome Biology, 6(3), 213. https://doi.org/10.1186/gb-2005-6-3-213
True, J. R., & Carroll, S. B. (2002). Gene co-option in physiological and morphological evolution. Annu Rev Cell Dev Biol, 18, 53-80. https://doi.org/10.1146/annurev.cellbio.18.020402.140619
Weinberg, R. A. (1995). The retinoblastoma protein and cell cycle control. cell, 81(3), 323-330.
Yaakob, M. A., Mohamed, R., Al-Gheethi, A., Aswathnarayana Gokare, R., & Ambati, R. R. (2021). Influence of Nitrogen and Phosphorus on Microalgal Growth, Biomass, Lipid, and Fatty Acid Production: An Overview. Cells, 10(2). https://doi.org/10.3390/cells10020393
Yu, X., Liu, H., Klejnot, J., & Lin, C. (2010). The CRY blue light receptors. The Arabidopsis book / American Society of Plant Biologists, 8, e0135. https://doi.org/10.1199/tab.0135