Abstract
Fundamentally, foraging is the action of searching and collecting for food in nature. Unlike hunting, which is distinguished by active pursuit and flight, foraging centers around detection, gathering and transport. Every animal has their individualized method for foraging, nevertheless, these methods are either designed to be performed individually or collaboratively. Furthermore, this seemingly simple, essential concept of obtaining food can be examined through its complex physical implications. In fact, the various foraging methods investigated in this essay will unpack just that, looking into detection plans concerning the vision and flocking behaviours of birds as well as the use of sponges by Bottlenose Dolphins. The essay will then examine the gathering element of foraging with Woodpeckers’ food retrieving drilling techniques, followed by the investigation of ants’ transportation of food source, whether it be with the Myrmicine ant species who perform alone, or the Paratrechina longicornis and Solenopsis invicta ants who choose to forage collaboratively. In this article, we will expand on six examples of foraging and their ties with varying physical properties.
Introduction
Foraging is critical for the survival of any species, as the vital need of any living being is to feed. Foraging, defined as the search for food or provisions, is practiced in different ways by various species. To accomplish this basic need, certain tools and strategies are used by the foraging animals. Some of them are specific to one species because they depend on the animals’ morphologies and capacities, as illustrated later in the essay for woodpeckers, myrmicine ants and goshawks. Others have been greatly influenced by the animal’s habitat, as in the case of bottlenose dolphins living in Shark Bay.
As much as foraging can be a collective process involving a group within a species, it can also consist of independent tasks executed by one individual. Foraging behaviours differ from one species to another, depending on whether the animal engages in foraging activities solitarily or if individuals cooperate with one another as a group. In case of group foraging, effective communication is extremely important in order to share information about the food source locations. These group efforts are illustrated with pelicans, as well as Paratrechina longicornis and Solenopsis invicta ants below. What brings these species together however is the fact that their behaviours reflect the Optimal Foraging Theory, an ecological model formulated by R.H. MacArthur and E.R. Pianka in 1966. The OFT states that animals forage in a manner that maximizes its net energy intake at the lowest energy cost (MacArthur & Pianka, 1966). This essay examines different foraging behaviours exhibited by a variety of animal species, all with a common goal of satisfying the Optimal Foraging Theory. The essay will focus on three stages of foraging: detection, gathering and transport of food.
Detection of Food Resource
Group Foraging: Flocking Behaviour of Birds
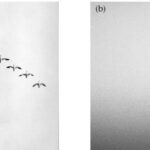
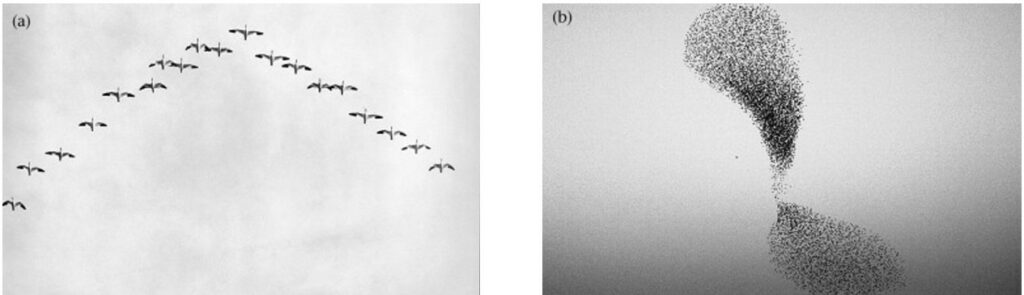
Flocking is a strategy used by birds to support their foraging activity, more specifically to detect prey. Flocking can be defined as the behaviour exhibited when a group of birds, called a flock, come together to forage as a group. A flock is therefore the gathering of individuals attracted by a common objective, which in case of foraging is feeding. There are two main organization patterns when describing flocks. One is the “cluster” where birds fly together in a loose, three-dimensional cloud in order to approach feeding grounds, but also to confuse predators (Figure 1 (b)). The second kind of pattern is the V-shape formation where birds fly at the same altitude but behind each other and positioned about 120 degrees from the bird in front (Figure 1 (a)).
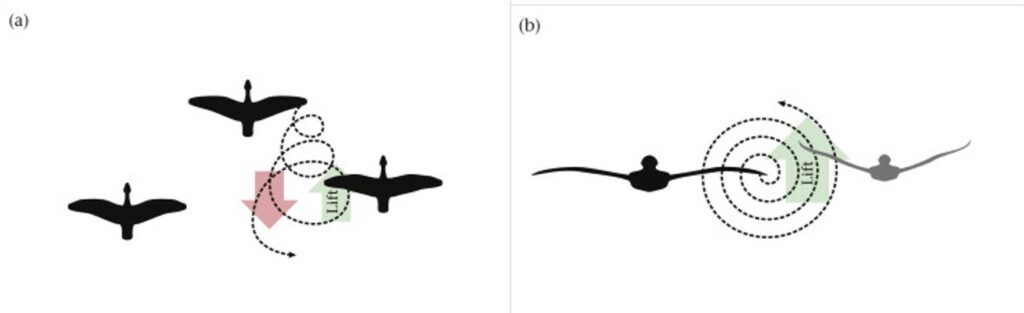
The V-shape formation makes the flight more efficient as birds in the formation ride off the lift created by the birds in front. Indeed, as air streams over the bird wing, it generates lift but also creates vortices at the wing tip. These vortices are essentially horizontal tornadoes (Lebar Bajec & Heppner, 2012) and have a rising and a falling component (Figure 2 (a) and (b)). If the following bird is well positioned behind the flying bird, as in the V-shape formation, the energy may be partially recaptured and utilized by the bird behind. In other words, the upward lift created by the horizontal vortices is captured by the wing of the bird behind. The study by Lissaman & Schollenberger (1970) provides a first quantitative analysis to the benefits of V-shape formation and estimates that a flock of birds may be able to cover 71% more range than a single bird which makes them better suited for foraging activity.
For example, pelicans are great users of the V-shape formation and it’s benefit in terms of aerodynamic factors that save energy, to allow long trips between two feeding grounds. On the US coastal water, the Brown Pelicans (Pelecanus occidentalis) fly low over the waves in V-shape formation to change their sheltering bay and feeding zones. They are known to change their feeding location three to four times per day which makes it quite energy intensive, thus the use of flocking when traveling.
The benefit of the flocking behaviour, most importantly, is that it helps groups of birds identify opportunities to find food more easily, while improving chances of detecting a predator in time (Friederici, 2009). The identification of forage patches is key to the survival and the expansion of the bird community. Finding a new forage patch has the short-term benefit of finding food, but also provides a long-term benefit with information on how to improve future foraging success (Clark & Mangel, 1984). Birds flocking for foraging therefore increases individual success in their quest for food and individuals’ safety against predators which makes it an optimal strategy.
Birds Vision in Relation to Foraging
Birds gather information about their environment mainly through vision by scanning their surroundings. The eyes of birds are relatively large in proportion to their body size due to the need of birds for high precision at various distances. Assuming that the quality of the photoreceptor in a bird eye is the same for all of its species, the species having the larger eye size have a greater ability to identify objects at any distances. For instance, raptors such as the goshawk (Accipiter gentilis) have large eyes for their size, up to 1.4 times greater than the average for birds of the same weight (Figure 3). Moreover, their eyes are tube-shaped which enables them to produce a larger retinal image. Finally, the combination of large and the tube-shaped eyes provide an enhanced long-distance vision for the raptors, when foraging for carrions.

Furthermore, many raptors have foveas, which are essential for fine visual discrimination. Indeed, some species of raptors have two foveas, a deep central fovea and a shallower temporal fovea (Mitkuset al., 2017). Raptor foveas have more rods and cones than the human foveas which provides raptors with exceptional long-distance vision (Esteban Fernández-Juricic et al., 2004). As some raptors are carrion eaters, usually searching from a long distance for large carcasses or aggregations of other species, the quality of their eyesight is critical for their survival. Birds’ eyes are aligned laterally and each eye gives a lateral and a frontal view. The lateral visual field serves to monitor predators, but most importantly, to detect food. In the case of goshawk, their eyes are located on each side at 30 degrees from the centre which provides them a visual field of 340 degrees and, thanks to their enhanced foveas, they can capture a large spectrum of colours, including UVs. It provides them the ability to identify any traces from their prey, such as urine, blood or sweat.
Tool Use: Sponging by Bottlenose Dolphin
Sponge carrying is a foraging tactic observed mostly in female Bottlenose dolphins living in Shark Bay, Western Australia. These so-called spongers were observed carrying conical marine sponges over their beaks in the process of detecting food resources (Figure 4). The sponge covers a large portion of the dolphins’ face and protects the dolphin from damages caused by sharp rock and shells when foraging along the seafloor (Smolker et al., 1997). Spongers move slowly in an undirected pattern along the sandy seafloor, their protected rostrum (beak) occasionally touching the sand.

The behaviour of the spongers differed from the non-spongers in that 93% of their dives consisted of the type ‘tail-out’, as compared with 37% for non-spongers (Mann et al., 2008). The tail-out dives are deep dives performed by the dolphins consisting of putting the head down at a steep angle. These observations can be explained by the fact that the foraging area for the sponge-carrying dolphins is located deep in the ocean. In fact, the foraging habitat for sponge-carriers ranges from the shallow sand and seagrass flat, approximately 4 m deep, to deeper basins and channels of about 12 m deep (Smolker et al., 1997). Sponges were typically used by the Bottlenose dolphins when searching for bottom-dwelling prey that lack swimbladders (Patterson, 2021). Given that fishes without swimbladders have weaker acoustic signals, it is more difficult for dolphins to detect prey by echolocation. Alternatively, Bottlenose dolphins probe the seafloor to find the targeted prey. Nevertheless, the Shark Bay channels are characterized by frequent rock and sharp shell, which is likely to damage the dolphins’ rostra. Hence, Bottlenose dolphins benefit largely from the presence of the beak-covering marine sponge.
The wearing of the sponge contributes to hydrodynamic drag, by resisting the motion of the dolphin. The hydrodynamic drag is proportional to the surface area of the object facing water (Babenko & Carpenter, 2003). As a result of an increase in the surface area of the moving dolphin, the drag force opposing its movement increases when the dolphin’s rostrum is covered with the sponge. Consequently, the dolphin is required to deliver more power per unit mass in order to move forward. The energetic cost is increased, which seems to contradict the Optimal Foraging Theory which claims that individuals use energetically favourable foraging tactics. Due to the significant energetic cost caused by the wearing of the sponge, Bottlenose dolphins drop the sponge during activities that require them to move intensely, such as during active chase. Sponges are foraging tools employed uniquely in the search phase and not during active chase.
Gathering of Food
Drilling Motion of Woodpeckers
Woodpeckers are birds living in woodland habitat whose foraging behaviour is characterised by distinctive drilling motion. Woodpeckers use their chisel-like beak to make holes of various sizes into trees (All About Birds, 2009). They have the ability to extend their sticky tongue to a considerable distance, in order to collect ants and insect larvae. In the process of making a hole, the Woodpecker’s head moves back and forth thus contributing to its characteristic drilling motion. While they forage for small insects, the resulting drumming sound is used for communication with other individuals (Gibson, 2006).
Scientists have been puzzled by their drumming behaviour: how can their brain tolerate intense acceleration for such a long duration? After examining their anatomical structure, scientists agree on two physical factors that allow their brain to withstand repetitive acceleration without injury. First, the small size of their brain increases tremendously their tolerance toward acceleration. In fact, it decreases the ratio of brain mass to surface and therefore reduces the pressure on the nervous tissue of the brain. A.H.S. Holbourn proposed the following relationship between the brain mass (M) and the tolerable rotational acceleration. By observing this relationship, we can realize that tolerable acceleration is inversely proportional to the mass ratio to the 2/3 (Gibson, 2006).
\frac{\ddot \theta_1}{\ddot \theta_2} = (\frac{M_2}{M_1})^{2/3}In comparison, the average human brain mass is 1600 g, whereas a typical acorn woodpecker has a brain mass of 2.5 g. As a result, acorn woodpeckers can withstand 16 times more acceleration than human.
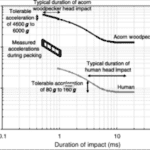
Furthermore, the tolerable acceleration depends on the duration. The shorter duration allows woodpeckers to withstand higher acceleration. In fact, according to Figure 5 provided by the Japan Brain Association, the acceleration tolerance increases by 5 times if the duration goes down from 10 ms to 1 ms. The acceleration duration of woodpeckers during its drilling motion is remarkably short: between 0.5 ms and 1 ms. Its short duration allows them to tolerate an acceleration of masses ranging from 4 600 g to 6 000 g (Gibson, 2006).
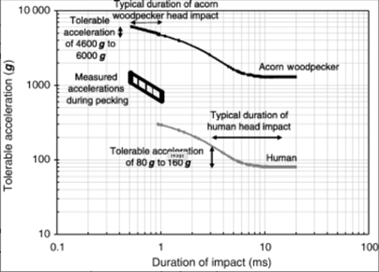
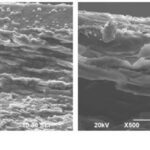
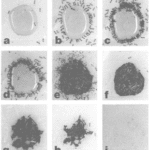
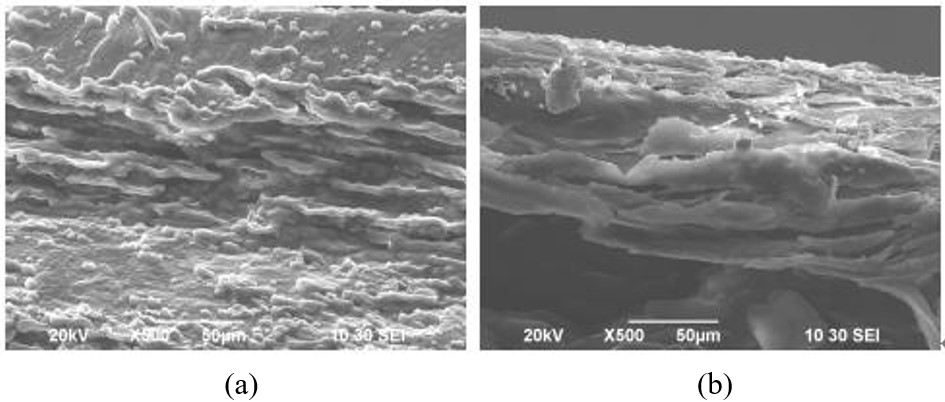
In addition, woodpeckers’ beaks have some interesting mechanical properties which enable them to withstand acceleration. Comparing the beak bone structure of a woodpecker (Figure 6 (a)) and that of a lark (Figure 6 (b)), the woodpecker’s beak is much better at absorbing impact due to its rod-like structures and thinner trabeculae (Wang et al., 2011). The beak bone of the woodpeckers is structured in a way that absorbs shock optimally, leading to a decreased amount of shock being transmitted to the brain. Woodpecker’s bones are also characterized by a higher proportion of organic materials and a smaller proportion of minerals. These properties are examined by testing the ignition loss, taking into consideration the fact that organic materials have greater tendency to burn due to its richness in carbon, hydrogen, and oxygen. The high ignition loss of woodpecker’s beak bone indicates that it contains more organic material, which increases its flexibility and its shock-absorbing tendency. Hence, woodpeckers have developed physical and behavioural adaptations which allow them to effectively collect their food without brain damage.
Transport of Food
Tool-Assisted Liquid Transport by Myrmicine Ants
Ants are foragers whose liquid-feeding performances are fascinating to watch. Ants collect liquid food resources and store them within a pouch called crop. Once arrived at the colony after the foraging activities, ants share the content of the crop with the nestmates. Myrmicinae is a subfamily of ants characterized by a chitin-rich abdomen and inflexible crop. These characteristics of Myrmicinae have significant implications, as it prevents its species from transporting large amounts of liquid food inside their bodies (Máak et al., 2016). These limitations have favoured the evolution of a particular foraging behaviour of Myrmicine ants: the tool-assisted transport of food.
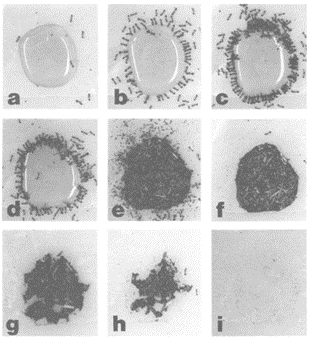
The strategy used by the Myrmicine ants consists of dropping the debris into the liquid food source and transporting the liquid-containing debris to the colony. Ants use materials with different physical properties, such as sand grains, pine needles and dead leaves. The debris is placed in the liquid first around the periphery, with the debris building gradually inwards until the area is entirely covered (Barber et al., 1989) (Figure 7). As a result, the transport of liquid to the colony is facilitated. According to the experiment conducted by Banschbach et al. in 2006 with the Myrmicine species Aphaenogaster rudis, the same ants were responsible for cutting the debris into small pieces, dropping pieces of debris into the liquid, as well as retrieving the water-soaked debris from the food source. These ants belonged to a small subset within the group of foragers, implying the existence of task specialization within the colony (Banschbach et al., 2006). Observations of A.Rudis have also demonstrated that ants acquired tools at a very short distance from the food resource, which increases foraging efficiency despite a relatively small number of foragers.
Experiment carried out by Lorinczi et al. in 2018 have shown that ants are able to optimize their foraging effort by exhibiting tool selectivity at 2 stages: when dropping the tool into the food and when retrieving it. The overall foraging success of Myrmicine ants is determined by how fast the food source is transported to the nest. For instance, since tools that are smaller than the maximum mandible opening of the ants are handled more easily than that of larger tools, the frequency of tool use increased when the size of tools was less than 2 mm (Tanaka & Ono, 1978). At the stage of dropping the tool, in general, the absorbent property of the substance was the determinant factor influencing tool selection. Nevertheless, when retrieving the dropped tool from a highly dense or viscous food resource, the weight/buoyancy of the tool became a more important factor. The evidence supporting the latter statement is that sponge was used more frequently by Aphaenogaster subterranea than the small soil grain that has a higher soaking power, when the encountered food is honey (Máak et al., 2016). The behaviour of A. subterranea can be explained by the fact that the lighter and buoyant sponge can be grasped by the ant more easily, and minimizes the tensile force required to lift the dense, viscous honey.
Cooperative Transport in Ant Teams
In order to fuel themselves, ants must leave their nest to forage for food. In doing so, they must locate their food source and determine an optimal path back to their nest (Li et al.,2014). The foraging activities of Myrmicinae, the subfamily of ants previously described, can be categorized into diffuse foraging, where foragers leave the colony independently and engage in foraging activities alone (Traniello, 1989). However, other subfamilies of ants come together to transport their food, hence partaking in cooperative transport. This group behavior relies mainly on the fundamentals of mechanical forces and occurs when animals come together in order to carry a relatively large object (Feinerman et al.,2018). To further examine this foraging tactic, one can observe the trajectories performed by Paratrechina longicornis, also known as the longhorn crazy ant (Feinerman et al.,2018).
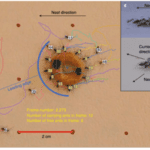
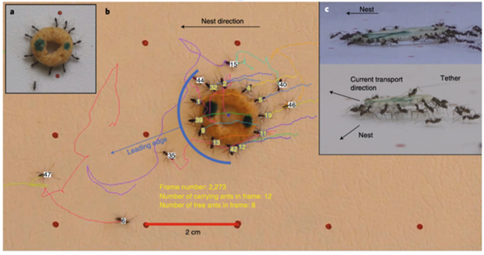
As seen in Figure 8, multiple ants are carriers, therefore, in order for the speed to be directly proportional to the total force, the P. longicornis must align their respective forces (Feinerman et al.,2018). To achieve this alignment, the ants do one of two things: align their force to the nest or mimic a fellow carrier’s force (Feinerman et al.,2018). Hence, either the ant knows the direction to the nest or simply follows the existing direction of the motion. The distinction between the two types of alignments originates from the ant’s distinctive abilities, consequently making it possible for a cohesive collaborative movement to take place (Feinerman et al.,2018). Moreover, in Figure 8 (c), the P. longicornis carry the food item at a slight angle. In fact, this positioning is due to the mimicking alignment. Each ant has a point of attachment where they can sense force, this point is what relates them to the other ant they would be “coupling” with, or mimicking (Feinerman et al.,2018). The ant reacts in consequence of the sum of all forces (Ftotal) applied on the food item (Feinerman et al.,2018). Thus, the force (Findividual) that the ant senses is necessary depending on their attachment position; ants leading the motion tend to pull, while those at the tail tend to lift and reduce friction (Feinerman et al.,2018). When the Ftotal< Findividual, the ant will pull in the same direction as Ftotal, but when Ftotal> Findividual the ant will sporadically lift or pull in the opposite direction of Ftotal (Feinerman et al.,2018). Notably, Ftotal increases as the number of carrier ant increases, keeping the entire group alert in order to coordinate their motion, whereas in smaller groups that coordination is easier to maintain. Thus, this process heavily reflects the collective intelligence of these P.longicornis (Li et al. 2014). All in all, as ants forage for food, they implement a group strategy of transportation that depends on the manipulation of mechanical forces.
Similarly, to P. longicornis, Solenopsis invicta ants, also known as red fire ants, cooperatively transport food as means of foraging. However, they do their transport on the vertical axis, as seen foraging mostly on trees (Qin et al., 2019). Even with extra challenges, such as the large effect of gravitational force, the S. invicta nonetheless effectively transports large food pieces up and down tree trunks (Qin et al., 2019).
Conclusion
Animal species, be it terrestrial or aquatic, have developed different tactics to optimize their foraging activities. The animals whose behavioural adaptations have been examined in the essay are birds, ants and dolphins. Foraging activities were divided into three phases, namely the detection phase, food gathering phase and food transport phase. Animals have developed foraging behaviours in a way that favours energy intake, which is in line with the Optimal Foraging Theory. According to our observations, the optimal foraging strategies differed from one species to another depending on if individuals within the species forage individually or collectively as a group. For instance, bottlenose dolphins in Shark Bay, as well as Myrmicine ants, are independent foragers that rely on tools or their physical adaptations to optimize foraging activities. On the other hand, group foragers, such as the birds in a flock, benefitted from the increased amount of information shared among individuals, especially when searching for forage patch. A noteworthy observation is that many independent foragers use material in their environment as a tool to enhance their foraging activities, whereas group foragers have developed tactics mostly to communicate information within the group. All in all, even a concept as simple and essential as acquiring food is one that actively relies on the unavoidable physical properties involved in its various methods.
References
All About Birds. (2009, April 1). Why Do Woodpeckers Like To Hammer On Houses? And What Can I Do About It? TheCornellLab. https://www.allaboutbirds.org/news/why-do-woodpeckers-like-to-hammer-on-houses-and-what-can-i-do-about-it/
Babenko, V. V., & Carpenter, P. W. (2003). Dolphin Hydrodynamics. In P. W. Carpenter & T. J. Pedley (Eds.), Flow Past Highly Compliant Boundaries and in Collapsible Tubes (pp. 293-323). Springer Netherlands.
Banschbach, V. S., Brunelle, A., Bartlett, K. M., Grivetti, J. Y., & Yeamans, R. L. (2006). Tool use by the forest ant Aphaenogaster rudis: Ecology and task allocation. Insectes Sociaux, 53(4), 463-471. https://doi.org/10.1007/s00040-006-0897-2
Barber, J. T., Ellgaard, E. G., Thien, L. B., & Stack, A. E. (1989). The use of tools for food transportation by the imported fire ant, Solenopsis invicta. Animal Behaviour, 38(3), 550-552. https://doi.org/https://doi.org/10.1016/S0003-3472(89)80052-1
Caney, M. (2012, October 16). Dolphins pass fishing trick from mother to daughter. Dolphin Way. https://www.dolphin-way.com/2012/10/dolphins-pass-fishing-trick-from-mother-to-daughter/
Clark, C. W., & Mangel, M. (1984). Foraging and Flocking Strategies: Information in an Uncertain Environment. The American Naturalist, 123(5), 626-641. http://www.jstor.org/stable/2461242
Emlen, J. T. (1952). Flocking Behavior in Birds. The Auk, 69(2), 160-170. https://doi.org/10.2307/4081266
Feinerman, O., Pinkoviezky, I., Gelblum, A., Fonio, E., & Gov, N. S. (2018). The physics of cooperative transport in groups of ants. Nature Physics, 14(7), 683-693. https://doi.org/10.1038/s41567-018-0107-y
Fernández-Juricic, E., Erichsen, J. T., & Kacelnik, A. (2004). Visual perception and social foraging in birds. Trends Ecol Evol, 19(1), 25-31. https://doi.org/10.1016/j.tree.2003.10.003
Friederici, P. (2009). How a Flock of Birds Can Fly and Move Together. Audubon Magazine. https://www.audubon.org/magazine/march-april-2009/how-flock-birds-can-fly-and-move-together
Gibson, L. J. (2006). Woodpecker pecking: how woodpeckers avoid brain injury. Journal of Zoology, 270(3), 462-465. https://doi.org/https://doi.org/10.1111/j.1469-7998.2006.00166.x
Jones, M. P., Pierce, K. E., & Ward, D. (2007). Avian Vision: A Review of Form and Function with Special Consideration to Birds of Prey. Dentistry, 16(2), 69-87. https://doi.org/https://doi.org/10.1053/j.jepm.2007.03.012
Lebar Bajec, I., & Heppner, F. (2012). Organized flight in birds. Animal Behaviour, 78, 777-789. https://doi.org/10.1016/j.anbehav.2009.07.007
Li, L., Peng, H., Kurths, J., Yang, Y., & Schellnhuber, H. J. (2014). Chaos-order transition in foraging behavior of ants. Proceedings of the National Academy of Sciences, 111(23), 8392-8397. https://doi.org/doi:10.1073/pnas.1407083111
Liguori, J. (1998, September 25). Northern Goshawk. Macaulay Library. https://macaulaylibrary.org/asset/246756081
Lissaman, P., & Shollenberger, C. (1970). Formation Flight of Birds. Science (New York, N.Y.), 168, 1003-1005. https://doi.org/10.1126/science.168.3934.1003
Lucas, J. R. (1982). The biophysics of pit construction by antlion larvae (Myrmeleon, Neuroptera). Animal Behaviour, 30(3), 651-664. https://doi.org/https://doi.org/10.1016/S0003-3472(82)80135-8
Maák, I., Lőrinczi, G., Le Quinquis, P., Módra, G., Bovet, D., Call, J., & d’Ettorre, P. (2017). Tool selection during foraging in two species of funnel ants. Animal Behaviour, 123, 207-216. https://doi.org/https://doi.org/10.1016/j.anbehav.2016.11.005
MacArthur, R. H., & Pianka, E. R. (1966). On Optimal Use of a Patchy Environment. The American Naturalist, 100(916), 603-609. http://www.jstor.org/stable/2459298
Mann, J., Sargeant, B. L., Watson-Capps, J. J., Gibson, Q. A., Heithaus, M. R., Connor, R. C., & Patterson, E. (2008). Why do dolphins carry sponges? PLOS ONE, 3(12), e3868. https://doi.org/10.1371/journal.pone.0003868
Mitkus, M., Olsson, P., Toomey, M. B., Corbo, J. C., & Kelber, A. (2017). Specialized photoreceptor composition in the raptor fovea. Journal of Comparative Neurology, 525(9), 2152-2163. https://doi.org/https://doi.org/10.1002/cne.24190
Patterson, E. M. (2012). Ecological and life history factors influence habitat and tool use in wild bottlenose dolphins (Tursiops sp.) [Doctoral dissertation, Georgetown University]. repository.library.georgetown.edu.
Pierce, J. D. (1986). A Review of Tool Use in Insects. The Florida Entomologist, 69(1), 95-104. https://doi.org/10.2307/3494748
Potier, S., Mitkus, M., Bonadonna, F., Duriez, O., Isard, P. F., Dulaurent, T., Mentek, M., & Kelber, A. (2017). Eye Size, Fovea, and Foraging Ecology in Accipitriform Raptors. Brain Behav Evol, 90(3), 232-242. https://doi.org/10.1159/000479783
Qin, W., Lin, S., Chen, X., Chen, J., Wang, L., Xiong, H., Xie, Q., Sun, Z., Wen, X., & Wang, C. (2019). Food Transport of Red Imported Fire Ants (Hymenoptera: Formicidae) on Vertical Surfaces. Scientific Reports, 9(1), 3283. https://doi.org/10.1038/s41598-019-39756-4
Sinkovits, D. W. (2006). Flocking Behavior. University of Illinois. https://guava.physics.uiuc.edu/~nigel/courses/569/Essays_Spring2006/files/Sinkovits.pdf
Smolker, R., Richards, A., Connor, R., Mann, J., & Berggren, P. (1997). Sponge carrying by dolphins (delphinidae, Tursiops sp.): A foraging specialization involving tool use? Ethology, 103(6), 454-465. https://doi.org/10.1111/j.1439-0310.1997.tb00160.x
Stephens, D. W., Brown, J. S., & Ydenberg, R. C. (Eds.). (2007). Foraging: Behavior and Ecology. (2007). University of Chicago Press.
The Editors of Encyclopaedia Britannica. (2019, May 3). Antlion. Encyclopaedia Britannica. Retrieved October 8, 2021, from https://www.britannica.com/animal/antlion
Traniello, J. F. A. (1989). Foraging Strategies of Ants. Annual Review of Entomology, 34(1), 191-210. https://doi.org/10.1146/annurev.en.34.010189.001203
Wang, L., Zhang, H., & Fan, Y. (2011). Comparative study of the mechanical properties, micro-structure, and composition of the cranial and beak bones of the great spotted woodpecker and the lark bird. Science China Life Sciences, 54(11), 1036-1041. https://doi.org/10.1007/s11427-011-4242-2
Zhou, A., Du, Y., & Chen, J. (2020). Ants adjust their tool use strategy in response to foraging risk. Functional Ecology, 34(12), 2524-2535. https://doi.org/https://doi.org/10.1111/1365-2435.13671