Introduction
Often, only the sense of sight and hearing are considered essential to understanding the world in which humans live in. In practice, however, the sense of smell, or olfaction, plays an important role in everyday tasks that are taken for granted. For example, the ability to taste food is highly dependent on the nose’s capacity to analyze the food’s smell (Spence, 2015).
It is interesting however to note that the human’s sense of smell is very underdeveloped when compared to other species whose survival depends much more on their olfaction (Conover, 2017). This paper analyzes the general structures of the olfactory system, the steps for the molecules in the environment to be transformed into an action potential, the characteristics of undetectable molecules, and specific examples of the role of olfaction in animal interactions.
General Overview of the Organization of the Mammalian Olfactory System
The mammalian olfactory system is composed mainly of the olfactory sensory neurons (OSNs), supporting cells, glomeruli, olfactory bulb, and mitral cells. Odorant molecules first reach the cilia of the OSN which project into the mucus and induce a signal transduction pathway that leads to an action potential along the axon of the OSN. The axons of OSNs that express the same receptor, and are thus receptive to the same odorants, converge to one or a few glomeruli (Treloar et al., 2002; Vosshall, 2003). The collection of glomeruli is called the olfactory bulb. Inside each glomerulus, the OSN axons synapse with dendrites of mitral cells (or second-order olfactory neurons) that relay the information to the brain, allowing us to smell (Mori et al., “The olfactory bulb”).
What follows is an in-depth discussion on odorant molecules, olfactory receptors, signal transduction process, functions of the cilia, and the glomeruli of this system.

Parts of the Olfactory System Odorant Molecules
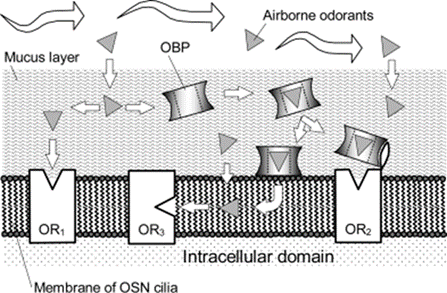
Different theories speculate as to where odorant molecules bind to the receptor and how they reach the receptor. Since some odorants are hydrophilic, they can diffuse through the hydrophilic mucus layer (shown by the left part of Fig. 2 ending in OR1); others are hydrophobic and may require the help of odorant-binding proteins (shown by the right part of Fig. 2 ending in OR2). For instance, the use of odorant-binding proteins has been identified in species like the porcupine (Felicioli et al., 1993), bovine (Pevsner et al., 1985), and rats (Löbel et al., 1998). Depending on the species, these proteins may or may not be specific to particular hydrophobic compounds and can potentially prevent saturation at the binding site when odorant concentration is high (Matarazzo et al., 2002). The third case involves hydrophobic odorants that may bind to the receptor inside the hydrophobic interior of the lipid bilayer of the OSN cilia membrane (shown in the middle part of Fig. 2) (Hau et al., 1999; Hubert et al., 2005, as cited in Zarzo, 2007). Proposed for odorants that are acetates, alcohols, and ketones, this hypothesis still requires more research (Hau et al., 1999).
Attached to a receptor, odorants can either initiate a response, acting as agonists, or block or dampen a response, acting as antagonists (Oka et al., 2004). As well, a mixture of two odorants can stimulate cortical neurons that lack stimulation from the individual odorants (Zou and Buck, 2006). These characteristics of odorants imply that they modulate the perceived odor quality and introduce new odor qualities.
Removing odorants from the receptor after binding is just as important as ensuring odorants reach the receptor in the first place. Odorants need to be quickly inactivated so that incoming odorants can be detected. Enzymes in the epithelium are involved in the degradation and biotransformation of the attached odorant (Breer, 2003). Examples of these enzymes include the cytochrome P-450 (CYP) family—specifically CYP1B1, CYP2F1, and CYP4B1—and aldehyde dehydrogenase (ALDH6) (Nef et al., 1989; Su et al., 2000; Zhang et al., 2005). In particular, Nef and colleagues (1989) predicted that the CYP family of enzymes catalyze the conversion of many hydrophobic odorants by turning off the original signal or by generating a more active stimulus.
Olfactory Receptors (ORs)
Olfactory receptors are key to initiating the detection process as these proteins are what odorant molecules bind to. ORs are transmembrane proteins that belong to the family of G-protein-coupled receptors (Buck and Axel, 1991). On the molecular level, ORs are composed of 7 transmembrane domains with mostly amino acid residues that are assumed to form alpha-helix structures in the lipid bilayer membrane (Buck and Axel, 1991). Since the third, fourth and fifth transmembrane domains exhibit variable sequences in different variants of the gene, this suggests that they are involved in the odorant binding site (Buck and Axel, 1991; Liu et al., 2003; Pilpel and Lancet, 1999). This is because the ability for these domains to vary can allow different odorants to bind.

Receptors do not act alone and can be paired, or work in systems. An expression system of odorant receptors must have a set of receptors targeting the plasma membrane, which can also interact and couple with other odorant receptor systems through olfactory neurons, insofar as both can experience ligand stimulation (Zhao et al., 1998). Olfactory receptors can constitute extremely large multigene families; the sheer number of variants and relative structures explain how a diverse array of odorants can be detected, especially in more odor sensitive species such as rats (Buck and Axel, 1991).
Upon binding, olfactory receptors will undergo conformational change. Although the details are not known, it is theorized to be close to other G‐protein‐coupled receptor conformation mechanisms (Zarzo, 2007). Photoactivation of rhodopsin will rotate and tilt the amino acid TM6 to TM3, and the loop between amino acids TM3 and TM4 containing the DRY motif aspartic acid-arginine-tyrosine plays an important role in activating receptors and triggering the trading of guanosine diphosphate for guanosine triphosphate (Zarzo, 2007), in studies featuring the H2 (Alewijnse et al., 2000), α1b‐adrenergic (Scheer et al., 2000), and AT1A angiotensin (Gáborik et al., 2003) receptors.
For a molecule to be able to activate receptors, it must be lipophilic, vaporizable, and water soluble (Cotton, 2009). Most of the molecules we smell are small with molecular weights under 300 (Cotton, 2009).
What Determines Olfactory Receptor Activation?
Despite a history of contested theories on what links molecules to smell, as details of olfactory receptor activation at the molecular level are poorly understood (Zarzo, 2007), there are such theories as Malcolm Dyson’s 1938 ‘vibrational theory’, and Linus Pauling’s 1946 ‘shape theory’, and the modern biochemical understanding of olfactory quantum mechanics that can offer a clear image on molecule – receptor activation.
In 1946, Linus Pauling asserted that a molecule’s odor was determined by its shape and by its size, meaning odorless molecules were odorless because their shape would not fit into the olfactory receptors. Pauling believed different shaped receptors recognized their appropriate odorant(s), implying that odorant molecules worked in the same way as it was once believed enzyme activation worked: by the lock-and-key model (Cotton, 2009). By the mid-1960s, shape theory was overtaken by odotype theory, which suggested that receptors will probe the shape and geometry of the molecule, and that the ‘net smell’ comes from the combination of receptor stimuli (Cotton, 2009). However, there are instances of similarly shaped molecules that smell very different. For example, 1,1-dimethylcyclohexane is described as ‘powdery, faint and fruity’, while 1,1-dimethyl-1-silacyclohexane, its silicon substituted analogue, has an intense ‘grassy green, freshly cut grass’ odour (Cotton, 2009).

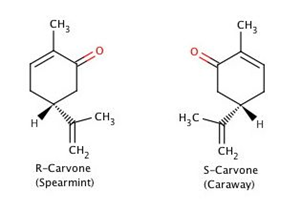
In 1938, Malcom Dyson proposed (in a theory that has since been modified by Turin, 1966), that olfactory receptors can detect frequencies from odor molecules, and that the olfactory receptor proteins behave like vibrational spectrometers through electron tunneling; the electrons will excite vibrational modes as they travel through a zinc ion to a G-protein, activating the receptor. For example, Dyson proposed that guaiacol mixed with benzenecarbaldehyde would produce a ‘vanillin’ like smell, since they contain the same functional groups – and hence vibrational frequencies – as vanillin (Cotton, 2009). Simon Gate, a nanotechnology researcher and surgeon of the University College of London is quoted: “Changing the mass of some atoms inside certain molecules can affect the way they smell to humans, even though that does not change their shape in a large way. This is difficult to explain without the vibrational theory” (Ryan, 2013). Despite this, it has been suggested that Wright’s proposed vibration theory has just as much predictive ability as shape theory (Hoehn et al., 2018) – a study completed by Eriksson et al. used substituted pyridine and the perseverance of pure pyridine odor to test the predictive nature of vibrational olfactory theory and found that it was just as predictive as molecular shape and electron density orientation (Eriksson et al., 1981). Although most enantiomer pairs have similar smells to humans (Cotton, 2009), vibrational theory cannot explain how some enantiomers will produce different smells (Boelens et al., 1993).
The most recent olfactory theory states that the Van der Waal electrostatic forces between the odorant and receptor will permit binding, which will become active and carry through the previously mentioned pathways (Hoehn et al., 2018). Indeed, the answer to how and why certain molecules bind to receptors may even lie with quantum mechanics, specifically the possibility of inelastic electronic transfer (IET). In his article “Quantum coherence in biological systems”, Seth Lloyd states plainly: ‘the only known mechanism that can explain this vibrational sensitivity of the sense of smell is quantum mechanical’ (Lloyd, 2011). When an odorant molecule binds to the olfactory receptors, electrons can pass through the molecule by emitting a phonon (an elementary vibrational motion) of a certain frequency to a vibrational mode (Lloyd, 2011). This is supported by the fact that common fruit flies can discriminate between an organic molecule with hydrogen, and with deuterium instead – by the shape theory, this would not be possible (Lloyd, 2011).
This is not to say that the shape or vibrational theories are of no relevance and to be thrown out, just that they are not sufficient to explain olfactory receptor activation by themselves. Once they are analyzed through the lens of quantum mechanics, we get a clearer picture of what determines receptor binding and activation (Lloyd, 2011).
Signal Transduction in an Olfactory Sensory Neuron (OSN)
Like many signal transduction pathways, the signal transduction pathway in OSNs transform a chemical interaction into an electrical signal, beginning with the odorant molecule (ligand) binding to the receptor on the cilia and culminating to an action potential generated at the axon hillock region of the OSN.

The detection of smell is initiated by an odorant molecule binding to a specific olfactory receptor (OR) on the external surface of the cilia. This binding activates olfactory-specific G protein (referred to as G-protein in this paper) (Jones and Reed, 1989) that likely trades guanosine diphosphate (GDP) for guanosine triphosphate (GTP) (Bakalyar and Reed, 1990). The GTP attached G-protein then activates the olfactory-specific adenylate cyclase type III (referred to as adenylate cyclase in this paper) (Bakalyar and Reed, 1990). The catalytic region of the adenylate cyclase proceeds to convert adenosine triphosphate (ATP) to cyclic adenosine monophosphate (cAMP) which is a second messenger that is found in the transduction pathway for many odorants and plays an integral role in regulation of the cyclic nucleotide-gated channels (CNG) (Brunet et al., 1996; Lowe et al., 1989; Takeuchi et al., “Cross-adaptation between olfactory responses”).
Cyclic nucleotide-gated channels are gated by cAMP. Here, cAMP opens the gated channel to allow the influx of mostly Ca2+ and some Na+ (Nakamura and Gold, 1987). Both these ions carry the primary depolarizing receptor current which contributes to the depolarization of the cilia (Nakamura and Gold, 1987). When there is a sufficient amount of accumulated Ca2+ in the ciliary, the Ca2+ gated Cl(Ca) channels are opened and there is a movement of Cl– out of the cilia, further depolarizing it (Firestein et al., 1990; Kleene, “Origin of the chloride current in olfactory transduction”; Kurahashi and Yau, 1993; Lowe and Gold, 1993; Zhainazarov and Ache, 1995). Thus, Cl– forms the secondary depolarizing current. Since the concentration of intracellular Cl– is in a similar range as that of mucus Cl–, there would be an efflux of Cl– once the channel opens (Kaneko et al., 2001; Reuter et al., 1998). Interestingly, the Cl(Ca) channel amplifies the CNG primary current as shown in rats and mice; the combination of a high open probability (0.97) and a very small unit conductance from the analysis of macroscopic currents (1.3 picosiemens (pS) for rats (Matthews and Reisert, 2003) and 1.6 pS for mice [Pifferi et al., “Bestrophin-2 is a candidate calcium-activated chloride channel”]) allows for high amplification of the primary current without an increase of “noise” (or stimulus) (Kleene, “High-gain, low-noise amplification in olfactory transduction”). In fact, Cl– can contribute up to 90 % of total induced current in mice (Bhandawat et al., 2005; Boccaccio and Menini, 2007).
Finally, the depolarization is conducted passively from the cilia to the axon hillock part of the OSN where the action potentials are generated and are directed along the axon to the olfactory bulb (Lagostena and Menini, 2003). An action potential is generated once the receptor potential reaches the threshold level. It is interesting to note that the frequency of action potentials sent to the olfactory bulb increase with concentration of the odorant since the amplitude of the receptor potential grows (Imanaka and Takuchi, 2001; Ma et al., 1999). Thereby, one can distinguish a mild and a strong scent.
To restore the balance of ions, especially Ca2+, is just as important in the transduction process itself so that a new signal can be transmitted once another odorant binds. To achieve this balance, Ca2+ is extruded by the Na7Ca2+exchanger (denoted Na/Caex in Fig. 2) due to the Na+ driving force and the Ca2+-ATPase. The former method was discovered by Reisert and Matthews (1998) as an absence of external Na+ prolonged the current produced by the Cl(Ca) channel which suggested that Ca2+ was not extruded. The latter method was shown by Castillo et al. (2007) as the current relaxation time was also lengthened without intracellular ATP and the blocking of Ca2+-ATPase, specifically in rats and toads.
As well, it is important that particular OSNs do not fire action potentials without any odorants present and when there are fluctuations in cAMP concentrations. These nonspecific responses are prevented by high concentrations of Ca2+ and Mg2+ in the mucus that block CNG channels at the resting potential (Purves et al., 2001). Thus, several channels need to be opened at once which happens when the correct odorant binds to the receptors (Purves et al., 2001).
Function of Olfactory Cilia
Olfactory cilia not only play a major role in odorant detection and signal transduction by housing the channels necessary for action potential generation, these microtubule-based structures are also essential for signal amplification. The first type of amplification is molar amplification which involves increasing the number of active molecules (Takeuchi et al., “Mechanism of Signal Amplification in the Olfactory Sensory Cilia”). The second is nonlinear amplification in which a small change in concentration of odorant causes a large change in the response (Takeuchi et al., “Mechanism of Signal Amplification in the Olfactory Sensory Cilia”).
Furthermore, the adaptation and desensitization of the OSN occur in the cilia. When the OSN is stimulated repeatedly or for long periods of time by an odorant, the sensation of the neuron gradually lowers (Getchell and Sheperd, 1978). If the neuron is stimulated for a prolonged time, the receptor current decreases with time (Firestein et al., 1990; Kurahashi and Shibuya, 1990; Menini, 1995). To adapt, cilia are expected to “reset” the neuron so that it can discriminate high odor concentrations without the saturation of the transduction process (Pifferi et al., “Signal Transduction in Vertebrate Olfactory Cilia”). If there is a repeated stimulus, a reduction in the amplitude of response for the following pulse ensues, which is seen more when the pulses are closer together (Kurahashi and Shibuya, 1990; Kurahashi and Menini, 1997). Thus, to deal with this desensitization, cilia have adapted to shift the range of stimulus concentrations that the neuron can respond to higher odorant concentrations (Boccaccio et al., 2006; Kurahashi and Menini, 1997). This adaptation is regulated by CNG channels by changing the channel sensitivity to cAMP through the activation of apocalmodulin or another Ca2+-responsive endogenous factor (Bradley et al., 2004; Kleene, “Both external and internal calcium reduce the sensitivity of the olfactory cyclic-nucleotide-gated channel to CAMP”; Kramer and Siegelbaum, 1992).
Often serving as a benefit, the cilia is involved in masking of unpleasant odors using flavors and fragrances or by the stimulant itself (Kurahashi et al., 1994). In fact, this process is also regulated by the CNG channel as it is directly suppressed by the odorant or experiences a reduction in current (Takeuchi et al., “Mechanism of olfactory masking in the sensory cilia”). As an effect, the Cl(Ca) channels carry out the signal reduction through the entire cilia.
The functions explored above are performed on the condition that the odorant molecule binds to the receptor. With the OSN having numerous cilia that are thin, there is a higher chance of the odorant molecule binding to the receptor due to the increase in surface area (SA) that is exposed to the external environment (Menco, 1980). As well, because of cilia’s long thin structure, the membrane SA to cytoplasmic volume ratio is large. This plays a role in limiting the number of molecules that are capable of generating a large concentration change which is important in the transduction process (Pifferi et al., “Signal Transduction in Vertebrate Olfactory Cilia”).
Glomeruli
The action potential generated from the depolarization of the cilia described above leads to a synapse made by the OSN axons in the glomerulus (Mori et al., “The olfactory bulb”). Inside glomeruli, many synapses occur—such as those between axons of OSNs and dendrites of mitral cells and between tufted or mitral cells and periglomerular cells—characterizing them as the functional units of olfactory processing (Allison, 1953; Aungst et al., 2003; Johnson and Leon, 2007; Mori et al., “The olfactory bulb”). Because there are numerous axons extending from the OSNs, glomeruli can also be considered to be a developmental solution to the problem of organizing axons (Zou et al., 2009).
As mentioned above, all ONS expressing the same OR converge to one or a few glomeruli so that the signal-to-noise ratio is improved and the stimulus sensitivity is increased (Treloar et al., 2002; Vosshall, 2003). This phenomenon is achieved by the neuron expressing the receptor in the axon terminal growth cone to detect cues in the target area (Barnea et al., 2004; Dubacq et al., 2009). In fact, a potential ligand of the axonal odorant receptors are phosphatidylethanolamine-binding protein1, a molecule expressed in the olfactory bulb. In addition, there seems to be a growth in both the activity of individual glomeruli and number of activated glomeruli when there is increasing odorant concentration (Bozza et al., 2004; Wachowiak and Cohen, 2001) Further, odorants exhibiting particular structural features activate glomeruli that are found in close proximity and form molecular-feature clusters (Mori et al., “Maps of odorant molecular features in the mammalian olfactory bulb”). However, the glomerular position is not stereotypic and invariant, so different species and individuals have different glomerular maps (Ishii et al., 2001; Norlin et al., 2001; Serizawa et al., 2000; Vassalli et al., 2002). What determines glomerular positioning has yet to be concluded.
Unique Utilizations of Olfaction in Animal Interactions
While the general chemical mechanism for olfaction remains the same across most species, specialization and differentiation have risen as a result of unique ways in which different animals use olfaction. What follows are three examples of how different animals use olfaction in unique ways.
Salmon and Olfactory Imprinting
The olfactory system of salmon includes the same basic mechanism as that described previously. However, salmon use this olfaction as a mechanism for finding their way back to the river that they were spawned in. To achieve this, young salmon undergo olfactory imprinting during early stages in their development (Hasler et al., 1978). This can be likened to a kind of olfactory driven memory. Each stream has a unique chemical composition given by the specific vegetation, soil, and other environmental factors giving it a unique odor (Hasler et al., 1978). During imprinting, young salmon become “attuned” to their stream’s unique odor. Eventually, the salmon leave the stream for the open ocean, travelling thousands of kilometers. When the time comes to mate, the salmon may return unerringly to the stream in which they were born, guided by the unique smell of their native stream (Hasler et al., 1978).
In one study performed on coho salmon, researchers found that the olfactory receptor neurons of salmon reared in environments with a chemical called PEA demonstrated a six-fold increase in responsiveness of olfactory receptor neurons to the chemical compared to salmon raised in the absence of PEA. This is attributed to PEA inducing more cGMP activity in imprinted salmon (Nevitt and Dittman, 1998). This demonstrates how environmental conditions change and modify the salmon’s olfactory response in a way that allows them to identify their native stream.
Furthermore, researchers found that small exposure to PEA led to dramatic increase in sensitivity later in the salmon’s life. In addition, the olfactory receptor neurons of salmon in different life-stages had different electrical properties. In pre-smolts the Ca2+ dependent K+ current dominated the outward current; however, in smolted salmon, a transient K+ current was more prominent (Nevitt and Dittman, 1998). Because this imprinting occurs during smolting, which is marked by high thyroid hormone levels, researchers suggest that these changes in sensitivity and morphology may be attributed to “hormonally driven modulation in the expression of particular olfactory receptor proteins” (Nevitt and Dittman, 1998, p. 220). These findings demonstrate how olfactory receptor neurons are not static odor detectors but are rather dynamic and changing to accommodate the salmon’s needs: they must be imprintable and conditionable during early stages of the salmon’s life, and then demonstrate an increased response to native stream odorants later when the salmon must return to its native stream. This is just one example of how salmons’ unique modulation of olfactory neurons allows for a highly effective means of olfactory driven memory and navigation.
Moths and Pheromones
Moths use a combination of chemicals, known as pheromones, as a means of attracting, for females, and finding, for males, a mate. Pheromones are volatile and thus, when released by a female moth, form elongated plumes called an active space (Yoshimura, 1996). When a male moth senses pheromones of a female moth of the same species, he flies sinusoidally upwind along the longitudinal axis of the active space (Yoshimura, 1996). If the male moth no longer senses the pheromone, he flies in a left-right zigzag manner until his flight pattern is ninety degrees to the wind direction, effectively remaining in place, until he catches a whiff of the pheromone again and can continue on the path to the female (Yoshimura, 1996). In this way moths use their olfaction capabilities as a means for communication between individuals across long distances and plays a key role in mating and species procreation.
Olfaction in moths occurs on their sensilla: hair-like organs which contain sensory neurons. Some of these olfaction neurons are specialized for sensing the components in the moths’ sex pheromone (Yoshimura, 1996). One study examined in detail the pheromone receptor capabilities of Mamestra brassicae L., commonly known as the cabbage moth. By analyzing the amplitude of the action potential of receptor cells in response to different chemicals, researchers found three unique receptor cells that were tuned to the pheromone compounds of this species (Renou and Lucas, 1993). The first two receptor cells were always found in pairs: the first cell being tuned to the main component of the M. brassicae pheromone and the second to two compounds that decrease the moth’s flight response to the pheromone source (Renou and Lucas, 1993). The third cell is tuned to respond to another two components of the pheromone (Renou and Lucas, 1993). These findings support the idea that specific pheromone receptor cells for sensing different compounds are associated with different morphologies and positions of the sensilla on the antenna.
The Role of Olfaction in Hunting
The relation between prey and predator is an animal interaction in which olfaction plays a big role. Many predators use their sense of smell to be able to localize their prey. The following paragraphs will analyze specific examples of how certain species use olfaction to track down their prey.
Origins of odorants
First, it is important to understand the origin of odorants in mammals and birds, as well as the information that can be known from these molecules. Both birds and mammals are very rich in odorants whose sources are quite diverse. Some odorants are products of metabolic reactions and escape the body by exhaling. These metabolic odorants include carbon dioxide, acetone, and multiple phenolic compounds (Conover, 2007).
Other odorants are products of an array of glands. Skin glands produce odorants that result from these glands’ primary functions. Many mammals have sweat glands whose roles are to regulate body temperature, like in humans and some primates. However, most mammals have sweat glands on their feet to prevent the animal from slipping (Aldeman et al., 1975, as cited in Conover, 2007). These sweat glands’ by-products are a large number of odorants. For example, human sweat contains up to 300 odorants (Sommerville and Gee, 1984, as cited in Doving, 1990 and Conover, 2007).
Salivary glands, eye glands and anal sacs secrete odorants used to communicate important information about the animal, such as its age, sex, health, position in the social hierarchy, reproductive condition, and territorial status, to other individuals of the same species (Conover, 2007). Odorants from the anal sacs, found in urine and feces, are often used for scent marking with the intention of informing conspecifics on pack membership and the boundaries of their territories (Barrette and Messier, 1980; Singer et al., 1997, both as cited in Conover, 2007). While the main purpose of these odorants is to communicate with conspecifics, these odorants also leave a trace for their predators to localize them and hunt them down.
Some odorants are produced by the decomposition of larger molecules. Most mammals and birds have soft and waterproof surfaces that can be either composed of fur, skin, or feathers. To maintain these properties, these animals have glands on their surfaces whose role is to secrete nonpolar molecules, such as lipids, oil, fatty acids, and wax. However, when grooming themselves, these animals use their saliva, which, on its own, is rich in odorants, but is also rich in proteins, such as lipases, amylases, ribonucleases, peroxidases, proteinases, peptidases, glycoproteins, tyrosine-rich proteins, lysozymes, and peptides (Ellison, 1979; Barka, 1980; Bradley, 1991, all as cited in Conover, 2007). The grooming process puts these proteins in contact with the organic molecules found on their skin as well as with the nonpolar molecules mentioned above and breaks them down into smaller, more volatile molecules, which have the potential of being odorants (Conover, 2007).
Some odorants originate from microorganisms, such as parasites and bacteria, that live on the animal’s surface as well as in their digestive system. These microbes produce smells by decomposing organic compounds into more volatile chemicals, especially in anaerobic (where there is no oxygen) environments, such as phenols and ammonia. These smells tend to be stronger when the animal gets sick, which puts the sick individuals more at risk to be located by their predators. An example of this is presented in the 1971 article by Cook et al., where it was noted that ill deer fawns were more likely to be located by coyotes because their bodies discharged fluids with strong scents, such as blood and mucus (Conover, 2007).
Examples of Olfactory Predators
Not all animals use the same modality to chase their prey. Humans use vision the most, but other species benefit more from olfaction. This section will look at a couple of species that use olfaction to catch their prey: the olfactory predators.
For most birds, the use of olfaction as the main modality to get food would be disadvantageous since they mostly consume flying insects. However, there is a category of birds that feed on fish and fish carcasses called Procellariiformes, also known as tube-nosed seabirds (Fig. 7). These birds are considered olfactory predators because they find their prey by following the odorants they release. A great example is Antarctic krill that live in schools. Sea birds feed on these krill, but also on the animals that feed on them, such as fish. When digesting the crustaceans, these fish release odorants that help the sea birds find them (Conover, 2007). It is important to note, however, that these birds do not solely rely on olfaction, and that they do use their vision to get the food from the water: olfaction and vision work together, but at different scales.

Olfaction is used differently by snakes to hunt their prey. They use both visual and olfactory cues to locate and identify their prey. However, they inject venom in some of their victims, such as mammals, and then release them. Once the venom kills or paralyses the prey, the snakes then use their olfaction to locate them (Conover, 2007). It is clear to say that while olfactory predators have a heightened sense of smell, which allows them to track down their prey by their odorants, they still use their vision to maximize their hunting abilities.
Conclusion
Altogether, looking at olfaction through a molecular lens magnifies the complexity and intricacy of the olfactory system and the many parts that work together to improve and enhance the lives of numerous species. Above, we illustrate some characteristics of odorant molecules, receptors, cilia and glomeruli and break down the crucial signal transduction process. As important as it is to appreciate what permits us to detect some odors, it is as imperative to understand what prevents us from detecting others by investigating receptors and properties of the odorant molecules themselves. Putting the olfactory system into perspective, we then explored olfaction in the imprinting of salmon and the communication between moths and how both are achieved through a molecular level analysis. Finally, we highlighted the significance of olfaction within a species as well as when predators hunt for their prey before taking a closer look at some of these predators. Though we have discussed quite a few topics, there are still many unknowns that have yet to be discovered surrounding the mechanisms of olfaction in general and specifically in various species. By elucidating the current knowns, we can not only further our understanding, but also open the door to discover novel implications for the field of bioengineering.
References
Alewijnse, A. E., Timmerman, H., Jacobs, E. H., Smit, M. J., Roovers, E., Cotecchia, S., & Leurs, R. (2000). The Effect of Mutations in the DRY Motif on the Constitutive Activity and Structural Instability of the Histamine H<sub>2</sub>Receptor. Molecular Pharmacology, 57(5), 890-898. Retrieved from https://molpharm.aspetjournals.org/content/molpharm/57/5/890.full.pdf
Allison, A. C. (1953). The structure of the olfactory bulb and its relationship to the olfactory pathways in the rabbit and the rat. Journal of Comparative Neurology, 98(2), 309-353. doi:10.1002/cne.900980206
Aungst, J. L., Heyward, P. M., Puche, A. C., Karnup, S. V., Hayar, A., Szabo, G., & Shipley, M. T. (2003). Centre-surround inhibition among olfactory bulb glomeruli. Nature, 426(6967), 623-629. doi:10.1038/nature02185
Bakalyar, H. A., & Reed, R. R. (1990). Identification of a specialized adenylyl cyclase that may mediate odorant detection. Science, 250(4986), 1403-1406. doi:10.1126/science.2255909
Barnea, G., O’Donnell, S., Mancia, F., Sun, X., Nemes, A., Mendelsohn, M., & Axel, R. (2004). Odorant Receptors on Axon Termini in the Brain. Science (New York, N.Y.), 304, 1468. doi:10.1126/science.1096146
Bhandawat, V., Reisert, J., & Yau, K. W. (2005). Elementary response of olfactory receptor neurons to odorants. Science, 308(5730), 1931-1934. doi:10.1126/science.1109886
Boccaccio, A., Lagostena, L., Hagen, V., & Menini, A. (2006). Fast adaptation in mouse olfactory sensory neurons does not require the activity of phosphodiesterase. Journal of General Physiology, 128(2), 171-184. doi:10.1085/jgp.200609555
Boccaccio, A., & Menini, A. (2007). Temporal development of cyclic nucleotide-gated and Ca2+ -activated Cl- currents in isolated mouse olfactory sensory neurons. Journal of Neurophysiology, 98(1), 153-160. doi:10.1152/jn.00270.2007
Bochenkov, V., & Sergeev, G. (2010). Sensitivity, selectivity, and stability of gas-sensitive metal-oxide nanostructures. In Metal Oxide Nanostruct. Appl. (Vol. 3, pp. 31-52).
Boelens, M. H., Boelens, H., & van Gemert, L. J. (1993). Sensory Properties of Optical Isomers. Perfumer & Flavorist, 18, 1-16. Retrieved from https://www.perfumerflavorist.com/flavor/rawmaterials/natural/15546447.html
Bozza, T., McGann, J. P., Mombaerts, P., & Wachowiak, M. (2004). In vivo imaging of neuronal activity by targeted expression of a genetically encoded probe in the mouse. Neuron, 42(1), 9-21. doi:10.1016/s0896-6273(04)00144-8
Bradley, J., Bönigk, W., Yau, K. W., & Frings, S. (2004). Calmodulin permanently associates with rat olfactory CNG channels under native conditions. Nature Neuroscience, 7(7), 705-710. doi:10.1038/nn1266
Breer, H. (2003). Olfactory receptors: molecular basis for recognition and discrimination of odors. Anal Bioanal Chem, 377(3), 427-433. doi:10.1007/s00216-003-2113-9
Brunet, L. J., Gold, G. H., & Ngai, J. (1996). General Anosmia Caused by a Targeted Disruption of the Mouse Olfactory Cyclic Nucleotide–Gated Cation Channel. Neuron, 17(4), 681-693. doi:10.1016/S0896-6273(00)80200-7
Buck, L., & Axel, R. (1991). A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell, 65(1), 175-187. doi:10.1016/0092-8674(91)90418-x
Castillo, K., Delgado, R., & Bacigalupo, J. (2007). Plasma membrane Ca2+-ATPase in the cilia of olfactory receptor neurons: possible role in Ca2+ clearance. European Journal of Neuroscience, 26(9), 2524-2531. doi:https://doi.org/10.1111/j.1460-9568.2007.05863.x
Conover, M. R. (2007). Olfactory predators and odorants. In Predator-Prey Dynamics: The Role of Olfaction (1 ed.). Boca Raton: CRC Press. Retrieved from https://doi-org.proxy3.library.mcgill.ca/10.1201/9781420009125
Cotton, S. (2009). If it smells – it’s chemistry. Retrieved from https://edu.rsc.org/feature/if-it-smells-its-chemistry/2020168.article
De Vito, S., Piga, M., Martinotto, L., & Di Francia, G. (2009). CO, NO2 and NOx urban pollution monitoring with on-field calibrated electronic nose by automatic bayesian regularization. Sensors and Actuators B: Chemical, 143(1), 182-191. doi:10.1016/j.snb.2009.08.041
Felicioli, A., Ganni, M., Garibotti, M., & Pelosi, P. (1993). Multiple types and forms of odorant-binding proteins in the Old-World porcupine Hystrix cristata. Comparative Biochemistry and Physiology. B: Comparative Biochemistry, 105(3-4), 775-784. doi:10.1016/0305-0491(93)90119-p
Firestein, S., Shepherd, G. M., & Werblin, F. S. (1990). Time course of the membrane current underlying sensory transduction in salamander olfactory receptor neurones. Journal of Physiology, 430, 135-158. doi:10.1113/jphysiol.1990.sp018286
Gáborik, Z., Jagadeesh, G., Zhang, M., Spät, A. s., Catt, K. J., & Hunyady, L. s. (2003). The Role of a Conserved Region of the Second Intracellular Loop in AT1 Angiotensin Receptor Activation and Signaling. Endocrinology, 144(6), 2220-2228. doi:10.1210/en.2002-0135
Getchell, T. V., & Shepherd, G. M. (1978). Adaptive properties of olfactory receptors analysed with odour pulses of varying durations. The Journal of Physiology, 282(1), 541-560. doi:https://doi.org/10.1113/jphysiol.1978.sp012480
Hasler, A., Scholz, A. T., & Horrall, R. M. (1978). Olfactory imprinting and homing in salmon. American Scientist, 66(3), 347-355.
Hau, K. M., Connell, D. W., & Richardson, B. J. (1999). Quantitative structure-activity relationships for nasal pungency thresholds of volatile organic compounds. Toxicological Sciences, 47(1), 93-98. doi:10.1093/toxsci/47.1.93
Hoehn, R. D., Nichols, D. E., Neven, H., & Kais, S. (2018). Status of the Vibrational Theory of Olfaction. Frontiers in Physics, 6(25). doi:10.3389/fphy.2018.00025
Imanaka, Y., & Takeuchi, H. (2001). Spiking Properties of Olfactory Receptor Cells in the Slice Preparation. Chemical Senses, 26(8), 1023-1027. doi:10.1093/chemse/26.8.1023
Ishii, T., Serizawa, S., Kohda, A., Nakatani, H., Shiroishi, T., Okumura, K., . . . Sakano, H. (2001). Monoallelic expression of the odourant receptor gene and axonal projection of olfactory sensory neurones. Genes to Cells, 6(1), 71-78. doi:10.1046/j.1365-2443.2001.00398.x
Johnson, B. A., & Leon, M. (2007). Chemotopic odorant coding in a mammalian olfactory system. Journal of Comparative Neurology, 503(1), 1-34. doi:10.1002/cne.21396
Jones, D. T., & Reed, R. R. (1989). Golf: an olfactory neuron specific-G protein involved in odorant signal transduction. Science, 244(4906), 790-795. Retrieved from https://science.sciencemag.org/content/sci/244/4906/790.full.pdf
Kaneko, H., Nakamura, T., & Lindemann, B. (2001). Noninvasive measurement of chloride concentration in rat olfactory receptor cells with use of a fluorescent dye. American Journal of Physiology: Cell Physiology, 280(6), C1387-1393. doi:10.1152/ajpcell.2001.280.6.C1387
Kleene, S. J. (1993). Origin of the chloride current in olfactory transduction. Neuron, 11(1), 123-132. doi:10.1016/0896-6273(93)90276-w
Kleene, S. J. (1997). High-gain, low-noise amplification in olfactory transduction. Biophysical Journal, 73(2), 1110-1117. doi:10.1016/s0006-3495(97)78143-8
Kleene, S. J. (1999). Both external and internal calcium reduce the sensitivity of the olfactory cyclic-nucleotide-gated channel to CAMP. Journal of Neurophysiology, 81(6), 2675-2682. doi:10.1152/jn.1999.81.6.2675
Kramer, R. H., & Siegelbaum, S. A. (1992). Intracellular Ca2+ regulates the sensitivity of cyclic nucleotide-gated channels in olfactory receptor neurons. Neuron, 9(5), 897-906. doi:10.1016/0896-6273(92)90242-6
Kurahashi, T., Lowe, G., & Gold, G. H. (1994). Suppression of odorant responses by odorants in olfactory receptor cells. Science, 265(5168), 118-120. doi:10.1126/science.8016645
Kurahashi, T., & Menini, A. (1997). Mechanism of odorant adaptation in the olfactory receptor cell. Nature, 385(6618), 725-729. doi:10.1038/385725a0
Kurahashi, T., & Shibuya, T. (1990). Ca2(+)-dependent adaptive properties in the solitary olfactory receptor cell of the newt. Brain Research, 515(1-2), 261-268. doi:10.1016/0006-8993(90)90605-b
Kurahashi, T., & Yau, K. W. (1993). Co-existence of cationic and chloride components in odorant-induced current of vertebrate olfactory receptor cells. Nature, 363(6424), 71-74. doi:10.1038/363071a0
Lagostena, L., & Menini, A. (2003). Whole-cell Recordings and Photolysis of Caged Compounds in Olfactory Sensory Neurons Isolated from the Mouse. Chemical Senses, 28(8), 705-716. doi:10.1093/chemse/bjg063
Liu, X., Cheng, S., Liu, H., Hu, S., Zhang, D., & Ning, H. (2012). A survey on gas sensing technology. Sensors (Basel, Switzerland), 12(7), 9635-9665. doi:10.3390/s120709635
Lloyd, S. (2011). Quantum coherence in biological systems. Journal of Physics: Conference Series, 302, 012037. doi:10.1088/1742-6596/302/1/012037
Löbel, D., Marchese, S., Krieger, J., Pelosi, P., & Breer, H. (1998). Subtypes of odorant-binding proteins–heterologous expression and ligand binding. European Journal of Biochemistry, 254(2), 318-324. doi:10.1046/j.1432-1327.1998.2540318.x
Lowe, G., & Gold, G. H. (1993). Nonlinear amplification by calcium-dependent chloride channels in olfactory receptor cells. Nature, 366(6452), 283-286. doi:10.1038/366283a0
Lowe, G., Nakamura, T., & Gold, G. H. (1989). Adenylate cyclase mediates olfactory transduction for a wide variety of odorants. Proceedings of the National Academy of Sciences, 86(14), 5641-5645. Retrieved from https://www.pnas.org/content/pnas/86/14/5641.full.pdf
Ma, M., Chen, W. R., & Shepherd, G. M. (1999). Electrophysiological characterization of rat and mouse olfactory receptor neurons from an intact epithelial preparation. Journal of Neuroscience Methods, 92(1-2), 31-40. doi:10.1016/s0165-0270(99)00089-8
Matarazzo, V., Zsürger, N., Guillemot, J.-C., Clot-Faybesse, O., Botto, J.-M., Farra, C. D., . . . Ronin, C. (2002). Porcine Odorant-binding Protein Selectively Binds to a Human Olfactory Receptor. Chemical Senses, 27(8), 691-701. doi:10.1093/chemse/27.8.691
Matthews, H. R., & Reisert, J. (2003). Calcium, the two-faced messenger of olfactory transduction and adaptation. Current Opinion in Neurobiology, 13(4), 469-475. doi:10.1016/s0959-4388(03)00097-7
Menco, B. P. (1980). Qualitative and quantitative freeze-fracture studies on olfactory and nasal respiratory structures of frog, ox, rat, and dog. I. A general survey. Cell Tissue Res, 207(2), 183-209. doi:10.1007/bf00237805
Menini, A. (1995). Cyclic nucleotide-gated channels in visual and olfactory transduction. Biophysical Chemistry, 55(3), 185-196. doi:10.1016/0301-4622(94)00153-b
Mori, K., Nagao, H., & Yoshihara, Y. (1999). The olfactory bulb: coding and processing of odor molecule information. Science, 286(5440), 711-715. doi:10.1126/science.286.5440.711
Mori, K., Takahashi, Y. K., Igarashi, K. M., & Yamaguchi, M. (2006). Maps of odorant molecular features in the Mammalian olfactory bulb. Physiological Reviews, 86(2), 409-433. doi:10.1152/physrev.00021.2005
Nakamura, T., & Gold, G. H. (1987). A cyclic nucleotide-gated conductance in olfactory receptor cilia. Nature, 325(6103), 442-444. doi:10.1038/325442a0
Nef, P., Heldman, J., Lazard, D., Margalit, T., Jaye, M., Hanukoglu, I., & Lancet, D. (1989). Olfactory-specific cytochrome P-450. cDNA cloning of a novel neuroepithelial enzyme possibly involved in chemoreception. Journal of Biological Chemistry, 264(12), 6780-6785. doi:10.1016/s0021-9258(18)83497-4
Nevitt, G., & Dittman, A. (1998). A new model for olfactory imprinting in salmon. Integrative Biology: Issues, News, and Reviews, 1(6), 215-223. doi:https://doi.org/10.1002/(SICI)1520-6602(1998)1:6<215::AID-INBI3>3.0.CO;2-V
Norlin, E. M., Alenius, M., Gussing, F., Hägglund, M., Vedin, V., & Bohm, S. (2001). Evidence for gradients of gene expression correlating with zonal topography of the olfactory sensory map. Mol Cell Neurosci, 18(3), 283-295. doi:10.1006/mcne.2001.1019
Oka, Y., Omura, M., Kataoka, H., & Touhara, K. (2004). Olfactory receptor antagonism between odorants. EMBO Journal, 23(1), 120-126. doi:10.1038/sj.emboj.7600032
Pevsner, J., Trifiletti, R. R., Strittmatter, S. M., & Snyder, S. H. (1985). Isolation and characterization of an olfactory receptor protein for odorant pyrazines. Proceedings of the National Academy of Sciences, 82(9), 3050-3054. Retrieved from https://www.pnas.org/content/pnas/82/9/3050.full.pdf
Pifferi, S., Menini, A., & Kurahashi, T. (2010). Signal Transduction in Vertebrate Olfactory Cilia. In A. Menini (Ed.), The Neurobiology of Olfaction. Boca Raton, Florida: CRC Press and Taylor & Francis. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK55986/
Pifferi, S., Pascarella, G., Boccaccio, A., Mazzatenta, A., Gustincich, S., Menini, A., & Zucchelli, S. (2006). Bestrophin-2 is a candidate calcium-activated chloride channel involved in olfactory transduction. Proceedings of the National Academy of Sciences, 103(34), 12929-12934. Retrieved from https://www.pnas.org/content/pnas/103/34/12929.full.pdf
Pilpel, Y., & Lancet, D. (1999). The variable and conserved interfaces of modeled olfactory receptor proteins. Protein Science, 8(5), 969-977. doi:10.1110/ps.8.5.969
Purves, D., Augustine, G. J., & Fitzpatrick, D. (2001). The Transduction of Olfactory Signals. In Neuroscience (2 ed.). Sunderland, MA: Sinauer Associates. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK11039/
Reisert, J., & Matthews, H. R. (1998). Na+-dependent Ca2+ Extrusion Governs Response Recovery in Frog Olfactory Receptor Cells. Journal of General Physiology, 112(5), 529-535. doi:10.1085/jgp.112.5.529
Renou, M., & Lucas, P. (1994). Sex pheromone reception in Mamestra brassicae L. (Lepidoptera): Responses of olfactory receptor neurones to minor components of the pheromone blend. Journal of Insect Physiology, 40(1), 75-85. doi:10.1016/0022-1910(94)90114-7
Reuter, D., Zierold, K., Schröder, W. H., & Frings, S. (1998). A depolarizing chloride current contributes to chemoelectrical transduction in olfactory sensory neurons in situ. Journal of Neuroscience, 18(17), 6623-6630. doi:10.1523/jneurosci.18-17-06623.1998
Ryan, C. (2013). Secret of scent lies in molecular vibrations. Retrieved from https://www.ucl.ac.uk/news/2013/jan/secret-scent-lies-molecular-vibrations
Scheer, A., Costa, T., Fanelli, F., De Benedetti, P. G., Mhaouty-Kodja, S., Abuin, L., . . . Cotecchia, S. (2000). Mutational Analysis of the Highly Conserved Arginine within the Glu/Asp-Arg-Tyr Motif of the α<sub>1b</sub>-Adrenergic Receptor: Effects on Receptor Isomerization and Activation. Molecular Pharmacology, 57(2), 219-231. Retrieved from https://molpharm.aspetjournals.org/content/molpharm/57/2/219.full.pdf
Serizawa, S., Ishii, T., Nakatani, H., Tsuboi, A., Nagawa, F., Asano, M., . . . Sakano, H. (2000). Mutually exclusive expression of odorant receptor transgenes. Nature Neuroscience, 3(7), 687-693. doi:10.1038/76641
Spence, C. (2015). Just how much of what we taste derives from the sense of smell? Flavour, 4(1), 30. doi:10.1186/s13411-015-0040-2
Su, T., Bao, Z., Zhang, Q. Y., Smith, T. J., Hong, J. Y., & Ding, X. (2000). Human cytochrome P450 CYP2A13: predominant expression in the respiratory tract and its high efficiency metabolic activation of a tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Research, 60(18), 5074-5079.
Takeuchi, H., Imanaka, Y., Hirono, J., & Kurahashi, T. (2003). Cross-adaptation between olfactory responses induced by two subgroups of odorant molecules. Journal of General Physiology, 122(3), 255-264. doi:10.1085/jgp.200308867
Takeuchi, H., Ishida, H., Hikichi, S., & Kurahashi, T. (2009). Mechanism of olfactory masking in the sensory cilia. Journal of General Physiology, 133(6), 583-601. doi:10.1085/jgp.200810085
Takeuchi, H., & Kurahashi, T. (2005). Mechanism of Signal Amplification in the Olfactory Sensory Cilia. The Journal of Neuroscience, 25(48), 11084-11091. Retrieved from https://www.jneurosci.org/content/jneuro/25/48/11084.full.pdf
Treloar, H. B., Feinstein, P., Mombaerts, P., & Greer, C. A. (2002). Specificity of glomerular targeting by olfactory sensory axons. Journal of Neuroscience, 22(7), 2469-2477. doi:10.1523/jneurosci.22-07-02469.2002
Vassalli, A., Rothman, A., Feinstein, P., Zapotocky, M., & Mombaerts, P. (2002). Minigenes impart odorant receptor-specific axon guidance in the olfactory bulb. Neuron, 35(4), 681-696. doi:10.1016/s0896-6273(02)00793-6
Vosshall, L. B. (2003). Putting smell on the map. Trends in Neurosciences, 26(4), 169-170. doi:10.1016/S0166-2236(03)00037-7
Wachowiak, M., & Cohen, L. B. (2001). Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron, 32(4), 723-735. doi:10.1016/s0896-6273(01)00506-2
Yoshimura, T. (1996). Pheromone Searching in the Oriental Silkworm Moth. Retrieved from http://nelson.beckman.illinois.edu/courses/neuroethol/models/pheromone_searching/pheromone_searching.html
Zarzo, M. (2007). The sense of smell: molecular basis of odorant recognition. Biological Reviews, 82(3), 455-479. doi:https://doi.org/10.1111/j.1469-185X.2007.00019.x
Zhainazarov, A. B., & Ache, B. W. (1995). Odor-induced currents in Xenopus olfactory receptor cells measured with perforated-patch recording. Journal of Neurophysiology, 74(1), 479-483. doi:10.1152/jn.1995.74.1.479
Zhang, X., Zhang, Q. Y., Liu, D., Su, T., Weng, Y., Ling, G., . . . Ding, X. (2005). Expression of cytochrome p450 and other biotransformation genes in fetal and adult human nasal mucosa. Drug Metabolism and Disposition: The Biological Fate of Chemicals, 33(10), 1423-1428. doi:10.1124/dmd.105.005769
Zhao, H., Ivic, L., Otaki, J. M., Hashimoto, M., Mikoshiba, K., & Firestein, S. (1998). Functional Expression of a Mammalian Odorant Receptor. Science, 279(5348), 237-242. Retrieved from https://science.sciencemag.org/content/sci/279/5348/237.full.pdf
Zou, D. J., Chesler, A., & Firestein, S. (2009). How the olfactory bulb got its glomeruli: a just so story? Nature Reviews: Neuroscience, 10(8), 611-618. doi:10.1038/nrn2666 Zou, Z., & Buck, L. B. (2006). Combinatorial Effects of Odorant Mixes in Olfactory Cortex. Science, 311(5766), 1477-1481. Retrieved from https://science.sciencemag.org/content/sci/311/5766/1477.full.pdf







