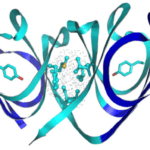Introduction
In “The Optical Properties of Camera Eyes” the optical properties of the camera-type eye and how light can properly converge light onto the retina to endow an organism with a clear picture of its surroundings were discussed (Gedik et al., 2020). To summarize, the camera eye of many animal species may adjust its focal length through a process known as accommodation; this process allows an organism to clearly focus on an object, whether it be located close or far away. However, the complexity of the eye spans beyond its optical properties; millions of years of evolutionary adaption have provided the eye with a highly specialized molecular structure and biochemical composition that not only permits the transformation of light into chemical signals that can be interpreted by the brain, but also provides the eye with mechanisms through which it can protect itself from chemical damage. We will start off this paper with an explanation of how nerve impulses are created from light hitting retinal cells in animals. We will then move on to the cellular and molecular structure of the lens to ultimately end with a section on the enzymatic defensive mechanisms that exist in the cornea. As you can see in Fig. 1, we will actually be following the inverse path that light takes in the eye to explain the cause-and-effect relation between the various ocular components.
The Chemistry of Photoreception
The visual pathway is initiated by light coming from or bouncing off an object, which has an activation effect on the photoreceptors in the retina. These photoreceptors are essential to generate a nerve impulse to the brain (Casiday and Frey, 2000). The retina contains millions of these cells, which are divided into two categories: cones and rods.
Cones and Rods: A Collaborative Effort
While evolution has permitted the adaptation of today’s animal species to their respective biological needs, it is obvious that a “perfectly designed species” does not exist. Investigating more specifically the many species in the animal kingdom, one can notice that in terms of vision at night, most animals are unable to distinguish colors. The reason behind this phenomenon is the imperfect design of photoreceptors present in the retina (Manning and Brainard, 2009). To reiterate, it is important to know that photoreceptor cells are categorically organized into cones and rods. The formers provide sharpness of images and the latter are essential for night vision. However, even though rods (Fig. 2) are essential for night vision, they do not detect colors (Chudler, 2017). On the other hand, cones are not efficient when the brightness of the surrounding environment is low. Consequently, the design flaw of photoreceptors resides in the fact that the cones and rods inexorably complete each other; taking only one at a time will not grant good vision in every situation.

An Overview of Photoreception
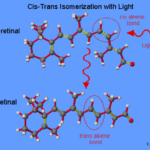
Looking more in detail at the outer layers of the retinal photoreceptors, there are proteins bound to the chromophore 11-cis-retinal. A chromophore is an atom that is responsible for the color displayed by a compound. “The color arises because the extended π system absorbs a certain wavelength of visible light and excites a π electron from its ground state to a higher-energy orbital” (Kuball et al., 2017). When the chromophore is exposed to light, it undergoes an isomerization, also known as a change in molecular arrangement, from cis to trans, which will be further developed in the next sections. This isomerization results in the modification of the protein shape and, further, into a chain of biochemical reactions.
The closing of sodium ions channels (Na+) in the cell membrane is the direct outcome of the abovementioned changes. As a consequence of this closing, the inside of the cell becomes more negative while the outside becomes more positively charged; a large potential difference is thus built across the plasma membrane. The potential difference travels as an electrical impulse at the synaptic terminal where a nerve cell carries this impulse to the brain. In the brain, the visual information is interpreted (Casiday and Frey, 2000).
Isomerization in Detail
Cis-retinal is isomerized to all-trans retinal when a photon of light comes in contact with a rod cell. As can be seen in Fig. 3, the structural difference between the cis and trans isomer resides in the arrangement of molecules around the double bound. In cis-retinal, the hydrogens, illustrated as the white compounds in the aforementioned figure, are on the same side of the double bond, which is illustrated in yellow. When isomerization occurs, the hydrogens get placed on opposite sides of the double bond. As a result of this structural change, the cis-retinal, originally in a bent form, becomes more linear in the trans-retinal conformation (Ophardt, 2020). As explained by Rachel Cassidy, “when an atom or molecule absorbs a photon, its electrons can move to higher-energy orbitals, and the atom or molecule makes a transition to a higher-energy state. In retinal, absorption of a photon promotes a p electron to a higher-energy orbital (a p-p* excitation). This excitation “breaks” the p component of the double bond, thus allowing free rotation about the bond between carbon atom 11 and carbon atom 12. Thus, when 11-cis-retinal absorbs a photon in the visible range of the spectrum, free rotation about the bond between carbon atom 11 and carbon atom 12 can occur and the all-trans-retinal can form” (Casiday and Frey, 2000).

The last important consideration to be made regarding the isomerization process is that it occurs in the presence of light. In fact, while in the presence of light, isomerization occurs about 50 % of the time; contrarily, in the dark, spontaneous isomerization occurs only once in 1000 years (Casiday and Frey, 2000).
Effect of Isomerization on Proteins in the Eye
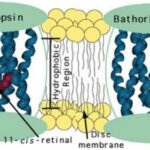
The ultimate effect of isomerization is that it modifies proteins in the rod photoreceptors to change their shape. The generation of a nerve impulse is the direct result of this modification of shape. When looking closely at the rod photoreceptors, there exists a complex known as rhodopsin. The latter is composed of a protein called opsin and the chromophore 11-cis-retinal. Have you ever wondered why some animals cannot see ultraviolet light? Isolating 11-cis-retinal, which has a maximum absorbance in the ultraviolet part of the spectrum, animals should be able to see UV light. However, the maximum absorbance of rhodopsin is 500 nm, which is in the green part of the absorbance spectrum.
It is primordial to recall that living entities with the capacity of vision only see colored objects as they are because of the reflected light, but not the colors absorbed. For example, when sunlight is shined on a green leaf, the violet, red and orange wavelengths are absorbed. The reflected wavelengths appear green. “In each case the complementary colors to the ones absorbed are the ones seen” (“Seeing Colors” | Harper College, 2016).

Integrating this concept back to our discussion, it is essential to note that rhodopsin absorbs in the ultraviolet region of the spectrum; however, the lens of the eye absorbs most ultraviolet light. Consequently, most of the ultraviolet light is prevented from reaching rhodopsin, and the little that does is absorbed by this protein in the retina; this is why most of the animal kingdom cannot see ultraviolet light (Casiday and Frey, 2000).
Formation of Bathorhodopsin
When light is absorbed by rhodopsin, 11-cis-retinal molecules undergo a reaction which isomerizes them to the trans structure. The protein constructed of rhodopsin and the all-trans configuration is referred to as Bathorhodopsin. A problem arises from following the isomerization: the trans structures, which have a linear shape, do not fit well into the protein structure and, consequently, they undergo folding and twisting. However, the twisted conformation is extremely energetically unfavorable, and the latter leads to the expulsion of the chromophore from the protein (Yoshizawa and Wald, 1963).

Finally, to get a nerve impulse to the brain, an essential intermediate, called metarhodopsin II, has the task to activate an enzyme which launches a signal transduction cascade leading to the production of a nerve impulse to the brain.
UV Spectrum Vision in Certain Animals
Although some members of the animal kingdom are unable to see ultraviolet light, a select few, such as certain fish, insects and mammals, have developed this trait. The reason behind this particular adaptation is still unknown to scientists, but some hypotheses have been suggested. In fact, the ability to see UV light might be useful for the survival and reproduction of a many animal species. For a first example, we can look at reindeers; analyzing the migration of reindeers to the Antarctic about 10 000 years ago, it is evident that they have been able to adapt quickly and efficiently. As explained Jessica Griggs, the snow in the Arctic has an impressive reflective property in regard to UV light, only 10 % is absorbed by frozen wastes (Griggs, 2011). The diet of reindeers is mainly composed of lichens, which are abundant in the Antarctic area. These slow-growing plants absorb UV light and therefore appear black to the reindeers in comparison to the surrounding snow and ice.

With regards to insects, butterflies are another species that may see UV light. They use this ability to find appropriate mates for reproduction; this is executed through the production of specific UV markings by certain butterflies, such as the Cleopatra species. Additionally, these marking allow them to create effective disguises that aid them in their survival. In fact, Cleopatra butterflies can, with the help of their UV markings, take on the appearance of certain predators (McFadden 2020).

All in all, it is clear that without retinal photoreception, the vision-equipped organisms of the animal kingdom would be unable to use surrounding light to their advantage. However, you are probably wondering: how does light even get to the retina uninterrupted? As it was mentioned in the introduction, it first must pass through the lens, which has as a primary function to properly direct light onto the retina. Consequently, it is crucial to discuss how the biological design of the lens allows it to complete its role with incredible efficiency.
The Anatomy and Architecture of the Ocular Lens
The lens is one of the most crucial components of the eye, playing a vital role in the refraction of light for eye accommodation. It consists of the lens capsule, the lens epithelium, and the lens fibers. The lens fibers, also called fiber cells, make up most of the lens’ internal structure. Additionally, they do not possess any organelles; this includes a lack of a vascular system, a lymphatic system, or a nerve supply to support itself. Thus, the lens depends on the aqueous humor in front of it to receive nutrients and remove waste that is produced (Hudson and Graubart, 2020). This section will focus on the lens fibers’ lack of organelles and its importance for the proper functioning of this ocular component. However, it is essential to first discuss the development of the lens to better understand its function.

The Development of the Lens
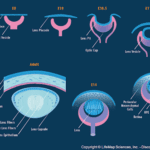
For this subsection, we will be repeatedly referring to Fig. 9 to better illustrate and explain the different stages of lens formation. The development of the lens in an embryo starts with induction. Early in embryo development, three types of germ layers exist: the ectoderm, the mesoderm, and the endoderm (“Development of the Eye part 2; Development of the Lens (Ophthalmology by J.D)” | Medicose lectures by J.D | YouTube, 2019) (Fig. 8). An outward growth of the forebrain, which is also called the optic vesicle, triggers the morphogenesis of the lens. Since scientists know more about the process of induction than the molecules causing it, a “presumptive lens ectoderm” is assumed to exist close to the optic vesicle (McAvoy et al., 1999) (refer to E8 of Fig. 9).
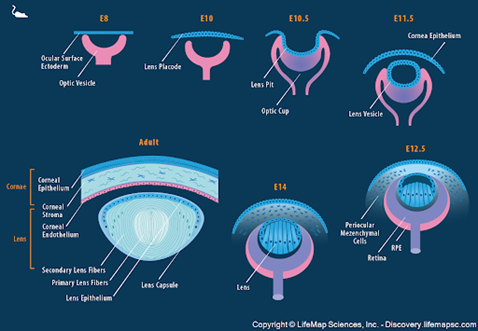
The optic vesicle does not touch the presumptive lens, but their cytoplasmic processes initiate their interaction. The ectoderm gets thicker and becomes the lens placode (McAvoy et al., 1999) (refer to E10 of Fig. 9). The placode folds on itself to form a cavity with the optical vesicle, which forms the lens pit (refer to E10.5 of Fig. 9). This invagination continues until the cavity separates from ectoderm through the apoptosis of the cells at the point of invagination (“Development of the Eye part 2; Development of the Lens (Ophthalmology by J.D)” | Medicose lectures by J.D | YouTube, 2019). Apoptosis refers to the programmed death of cells (Alberts et al., 2002) and, in lens development, results in the formation of the structure of this ocular component (“Development of the Eye part 2; Development of the Lens (Ophthalmology by J.D)” | Medicose lectures by J.D | YouTube, 2019). This concludes the induction phase.
The next phase in lens development is the differentiation of its cells. The lens moves inside the optic cup, a crescent like structure facing the opening of the eye, as can be seen in Fig. 9. It then develops an anterior wall (the front of the lens) and a posterior wall (the back of the lens); the anterior portion faces the opening of the optic cup, which will become the opening of the eye, while the posterior wall faces the retina (Hudson and Graubart, 2020). Additionally, since the lens is formed from the ectoderm, it consists of cuboidal epithelial cells (Fig. 10).
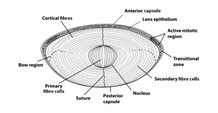
The cells on the posterior wall of the lens elongate and lose their nuclei to form the primary lens fibers, which make up the nucleus of the lens (Hudson and Graubart, 2020). On the other hand, the cells of the anterior wall contain the lens stem cells. They elongate similarly to form the secondary lens fibers. The lens stem cells keep differentiating throughout the lifespan of the organism, so new layers of secondary lens fibers are continuously added to the equator. As can be seen in Fig. 10, this region actively participates in mitotic cell division; it is called the germinative zone (McAvoy, 1999). This contributes to the growth of the lens, which adopts an onion-like shape. As new fibers are added to the outside of the lens, the older remaining fibers are pushed inwards. In addition to the growth of the lens, the outermost membrane of the cuboidal epithelium, called the basement membrane, thickens to form the lens capsule (“Development of the Eye part 2; Development of the Lens (Ophthalmology by J.D)” | Medicose lectures by J.D | YouTube, 2019) (Fig.10).
Differentiation and Transparency
One of the most important functions of the lens involves its ability to refract light rays so that they fall on the fovea of the retina. This ability of the lens to adapt its shape depending on the incoming rays’ angle is called eye accommodation, which was discussed in the previous paper. For the lens to be able to refract light, light rays must cross the lens without being obstructed. As such, the lens, and thus the lens fibers making up most of its internal structure, must be transparent.
The cytoplasm alone has a refractive index of around n = 1.37. For comparison, water has a refractive index of n = 1.33. However, when the cytoplasm has organelles in it, the refractive index increases dramatically (Bassnett, 2009). To prevent light rays from being scattered, the lens employs a variety of strategies, including the degradation of fiber cell organelles.
The Loss of Organelles During Differentiation
The main way in which the effect of light scattering is minimized by the lens is through fiber cells losing their organelles during cell differentiation. Because new lens fibers are continuously added onto older lens fibers, each fiber cell is at different stages of differentiation at any given time. For example, the innermost lens fiber cells lack their nuclei, as the chromatin in the nucleus is disintegrated. However, in the newly added outer lens fibers, nuclei exist; these cells consequently adopt a more spherical shape before the disintegration of their nucleus. Research on cattle (Dahm et al., 1998) and chicken lenses (Bassnett and Mataic, 1997) show that before the nucleus changes its shape, the nuclear lamina, which is involved in DNA replication and many other nuclear activities, must break down (Bassnett, 2009).
DNA transcription is the first stage of DNA-based based gene expression; lens fiber cells most probably lose their ability to transcribe DNA just before the disintegration of the nucleus (Bassnett, 2009). This became apparent when research conducted by Shestopalov and Bassnett revealed that many lens fiber cells without a disintegrated nucleus may perform DNA transcription (Bassnett, 2009).
The biochemical process of nuclear degradation in mammals involves two types of DNase enzymes with optimum pH acidity: DNase IIα and DNase IIβ. These two DNases are lysosomal enzymes. These types of enzymes are involved in the breakdown of large biomolecules (Cooper, 2019). In this particular case, DNase IIα and DNase IIβ are involved in the degradation of DNA. While these two genetically similar lysosomal enzymes are found in similar quantities, their concentration in lens fiber cells changes during lens fiber differentiation. According to Bassnett (2009), Quantitative Polymerase Chain Reaction measurements show that during differentiation, DNase IIα is decreased while DNase IIβ is increased, causing the creation of a 1:1800 ratio in favor of DNase IIβ. This revealed to researchers that DNase IIβ is the main enzyme taking part in the degradation of the nucleus (Bassnett, 2009).
The two lysosomal enzymes are believed to come in contact with genetic material by first fusing with the nuclear envelope, the membrane of the nucleus, to ultimately get released into the nucleus. This sequence of events resembles autophagy, “a mechanism that clears intracellular superfluous or damaged macromolecules or organelles via the lysosome degradation pathway” (Zhang et al., 2017). Once the enzymes are released, DNase IIα and DNase IIβ cause chromatin to split, which results in the formation of 3′- OH ends; the importance of these ends are discussed below (Bassnett, 2009).
The disintegration of the nucleus occurs differently in different species during embryonic development. In cattle lenses, the chromatin condenses and takes the shape of small balls of genetic material. An experimental technique known as the TUNEL technique can used to determine the DNA’s structural state by tracking the presence of 3’ -OH ends (Bassnett, 2009). Through the TUNEL technique, researchers have been able to determine that these balls of chromatin form a single large structure before disintegration (Bassnett, 2009). In contrast, in chicken lens, the condensed chromatin disappears after some days, without the formation of a single structure. In mice, the chromatin does not condense, but genetic material is let out into the cytoplasm (Bassnett, 2009). Similarly, the time period of disintegration differs between species, with disintegration starting at day 12 of embryonic development in chicken and day 17 in mice (Bassnett, 2009).
After the nucleus is disassembled, the quick decay of ribosomal RNA molecules follows due to their short lifespan. The decay of mRNA takes a little longer than a few weeks (Bassnett, 2009).
In most lens fiber cells, the only organelle that remains after differentiation is the plasma membrane, which possesses a refractive index that is almost equal to the refractive index of the cytoplasm. However, there are some exceptions: not all lens fibers lack their organelles! Some undifferentiated epithelial cells can be found on the periphery of the lens, close to the iris. Because of their location, they have minimal impact on light refraction; rather, their functions involve the regulation of homeostasis and osmotic pressure in the lens (Hudson andand Graubart, 2020). Similarly, a very thin layer of possessing organelles remains on the central lens epithelium. Due to their thin length, they have an almost negligible impact on the passing light rays (Bassnett, 2009).
Indeed, internal lens fiber cells have removed many of the components that could hinder and diverge the passage of light before it reaches the retina. However, these cells are not completely empty either; their cytoplasm are packed with highly specialized crystallin proteins. The optical properties of these proteins were extensively studied in our previous paper. However, their molecular structure is key to providing them with the necessary characteristics to properly execute their refractive functions.
The Molecular Design of Crystallin Proteins
Crystallin proteins constitute the primary structural components of the vertebrate eye lens; they make up approximately ninety percent of the lens’ dry mass and provide this ocular component with the necessary refractive power to properly converge light onto the retina (Horwitz et al., 1999). There are three main types of crystallin proteins that are found throughout the animal kingdom: α-crystallins, β-crystallins, and γ-crystallins. This section of the paper will focus on explaining the molecular design of these lens proteins and how they are crucial to providing an organism with clear vision throughout its lifetime.
An Introduction to Protein Structure
Proteins, also known as polypeptides, compose an incredibly diverse group of biomolecules which can play a variety of roles in an organism. As such, they are unequivocally essential to the functioning of cells possessing a specific role in the body (Haurowitz and Koshland, 2020). In the context of the ocular lens, as discussed in “The Optical Properties of Camera Eyes”, crystallin proteins are the reason behind the refractive nature of lens fiber cells (Gedik et al., 2020).
In their most basic form, known as the primary structure, proteins exist as a specific sequence of amino acids that are bonded to each other through peptide bonds (Haurowitz and Koshland, 2020) (Fig. 11).
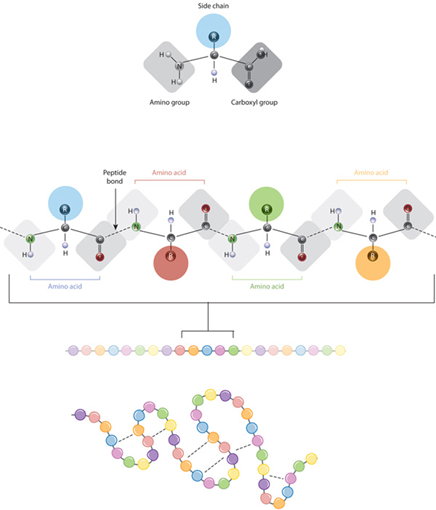
The primary structure of a polypeptide is determined by an organism’s DNA: distinct regions in specific genes contain the code required to synthesize the amino acid sequence through a process known as DNA transcription and mRNA translation (Haurowitz and Koshland, 2020). However, for proteins to be able to conduct their assigned functions, they must fold into specific three-dimensional arrangements determined by interactions between their amino acids (Haurowitz and Koshland, 2020). The secondary and tertiary structures are determined by such interactions and, as can be seen in Fig. 12, the latter is key to forming the folded polypeptide chain (Haurowitz and Koshland, 2020). Additionally, some proteins possess a quaternary structure in which they exist as a combination of many polypeptide chains; it is held together by similar interactions as in the tertiary structure, but these instead form between amino acids of different chains (Haurowitz and Koshland, 2020). A dimer is a protein complex made of two polypeptide chains, a trimer, three, and so forth. In this section, we will be discussing oligomerization, which defines the process of creating an oligomer, a protein consisting of a few to many polypeptide chains, which are also called subunits (Haurowitz and Koshland, 2020)

However, it is crucial to remember that cells are dynamic systems. Proteins may sometimes be subject to various environmental changes, such as variation in pH and temperature, which may result in the disruption of the weak bonds holding the tertiary and quaternary structures together. This process is known as denaturation and results in the unfolding of the protein, as well as the disruption or complete loss of its assigned function (Haurowitz and Koshland, 2020).
The Molecular Design of βγ-Crystallins
A protein family constitutes a group of proteins that perform a similar function, consequently allowing members of a family to interact in similar ways with substances in the surrounding environment (“Protein Structure” | Scitable by Nature Education, 2014). Both β- and γ-crystallins are part of the same protein superfamily of βγ-crystallins (Slingsby et al., 2013). Unfortunately, very little is known about their functions in the ocular lens other than light refraction (Andley, 2007). The atypical but essential refractive properties of these lens proteins were discussed extensively in our previous installment. To summarize, crystallins, notably those of βγ superfamily, have a highly specialized primary structure that is low in alanine, threonine, valine, and leucine amino acids, while possessing large amounts of aromatic tryptophan and tyrosine residues. The unusual amino acid composition of these proteins is due to the formers possessing very low molecular refractive index increments and the aromatic amino acids possessing very high molecular refractive indices (Slingsby et al., 2013).
Recall however that the ocular lens is required to remain clear throughout an organism’s lifetime to prevent vision impairment. Ordinarily, this would be a difficult task considering that there is no protein turnover in fiber cells; defective proteins are unable to be replaced and regenerated. Consequently, crystallin proteins must be extremely soluble and thermodynamically stable, as they can be subject to various stresses, including ultraviolet radiation, that may risk causing their denaturation; if this denaturation were to occur, it would result in the aggregation of unfolded proteins and clouding of the lens (Saha and Das, 2004). Members of the βγ-crystallin family have a very distinctive molecular structure that has evolved as a means of providing these proteins with the necessary properties to resist denaturation and aggregation.
β- and γ-Crystallin chains possess a tertiary structure that forms a symmetrical arrangement of four three-dimensional structures known as Greek key motifs, which are equally separated between two sections, known as domains (Slingsby et al., 2013) (Fig. 13). This motif is one of the primary reasons behind the high stability of these proteins, as it creates highly compact structures with decreased propensity for denaturation (Vendra et al., 2013).
To understand how the Greek key motifs of βγ-crystallins behave, it is first necessary to understand how proteins interact with the surrounding environment. Firstly, recall that the cytoplasm is aqueous in nature and that it easily dissolves hydrophilic compounds (Haurowitz and Koshland, 2020). However, proteins are composed of a variety of hydrophobic and hydrophilic amino acids (Haurowitz and Koshland, 2020). Consequently, the tertiary structure of a cytoplasmic protein will arrange itself in such a way as to minimally expose the hydrophobic residues to the aqueous environment by placing them in the internal regions of the folded protein structure as well as by exposing the hydrophilic regions so they can interact with the surrounding environment (Haurowitz and Koshland, 2020).
As can be seen in Fig. 13, most of the hydrophobic residues of βγ-crystallins, including the aromatic amino acids that supply these proteins with a high refractive index, are effectively concentrated at the center of the polypeptide, allowing for very minimal interactions with the aqueous environment (Slingsby and Clout, 1999). Additionally, the outer regions of these proteins, which are exposed to the cytoplasmic surroundings, are highly concentrated in hydrophilic amino acids; this allows for the formation of ionic interactions and hydrogen bonds with the aqueous environments, thus explaining the incredible solubility of βγ-crystallin proteins (Slingsby et al., 2013).

The Molecular Design of α-Crystallins
As mentioned in “The Optical Properties of Camera Eyes”, it is widely accepted that crystallin proteins in the ocular lens originated from bodily proteins that initially had functions unrelated to light refraction (Gedik et al., 2020). Notably, α-crystallins, which make up approximately 40 % of the protein content of the lens, belong to the family of small heat shock proteins (sHSP) (Haslbeck et al., 2015). Their unique ancestry in comparison to β- and γ-crystallins has endowed them with a function that plays a crucial role in maintaining ocular lens transparency throughout an organism’s lifetime. All members of the sHSP family, including α-crystallins, are capable of behaving as molecular chaperones when nearby proteins are under some form of stress that puts them at risk of denaturing (Saha and Das, 2004).
The ability to chaperone defines the property of some proteins to assist in the proper folding of the tertiary and quaternary structures of nearby polypeptides (Goodsell, “Chaperones”). When proteins get denatured, as mentioned in the introductory descriptions of protein structure, they unfold; this may result in the exposure of their hydrophobic regions. Consequently, when proteins are unfolded, they are prone to interacting with each other and forming aggregates (Saha and Das, 2004). Molecular chaperones act by wrapping around a denatured protein and shielding it from the surrounding environment to ultimately assist it in reforming the weak bonds between its amino acids (Goodsell, “Chaperones”).
In relation to the ocular lens, as we have detailed in our previous work, βγ-crystallins provide the greatest amount of refractive power to fiber cells. However, regardless of their incredible stability and resistance to denaturation, they may still unfold at the exposure of harsh stresses, including oxidation, disulfide bond cleavage and ultraviolet radiation (Saha and Das, 2004). Due to fiber cells being unable to destroy and replace these dysfunctional proteins, α-crystallin chaperones are the only mechanism by which the eye can prevent the clouding of the ocular lens that would result from the aggregation of denatured β-crystallins and γ-crystallins (Haslbeck et al., 1999). As can be seen in Fig. 14, α-Crystallins possess an internal cavity, just as many other molecular chaperones, that can bind to and cover an unfolded protein so as to prevent it from aggregating with other denatured polypeptides (Goodsell, “Chaperones”). In other words, α-crystallins prevent lens clouding by behaving as a “pit” that traps denatured proteins before they begin to aggregate (Andley, 2007).

Just as with the members of the βγ-crystallin family, α-crystallins must also be extremely soluble and thermodynamically stable to resist stress-induced unfolding. However, their origin is distinct from that of the other two ubiquitous proteins; α-crystallins do not organize themselves in the characteristic stabilizing Greek key motif (Zhao et al., 2011). The following question thus naturally arises: what is it about the design of α-crystallins that bestows them such high molecular stability?
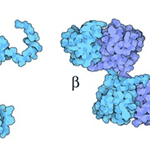
There are two α-crystallin genes that code for their respective homologues of α-crystallin: αA and αB (Horwitz et al., 1999) (Fig. 15). As can be seen in Fig. 14, α-crystallins in the lens exist as oligomeric structures formed through an assemblage of various αA- and αB-crystallin peptide chains (Horwitz et al., 1999). α-Crystallin complexes, which are simply the names given to the complete oligomeric assemblage of the different subunits, are also extremely heterogeneous in size and composition: the various α-crystallin complexes in the lens can be composed of anywhere between 20 and 45 different αA and αB subunits (Haslbeck et al., 2015).

Veritably, the existence of α-crystallin complexes as heterogeneous combinations of αA and αB peptides provides an extensive amount of benefit to the lens. While the two types of α-crystallins do possess similar chemical properties, they still possess a significant degree of difference including, but not limited to, their hydrophobicity, binding ability to denatured proteins, and thermodynamic stability. However, it has been shown that oligomeric α-crystallin complexes are significantly more thermodynamically stable than αA- and αB- crystallins alone (Horwitz et al., 1999). In fact, in the ocular lens, there is roughly a 3:1 ratio of αA and αB crystallin; complexes will form by more-or-less respecting this ratio regardless of their size or number of subunits (Horwitz et al., 1999). This specialized oligomerization condition has evolved due to it being able to provide α-crystallin complexes with the greatest possible degree of thermodynamic stability (Horwitz et al., 1999). Hence, the quaternary structure of α-crystallins endow these protein complexes with extreme stability, consequently allowing them to better complete their functions as molecular chaperones in the ocular lens.
In sum, the molecular designs of crystallin proteins play a key role in providing them with the chemical properties that are crucial to maintaining transparency in the ocular lens. Notably, βγ-crystallins are primarily structural refractive proteins that achieve high thermodynamic stability and solubility through their characteristic Greek key motifs while α-crystallins are molecular chaperones that are stabilized by their heterogeneous oligomeric organization.
We have now extensively studied the lens and its biochemical composition. However, although this ocular component is undeniably crucial to the proper functioning of the eye, it would be unable to work as effectively as it does without the cornea, which is in direct contact with the surrounding environment. Let us consider the following analogy: if an engineer were to design a bridge, it is obvious that they would need to create a system that may hold its own weight; however, the effects of external forces, such as winds, tectonic plate activity in earthquake-prone regions, and such, also need to be considered. An effective system or machine may complete its function while withstanding external effects that may threaten to hinder it. Thus, nature truly proves itself to be a formidable engineer when we consider that it has developed effective biological mechanisms to counteract the many environmental stresses that the eye is exposed to.
Defensive Enzymatic Reactions in the Eye
While we have previously briefly discussed enzymatic activity with the lysosomal enzymes DNase IIα and DNase IIβ, it is important to understand that there are many more enzymes involved in the proper functioning of the eye. In fact, enzymes are crucial to the biological operation of living organisms. They are typically proteins, although some enzymes are RNA-based, that act as catalysts for biochemical reactions (Editors of Encyclopedia Britannica, “Refractive index”). They do so in a wide variety of ways and are present throughout the body; their roles and functions can range anywhere from helping in breaking down food molecules into smaller biochemically useful compounds, to protecting the body against biological threats (Editors of Encyclopedia Britannica, “Refractive index”). This section of the paper will focus on explaining how the enzymatic constitution of eye may protect it from external chemical stresses that could hinder the organism’s vision.
Glutathione Reductase
Oxidative stress is a form of chemical stress that exists as an “imbalance between [free radicals] and antioxidants” (Fink, 2019). Free radicals are atoms or molecules, primarily those containing oxygen, that possess one or more unpaired electrons; these molecules are extremely reactive and can thus cause many unwanted chemical reactions in the body (Krant, 2017). Many different environmental factors can result in the formation of free-radicals, one of which is ultraviolet light (Krant, 2017). In terms of the eye, its frequent exposure to ultraviolet radiation would ordinarily cause the formation of a large number of free radicals. However, nature has engineered these highly specialized ocular structures to effectively combat oxidative stress through the prevalence of a specific bodily enzyme called Glutathione Reductase (GR) (Ganea and Harding, 2006). GR is present in all six biological kingdoms (Kanzok et al., 2001); in the eye, it is an enzyme that is found at every single ocular component that is exposed to light, notably the cornea, the lens, and the retina (Ganea and Harding, 2006). This protein is crucial in defending the eye against oxidative stress; it participates in the neutralization of free radicals to prevent their damaging effects (Kanzok et al., 2001). However, how exactly does Glutathione Reductase assist the eye in protecting itself from the harmful effects of ultraviolet radiation?
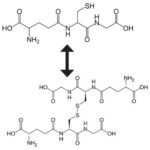
Most cells, including those in the eye, contain significant concentrations of a small antioxidant protein known as glutathione (Pizzorno, 2014). Antioxidants are molecules that may fight free radicals (“Antioxidants” | Harvard School of Public Health 2019); glutathione is a prominent one in the body that is made of three amino acids: cysteine, glycine, and glutamic acid (Pizzorno, 2014). In fact, this molecule exists in two states in cells; reduced, where it is known as GSH and oxidized, where it is known as GSSG (Fig. 16). The following chemical reaction explains how Glutathione’s ability to switch from its reduced state to its oxidized state allows for the neutralization of free radicals, which are represent as R• in their reactive form, and RH in their neutralized form (Wu et al., 2004):
2 GSH + 2 R• \to GSSG + RH

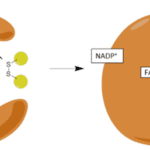
The enzyme Glutathione Reductase is directly involved in regenerating the reduced form of Glutathione (GSH) from its oxidized form (GSSG), thus allowing for the further neutralization of free radicals in cells (Couto et al., 2016). This occurs through an exchange of electrons from the GR enzyme to GSSG, which results in the oxidation of the enzyme and the reduction Glutathione (Berkholz et al., 2008) (Fig. 17). Through this occurrence, the newly reduced Glutathione can go back to trapping other free radicals; this cycle continues over and over in the cell (Couto et al., 2016).

As was mentioned previously, the cornea is the ocular component that is directly exposed to the environment, and consequently is very prone to ultraviolet damage from sunlight (Ganea and Harding, 2006). Consequently, when the cornea is under high oxidative stress due to the formation of a large number of free radicals, the concentration of GR in corneal cells is increased so as to reduce the oxidized glutathione (Ganea and Harding, 2006). Actually, this process also occurs in the ocular lens and the retina, which are also relatively prone to oxidative damage (Ganea and Harding, 2006).
Conclusion
In sum, while the optical properties of the different components of the eye are unequivocally crucial to vision, their biochemical compositions are the reason behind their harmonious and coordinated functionality. The cornea acts as a primary layer of armor to defend the eye against ultraviolet damage that can result from light passing through it. The lens, which is the next destination in the light rays’ trajectory, is made up of specialized fiber cells that lost their organelles to reduce light scattering. These cells are packed with highly refractive proteins known as crystallins, which have developed a durable structure and powerful repair mechanism to combat chemical stresses that could impair the lens’ transparency and functionality. Finally, once light rays have crossed the lens, they arrive at their final destination, the retina, which converts the message carried by photons into chemical signals that can be interpreted by the brain as an image of the surrounding environment. Now, future bioengineers, here is some food for thought: how may we take our newly found knowledge of this complex, almost machine-like, structure created by nature and utilize it in our designs and creations?
Appendix
“Development of the Eye part 2; Development of the Lens (Ophthalmology by J.D).”, 22 May 2019,
Medicose lectures by J.D, YouTube, https://www.youtube.com/watch?v=fmzWYz3gvUE.
References
Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K., & Walter, P. (2002). Molecular biology of the cell. New York: Garland Science.
Alexander, C. (2011). Reindeer see a wonderful world of ultraviolet light. In. constantinealexander.net. Retrieved from https://www.constantinealexander.net/2011/05/reindeer-see-a-wonderful-world-of-ultraviolet-light.html
Andley, U. P. (2007). Crystallins in the eye: Function and pathology. Progress in Retinal and Eye Research, 26(1), 78-98. doi:10.1016/j.preteyeres.2006.10.003
Bassnett, S. (2009). On the mechanism of organelle degradation in the vertebrate lens. Experimental Eye Research, 88(2), 133-139. doi:10.1016/j.exer.2008.08.017
Bassnett, S., & Mataic, D. (1997). Chromatin degradation in differentiating fiber cells of the eye lens. Journal of Cell Biology, 137(1), 37-49. doi:10.1083/jcb.137.1.37
Berkholz, D. S., Faber, H. R., Savvides, S. N., & Karplus, P. A. (2008). Catalytic cycle of human glutathione reductase near 1 A resolution. Journal of Molecular Biology, 382(2), 371-384. doi:10.1016/j.jmb.2008.06.083
Biologywise. (2014). Information About the 3 Germ Layers in Animals. Retrieved from https://biologywise.com/information-about-the3-germ-layers-in-animals
Britannica, E. o. E. (2019, 23 Dec 2019). Refractive index. Retrieved from https://www.britannica.com/science/refractive-index
Casiday, R., & Frey, R. (2000). “I Have Seen the Light!” Vision and Light-Induced Molecular Changes. In: Washington University. Retrieved from http://www.chemistry.wustl.edu/~edudev/LabTutorials/Vision/Vision.html
Chudler, E. H. (2017). The Retina. Retrieved from https://faculty.washington.edu/chudler/retina.html
College, H. (2016). Seeing Colors Retrieved from http://dept.harpercollege.edu/chemistry/chm/100/dgodambe/thedisk/spec/5back3.htm
Cooper, G. M. (2019). The cell : a molecular approach.
Couto, N., Wood, J., & Barber, J. (2016). The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radical Biology and Medicine, 95, 27-42. doi:10.1016/j.freeradbiomed.2016.02.028
Dahm, R., Gribbon, C., Quinlan, R. A., & Prescott, A. R. (1998). Changes in the nucleolar and coiled body compartments precede lamina and chromatin reorganization during fibre cell denucleation in the bovine lens. European Journal of Cell Biology, 75(3), 237-246. doi:10.1016/s0171-9335(98)80118-0
Discovery, L. M. (n.d.). Mouse Lens and Cornea Development. In. discovery.lifemapsc.com. Retrieved from https://discovery.lifemapsc.com/library/images/mouse-lens-and-cornea-development
Education, S. b. N. (2014). Protein Structure. Retrieved from https://www.nature.com/scitable/topicpage/protein-structure-14122136/
Fink, G. (2019). Stress: physiology, biochemistry, and pathology : handbook of stress. Volume 3 Volume 3. Retrieved from http://search.ebscohost.com/login.aspx?direct=true&scope=site&db=nlebk&db=nlabk&AN=1852728
Francisco, U. o. C. S. (2011). Integrated Analysis of Molecular Assemblies Using Chimera: A Demonstration with Alpha Crystallin. In. cgl.ucsf.edu. Retrieved from http://www.cgl.ucsf.edu/chimera/data/acryst2011/acrystdemo.html
Franson, J. (2014). Electromagnetic Spectrum. Retrieved from https://sites.google.com/a/coe.edu/principles-of-structural-chemistry/relationship-between-light-and-matter/electromagnetic-spectrum
Ganea, E., & Harding, J. J. (2006). Glutathione-related enzymes and the eye. Current Eye Research, 31(1), 1-11. doi:10.1080/02713680500477347
Gedik, T., Hassouna, N., Sharma, M., & Yazbek, M. S. (2020). The Optical Properties of Camera Eyes. Bioengineering McGill University. Montreal, Canada. https://bioengineering.hyperbook.mcgill.ca/1292/
Goodsell, D. (2002, Aug 2002 ). Chaperones. Retrieved from https://pdb101.rcsb.org/motm/32
Goodsell, D. (2010). Crystallins. Retrieved from https://pdb101.rcsb.org/motm/127
Griggs, J. (2011). Reindeer gained UV vision after moving to the Arctic. Retrieved from https://www.newscientist.com/article/dn20519-reindeer-gained-uv-vision-after-moving-to-the-arctic/?ignored=irrelevant
Haslbeck, M., Peschek, J., Buchner, J., & Weinkauf, S. (2016). Structure and function of α-crystallins: Traversing from in vitro to in vivo. Biochim Biophys Acta, 1860(1 Pt B), 149-166. doi:10.1016/j.bbagen.2015.06.008
Haurowitz, F., & Koshland, D. E. (2020, 1 Dec 2020). protein. Retrieved from https://www.britannica.com/science/protein
Health, H. S. o. P. (2019). Antioxidants. In. hsph.harvard.edu: Harvard University. Retrieved from https://www.hsph.harvard.edu/nutritionsource/antioxidants/
Horwitz, J., Bova, M. P., Ding, L. L., Haley, D. A., & Stewart, P. L. (1999). Lens alpha-crystallin: function and structure. Eye (London, England), 13 ( Pt 3b), 403-408. doi:10.1038/eye.1999.114
Hudson, L., & Graubart, E. (2020). Lens Anatomy Retrieved from http://cataractcourse.com/lens-anatomy-and-development/lens-anatomy/
Kanzok, S. M., Fechner, A., Bauer, H., Ulschmid, J. K., Müller, H. M., Botella-Munoz, J., . . . Becker, K. (2001). Substitution of the thioredoxin system for glutathione reductase in Drosophila melanogaster. Science, 291(5504), 643-646. doi:10.1126/science.291.5504.643
Krant, J. (2017, 1 Sept 2017). Can Antioxidants Help to Prevent UV-induced Free Radical Damage? Retrieved from https://artofdermatology.com/can-antioxidants-help-prevent-uv-induced-free-radical-damage/
Kruszelnicki, K. S. (2015). Stars look pointy because … Retrieved from https://www.abc.net.au/science/articles/2015/06/16/4253961.htm
Kuball, H. G., Höfer, T., & Kiesewalter, S. (2017). Chiroptical Spectroscopy, General Theory☆. In J. C. Lindon, G. E. Tranter, & D. W. Koppenaal (Eds.), Encyclopedia of Spectroscopy and Spectrometry (Third Edition) (pp. 217-231). Oxford: Academic Press.
Manning, J. R., & Brainard, D. H. (2009). Optimal design of photoreceptor mosaics: why we do not see color at night. Visual Neuroscience, 26(1), 5-19. doi:10.1017/s095252380808084x
McAvoy, J. W., Chamberlain, C. G., de Iongh, R. U., Hales, A. M., & Lovicu, F. J. (1999). Lens development. Eye (London, England), 13 ( Pt 3b), 425-437. doi:10.1038/eye.1999.117
McFadden, C. (2020). 9 Animals That Can Actually See in UV. In. interestingengineering.com. Retrieved from https://interestingengineering.com/9-animals-that-can-actually-see-in-uv
Ophardt, C. (2020, 10 Aug 2020). Cis-Trans Isomerization of Retinal. Retrieved from https://chem.libretexts.org/Bookshelves/Biological_Chemistry/Supplemental_Modules_(Biological_Chemistry)/Photoreceptors/Chemistry_of_Vision/Cis-Trans_Isomerization_of_Retinal
Pizzorno, J. (2014). Glutathione! Integrative Medicine (Encinitas, Calif.), 13(1), 8-12. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4684116/
Rye, C., Wise, R., Jurukovski, V., DeSaix, J., Choi, J., & Avissar, Y. (2016). Proteins. In Biology (pp. 85-94). Retrieved from https://openstax.org/books/biology/pages/3-4-proteins
Saha, S., & Das, K. P. (2004). Relationship between chaperone activity and oligomeric size of recombinant human αA- and αB-crystallin: A tryptic digestion study. Proteins: Structure, Function, and Bioinformatics, 57(3), 610-617. doi:https://doi.org/10.1002/prot.20230
Schulte, C. (2018). Zebrafish Help Cure Blindness. Retrieved from https://azretina.sites.arizona.edu/node/342
Séret, B., Blaison, A., Dagorn, L., & Filmalter, J. (2012). Fin to carcass weight ratio for the silky shark Carcharhinus falciformis in the western Indian Ocean.
Slingsby, C., & Clout, N. J. (1999). Structure of the crystallins. Eye (London, England), 13 ( Pt 3b), 395-402. doi:10.1038/eye.1999.113
Slingsby, C., Wistow, G. J., & Clark, A. R. (2013). Evolution of crystallins for a role in the vertebrate eye lens. Protein Science, 22(4), 367-380. doi:10.1002/pro.2229
Splettstößer, T. (2007). Glutathione Reductase. In G. reductase.png (Ed.), (Vol. 857 × 755, pp. Cartoon representation of the atomic structure of Glutathione Reductase, based on PDB ID: 1GRE). commons.wikimedia.org
Vendra, V. P., Agarwal, G., Chandani, S., Talla, V., Srinivasan, N., & Balasubramanian, D. (2013). Structural integrity of the Greek key motif in βγ-crystallins is vital for central eye lens transparency. PloS One, 8(8), e70336. doi:10.1371/journal.pone.0070336
Wu, G., Fang, Y. Z., Yang, S., Lupton, J. R., & Turner, N. D. (2004). Glutathione metabolism and its implications for health. Journal of Nutrition, 134(3), 489-492. doi:10.1093/jn/134.3.489
Yoshizawa, T., & Wald, G. (1963). Pre-lumirhodopsin and the bleaching of visual pigments. Nature, 197, 1279-1286. doi:10.1038/1971279a0
Zhang, Y., Choksi, S., & Liu, Z. (2017). Chapter 4 – Role of Autophagy in the Transition From Monocyte to Differentiation. In M. A. Hayat (Ed.), Autophagy: Cancer, Other Pathologies, Inflammation, Immunity, Infection, and Aging (pp. 149-159): Academic Press. Zhao, H., Brown, P. H., Magone, M. T., & Schuck, P. (2011). The molecular refractive function of lens γ-Crystallins. Journal of Molecular Biology, 411(3), 680-699. doi:10.1016/j.jmb.2011.06.007