Analysis of the Survival Mechanisms of Hydra
Jessica Coulson, Magnus Duffy, Ruolin Hu, Shilong Zheng
Abstract
This paper aims to get a better understanding of the physical properties of Hydras. It describes the structure of the body of the Hydra as well as its behavior and interactions with its environment, with a focus on physics. Despite its relatively simple nervous system, characterized by a nerve net without the complexity of a brain, this organism has developed fascinating mechanisms that allow it to not only survive but thrive in its freshwater environment. The organism also has a versatile basal disk which allows it to attach and detach itself to surfaces. Additionally, the structural and physical properties of the mesoglea will be examined to explain the animal’s surprising flexibility and mobility. Moreover, Hydras exhibit contractions in response to light of varying wavelengths and intensities. This phenomenon is believed to influence their feeding and sleeping behaviors. Lastly, this study delves into the necessary mechanochemical and photochemical inputs which act as complex three-way filter for nematocyst discharge in predation, as well as the mechanisms and optimization strategies behind the impressive force involved in this process, one of the fastest in the animal kingdom.
Introduction
Hydra is a genus of freshwater polyps that first appeared over 600 million years ago in the phylum Cnidaria. The organisms were first observed by Antoni van Leeuwenhoek in 1702 (Moore, 2014). Unlike other organisms in the class Hydrozoa, Hydras do not have a medusoid stage. Equally, their polyp form is rather simple with a long flexible body column leading to various tentacles at the oral end and, towards the aboral end, a basal disk. Consequently, their simple and inoffensive appearance caused the Swiss naturalist Abraham Trembley to mistake them for plants. However, their ability to perform complex movements such as somersaulting through contractions and extensions of their bodies resembled motion of animals. This ambiguity led to the famous experiment where Trembley cut the Hydra in two and observed that each end regenerated fully into two distinct polyps. Such regeneration abilities, most often found in plants, perplexed the naturalist further, and he eventually sent a letter to Reaumur, a renowned biologist, who, in a response letter, declared it an animal and named it a polyp, thus, creating the term as it is known today (Naik et al., 2020).
Basal disc
Attachment and detachment
This organism consists of three major parts, the head, the gastric column and the foot, as seen in Figure 1 below (Seabra et al., 2022).
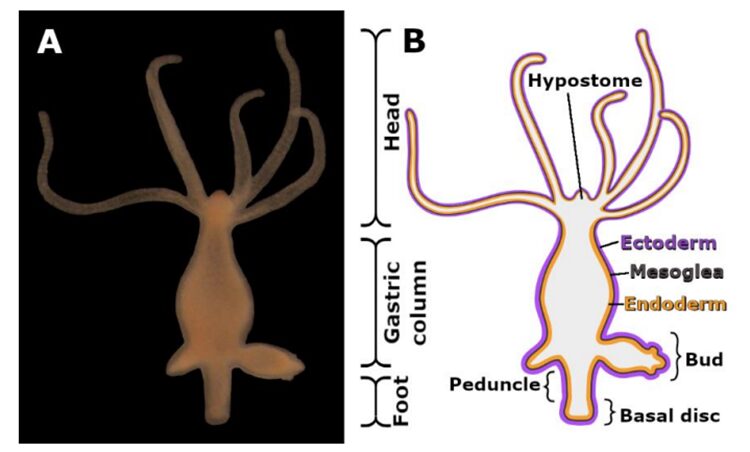
Fig. 1A. Image of Hydra. 1B. Morphological Scheme of Hydra
The basal disk is part of the foot and serves a role in the attachment of the polyp to a substrate. A 2022 paper by Seabra et al. found that the organism was able to attach itself due to the secretion of an adhesive material by the basal disc. This material is likely excreted through uniformly distributed pores between the basal disc cells, discussed in a 2016 article (Rodrigues et al., 2016). However, the Seabra research team found that the detachment process was much less rooted in chemistry and was rather a mechanical process of the polyp. This is because the Hydra does not have a duo-gland adhesive system, and only secretes material to attach itself to substrates, not to detach. The detachment is achieved through a muscular process where the basal disc cells on the outer rim pull themselves away from the surface, followed by the cells found nearer to the center. This method is more beneficial than pulling away with the cells in the center first since this would then involve another force from pressure. Pulling up from the middle first would create a small pocket between the Hydra and the surface it is attached to. Since the volume of this pocket was initially very small, expanding the pocket will increase the volume and thus decrease the pressure. This is due to the inverse proportionality of volume and pressure from Boyle’s Law: P1V1 = P2V2. In this formula, P1 and V1 are the initial pressure and volume of the system, respectively and P2 and V2 are the final pressure and volume of the system. However, the pressure of the surrounding water or the rest of the body of the polyp will have stayed the same. Since force is proportional to pressure and acts perpendicularly to the surface on which the pressure is exerted, the surroundings will exert the same force onto the air pocket. This air pocket will apply less force on the surface since its pressure has decreased. Therefore, the Hydra would need to compensate for this by using more energy to maintain this pocket and prevent it from collapsing, which would keep the Hydra attached to the surface. Thus, the method to use the minimal energy is by first retracting the outer edges of the basal disk, as this eliminates the complications that ensue from the creation of an air pocket.
The aforementioned 2016 paper provides more information on the physical properties of the basal disc cells (Rodrigues et al., 2016). Rodrigues et al.y observed differences in actin filament distribution in the ectodermal cells and the endodermal cells. In the ectodermal layer there were radial myonemes which branched apically towards the base of the polyp. In contrast, the endodermal layer consisted of circular actin filaments, leading the researchers to believe in the involvement of both layers in the muscular process which allows the Hydra to detach itself from a substrate. At the very bottom of the basal disc, where the polyp attaches itself to a base, cytoplasmic extensions with a diameter of 0.4-1 micrometers protrude. These extensions are similar to filopodia, structures composed of microfilaments capable of contracting and moving an organism (“Means of locomotion,”).
The foot, as seen in the figure below (Fig. 2.), leaves a footprint behind when the animal detaches itself from the surface. This article also considered the physical properties of the footprint itself, which they described as a “meshwork of nanopores,” as seen in the image to the left below (Fig. 3a), where the denser regions have a darker color, seen using atomic force microscopy. The pores are accounted for by the cytoplasmic extensions, which are similar in diameter.
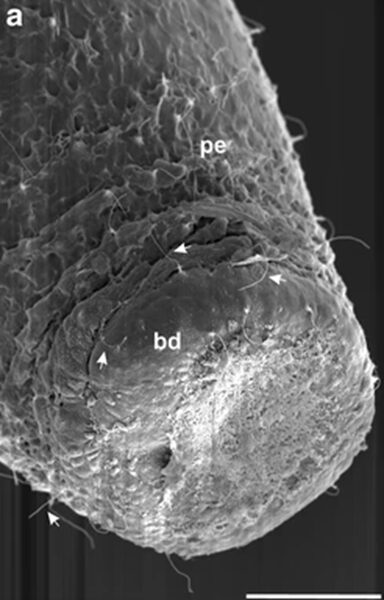
Fig. 2. Outer aspect of basal disc.
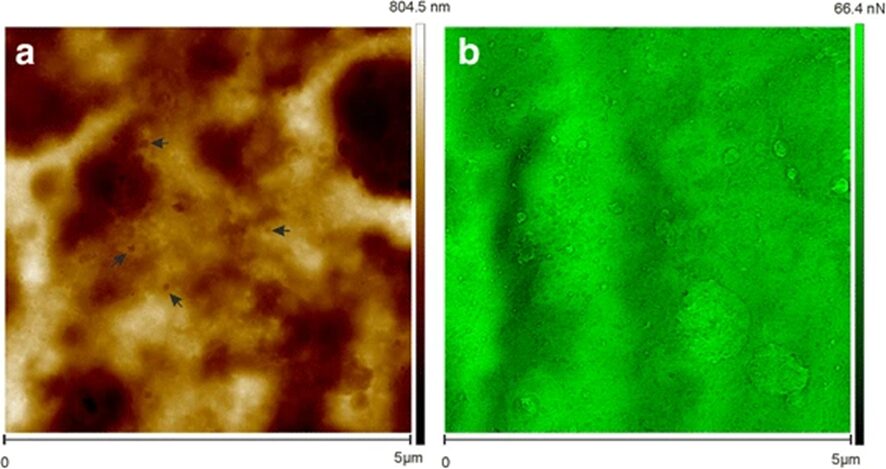
Fig. 3. The figure on the left (Fig. 3b) illustrates the adhesion forces. The highest forces were in the thinnest areas, and they were found to be as strong as 66.4nN. These findings led the team to conclude that the secreted material is, in fact, adhesive and is what allows the Hydra to attach itself to surfaces.
Bubble formation
Hydra have also been observed to form a gas bubble at the base of their foot, and though the mechanism of this process does not seem to have been studied, the circumstances surrounding it have. These were studied in a 1966 article (Lomnicki & Slobodkin, 1966), where researchers noticed that this was a method used by hydra to float to the surface of water. The Hydra would form a gas bubble from their foot and float to the surface of the water. Here, the bubble would usually pop and the animal would hang from the water film, with its head facing downwards. Interestingly, the researchers observed that the likelihood of a Hydra forming a bubble was related to how much it had been fed; poorly-fed hydra were the ones that would create this bubble to float to the surface. Hydra may have evolved this behavior since it allows them to move away from areas with little food, which should eventually lead to them stumbling upon areas with more abundant resources. When they float up to the surface, they are removed from the microenvironment, and can be brought to new areas due to the current of the water. Similar logic applies as to why richly fed hydra do not produce this bubble; it is advantageous for them to stay in an area where there is enough food for them to thrive, instead of floating away.
Mesoglea
Mesoglea mechanism
The mesoglea of hydra is an incredible extracellular matrix sandwiched between the inner and outer cell walls of the animal. An article from 1969 (Hausman & Burnett, 1969) covers the stresses that it is exposed to. There are stresses parallel and perpendicular to the oral-aboral axis when the hydra extends or contracts itself. From its resting length, it can contract to one quarter of this length, or extend to three or four times its resting length. However, this also leads to a modification of the width of the hydra, which can be doubled or halved when the length is respectively shortened or extended. There are many fibrils that are present along the Hydra’s oral-aboral axis, but also some perpendicular to it. The researchers believe that there must be some secondary arrangement to allow for the mesoglea to undergo such stress, especially considering these tensile and compressive stresses are not the only ones acting on the structure. There are also epithelial cells that extend through the mesoglea and lead to twisting stress since the muscle fibers often pull in opposing directions. Therefore, the mesoglea acts as a structural reinforcement to the Hydra to withstand the mechanical stresses exerted on the organism and keep it intact and functional.
However, this structure also serves another purpose, as discussed in the same article (Hausman & Burnett, 1969). It can also be used as an attachment site for cells, allowing them to move along it. An article by Shostak et al. discusses that epitheliomuscular and digestive cells are created through mitosis near the head region of the Hydra and then move towards the foot, where they are left behind as the Hydra moves (Shostak et al., 1965). Since these two cell layers were found to sometimes move apically at different rates, it was determined that they must have been moving down a layer between them, and they could not be cemented to each other. The only possible layer could be the mesoglea, a surface to which the cells could independently attach and move. Since the rest of the cells move along the mesoglea, it must possess the structural properties to resist stress without the contribution of the surrounding cell layers. The cells that separate from the mesoglea and the mesoglea itself contain collagen proteins, which are very strong and are found in connective tissues of mammals. This feature of the mesoglea further implies that it has the properties required for it to be the structure that the cells move along. The mesoglea is also tough and elastic, which would allow it to keep the shape of the Hydra even without additional support from the surrounding layers. These layers are not fixed too firmly to the mesoglea as this would hinder their movement from the distal end of the organism to the apical end, though this means that they can contribute less to the structural integrity of this animal. Therefore, mesoglea is found to be the substratum for cell movement in Hydra.
Tensile strength through structure
Hydra genus members are flexible creatures that can stretch up to 7-8 times their body length without permanent deformation. This is due to their mesoglea, an extracellular matrix (ECM) between the ectodermal and endodermal cell walls (Fig. 4). More precisely, the Hydra mesoglea is a tri-laminar structure made of two basal laminar layers and, in between, an interstitial matrix composed of collagen I fibrils bundled together (Veschgini et al., 2023).
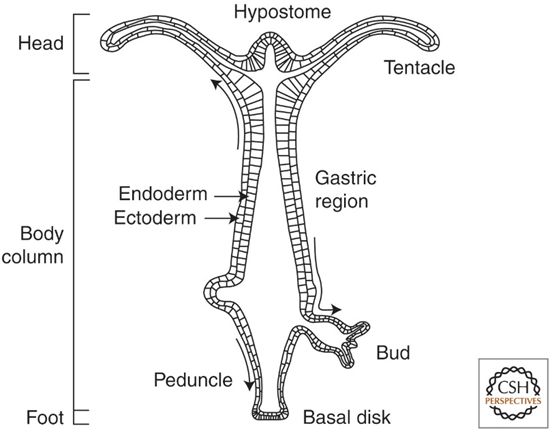
Fig. 4.. Longitudinal Cross-section of Adult Hydra (Bode, 2009).
By looking at the interstitial matrix on a micrometer scale with immunofluorescence confocal microscopy, it is shown that these collagen bundles form grid-like patterns that differ, depending on the location of the body, in size, angle and length (Veschgini et al., 2023). This is seen in confocal images of 50x50 µm2 of the isolated mesoglea in the hypostome, the middle gastric region and the peduncle (Figure 5C, E and G). Indeed, in all three regions, the collagen I gathers in parallel bundles that intersect to form a grid, as highlighted with the broken white lines (Fig. 5D, F and H). In addition, the hypostome region shows collagen grouping in a parallel manner, with a 5 ± 1 µm space between each column of bundles (Fig. 5D). However, the larger structure does not seem to follow the oral-aboral (OA) axis given that there is a 55° angle between the columns and the axis. Comparatively, in the middle gastric region and in the peduncle, the overall structure is aligned with the OA axis, but also offers higher collagen bundle density along it rather than perpendicular to it. Notably, the grid lines most parallel to the OA axis (<10°) are usually around 10 ± 2 µm, while the almost perpendicular lines (100 ± 10°) are two times smaller with a length of 5 ± 1 µm (Fig. 5F and H). This means that the spacing between each column is much smaller compared to the length of the columns, therefore creating, at the micrometer scale, an anisotropic pattern that adapts well to tensile stress often experienced by Hydras when extending and contracting (Veschgini et al., 2023).
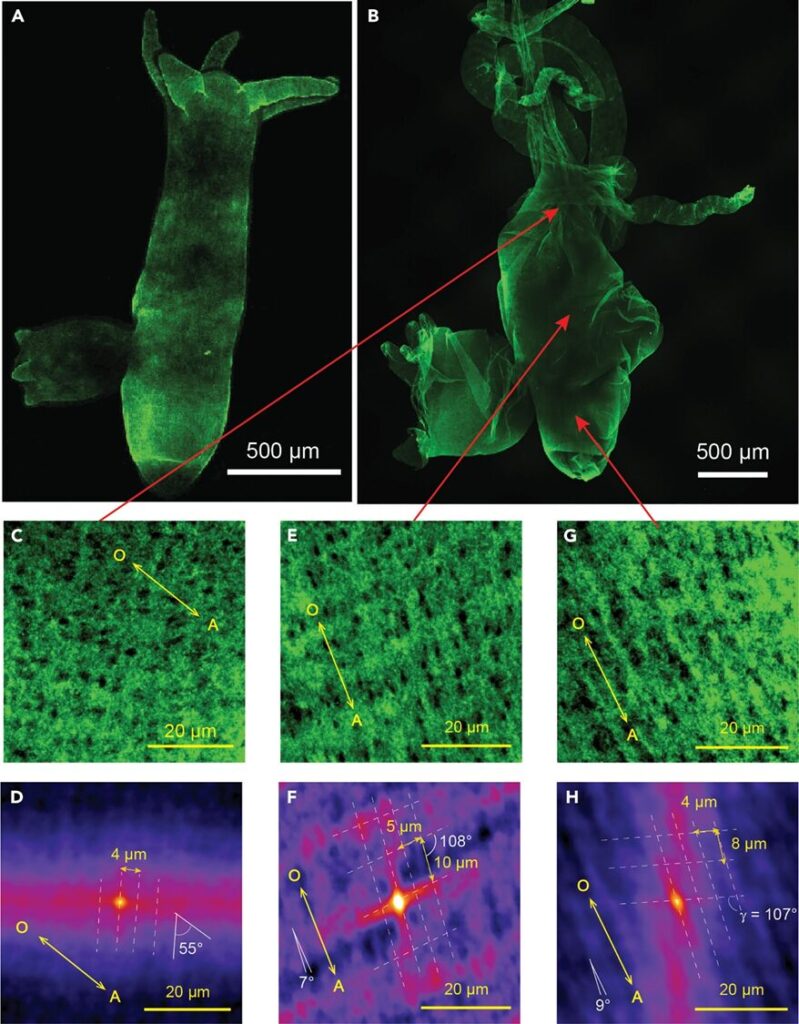
Fig. 5. Confocal images of Hydra ECM on the micrometer-scale. (A and B) Images of isolated, whole Hydra mesoglea stained with type I collagen antibodies in vivo (a)and ex vivo (b). (C, E and G) Confocal images of the regions indicated by the red arrows, with an area of 50x50 µm2. (D, F and H) Maps calculated from the confocal images using autocorrelation analysis. They show the OA axis, the angle and distance between the columns and rows that form the grid pattern representing the collagen bundle network (Veschgini et al., 2023).
Furthermore, on the nanometer scale, the structural organization of individual collagen fibrils is also anisotropic. Indeed, by analyzing the scattering of X-rays perpendicular and parallel to the OA axis through nano-Grazing-Incidence Small-Angle X-ray Scattering (Fig. 6), researchers calculated the real space lattice parameters (a, b, γ) of collagen fibrils and demonstrated that, in both directions, the rod-like fibrils organized into a slanted and compressed hexagonal pattern (Veschgini et al., 2023). However, the parallel hexagonal lattice is slightly bigger, with parameters of a|| = (17.6 ± 3.6) nm, b|| = (6.7 ± 0.8) nm, compared to the perpendicular lattice, with parameters of a⊥ = (11.8 ± 0.5) nm, b⊥ = (4.3 ± 0.4) nm (Fig. 6D). This demonstrates again a higher organization of collagen I parallel to the OA axis to help the Hydra ECM resist tensile stress when it extends or contracts. As such, the arrangement of collagen I fibrils on a nanoscale as well as how its bundles form on a microscale both demonstrate an evolutionary adaptation to improve the Hydra’s tensile strength along its axis of elongation, allowing this mostly sedentary creature to explore and feel its surroundings with its unique body (Veschgini et al., 2023).
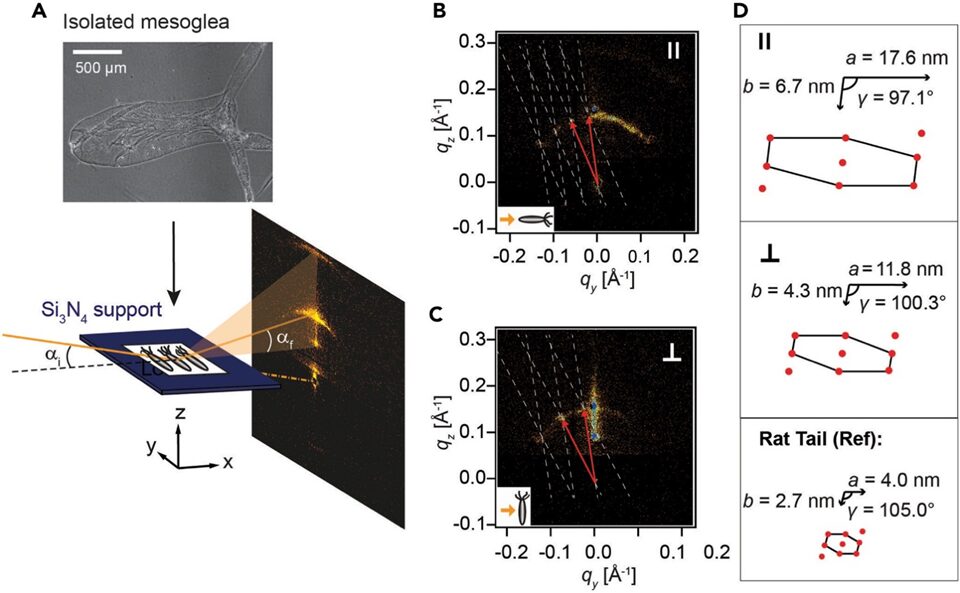
Fig. 6. Using nano-GISAXS to determine the nanometer-scale arrangement of collagen I. (A) Top: phase-contrast microscopy image of mesoglea. Bottom: experimental setup for nano-GISAXS, where the isolated mesoglea is put on a Si3N4 window and lit up with a nano-focused X-ray beam at a grazing incidence angle αi = 0.46°. (B) The resulting scattering pattern with the mesoglea OA axis placed parallel to the beam. (C) The scattering pattern with the body axis perpendicular to the beam. (B-C) The white dotted lines show the reciprocal lattice, and the red arrows indicate the lattice vectors. (D) Lattices in real space with parameters (a, b, γ) parallel (top) and perpendicular (middle) to the body axis. Bottom: comparison with lattice parameters of collagen I from rat tail tendon (Veschgini et al., 2023).
Body stiffness and somersaults
While tensile strength helps the animal extend further without tearing, the stiffness of the Hydra body dictates how much energy is expended for a given motion. Consequently, researchers proved that Hydra’s ability to perform somersaults, a complex and energy costly behavior, is made possible due to the stiffness in the shoulder or upper gastric region of the animal, which acts like a spring that stores elastic energy, allowing it to finish the maneuver (Naik et al., 2020).
To do a somersault, the animal first stretches down to around twice its normal length and attaches itself to the floor with its tentacles and hypostome (Fig. 7.1). This causes the shoulder to be bent at around 900, therefore storing up spring energy. Finally, the basal disc is detached from the other end and the body is relaxed and lifted, allowing the organism to turn upside down (Fig. 7. 2-6) (Naik et al., 2020).
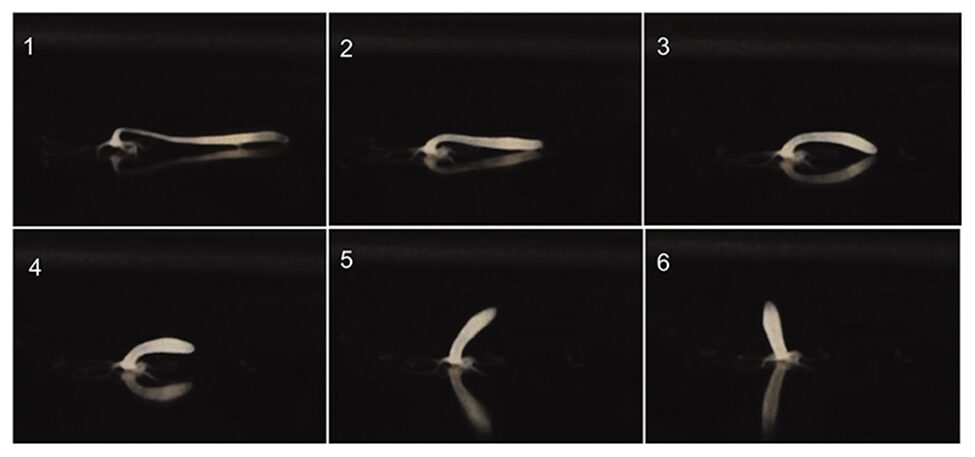
Fig. 7. Hydra somersault (Naik et al., 2020)
Throughout the movement, there is the force of gravity acting on the Hydra body, which is opposed by the force of buoyancy (Fig. 8B). Equally, below the base of the tentacles, the bending force that helps the body column of the polyp return to a vertical stance is reduced by the drag force of water (Fig. 8B). Notably, when the basal disc is released, both forces act parallel to the floor as the body column is contracted along the horizontal axis. However, the bending force subsequently gains a vertical component as the body shifts back to its original vertical axis (Naik et al., 2020).
Additionally, the energy of the arced shoulder can be calculated with the formula:
![]()
Equation 1: Spring potential energy stored in shoulder.
Here, I is the second moment of inertia, R is the radius of the circle that approximates the curvature, L is the length of the upper gastric area, and Y is the experimental Young’s modulus at that region (Fig. 8C) (Naik et al., 2020).
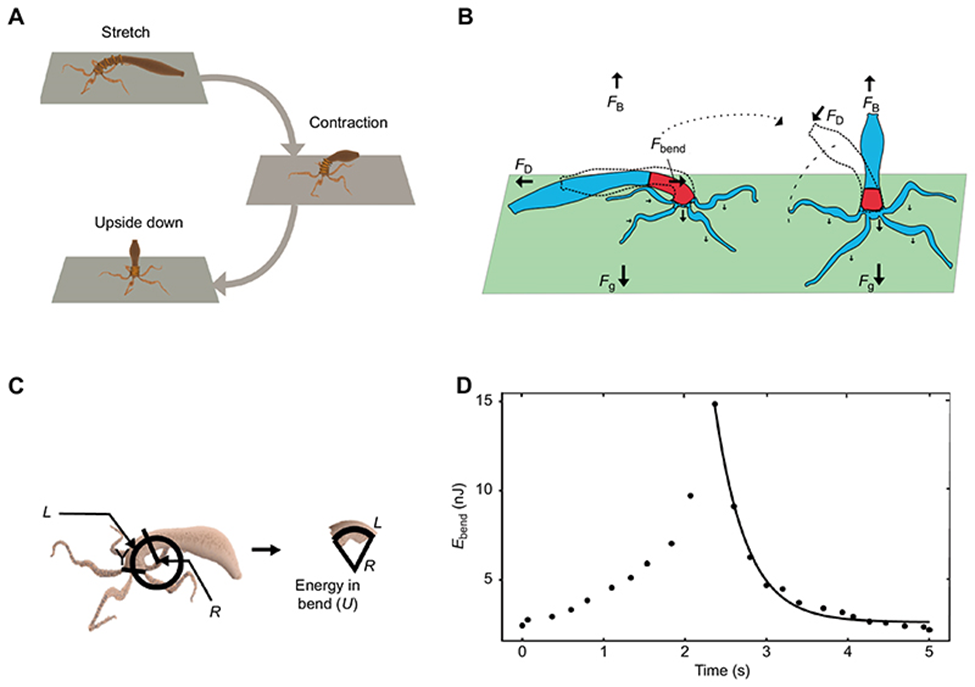
Fig. 8. Diagram illustrating the forces acting on Hydra during a somersault and the energy stored in the shoulder region (Naik et al., 2020)
Young’s modulus (E) is the stress (𝜎) over strain (ε) ratio and is proportional with the stiffness of a material:
![]()
Equation 2:Young’s modulus.
Moreover, stress describes the force distributed uniformly to the material’s area, whereas the strain is the change in length due to deformation compared to the original length.
Researchers were then able to map the elasticity of the Hydra body column using atomic force microscopy. The measurement revealed a uniform stiffness of Y=450±6 Pa throughout the body except for the shoulder, where the Young’s modulus was measured to be Y=1480±20 Pa, a value that is three times higher than compared to the rest of the body (Naik et al., 2020). Thus, when bending down, this differential stiffness in the shoulder allows the animal to store its kinetic energy as spring potential energy, whose recoil pushes it into the upright position.
Ultimately, the change in energy in the upper gastric area is described in Fig. 8D, where the left curve ranging from t=0s to around t=2.3s represents the initial extension prior to the release, thus creating a buildup of elastic energy, which is then released to facilitate the vertical rotation of the lower body, as shown on the rightmost curve. More specifically, by approximating the organism as an elastic cylinder composed of 50 rings with, each, 10 beads and using it to simulate a somersault, it was shown that under uniform stiffness, not only would the elastic ring be unable to stand upside down, but it would also transfer 50% less energy to the shoulder area as compared to the normal Hydra stiffness distribution. However, the lower young’s modulus did initially allow more potential energy to be stored as the elastic cylinder was able to stretch further (Naik et al., 2020). Consequently, without the stiffness in the upper body, a somersault would be much more energetically taxing to perform, thereby impairing the organism’s mobility.
Porous structure of the mesoglea
Movement in multicellular organisms is a complex task and, though physical characteristics such as strength and stiffness are important, it still relies on the synchronous movement of the muscular system. In Hydra, muscle cell processes are in the epithelial walls around the mesoglea. As such, to allow the tubular body to extend, the outer ectodermal layer that is vertically aligned with the OA axis must relax, whereas the inner endodermal layer that is arranged perpendicular to the OA axis in a circumferential manner must contract (Shimizu et al., 2008). While the exact mechanism behind this synchronization is unclear, researchers hypothesized that the mesoglea might be involved.
Indeed, to further the understanding of the tri-laminar structure of the mesoglea, researchers used single and double immunofluorescence staining to highlight and compare collagen I, IV and laminin, the main protein constituents found in the interstitial matrix and basal lamina (Shimizu et al., 2008).
In doing so, they found dots, which, at high magnification, appeared as rings and pores, in the images of individually stained laminin and collagen IV (Fig. 9A-D). As for collagen I, the images showed an organized grid aligned with the OA axis (Fig. 9E and F), with fibers parallel to the axis being more numerous than those perpendicular, an observation that resembles and agrees with previous structural analysis of Hydra type I collagen (Shimizu et al., 2008; Veschgini et al., 2023).
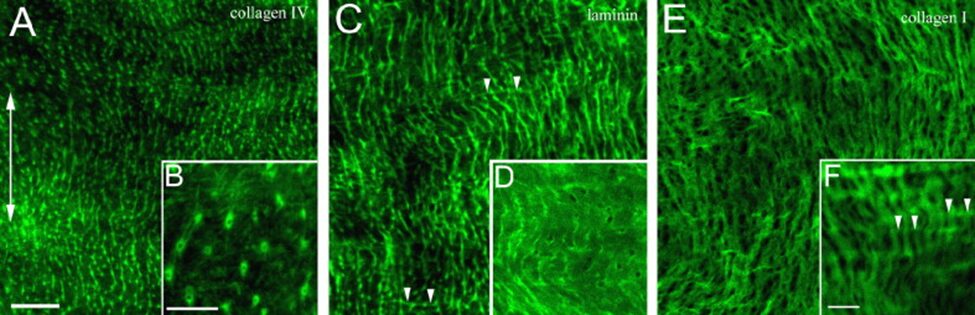
Fig. 9.. Immunofluorescent labeling of Hydra mesoglea components in the mid-section, viewed from the ectoderm. The double headed arrow (A) indicates the OA axis for all panels. (A, C and E) Collagen IV, laminin, and collagen I labeling. (B, D and F) Higher magnification of the fluorescent components. (C and F) Arrowheads reveal perpendicular fibers. Bar in A = 20 µm for A, C, E. Bar in B = 10 µm for B, D. Bar in F = 10 µm. (Shimizu et al., 2008)
However, after staining together collagen I with either laminin or collagen IV (Fig. 10A-L), the resulting images revealed that the dots formed by the basal lamina proteins (Fig. 10B and H) matched the grid spacing of the collagen I network. In fact, the black points in collagen I images (Fig. 10A and G), when visualized with the other protein, showed bright green, fluorescent spots filling them (Fig. 10C and I). Additionally, a cross-sectional view of the mesoglea revealed lines crossing the structure, much like tunnels going through the interstitial matrix and connecting both sides of the basal laminar layers (Fig. 10D-F and J-L). Indeed, the fact that Fig. E and K feature a highlighted contour around and through the column, whereas Fig. D and J lack that clearly defined edge and streak indicates that the basal lamina not only surrounds the interstitial matrix, but that its components also line the inner channels going across it (Shimizu et al., 2008).
Comparatively, the double staining of collagen IV with laminin results in almost identical photographs of the proteins when viewed alone and when overlapped (Fig. 10M-O). While the cross-section for the staining of lamina is blurrier, it nonetheless depicts the same trans-mesoglea channels as the ones in the cross-section of collagen IV, proving that both proteins occur at the same place (Fig. 10O-R) (Shimizu et al., 2008).
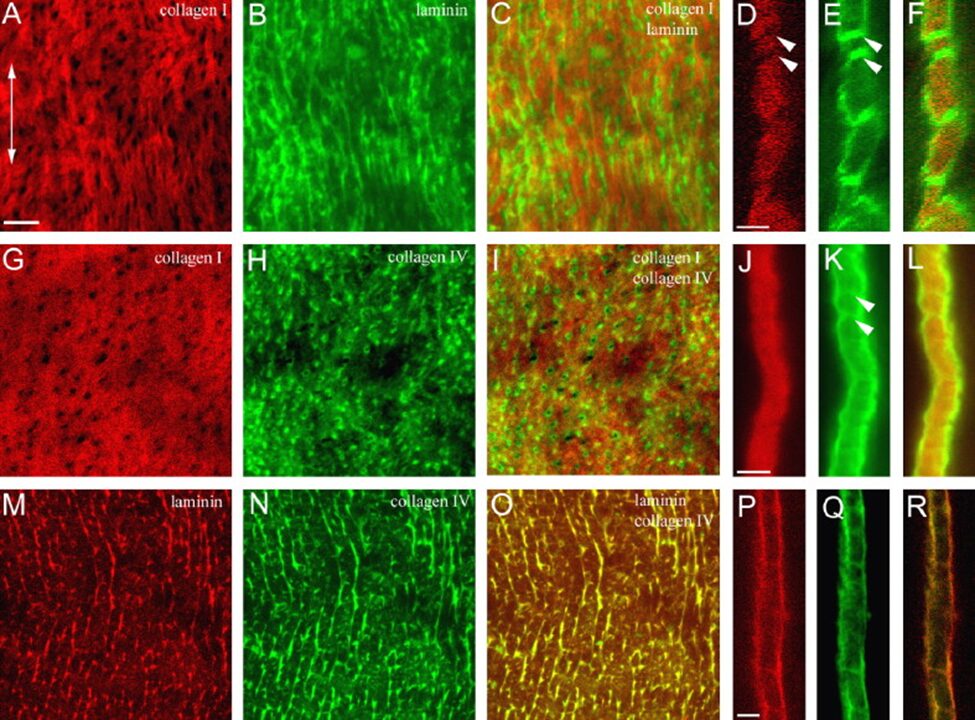
Fig. 10. Double Immunofluorescent labeling of hydra mesoglea components. (A-F) Double labeling of collagen I and laminin. (G-L) Double labeling of collagen I and collagen IV. (M-R) Double labeling of laminin and collagen IV. (A-C, G-I, M-O) Mesoglea is viewed from the ectoderm. (D-F, J-L, P-R) Cross-sectional view of the mesoglea. Double headed arrow in (A) indicates the OA axis across all panels. Double arrowheads in D, E and K shows trans-mesoglea channels in the interstitial matrix. Bar in A = 10 µm for A-C, G-I, M-O. Bar in D = 5 µm for D-F. Bar in J = 5 µm for J-L. Bar in P =5 µm for P-R (Shimizu et al., 2008).
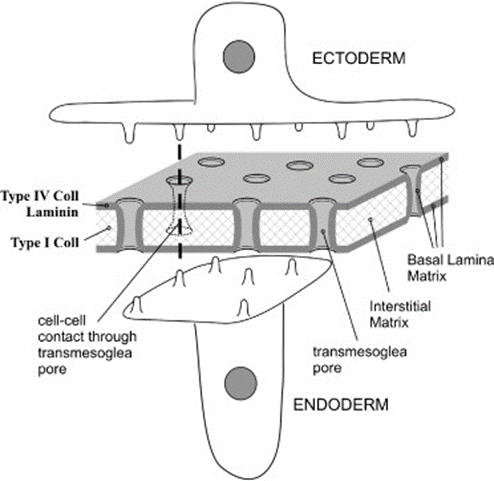
Fig. 11. Diagram of hydra mesoglea and epithelia (Shimizu et al., 2008)
Therefore, through immunofluorescence staining, researchers concluded that collagen IV does co-localize with laminin and, together, they form the basal laminar layers which cover the surface of the interstitial matrix as well as the trans-mesoglea pores found inside. These pores consequently connect the endodermal cell wall to the ectodermal cell wall, allowing cell processes to reach either side (Fig. 11.). This connection between the two epithelia may, as a result, explain the synchronous contraction and relaxation of muscles on opposite ends that cause movement in Hydra (Shimizu et al., 2008).
Heliotropism
Extraocular photoreception
Hydra are part of the phylum Cnidaria in which there are two types of photosensitive organizations: ocelli and extraocular photoreception (EOP). Ocellus is a sensor of ambient light intensity with a lens, pigment cells, and retinal sensory cells in an organized structure. This structure is similar to that of an eye and can be found in species of jellyfish. On the other hand, the eyeless Hydra have extraocular photoreception where their photosensitive cells are not organized organs like an ocellus (Taddei-Ferretti & Musio, 2000). From an evolutionary perspective, Hydra’s photo response system can be considered one of the first steps of photoreception in Cnidaria towards organized photoreceptive organs.
The head of the hydra consists of the hypostome which is a protuberance surrounded by tentacles. In order to determine the location of photosensitive cells in the hydra’s body, the tentacles were cut from the bodies of the hydra while ensuring the hypostome was intact (Guertin & Kass-Simon, 2015). The amputation of the tentacles did not affect the hydra’s contractions in response to light. However, once the hypostome was removed, the hydras were no longer able to contract in response to light. Since the tentacle contractions are determined by the presence of the hypostome, researchers hypothesized that the hypostome contains the photoreceptor cells of hydra. Other research has suggested that sensory neurons are responsible for controlling local myoepithelial contractions and influencing neural net contractions (Taddei-Ferretti & Musio, 2000). Kass-Simon and Diesl recorded an increase in spontaneous oscillation frequency of the resting potential in the endodermal epitheliomuscular cells induced by light in Hydra (Kass-Simon & Diesl, 1977).
Although hydra lack a brain, they have a nervous system with neurons spread across the body that can create behavior. The neural activity triggers calcium dynamics which leads to muscle contractions. The periodic bioelectric activities create reflex responses quantified as phase shifts (Fig. 12.). The phase shifts depend on the intensity and polarity of the light stimulus but show the contraction response to light stimulation.
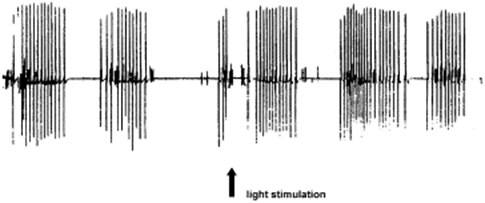
Fig. 12. Phase shift measurement of the periodic bioelectric activities of a Hydra vulgaris contraction after a 10 s white light stimulation (Taddei-Ferretti & Musio, 2000).
The nervous system is connected to different parts of the body, one of which is the hypostome where the photosensitive cells are assumed to be located. The cells are responsible for signaling the hydra’s body to react to light stimuli. Despite their lack of traditional photoreceptor structures, Hydra have a highly developed photo response. A Hydra’s response to high intensive sunlight occurs within 15 to 30 seconds and consists of body shortening and elongation by polyp contraction of the ectoderm and endoderm myofibrils (Rushforth et al., 1963). The response to artificial light is less intense and takes 1-2 minutes to occur. These contractions allow the hydra to react and move towards the light as the organisms exhibit phototaxis. These contractions consist of a successive series of partial body contractions of the Hydra, forming a tight ball with contracted tentacles. The reaction time was also found to be inversely proportional to temperature with the mean being 42.5 ± 10 seconds (Rushforth et al., 1964).
Light-driven feeding behavior
These freshwater organisms have been shown to exhibit a response to various wavelengths in the visible spectrum. Hydra’s ability to react to varying wavelengths is hypothesized to allow them to perceive the progress of the sun across the sky. This would assist them in anticipating prey and predator movements. Of these wavelengths, researchers have observed that hydra are most sensitive and attracted to blue light with wavelengths ranging from 400-450 nm independent of light intensity. Hydra have shown no reactions to red light with wavelengths greater than 600 nm in the visible light spectrum. This heightened responsiveness to blue light has led to hypotheses regarding its impact on the hydra’s feeding behavior (Guertin & Kass-Simon, 2015). Copepods, planktonic crustaceans, are similar to typical hydra prey with a transparent shell that refracts blue light under water. The dynamic movement of the small organisms provokes a change in light perceived by Hydra, indicating signs of prey and thereby inducing a contraction. The contractions in reaction to the detection of prey include tentacle writhing, tentacle ball formation, and mouth opening (Meech, 2019). Tentacle pulses from the reaction to light ranged from 200 to 1000 μV in size following a bursting pattern (Guertin & Kass-Simon, 2015). This pattern groups 4 tentacle pulses together followed by an extended period of silence.
The changes in light due to prey are detected by the light sensitive protein opsin in sensory cells that regulate the firing of Hydra’s cnidocytes – specialized cells found in the head of Hydra that are used to capture prey (David C Plachetzki et al., 2012). Opsins are universal photoreceptor molecules of all visual systems in the animal kingdom. They can change their conformation from a resting state to a signaling state upon light absorption. Studies found that significantly fewer cnidocytes were recovered from assays conducted in bright blue light with the wavelength 470 nm and light intensity of 2.8 W/cm2 than in dim trials with the wavelength 470 nm and light intensity of 0.1 W/cm2 (David C Plachetzki et al., 2012). It was found that under bright light, cnidocyte discharge was significantly lower than under dim light conditions. A prey animal would trigger a series of responses in the tentacles of the Hydra composed of battery cells. The battery cells consist of sensory neurons, ganglion cells, and nematocysts with the sensory neurons inducing the contraction in the tentacles in response to light. Response to light stimuli was inhibited once the Hydra began feeding and would take 50 to 65 minutes to be restored (Rushforth et al., 1964).
Light Response Contraction Mechanism. Contraction bursts (CB) are a distinct electrical event along with pre locomotion bursts (PB) and rhythmic potentials (RP). CBs have a correlation with motor output and involve the whole body of the Hydra to contract in response to environmental stimuli including light (Wang et al., 2023). A big slow wave was recorded at the base of a Hydra corresponding to the biometric activities with a contraction pulse, contraction bursts, and a series of rhythmic pulses (Fig. 13.). The electrical potentials were detected using a polyethylene Ag/AgCl suction electrode.
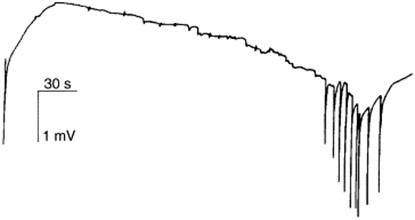
Fig. 13. A big slow wave corresponding to a full period of bioelectric activities for Hydra vulgaris contractions (Taddei-Ferretti & Musio, 2000).
Calcium imaging signals found that a rapid global activation of the muscle layer drives the whole-body contraction. This contraction is affected by activating the longitudinal ectodermal layer. Nerve nets contain the neural activity which triggers calcium dynamics in the muscle layers which drive muscle contractions that simulate Hydra behaviors. Calcium dynamics control muscle contractions following two pathways (Fig. 14.). The first involves IP3 induced calcium ion release from internal calcium stores creating a slow pathway. This pathway triggers the slow propagation of body-column waves and bending waves in the Hydra body. Hydra’s CB causes a fast calcium synchronization to activate the muscle cells in both the ectoderm and endoderm following the fast pathway with membrane ion channels (Wang et al., 2023). The contractions simultaneously activate the ectoderm and endoderm at specific time intervals. The duration of the synchronized activity of the inner and outer cell layers changes with time depending on the Hydra’s specific behavior.
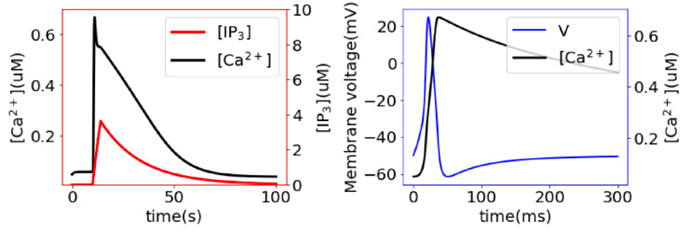
Fig. 14.. Slow (left) and fast(right)dynamics of the change in calcium ion concentrations, leading to body contractions (Wang et al., 2023).
Impact of light on sleep-wake cycles
Hydrae have a primitive nervous system with their sleep patterns lacking circadian rhythms. Studies detected 4-hour rhythms that might be generated by ultradian oscillators (Kanaya et al., 2020). To investigate their sleep patterns, experiments were conducted in controlled environments where movement was recorded for 24 hours, 12 hours light and 12 hours dark (LD). Movement was also measured for 24 hours of light (LL) and 24 hours of darkness (DD) to compare. It was determined that Hydras display behavioral responses to light transitions. The movement of Hydra was highest during the day and lowest at night with feeding taking place at dusk, following a diurnal sleep pattern (Fig. 15.). Hydra displayed overt rhythms in their daily sleep-wake cycles in LD conditions, these rhythmic behaviors disappeared in either LL or DD conditions.
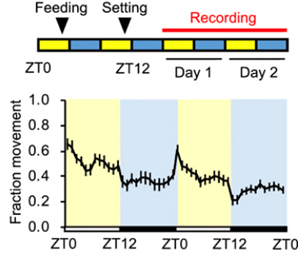
Fig. 15.. Measured Hydra movement following diurnal behaviors with feeding more than 24 hours before recording (Kanaya et al., 2020).
Cnidocytes in predation
Introduction of cnidocytes and nematocysts
As an evolutionary feature unique to Cnidarians, cnidocytes (also known as nematocytes) are stinging cells present around the organism’s mouth and tentacles used to capture and intoxicate preys, which for Hydra include small crustaceans, fish eggs, young insects, and larval mollusks. Cnidocytes could also function in defense against predators, and as a means of locomotion by adhering to substrates. However, our emphasis in this discussion will be on the mechanisms of cnidocytes in the context of predation. The basic structure of a cnidocytes includes: a membrane enclosed cyst which contains an organelle termed nematocyst (stingers), itself consisting of a central shaft and coiled tubule around the shaft, an opening called operculum, a hairlike mechanochemical receptor called cnidocil, and stereocilia enclosing the cnidocil (Karabulut et al., 2022; David C. Plachetzki et al., 2012).
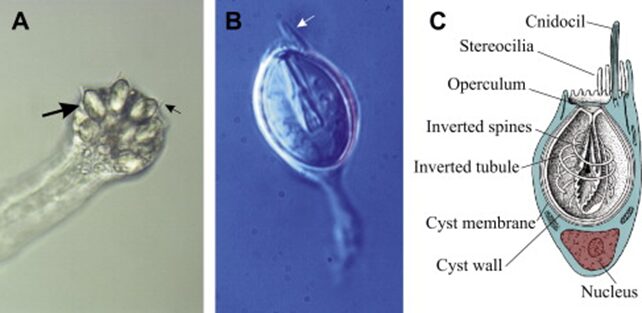
Fig. 16.. (A) End of a tentacle in Cladonema. Individual cnidocytes (large arrow) and cnidocils (small arrow) clearly visible. (B) A single stenotele (penetrant) cnidocyte isolated from Cladonema. (C) A diagram of a cnidocyte. (Anderson & Bouchard, 2009)
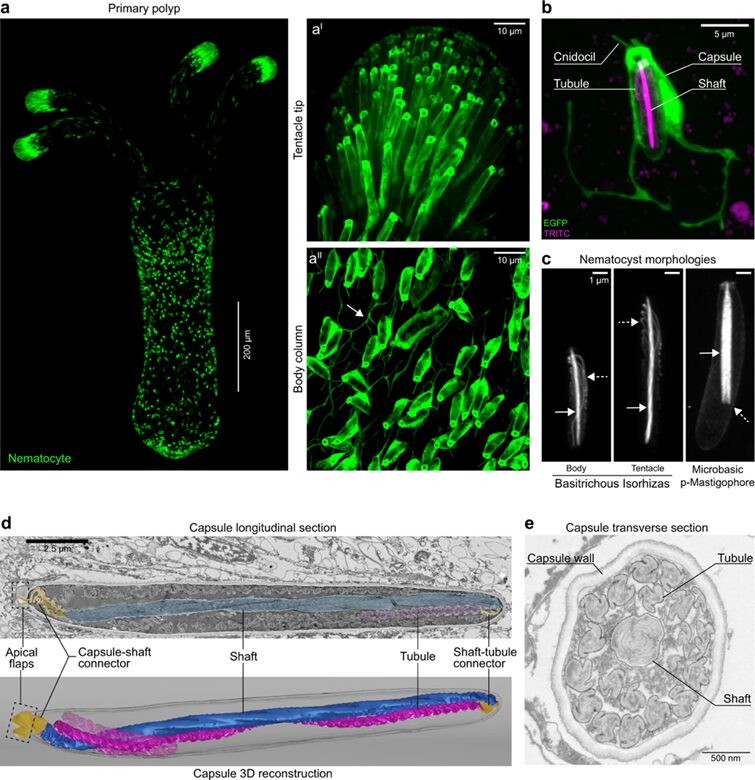
Fig. 17. (A) Cnidocytes (also called nematocytes) of a transgenic N. vectensis primary polyp expressing Enhanced Green Fluorescent Protein (EGFP) under the control of the nematogalectin promoter. (B) Close up view of a single nematocyte. The cnidocil (sensory organelle), cell body, and capsule expressing EGFP, as well as the tubule and shaft labeled in magenta are shown. (C) Nematocyst morphologies. (D) Longitudinal section of a nematocyst showing densely coiled shaft (blue) and tubule (magenta), the capsule-shaft connector, and the shaft-tubule connector (both in yellow). (E) Transverse section of a nematocyst showing the propeller shaped tubule, optimized to displace water behind itself to generate thrust during tubule eversion (Karabulut et al., 2022).
When stimulated, the nematocyst ejects the shaft attached to the tubule under the pressure of 7.7 GPa and at a velocity of 18.6 m/s. This punches a hole into the prey’s integument and neurotoxins are injected (Zhang et al., 2019). The initial phase of pressure-driven capsule explosion and subsequent thread ejection occurs in as fast as 700 nanoseconds, and sufficient force is generated to penetrate the prey in less than 3 ms (Anderson & Bouchard, 2009; Karabulut et al., 2022). Across the eukaryotic tree of life, nematocyst discharge ranks top in both extension rate (discharge speed) and strain (change in length/length) (Flaum & Prakash, 2023). There has been much interest in understanding the nematocyst firing mechanism to model nematocysts for applications such as microinjector design for targeted drug delivery.
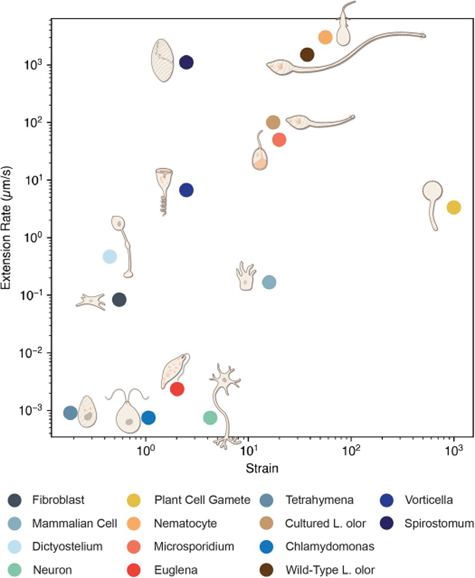
Fig. 18. Hyper extensible single cells with large strain and strain rates (Flaum & Prakash, 2023).
Sensory inputs for nematocyst discharge
Since nematocyst discharge is irreversible and discharged cysts are not replaceable (thus cnidocytes are single use), the need for energy efficiency dictates that nematocyst firing is meticulously controlled to ensure activation only under suitable circumstances, when success rate is high for prey capture. A study conducted by Glen in 1989 sheds light on the mechanisms of cnidocyte activation in sea anemones (Watson & Hessinger, 1989). Chemoreceptors specialized for detecting specific substances fine-tune the mechanoreceptors to the vibrational frequencies of targets emitting those substances. It was observed that cnidocytes discharge preferentially into targets at specific frequencies: 30, 55, and 65 to 75 hertz. However, when exposed to concentrated chemosensitizers like N-acetylated sugars and mucin, these preferences shift to 5, 15, 30, and 40 hertz, aligning with the rhythmic movements of swimming prey. Furthermore, cnidocyte mechanoreceptors increased in length by up to 70% when exposed to mucin (Watson & Hessinger, 1989).
Similarly, in Hydra, the collaboration or interplay of mechanical and chemical receptors provides a twofold filter for nematocyst activation. When chemical stimuli are present alone, no discharge occurs; when mechanical stimuli are present, baseline discharge occurs. Only when both are present is maximal discharge fired. In Hydra, chemical receptors interact with polysialic acid fragments and protein-like contact molecules found on preys, enabling mechanosensitive-triggered attacks (Anderson & Bouchard, 2009; Weir et al., 2020; Zhang et al., 2019).
Furthermore, light regulation through opsin-based phototransduction could also be involved in cnidocyte discharge. Separate research on the Hydra and other Cnidarian groups found that discharge occurs significantly more when exposed to dim blue light compared with bright blue light. For one, the feeding behavior of many aquatic organisms is tuned to diurnal cycles that peak at dusk, since prey items could be scarce during daylight hours. Additionally, light information in the form of a shadow cast locally by the prey could provide another layer of sensory precision, thus enhancing the likelihood that the tubule reaches it (Picciani et al., 2021; David C. Plachetzki et al., 2012).
Discharge mechanism and force generation
As previously mentioned, during nematocyst discharge, an average velocity of the stylet tip of 18.6 m/s is generated. By assuming constant acceleration, researchers calculated that 5,413,000 g is required to produce this average velocity from a standing start (Ozbek et al., 2009). The release of pressure and elastic energy stored in the capsule, as well as the elastic energy stored in the coiled shaft and tubule is believed to generate the force behind such high acceleration. Each phase of the discharge will be described along with discussion of the forces involved and nature’s optimization strategy.
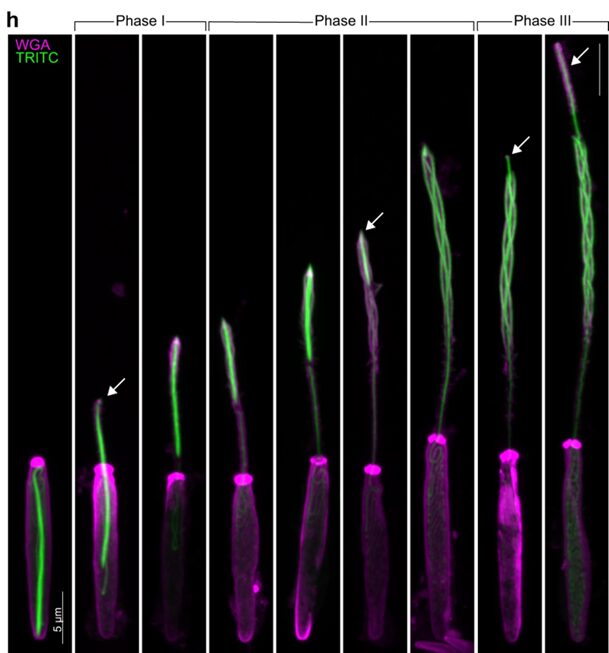
Fig. 19.. Geometric transformation of a nematocyst during discharge shown by fluorescent staining, with arrows highlighting the apex of the thread at various phases. (Karabulut et al., 2022)
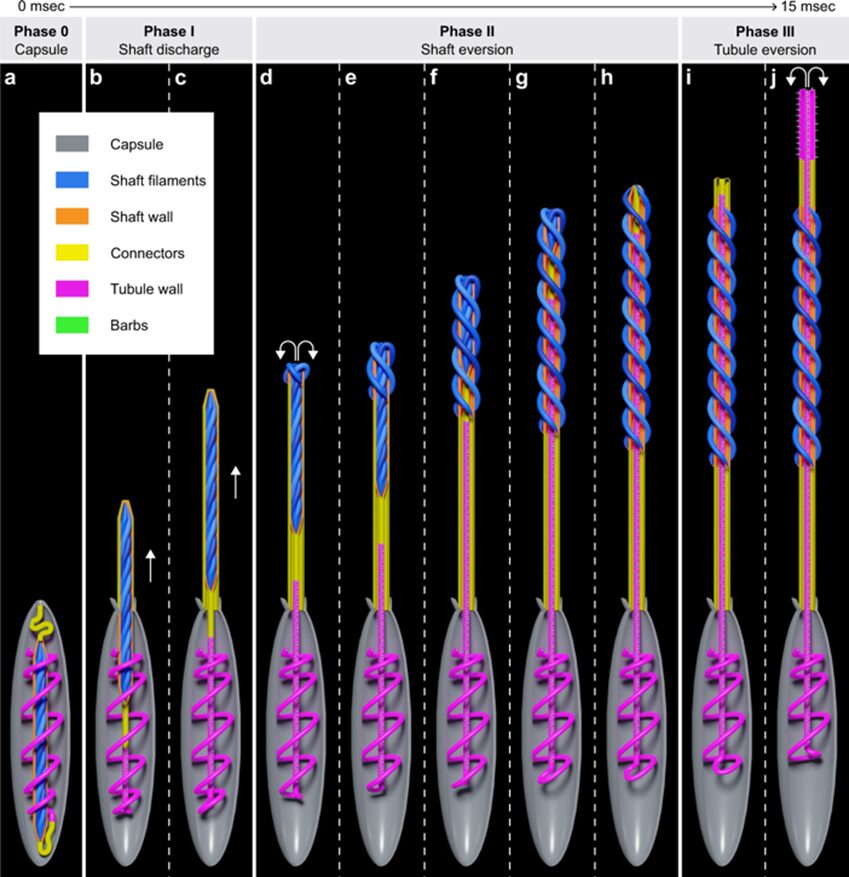
Fig. 20. Modeled geometric transformation of a nematocyst during discharge. Phase 0: Undischarged capsule with tightly coiled shaft surrounded the coiled tubule. During the entire discharge process the organelle is efficiently anchored by the cytoskeleton and does not alter its position inside the cell. Phase 1: Initial stage of shaft discharge. Phase 2: Geometric eversion/uncoiling of the compressed shaft filaments. Phase 3: Geometric eversion of the tubule (Karabulut et al., 2022).
Phase 0
A high initial osmotic pressure of up to 150 bar is built up at the end of capsule morphogenesis by a concentration difference of poly-γ-glutamate and anions (2.80 ± 0.20 μmol per milligram of isolated and purified dry nematocysts). When sensory receptors detect a stimulus, they trigger an increase in pressure and volume inside the capsule, and it is proposed that the pressure and volume must pass a critical threshold value to discharge. It is also worth noting that in the majority (80%) of all records, discharge occurred without any visible changes in the capsule prior to stylet ejection, which indicates that the cyst or capsule is in its maximally expanded state, and only a minimal increase in pressure is sufficient to elicit discharge. Upon triggering, proteins in the operculum might also loosen their supra-molecular architecture. Thus, the release of the increased osmotic pressure would be directed to the opening instead of a random rupture of the wall structure. Furthermore, on a molecular level, nematocyst walls are designed to withstand extreme mechanical stress and store high elastic energy by combining high resistance and flexibility. Consequently, an unusually stable protein network is required to withstand the involved physical forces, and it is believed that the collagens termed mini collagens in nematocysts capsule walls are great contributors to this property (Karabulut et al., 2022).
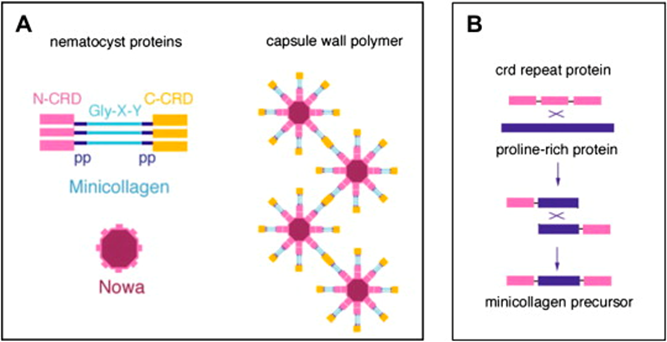
Fig. 21. Protein structures in nematocysts (Karabulut et al., 2022).
Phase 1 and phase 2
Based on still images and movies, researchers found that shaft eversion occurs after complete ejection of the shaft (shaft eversion refers to the process where the shaft uncoils and turns inside-out). Thus, it is postulated that the capsule-shaft connector accumulates maximal elastic stress when the ejected shaft reaches its maximal distance from the capsule (Karabulut et al., 2022).
Phase 3
It has been demonstrated that hydrating ruptured capsules results in extrusion and untwisting of the tubule without it undergoing eversion. This suggests that the twisted tubule stores elastic energy which transforms into kinetic energy by acting as a spring that is released by relaxation to a cylindrical state. Additionally, the tubule structure likely allows the flow of poly-γ-glutamate matrix from the capsule into the lumen, recharging the forces that push the tubule forward. After discharge, the elastically stretched nematocyst capsule shrinks to 50% of its original volume, signifying the release of kinetic energy during the discharge. The tubule’s tip punches a hole into the prey’s cuticle through which the tubule enters, releasing hemolytic and neurotoxic substances (Karabulut et al., 2022).
Purposes and advantages of high-acceleration discharge
Researchers investigated the relationship between the puncture performance (depth) and the tool sharpness (cusp angle) across a wide range of bio-relevant puncture speeds (quasi-static to ~20m/s) and found that sensitivity of puncture performance to variations in tool sharpness reduced at higher puncture speeds. Organisms with passive or low-speed puncture mechanisms depend on sharp tools for effective penetration and function retention, while higher-speed puncture systems may allow for greater variability in tool shape, enabling higher adaptability during the evolutionary process to other mechanical factors (Zhang & Anderson, 2023).
High initial accelerations are required for the barb to reach a velocity upon impact sufficient to puncture the cellular membrane or other external covering of the prey, since final velocity is proportional to the force available for puncture. Indeed, a small mass cnidarian nematocyst can effectively pierce through a 5 μm thick solid cuticle of a crustacean prey (Hamlet et al., 2020).
The physics governing the motion of structures in water at the microscale are usually over-damped because the fluid environment is dominated by viscous forces rather than inertial forces. High accelerations are needed to reach a Reynolds number sufficient for the barb to successfully reach the prey. Reynolds number is the ratio of inertial (resistant to change or motion) forces to viscous (heavy and gluey) forces given by the formula:
![]()
Where ρ is the density of the fluid (SI units: kg/m3), u is the flow speed (m/s), L is a characteristic length (m), μ is the dynamic viscosity of the fluid (Pa·s or N·s/m2 or kg/(m·s)), and ν is the kinematic viscosity of the fluid (m2/s) (Hamlet et al., 2020).
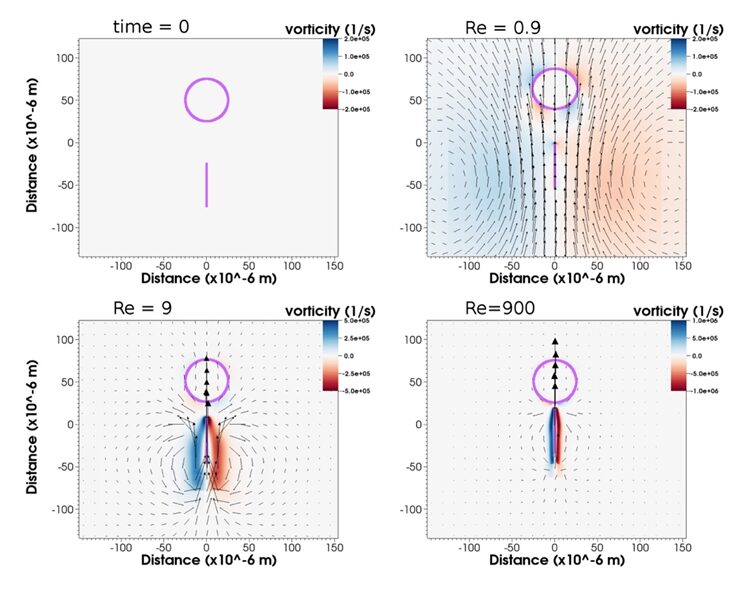
Fig. 22. Vorticity, velocity, barb, and prey position at time 5.5*10-6 s, approximately three-quarters of the way through the software simulation with Reynolds numbers (Re) manipulated to be 0.9, 9, and 900. From Re=0.9 to Re=9, and to Re=900, there is noticeable reduction in fluid volume entrained by the movement of the barb (Hamlet et al., 2020).
Nematocyst firing is in the intermediate range at 𝑅𝑒∼900 where inertial forces are also significant. In a study, researchers numerically simulated the dynamics of a barb-like structure and manipulated the Reynolds number to range from 0.9-900 by changing μ, taken to be the dynamic viscosity of the fluid (Hamlet et al., 2020). Findings suggest that acceleration followed by coasting at lower Reynolds numbers is not sufficient for a nematocyst to reach its target. At small Re, the simulated barb-like projectile faces high viscous damping effects normally encountered at small cellular scales and forms around itself a large boundary layer which pushes the prey out of the way. At higher Re comparable to real nematocyst discharge and at its current high velocity that help it overcome the viscous regime and stay in the inertial regime, the barb can reach the target due to the reduced boundary layer size (Hamlet et al., 2020).
Conclusions
Evolution has had billions of years to come up with many of its own design solutions to the problems that species face. For example, the bubble formation from the basal disk can remove a Hydra from its microenvironment and allow the organism to float somewhere with a more abundant source of food. Since this behavior is only observed in poorly fed Hydra, we can conclude that it is a solution to prevent Hydra starving by staying in an area where little food is to be found. On the other hand, the mesoglea, through its porous tri-laminar and anisotropic structure, serves as the skeleton of the epithelial-muscular system, offering both tensile strength and stiffness. Equally, the heightened stiffness in the shoulder region of Hydra is cleverly used to store kinetic energy and allow the animal to recoil back to the upright position at the end of a somersault. Hydra’s ability to respond to varying intensities and wavelengths of light, even in the absence of traditional eyes, represents an intriguing example of nature’s design solutions. Their heightened sensitivity to blue light allows them to quickly react to prey that reflect blue light in water, triggering a series of behavioral responses that result in efficient predation. This adaptation enhances their survival by allowing them to sense and react to environmental changes, predators, or potential prey. The three-fold filter for prey detection and the subsequent firing of single use stinging cells represent a finely tuned and energy expenditure minimizing mechanism. Then, by investigating the osmotic pressure and elastic energy involved in nematocyst discharge, this paper looked at the mechanism of force and speed generation which researchers hope to gain insight into to model biomedical devices and tools on cnidocytes, arguably one of the most complex cells in the eukaryotic tree, even though, with its radial symmetry, Hydra is more basal than Bilaterians. The intricate mechanisms of ejection and eversion of the shaft and tubule, their sequential release, and the potential energy replenishment processes examined in this paper have emerged as critical adaptive strategies employed by the Hydra to effectively mitigate the challenges posed by increased effect of boundary layers at the micrometer scale, as well as to secure the successful penetration of prey surfaces. These mechanisms are an integral part of the Hydra and its ability to survive with such a primitive structure.
References
Anderson, P. A. V., & Bouchard, C. (2009). The regulation of cnidocyte discharge. Toxicon, 54(8), 1046-1053. https://doi.org/https://doi.org/10.1016/j.toxicon.2009.02.023
Bode, H. R. (2009). Axial Patterning in Hydra. Cold Spring Harbor Perspectives in Biology, 1(1), a000463-a000463. https://doi.org/10.1101/cshperspect.a000463
Flaum, E., & Prakash, M. (2023). Curved crease origami and topological singularities at a cellular scale enable hyper-extensibility of<i>Lacrymaria olor</i>. Cold Spring Harbor Laboratory. https://dx.doi.org/10.1101/2023.08.04.551915
Guertin, S., & Kass-Simon, G. (2015). Extraocular spectral photosensitivity in the tentacles of Hydra vulgaris. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 184, 163-170.
Hamlet, C., Strychalski, W., & Miller, L. (2020). Fluid Dynamics of Ballistic Strategies in Nematocyst Firing. Fluids, 5(1), 20. https://www.mdpi.com/2311-5521/5/1/20
Hausman, R. E., & Burnett, A. L. (1969). The mesoglea of hydra. I. Physical and histochemical properties. Journal of Experimental Zoology, 171(1), 7-13. https://doi.org/https://doi.org/10.1002/jez.1401710103
Kanaya, H. J., Park, S., Kim, J.-h., Kusumi, J., Krenenou, S., Sawatari, E., Sato, A., Lee, J., Bang, H., & Kobayakawa, Y. (2020). A sleep-like state in Hydra unravels conserved sleep mechanisms during the evolutionary development of the central nervous system. Science advances, 6(41), eabb9415.
Karabulut, A., McClain, M., Rubinstein, B., Sabin, K. Z., McKinney, S. A., & Gibson, M. C. (2022). The architecture and operating mechanism of a cnidarian stinging organelle. Nature Communications, 13(1). https://doi.org/10.1038/s41467-022-31090-0
Kass-Simon, G., & Diesl, V. (1977). Spontaneous and evoked potentials from dissociated epithelial cells of Hydra. Nature, 265(5589), 75-77.
Lomnicki, A., & Slobodkin, L. B. (1966). Floating in Hydra Littoralis. Ecology, 47(6), 881-889. https://doi.org/https://doi.org/10.2307/1935636
Means of locomotion. In. Britannica. https://www.britannica.com/science/protist/Fossil-protists-and-eukaryotic-evolution
Meech, R. W. (2019). Electrophysiology and behavior of Cnidarian nervous systems. In Oxford Research Encyclopedia of Neuroscience.
Moore, K. (2014). Hydra meets Handel. https://royalsociety.org/blog/2014/03/hydra-meets-handel/
Naik, S., Unni, M., Sinha, D., Rajput, S. S., Reddy, P. C., Kartvelishvily, E., Solomonov, I., Sagi, I., Chatterji, A., Patil, S., & Galande, S. (2020). Differential tissue stiffness of body column facilitates locomotion of Hydra on solid substrates. Journal of Experimental Biology, 223(20), jeb232702. https://doi.org/10.1242/jeb.232702
Ozbek, S., Balasubramanian, P. G., & Holstein, T. W. (2009). Cnidocyst structure and the biomechanics of discharge. Toxicon, 54(8), 1038-1045. https://doi.org/10.1016/j.toxicon.2009.03.006
Picciani, N., Kerlin, J. R., Jindrich, K., Hensley, N. M., Gold, D. A., & Oakley, T. H. (2021). Light modulated cnidocyte discharge predates the origins of eyes in Cnidaria. Ecology and Evolution, 11(9), 3933-3940. https://doi.org/10.1002/ece3.7280
Plachetzki, D. C., Fong, C. R., & Oakley, T. H. (2012). Cnidocyte discharge is regulated by light and opsin-mediated phototransduction. BMC biology, 10(1), 1-10.
Plachetzki, D. C., Fong, C. R., & Oakley, T. H. (2012). Cnidocyte discharge is regulated by light and opsin-mediated phototransduction. BMC Biology, 10(1), 17. https://doi.org/10.1186/1741-7007-10-17
Rodrigues, M., Leclère, P., Flammang, P., Hess, M. W., Salvenmoser, W., Hobmayer, B., & Ladurner, P. (2016). The cellular basis of bioadhesion of the freshwater polyp Hydra. BMC Zoology, 1(1), 3. https://doi.org/10.1186/s40850-016-0005-7
Rushforth, N. B., Burnett, A. L., & Maynard, R. (1963). Behavior in Hydra: contraction responses of Hydra pirardi to mechanical and light stimuli. Science, 139(3556), 760-761.
Rushforth, N. B., Krohn, I. T., & Brown, L. K. (1964). Behavior in Hydra: inhibition of the contraction responses of Hydra pirardi. Science, 145(3632), 602-604.
Seabra, S., Zenleser, T., Grosbusch, A. L., Hobmayer, B., & Lengerer, B. (2022). The Involvement of Cell-Type-Specific Glycans in Hydra Temporary Adhesion Revealed by a Lectin Screen. Biomimetics, 7(4), 166.
Shimizu, H., Aufschnaiter, R., Li, L., Sarras, M. P., Borza, D.-B., Abrahamson, D. R., Sado, Y., & Zhang, X. (2008). The extracellular matrix of hydra is a porous sheet and contains type IV collagen. Zoology, 111(5), 410-418. https://doi.org/10.1016/j.zool.2007.11.004
Shostak, S., Patel, N. G., & Burnett, A. L. (1965). The role of mesoglea in mass cell movement in Hydra. Developmental Biology, 12(3), 434-450. https://doi.org/https://doi.org/10.1016/0012-1606(65)90008-4
Taddei-Ferretti, C., & Musio, C. (2000). Photobehaviour of Hydra (Cnidaria, Hydrozoa) and correlated mechanisms: a case of extraocular photosensitivity. Journal of Photochemistry and Photobiology B: Biology, 55(2-3), 88-101.
Veschgini, M., Suzuki, R., Kling, S., Petersen, H. O., Bergheim, B. G., Abuillan, W., Linke, P., Kaufmann, S., Burghammer, M., Engel, U., Stein, F., Özbek, S., Holstein, T. W., & Tanaka, M. (2023). Wnt/β-catenin signaling induces axial elasticity patterns of Hydra extracellular matrix. iScience, 26(4), 106416. https://doi.org/https://doi.org/10.1016/j.isci.2023.106416
Wang, H., Swore, J., Sharma, S., Szymanski, J. R., Yuste, R., Daniel, T. L., Regnier, M., Bosma, M. M., & Fairhall, A. L. (2023). A complete biomechanical model of Hydra contractile behaviors, from neural drive to muscle to movement. Proceedings of the National Academy of Sciences, 120(11), e2210439120.
Watson, G. M., & Hessinger, D. A. (1989). Cnidocyte Mechanoreceptors Are Tuned to the Movements of Swimming Prey by Chemoreceptors. Science, 243(4898), 1589-1591. https://doi.org/10.1126/science.2564698
Weir, K., Dupre, C., van Giesen, L., Lee, A. S. Y., & Bellono, N. W. (2020). A molecular filter for the cnidarian stinging response. eLife, 9, e57578. https://doi.org/10.7554/eLife.57578
Zhang, B., & Anderson, P. S. L. (2023). Investigation of the rate-mediated form-function relationship in biological puncture. Scientific Reports, 13(1), 12097. https://doi.org/10.1038/s41598-023-39092-8
Zhang, R., Jin, L., Zhang, N., Petridis, A. K., Eckert, T., Scheiner-Bobis, G., Bergmann, M., Scheidig, A., Schauer, R., Yan, M., Wijesundera, S. A., Nordén, B., Chatterjee, B. K., & Siebert, H.-C. (2019). The Sialic Acid-Dependent Nematocyst Discharge Process in Relation to Its Physical-Chemical Properties Is a Role Model for Nanomedical Diagnostic and Therapeutic Tools. Marine Drugs, 17(8), 469. https://doi.org/10.3390/md17080469