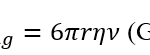Abstract
Coccolithophores are one of the world’s most important calcifying organisms. Populating the greater light-intensive areas of the ocean, these unicellular haptophytes are more broadly classified as photosynthetic phytoplankton. These organisms’ anatomy, physiology, and various processes make them irreplaceable contributors to the world’s carbon cycle. They are responsible for modifying upper-ocean alkalinity and affecting air/sea carbon exchange, which is accomplished by two main processes: photosynthesis and calcification. Coccoliths, the calcified exterior shell and identifying features of coccolithophores, are involved in many aspects of the unicellular organism’s life. Having been seen as a means of protection from mechanical stress thanks to excellent compression resistance, protection from ultraviolet radiation, and buoyancy, coccoliths, and the encompassing coccosphere, drastically impact the group of species for the better. Coccolithophores are also equipped with structures known as vestigial haptonema, a flagella-like structure that is involved in the maneuvering of the organism, as well as in prey capture, aspects which are important considering they are otherwise distributed vertically, geographically, and seasonally based on ocean currents. Additionally, thanks to their remarkable optical properties, we can track particulate inorganic and organic carbon. Overall, this group of species has adapted phenomenally to the complex forces and problems they face in the ocean, and seeing as they are at the bottom of the marine food chain, their success allows for the prosperity of Earth’s oceans.
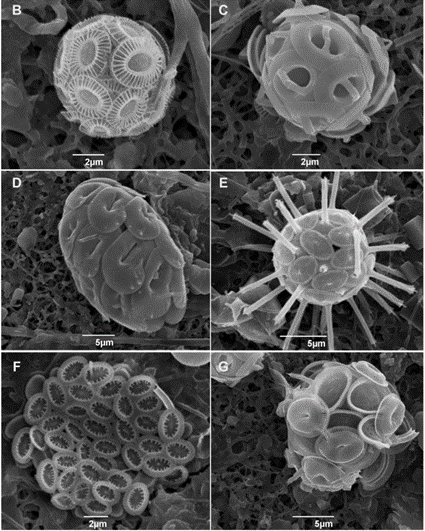
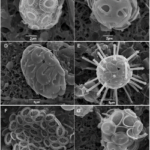
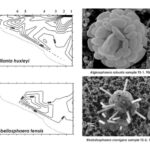
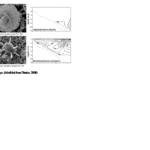
Fig. 1 SEM micrographs of different coccolithophore specimens. B. Emiliania huxleyi, C. Gephyrocapsa oceanica, D. Helicosphaera carteri, E. Rhabdosphaera clavigera, F. Syracosphaera protrudens, G. Syracosphaera pulchra (Dimiza et al., 2020).
Introduction
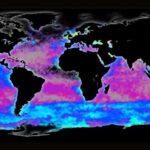
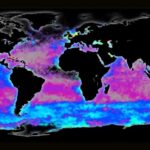
Phytoplankton are microorganisms that are the base of the aquatic food web. Being primary producers and at the base of the aquatic food web, the whole aquatic ecosystem is dependent on phytoplankton to sustain and develop. There are many species that compose oceanic phytoplankton, a few key species are diatoms, dinoflagellates, chlorophytes, and green algae. But one that plays a central role in the marine carbon cycle, flourishing the most in nutrient-poor environments is the coccolithophore (Fig. 1). Surrounded by microscopic calcite plates called coccoliths, forming an enclosed coccosphere, coccolithophores are a unique type of unicellular algae. Although small, these algae are incredibly important to marine ecosystems as well as the general health of the planet. In this paper, we will explore the physical properties of these little but significant species, as well as their interactions with the environment. In fact, as will be further discussed, the accumulation of their shed coccoliths in the ocean and add up to more than 1.5 million tons of calcite per year, making them the leading calcite producers of the marine world (Weier, 1999). It is said that Earth’s inorganic and organic carbon system has been transformed by the evolutionary and ecological success of these species over the last 220 million years (de Vargas, 2007 #22). The observed calcite belt (Fig. 2) in the Southern Ocean is a piece of evidence that shows coccolithophore’s important contribution to Earth’s inorganic and organic carbon systems.
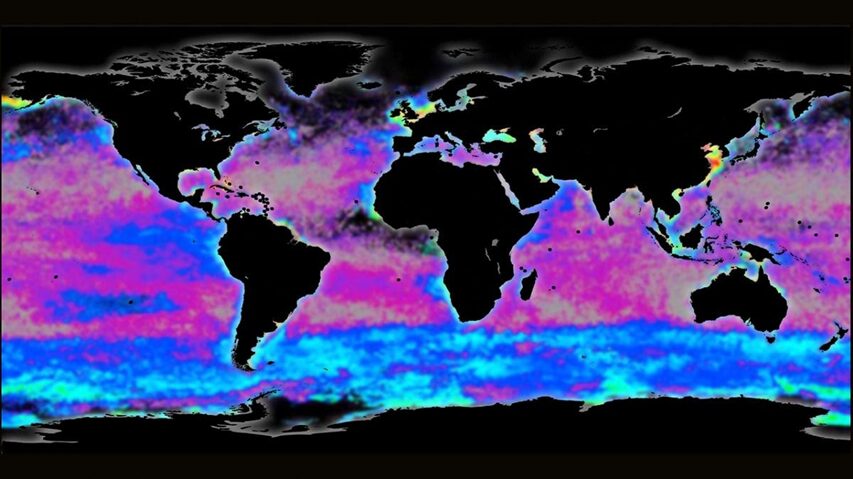
Fig. 2 Map of the Great Calcite Belt in the Southern Ocean. The blue colouration indicates high calcite concentrations (Rosengard, 2017).
Coccolith and Coccolithosphere Structure
For a number of years now, researchers have hypothesized the function and purpose of a coccolithophore’s calcified shell: the coccosphere. Some current theories suggest that it could be for protection against mechanical stress, diffusion of light, and blocking UV radiation, as well as buoyancy control (Young, 1987).
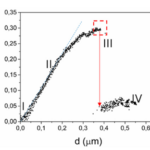
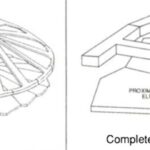
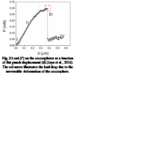
The hypothesis that the shell of coccolithophores provides mechanical protection has been proven to be true for the most common species of coccolithophores, Emiliania huxleyi (E. huxleyi). Similar to many other species, they are equipped with a coccosphere composed of interlocking calcium carbonate (CaCO3) coccoliths, glued together by a polysaccharide matrix. Consisting of several layers of coccoliths, the structure measures around 5-10 μm (Jaya et al., 2016). A study conducted with an E. Huxleyi strain has confirmed that the complex coccosphere confers mechanical protection to the cell. A total of 23 coccospheres possessing an average of 20 coccoliths forming 2-3 coccolith layers were tested; placed inside a scanning electron microscope (SEM), each of them was individually subject to compression by a flat punch indenter acting as a micro-crusher (Jaya et al., 2016). The coccospheres were compressed under displacement control at a rate of 10 nm/s, and their deformation process was monitored under the SEM until fracture occurred (Jaya et al., 2016). A load (P) as a function of displacement (d) graph was constructed, clearly illustrating the successive events characterizing the deformation behavior of the coccosphere until complete failure (Fig. 3).
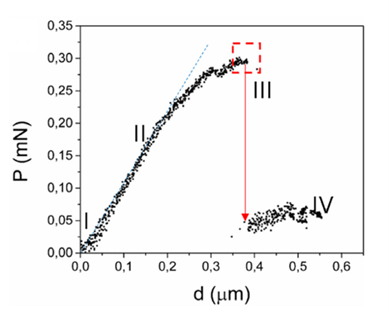
Fig. 3 Load (P) on the coccospheres as a function of flat punch displacement (d) (Jaya et al., 2016). The red arrow illustrates the load drop due to the irreversible deformation of the coccosphere.
After the initiation of compression by the flat punch (I), the load on the coccosphere increases linearly, indicating a pure elastic (fully reversible) deformation. A deviation from the linear pattern (II) signals the onset of plastic (irreversible) deformation or crack initiation. Then, the load instantly drops (III), signalling that the coccosphere has lost its structural integrity. Finally, a low and constant force is sustained by the coccosphere (IV). The maximum load that the coccospheres took before failure ranged between 0.07 mN and 0.37 mN with a mean value of 0.20 mN ± 0.07 mN (Jaya et al., 2016).
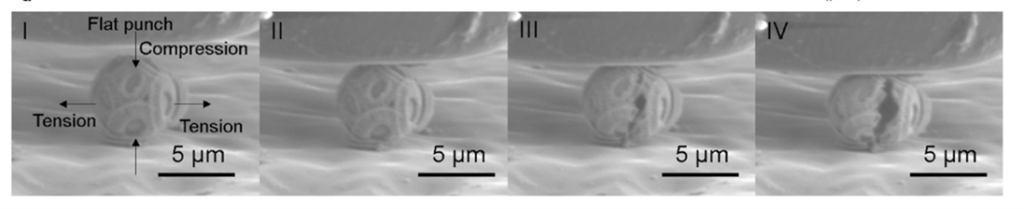
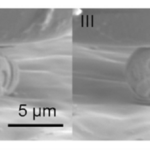
Fig. 4 Snapshots illustrating the different stages of coccosphere deformation and fracture. The numbers I-IV link the images with the load (P) vs displacement (d) graph (Jaya et al., 2016).
It is also important to note that during the test, the compression force applied on the coccosphere was uniaxial in the vertical direction, leading to tensile stresses in the horizontal direction (Fig. 4 I), whereas underwater, the coccolithophores are subject to hydrostatic (tri-axial) stress conditions; the stress sustained by the coccospheres under a uniaxial testing condition is, therefore, greater than the hydrostatic stress it is normally under, meaning that the maximum loads obtained from the test are the lower limit of the load coccospheres can truly support in their natural habitat (Jaya et al., 2016).
The coccospheres can accommodate an important load through linear elastic deformation before irreversible fracture, which sets them apart from other purely calcite materials, consolidating the idea that the coccosphere can serve as mechanical protection for the cell (Jaya et al., 2016). The impressive damage tolerance of the coccosphere could be explained by the architecture of the interlocked coccoliths and the polysaccharides that glue them together. In fact, the polysaccharide matrix gives room for the coccoliths to move relative to each other when subjected to an external force (Jaya et al., 2016). In addition, the coccolith is shaped like a cantilever, optimizing the protection of the cell (Fig. 5). In fact, the cantilever shape ensures that the individual coccoliths always start cracking at the point where the distal shield element is attached to the outer and inner tube elements; the contact with the flat punch imposes a bending force on the distal element, where the stress is the highest near its junction with the tube elements (Jaya et al., 2016). The fracture then propagates along the fixed ends of the cantilever, and if loading persists, the coccosphere separates into two hemispheres that can continue taking a lower load (Jaya et al., 2016).
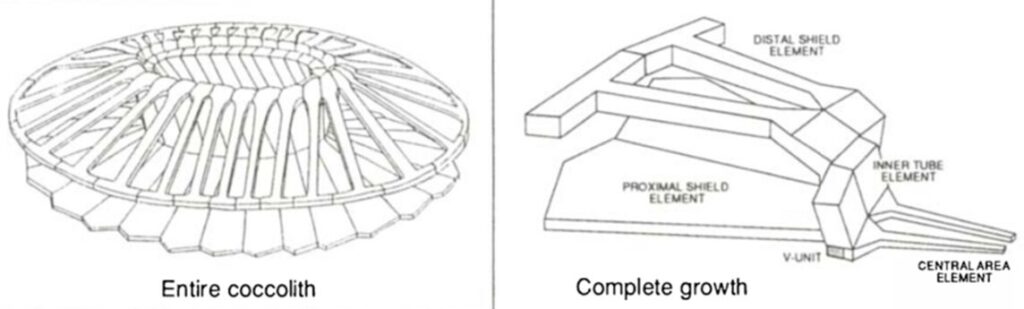
Fig. 5 A model of the coccolith and the cantilever structure forming the coccolith (Paasche, 2002).
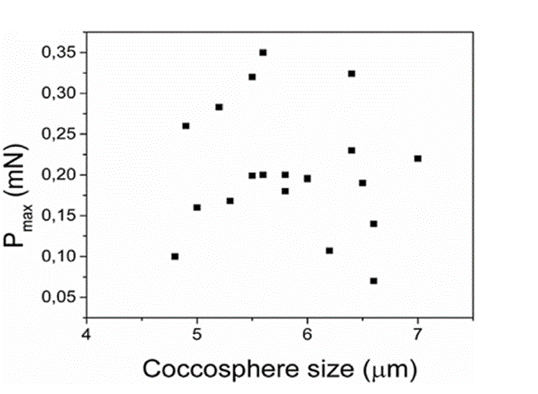
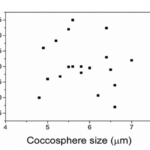
Fig. 6 Maximum load (Pmax) as a function of coccosphere size, showing that there is no correlation between the two (Jaya et al., 2016).
Data indicates that there is no apparent link between the maximum load and the number of coccolith layers and therefore the size of the coccosphere (Fig.6). However, the number of layers does play a significant role in mechanical protection due to the fact that when the outer layer of coccoliths is damaged, the layers underneath can still withstand compression (Jaya et al., 2016).
In short, the complex architecture of coccospheres enables them to support compression force fairly large compared to their size, despite being composed of more than 90% ceramic constituents (Jaya et al., 2016), which confirms the fact that coccoliths have evolved to confer mechanical protection.
Protection against light and UV radiation
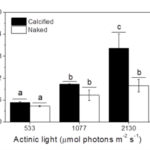
While the calcification in coccolithophores can be interpreted as an evolutionary adaptation to protect them from mechanical stress, that is not the only possible explanation for the coccolith and coccosphere morphologies. A study has examined the effect of photosynthetically active radiation (PAR) and ultraviolet radiation (UVR) on calcified and naked E. huxleyi strains, revealing that coccoliths play an important role in reducing the potentially harmful effects of light and UVR on the species (Xu et al., 2016).
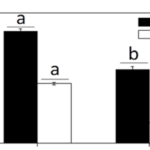
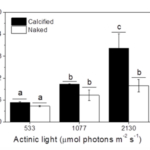
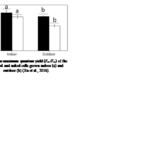
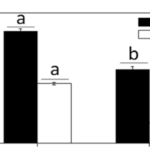
Many factors were evaluated to observe the behavior of the coccolithophores when incubated in low-light environments and when subjected to visible radiation (400-700 nm), UV-B radiation (280-315 nm), and UV-A radiation (315-400 nm) (Xu et al., 2016). The growth rate was tracked based on cell concentration before and after experimental incubations. Interestingly, it was found that both calcified and naked cells had decreased growth rates when moved to an outside environment with the presence of natural solar radiation; yet, the cells with coccoliths had growth rates 3.5 times higher than those of naked cells (Fig. 7).
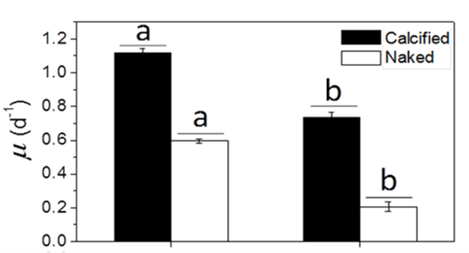
Fig. 7 The specific growth rate (µ) of the calcified and naked cells grown in indoor (a) and outdoor (b) conditions (Xu et al., 2016).
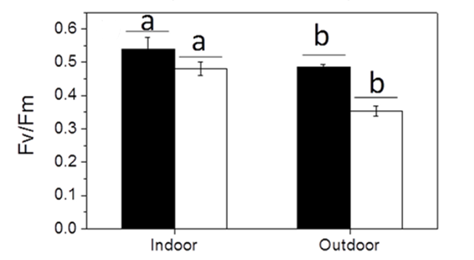
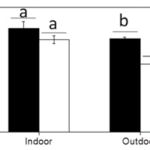
Fig. 8 The maximum quantum yield (Fm /Fm) of the calcified and naked cells grown indoor (a) and outdoor (b) (Xu et al., 2016).
To continue, the maximum quantum yield of photosystem II of the cells was measured to compare the photochemical performance of the dark-adapted cells. The quantum yield of the cells refers to their efficiency in converting absorbed light into emitted light, which gives insight into their rate of photosynthesis and is measured through the fluorescence of chlorophyll a molecules (Xu et al., 2016). The maximum quantum yield was calculated as Fv /Fm = (Fm – F0)/Fm , where F0 is the basal fluorescence under a photon flux density of 0.2 µmol m-2 s-1, Fv is the variable fluorescence, and Fm is the maximum fluorescence with a light pulse of 5000 µmol m-2 s-1 (Xu et al., 2016). The maximal quantum yield decreased for both types of cells when they were transferred to outdoor conditions, though, as the trend was more significant for naked cells (Fig. 8); it is clear that solar radiation has a detrimental effect on the cells, but those that were covered by coccoliths were better protected (Xu et al., 2016).
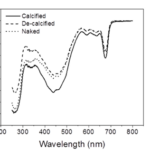
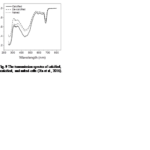
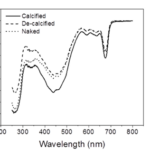
In order to understand coccolith absorptivity, the study also measured the transmission of both visible and UV radiation through the coccoliths. The results revealed that relative to decalcified cells, the presence of coccoliths led to the reduction of transmission of visible radiation by 7.5%, that of UV-A by 14.1%, and that of UV-B by 18.4% (Fig. 9). The coccolith, therefore, serves as a light-mitigating tool, possibly functioning like a UV light filter that limits the amount of harmful light that reaches the inside of cells and hinders their photochemical performance.
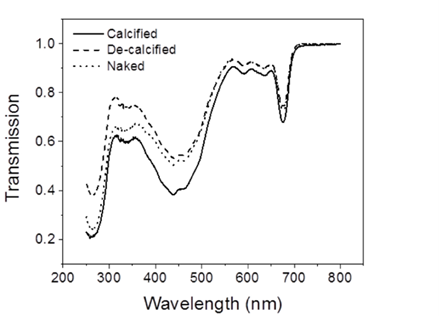
Fig. 9 The transmission spectra of calcified, decalcified, and naked cells (Xu et al., 2016).
Finally, another factor that was taken into account was the non-photochemical quenching (NPQ) of the cells measured under different light conditions. NPQ is a photoprotective mechanism employed by aquatic photoautotrophs to dissipate excess energy as heat (Xu et al., 2011). The results indicated that calcified cells showed higher NPQ values compared to naked cells (Fig. 10), meaning that the presence of a coccolith shell facilitated the NPQ process, making it apparent again that coccoliths confer protection against harmful light and UV radiations. Perhaps, the interlocking coccoliths distributed on many layers provide a greater surface area for energy dissipation.
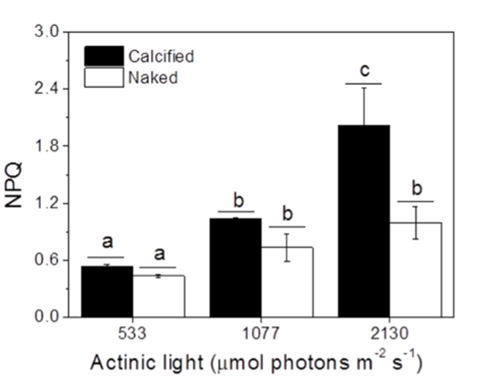
Fig. 10 The non-photochemical quenching (NPQ) of calcified and naked cells grown indoors under different light levels (Xu et al., 2016).
Vestigial Haptonema
Considered to be calcifying haptophytes, coccolithophores, like many others in this family, have a unique structure; the vestigial haptonema. This filamentous appendage is an organelle unique to haptophytes and is sometimes mistaken for a flagellum under the light microscope when in fact, it is not (Fig. 11) (Ha et al., 2023). Much like the flagella, this organelle’s origin is the anterior tip of haptophyte cells, such as a coccolithophore, and the organelle’s behaviours are coordinated (Tsuji & Yoshida, 2017). However, the haptonema distinguishes itself by its internal structure and the several roles it plays, including prey capture and obstacle avoidance (Tsuji & Yoshida, 2017).

Fig. 11 Similar appearance between two flagella (left) and a vestigial haptonema (right) on a haptophyte (Gregson, Green & Leadbeater, 1993).
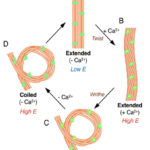
In terms of structural differences, the haptonema are composed of around six or seven microtubules in the tip of the organelle, and an additional two at the base, all of which are surrounded by endoplasmic reticulum (Tsuji & Yoshida, 2017) as well as microtubule-associated proteins (MAPs) (Nomura et al., 2019). It was also shown, through electron microscopy, that in the extended state, all microtubules but one are arranged in parallel. The exception is often found to be wrapped around the other microtubules (Nomura et al., 2019). This is unique in terms of structural composition as it allows for the haptonema to bend and coil which is useful for some of its roles such as obstacle avoidance and prey capture, among others.
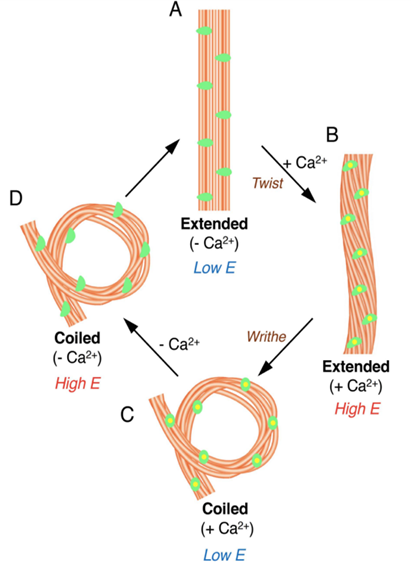
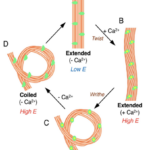
Fig. 12 Model for configurational change in microtubules of haptonema responsible for coiling (Nomura et al., 2019).
First, let us discover the mechanism by which the haptonema coils and extends. According to Nomura et al. (2019), the whole process is triggered by mechanical stimulation (such as contact between the haptonema and another object) but the motor-independent mechanism in which this stimulation takes place remains unknown. Despite this, Nomura et al. (2019) were able to successfully analyze the whole process of haptonema coiling. There are several steps in this action, starting with the left-handed twisting of the haptonema and then a writhing motion from the tip of the structure to form a helix (Nomura et al., 2019). The helix then uncoils upon recovery. Recall the unusual arrangement of the microtubules in the haptonema; upon electron microscopy, Nomura et al. (2019) determined that the microtubules were unusually flexible. In addition, they concluded that there is a Ca2+-dependent coiling inhibitor, paclitaxel, which is a molecule stabilizer, and, in the absence of Ca2+, induces a right-handed twist of the haptonema (Fig. 12) (Nomura et al., 2019). This, along with the discovery that the addition of Ca2+ causes the propagation of a bend to the proximal region of the structure, suggests that the incredibly quick coiling of the haptonema is due to changes in the microtubule surface lattice and thus, conformational changes of microtubules, all regulated by calcium cations (Nomura et al., 2019). These microtubules have different configurations depending on the state in which the haptonema is presented (Fig. 13-14): In the coiled state, the structure of the microtubules is highly curved, sometimes exceeding the curvature limits of microtubules (past the point of breakage). The high amount of bending stress suggests that ions are responsible for the configurational change of the microtubules as the binding induces a mechanical strain on the microtubules (Nomura et al., 2019). The release of calcium cations, on the other hand, ends the mechanical stress applied to the microtubules, thus returning the haptonema to its extended state (Fig. 12-13) (Nomura et al., 2019). This may suggest that this entire mechanism is a design approach to storing elastic energy in coiled microtubules since Ca2+ ions are abundant in seawater. According to Gregson, Green & Leadbeater (1993), the haptonema coiling is dependent on an external concentration of Ca2+ ion between 10-6 and 10-7 M. This concentration range is a threshold below which the haptonema coiling frequency is reduced upon cell death (Gregson, Green & Leadbeater, 1993). This property of haptonema coiling upon cell death is one that Gregson, Green & Leadbeater (1993) used to show that haptonema coiling is dependent on external calcium ions concentrations. They applied four different compounds inhibiting the uptake of external Ca2+ ions to organisms with haptonema and discovered that upon cell death, the haptonema would remain erect, indicating the absence of Ca2+ ions in the haptonema, thus demonstrating the dependence on external calcium ions (Gregson, Green & Leadbeater, 1993).
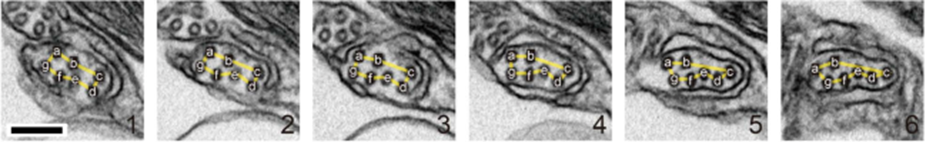
Fig. 13 Organization of microtubules in extended haptonema (Nomura et al., 2019).
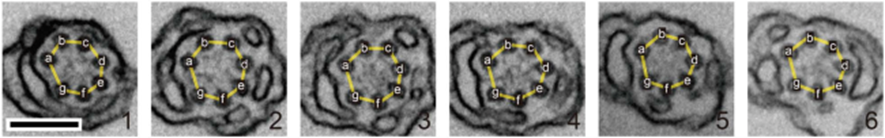
Fig. 14 Organization of microtubules in coiled haptonema (Nomura et al., 2019).
Furthermore, it was concluded that unstimulated haptophytes can control the intracellular calcium ion concentration independently of the extracellular Ca2+ ion concentration (Gregson, Green & Leadbeater, 1993). The discoveries point to the possibility of these organisms relying on potential energy stored in extracellular ions, and the haptonema coiling being a response similar to that of an action potential insofar as there is a stimulus that causes a change of permeability of the membrane, leading to an influx of ions. In this case, the ion rushing in is Ca2+ instead of Na+. This also supports the belief that unstimulated haptophytes can control the intracellular concentration of calcium ions, as they would need to maintain the intracellular ion levels lower than the extracellular ones to create a concentration gradient for calcium when there is a stimulus.
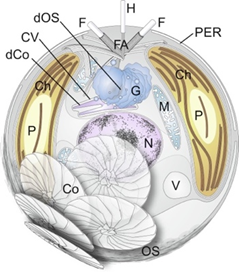
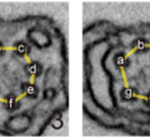
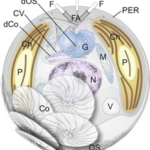
Fig. 15 A detailed diagram of main structures of haptophyte algae, member of the Isochrysidales and Coccolithales orders (both of which are coccolithophores)) (Tsuji & Yoshida, 2017). Circled is the flagellar apparatus (FA) which includes two flagella (F) and one haptonema (H).
Now that the mechanism by which the haptonema coils and extends has been clarified, let us take a deeper look into the functions of this structure: obstacle avoidance is one of the primary purposes of this mobile organelle (Nomura et al., 2019). The haptonema, along with flagella and the basal bodies of the aforementioned structures, come together to form a complex known as the flagellar apparatus. (Fig. 15) (Tsuji & Yoshida, 2017). One can think of this complex as a boat propulsion system. The beating of the flagella can be thought of as dual motors propelling the vessel, while the bending and coiling of the haptonema are akin to the rudder of marine vessels. Together, they are responsible for moving and steering the boat, which in this case, is the rest of the cell. The coiling of the haptonema is incredibly fast, between 1/60 to 1/100 seconds (Tsuji & Yoshida, 2017), and allows for incredibly responsive adjustments. If the cell was rapidly approaching an obstacle, the haptonema would coil and the flagella would beat in the opposite direction, allowing the maneuvering of the cell in a manner that avoids the obstacle (Tsuji & Yoshida, 2017).
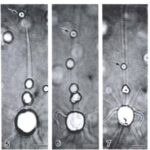
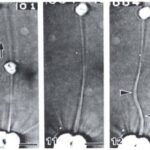
The feeding process of coccolithophores with vestigial haptonema also utilizes this organelle’s unique bending and coiling features. According to Kawachi et al. (1991), to capture food, the haptonema must be fully extended forward and the flagella beat backward, close to the cell body. Once the required flagellar apparatus configuration is achieved, the coccolithophore is in a suitable position to acquire nutrients. This acquisition is done in several consecutive steps: firstly, there are the food particles attaching and adhering to the haptonema, (Fig. 16 A-B) before descending to what Kawachi et al. (1991) call the particle-aggregating center (Fig. 16 B-E) which, as the name suggests, is an area near the base of the haptonema where food particles aggregate. The particles are also tightly packed in this region (Fig. 16 E, 17 A) before the aggregate makes its way to the tip of the haptonema (Fig. 17 A-C). This may only occur, however, once the cell has ceased to swim. Once at the tip, the haptonema bends (Fig. 17 D-E, 18 A-E) and the food is delivered to the cell’s lateral posterior surface (Fig. 18 F-G) where it is subsequently translocated and ingested into the cell via phagocytosis (Kawachi et al., 1991), before the haptonema returns to its erect state (Fig. 18 H-J).
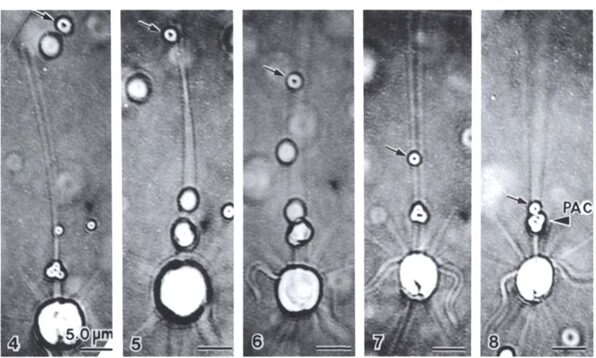
Fig. 16 A-E Video images of haptonema capturing food particles and collecting them at the “particle-aggregating center” (Kawachi et al., 1991).
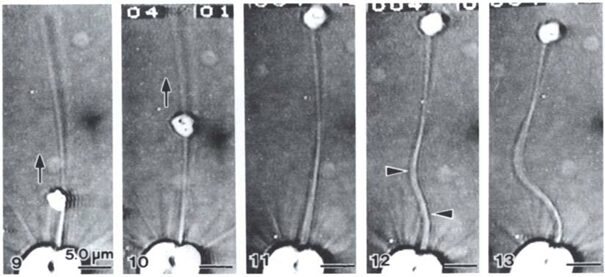
Fig. 17 A-E Video images of the process of haptonema moving food aggregate to tip of organelle and beginning of bend of haptonema (Kawachi et al., 1991).
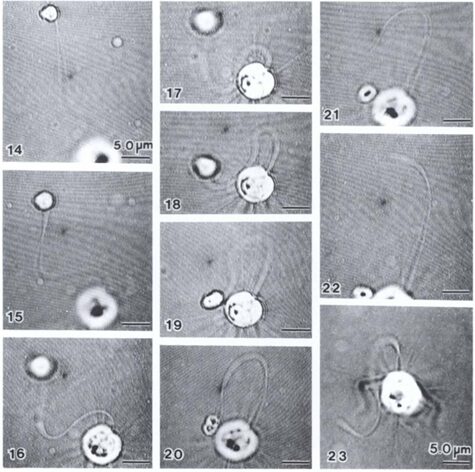
Fig. 18 A-J Video Images of haptonema bending and translocating food aggregate to lateral posterior surface of cell (Kawachi et al., 1991).
Overall, the vestigial haptonema is an incredibly fascinating and useful organelle unique to haptophytes (of which coccolithophores make up 6/7 of the total species of haptophytes). The combination of all these evolutionary systems results in a lightning-fast coiling mechanism, allowing the unicellular organism to maneuver and avoid obstacles in an efficient way and capture nutrients.
Coccoliths and the Environment
Refractivity of coccoliths
In order to measure the ocean carbon input and output, researchers can rely on measuring the particulate inorganic carbon (PIC) and particulate organic carbon (POC). With coccoliths having high indices of refraction, they can easily be seen from space using remote sensing, they must be considered in PIC algorithms (Balch, 2018). In fact, the morphology of coccoliths, and how it changes based on nutrient availability must be considered when using satellite remote sensing as it could change their reflectance per mass of calcite, leading to skewed PIC values (Balch, 2018).
Coccoliths have further been seen to rotate the plane of linearly polarized light depending on their varying levels of calcite (Balch, 2018). Researchers have in fact seen that forward scatter polarization is related to the number of coccoliths per cell or degree of calcification (calcite mass) in fully plated coccolithophores (Balch, 2018), (Von Dassow, 2012). Using this, it is possible to distinguish calcite-containing particles, or cells, from non-calcified particles, because calcite-containing particles will have higher values of forward scatter light, which are polarized orthogonally to the incident laser (Von Dassow, 2012). This, however, was not observed for individual coccoliths – potentially due to the orientation relative to the incident laser in contrast to a roughly spherical coccosphere where orientation does not matter (Balch, 2018). The intensity of forward scatter polarization was not dependent on calcite weight for detached coccoliths; in this case, however, the mass showed a linear relationship with side scatter though to a lower extent (Von Dassow, 2012). For this reason, it may be the ratio of coccolith calcite mass to organic cells that determines the forward depolarization of light (Von Dassow, 2012).
Buoyancy and vertical displacement
Not only do coccolithophores vary in their geographic distribution, but their vertical positioning in the ocean water column also differs based on species, temperature, and access to nutrition (Dimiza, 2008) For all phytoplankton, it can prove beneficial to inhabit deeper regions of the ocean, below the photic zone, during periods of nutrient depletion (Honjo, 1976). Due to the high availability of light energy, the uppermost layers of the photic zone are often teeming with life, leading to high competition, and nutrient depletion. However, spanning the photic zone globally, coccolithophores seemingly favor these warm, low-productivity areas (Margarita D. Dimiza, 2008).
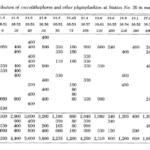
One study by Honjo showed that in sub-Arctic zones, coccolithophores are distributed pretty exclusively in the upper 50 m of water, whereas, in moderate temperature and tropical water, Coccolithophores are most abundant between 50 and 100 m, coinciding with the chlorophyll maximum layer (Honjo, 1976). A study performed in the Northwestern Sargasso Sea showed similar data with a majority of coccolithophores being within a 50 m depth. Though, as mentioned, the vertical displacement of coccolithophores is highly dependent on the species. Marshall noticed that certain species such as Calcioconus vitreus, and Calciosolenia granii were completely absent below 100 m depth while Coccolithus E. huxleyi was found at depths of 1,200 m and Michaelsarsia asymmetrica at 1,500 m (Marshall, 1966). The results of this study can be seen below in Fig. 19.
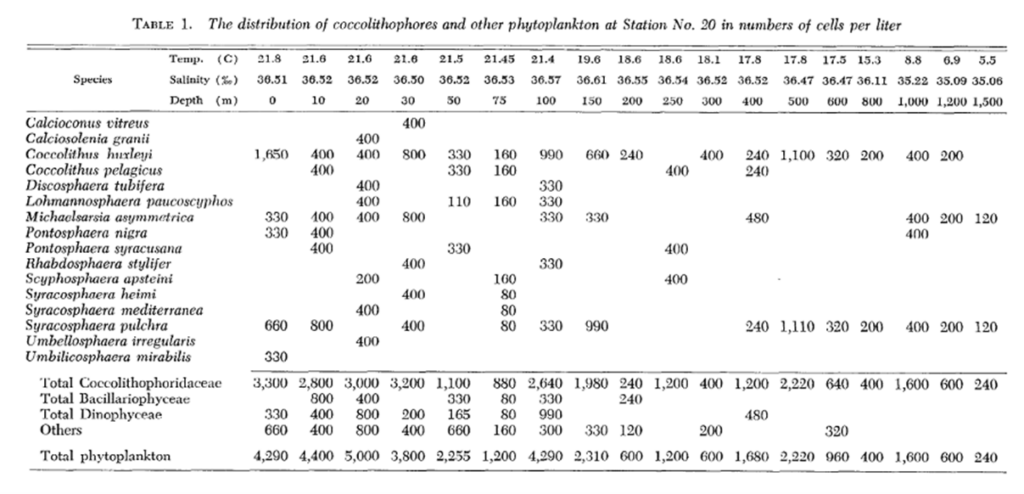
Fig. 19 Distribution of coccolithophores and other phytoplankton in cells per liter (Marshall, 1966).
Another study by Dimiza, Triantaphyllou & Dermitzakis sampled coccolithophore cell density from 0-120 meters at 8 different stations in the Gulf of Korthi and assessed the distribution of different species. The group saw cell density vary between 14.7×103 cell/L to 1.9×103 cell/L, finding higher densities in the upper and middle photic zones (Margarita D. Dimiza, 2008). Much like the other studies, they also found that many species prefer to live in specific depth ranges as seen in Fig. 20.
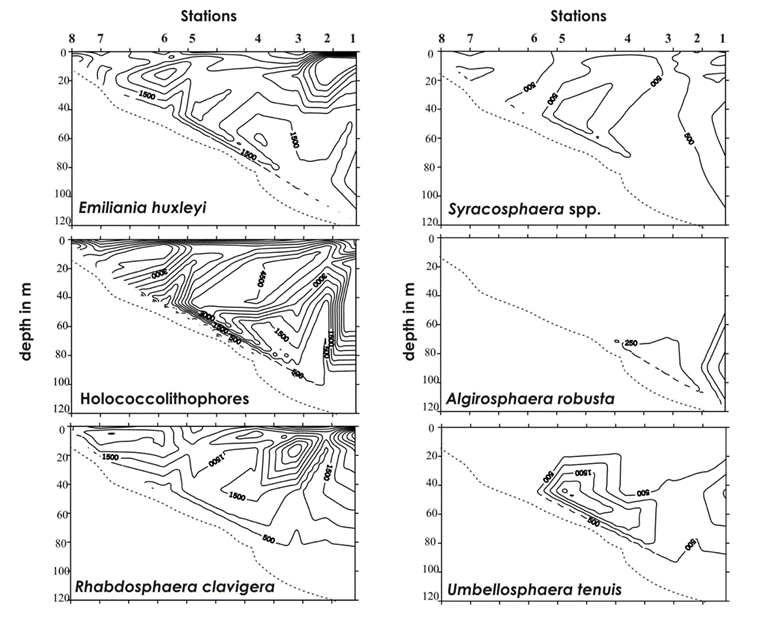
Fig. 20 Vertical distributions (cells/L) the species Emiliania huxleyi, Syracosphaera spp., Holococcolithophores, Algirosphaera robusta, Rhabdosphaera clavigera, and Umbellosphaera tenuis (Dimiza, 2008).
This species-specific vertical localization, as well as a sharp decrease in living coccolithophores between 200 and 400 m, suggest that they are able to control their position in the vertical column (Honjo, 1976). Though there is not much data to prove this theory, it is possible that their descending speed is controlled using their coccoliths as ballasts f or the cell and other descending materials (Honjo, 1976). As the majority of coccolithophores are consumed by grazers, the remaining forms of coccolith death would not significantly lead to large numbers of disintegrated coccoliths, thus, the origin of suspended free coccoliths may result from shedding to maintain buoyancy (Honjo, 1976).
During these samplings, Dimiza was also able to photograph the different coccolith species they came across (Fig. 21). When we compare these next to the different vertical distribution patterns, we can infer that their vertical placements could depend on their shape and size. Buoyancy of an object is dependent on the volume of an object as well as how their density compares with that of the fluid they are in, this ratio is known as specific density. Looking specifically at Algirosphaera robusta, its highest cell density is at around 70 – 110 m depth, while the E. huxleyi and Umbellosphaera tenuis are quite concentrated at 30-80 m and Rhabdosphaera clavigera at the upper 40 m. Looking at the microscopic imaging of these four samples, we see that they all roughly occupy the same volume, except for R. clavigera, which is about two times the size of the others. This increased volume leads to increased water displacement and thus greater buoyancy, potentially explaining why it is mainly found in shallow areas. Conversely, although similar, if not, larger in size to E. huxleyi and U. tenuis, A. robusta seems to have many coccoliths tightly packed onto its cell, potentially increasing the mass more gravely than its increased volume, leading to higher density, and lower buoyancy – explaining why cells are more concentrated at lower depths. These characteristics may have been adopted by each species in order to escape competition and acquire their own niche.
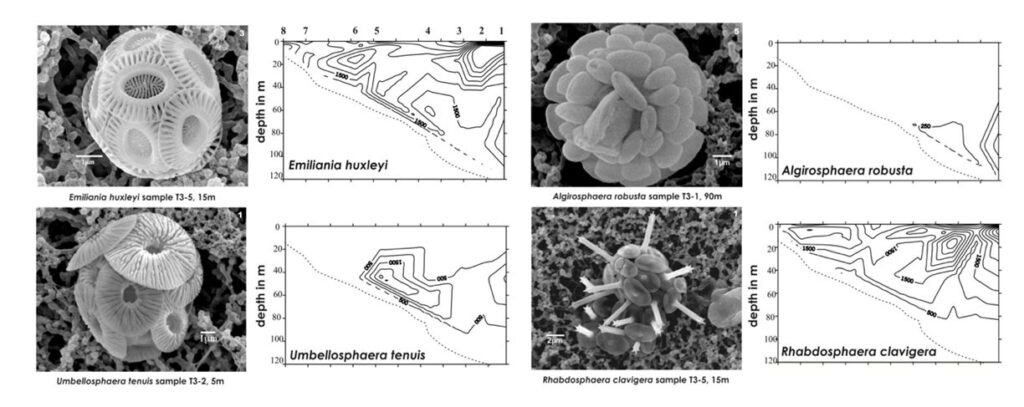
Fig. 21 Comparing vertical distribution and coccosphere shape (Modified from Dimiza, 2008).
Sinking of Coccoliths
Due to their importance regarding ocean carbon, the descent of shed coccoliths has also been extensively studied. From the point of view of Stokesian dynamics, assuming the coccolith to be a calcite sphere, they sink very slowly. See Stoke’s Law below where r is the radius of the object, η the viscosity of the liquid, and ν the velocity.
Fdrag=6πrην
Stokes Law (Gregerson, 2021).
However, in the field, as with vertical variation, the sinking speed of coccoliths changes from one species to the next. From laboratory measurements, it has been shown that the descending speed of E. huxleyi was about 1.6 µm/sec at 18 ℃ and about 1 µm/sec at 10 ℃ while Coccolithus neohelis sunk slightly faster. These laboratory results were a whole magnitude slower than the sinking speed calculated from Stokes law (Honjo, 1976). With these measurements, it was calculated that the residence time of a common coccolith can be between 50 years for large coccoliths such as Cyclococcolithina and 150 years for smaller coccoliths such as Gephyrocapsa ericsonii (Honjo, 1976). During this time, due to eddy diffusion (irregular, and turbulent mixing of a fluid by eddy motions), advection, and deep-water turbulence, coccoliths that reach the deep seafloor, 5,000 m below, could have displacement on the order of 105 km, about twenty-five times the circumference of the Earth, which is astronomical in comparison to 2 µm, the typical size of a coccolithophore. Though, with an average surface area of about 80 µm2, and the dissolution rate of calcite being about 0.03 mg cm2/year, a coccolith should dissolve in a single year, sinking less than 100 m (Honjo, 1976). Thus, there should be no singular coccoliths suspended in the calcite-undersaturated water column except for the uppermost layer due to their probabilistic dissolution (Honjo, 1976). The development of the deep-sea floor coccolith ooze would be impossible if coccoliths were to rain individually (Honjo, 1976). However, there are well-preserved coccoliths found in suspension in most of the oceans, telling us that the dissolution rates of a coccolith’s calcite must be at least two orders less than that of inorganic calcite (Honjo, 1976). Although this may be explained by what is discussed in the next paragraph, lower dissolution rates of organic calcite protect the coccolithophores by mitigating the dissolution of their coccosphere, which, as previously discussed, provides mechanical and photic protection.
To mediate this, the long-range downward transport of coccolith calcite is achieved by the formation of fecal pellets and agglutination of coccospheres and coccoliths (Paasche, 2019). Typically grazed on by zooplankton, coccoliths, which are not normally dissolved or crushed when ingested aggregate into fecal pellets (Honjo, 1976). These fecal pellets are often covered by pellicles so that the contents are protected from immediate chemical dissolution (Honjo, 1976). Within a single pellet, about 105 coccoliths, or about 1 µg of CaCO3 can descend at the speed of 160 m/day, reaching the sea floor 5000 m below within a month’s time while the dissolution of the coccolith is negligible (Honjo, 1976). In fact, through phytoplankton excrement, about 92% of coccoliths produced in the euphotic layer reach the deep-sea floor (Honjo, 1976). Although this does not necessarily prove advantageous to the coccolithophores, as will be discussed in the next session, it can be highly regarded as a design solution for nature. A summary of the process is shown in Fig. 22.
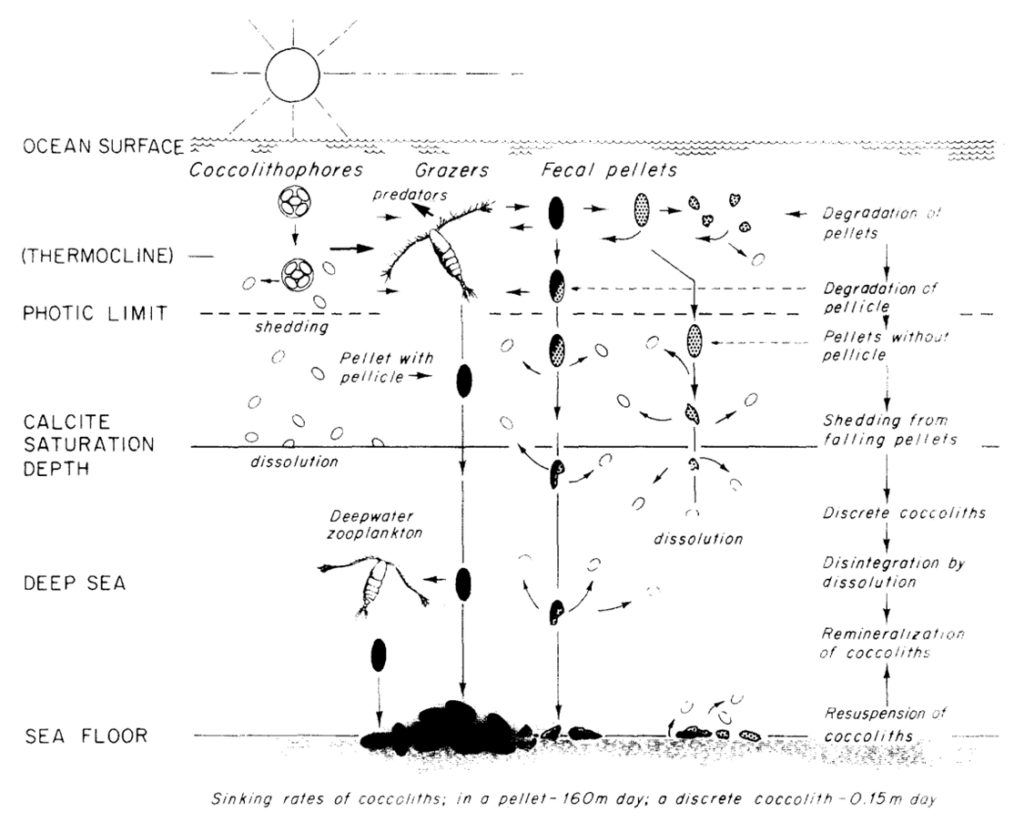
Fig. 22 Model of the production, transportation, dissolution and deposition of coccoliths in deep ocean (Honjo, 1976).
Marine Carbon Cycle
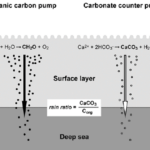
Coccolithophore plays an important role in the global marine carbon cycle. The formation of calcite coccolith in the upper sea layer and their subsequent sinking to the deep modifies upper-ocean alkalinity and directly affects air/sea carbon dioxide exchange. This process involves two main mechanisms of coccolithophore, photosynthesis and calcification reaction. First, the fixation of inorganic carbon via photosynthesis in the euphotic layer of the sea transforms carbon dioxide into an organic compound. This organic compound is then exported vertically down to the deep sea. On its way to the depths, the remineralization reaction of this organic compound releases carbon dioxide, which then accumulates in the deeper layers, sequestering atmospheric carbon into the deep sea. This process is termed the “organic carbon pump”. In contrast, the production and export of calcium carbonate has the opposite effect on air/sea CO2 exchange, causing a net release of CO2 to the atmosphere. Due to its counteracting effect on CO2 flux, this process is often referred to as the “carbonate counter pump” (Fig. 23). The ratio between the strength of these two processes is called the rain ratio (or PIC:POC ratio), which determines the flux of CO2 between the surface ocean and the overlying atmosphere. As such, calcite overproduction, typically seen in blooms of Emiliania huxleyi, can even turn into a net source of CO2 to the atmosphere. However, the coccoliths, that are formed from the synthesis of calcium carbonate, will accumulate into marine snow and act as ballast that will carry other organic matter into the deep sea. The presence of these coccoliths could thus enhance the sedimentation of particulate organic carbon such as in fecal pellets, therefore lessening the effect of CO2 release (Rost & Riebesell, 2004). In fact, the effect
of coccolith ballasting on atmospheric CO2 concentration could outweigh CO2 output from biomineralization. On geological time scales, certain types of coccoliths that are resistant to dissolution slowly accumulate in deep-oceanic sediments, at rates of less than 10 mm/1,000 years to more than 100 mm/1,000 years. Since the Cretaceous, coccoliths have been the prime contributors to the kilometers-thick accumulation of calcareous ooze-covering deposits that will eventually be subducted into the mantle of the Earth, thus depleting carbon from its surface for millions of years (De Vargas et al., 2007).
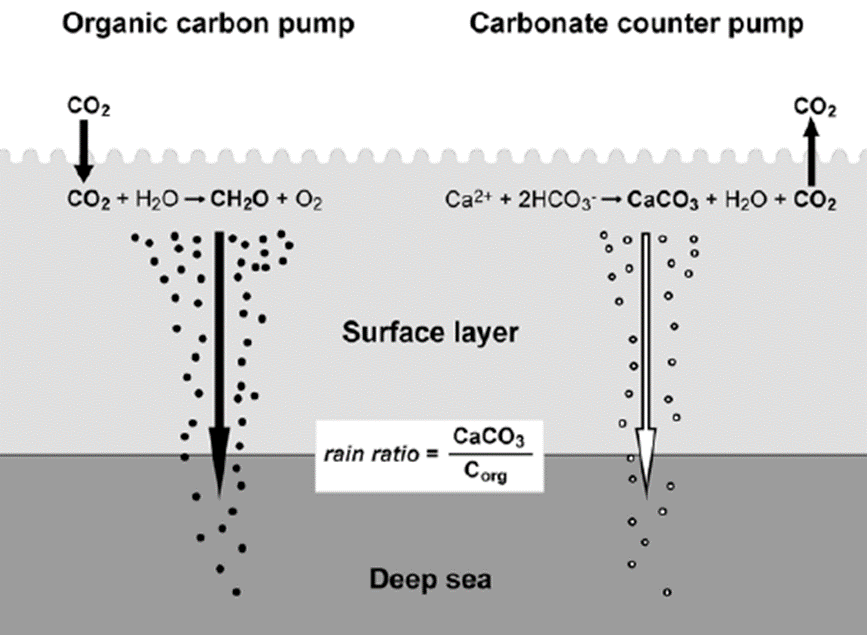
Fig. 23 Illustration of the two main mechanisms in coccolithophore that play an important role in the marine carbon cycle: organic carbon pump and carbonate counter pump (Rost & Riebesell, 2004).
Cliffs of Dover
The Cliffs of Dover is a natural scenery that shows evidence of coccolith subduction in the Earth’s mantle. The sheer cliffs are composed of white chalk, or calcite, made by coccolithophores, unicellular algae (Fig. 24). Coccoliths sink and accumulate on the sea floor, compacting and hardening into chalk (calcium carbonate). The White Cliffs’ chalk was laid down in a shallow sea above present-day England almost 100 million years ago and thrust upward by movements of the Earth’s crust. Nowadays, a new calcite plate formation is happening along the Great Calcite Belt. However, it is difficult to say whether the plates from the Great Calcite Belt will form chalk and produce a large structure like the Dover Cliffs. To build a Dover-like structure, many layers of calcite would need to be deposited on the seafloor over millions of years (William M. Balch, 2016).

Fig. 24 White Cliffs of Dover (Immanuel Giel from Wikipedia).
Response to Environmental Drivers
Many factors are known to have an effect on the photosynthesis of coccolithophores. In this part, we will refer to them as environmental drivers, including light intensity, light fluctuating frequency, CO2, temperature, and ultraviolet radiation (UVR). We will explore the effects of these drivers on coccolithophore’s physiological performance.
One of the major pressures that coccolithophore and any oceanic micro-organism must face is the shoaling of upper mixed layers in future ocean environments. The shoaling of upper mixed layers refers to the shallowing of the ocean mixed layer due to climate change. Consequently, as the mixed layer becomes shallower, coccolithophore cells are exposed to higher light intensity, and therefore high level of photoinhibition, a form of light-induced loss of photosynthetic activity. In particular, since the photosystems II are more sensitive to light than the rest of the photosynthetic machinery, photoinhibition often refers to the closing of PSII due to light damage. In order to respond to this phenomenon, coccolithophore cells will increase their effective absorption cross-section. To do so, when a photosystem II (PSII) reaction center closes or becomes photo-inactivated for protection against high light intensity, its antenna complex may serve other functional PSII centers via adjacent antennae, causing a dynamic and increasing effective antenna size. Coccolithophores have adapted this mechanism to protect themselves against PSII photoinhibition and cope with high light intensities. This negative effect of high light intensity, however, can be offset by lowering the fluctuating frequency of light. At lowly fluctuating light, more energy will be used for photo-damage repair processes, this leads to an increase in the maximum potential quantum efficiency of Photosystem II, measured by the Fv /Fm indicator, a normalized ratio created by dividing variable fluorescence by maximum fluorescence.
The combination of increased CO2 level and increased temperature act additively to increase the rate of carbon fixation. For example, the species Gephyrocapsa oceanica, while grown under elevated temperature and CO2 conditions, has many downregulated proteins. The energy saved from downregulation can be used for the repair of photodamages. On the other hand, the stimulated photosynthetic performance appeared to be attributed to the extra carbon loss which provides a protective role. The increase in ultraviolet radiation has the opposite effect, it decreases the photosynthetic carbon fixation rates of G. oceanica. It is shown that the presence of UVR increased the effective absorption cross sections, suggesting that PSII reaction centers close or become photo-inactivated not only due to the high light intensity but also due to the high level of UVR. Similarly, to high light intensity, the antenna complex serves adjacent antennae connected to open functional PSII centers, causing a dynamically increased effective antenna size (Jin, Liu, & Gao, 2019).
Geographical/seasonal distribution:
The composition and species richness of coccolithophores varies greatly between oceans and seasons. For a better understanding of this displacement of coccolithophores, we will look at the seasonal distribution of coccolithophores in two important seas: the Southern China Sea and the Northwest Aegean Sea.
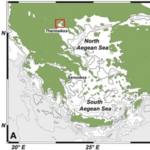
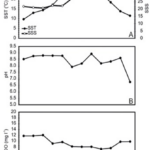
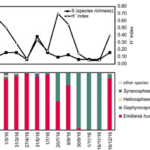
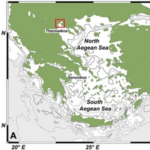
One of the study areas is Thessaloniki Bay, situated at the northern part of the Inner Thermaikos Gulf, Northwest Aegean Sea (Fig. 25), which is a semi-enclosed and shallow-water area, with an average depth of 30 m. The maximum annual sea surface temperature (SST) is 26 degrees Celsius in the summer and the minimum is 11 degrees Celsius in the winter season. The sea surface salinity (SSS) ranged from 35 at its highest during the winter, to around 22 from January to May (Fig. 26A). The pH ranges from 8.9 in September to 6.8 in December (Fig. 26B). Generally, high dissolved oxygen (DO) concentrations (Fig. 26C) were measured during the whole sampled year. The coccolithophore community consisted of 32 different species, such that the species richness is observed quite consistently, fluctuating between 2, from September to November, with the highest value at 15 during May (Fig. 27A). Meanwhile, the species diversity (H’ index) is highest in January and July (Fig. 27A). The two most abundant species of coccolithophore in this area are E. huxleyi and G. oceanica. The annual average abundance of E. huxleyi is 64% and of G. oceanica is 32%. The highest density of E. huxleyi (2.1 × 105 cells/liter) occurred during late spring–early summer (from April to July), and the lowest (0.6 × 103 cells/liter) was recorded during autumn (from September to November). On the other hand, the density peak of G. oceanica (2.1 × 105 cells/liter) was observed in July, but high values (above 1 × 104 cells/liter) persisted till November. Among the less abundant species, Helicosphaera carteri (H. careri) represented about 25% (2.7 × 103 cells/liter) of the January assemblage, and Syracosphaera spp. contributed up to 6% (1.4 × 104 cells/liter) of the total assemblage during May. In Thessaloniki Bay, we can see the dominance of the species E. huxleyi in late spring and early summer, with low salinity and high phosphorus concentration (Fig. 26 and 27), while the strong dominance of G. oceanica starts in late summer and persists during autumn intervals, with higher salinity and nutrient levels (Fig. 26 and 27) (Dimiza et al., 2020).
Fig. 25 Geographic mapping of the Thermaikos Gulf, Northwest, Aegean Sea (Dimiza et al., 2020).
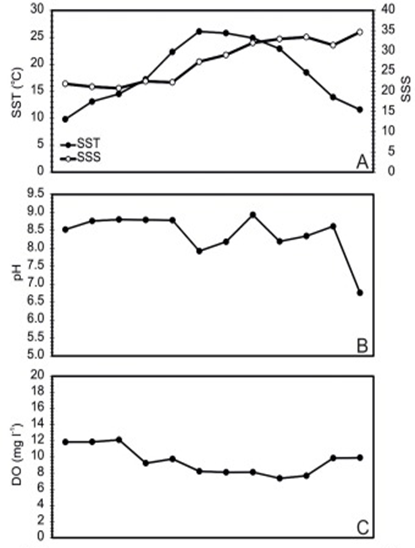
Fig. 26 Mapping of Thermaikos Gulf surface sea temperature and salinity (A), pH (B) and dissolved oxygen level (C) (Dimiza et al., 2020).
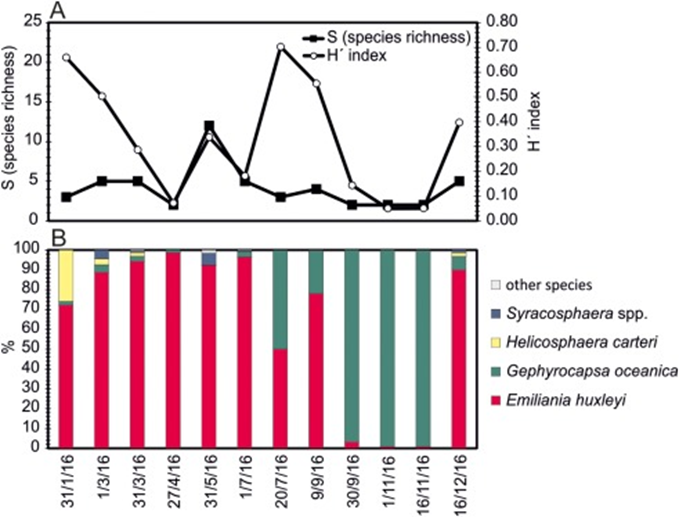
Fig. 27 Seasonal species richness and diversity (A), seasonal relative abundance (B)in Thermaikos Gulf (Dimiza et al., 2020).
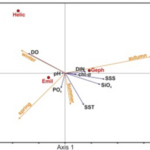
To better explain this seasonal distribution of different coccolithophore species, a canonical correspondence analysis was performed to plot the seasonal variation and environmental variability (Fig. 28). The three most abundant species are shown in this graph: “Emil” for E. huxleyi, “Geph” for G. oceanica and “Helic” for H. careri. It is shown in this graph that the dominance of different species of coccolithophore corresponds to an increase in an environmental variable. E. huxleyi is the most abundant species in this area and its abundance persists throughout the majority of the year; its bloom in the spring season, however, does not indicate a significant increase of variable, meaning this species is quite well-suited for the ambient condition of this sea. However, with the drastic change in surface sea salinity (SSS), an increase in the concentration of dissolved inorganic nitrogen (DIN) as well as orthosilicate (SiO4) during the autumn season corresponds to the dominance of G. oceanica over E. huxleyi. Similarly, in the winter season, the bloom of H. careri is associated with an increase in dissolved oxygen (DO) (Fig. 28) (Dimiza et al., 2020). Overall, G. oceanica has a high salinity tolerance and is more active in nutrient acquisition than E. huxleyi, correlating with the increase of salinity, and increase in the concentration of DIN and SiO4. Meanwhile, E. huxleyi is more adapted to cold temperatures than G. oceanica, which explains its returned dominance when approaching winter time. On the other hand, H. careri is an opportunistic species that will only grow in the high-nutrient season, especially with the increase in dissolved oxygen.
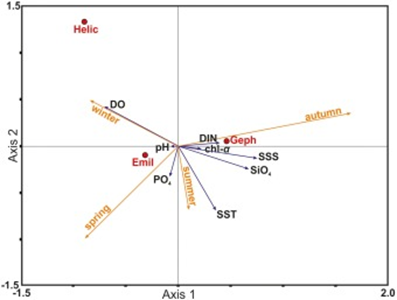
Fig. 28 Canonical correspondence analysis (CCA) plots showing the seasonal variation between coccolithophore species and environmental variables.
The partition in tolerances and behaviour of these coccolithophore species during different seasons of the year represent an evolutionary adaptation to minimize competition. Living in the same ocean area, different species must have different niches, or otherwise, one species will become extinct. In the case of Thessalonika Bay, G. oceanica competes with E. huxleyi by developing a better tolerance to salinity, taking advantage of the increase in nutrients to take over E. huxleyi. Meanwhile, H. careri develops an opportunistic behaviour to only grow when dissolved oxygen is abundant. Since neither G. oceanica nor H. careri can outcompete E. huxleyi in ambient conditions, these two solutions have adapted to take advantage of the environmental variability within different seasons of the year to avoid extinction.
In the South China Sea, the distribution of coccolithophore species’ is a lot more diverse. Remarkably, we see that the species E. huxleyi is outcompeted even during its bloom season. In this area, the dominance is also composed of three main species: E. huxleyi, G. oceanica, and Fraxinus profunda (F. profunda). G. oceanica shows the same trend while its bloom starts during the autumn season, taking advantage of the warm temperature and high salinity. Meanwhile, E. huxleyi is outcompeted by F. profunda during its bloom season from spring to summer. Most notably, we see the lowest abundance of E. huxleyi in the summers of 2009-2010, while F. profunda has its highest abundance during this time (Fig. 29 and 30) (Priyadarshani et al., 2019). Unlike the opportunistic behaviors of G. oceanica or H. careri in the Thessalonika Bay where these two species take advantage of the seasonal variability to compete with E. huxleyi, F. profunda instead develops to adapt better in this oceanic area and directly compete with E. huxleyi for nutrients. F. profunda has a different design solution that is also successful in competing against the most abundant coccolithophore species E. huxleyi.
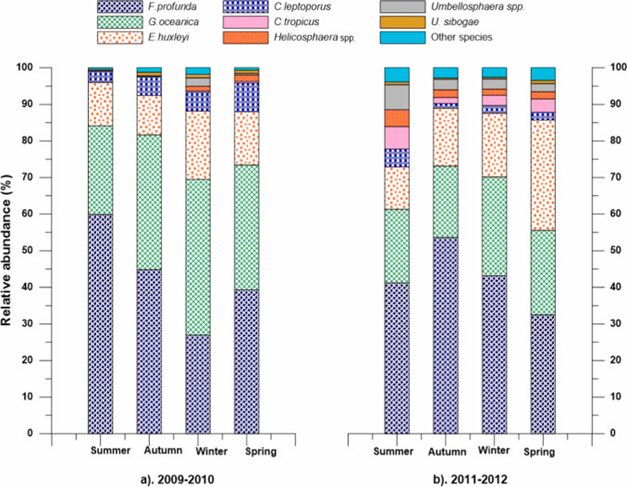
Fig. 29 Seasonal relative abundance of key coccolithophore species in four seasons during 2009–2010 and 2011–2012 in the northern South China Sea (Priyadarshani et al., 2019).
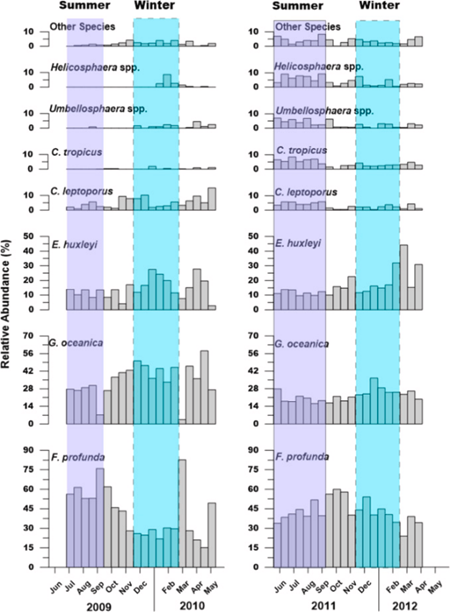
Fig. 30 Temporal variation in relative abundance of key coccolithophore species during 2009–2010 and 2011–2012 in the northern South China Sea (Priyadarshani et al., 2019).
To summarize, E. huxleyi is known to be the most frequent and abundant coccolithophore in modern oceans (Dimiza et al., 2020), it is obvious that different species will need to develop a design solution to compete with E. huxleyi to survive. The most common method, observed particularly in Thessaloniki Bay, is taking advantage of seasonal variability by adapting to the condition of a specific season to outcompete E. huxleyi. However, a more interesting approach was seen in F. profunda, when they developed the ability to adapt to the environment in the South China Sea to directly compete with E. huxleyi in this sea. In the end, despite E. huxleyi being the most abundant species of coccolithophore species, different coccolithophores can also survive by developing design solutions to succeed in this interspecific competition.
CONCLUSION
Evolving what can be referred to as ‘design solutions’, coccolithophores have developed over the ages into an affluent, long-lasting, and generally successful group of species that have a major role in the marine ecosystem and the Earth’s carbon cycle. At the base of the aquatic food chain, these photosynthetic unicellular algae directly and indirectly provide sustenance to all other ocean organisms. Not to mention their pivotal role in carbon exchange between the atmosphere and ocean, through the production of calcite. Overall, coccolithophores and these design solutions discussed have not only allowed for their establishment but have aided the establishment of many important systems and cycles.
One of such wonderous features coccolithophores have come to evolve is a calcite (CaCO3) protective shell, formed by individual interlocked coccolith plates, forming a coccosphere. With the ability to accommodate compression and tensile loads through linear elastic deformation, the shell of coccoliths protects coccolithophores from mechanical stresses, despite being mostly composed of brittle ceramic. The interlocking of the cantilever-shaped coccoliths allows the coccosphere to break at specific points such that the remaining individual coccoliths can continue to protect the cell, providing an ingenious solution to the constant mechanical pressure they face in the ocean. By acting like a UV light filter, this coccosphere has also been seen to minimize the harmful effects of light and UV radiation on the cell’s growth rate, maximum quantum yield, and non-photochemical quenching ability. Seeing as coccolithophores tend to live in the upper photic zone, and are constantly bombarded by intense UV radiation, having a shell to filter it out becomes extremely important and clever. Furthermore, as a response to high amounts of light, coccolithophores have developed the ability to increase their effective absorption cross-section to counter the photoinhibition phenomena and repair radiation damages during decreased fluctuating light to increase the maximum quantum efficiency of photosystem II. As such, coccolithophores have resourcefully used their access to high calcium and bicarbonate concentrations to create biological armor, protecting them from mechanical and photic stresses.
Another essential design solution coccolithophores have developed is their vestigial haptonema. Being unicellular, these coccolithophores are unable to travel large distances on their own and mostly rely on the ocean currents. So, in order to capture nutrients and avoid obstacles, they rely on this effective and rapid coiling structure.
Furthermore, it has been found that the abundance and diversity of coccolithophore communities vary depending on the season, the sea surface salinity, the water temperature, and pH. A study conducted in the Northwest Aegean Sea showed that species richness is highest in May and lowest from September to November. E. huxleyi and G.oceanica are the most abundant species in the area; E.huxleyi had the highest density during spring-early summer when salinity is low and phosphorus concentration is high, while G.oceanica density was highest in July and maintained elevated during the fall when salinity and nutrient levels are high. Another study conducted in the South China Sea indicated that more diverse coccolithophore species were present, while 3 species were the most dominant: E.huxleyi, G.oceanica, and F.profunda. Large variations in the vertical distribution of coccolithophore species have also been observed, showing that they may be able to control their position in the ocean water column. This may be a product of their ability to control density by shedding or producing coccoliths, using them as a ballast. As such, it is apparent that different coccolithophore species acquire their own niches and develop tolerance to varying sea conditions in order to avoid competition with other species and to survive.
It has also been discovered that the aggregation of sinking coccoliths provides a crucial role in the downward movement of biological materials, again behaving as a ballast. Though not necessarily beneficial to coccolithophores, this design solution of nature provides nutrients to the ocean floor ecosystem consisting of millions of species.
Knowing the crucial role coccolithophores play in the environment and knowing the crucial roles that the calcified coccospheres play in cell protection, it is essential to think about the detrimental effects that progressive seawater acidification and global warming can have on the future of the species. These environmental changes can lead to the reduction of growth and calcification rates of the coccoliths, which will disturb many of their functions described above; the thickness of the coccolith shell as well as the number of coccoliths per cell could be reduced, leading to less effective protection against UVR exposure (Xu et al., 2016). Coccolith malformations could also increase, which would threaten the structural integrity of the coccosphere (Jaya et al., 2016). Without their protective armor, coccolithophores become subject to the intense mechanical, chemical, and photic stresses of the ocean environment and may easily die out. While the other design solutions may potentially evolve and protect them further, these complex and astute mechanisms take many generations to develop and with the current rate of ocean acidification, coccolithophores will most likely not have the time to adjust. With the loss of these small but important organisms, we lose an essential part of the oceanic food web, carbon cycle, and nutrient distribution systems. There is so much elegance in the design of all organisms, as pointed out for coccolithophores in this essay, we must be wary of the impact we as humans have and ensure we do not destroy what has taken billions of years to come to the way it is now.
REFERENCES
Balch, W. M. (2018). The ecology, biogeochemistry, and optical properties of coccolithophores. Annual review of marine science, 10, 71-98. https://www.annualreviews.org/doi/10.1146/annurev-marine-121916-063319
de Vargas, C., Aubry, M.-P., Probert, I., & Young, J. (2007). Origin and evolution of coccolithophores: from coastal hunters to oceanic farmers. In Evolution of primary producers in the sea (pp. 251-285). Elsevier.
Gregerson, E. (2021, 2021). Stokes’s Law. https://www.britannica.com/science/turbulent-flow
Gregson, A. J., Green, J. C., & Leadbeater, C. (1993). STRUCTURE AND PHYSIOLOGY OF THE HAPTONEMA INCHRYSOCHROMULINA(PRYMNESIOPHYCEAE). II. MECHANISMS OF HAPTONEMATAL COILING AND THE REGENERATION PROCESS1. Journal of Phycology, 29(5), 686–700. https://doi.org/10.1111/j.0022-3646.1993.00686.x
Hay, W. (1968). Coccoliths and other calcareous nannofossils in marine sediments in Cyrenaica. BARR, FT: Geology and archaeology of Northern Cyrenaica, Libya, Petroleum Exploration Society of Libya, 149-157.
Hutchinson, G. E. (1967). A Treatise on Limnology, Introduction to Lake Biology and the Limnoplankton. J. Wiley & Sons. https://books.google.ca/books?id=MGTdREaXHOEC
Honjo, S. (1976). Coccoliths: Production, Transportation and Sedimentation. Marine Micropaleontology, 1, 65-79. https://www.sciencedirect.com/science/article/pii/0377839876900050
Jaya, B. N., Hoffmann, R., Kirchlechner, C., Dehm, G., Scheu, C., & Langer, G. (2016). Coccospheres confer mechanical protection: New evidence for an old hypothesis. Acta Biomaterialia, 42, 258-264. https://doi.org/https://doi.org/10.1016/j.actbio.2016.07.036
Jin, P., Liu, N., & Gao, K. (2019). Physiological responses of a coccolithophore to multiple environmental drivers. Marine Pollution Bulletin, 146, 225-235. https://doi.org/10.1016/j.marpolbul.2019.06.032
Margarita D. Dimiza, M. V. T. M. D. D. (2008). Vertical distribution and ecology of living coccolithophores in the marine ecosystems of Andros Island (Middle Aegean Sea) during late summer 2001. Hellenic Journal of Geosciences, 43, 7-20 7-20. http://www.hellenjgeosci.geol.uoa.gr/43/GEOL%2043%20PAGES%207-20.pdf
Marshall, H. G. (1966). OBSERVATIONS ON THE VERTICAL DISTRIBUTION OF COCCOLITHOPHORES IN THE NORTHWESTERN SARGASSO SEA 1. Limnology and Oceanography, 11(3), 432-435. https://aslopubs.onlinelibrary.wiley.com/doi/epdf/10.4319/lo.1966.11.3.0432?src=getftr
Paasche, E. (2019). A review of the coccolithophorid Emiliania huxleyi (Prymnesiophyceae), with particular reference to growth, coccolith formation, and calcification-photosynthesis interactions. Phycological Reviews, 20, 503-529. https://www.tandfonline.com/doi/pdf/10.2216/i0031-8884-40-6-503.1?casa_token=-sXzwwOkeWEAAAAA:9AP3ipNjL6BeeWUlNNh2iSCNlBkimOnM0jlFmDLcJzcCEvSwugN44fGsofufnRbuHFgujW9BQENrbg
Priyadarshani, W. N. C., Ran, L., Wiesner, M. G., Chen, J., Ling, Z., Yu, S., & Ye, Y. (2019). Seasonal and interannual variability of coccolithophore flux in the northern South China Sea. Deep Sea Research Part I: Oceanographic Research Papers, 145, 13-30. https://doi.org/10.1016/j.dsr.2019.01.004
Rosengard, S. (2017, January 22). The Great Calcite Belt. Https://Www.whoi.edu/; Woods Hole Oceanographic Institution. https://www.whoi.edu/multimedia/great-calcite-belt/
Rost, B., & Riebesell, U. (2004). Coccolithophores and the biological pump: responses to environmental changes. In H. R. Thierstein & J. R. Young (Eds.), Coccolithophores: From Molecular Processes to Global Impact (pp. 99-125). Springer Berlin Heidelberg. https://doi.org/10.1007/978-3-662-06278-4_5
Von Dassow, P., van Den Engh, G., Iglesias-Rodriguez, D., & Gittins, J.R. (2012). Calcification state of coccolithophores can be assessed by light scatter depolarization measurements with flow cytometry. Journal of plankton research, 34(12), 1011-1027. https://academic.oup.com/plankt/article/34/12/1011/1418292
Weier, J. (1999). What Is a Coccolithophore? Earth Observatory. https://earthobservatory.nasa.gov/features/Coccolithophores
William M. Balch, B. S. T., Bruce C. Bowler, Dave T. Drapeau, Laura C. Lubelczyk, Catherine Mitchell, Sara Rauschenberg et al. (2016). Giant algal bloom sheds light on formation of White Cliffs of Dover. American Geophysical Union. https://news.agu.org/press-release/giant-algal-bloom-sheds-light-on-formation-of-white-cliffs-of-dover/
Xu, J., Bach, L. T., Schulz, K. G., Zhao, W., Gao, K., & Riebesell, U. (2016). The role of coccoliths in protecting Emiliania huxleyi against stressful light and UV radiation. Biogeosciences, 13(16), 4637-4643. https://doi.org/10.5194/bg-13-4637-2016
Xu, K., Gao, K., Villafañe, V. E., & Helbling, E. W. (2011). Photosynthetic responses of Emiliania huxleyi to UV radiation and elevated temperature: roles of calcified coccoliths. Biogeosciences, 8(6), 1441-1452. https://doi.org/10.5194/bg-8-1441-2011
Young, J. (1987). Possible Functional Interpretations of Coccolith Morphology. Abh Geol, 39.