Abstract
Pincers, or chelae, are a highly advantageous biological tool possessed by many arthropods. The chelae are useful and necessary for the survival of many species from arachnids to crustaceans. The scorpion’s pincers have a variety of purposes, including grasping and capturing prey, and deterring predators. Chelae are a highly functional and morphologically diverse group of structures found in crustaceans, with uses ranging from breaking coconuts in coconut crabs to acting as a protective gate to the shell it inhabits in hermit crabs. To complete these tasks the chelae are composed of a reinforced hierarchical layered structure, with much of the composition based upon Bouligand architecture. However, there is some variable composition between different components of the chelae from the cuticle to the muscles, that allows each component to have its own unique properties and undergo different processes such as molting and mineralization. This structural design creates a tool that can endure the powerful stresses created during the chela’s adduction as well as environmental damage. The following paper explores the details of the pincer’s chemical structure, processes, and possible applications.
Introduction
Biological tools have been developed and refined over hundreds of thousands of years to maintain species’ survival. As natural resources are limited, competition among species is inevitable. Darwin’s mechanism of natural selection selects features that increase a species’ chances of survival and reproduction cyclically as the features are passed down through generations. These traits continue to pass down until they are no longer favorable due to an external change, or a more opportune feature is introduced. When opportune features enter a gene pool, they lead to adaptation and evolution in a species (Ashraf & Sarfraz, 2016). One example of this is the emergence of pincers or chelae.
Chelae are significant in the later evolution of crustaceans. These pincers were produced by asymmetrical growth in their limbs’ second to last joint. This growth became the fixed part of the pincer, while the last joint was allowed movement in order to close and grip. The evolution of these organs led to a multitude of adaptation possibilities for the arthropods who used them and further increased the diversity of these species (Clarkson, 1985). One of the earliest cases of pincers is shown in Figure 1, the fossil of Sidneyia inexpectans, a pincer-wielding species from approximately 5 million years ago. The ancient arthropod appears to have at least three pincer-like structures, which may have a grasping function. These early pincers may have had the capacity to push and direct prey and food for ingestion (Zacaï et al., 2016). These pincers are appendages that can be seen on the upper left and right of the major body of the species.

Fig 1. General morphology of Sidneyia inexpectans (Zacaï et al., 2016)
Over the past 500 million years, pincers have significantly evolved in many different species. Chelae are seen frequently in modern arthropods, from crustaceans to arachnids and insects; pincers have a myriad of functions in today’s world. Chelae-wielding species use this biological adaptation as a weapon, defense, and sexual ornaments.
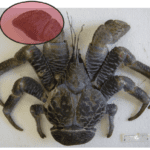
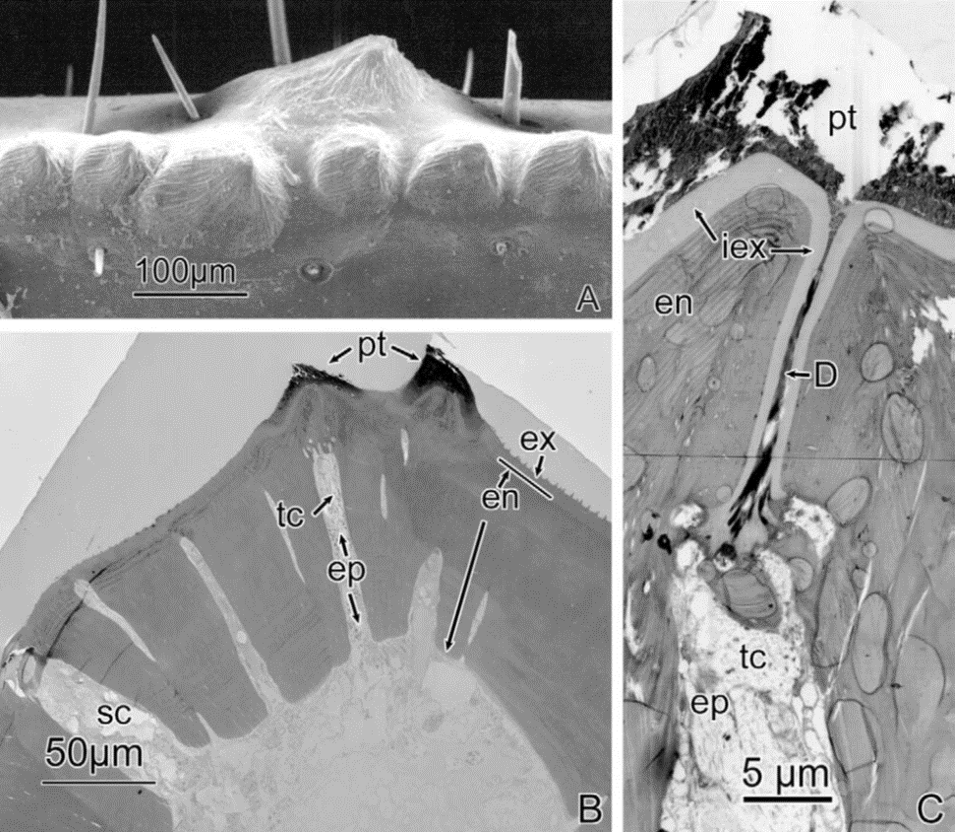
Chelae are most often used as weapons and hunting tools by chelae-possessing organisms. These weapons are often utilized to capture or seize prey via mechanical or chemical means. A notable species that uses its chelae as a weapon is the scorpion. Scorpions are predatory arachnids that use their chelae to seize their prey and orient their prey for consumption (Simone & Van Der Meijden, 2021). Many crustaceans also use their chelae for hunting, such as the Birgus latro, or the coconut crab. As the largest terrestrial crustacean, the coconut crab’s pincers are enormous. They have adapted to acquire food in plants with tough exteriors and as a weapon against other terrestrial species. These mighty creatures are known to have the strongest chelae with the ability to exert forces up to 800 N. These substantial chelae of the coconut crab are shown in Figure 2 (Oka et al., 2016).
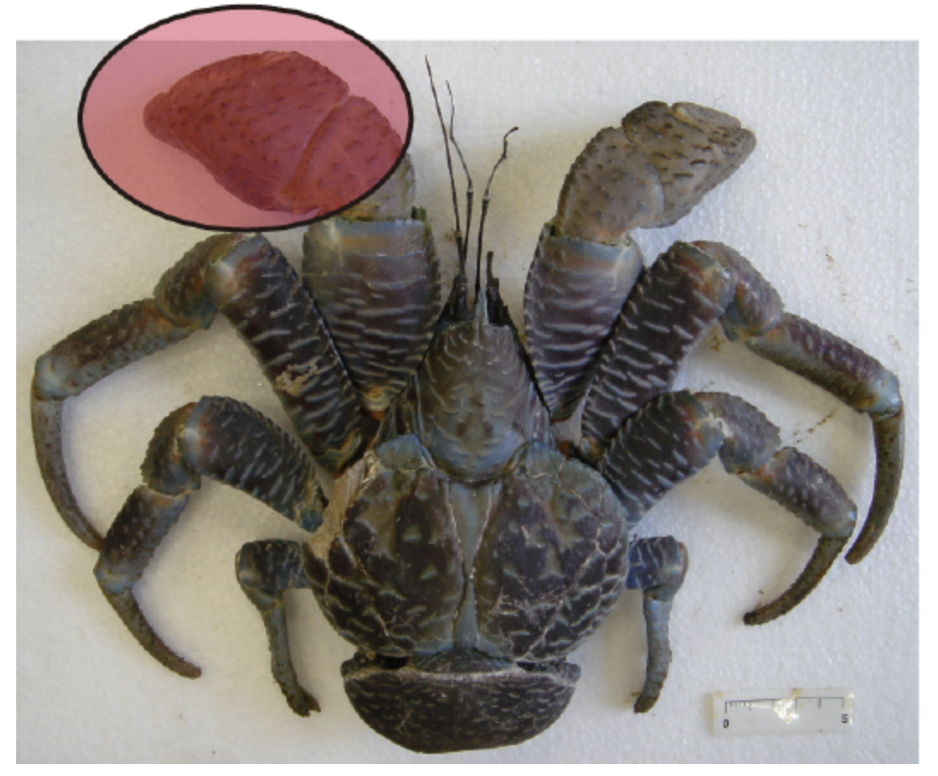
Fig 2. Coconut crab, Birgus latro, highlighting the chela (Oka et al., 2016)
Many organisms also use their chelae for defence. While scorpions are known to be predatory creatures, some scorpions have been known to use their chelae as a shield. When faced with frontal attacks, some scorpions will focus their stout pincers on their head for protection (Simone & Van Der Meijden, 2021). Another species that uses its chelae as a biological shield is the Coenobita brevimanus, a species of hermit crab. This species is shown in Figure 3, which highlights the notable feature of its chelae, the left chela is much larger than the right chela. When in a defensive position, the hermit crab will use its excessively large left chela as a blockade by covering the entrance of the shell (Reshmi & Bijukumar, 2010).
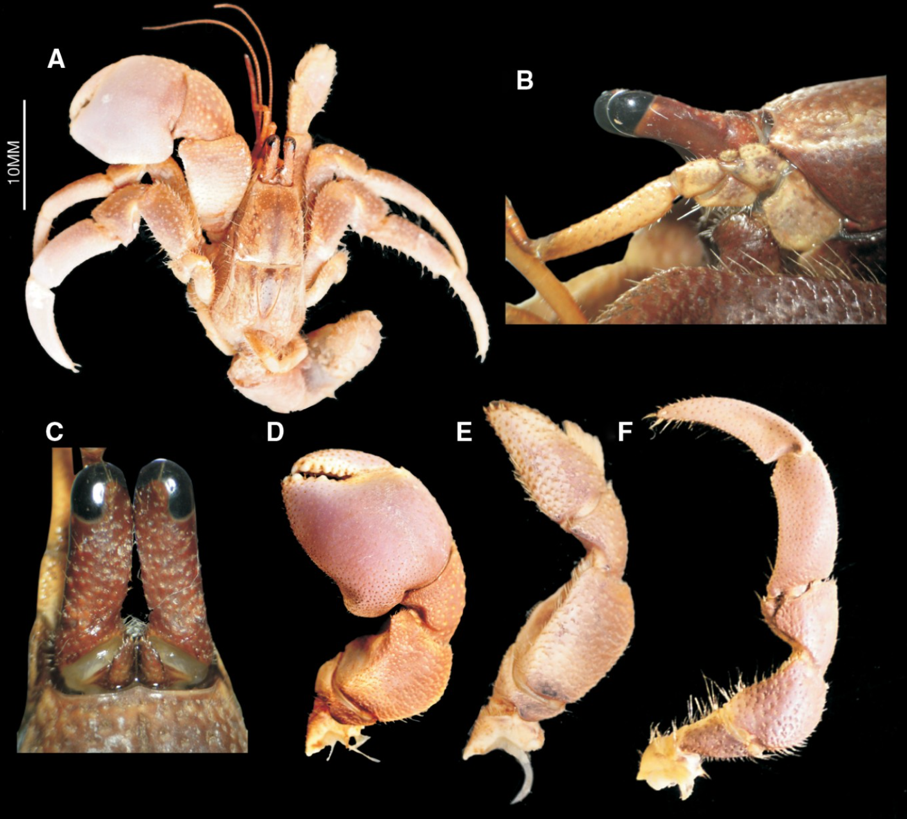
Fig 3 (A) Anatomy of the Coenobita brevimanus hermit crab; (B) ocular penduncle; (C) ocular acicle; (D) left chela; (E) right chela; (F) third pereopod (Reshmi & Bijukumar, 2010).
Sexual contests and communication between a chelae-wielding species are frequent. They are used especially in mating signals and in competition with others of the same sex. For example, Uca pugnax, or fiddler crab, uses its major (larger) pincers for fighting and mating cues (Levinton & Allen, 2005). The generous size and bright color of these large chelae are key traits that not only attract potential mates, but also demonstrate the crab’s ability to fight and intimidate enemies.
Chelae have evolved into a highly adaptable tool with many roles, giving arthropods the ability to perform a wide variety of tasks. This morphological feature continues to ensure the survival of different species in different ways, however, most pincers possess a set of similar characteristics related to composition, mechanics, and structure (Lane, 2018). In arthropods, the chelae function as a closed kinematic chain. A closed kinematic chain can be defined as a series of connected links and pivot points that form a closed loop (Olsen, 2019).
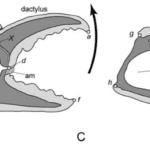
There is a fixed finger on each chela which is connected to a series of rigid joints and pivots within the chela, and the mobile finger whose movement creates mobility throughout the appendage. This movement is controlled by muscles in the chelae which open and close the moving finger. In scorpions the fixed chela is known as the tibia and the moveable finger as the tarsus. In crustaceans these are referred to as the propodus and the dactylus, respectively. In the case of crab pincers, the dactylus, propodus, opener and closer muscles form a permanent closed kinematic chain that allows mobility for the dactylus as it pivots on the propodus while providing stability for the mechanism during extension (Glaeser & Nachtigall, 2019). The crustacean pincer, and its respective components, are shown in Figure 4, along with the movement they create (Fujiwara & Kawai, 2016).

Fig 4. Illustration of the chela of Baptoziuszius vinosus. Diagrams C and D illustrate the retraction and closing of the dactylus. Point (X) indicates the position of the propodus-dactylus joint comprising the lateral and medial fulcra. Points (d) and (e) locate the positions of insertion of the closer apodeme (ca) and the opener apodeme (oa) to the ventral and dorsal margins of the proximal dactylus, respectively. The arrows in diagrams C and D indicate the direction of the opener and closer muscles, with contraction of the opener muscle in the opener apodeme causing the dactylus to actuate away from the propodus and contraction of the closer muscle forcing the dactylus to pinch against the propodus (Fujiwara & Kawai, 2016).
This multitude of mechanical functions of the chelae is viable due to their individual structure and composition. Every substance in the universe, from planets in the solar system to the chelae of a hermit crab, is composed of chemicals. The chemical composition of chelae not only builds their individual hierarchical structures but also creates unique properties that have allowed the chelae to remain an opportune feature in arthropods for millions of years. An analysis of chelae’s chemical structure, coupled with their mechanical properties, can offer insight into their biological and adaptive functions. The mechanical functions of the chelae are able to be carried out by their structure, which is continuously viable due to their unique chemical and physical properties formed from their molecular composition and interactions (Lin et al., 2021).
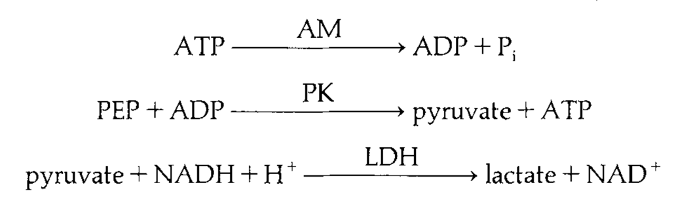
Although arthropods employ their pincers in various ways, the building blocks of their chelae are similar. The chela is composed of mainly chitin-protein complexes, where the protein consists of various proteins, glycoproteins, and proteoglycans. The chela is also rich in calcium carbonate, organized in rigid hierarchical layers (Zhou et al., 2010). Within the distinct layers of the pincer cuticle, there is some differentiation in the hierarchy, yet the pincer cuticle is usually composed of 3-4 layers. The various layers are distinguishable according to their fiber arrangement and morphology: the epicuticle, the exocuticle, an intermediate layer, and the endocuticle (Kellersztein et al., 2021). The layers of the cuticle of the fixed chela, or the tibia, are shown in Figure 5, which illustrates the four unique layers of the scorpion cuticle.
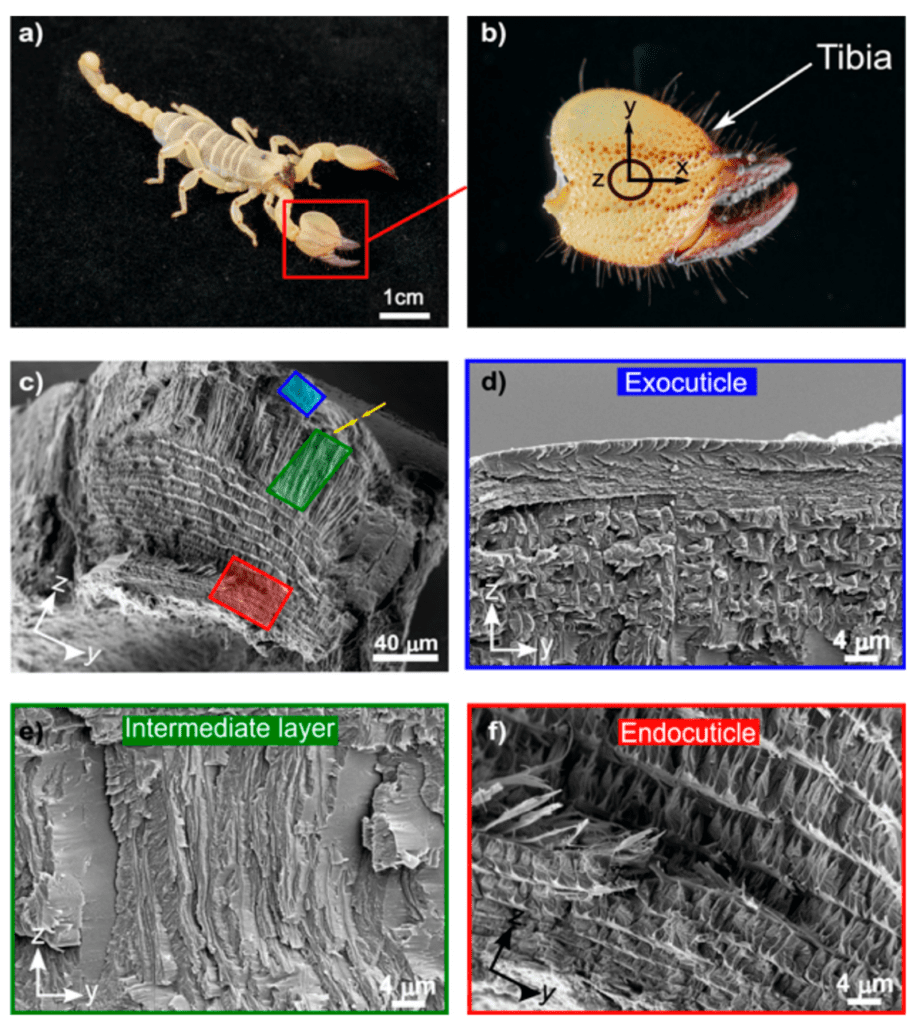
Fig 5. Hierarchical structure of the scorpion cuticle: (c) Cross-section of the tibia exposing the epicuticle, the exocuticle (blue), the intermediate layer (green), and the endocuticle (red); (d) The exocuticle is a multilayer structure; (e) the intermediate layer of oriented fibers; and (f) the endocuticle (Kellersztein et al., 2021).
In this paper, we will be exploring the unique chemical structure of the chela, specifically the cuticle and muscles. Additionally, we will be discussing the chemical processes throughout the chelae that create the unique physical properties of this highly adaptive tool, such as molting and calcification. Lastly, we will study the effects of these chemical properties, structure, and reactions on chelae, the chelae-wielding organism, and further applications.
Structural Overview
The structure of the chelae and its cuticle is generally continuous across arthropod species with some exceptions and variations. The chela is a multifunctional body component of the exoskeleton that leads to the development of different stresses in the cuticle. This chitin-based exoskeleton develops attachment for muscles, a water barrier, and environmental protection (Meyers et al., 2008). The chela cuticle is further divided into 3-4 rigid, unique tiers mainly composed of a chitin-protein matrix and minerals arranged in hierarchical layers. The layers comprise the epicuticle, the exocuticle, the extra-layer stacked lamella, and the endocuticle shown in Figure 6 (Kellersztein et al., 2019). Figure 6 shows the hierarchical structure of the chitin fibers creating twisted planes and the layers of the cuticle, which compose the chelae. While similar in composition, each of these layers has a unique structure and variations in composition that provide their unique properties in the function of the chelae.

Fig 6. Schematic illustration of the hierarchical structure in the chela cuticle of the Scorpio maurus Palmantus and the Buthus occitanus israelis respectively (Kellersztein et al., 2019).
Epicuticle
The epicuticle is the outermost layer of the pincer cuticle. This is the thinnest layer of the pincer, and its thickness is measured in the hundreds of nm. This layer has an absence of chitin and its texture is known to be waxy. In some species, the epicuticle is further divided into the inner and outer epicuticles. The outer epicuticle is made of a paraffin membrane, and the inner cuticle is rich in disulfide bonds. Due to its lack of chitin, this layer is not applied mechanically in the chela, however, its waxy texture is responsible for waterproof protection (Kellersztein et al., 2019).
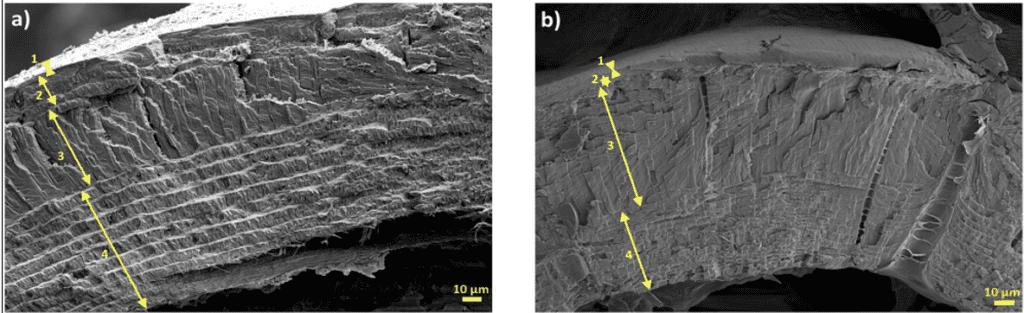
Fig 7. Transversal cross-section of the epicuticle of two scorpion species: (a) Scorpio maurus palmatus and (b) Buthus occitanus israelis (Kellersztein et al., 2019).
Exocuticle
The exocuticle is a thick layer primarily composed of Bouligand layers, creating tough structural properties for the cuticle. The Bouligand structure is a hierarchical composite composed of a helicoidal arrangement of strong fibers. This extremely dense layer is found in all arthropods and exhibits high resistance to external forces. The differences in this layer between species lead to unique mechanical advantages in the cuticle. In many scorpion species, the exocuticle is divided into 2-4 sublayers, however, in crustaceans, the exocuticle is continuous in Bouligand morphology (Kellersztein et al., 2019). The Bouligand structures that form this layer are made up of chitin-protein complexes in an asymmetric helicoid stacking pattern. This layer has also been found to be rich in calcium carbonate. Figure 8 shows the exocuticle of two species of scorpion Scorpio maurus palmatus (SP) and buthus occitanus Israelis (BO) (Kellersztein et al., 2019). In the SP species, the exocuticle is approximately 20 μm thick and is divided into four distinct layers, an angled fibrous layer, a generous layer of horizontal structures, a vertically arranged layer high in fiber density, and a Bouligand layer. However in the BO, there were only two unique layers observed, and its thickness is approximately 10 μm (Kellersztein et al., 2019).
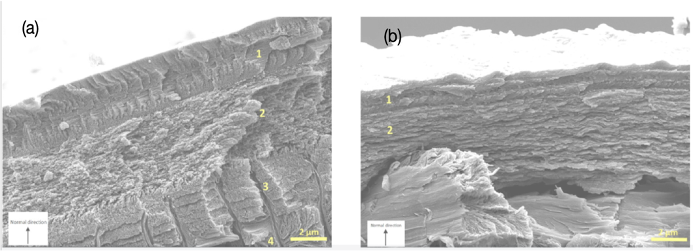
Fig 8. The exocuticle of two species of scorpion (a) Scorpio maurus palmatus and (b) Buthus occitanus israelis (Kellersztein et al., 2019).
Extra Layer
The layer scarcely observed in arthropods, yet seen in a multitude of scorpions is the extra layer. Neither crabs nor lobsters possess this layer. This layer is another contributor to the mechanical properties of the chelae, particularly stability. The extra layer is a part of the chelae which provides resistance to external forces and loads and is a protective barrier when the pincer is used as a defensive weapon. The layer is composed of unidirectional chitin fibers that form tight, vertically stacked laminae. Additionally, the extra layer is a dominant layer of the scorpion chela.
The SP extra-layer is approximately 50 μm thick and constitutes 30% of its chela cuticle, while the BO extra-layer is about 60 μm and composes 50% of the entire structure. These extra layers of the SP and BO specifically are shown in Figure 9 with the yellow arrows pointing to the pore canals. Like the lobster exocuticle, the extra layer of those scorpions has pore canals. These canals are directed, perpendicular to the surface (Kellersztein et al., 2019).

Fig 9. The extra-layer of stacked lamella in the (g) Scorpio maurus palmatus and (h)Buthus occitanus israelis chela, with arrows pointing to the pore canals (Kellersztein et al., 2019).
Endocuticle
The final and innermost layer of the chela cuticle is the endocuticle. This layer is common to all arthropods and is composed entirely of Bouligand structures further arranged in a horizontal stack of laminae. In Figure 10 the Bouligand structures forming the endocuticle of the SP scorpion species are shown; this endocuticle specifically has a total thickness of 80 μm, but this value varies between species (Kellersztein et al., 2019). The endocuticle is composed of a network of parallel chitin fibers that divide to form a stacked structure in Bouligand structure. The Bouligand structure will be reviewed further in the paper. The Bouligand layers have a parabolic shape in the scorpion endocuticle. The jagged parabola is a result of the oblique cuts in the twisted helical plywood structure, a cut where the structure is left with two angled sides (Kellersztein et al., 2019).

Fig 10. (a) An image of a singular lamella, illustrating the Bouligand structure in the cuticle of the scorpion species Scorpio maurus palmatus (Kellersztein et al., 2019).
Composition
Chitin
The scorpion chela cuticle is composed of α-chitin (poly(N-acetyl-d-glucosamine) fibers embedded in a protein matrix. This chitin-protein complex is essential for the structure and function of the cuticle, as it demonstrates a strain-stiffening behaviour (Kellersztein et al., 2019). This is due to strain hardening, which is the increase of elasticity under conditions of stress or stain. Chitin allows for shear-induced interactions to elicit nonlinear elasticity and therefore a progressive resistance to deformation (Furlani et al., 2021).
Chitin, β-(1-4)-2-acetamido-2-deoxy-D-glucose, is a linear polysaccharide, also known as sugars, and is the second most abundant natural polymer on earth. In nature, chitin tends to form a co-polymer with a polymer derivative of itself called chitosan, β-(1-4)-2-amino-2-deoxy- D-glucose. As observed in the Figure 11 below, the two structures differ at the #2 position on the ring; where the R group attached to the amino group is an acyl group (O=CCH3) for chitin and hydrogen on chitosan (Meyers et al., 2008).

Fig 11. Chemical structure of the chitin and chitosan copolymer (Meyers et al., 2008).
The chitin structure can be found in 3 polymorphic forms (different arrangements of a substance): α-, β-, and γ-chitin. The α-chitin seen in the arthropod chela cuticle is the most rigid of these 3 forms. As shown in Figure 12 below, α-chitin has an anti-parallel configuration while β-chitin is parallel and γ-chitin is a mix of both. The arrangement of the α-chitin allows the chitin to form strong hydrogen bonds between its chitin chains and creates a highly ordered crystalline structure. The numerous hydrogen bonds can be seen in the figure below between alcohols (-OH), amino groups (-NH), carbonyl groups (-C=O) and cyclic oxygens on the chitin chains. These strong hydrogen bonds are what make the α-chitin rigid and insoluble (Meyers et al., 2008).

Fig 12. The 3 polymorphic structures of chitin: a) α-chitin, b) β-chitin, and c) γ-chitin (Meyers et al., 2008).

Fig 13. Hydrogen bond network between α-chitin chains (Meyers et al., 2008).
In the arthropod exoskeleton, the linear chitin chains align anti-parallel to form α-chitin crystals. At the next level of the hierarchy, these crystals are found enveloped by several different brittle proteins and will pack together to make strong nanofibrils; where the bundle of these protein-chitin nanofibrils creates fibers. In the cuticle, these fibers are found in twisted stacks, forming a structure known as the Bouligand (Meyers et al., 2008), which will be discussed further for its mechanical strength. The chitin polysaccharide composition of the cuticle thus serves as the backbone reinforcer for the entire arthropod exoskeleton.
Bouligand (protein-chitin fibers)
The rigid chitin-protein complex seen throughout the exoskeleton of arthropods is called the Bouligand structure. Species containing this hierarchical architecture, such as arthropods, are shown to demonstrate exceptional toughness and damage-tolerant properties. The tightly packed structure prevents bending and deflects damage by maintaining a packed strong structure. The chitin-protein complex is responsible for the brittle fracture in the cuticle of the chelae, as the fracture splits into these tightly packed and highly structured layers when damage is inflicted. The Bouligand structure is a hierarchical composite composed of a helicoidal arrangement of strong fibers in a weak complex (Suksangpanya et al., 2018). This arrangement is formed by stacking highly aligned layers, such that each layer is skewed by a small angle from the layer above it. Each mineral rod is composed of chitin-protein fibrils arranged in a helical pattern. Each rod is approximately 60 nm in diameter and comprises 3 nm diameter segments. Figure 14 shows channels (0.5-1 μm) linking the inside and outside of the shell that are bounded by tubules (Meyers et al., 2008).

Fig 14. Hierarchy of the crab exoskeleton, entire specimen, cuticle sections, and Bouligand structure (Meyers et al., 2008).
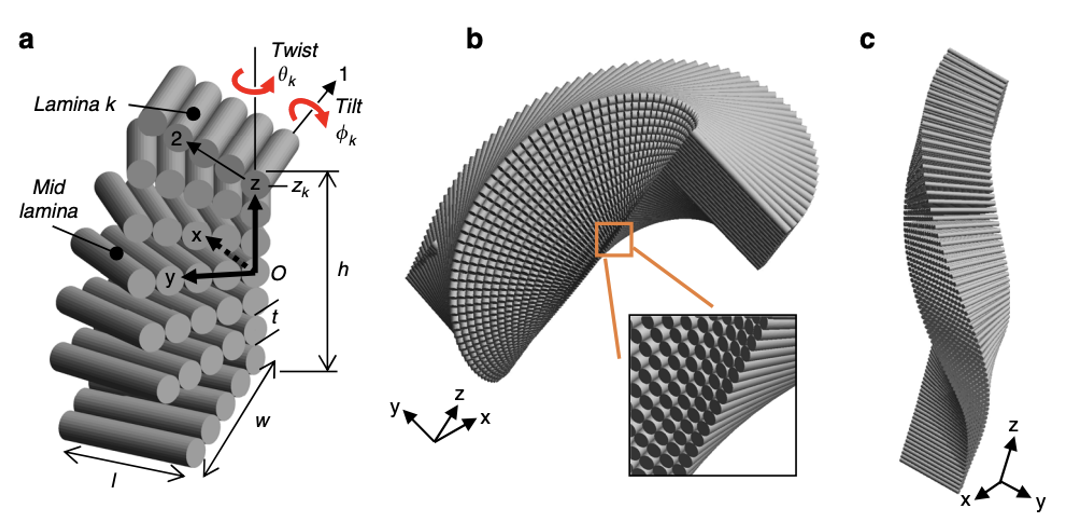
The intricate Bouligand laminate unit (BLU) resembles laminated composites, and has the ability to inspire highly rigid, tougher synthetic structures. Figure 15 illustrates the hierarchical structure of the stacking and twisting of BLU’s, and how they build to create large, highly strong structures. BLU’s were known to have infinite helical symmetry, as its laminae are stacked progressively, twisting in plane around an axis located at the lamina center (Greenfeld et al., 2020). However, a recent study of scorpion chelae revealed that the twisting axis is rather located at the lamina corner, and the lamina are progressively twisted resulting in a warped helicoid. This helicoid does not possess symmetry, and its appearance is variable along the rotation axis. This asymmetric, tilted structure allows each BLU to create a contiguous interlocked structure through entanglements with surrounding BLU’s.

Fig 15. Hierarchical structure of the Scorpion via cross sections: (a) Scorpio maurus palmatus; (b) Chela (pincer), tarsus (moving finger) and axes definition; (c) YZ transversal cross-section of the lower part of the taurus; (d) XZ longitudinal cross-section of the upper part of the taurus; (e) magnification of the YZ cross section showing the endocuticle and BLU; (f) definitions of layers (Greenfeld et al., 2020).
Each of the 40 to 100 laminae that compose the BLU is approximately 50 to 100 nm thick. A single layer of unidirectional chitin fibers immersed in a protein-rich matrix constitutes each laminae. Each BLU gradually rotates its laminae and makes one-half of a revolution to generate the left-handed helical prism of laminae structure depicted in Figure 16. On a larger scale, the twist and tilt are identified and further modelled after being discovered (Greenfeld et al., 2020). Due to rotation around the lamina corner rather than the centre, the model has no apparent symmetry about the three primary planes. Additionally, there is a divide between the varied tilting of the upper half of the BLU compared to that of the lower half, due to the effect of tilting. The lower half of the BLU has a narrower cross-section as a result of the tilting’s geometrical interference. Each BLU’s off-axis helicoidal shape creates the counter-shape of its adjacent BLUs, resulting in a tight fit between the BLUs. Shear-interlocking and damage resistance is evident in all three of the primary planes due to the fact that the BLU’s are able to mesh together as if they are pieces of a jigsaw puzzle. When an external tensile force is applied to these highly linked BLU’s the shear-interlocking between the BLU’s and the inter- and intra- layers surrounding them in three dimensions inhibits shear in all directions and contributes to toughness by deflecting cracks at the various angular directions of the laminae (Greenfeld et al., 2020).
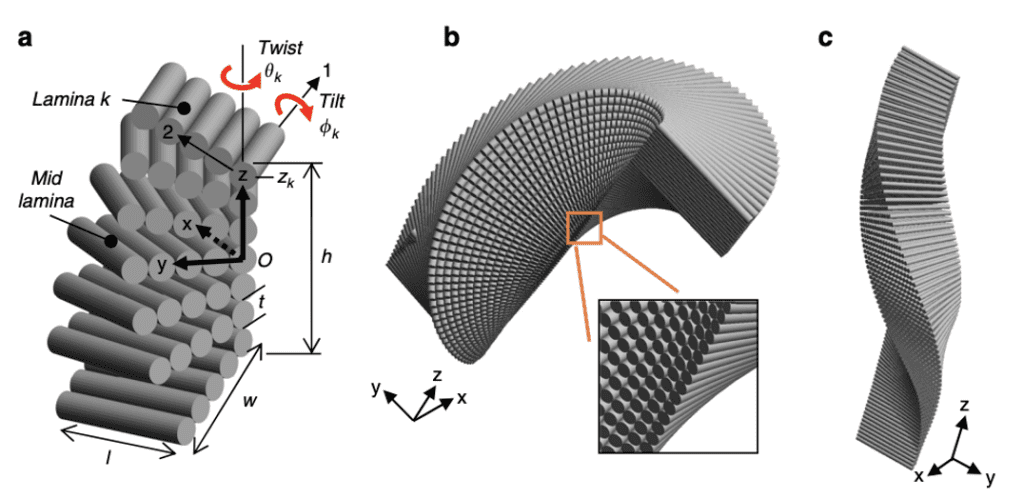
Fig 16. BLU (Bouligand laminate unit) geometric model. (a) Coordinate systems- layer/laminate (x,y) and lamina (1,2); lamina k twist angle is 𝛳k, tilt angle is ɸk and z-position is zk; l and w are lamina length and width respectively; (b) and (c) modeled view of BLU with closer perspective of rough edge (Greenfeld et al., 2020).
Processes of the Exoskeleton
Molting
One unique adaptation of arthropods is their ability to shed their tough exoskeleton. This process is known as molting and is essential for arthropod growth. Because minerals toughen crustacean skin and arthropods have hard chitin shells, the creature cannot expand (Nagasawa, 2012). In molting, the arthropod creates a new exoskeleton and sheds its old one, leaving behind a hard shell (Roer & Dillaman, 1984), as observed in Figure 17 below. By studying the cuticle’s molting process, a better understanding of the different phases of the pincer structure throughout a crustacean’s life can be developed.

Fig 17. Molted exoskeleton of a crab (Michael, 2021)
The exoskeleton undergoes a continuous cycle of shedding and reforming. Molting will occur periodically in crustaceans; where most decapods will molt 1-2 times a year (Roer & Dillaman, 1984). The shedding of the cuticle will cycle through these stages: the intermolt, early premolt, late premolt, post-molt, and finally, will return to the intermolt integument (or outer layer). These stages are illustrated in Figure 18 below.
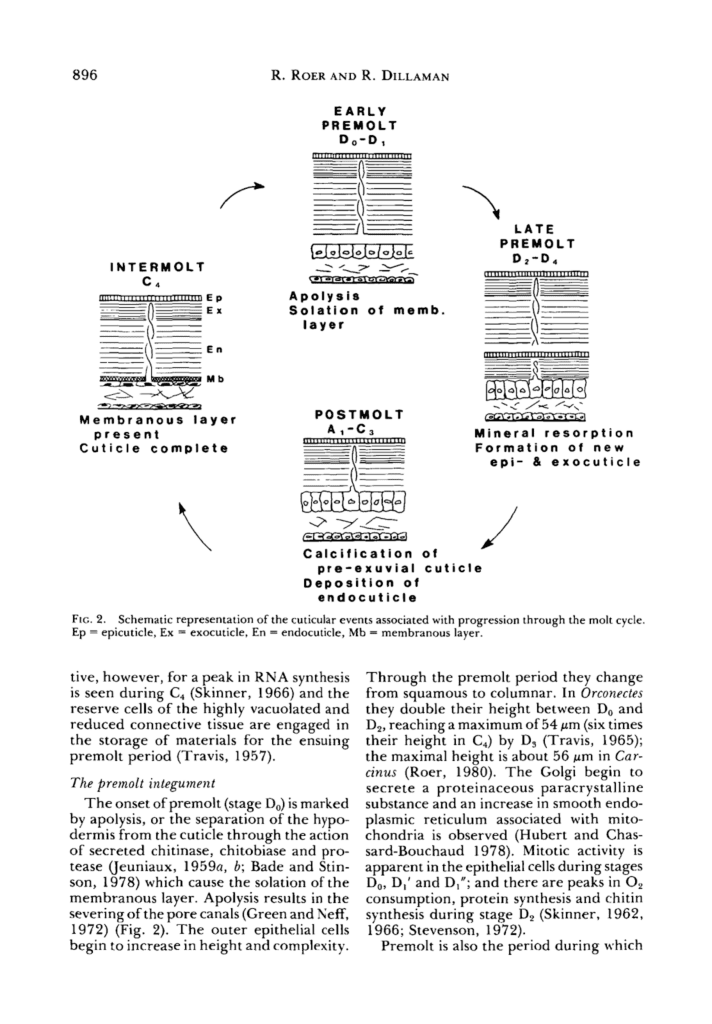
Fig 18. Schematic representation of the process of shedding and reformation of the crab exoskeleton (molting) and processes throughout the molt cycle. Ep = epicuticle, Ex = exocuticle, En = endocuticle, Mb = membranous layer (adapted from Roer & Dillaman, 1984).
The intermolt is the resting state of the molting cycle, where no active changes occur. Parallels can be drawn to the cell cycle, where interphase is the stable state of the cell; it spends most of its life in interphase and is not dividing. The four layers of the exoskeleton we will be discussincg are the epicuticle, exocuticle, endocuticle, and membranous layer, which is the layer of epithelial cells. During intermolt, these layers are complete, and the first three are calcified. Calcification, also known as mineralization, occurs in the crustacean cuticle to create more rigid structures (Roer & Dillaman, 1984). We will go into more depth into what calcification, or mineralization, of the cuticle, entails. Additionally, in the intermolt, the epithelial cells forming the membranous layer, or hypodermis, are flat and thin (Roer & Dillaman, 1984).
The early premolt integument is the beginning of the arthropod exoskeleton shedding. In this stage, the hypodermis layer separates from the cuticle, a process called apolysis. This separation from the cuticle results in the severing of the pore canals. The outer epithelial cells, which were previously squamous, meaning flat and thin, become columnar and taller (Roer & Dillaman, 1984).
In the late premolt stage, the new epicuticle and exocuticle layers form underneath the old cuticle. These layers are referred to as the pre-exuvial layers because they form before the molt. In this stage, the new layers are not calcified and only occur once the old cuticle is shed; however, the epicuticle is tanned at this stage. Minerals and organic portions of the old cuticle are partially reabsorbed and reused into the forming layers (Roer & Dillaman, 1984).
After shedding the old cuticle, the layers’ calcification and the endocuticle’s formation occur in the post-molt integument. As calcification begins, calcium carbonate CaCO3 crystals will form within pore canals, and between and within chitin-fibrils of the exocuticle and endocuticle (Nagasawa, 2012). The columnar epithelial cells will return back to their standard small height (Roer & Dillaman, 1984). This ends the cycle of molting, where the crustacean now has a new adapted shell to protect itself.
Calcification
Calcification is a process that occurs in the crustacean cuticle after the molt and creates a harder exterior. As of now, the only arthropods known to have calcified exoskeletons are the crustaceans (Nagasawa, 2012). Although providing extra strength, the added minerals to the cuticle adds extra weight to the body. This consequence, however, does not appear to be problematic for the crustacean’s ability to function (Nagasawa, 2012), but could explain why other smaller pincer-wielding arthropods have not evolved to possess this attribute. Calcification of the crustacean means that the cuticle is deposited with calcium carbonate (CaCO3). After the crustacean has molted its shell, the new exoskeleton will need to reinforce itself with this mineral. The reaction for the formation of CaCO3, as shown below in Equation (i), is an acid-base reaction between a calcium (Ca2+) ion and HCO3–:
Ca2+ + HCO3-→ CaCO3 + H+ (i)
This reaction will lead to a buildup of hydrogen ions (H+) which will be transported out from the cuticle. To calcify, the crustaceans must have an uptake of Ca2+ and HCO3–. These ions are found in their aquatic environment and HCO3– is also produced metabolically by the crustacean itself (Cameron, 1985). The latter is shown through the following reaction Equation (ii) with carbon dioxide (CO2):
CO2+ H2O → HCO3- + H+ (ii)
Where more hydrogen ions must be exported. This inflow and outflow of molecules from the cuticle is possible because of the pore canals found throughout the cuticle (Cameron, 1985). During the post-moult phase, where new cuticle layers are forming, an influx of Ca2+ and HCO3– is transported through these canals. In addition, the pore canals allow the exodus of the hydrogen waste products, as shown in Figure 19 below.

Fig. 19 Diagram of transportation of Ca2+and HCO3– and H+ ions in the post-molt blue crab (Cameron, 1985).
Metals incorporated into cuticular tools
A large number of species of arthropods, such as spiders, ants, and scorpions, have been found to have high concentrations of zinc and to a lesser degree, manganese, calcium, chlorine and even iron, incorporated in the cuticle of the working regions of their cuticular weapons, mandibles, pincers, legs, and stingers (Schofield et al., 2003).
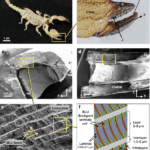
This accumulation comes with a significant increase in hardness, up to three times in some organisms (Schofield et al., 2003). This accumulation occurs very early throughout the initial stages of the organism’s life, usually starting after the beginning of pre-ecdysial darkening (hardening of the cuticle). Scanning transmission ion microscopy (STIM) and particle-induced X-ray emissions (PIXE) images in Figure 20 show how the zinc concentration increases throughout the development of the scorpion’s cuticle.

Fig 20. Visible difference in zinc concentration (higher concentration in white) of cuticular tools of scorpions, V. spinigeris, at different stages of their early development. The body parts of scorpions at different developmental stages are shown in each image; starting clockwise from the top left in each image the scorpions are 38, 90 and 160 hours post-ecdysis. The images of the first column are of the pincer fingers, the column to the right of that shows the tarsal pincers, and the rightmost one, shows the chelicerae. The images in the top row were created using STIM; whiter shades indicate greater mass density. The bottom row is a matching PIXE image showing the origin of zinc X-rays in nearly the same field; whiter shades indicate greater numbers of X-ray counts. The total zinc X-ray count (approximately proportional to content) for the distal 200 μm of the toothed regions of the pedipalps of the 38, 90 and 160 h old specimens was 9, 89 and 330 respectively; for the distal 50 μm of both lateral tarsal pincers the count was 25, 38 and 214 respectively, and for the distal 50 μm of the right cheliceral finger the count was 18, 27 and 331 respectively. The frame edge lengths for the images of the pedipalps, tarsal pincers, and chelicerae are, respectively, 1.1, 0.55 and 0.82 mm (Schofield et al., 2003).
In scorpions, the chela teeth, the mandibles and the stinger are the most zinc enriched parts of the body, with zinc concentrations reaching as high as 25% of the dry mass of the modified outer exo-cuticle. The zinc concentration of the teeth of the pincer forms a gradient, increasing from the basal region to the tip (Schofield et al., 2003).
Although we do not yet have a clear understanding of the chemistry and biochemistry of the zinc-enrichment process, a detailed ultrastructural study of the scorpion’s zinc-reinforced cuticle teeth revealed that a complex system of nanopores, tooth canals, ducts and ductules (Figure 21) develops in the scorpion’s pedipalp teeth before zinc deposition. This suggests that an active zinc transport system is turned on during the late development stages of the cuticle and allows for the incorporation of the zinc (Schofield et al., 2003). Interestingly, this zinc and the other cuticle-enriching elements appear to be coming from internal stores and can be developed before the scorpion is fed (Schofield et al., 2003).

Fig 21. (A) SEM image of 7 pedipalp finger teeth of a scorpion (V. spinigeris). Six of the teeth are lined up in a row and the 7th tooth is slightly off to the side. Numerous sensory bristles can be seen in the image. (B) TEM image of the cross-section of a scorpion pedipalp finger (Centruroides exilicauda) cut through a main row tooth and an auxiliary tooth. (tc): tooth canal extending through the endocuticle (en) from the lumen toward the base of the tooth. It is filled with epidermal tissue (ep). (pt): zinc-enriched region of each tooth, distinguished by an increased electron-density, show in a darker colour. (sc): canal belonging to a sensory bristle. (C) Higher magnification TEM image of the distal end of the same tooth canal (tc). D: indicates the cellular material filling the duct complex, extending to the base of the metal-enriched tooth matrix (pt). (iex): Inner exocuticle layer, lines the wall of the duct complex. (en): endocuticle. (ep): epidermal tissue (Schofield et al., 2003).
Invertebrate Chelae Muscle
Muscles are essential to invertebrate pincer functionality
Within the invertebrate (crustacean specifically) pincer, there are two muscles: the closer and the opener muscles. Of particular interest to many researchers is the closer muscle due to the varying characteristics between different arthropods (Longo & Díaz, 2013). The functional and structural properties of arthropod musculatures are analogous to vertebrate skeletal muscles with a striated pattern (Royuela et al., 2000). Thus, to explain the basic process of muscle contraction, we will examine the vertebrate skeletal muscle construction due to its similarities with invertebrate striated muscle.
Muscle Anatomy
A primary identifying characteristic of skeletal musculature is the transverse striations arising from its longitudinal arrangements of muscle fibers (Betts et al., 2013). The fundamental cellular unit of the muscle, the muscle fiber, is formed during early development when embryonic myoblasts fuse with other cells to form multinucleated skeletal muscle fibers. Parallel muscle fibers span across the length of the muscle and house the essential organelles and macromolecules required for muscle contractions. The multinucleated nature of muscle fibers is necessary for synthesizing the vast quantities of proteins needed for muscle contractions (Betts et al., 2013). Multinucleation is also thought to potentially increase transcriptional diversity to regulate specialized fiber regions, with the increased DNA content also aiding in optimal cellular function and structure. Skeletal muscle regeneration is also believed to benefit from multinucleation (Prasad & Millay, 2021). Skeletal muscle contains a population of stem cells (satellite cells) which upon stimulus, will fuse and regenerate damaged muscle fiber (Petrany et al., 2020).
The two organelles essential for muscle fiber contraction are the myofibrils and the mitochondria. The mitochondria are responsible for synthesizing adenosine triphosphate (ATP), which is hydrolyzed to provide energy during muscle contractions while myofibrils serve as the contractile organelle. Figure 22a depicts the cross-section of the muscle fiber with tightly bundled myofibrils and interspersed mitochondria and nuclei. The myofibril is composed of smaller subunits known as sarcomeres. The sarcomeres are the primary contractile unit of the muscle. Within each sarcomere is an alternating arrangement of actin and myosin myofilaments. The actin filaments, the primary component of so-called “thin” filaments, are connected to the Z-discs, separating each sarcomere. In between the actin filaments are the thick filaments comprising myosin. This can be seen in Figure 22b, with the thin filaments forming an interlocking interaction with the central thick myosin filaments.

Fig. 22 Illustration of muscle fiber (a) and myofibril composition (b). In graphic (a), a cross-section of the muscle fiber indicates the localization of myofibrils, mitochondrion, and nuclei. In the zoomed-in picture of the myofibril (b), the essential contractile unit–the sarcomere–is shown featuring the thick and thin filaments. A band: section of sarcomere containing myosin thick filaments. I band: space between thick filaments of adjacent sarcomeres. H zone: area between opposing thin filaments (Betts et al., 2013).
Muscle Contraction Mechanism
Sliding Filament Theory
The mechanism of muscle contraction can be explained by the sliding filament theory, which proposes the sliding action of actin along the myosin filaments (Huxley & Hanson, 1954). By pulling opposing actin filaments towards each other, the Z discs are brought closer together, thus shortening the length of the sarcomere. The shortening sarcomere, in turn, results in a net contraction of the myofibril, which, combined with the concurrent myofibril contractions, yields a cumulative contractile force from the muscle.
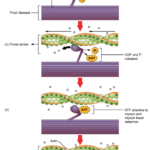
To achieve this sliding action, the myosin heads on the thick filament are first bound to an active binding site on the actin filament. This forms a cross-bridge linkage between the myosin head and the actin filament (Huxley, 1969). Following the binding of the myosin head is the power stroke, where the myosin head translocates the actin filament toward the M-line (Spudich, 2001). During the power stroke, the hydrolyzed ATP is released as adenosine diphosphate (ADP) and phosphate from the myosin, which remains bound to the actin. To release the myosin head from the thin filament, an ATP binds to the myosin, detaching it from the thin filament. To re-cock the myosin head into a position ready for binding to the thin filament, the recently bound ATP is hydrolyzed, providing the energy necessary to return the myosin head to the cocked position. This cross-bridge cycle is repeated until neural stimulus is terminated or the muscle fatigues (Fitts, 2008). The process of completing one cycle of cross-bridge cycling is shown below in Figure 23.

Fig 23. Process of cross-bridging cycling. The first step of cycling (a) is binding the myosin head to the actin filament to form the cross-bridge (b). Notice the troponin (yellow spheres) attached to tropomyosin filaments (orange) with bound calcium ions (white sphere). The calcium ion binding shifts the shielding proteins, revealing the active binding sites on actin. Step (c) shows the power stroke of the myosin head translocating the actin filament towards the M-line (not shown) and the disassociation of the ADP and phosphate. To detach the myosin head from the active site, an ATP is bound to myosin (d) and hydrolyzed to cock the myosin head in preparation for another cycle (e) (Betts et al., 2013).
Excitation-Contraction Coupling
To initiate a skeletal muscle contraction, a nerve impulse is necessary to prepare the sarcomere for cross-bridging cycling. An action potential is transmitted from a motor neuron to the sarcolemma (the outer membrane surrounding the muscle fiber) which then propagates down the sarcolemma and transmits the action potential to the T-tubules (Betts et al., 2013). The T-tubules are invaginations of the sarcolemma that allow transmission of the action potential to the interior of the muscle fiber, which triggers the opening of calcium release channels in the sarcoplasmic reticulum (Fig. 24). This releases the calcium ions stored in the sarcoplasmic reticulum into the myofibrils (Betts et al., 2013).
Interspersed in the thin filaments of the sarcomere are troponin and tropomyosin proteins. Troponin and tropomyosin proteins are responsible for activating and inhibiting the cross-bridging cycle (Huxley, 1969). When the sarcomere is inactive, tropomyosin blocks the active sites of the actin filament until the sarcoplasmic reticulum releases calcium ions. When calcium ions are concentrated in the sarcomere, the calcium ions bind to troponin, triggering a conformational change that shifts the tropomyosin strands unblocking the actin filament active sites (Fig. 23a). The uninhibited active sites are then available for binding the myosin heads to initiate muscle contraction (Betts et al., 2013).
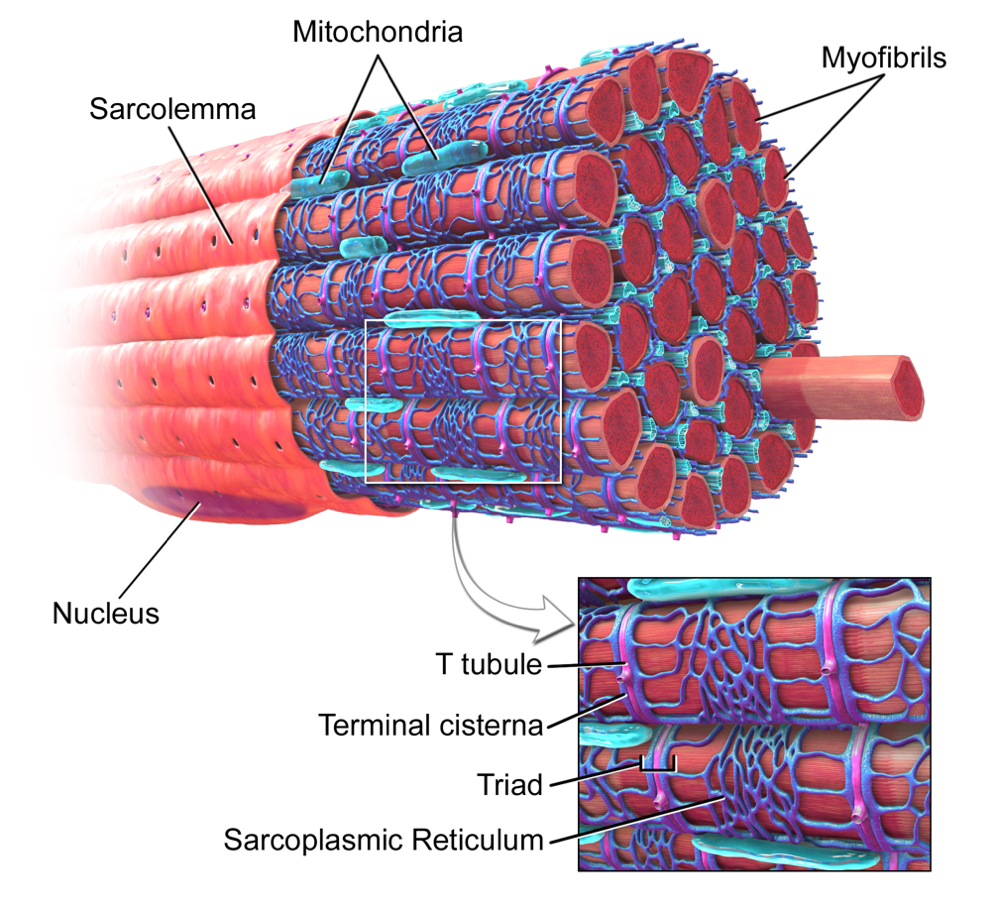
Fig 24. Skeletal Muscle Fiber with a zoomed-in view of the T-Tubule (pink) and Sarcoplasmic Reticulum (dark blue) (Blaus, 2014).
Muscle Chemistry and Crabs
Sarcomere Lengths
One of the distinctive characteristics of the crustacean striated musculature is the variety of muscle fiber sizes (Longo & Díaz, 2013). The plethora of muscle fiber lengths and diameters contribute significantly to the functionality of the skeletal muscle. Muscle fibers large in diameter are associated with greater force generation and faster contraction velocity (Mellon, 1992). The length of the sarcomere also plays a significant role in the mechanical stress generated during muscle contraction.
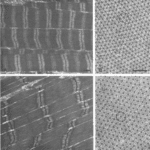
Muscle fibers containing long sarcomeres are observed to exhibit greater force relative to short sarcomere muscle fibers (Longo et al., 2011). This is thought to be associated with the higher ratio of thin to thick filaments in long sarcomeres as seen in the electron micrograph in Figure 25. With a larger proportion of thin filaments, long sarcomeres are able to establish a greater number of cross-bridge interactions between thin and thick filaments, eliciting greater force production.
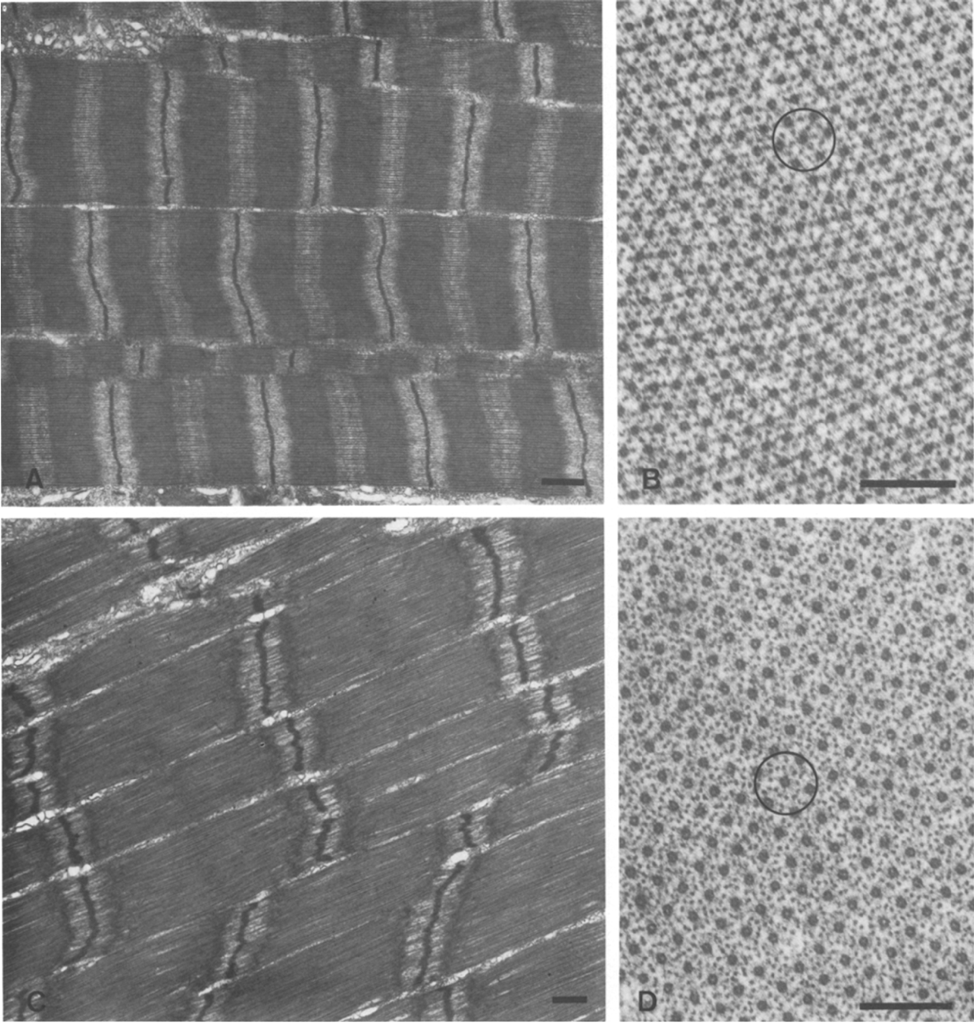
Fig. 25 Myofibrils from Cherax destructor: (A) Longitudinal view of the short sarcomere muscle fiber, bar = 1 μm; (B) Transverse view of short sarcomere muscle fiber, bar = 2 μm: (C) Longitudinal view of the long sarcomere muscle fiber, bar = 1 μm; (D) Transverse view of long sarcomere muscle fiber, bar = 2 μm. In images (B) and (D), there is a higher density of dark spots in (C) compared to (D) indicating a higher ratio of thin filaments in (D) (West et al., 1992).
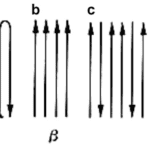
In a study of myofibrils from Cherax destructor by West et al. (1992), longer sarcomere myofibrils were observed to contain approximately a six-to-one ratio of thin filaments to thick as opposed to just three-to-one in short sarcomeres. After introducing both long sarcomere and short sarcomere muscle fibers to calcium and strontium ion-activating solutions, it was found that long sarcomeres were able to generate approximately twice the maximum tension (Figure 26).
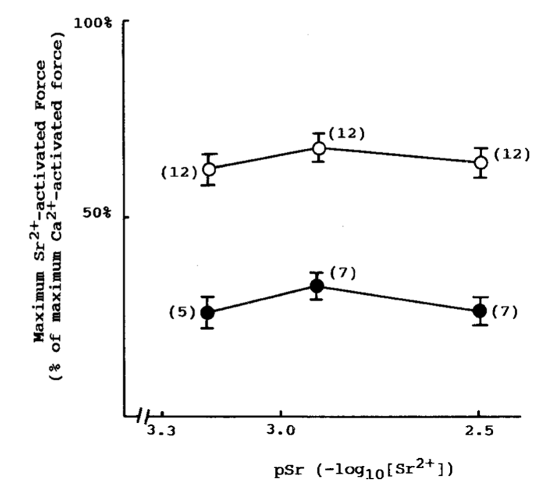
Fig. 26 Long sarcomeres (hollow dots) exhibit greater maximum force production compared to short sarcomeres (solid dots) after activation in strontium ion solution (West et al., 1992).
It is suggested that the rate of ATP hydrolysis does not contribute to the disparity between maximal force production in long and short-sarcomere myofibrils. While analyzing maximal force production, MgATPase activity was also recorded revealing that short sarcomeres exhibited higher MgATPase activity (Fig. 27). However, the difference was statistically insignificant and likely suggests that the MgATPase activity is similar in both lengths (West et al., 1992). Thus, the cycling rate of myosin cross-bridging is likely to be approximately the same between long and short sarcomeres.

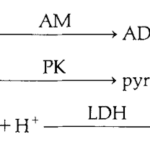
Fig 27. MgATPase activity linked to enzyme cascade. ATP is hydrolyzed by actomyosin to ADP and phosphate. ADP byproduct is metabolized by pyruvate kinase to synthesize pyruvate which is further processed by lactate dehydrogenase to lactate using NADH. By measuring NADH concentration rather than ATP concentration, error from ATP dependent membrane pumps is excluded to provide a more accurate rate of ATP hydrolysis by actomyosin complex (West et al., 1992).
The proportion of long and short sarcomeres is also suggestive of the behavior of crustacean species. Species that tend to require high-force generation, are found to contain higher proportions of longer sarcomeres (Longo et al., 2011). For example in the chelae of the coconut crab, Birgus latro, longer sarcomeres are observed as a result of the coconut crab’s primary diet of coconuts requiring tremendous force generation to open. Likewise, Cherax destructor also contains high proportions of long sarcomere muscle fibers in the closer muscle suggesting that the behavior is biased towards high-force production tasks and is further supported by its aggressive behavior during territorial defense and burrowing (West et al., 1992).
Biomimetics: Applications of Cellulose, Chitin, Chitosan, and Silk Fibroin Composites
Naturally occurring biological structures, such as the exoskeletons of crustaceans and the cuticles of arthropods, possess outstanding mechanical properties and functionality due to their hierarchical and ordered structure of polysaccharides, nanofibers, and proteins (Natarajan & Gilman, 2018; Tardy et al., 2019; Weaver et al., 2012; Xiong et al., 2017). The successful replication and artificial production of these biological materials can be promising for designing materials that would address modern challenges in the domains of bioengineering and medicine. In this section of the paper, we will focus our attention on the four major biopolymers, cellulose, chitin, chitosan, and silk fibroin that are considered the next-generation of green innovation materials (El Seoud et al., 2022).
These biomaterials are believed to have very promising applications in medicine, pharmacology, food industry, textile industry, and the decontamination of fluids. The number of such applications is bound to only expand due to the renewable, sustainable, biodegradable properties of these composites and the rising public concern about the environmental impact of the use of petroleum-based polymers.
Fibers and Nanofibers
It is possible to produce continuous fibers of cellulose, chitin, and silk fibroin by using various solution-spinning methods. The general process involves dissolving the biopolymers in a solvent, extracting them using a spinneret to form fibers, and then solidifying them through coagulation (El Seoud et al., 2022).

Fig. 28 SEM images of WPI-PEO electrospun nanofibers. Images in each column are in order of increasing magnification (top to bottom). The following fibers were obtained from solutions at: (a) pH 1, (b) pH 7, (c) pH 12 (Wilk & Benko, 2021).
It is possible to engineer the properties of the composite fibers by controlling the ratios of the dissolved biopolymers or by adding other compounds. For instance, nanocellulose, can be used as reinforcement to improve the mechanical properties, such as the elastic modulus and strength of chitin and silk fibroin nanofibers (Xu et al., 2021).
The main promising areas of application for fibrous matrices of biopolymers are pharmacology (drug-delivery systems) and regenerative medicine (wound healing) (Juncos Bombin et al., 2020). For example, combinations of chitin and silk fibroin have been shown to have the potential for being used as scaffolds in the repair of articular cartilage (Li et al., 2018). There are also examples of chitin and silk fibroin composite fibers that were used for antimicrobial wound dressings (Cai et al., 2010).
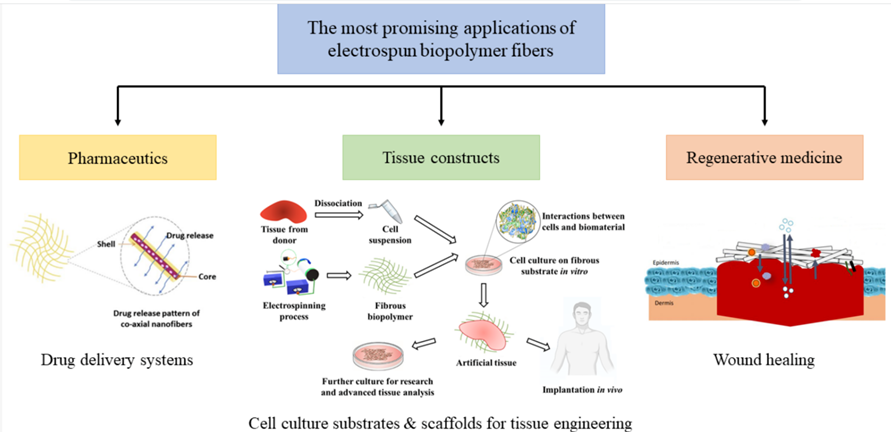
Fig. 29 Illustration of possible applications of electro spun fibrous biopolymer matrices (El Seoud et al., 2022).
Chitin has attracted significant interest in biomedical applications to produce fibrous materials with antibacterial properties, due to its inherent bacteriostatic and bactericidal properties (Kalantari et al., 2019). Cellulose and chitin are already in use in commercially available fibers: Chitopoly® and Crabyon® are textile technologies with antibacterial and odor-preventing properties (El Seoud et al., 2022).
Particles and Nanoparticles
The biopolymers mentioned above can also be useful in other physical forms. For instance, when formed into spherical-shaped beads with a diameter in the range of nanometers to micrometers, via nanoprecipitation (Nagpal et al., 2010; O’Neill & Reichardt, 1951) or electro-spraying from biopolymer solutions (Bock et al., 2011; Wu et al., 2014) these biopolymers have shown to be promising in several other applications.
Silk fibroin and chitosan microparticles have the potential for being used as drug carriers (Cheerarot & Baimark, 2015). Substances like Tetracycline hydrochloride, an antibiotic, human parathyroid hormone as well as plasmid DNA for vaccine delivery have successfully been loaded into silk fibroin and chitosan microparticles with promising results (Chung et al., 2011; Liu et al., 2014; Lv et al., 2018).
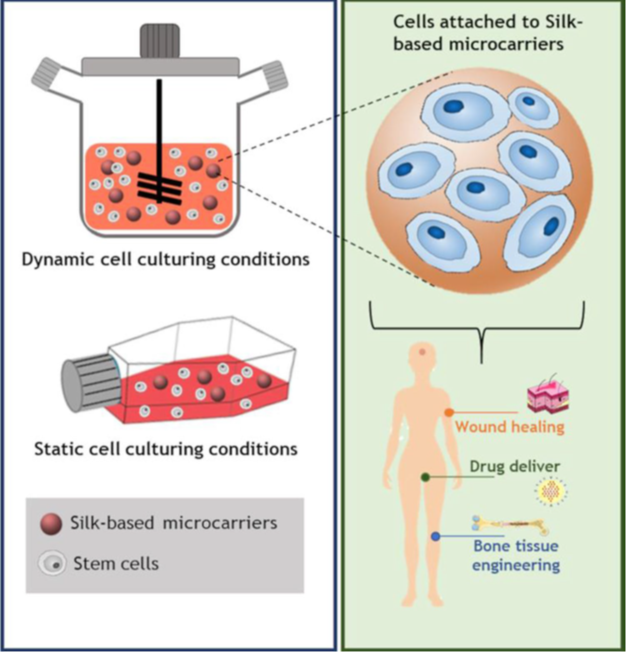
Fig. 30 Attachment of cells to biopolymer microcarriers, through static or dynamic cell culturing, and their applications (El Seoud et al., 2022).
The treatment of textiles, specifically of polyethylene terephthalate fabrics, with silk fibroin and chitosan microspheres, has been shown to have to improve their antibacterial and antistatic properties, resulting in improved wearing comfort (Zhang et al., 2019). Silk fibroin microparticles may also have potential use in wastewater treatment as they were shown to have very good adsorption capacity (Parushuram et al., 2021).
Most notably, however, due to the increasing concern for the risks posed by the omnipresent occurrence of petroleum-based plastic nano- and microparticles to the environment and to the health of living organisms (Srivastava et al., 2015), researchers are hopeful that biopolymer nanoparticles can be used to replace their petroleum-based counterparts in applications such as chromatography (as stationary phases), water treatment, protein immobilization, drug loading and release as well as nanotechnology (Gericke et al., 2013).
Composite Films
As discussed above, interest in biodegradable polymeric materials is due to, in part, the environmental problems associated with the use of petrochemical-based polymers (Cazón et al., 2018; Cazón et al., 2017). Proteins and polysaccharide-based materials are now used in the manufacturing of edible and biodegradable films (Sengupta & Han, 2013), which may have promising applications if integrated into food packaging, as well as in wastewater treatment membranes (Kanmani et al., 2017).
Numerous review papers have also highlighted the potential uses of such membranes in seawater desalination, drinking water purification and gas separation. Processes that are widely applicable in biomedical, food, beverage and pharmaceutical industries for concentration and separation of components (Galiano et al., 2018).
Chitosan Hydrogels
Hydrogels, 3D molecules composed of a cross-linked network of soft materials, have been successfully created using natural polysaccharides and have been extensively applied in the medical field. Due to their biocompatibility, degradability and low toxicity, chitosan hydrogels are useful carriers for drug release, both for rapid and sustained (Bacaita et al., 2014; Wang et al., 2017; Xu et al., 2019) as well as for the release of drugs in response to external stimuli, such as changes in pH, temperature, light, etc. (Huxley & Hanson, 1954; Sabourian et al., 2020; Singh et al., 2006).
The desired medication can be loaded into the hydrogel by passive diffusion (Fig. 31) a method shown to be very effective but restricted to the loading of small molecule drugs (Dhanikula et al., 2005; Wang et al., 2020).

Fig. 31 (A) Drug loading on chitosan hydrogels. The hydrogel is placed in a drug containing medium; the substance diffuses into the hydrogel. The rate of diffusion is dependent on hydrogel pore size and the molecular nature of the drug. (B) The drug release mechanism of smart chitosan Hydrogels (Tian et al., 2020).
Chitosan hydrogels are now common methods for antibiotic delivery, delivery of anesthetic drugs as well as anticancer drug delivery. Chitosan hydrogels also improve the performance of drugs in medical treatment by weakening toxicity, allowing for sustained release by reducing the side effects experienced by the organism (Tian et al., 2020).
Today, most work on composite biopolymers, other than chitosan hydrogel, is conducted at the research stage and while the materials have been successfully produced in the lab, scaling up the production of these materials still faces numerous challenges. However, as the need for their development and their applications will most certainly expand due to the attractive characteristics of these composites (biocompatibility, renewability, sustainability, biodegradation) as well as the increased public concern about the adverse environmental impact of petroleum-based polymers, these challenges are bound to be overcome (El Seoud et al., 2022).
Conclusion
In conclusion, the pincer exhibits several biochemical intricacies that contribute to its overall functionality. From its composition to the chemical processes that allow it to function, the pincer has acquired numerous unique characteristics during evolutionary processes. Creating an even more resistant cuticle, calcification occurs in crustaceans, where minerals of calcium carbonate (CaCO3) reinforce the exoskeleton. This process is seen as a reaction of calcium (Ca2+) and bicarbonate (HCO3–) ions to produce CaCO3. Although striated invertebrate musculature bears significant similarity to vertebrate skeletal muscle, there are several key differences. These include the variety of sarcomere lengths and sarcotubular constructions depending on the necessary properties. The superior strength of long sarcomere muscle fibers contributes to the muscle fiber composition of species depending on the species behavior. Due to their biochemical properties as well as their biocompatibility, renewability, sustainability, and biodegradation qualities, the materials composing the cuticle of the pincer are expected to replace petroleum-based polymers. As work is done to overcome the challenges involved with their larger-scale production, pincer cuticle-inspired materials are expected to yield sustainable innovations in various domains and industries and have a revolutionary impact on human lives in the foreseeable future.
References
Ashraf, M. A., & Sarfraz, M. (2016). Biology and evolution of life science [Editorial]. Saudi Journal of Biological Sciences, 23(1), S1-S5. https://doi.org/10.1016/j.sjbs.2015.11.012
Bacaita, E. S., Ciobanu, B. C., Popa, M., Agop, M., & Desbrieres, J. (2014). Phases in the temporal multiscale evolution of the drug release mechanism in IPN-type chitosan based hydrogels [Article]. Physical Chemistry Chemical Physics, 16(47), 25896-25905. https://doi.org/10.1039/c4cp03389b
Betts, J. G., Young, A. K., Wise, J. A., Johnson, E. J., B., P., Kruse, D. H., Johnson, J. E., Womble, M., & DeSaix, P. (2013). Anatomy and Physiology. OpenStax.
Blaus, B. (2014). Skeletal Muscle Fiber. In (2 ed., Vol. 3.26 MB). WikiJournal of Medicine.
Bock, N., Woodruff, M. A., Hutmacher, D. W., & Dargaville, T. R. (2011). Electrospraying, a reproducible method for production of polymeric microspheres for biomedical applications [Article]. Polymers, 3(1), 131-149. https://doi.org/10.3390/polym3010131
Cai, Z. X., Mo, X. M., Zhang, K. H., Fan, L. P., Yin, A. L., He, C. L., & Wang, H. S. (2010). Fabrication of chitosan/silk fibroin composite nanofibers for Wound-dressing Applications [Article]. International Journal of Molecular Sciences, 11(9), 3529-3539. https://doi.org/10.3390/ijms11093529
Cameron, J. N. (1985). Post-moult calcification in the blue crab (Callinectes sapidus): Relationships between apparent net H+ excretion, calcium and bicarbonate [Article]. Journal of Experimental Biology, VOL. 119, 275-285. https://www.scopus.com/inward/record.uri?eid=2-s2.0-0022371039&partnerID=40&md5=6a33b354287fa000714aeabdd7a9b199
Cazón, P., Vázquez, M., & Velazquez, G. (2018). Composite films of regenerate cellulose with chitosan and polyvinyl alcohol: Evaluation of water adsorption, mechanical and optical properties [Article]. International Journal of Biological Macromolecules, 117, 235-246. https://doi.org/10.1016/j.ijbiomac.2018.05.148
Cazón, P., Velazquez, G., Ramírez, J. A., & Vázquez, M. (2017). Polysaccharide-based films and coatings for food packaging: A review [Article]. Food Hydrocolloids, 68, 136-148. https://doi.org/10.1016/j.foodhyd.2016.09.009
Cheerarot, O., & Baimark, Y. (2015). Biodegradable silk fibroin/chitosan blend microparticles prepared by emulsification-diffusion method [Article]. E-Polymers, 15(2), 67-74. https://doi.org/10.1515/epoly-2014-0134
Chung, T. W., Chang, C. H., & Ho, C. W. (2011). Incorporating chitosan (CS) and TPP into silk fibroin (SF) in fabricating spray-dried microparticles prolongs the release of a hydrophilic drug [Article]. Journal of the Taiwan Institute of Chemical Engineers, 42(4), 592-597. https://doi.org/10.1016/j.jtice.2010.11.003
Clarkson, E. N. K. (1985). Carboniferous crustaceans [Article]. Geology Today, 1(1), 11-15. https://doi.org/10.1111/j.1365-2451.1985.tb00277.x
Dhanikula, A. B., Singh, D. R., & Panchagnula, R. (2005). In vivo pharmacokinetic and tissue distribution studies in mice of alternative formulations for local and systemic delivery of paclitaxel: Gel, film, prodrug, liposomes and micelles [Article]. Current Drug Delivery, 2(1), 35-44. https://doi.org/10.2174/1567201052772852
El Seoud, O. A., Jedvert, K., Kostag, M., & Possidonio, S. (2022). Cellulose, chitin and silk: the cornerstones of green composites [Review]. Emergent Materials, 5(3), 785-810. https://doi.org/10.1007/s42247-021-00308-0
Fitts, R. H. (2008). The cross-bridge cycle and skeletal muscle fatigue. Journal of Applied Physiology, 104(2), 551-558. https://doi.org/10.1152/japplphysiol.01200.2007
Fujiwara, S. I., & Kawai, H. (2016). Crabs grab strongly depending on mechanical advantages of pinching and disarticulation of chela [Article]. Journal of morphology, 277(10), 1259-1272. https://doi.org/10.1002/jmor.20573
Furlani, F., Marfoglia, A., Marsich, E., Donati, I., & Sacco, P. (2021). Strain Hardening in Highly Acetylated Chitosan Gels [Article]. Biomacromolecules, 22(7), 2902-2909. https://doi.org/10.1021/acs.biomac.1c00293
Galiano, F., Briceño, K., Marino, T., Molino, A., Christensen, K. V., & Figoli, A. (2018). Advances in biopolymer-based membrane preparation and applications [Review]. Journal of Membrane Science, 564, 562-586. https://doi.org/10.1016/j.memsci.2018.07.059
Gericke, M., Trygg, J., & Fardim, P. (2013). Functional cellulose beads: Preparation, characterization, and applications [Review]. Chemical Reviews, 113(7), 4812-4836. https://doi.org/10.1021/cr300242j
Glaeser, G., & Nachtigall, W. (2019). Shape, Movement, Lever. In The Evolution and Function of Biological Macrostructures (pp. 0-35). Springer Berlin Heidelberg. https://doi.org/10.1007/978-3-662-59291-5_1
Greenfeld, I., Kellersztein, I., & Wagner, H. D. (2020). Nested helicoids in biological microstructures [Article]. Nature Communications, 11(1), Article 224. https://doi.org/10.1038/s41467-019-13978-6
Huxley, H., & Hanson, J. (1954). Changes in the Cross-Striations of Muscle during Contraction and Stretch and their Structural Interpretation. Nature, 173(4412), 973-976. https://doi.org/10.1038/173973a0
Huxley, H. E. (1969). The Mechanism of Muscular Contraction. Science, 164(3886), 1356-1366. https://doi.org/doi:10.1126/science.164.3886.1356
Juncos Bombin, A. D., Dunne, N. J., & McCarthy, H. O. (2020). Electrospinning of natural polymers for the production of nanofibres for wound healing applications [Review]. Materials Science and Engineering C, 114, Article 110994. https://doi.org/10.1016/j.msec.2020.110994
Kalantari, K., Afifi, A. M., Jahangirian, H., & Webster, T. J. (2019). Biomedical applications of chitosan electrospun nanofibers as a green polymer – Review [Review]. Carbohydrate Polymers, 207, 588-600. https://doi.org/10.1016/j.carbpol.2018.12.011
Kanmani, P., Aravind, J., Kamaraj, M., Sureshbabu, P., & Karthikeyan, S. (2017). Environmental applications of chitosan and cellulosic biopolymers: A comprehensive outlook [Review]. Bioresource Technology, 242, 295-303. https://doi.org/10.1016/j.biortech.2017.03.119
Kellersztein, I., Cohen, S. R., Bar-On, B., & Wagner, H. D. (2019). The exoskeleton of scorpions’ pincers: Structure and micro-mechanical properties [Article]. Acta Biomaterialia, 94, 565-573. https://doi.org/10.1016/j.actbio.2019.06.036
Kellersztein, I., Greenfeld, I., & Wagner, H. D. (2021). Structural analysis across length scales of the scorpion pincer cuticle [Article]. Bioinspiration and Biomimetics, 16(2), Article 026013. https://doi.org/10.1088/1748-3190/abd2d2
Lane, S. M. (2018). What is a weapon? [Article]. Integrative and Comparative Biology, 58(6), 1055-1063. https://doi.org/10.1093/icb/icy083
Levinton, J. S., & Allen, B. J. (2005). The paradox of the weakening combatant: Trade-off between closing force and gripping speed in a sexually selected combat structure [Article]. Functional Ecology, 19(1), 159-165. https://doi.org/10.1111/j.0269-8463.2005.00968.x
Li, H., Hu, C., Yu, H., & Chen, C. (2018). Chitosan composite scaffolds for articular cartilage defect repair: A review [Review]. RSC Advances, 8(7), 3736-3749. https://doi.org/10.1039/c7ra11593h
Lin, W., Liu, P., Li, S., Tian, J., Cai, W., Zhang, X., Peng, J., Miao, C., Zhang, H., Gu, P., Wang, Z., Zhang, Z., & Luo, T. (2021). Multi-scale design of the chela of the hermit crab Coenobita brevimanus [Article]. Acta Biomaterialia, 127, 229-241. https://doi.org/10.1016/j.actbio.2021.04.012
Liu, Y., Lv, Z., Zhang, C., Zhu, X., Shi, T., Zhong, S., & Meng, Z. (2014). Preparation and immunogenicity of silk fibroin/chitosan microspheres for DNA vaccine delivery against infectious bursal disease virus [Article]. Shengwu Gongcheng Xuebao/Chinese Journal of Biotechnology, 30(3), 393-403. https://doi.org/10.13345/j.cjb.130344
Longo, M. V., & Díaz, A. O. (2013). Morphology of the claw closer muscle in two estuarine crab species (Crustacea, Varunidae): An ultrastructural study [Article]. Zoological Science, 30(8), 663-669. https://doi.org/10.2108/zsj.30.663
Longo, M. V., Goldemberg, A. L., & Díaz, A. O. (2011). The claw closer muscle of Neohelice granulata (Grapsoidea, Varunidae): A morphological and histochemical study [Article]. Acta Zoologica, 92(2), 126-133. https://doi.org/10.1111/j.1463-6395.2010.00484.x
Lv, B. H., Tan, W., Zhu, C. C., Shang, X., & Zhang, L. (2018). Properties of a stable and sustained-release formulation of recombinant human parathyroid hormone (rhPTH) with chitosan and silk fibroin microparticles [Article]. Medical Science Monitor, 24, 7532-7540. https://doi.org/10.12659/MSM.911203
Mellon, D. (1992). Microscopic anatomy of invertebrates. Vol. 10, Decapod Crustacea.
Meyers, M. A., Chen, P. Y., Lin, A. Y. M., & Seki, Y. (2008). Biological materials: Structure and mechanical properties [Review]. Progress in Materials Science, 53(1), 1-206. https://doi.org/10.1016/j.pmatsci.2007.05.002
Michael. (2021). Crabs and Molting Process. https://aquariumbreeder.com/
Nagasawa, H. (2012). The crustacean cuticle: Structure, composition and mineralization [Article]. Frontiers in Bioscience – Elite, 4 E(2), 711-720. https://doi.org/10.2741/e412
Nagpal, K., Singh, S. K., & Mishra, D. N. (2010). Chitosan nanoparticles: A promising system in novel drug delivery [Review]. Chemical and Pharmaceutical Bulletin, 58(11), 1423-1430. https://doi.org/10.1248/cpb.58.1423
Natarajan, B., & Gilman, J. W. (2018). Bioinspired Bouligand cellulose nanocrystal composites: A review of mechanical properties [Review]. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 376(2112), Article 20170050. https://doi.org/10.1098/rsta.2017.0050
O’Neill, J. J., Jr., & Reichardt, E. J. (1951). Method of Producing Cellulose Pellets (U. S. Patent No. U. S. P. a. T. Office.
Oka, S. I., Tomita, T., & Miyamoto, K. (2016). A mighty claw: Pinching force of the coconut crab, the largest terrestrial crustacean [Article]. PLoS ONE, 11(11), Article e0166108. https://doi.org/10.1371/journal.pone.0166108
Olsen, A. M. (2019). A mobility-based classification of closed kinematic chains in biomechanics and implications for motor control [Review]. Journal of Experimental Biology, 222(21), Article jeb195735. https://doi.org/10.1242/jeb.195735
Parushuram, N., Ranjana, R., Narayana, B., Mahendra, M., & Sangappa, Y. (2021). Facile fabrication of silk fibroin microparticles: their characterization and potential adsorption study [Article]. Journal of Dispersion Science and Technology, 42(10), 1513-1531. https://doi.org/10.1080/01932691.2020.1774383
Petrany, M. J., Swoboda, C. O., Sun, C., Chetal, K., Chen, X., Weirauch, M. T., Salomonis, N., & Millay, D. P. (2020). Single-nucleus RNA-seq identifies transcriptional heterogeneity in multinucleated skeletal myofibers. Nat Commun, 11(1), 6374. https://doi.org/10.1038/s41467-020-20063-w
Prasad, V., & Millay, D. P. (2021). Skeletal muscle fibers count on nuclear numbers for growth. Semin Cell Dev Biol, 119, 3-10. https://doi.org/10.1016/j.semcdb.2021.04.015
Reshmi, R., & Bijukumar, A. (2010). First report of the hermit crabs Coenobita brevimanus and Coenobita rugosus (Crustacea: Decapoda: Anomura) from the Indian coast. Marine Biodiversity Records, 3, e121, Article e121. https://doi.org/10.1017/S1755267210001089
Roer, R., & Dillaman, R. (1984). The structure and calcification of the crustacean cuticle [Article]. Integrative and Comparative Biology, 24(4), 893-909. https://doi.org/10.1093/icb/24.4.893
Royuela, M., Fraile, B., Arenas, M. I., & Paniagua, R. (2000). Characterization of several invertebrate muscle cell types: A comparison with vertebrate muscles [Article]. Microscopy Research and Technique, 48(2), 107-115. https://doi.org/10.1002/(SICI)1097-0029(20000115)48:2<107::AID-JEMT6>3.0.CO;2-U
Sabourian, P., Yazdani, G., Ashraf, S. S., Frounchi, M., Mashayekhan, S., Kiani, S., & Kakkar, A. (2020). Effect of physico-chemical properties of nanoparticles on their intracellular uptake [Review]. International Journal of Molecular Sciences, 21(21), 1-20, Article 8019. https://doi.org/10.3390/ijms21218019
Schofield, R. M. S., Nesson, M. H., Richardson, K. A., & Wyeth, P. (2003). Zinc is incorporated into cuticular “tools” after ecdysis: The time course of the zinc distribution in “tools” and whole bodies of an ant and a scorpion [Article]. Journal of Insect Physiology, 49(1), 31-44. https://doi.org/10.1016/S0022-1910(02)00224-X
Sengupta, T., & Han, J. H. (2013). Surface Chemistry of Food, Packaging, and Biopolymer Materials. In Innovations in Food Packaging: Second Edition (pp. 51-86). https://doi.org/10.1016/B978-0-12-394601-0.00004-7
Simone, Y., & Van Der Meijden, A. (2021). Armed stem to stinger: A review of the ecological roles of scorpion weapons [Review]. Journal of Venomous Animals and Toxins Including Tropical Diseases, 27. https://doi.org/10.1590/1678-9199-JVATITD-2021-0002
Singh, A., Narvi, S. S., Dutta, P. K., & Pandey, N. D. (2006). External stimuli response on a novel chitosan hydrogel crosslinked with formaldehyde [Article]. Bulletin of Materials Science, 29(3), 233-238. https://doi.org/10.1007/BF02706490
Spudich, J. A. (2001). The myosin swinging cross-bridge model. Nature Reviews Molecular Cell Biology, 2(5), 387-392. https://doi.org/10.1038/35073086
Srivastava, V., Gusain, D., & Sharma, Y. C. (2015). Critical Review on the Toxicity of Some Widely Used Engineered Nanoparticles [Article]. Industrial and Engineering Chemistry Research, 54(24), 6209-6233. https://doi.org/10.1021/acs.iecr.5b01610
Suksangpanya, N., Yaraghi, N. A., Pipes, R. B., Kisailus, D., & Zavattieri, P. (2018). Crack twisting and toughening strategies in Bouligand architectures [Article]. International Journal of Solids and Structures, 150, 83-106. https://doi.org/10.1016/j.ijsolstr.2018.06.004
Tardy, B. L., Mattos, B. D., Greca, L. G., Kämäräinen, T., Klockars, K. W., & Rojas, O. J. (2019). Tessellation of Chiral-Nematic Cellulose Nanocrystal Films by Microtemplating [Article]. Advanced Functional Materials, 29(25), Article 1808518. https://doi.org/10.1002/adfm.201808518
Tian, B., Hua, S., Tian, Y., & Liu, J. (2020). Chemical and physical chitosan hydrogels as prospective carriers for drug delivery: A review [Article]. Journal of Materials Chemistry B, 8(44), 10050-10064. https://doi.org/10.1039/d0tb01869d
Wang, H. Y., Wei, Z. G., & Zhang, Y. Q. (2020). Dissolution and regeneration of silk from silkworm Bombyx mori in ionic liquids and its application to medical biomaterials [Review]. International Journal of Biological Macromolecules, 143, 594-601. https://doi.org/10.1016/j.ijbiomac.2019.12.066
Wang, Y., Wang, J., Yuan, Z., Han, H., Li, T., Li, L., & Guo, X. (2017). Chitosan cross-linked poly(acrylic acid) hydrogels: Drug release control and mechanism [Article]. Colloids and Surfaces B: Biointerfaces, 152, 252-259. https://doi.org/10.1016/j.colsurfb.2017.01.008
Weaver, J. C., Milliron, G. W., Miserez, A., Evans-Lutterodt, K., Herrera, S., Gallana, I., Mershon, W. J., Swanson, B., Zavattieri, P., DiMasi, E., & Kisailus, D. (2012). The stomatopod dactyl club: A formidable damage-tolerant biological hammer [Article]. Science, 336(6086), 1275-1280. https://doi.org/10.1126/science.1218764
West, J. M., Humphris, D. C., & Stephenson, D. G. (1992). Differences in maximal activation properties of skinned short- and long-sarcomere muscle fibres from the claw of the freshwater crustacean Cherax destructor [Article]. Journal of Muscle Research and Cell Motility, 13(6), 668-684. https://doi.org/10.1007/BF01738256
Wilk, S., & Benko, A. (2021). Advances in fabricating the electrospun biopolymer-based biomaterials [Review]. Journal of Functional Biomaterials, 12(2), Article 26. https://doi.org/10.3390/jfb12020026
Wu, W., Gu, J., Zhou, G., Zhang, L., Gong, M., & Dai, H. (2014). Fabrication of natural cellulose microspheres via electrospraying from NaOH/Urea aqueous system [Article]. Journal of Applied Polymer Science, 131(16), Article 40656. https://doi.org/10.1002/app.40656
Xiong, R., Kim, H. S., Zhang, S., Kim, S., Korolovych, V. F., Ma, R., Yingling, Y. G., Lu, C., & Tsukruk, V. V. (2017). Template-Guided Assembly of Silk Fibroin on Cellulose Nanofibers for Robust Nanostructures with Ultrafast Water Transport [Article]. ACS Nano, 11(12), 12008-12019. https://doi.org/10.1021/acsnano.7b04235
Xu, K., Zhang, Y., Ye, Q., Wu, J., Li, Q., Su, G., Harper, D. P., Du, G., Ye, X. P., & Wang, S. (2021). Natural cuticle-inspired chitin/silk fibroin/cellulose nanocrystal biocomposite films: Fabrication and characterization [Article]. Materials Research Express, 8(3), Article 036402. https://doi.org/10.1088/2053-1591/abe974
Xu, S., Li, H., Ding, H., Fan, Z., Pi, P., Cheng, J., & Wen, X. (2019). Allylated chitosan-poly(N-isopropylacrylamide) hydrogel based on a functionalized double network for controlled drug release [Article]. Carbohydrate Polymers, 214, 8-14. https://doi.org/10.1016/j.carbpol.2019.03.008
Zacaï, A., Vannier, J., & Lerosey-Aubril, R. (2016). Reconstructing the diet of a 505-million-year-old arthropod: Sidneyia inexpectans from the Burgess Shale fauna [Article]. Arthropod Structure and Development, 45(2), 200-220. https://doi.org/10.1016/j.asd.2015.09.003
Zhang, Z., Zhao, Z., Zheng, Z., Liu, S., Mao, S., Li, X., Chen, Y., Mao, Q., Wang, L., Wang, F., Wang, X., Pan, Z., & Li, G. (2019). Functionalization of polyethylene terephthalate fabrics using nitrogen plasma and silk fibroin/chitosan microspheres [Article]. Applied Surface Science, 495, Article 143481. https://doi.org/10.1016/j.apsusc.2019.07.223
Zhou, F., Wu, Z., Wang, M., & Chen, K. (2010). Structure and mechanical properties of pincers of lobster (Procambarus clarkii) and crab (Eriocheir Sinensis) [Article]. Journal of the Mechanical Behavior of Biomedical Materials, 3(6), 454-463. https://doi.org/10.1016/j.jmbbm.2010.05.001