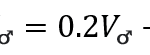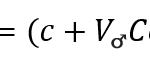Abstract
When it comes to the survival of some of nature’s more developed animals, it is often their uniquely designed appendages which allow them to make expert use of their environment. One such appendage is the tail and its many uses. Whether it be the thermoregulatory applications of the beaver and rat tail, the chameleon’s semi-prehensile tail, the fish’s propulsive tail, the squirrel’s balancing tail or the foraging fluke of aquatic mammals, there is a surprising amount of depth to the mechanical applications of the tail and its importance for many animals’ survival. More specifically, one can witness how tails are able to resist great crushing forces, to twist, to rotate to incredible degrees, to intuitively exchange heat between the body and environment, to allow for incredibly precise movements in water or on land, and much more.
Introduction
The tail is one of the most functionally diverse parts of the vertebrate body. In different species, its use ranges from locomotion or propulsion to thermoregulation, foraging, and even communication. Some species also have tails with no apparent use. The only uniting feature amongst all tails is their location on the body.
The tail, more technically referred to as the caudal region of vertebrates, extends along the main body’s axis, from the posterior (further back in position) to the anus (Mallo, 2021). While the caudal vertebrae typically define the morphology of the tail, this is not always the case. Bird tails are better understood by their caudal feathers, while fish tails are made up of a caudal fin attached to the final caudal vertebra. Additionally, aquatic mammals, such as cetaceans, have a fan-shaped extension beyond the vertebra.
As will be discussed, the evolved structures of the caudal region contain significant diversity among species. Each unique structure strongly correlates with each tail’s understood use and function.
Rodents – Thermoregulation
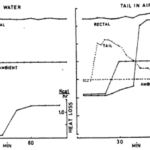
If there is one peculiar characteristic found in the tails of many of the world’s rodents such as beavers and rats, it is their completely hairless tail. Unlike most other mammals whose fur is required to keep their entire body insulated and thus warm as homeothermic animals (Fur, 2022), animals such as rats and beavers have completely hairless tails. As one might expect, this evolutionary trait is also related to the animal’s homeothermy and thus serves a thermoregulatory function. For example, the beaver’s tail displays an impressive combination of functions including aquatic movement and aquatic thermoregulation. Indeed, the lack of fur on its tail allows it to dissipate heat in both air and water, with the latter allowing for a significantly more pronounced dissipatory effect. This phenomenon can be observed below (Figure 1).
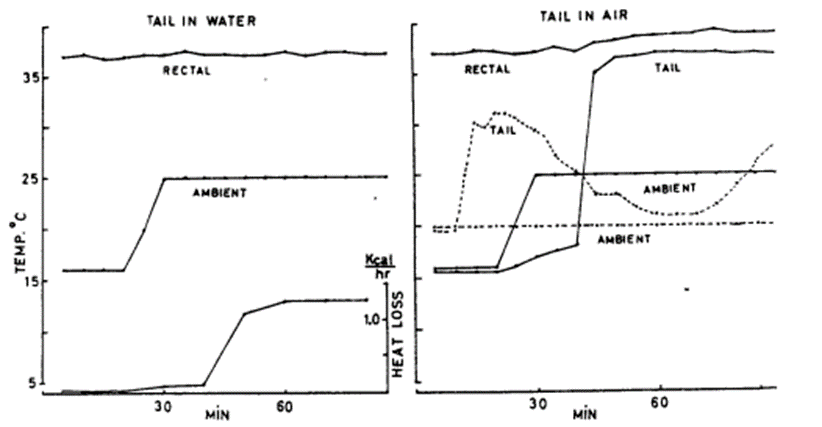
Fig. 1 Importance of beaver’s tail being immersed in water for thermoregulation shown through comparison (Steen & Steen, 1965).
It was experimentally determined that at ambient temperatures of 16 °C, the beaver can maintain its typical body temperature of 37°C with the tail’s temperature outside of water being slightly above 16°C. However, at ambient temperatures of 25 °C, the animal’s body temperature increases by 2°C above its normal value and the tail’s temperature significantly increases to 35°C. Conversely, this hyperthermia is not observed when the beaver’s tail is immersed in cool water (6°C ). At an ambient temperature of 16°C , the tail loses 0.1 kcal of energy per hour and at 25°C, the tail loses 1.2 kcal of energy per hour (Steen & Steen, 1965). As such, the beaver tail’s thermoregulatory function is revealed to be largely influenced and thus designed for its naturally aquatic habitat.
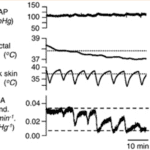
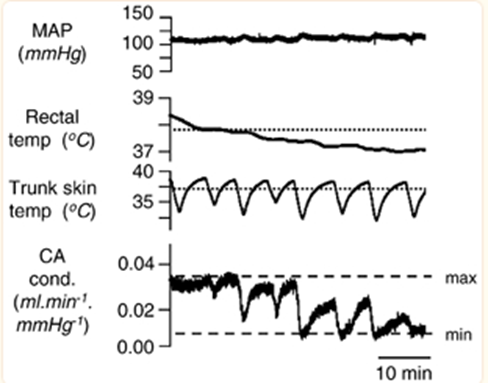
Focusing on the tail of the rat, another rodent with a similar tail but a non-aquatic environment, an interesting observation appears: the rat’s body temperature is in part regulated by the sympathetic fibers in its tail and the way it controls blood flow. For example, at skin and rectal temperatures above 39 °C, the fiber activity is low. When the tail is cooled by 2-7 °C with water, the tail’s sympathetic activity increases gradually. Additionally, repeated cooling of the tail’s trunk skin shows graded vasoconstriction of its circulation, which means a lower amount of blood flow at the tail resulting in heat loss. However, the constriction of blood vessels only reach significant levels when the rectal body temperature dips below a certain point (Owens et al., 2002). This creates a feedback loop which prevents over-adjustments of the rat’s body temperature. This general trend can be seen below (Figure 2).
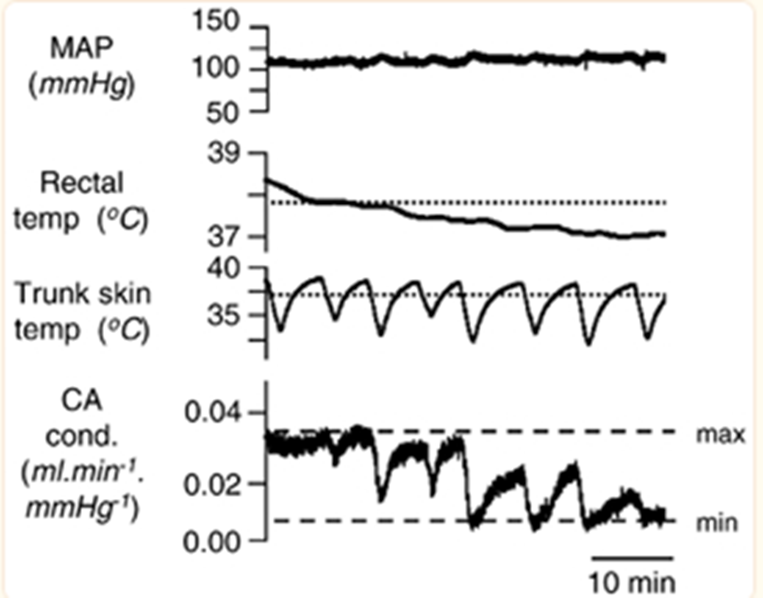
Fig. 2 This figure represents the mean arterial pressure (MAP), the rectal and trunk skin temperatures, as well as the caudal artery conductance which represents the amount of blood flowing to the tail (Owens et al., 2002).
Notice how both the interior and exterior temperatures of the rats influence the caudal artery conductance. These fluctuations seen on the exterior lead to varying levels of constriction, which sometimes reaches the minimum possible value. Ultimately, the minimal conductance is only maintained when the interior body temperature matches the average skin temperature. As such, one can see that the tail is designed to be sensitive and responsive to different sorts of thermal stimuli until the more important interior body temperature reaches its ideal level. Finally, whereas the beaver can impressively maintain its internal body temperature within large ranges of external temperatures as long as it has access to water, the rat’s tail is instead designed to provide necessary adjustments to its internal temperature by precisely responding to its external temperature. This distinction demonstrates how certain biological systems can accomplish identical objectives through incredibly similar means that only diverge slightly due to the natural environment these systems thrive in.
Seahorse – Square Prehensile Tail
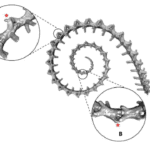
Syngnathids, such as seahorses and pipehorses (Fig. 3A), differ from most other teleost fish, mainly because of their prehensile tail (Neutens et al., 2014). Furthermore, the seahorse itself curiously has a tail with a square cross-section, seemingly allowing it to possess better functionality and strength than its round counterpart (Porter et al., 2015). This architecture allows for better mechanical performance in prehension and resistance to crushing forces. The tail has three primary joints allowing for motion: the ball-and-socket, the peg-and-socket, and the gliding. Although the square shape of the tail has little relation to the ball-and-socket joint, the peg-and-socket joints connecting the plates of adjacent segments substantially restrict twisting due to the tail’s square shape. This structure preserves articulatory organization and provides safety against torsion-induced damage while also assisting in relaxing the tail. Furthermore, the square architecture is flat, which increases surface contact and makes the tail undergo an exterior shape change when twisted, allowing for greater control when grabbing. If transverse compression and impact are exerted on the tail, the plates slide past one another with one degree of translation freedom therefore showing it to be stronger and more resilient than a cylindrical version, which would have its plates rotate and translate freely (Porter et al., 2015). A representation of the sea horse’s tail and some different tail models can be seen below (Fig 3B).



Fig. 3 Image of: a Cylix tupareomanaia, a pipehorse, (top left) (Adapted from Reef builders); a seahorse (top right) (Adapted from Seahorse 2022); a representation of the seahorse square tail (middle) juxtaposed with models meant to replicate its square shape (Middle-left and middle-right) and some with a cylindrical shape (bottom left an bottom right) (Adapted from Porter et al. 2015).
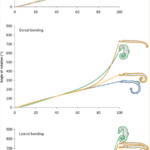
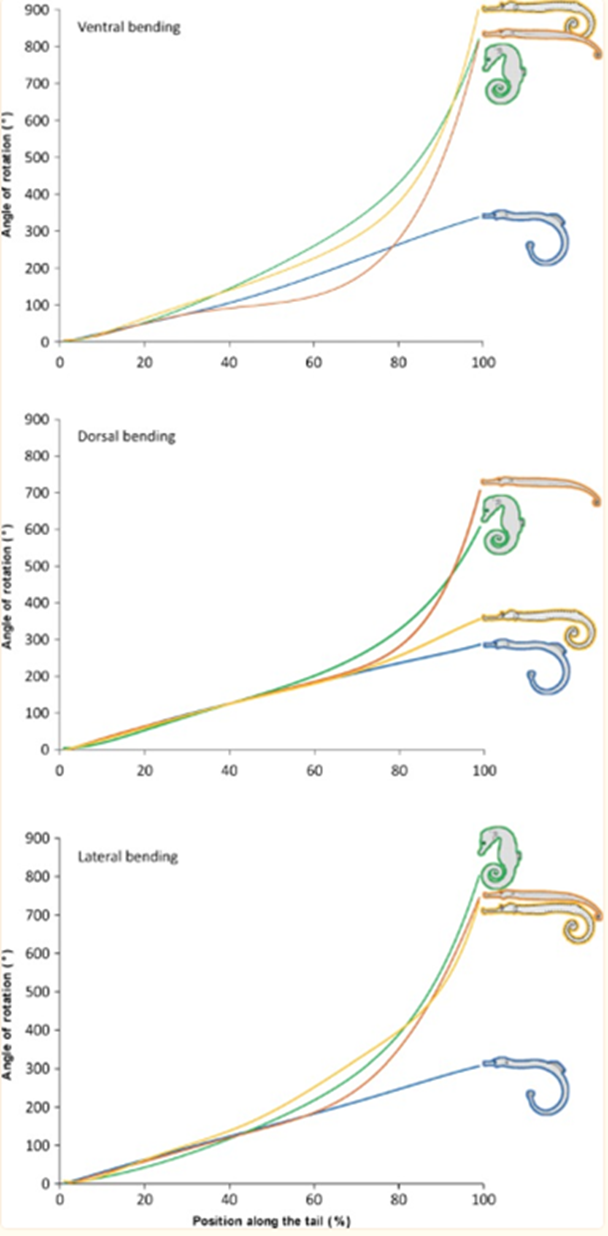
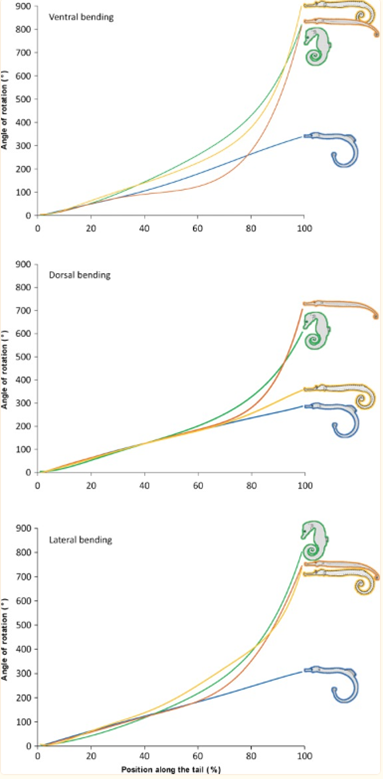
As such, there are clear benefits to the seahorse tail’s unusual shape. Taking a more general look at the tail’s peculiar function, one notices that it has evolved into a prehensile apparatus, unique among fishes that typically have caudal fins. This trait allows the animal to both grasp and hold on to substrates during feeding and hiding. This grasping requires a vertical posture in the water column, likely due to the seahorse’s habit of pivot feeding. More careful examination shows that the tail has four bony plates, one for each body segment, which surround the caudal muscles anchored to their central vertebral column. Although we previously established the extent of the tail’s resilience, it comes at the cost of its use in mobility; the seahorse instead relies mostly on its dorsal and pectoral fins to move. As such, the seahorse evolved in a way that allowed its tail to possess a unique function among its kind since most other fish could not afford the necessary sacrifice in mobility. Another important characteristic of the tail is the potential degree of rotation and bending allowed by its structure. Indeed, passive joint flexibility can be determined by observing the maximum angle of bending in both the ventral, dorsal and lateral directions (Neutens et al., 2014). One can observe this passive bending capacity in the seahorse, and some similar animals, below (Fig. 4).
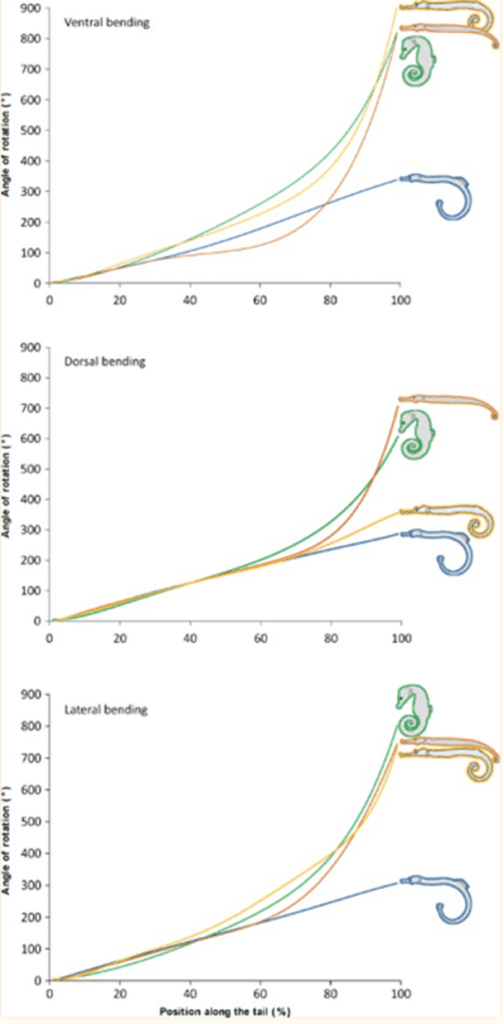
Fig. 4 Passive bending capacities of the pipefish (blue), seahorse (green), pipehorse (orange) and sea dragon (yellow) (Adapted from Neutens et al. 2014).
As one can see, the seahorse’s tail can rotate in all directions to an impressive degree. This characteristic can be explained as the result of multiple factors, such as the small size of the plates and their distally reduced positions. Another possible factor is the presence of large plates that lack sliding joints around a large posterior process. However, the effect of dermal ossification on bending capacities remains unclear (Neutens et al., 2014). In short, the structure of the horse’s square prehensile tail has an interesting array of capabilities due to its bone structure. It serves as an excellent example of how certain tails serving similar functions, such as prehensile tails generally useful for grabbing, can still distinguish themselves through some of their traits depending on the animal’s habitat and some of the concessions possible due to its morphology.
Chameleon – Semi-Prehensility
An investigation into the caudal region of a chameleon incites a study of changes in morphology due to different habitats and lifestyles. While the oldest members of the Chamaeleonidae family inhabited a terrestrial domain (Tolley, Townsend, & Vences, 2013), many different species of chameleons have developed an arboreal lifestyle (Figure 1; Luger et al., 2020). However, some species have evolved back to a terrestrial lifestyle from an arboreal ancestor, such as Chamaeleo namaquensis and Bradypodion occidentale (Luger et al., 2020). As the mechanical requirements for a tail in these two conditions differ, the evolutionary history of habitats among species creates a unique spectrum of tail usage and morphology. The tail of a veiled chameleon (Chamaeleo calyptratus) is shown below (Fig. 5).

Fig. 5 Image of a veiled (Chamaeleo calyptratus) (From Chris Kadet, 2008, Wikimedia, https://commons.wikimedia.org/wiki/File:Chris_Kadet_Veiled_Chalemeon_2008.jpg, CC by 3.0).
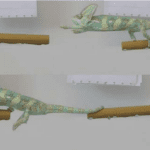
The structure and function of a chameleon tail varies across different species. However, the three most general functions are to aid in performing acrobatic maneuvers, in balancing while straddling perches (Fig. 6), and in stabilizing the body when using rapid tongue movements for food acquisition (Bergmann et al. 2003).
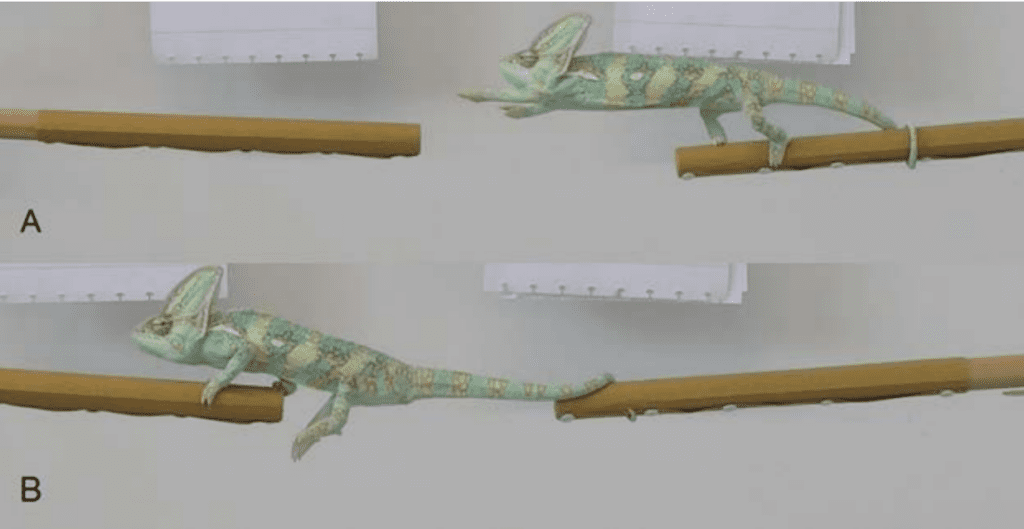
Fig. 6 Process of a chameleon performing a substrate crossing >1.25 times shoulder-hip length: (a) the chameleon coils its tail around the perch to free the front legs to grasp onto the next perch; (b) once front legs are on the next perch, the chameleon repositions the tail so the hind legs can move to the next perch (Luger, Vermeylen, et al. 2021).
The caudal vertebrae of arboreal chameleons can be divided according to two regions: the distal vertebrae and the proximal vertebrae (Luger et al., 2021). The proximal vertebrae are located closer to the base of the spine while the distal vertebrae extend to the tip of the tail. The proximal vertebrae are more robust and have a higher length to width ratio, while the distal vertebrae are more numerous and smaller (Figure 7). Additionally, the zygapophysial joints in the proximal region overlap more, which restricts the horizontal motion of the tail. On the other hand, zygapophysial joints are shorter in the distal region, which increases rotation ability towards the end of the tail (Luger et al., 2020). Luger et al. also propose that the more robust structure of zygapophysial joints in the proximal region ensures more stability and rigidity in the tail. However, in terrestrial chameleon species, the distal and the proximal regions are very similar in morphology, where both resemble the larger, more robust vertebrae found in the proximal region of arboreal chameleons (Luger, Watson, et al., 2021).
Luger, Watson, et al. (2021) also investigated the muscular system of the proximal and distal regions of Chamaeleo calyptratus, an arboreal species of chameleon, and found significant regional differences. They found that the proximal musculature cannot counteract as high as the distal musculature, regardless of the vertebral system. They concluded that the muscle arrangement impacts the mechanical efficiency of the musculoskeletal system. The proximal region is more rigid so that the tail remains off the ground when not in use, but the distal musculature aids in the prehensile qualities of the tail.
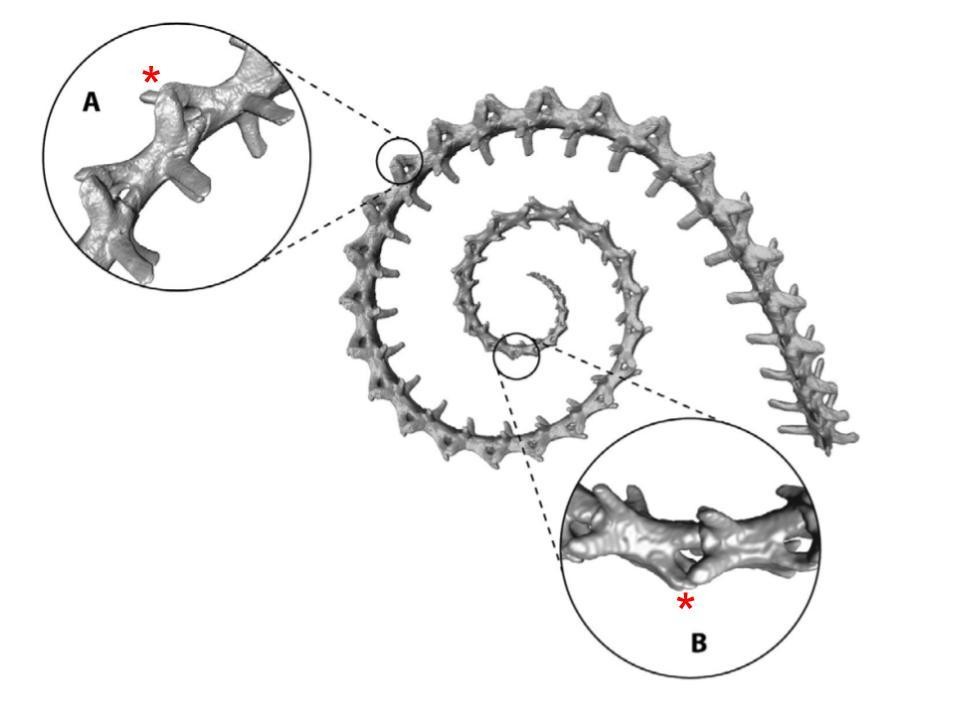
Fig. 7 3D-Visualization of caudal vertebrae of arboreal Chamaeleonidae, showing (a) a typical proximal vertebra and (b) a typical distal vertebra; red asterisks denote a zygapophysial joint in each region (Adapted from Luger et al. 2020).
In general, the prehensility of the tail for arboreal chameleon species has also made a longer tail advantageous, despite the extra energy and resources required to grow a longer tail, because a long tail can execute more spirals around a branch. This creates more contact between the tail and branch, which increases surface area and therefore friction making the chameleon’s body easier to support if needed.
Many studies of chameleon tails center on understanding how the difference in habitat and therefore tail usage in chameleons is correlated with the layout of the musculoskeletal system of the Chamaeleonidae family.
Fish – Propulsion
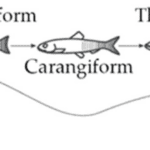
For finfish, the tail, or caudal fin, is a component of their propulsion system. A fish’s propulsion system generates thrust by the momentum transfer between the body of the fish and the surrounding water (Costa et al., 2020). Different factors, such as oscillation frequency of the body, shape of the fin, and stiffness of the fin, affect the locomotion properties of each species of fish. Additionally, Figure 8 shows the different modes of movement for body and caudal fin (BCF) swimmers. These modes define what portion of the body participates in the wave that creates propulsion.
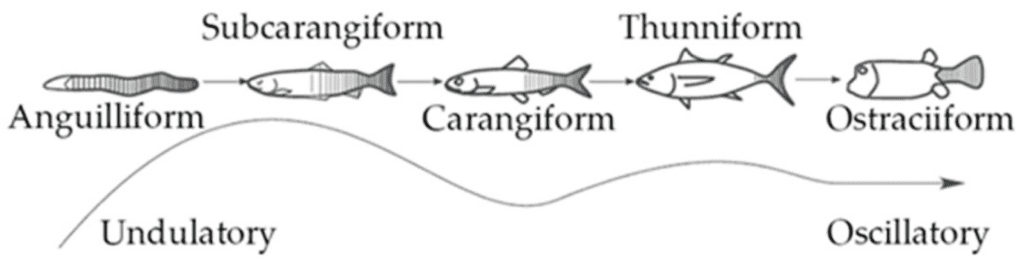
Fig. 8 Different propulsive mode classifications of BCF fish (Costa et al., 2020).
According to Costa et al. (2020), the thunniform mode has the highest propulsive efficiency, which consists of flapping solely the caudal fin. On the other hand, anguilliform swimmers have the most freedom of movement. For subcarangiform and carangiform, from one-half to one-third of the body participates in the propulsive movements.
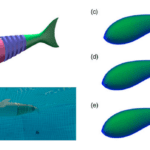
Zhong et al. (2022) aimed to study specifically how tail morphology played a role in the propulsive efficiency of fish. They investigated this relationship using carangiform motion and three different caudal fin shapes with different aspect ratios: round (similar to a snakefish), indented (similar to a saithe), and lunate (similar to tuna) (Fig. 9). An aspect ratio (AR) of a fin is the ratio of its span to its mean chord, meaning that a lunate fin has the highest AR while a round fin has the lowest AR.
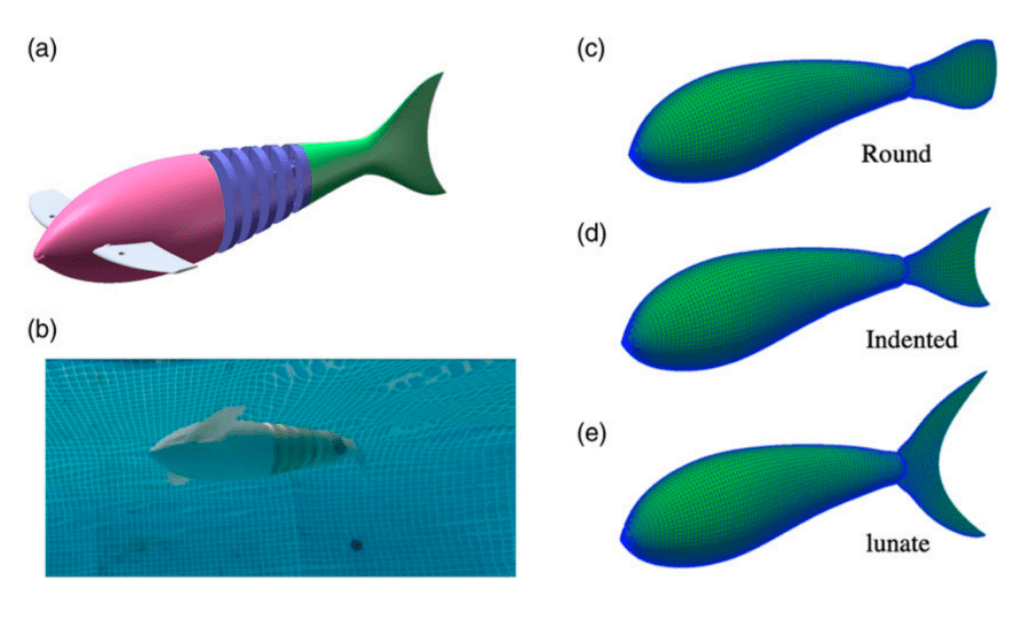
Fig. 9 Conformation of the tails used by Zhong et al.: (a-b) robotic model used, (c-e) shapes of the three tails used (Zhong et al., 2022).
Their findings were that indented tails produce the highest cruising speed at the base amplitude used. While the round tail is the most efficient, the indented tail is considered the best performer overall. This conclusion is consistent with carangiform swimmers, which commonly have indented tail shapes.
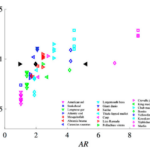
By definition, the magnitude of the propulsive wavelength indicates what mode classification a species uses. It has been found that a higher AR is associated with a longer propulsive wavelength such as a thunniform mode, while a low AR is associated with a shorter propulsive wavelength such as an anguilliform mode (Zhong et al., 2022). Figure 10 shows the correlation between propulsive wavelength and aspect ratio by plotting these characteristics for 22 different fish species. The plot shows that, as the aspect ratio increases, the propulsive wavelength is also longer. The relationship does not appear to be perfectly linear but nonetheless follows this general trend. Each species also has a range of corresponding propulsive wavelengths, while the aspect ratio remains the same.
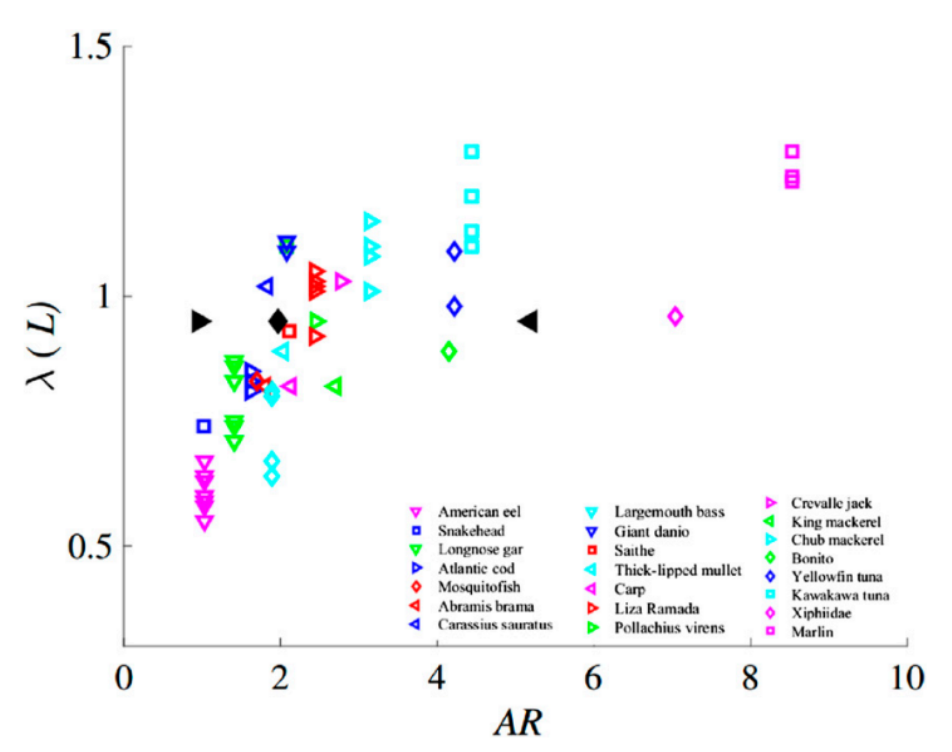
Fig. 10 Plot of 22 different fish species’ propulsive wavelength vs. tail aspect ratio; black points represent the three conditions of the study: right facing point triangle is round, diamond is indented, and left facing point triangle is lunate (Zhong et al., 2022).
While the morphology of many caudal fins and the propulsive wavelength, or mode, vary vastly across different fish species, Di Santo et al. (2021) found many kinematic similarities across the 44 species that they examined. For all species, the amplitude of the wave increases nonlinearly as it travels to the tail, with the majority of the increase occurring in the posterior (closer to the tail) region of the body.
The tail is a defining feature of a fish’s propulsion system, the characteristics of which are determined by the propulsive wavelength and the amplitude.
Squirrel – Communication, Righting, and Stabilization
Squirrels have adapted to possess a variety of different uses for their tail depending on lifestyle and habitat. Some of its uses are for stability and counterbalance while navigating an arboreal environment (Hofmann et al., 2021), for self-righting for falls (Fukushima et al., 2021), for intra- species communication (Hersek & Owings, 1993), and for communication with predators using flagging or infrared warnings (Blumstein, 2007).
In terms of squirrel tail anatomy, Hofmann et al. found that the caudal vertebrae arrangement of arboreal sciuromorpha species was similar to the arrangement of the caudal vertebrae of other arboreal non-prehensile mammals. It was proposed that this similarity is due to a similar lifestyle. Additionally, ground squirrels have a proportionally shorter tail compared to flying and arboreal squirrels, which could be due to their acrobatic lifestyle requiring more mechanical uses of the tail. Additionally, gliding squirrels have longer tails than arboreal squirrels (Hayssen, 2008).
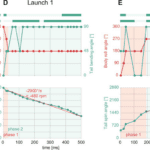
One of the fascinating uses of the tail is in a squirrel’s quick reflexes in self-righting during unexpected falls. Fukushima et al. (2021) studied the role of the tail in these instances by examining a “viral” video of a squirrel catapult, where squirrels were unexpectedly launched and still landed completely upright with no apparent harm (https://youtu.be/hFZFjoX2cGg?t=975, Launch 1- 16:19, Launch 2 – 17:49). The video captures two trials, showing that the squirrel uses successive adduction and abduction of the limbs along with rapid rotation of the tail to complete the landing. The squirrels use these movements to stabilize their head, find a safe space to land, rotate their body to align with the landing location, and finally to stabilize their body by abducting their limbs while still using tail rotation (Fig. 11). While the mass of the squirrel tail is only 3% of the total body weight, the area of the tail can be around 55%. Fukushima et al. propose that the size of the area can affect the aerodynamic properties of the tail and how it affects a squirrel’s righting technique.
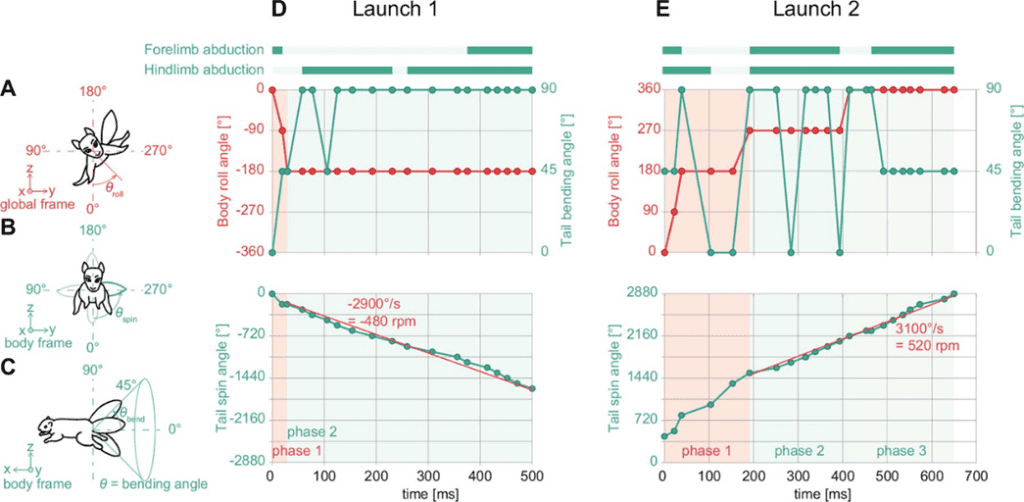
Fig. 11 Behavior analysis of 2 squirrel launches: (A-C) definitions of body roll angle, tail spin angle, and tail bending angle, respectively; (D) the body and tail motions as Launch 1 progresses; (E) the body and tail motions as Launch 2 progresses (Fukushima et al. 2021).
As mentioned above, another important function of squirrel tails is for communication. Different species of squirrels use their tails to communicate in different ways. Fox squirrels use their tails for precopulatory communication (McCloskey & Shaw, 1977). Males can either communicate an aggressive or non-aggressive intent, while females can communicate approval. California ground squirrels wave tails, sometimes with infrared signaling, to avoid predation by snakes (Blumstein, 2007). Aggression between dominant and subordinate gray squirrels is a function of how tight the bend in the tail is and what portion of the tail is bent.
Another interesting aspect of squirrel tail use in communication is the multimodal technique used when attempting to avoid predation by infrared-sensing rattlesnakes. Rundus et al. (2007) found that ground squirrels’ tails flag to avoid predation from snakes. However, when the communication is with a rattlesnake, which is infrared sensitive, the squirrel also heats its tail (Fig. 12). The squirrels do not heat their tail around gopher snakes, which are not infrared-sensing. The method of tail-heating is likely a combination of piloerection, the contraction of muscles at the base of hair follicles that bristles the tail hairs, and increased blood flow (Rundus et al., 2007).

Fig. 12 A ground squirrel tail flagging their infrared-heated tail to ward off a rattlesnake (Rundus et al., 2007).
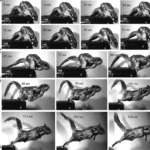
Essner (2002) also investigated the role of the tail in launching from platforms. Their study found varying levels of dorsiflexion of the tail during the propulsive phase of a launch for different sciuromorpha species (Fig. 13). It is suggested that this dorsiflexion counteracts the angular momentum in the pelvis created by the launch sequence (Essner, 2002).
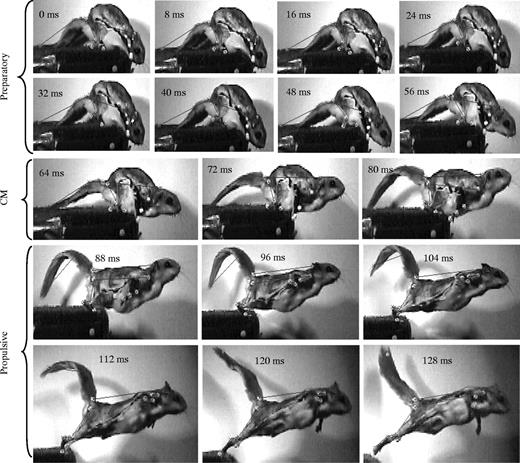
Fig. 13 Launch sequence of a flying squirrel; during the propulsive phase, the tail is dorsiflexed (Essner 2002).
Squirrel tails have a variety of important roles, including mechanical roles related to righting techniques and launch sequences in addition to multimodal communication such as flagging and infrared signaling.
Cetaceans – Foraging
In the past, whales, dolphins, and porpoises mostly fed themselves thanks to methods involving full-body hunting. Popular methods included: beaching, consisting of directing prey towards the coastline and subsequently catching it in shallow waters; bubble net feeding, consisting of trapping fish in bubbles; and echelon feeding, where the animals funnel prey by swimming in a V formation. While cetaceans are fully aquatic animals, they have much in common with humans—namely, their ability to share rich societal and cultural practices. Since the 1980s, researchers have noticed seemingly cultural trends resulting in the propagation of various methods of fluke-based feeding.
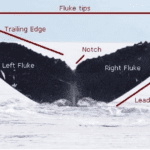
Cetaceans’ morphology consists of a long body, flippers, a dorsal fin, and a boneless tail (caudal fin) comprising a cartilaginous fluke (generally used for propulsion). From an outside perspective, cetaceans’ tails are of a symmetrical planform design. As seen in Figure 14, a whale fluke comprises a left and right fluke, each side with an irregular trailing edge and a smooth leading edge. The measurements of the fluke will vary from species to species with respect to hydrodynamic parameters that modulate energy consumption (Fish, 1998).
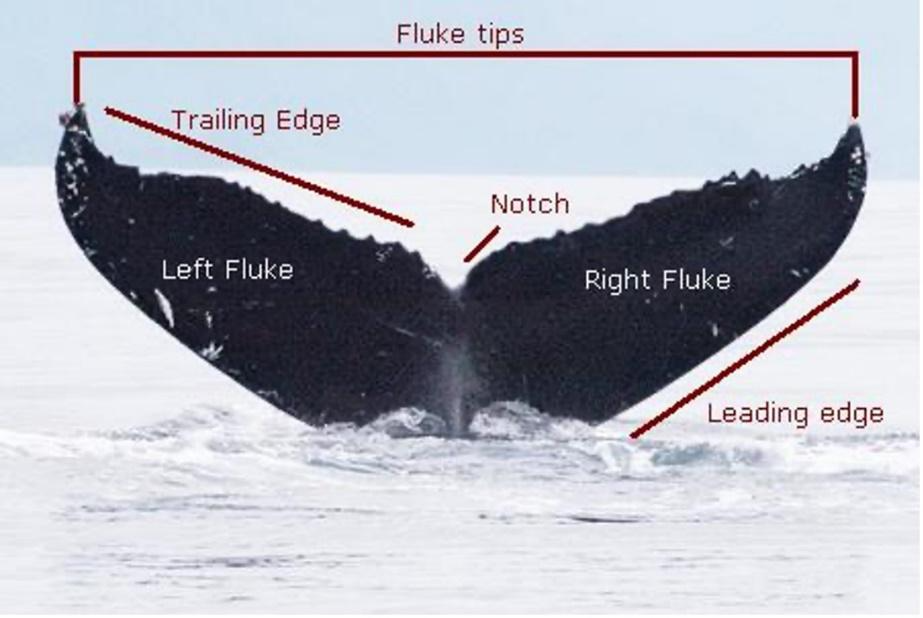
Fig. 14 Annotated picture of a whale fluke (alaskahumpacks.org).
Internally, this fluke is structured with layers like a natural composite sandwich: the skin’s upper layers are made of a cutaneous layer, a subcutaneous blubber layer, a ligamentous layer, and a core of dense fibrous tissue. Towards the trailing edge and tips, however, the fibrous tissue is thin, while the ligamentous layer is thickest. This arrangement dictates the fluke’s capacity to resist tension and limits bending – a species with a thicker trailing edge will have limited bending throughout a stroke (Fish, 1998).
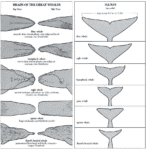
A cetacean’s fluke is unique, with identification factors ranging from pigmentation, ridges, and scarification or rake marks. Moreover, as a cetacean lobtails, one can observe both what type of dolphin, porpoise, or whale it is and its individuality – this is why the primary method of whale identification for marine biologists is fluke identification. Researchers have developed AI recognition software based on these specificities in the architecture of flukes – almost like fingerprint identification.
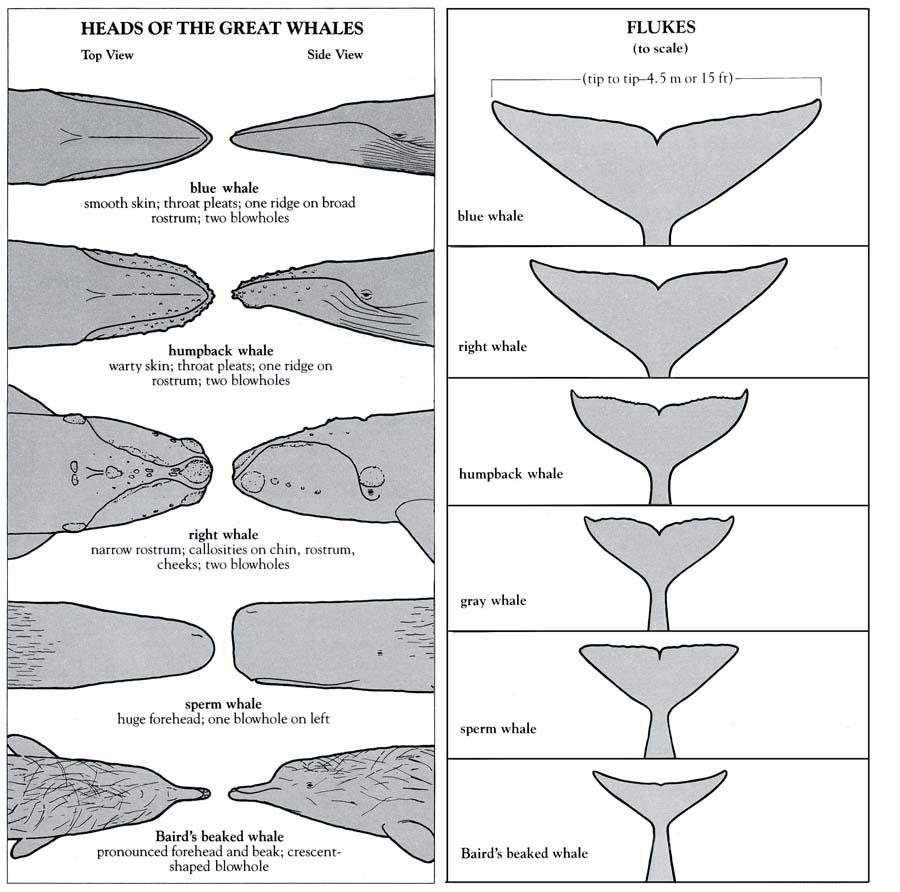
Fig. 15 Diagram of fluke identification amongst the cetacean family (Whaleopedia, 2020).
Since the beginning of the century, field researchers have observed bottlenose dolphins using a novel feeding method called “mud-ring feeding” (Engelby & Powell, 2019; Torres & Read, 2009). This cooperative foraging method consists of two main phases. During the first, one “ring-maker” dolphin strongly flicks the muddy substrate with its fluke to create a circular mud ring (Engelby & Powel, 2019). This is enabled by the strong but flexible material of the dolphin’s fluke. This mud plume encircles the school of fish which approaches the water’s surface, hoping to flee. During the second phase, the dolphins lunge to catch the fish leaping out of the water (Figure 16). This mud-ring feeding method is made clear in a popular youtube video from the BBC earth channel in which one sees a pod of dolphins go through the steps of mud-ring feeding (https://www.youtube.com/shorts/6FmH6YflxAw). This method of feeding has not been limited to a single region. Observations in the Florida bay (U.S.A) and the Chetumal-Corozal Bay (Belize) have shown that these feedings occur at a water depth inferior to 1m and almost always in groups.

Fig. 16 Three dolphins closing in on a mud-plume as fish are seen leaping out of the water trying to escape (Finn, 2018).
Another form of fluke-based foraging is lobtail feeding. According to one network-based diffusion analysis (Jenny Allen et al., 2013), lobtail feeding is a novel variation of the long-practiced bubble feeding; bubble feeding is a social foraging technique where a group of cetaceans swims below a school and blows bubbles in the hopes of stunning and trapping fish (National Marine Sanctuary Foundation, 2020). Contrastingly, “Lobtail feeding consists of striking the water’s surface one to four times with the ventral side of the fluke, followed by a bubble-feeding sequence” (Jenny Allen et al., 2013). With this method, the fish are first trapped by the movement of the fluke striking the water. The deflection of the water by the flukes generates a lift-induced drag: when the fluke slashes through the water it creates a difference in pressure between both ventral and dorsal surfaces of the fluke. In turn, this variation in pressure generates spanwise cross flows which form a spiraling vortical flow (Fish, 1998). Once the fluking ends, the whales blow bubbles where lobtailing occurred to further entrap their prey before eating it.
This behavior can be described as an “inside-loop” since the whale will lobtail the surface of the water and then swim away before swimming back to eat its prey (Hain et al., 1982). Furthermore, this action is repeated multiple times in a loop. In Figure 17, one can see this inside loop materialize as the whale goes through the steps of lobtail feeding.
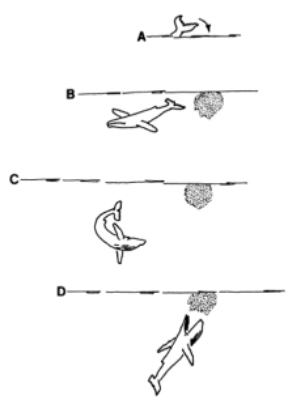
Fig. 17 Diagram representing a lobtail feeding inside-loop (Hain et al., 1982). First the whale lobtails the surface of the water (A) creating a bubble entrapping nearby fish (B). Then the whale swims away and comes back (C) to gulp the entirety of the bubble cloud (D).
Research has shown (Mason T. Weinrich et al., 1992) that this “lobtail feeding” is a hunting practice that has propagated culturally in humpback whales. Specifically, in New England’s Megaptera novaeangliae, the percentage of animals lobtail feeding, which was zero in 1980, increased regularly to 50.6% in 1989. It is hypothesized that the reason for this novel activity is a significant change in prey – to maximize efficiency, different prey require different foraging techniques. Specifically, there was a shift from an overwhelming herring population to a sand lance population. This is consistent as prior to the appearance of this new foraging technique, researchers noticed a significant drop in herrings in those regions (Andrus, 2013).
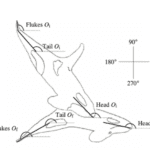
In Northern Norway, killer whales were found using underwater tail slaps to feed on a school of herrings. In this cooperative hunting technique, the whales first herd the school towards the surface and then swim under and around the school, systematically lunging and hitting the animals with their fluke (Domenici et al., 1999). As shown in Figure 18, the attack is composed of 2 phases. The first phase is preparatory with a “positive angle of attack,” according to Domenici et al. (1999). During the second phase, the fluke slaps submerged at a “mean maximum angle of attack of 47 degrees” (Domenici et al. 1999). This method seems to be one of the more cost-efficient methods for the whales, as they do not have to use their entire bodies.
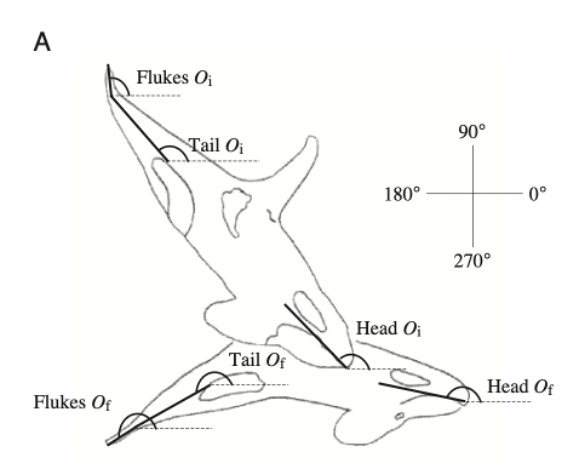
Fig. 18 Diagram of the whale’s position during the 2 phases (initial: Oi, final: Of) of tail slaps (Domeneci et al., 1999).
Bird tails – Natural Versus Sexual Selection and their Aerodynamic Properties
Bird tails are beautiful and diverse, varying in size, shape, and color. A few examples of spectacular tails in birds are: the bird with the longest tail – the male ribbon-tailed astrapia (Figure 19) – whose tail is more than twice the length of its body; Wilson’s bird-of-paradise (Figure 20), noted for its exceptional shape – its tail resembling a curled “W”; and the highly colorful peacock tail, which contains up to 175 feathers of brown, blue, turquoise, and green.

Fig. 19 Picture of the male ribbon-tailed astrapia (Robb, 2012).

Fig. 20 Picture of Wilson’s bird-of-paradise (Serhanoksay, 2011).
Bird tails are composed of bone structure and feathering. The tail feathers are referred to as the rectrices. These rectrices are composed at the core of an interlocking microstructure (Thompson, 2014). The tail, generally shaped in the form of an asymmetrical fan, helps the bird steer by shifting the direction of airflow or helps with balance while on the ground.
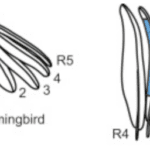
Many features of a bird’s tail play a role in courtship and sexual selection. Hummingbirds, for one, use their tails to sing. This mechanism of sound, intended by male birds, occurs when a hummingbird dives from a great height (Clark & Mistick, 2018). As the bird dives, it spreads its rectrices, which causes the outward-turned edge of the leftmost feather (R5 in Figure 21) to vibrate in the air, causing a sonar frequency (see Figure 21).
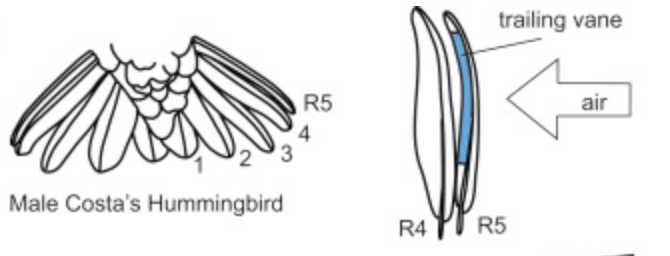
Fig. 21 Left: a diagram of the morphology of a male Costa’s Hummingbird tail; right: a visual representation of the air making the feathers vibrate (Clark & Mistick, 2018).
The bird can control the sound frequency and pressure levels during this dive, thanks to its velocity. The following equation models the frequency in kHz of the sound with respect to the bird’s velocity:
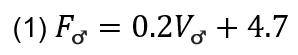
Additionally, the following equation models the perceived frequency of the female bird with respect to her position:
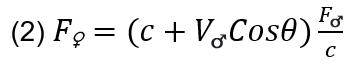
In the above equation, c represents the constant of the speed of sound (c=330 m/s) while θ represents the angle of the bird with respect to the horizontal.
However, it is essential to note that bird tails do not only contain aesthetic and audibly pleasing properties. Indeed, the presence of distinct characteristics in bird tails seems to be accredited to both sexual and natural selection (Balmford, 1995).
First, a bird’s tail can affect a bird’s cost-of-flight. This aerodynamic cost can be separated into two forces. The first, lift, refers to the force perpendicular to the direction of airflow. The lift provided by a bird’s tail is relatively minor compared to the lift provided by the bird’s wings, and an avian tail can only generate lift if its width increases in the direction of the flow (Balmford et al., 1995). The second force (and most related to a bird’s tail), drag, refers to the force opposing the bird’s lift force in the air. This drag is proportional to the lift, with an additional factor depending on the surface area. A longer tail will thus produce more drag than a shorter one making flying slightly more costly (see Figure 22). It is interesting to note that the drag produced by a tail can be useful in breaking the air upon landing.
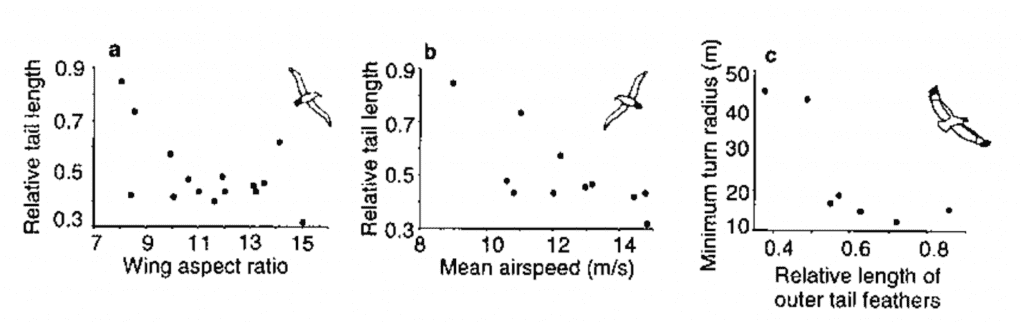
Fig. 22 Relationship between tail morphology and flight performance (Thomas, 1997).
A straight trailing edge is, in fact, the ideal shape for flight regardless of the angle of spread. Indeed, according to delta wing theory, “a forked tail that has a triangular planform when spread to just over 120° gives the best aerodynamic performance, and this may be close to a universal optimum” (Thomas, 1993). Interestingly, delta wing theory is also used to predict the aerodynamics of aircrafts, like the Concorde.
Thus, a bird’s capacity to fan and fork its tail is the most important factor when optimizing its flight. It seems logical that a shorter tail may fork more efficiently than a longer one. Therefore, it can be concluded that birds with shorter tails experience less drag and have an easier time flying.
An interesting experimental manipulation that occurred in the early 1990s on male scarlet-tufted malachite sunbirds confirms that birds with shorter tails are seemingly more agile in flight. Indeed, during this experiment, a group of birds had feather splints added to their tales, while another group had rings added to their legs to increase their mass (Evans & Thomas, 1992). After observing their behavior, the researchers concluded that the birds with shorter tails hawked faster than those with longer tails. Once again, a shorter tail can be assimilated with a more agile bird. Though it may not seem important, efficient hawking is primordial to a bird as it dictates its capacity to catch its prey.
A final interesting naturally selected property is bracing, whereby some birds such as woodpeckers and woodcreepers use the stiff feathers on their tails to brace themselves as they climb trees or tread the ground (Kazilek, 2009; Chen, 2012).
As Balmford et al. (1995) stipulated in their essay in the American Naturalist, while the role and presence of certain characteristics in birds have usually strictly been accredited to sexual selection, many birds’ tails contain naturally selected aerodynamic properties positively affecting their cost of flight, stability, and consequently their ability to nourish themselves and their overall chances of survival.
Conclusion
In conclusion, this paper has examined the extensive variety of structure and function of different tails. These unique functions include interior body temperature regulation through beaver and rat’s hairless tails, resistance to external forces and semi-prehensility through the seahorse tail, semi-prehensility as it relates to habitat for the chameleon tail, propulsive efficiency through the fish’s caudal tail, communication in addition to self-righting and a launching aid through squirrel tails, foraging methods through cetacean flukes, and natural and sexual selection through bird tails. From the extent of these investigations, one can begin to understand the sheer diversity of functions that a tail can fulfill. It has become apparent that the one defining characteristic of a vertebrate tail is its location in the caudal region of the body, while a discussion of structure and function indicates an impressive divergence. One can also see how environmental factors underline certain key adaptations in the tail’s design that have now become essential for each animal investigated. Finally, we can also observe the ingenuity of certain designs, which could very well serve as inspiration for future scientific and engineering developments or simply help improve existing ones. No shortage of inspiration can be derived from observing the results of nature and evolution at work.
References
Alaskahumpbacks.org. (n.d.). Annotated image of a whale fluke. How to Identify a Humpback Whale by Matching Photographs of the Flukes. Retrieved October 4, 2022, from https://www.alaskahumpbacks.org/matching.html
Balmford, A. (1995, December). How Natural Selection Shapes Birds’ Tails. The American Naturalist, 146(6), 848–868. https://doi.org/10.1086/285828
Balmford, A., Thomas, A. L. R., & Jones, I. L. (1993, February). Aerodynamics and the evolution of long tails in birds. Nature, 361(6413), 628–631. https://doi.org/10.1038/361628a0
Bergmann, P.J., Lessard, S. and Russell, A.P. (2003), Tail growth in Chamaeleo dilepis (Sauria: Chamaeleonidae): functional implications of segmental patterns. Journal of Zoology, 261: 417-425. https://doi.org/10.1017/S095283690300428X
Blumstein, D. T. (2007). Feeling the heat: Ground squirrels heat their tails to discourage rattlesnake attack. Proceedings of the National Academy of Sciences of the United States of America, 104(36), 14177-14178. doi:10.1073/pnas.0707286104
Clark, C. J., & Mistick, E. A. (2018b, April 23). Diagram from article Strategic Acoustic Control of a Hummingbird Courtship Dive. Current Biology. https://doi.org/10.1016/j.cub.2018.03.021
CJ Kazilek. (2009, September 30). 23 Functions of Feathers. ASU – Ask A Biologist. Retrieved October 4, 2022 from https://askabiologist.asu.edu/content/23-functions-feathers
Costa, D.; Palmieri, G.; Palpacelli, M.-C.; Scaradozzi, D.; Callegari, M. Design of a Carangiform Swimming Robot through a Multiphysics Simulation Environment. Biomimetics 2020, 5, 46. https://doi.org/10.3390/biomimetics5040046
Domenici, P., Batty, R. S., Simila, T., & Ogam, E. (2000). Killer whales (Orcinus orca) feeding on schooling herring (Clupea Harengus) using underwater tail-slaps: Kinematic analyses of field observations. Journal of Experimental Biology, 203(2), 283–294. https://doi.org/10.1242/jeb.203.2.283
DOMENICI, P., Batty, R. S., Similä, T., & Ogam, E. (1999, December 22). Diagram from kinematics of killer whale tail-slaps. https://cob.silverchair-cdn.com/cob/content_public/journal/jeb/203/2/10.1242_jeb.203.2.283/1/283.pdf?Expires=1667714541&Signature=xRJMq2u-G6Ado7EiFUxaWi~frY7oC99aNagW~w5CIu3Q3fkf2PSNv3N4L4bH9ccMINCkOj5dz2ONkG1B87-vYC87NCTqZ5tTaG6piZD5aLT2s6jlXMn-AH5EciZexTKJvqN4mYc5Tj4WODhXM-4XabVk~B8oKM8rY7g8wxpkAJjoQxh6Su2b93aKPTrOSOletInu9BB6jgmdjVhtw2rfLXMtIGoCozOIHThd6C84JpQcXjQMZT3b0LVQlFi9YF6J127A36SnrS3kd5SG9PJn-icOecao7PokLlwZZC2ivRSy2yRybPwm50VmRD-r4x-J5YxyfnIzHVx0YU-bN9wYZQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA
Essner, R. L., Three-dimensional launch kinematics in leaping, parachuting and gliding squirrels. J Exp Biol 15 August 2002; 205 (16): 2469–2477. doi: https://doi.org/10.1242/jeb.205.16.2469
Evans, M. R., & Thomas, A. L. R. (1992). The aerodynamic and mechanical effects of elongated tails in the scarlet-tufted malachite sunbird: Measuring the cost of a handicap. Animal Behaviour, 43(2), 337–347. https://doi.org/10.1016/s0003-3472(05)80229-5
Everything You Need To Know About Feathers. (2013, December 18). Bird Academy • the Cornell Lab. Retrieved October 4, 2022, from https://academy.allaboutbirds.org/feathers-article/
File:Wilson’s Bird of Paradise Best.jpg. (2020, December 18). Wikimedia Commons, the free media repository. Retrieved 02:53, October 5, 2022 from https://commons.wikimedia.org/w/index.php?title=File:Wilson%27s_Bird_of_Paradise_Best.jpg&oldid=519687318.
Finn, Q. (2018, June 26). Mud ring dolphin feeding. Twitter. https://twitter.com/Quad_Finn/status/1011765634793811968/photo/1
Fish, F. E. (1998). Biomechanical perspective on the origin of cetacean flukes. The Emergence of Whales, 303–324. https://doi.org/10.1007/978-1-4899-0159-0_10
Fukushima, T., Siddall, R., Schwab, F., Toussaint, S. L. D., Byrnes, G., Nyakatura, J. A., Jusufi, A., Inertial Tail Effects during Righting of Squirrels in Unexpected Falls: From Behavior to Robotics, Integrative and Comparative Biology, Volume 61, Issue 2, August 2021, Pages 589–602, https://doi.org/10.1093/icb/icab023
Georgia Marine Mammal Stranding Database. (n.d.). Feeding behavior of cetaceans. GAMMS: Behavior of Marine Mammals. Retrieved October 4, 2022, from https://marinemammal.uga.edu/index.php?page=information%2Fbehavior
Hain. (1982). Diagram from fishery Bulletin, Volume 80, Issue 2. https://books.google.ca/books?hl=en&lr=&id=4NEEDIQs1VIC&oi=fnd&pg=PA259&dq=fluke+bubble+feeding&ots=tiCNAFqOYt&sig=vbMxijUNuxlL46tYgJsk9FNjFaU#v=onepage&q=fluke%20bubble%20feeding&f=false
Hayssen, V., Patterns of Body and Tail Length and Body Mass in Sciuridae, Journal of Mammalogy, Volume 89, Issue 4, 15 August 2008, Pages 852–873, https://doi.org/10.1644/07-MAMM-A-217.1
Hersek, M. J., & Owings, D. H. (1993). Tail flagging by adult california ground squirrels: A tonic signal that serves different functions for males and females. Animal Behaviour, 46(1), 129-138. doi:10.1006/anbe.1993.1168
Hofmann, R., Lehmann, T., Warren, D. L., & Ruf, I. (2021). The squirrel is in the detail: Anatomy and morphometry of the tail in Sciuromorpha (Rodentia, Mammalia). Journal of Morphology, 282( 11), 1659– 1682. https://doi.org/10.1002/jmor.21412
Luger, A. M., Ollevier, A., De Kegel, B., Herrel, A., Adriaens, D. Is variation in tail vertebral morphology linked to habitat use in chameleons? Journal of Morphology. 2020; 281: 229– 239. https://doi.org/10.1002/jmor.21093
Luger, A. M., Vermeylen, V., Herrel, A., & Adriaens, D. (2021). Do substrate roughness and gap distance impact gap-bridging strategies in arboreal chameleons? Belgian Journal of Zoology, 151, 31-45. doi:10.26496/bjz.2021.83
Luger, A. M., Watson, P. J., Dutel, H., Fagan, M. J., Hoorebeke, L. V., Herrel, A., Adriaens, D. Regional Patterning in Tail Vertebral Form and Function in Chameleons (Chamaeleo calyptratus), Integrative and Comparative Biology, Volume 61, Issue 2, August 2021, Pages 455–463, https://doi.org/10.1093/icb/icab125
McCloskey, R. J., & Shaw, K. C. (1977). Copulatory Behavior of the Fox Squirrel. Journal of Mammalogy, 58(4), 663–665. https://doi.org/10.2307/1380016
Mallo, M. (2021). Of necks, trunks and tails: Axial skeletal diversity among vertebrates. Diversity, 13(7) doi:10.3390/d13070289
National Marine Sanctuary Foundation. (1970, February 5). Bubble net feeding: What is it? MarineSanctuary. Retrieved October 2, 2022, from https://marinesanctuary.org/blog/bubble-net-feeding-what-is-it/
Neutens, C., Adriaens, D., Christiaens, J., De Kegel, B., Dierick, M., Boistel, R., & Van Hoorebeke, L. (2014). Grasping convergent evolution in syngnathids: a unique tale of tails. Journal of anatomy, 224(6), 710–723. https://doi.org/10.1111/joa.12181
Norberg, U. M. (1995, February). How a Long Tail and Changes in Mass and Wing Shape Affect the Cost for Flight in Animals. Functional Ecology, 9(1), 48. https://doi.org/10.2307/2390089
Owens NC, Ootsuka Y, Kanosue K, McAllen RM. (2002 sep) Thermoregulatory control of sympathetic fibres supplying the rat’s tail. J Physiol. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2290547/
Porter, M. M., Adriaens, D., Hatton, R. L., Meyers, M. A., & McKittrick, J. (2015). Why the seahorse tail is square. Science, 349(6243) doi:10.1126/science.aaa6683
Ramos, E. A., Santoya, L., Verde, J., Walker, Z., Castelblanco‐Martínez, N., Kiszka, J. J., & Rieucau, G. (2021). Lords of the rings: Mud ring feeding by bottlenose dolphins in a Caribbean estuary revealed from sea, Air, and space. Marine Mammal Science, 38(1), 364–373. https://doi.org/10.1111/mms.12854
Rashid, D. J., Chapman, S. C., Larsson, H. C., Organ, C. L., Bebin, A. G., Merzdorf, C. S., Bradley, R., & Horner, J. R. (2014). From dinosaurs to birds: a tail of evolution. EvoDevo, 5(1), 25. https://doi.org/10.1186/2041-9139-5-25
Robb, L. (2012, January 6). Astrapia mayeri (male). Animal a Day! http://animaladay.blogspot.com/2012/01/ribbon-tailed-astrapia.html
Rundus, A. S., Owings, D. H., Joshi, S. S., Chinn, E., & Giannini, N. (2007). Ground squirrels use an infrared signal to deter rattlesnake predation. Proceedings of the National Academy of Sciences of the United States of America, 104(36), 14372-14376. doi:10.1073/pnas.0702599104
Santo, V. D., Goerig, E., Wainwright, D. K., Akanyeti, O., Liao, J. C., Castro-Santos, T., & Lauder, G. V. (2021). Convergence of undulatory swimming kinematics across a diversity of fishes. Proceedings of the National Academy of Sciences of the United States of America, 118(49) doi:10.1073/pnas.2113206118
Steen, I., Steen, J. B. (1965).Thermoregulatory importance of the beaver’s tail. Retrieved October 1, 2022, from https://www-sciencedirect-com.proxy3.library.mcgill.ca/science/article/pii/0010406X6590352X
Subaru. (n.d.). Diagram of fluke identification amongst the cetacean family. Whaleopedia. Retrieved October 2, 2022, from https://whaleopedia.org/enter/introduction-continued/
Thomas, A. L. R. (1997, April 1). On The Tails of Birds: What are the aerodynamic functions of birds’ tails, with their incredible diversity of form? BioScience, 47(4), 215–225. https://doi.org/10.2307/1313075
Thomas, A. L. R. (1997a, April). Correlations between tail morphology and measured flight performance. BioScience. https://doi.org/10.2307/1313075
Tolley, K. A., Townsend, T. M., & Vences, M. (2013). Large-scale phylogeny of chameleons suggests african origins and eocene diversification. Proceedings of the Royal Society B: Biological Sciences, 280(1759) doi:10.1098/rspb.2013.0184
Weinrich, M. T., Schilling, M. R., & Belt, C. R. (1992). Evidence for acquisition of a novel feeding behaviour: Lobtail feeding in humpback whales, Megaptera novaeangliae. Animal Behaviour, 44(6), 1059–1072. https://doi.org/10.1016/s0003-3472(05)80318-5
Wikimedia Foundation. (2022, September 1). Fur. Wikipedia. Retrieved October 1, 2022, from https://en.wikipedia.org/wiki/Fur#:~:text=Fur%20is%20a%20thick%20growth,that%20keeps%20the%20animal%20warm
Zhong Y, Wu J, Wang C, Li Y, Song J. Hydrodynamic effects of the caudal fin shape of fish in carangiform undulatory swimming. Proceedings of the Institution of Mechanical Engineers, Part C: Journal of Mechanical Engineering Science. 2022;236(12):6385-6394. doi:10.1177/09544062211069007
Reef Builders. 2 Views of a cylix tupareomanaia pipehorse. New Zealand’s new Species of Pygmy Pipehorse, Cylix tupareomanaia. Retrieved October 23, 2022, from https://reefbuilders.com/2021/09/20/new-zealands-new-species-of-pygmy-pipehorse-cylix-tupareomanaia/
Wikimedia Foundation. (2022, October 4). Seahorse. Wikipedia. Retrieved October 23, 2022, from https://en.wikipedia.org/wiki/Seahorse