Abstract
Scientists have investigated the cause of ageing for centuries, but there is no single source of age, rather, the ageing process occurs due to a combination of numerous factors. Examined in this paper are 4 categories of determinants of maximum lifespan in organisms: telomere dynamics and maintenance, mitochondrial repair and oxidative stress management, anti-inflammatory mechanisms, and tolerance and coexistence with viruses. Specifically, these mechanisms are examined in bats (order chiroptera). Bat species exhibit incredibly long lifespans for mammals of their body mass, defying near-linear trends between mammal body mass and maximum lifespans. Here we discuss the remarkable adaptations in chiroptera that make bats unique from ground-dwelling mammalian species.
Introduction
Ageing is a universal phenomenon observed by a multitude of physiological, chemical, and behavioural changes that every organism experiences. This process is extremely complex and links many factors, which is why scientists have not been able to find a clear theory that may explain completely the causes of ageing. Different environments, lifestyles, and circumstances have pushed organisms to evolve different characteristics that may have a positive or negative impact on their lifespans. Scientists have found many “hallmarks” of ageing in eukaryotic organisms that clearly are connected with longevity such as telomere attrition, loss of proteostasis, mitochondrial dysfunction, and many more (Gorbunova et al., 2020). There are also general trends that can be explained with basic physical or chemical principles that can be observed in the ageing processes of animals, however, when it comes to biology, there are always exceptions. One such exception is bats. Bats are considered outliers of the mammal community as they hold many peculiarities and differences with the general average, in particular, their longevity. Some species of bats are able to live “up to 10 times longer than expected given their body size” (Kacprzyk et al., 2017) and when compared to another mammal of similar size. Bats are also capable of powered flight, the only mammals capable of performing this feat, as well as live in highly dense populations, which facilitates the propagation of disease. When analyzing bats, scientists have found that they possess differences in those “hallmarks” of ageing such as telomere maintenance, oxidative stress, anti-inflammatory mechanisms, and tolerance of viruses, which all contribute to their extreme longevity.
Telomere Dynamics and Maintenance
When it comes to determining the age or biological degree of senescence of the average organism, telomere length is often considered as an efficient genetic biomarker. Telomeres are repetitive sequences of the nucleotides TTAGGG, which, along with different protein complexes, comprise the ends of the genetic code in an organism’s chromosomes (Ineson et al., 2020). They serve as caps for the encapsulated genes to protect them from genetic erosion caused by cell division and DNA replication. This steady detrition of the genetic code is due to the inability of cellular replication machinery to properly continue the 5’ end of the chromosomes’ nucleotide sequences, also known as the end-replication problem (Foley et al., 2020). As telomeres shorten and reach a critically low length, the inner genetic code itself is affected, and a DNA damage signal invokes cellular senescence, which is a process that propels the effects of ageing (Foley et al., 2018). To counter this erosion, most biological organisms benefit from certain biochemical agents to help maintain and even extend telomere length. A common enzyme, known as telomerase, is commonly used in cells to add nucleotide sequences to telomere ends, thus prolonging the cell’s ability to undergo division. This seemingly powerful tool has its setbacks however, especially considering that telomerase activity is prominent in cancer cells and drives their multiplication and growth. This is one of the reasons why organisms, notably those with larger body mass or lifespan, tend to minimize telomerase activity as a defense mechanism against cancer spread (M. Power, Foley, et al., 2021). To continue maintaining telomere length while reducing the threat of carcinogenic factors, organisms may develop and evolve alternative telomere maintenance and lengthening mechanisms that aid in providing better genomic stability. There is often a balance between alternative mechanisms and telomerase activity in organisms, which makes genetics a fascinating yet new field to study. Many factors are involved in telomere lengthening, and the length itself, especially when we look at different bat species worldwide.
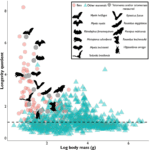
In most species, the length and shortening of their telomeres provides a relatively accurate indication of their age (Ineson et al., 2020) since telomere attrition is a sign that cellular replication is approaching its duplicative limits before commencing senescence. This accumulating evidence is visible as a general trend for most mammals and living organisms, where their relative telomere length, as well as their average body mass, is shown to have a positive correlation to expected and recorded longevity (M. Power, S. Power, et al., 2021). However, bats are a beautiful exception. This mammal is a truly fascinating topic of study because they are known as a complete outlier to studied senescence trends. Their telomere dynamics, activity and relative length vary constantly along with factors such as body size, and life expectancy. Scientists to this day find it difficult to properly estimate a bat’s age. This is exemplified in Figure 1, which plots the logarithmic value of hundreds of mammals’ body mass against their longevity quotient.
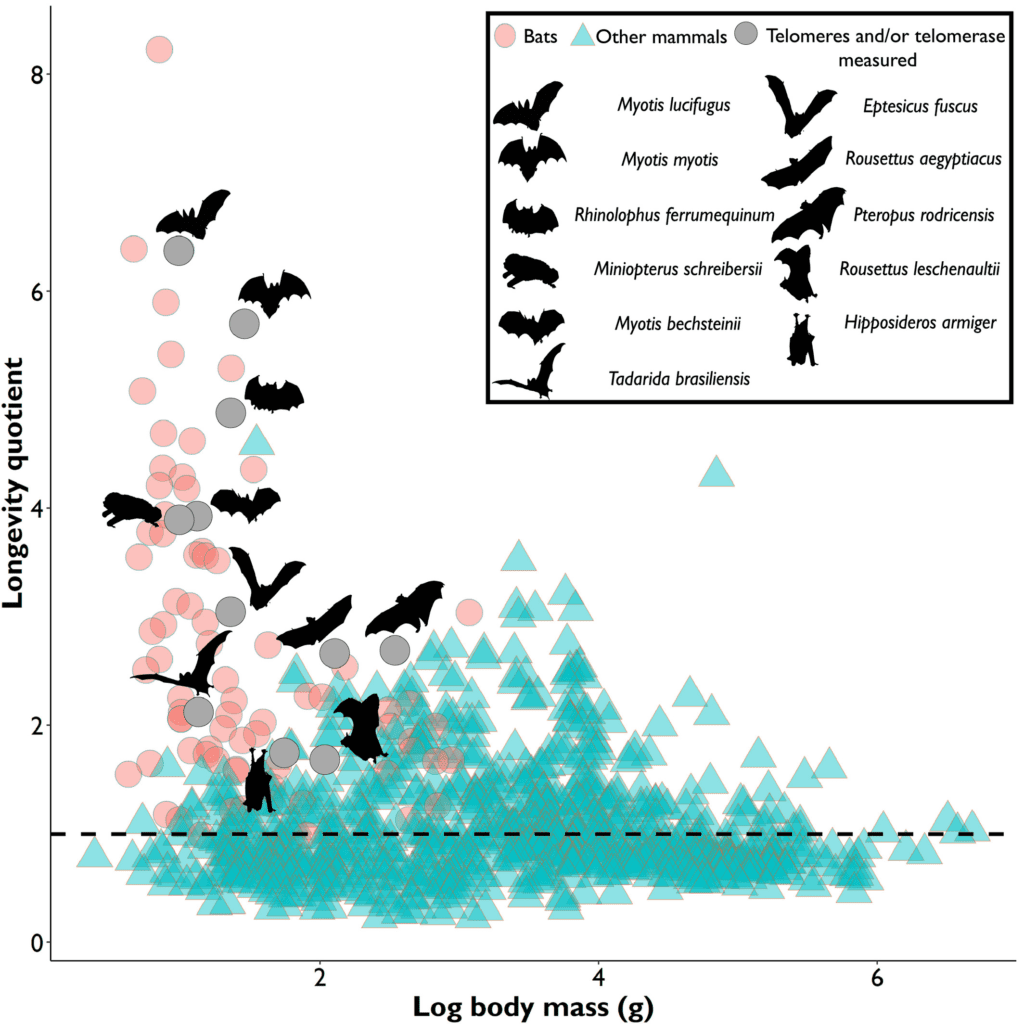
In Figure 1, it is clear to see that bats, shown in pink and gray circles as well as silhouettes, represent most outliers in the mammalian kingdom, with their small body mass and outstanding life expectancy, which counters the popular theory that larger-bodied specimens tend to have longer lives. This makes their telomere measurements not only unreliable when it comes to estimating their age, but also difficult to pinpoint what affects their relative telomere lengths. Nonetheless, even though bats are unique animals and show few clear trends, this unicity is also an interesting opportunity to learn about what factors affect their genetic activities, and an especially enticing case to identify different biomechanisms that they have developed to reach such high longevity and uniqueness, one of these adaptations being biochemical telomere maintenance mechanisms.
First, it is important to describe these different mechanisms that bats employ to maintain telomere activity and delay signs of senescence. For the record-holding long-lived bats, such as Brandt’s bat (M. brandtii), the little brown bat (M. lucifugus), and the greater mouse-eared bat (M. myotis) (Fig. 2), telomerase activity is greatly reduced, some species even showing no signs of it (M. Power, Foley, et al., 2021).


This tendency is remarkable, considering that smaller-sized species and mammals, such as the house mouse (M. musculus) and the Norway rat (R. norvegicus) have the tendency to express telomerase in almost all of their cells to dodge replicative senescence, due to their short lifespan eliminating most risks of cancer development. This specialty in bats indicates that agents other than telomerase maintain and elongate their telomeres, since the rate of diagnosed cancer in bats is some of the lowest ever recorded for mammals (Foley et al., 2020). In field studies, selection tests conducted upon telomere maintenance genes within various bat genomes of the genus Myotis have shown that 21 different telomere maintenance genes, including RAD50, KU80 and MDM2 are differentially expressed, 14 of which serve to repair DNA, and 5 of which function as telomere-lengthening agents (Foley et al., 2018). Two highlighted genes, ATM and SETX, are identified as potential telomere dynamics mediators in Myotis bats. They do so by silencing transcription at damaged DNA sectors and by prevention of DNA damage at replication forks. These genes are highly favorable for bats because reduced telomerase activity decreases cancer cells’ propagation abilities. Therefore, bats have evolved positive selection of these genes from their ancestors to favor alternative telomere maintenance mechanisms that improve their longevity and replicative efficiency (M. Power, Foley, et al., 2021). This selection even extends to the generational scale, where even species of birds such as the storm petrel (O. leucorhoa) have shown larger telomere length variability in the young, whereas adult populations generally had longer telomere on average, thus supporting the hypothesis that long-telomere individuals are favored by natural selection and thus grow to be long-lived specimens (Munshi-South & Wilkinson, 2010).
Now that the mechanisms have been proved to be favored in evolution and function smoothly, the question that arises amongst researchers is what factors are involved in this genetic selection, and what factors overall, whether intrinsic or extrinsic, affect the relative telomere length and genetic expression of bats. Since their chronological age and telomere lengths are difficult to measure and correlate, the logical next step is to study other significant parameters that affect our variables of interest.
Hibernation is an event present in many species where metabolic rate is reduced and telomere lengthening activity is increased. This is true for many animals like the edible dormouse (G. glis), the American black bear (U. americanus), and the grey mouse lemur (M. murinus), and bats are no exception. Species of bats that hibernate use this mechanism as an extreme form of somatic maintenance, where telomere length is increased, and the body is ‘reset’ and cleared of most of its genetic malfunctions before exiting the state of torpor. Diet also comes into play, especially when considering tissue-specific telomere activity. Different niches and habitats affect the intake of vitamins, minerals, nutrients, and proteins of different bats, which all affect their growth and the activity of different organs. Due to oxidative stress formed from organs and tissues that replicate and perform more rigorously, telomere lengthening and telomerase activity is accentuated in certain tissues, and this varies among species (Fig. 3) (wang et al., 2011).

Studies on different mammals and bats have shown that telomerase activity is favored mainly in the liver, spleen, and kidney of species such as the great roundleaf bat and Leschenault’s rousette, and that such activity spikes proportionately during the hibernation of the respective species. Additionally, homeothermic species also present different tissue-specific telomere dynamics when compared to heterothermic species, especially when considering their hibernation periods (Wang et al., 2011). And so, the bat’s diet naturally influences telomere lengthening in different parts of its body. This also suggests ethical and efficient ways to study bat genetics by providing data on which tissues are most fruitful for analysis to reduce the harm done to the sampled bat (M. Power, S. Power, et al., 2021).
On the other side of the spectrum, environmental and extrinsic factors have also been proven to be impactful on telomere dynamics. A major variable, predation risk, affects bats in different ecological niches, but their defense mechanisms, echolocation, ability of flight, nocturnal activity, and habitat, are used to their advantage to evade predators, and their tremendous metabolic needs do not appear to affect the bat’s long lifespan. This trend is even more apparent when comparing mainland populations against insular populations, where islands present lower predation threats (Munshi-South & Wilkinson, 2010).
Moreover, climate-related fluctuations in humidity, temperature, precipitation and wind speed are also shown to affect bats’ telomere dynamics, notably during their earlier stages in life and as well as during the spring transition period of the bat’s life cycle, where environmental conditions impact a bat’s operation considerably, as seen in Figure 4 (Foley et al., 2020). It is apparent in the four plots that specific relative telomere lengths aggregate around certain values of the environmental conditions, supporting the idea that there are ideal temperatures, precipitation levels and wind speeds where long-lived bats thrive better and genetically operate more efficiently, while also proving the correlation between telomere-lengthening dynamics and environmental variables. Therefore, not only are there alternative telomere maintenance mechanisms to add to telomerase activity, but there are also countless other extrinsic and intrinsic factors that affect how bats age and support their telomere lengthening and shortening fluctuations. The complexity of these studies and the uniqueness of bats’ lifestyles and niches make it so that researchers find great difficulty in pinpointing trends in bats’ ageing processes, but that does not remove from the fascination and excellent data that can be studied from these marvellous and distinct mammals.

Mitochondrial Repair and Oxidative Stress Management
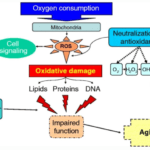
One of the most universally accepted theories of ageing is the free radical theory put forth by Denham Harman in the 1950s, which postulates that ageing is caused by the steady accumulation of damage caused by free radicals in the body, and this determines the maximum lifespan of species (Pole et al., 2016). The most significant contributor to this damage is radical oxygen species (ROS), the majority of which are produced by mitochondrial reactions through loose electrons in the electron transport chain. ROS are short-lived and highly reactive molecules that are produced during the partial decomposition of oxygen. The ageing process does not begin immediately, but rather when the amount of ROS in the body surpasses what the cells can decompose. ROS can lead to four different phenomena: necrosis (death), autophagy (digestion of the cell), apoptosis (self-destruction), and senescence (inability to proliferate, stuck in G1 phase). All four kinds of cell damage have negative effects on the health and lifetime expectancy of the organism. Figure 5 shows the process by which regular oxygen consumption produces the radical oxygen species that cause age. The figure also shows the three groups of organic macromolecules that can be damaged by ROS. ROS can be exogenous or endogenous, reaching the organism from its environment, or from its own cells respectively. Endogenous ROS contribute to the majority of oxidative damage and are the only form of ROS discussed here.
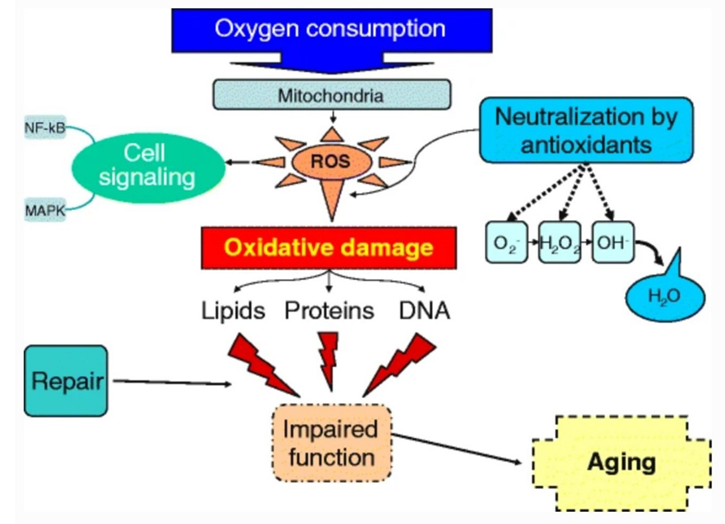
As the free radical theory of ageing suggests that senescence is a product of regular metabolic functions, metabolic rate is correlated with life expectancy of a species. Across living species, larger organisms tend to have slower basal metabolic rates and longer maximum lifetimes, however, there are exceptions. There are some species that have evolved to combat ROS and its damages, attaining life expectancies that defy this linear trend. Bats are an excellent example. With small body masses and the high metabolic demands of flight, bats have on average 3.5 times the lifespan of other eutherian mammals and can consume twice the energy in their lifetime as other mammals (Munshi-South & Wilkinson, 2010). The mechanism by which bats can prevent oxidative stress has been the subject of numerous studies. It has been suggested that bats possess certain characteristics in their mitochondrial genome that prevent damage to mitochondrial DNA (mtDNA). mtDNA is especially vulnerable to mutations due to its location, as mitochondrial processes are responsible for the majority of ROS production. mtDNA is especially vulnerable to deletion mutations, as it contains many direct repetitions. Some bats, however, possess much fewer of these direct repeats in mtDNA, resulting in reduced deletion mutations and increased longevity. However, this trend is not universal across bat species and cannot solely explain the extended bat lifespan.
Bats, like many other hibernating animals, exhibit relatively long lifespans due to periods of dormancy where metabolic rate is lowered, decreasing ROS production. On average, hibernating bats live for six years longer than non-hibernating ones (Lagunas-Rangel, 2019). During hibernation, metabolic rate is approximately 1/100th that of an active bat, greatly reducing ROS production. Hibernating bats are also known to possess higher levels of antioxidant proteins in their mitochondria, which reduce ROS production by inhibiting oxidation reactions.
Birds also exhibit extended lifespans, similar to bats. Comparison of birds and bats yields interesting conclusions as both evolved the ability of flight through separate evolutionary pathways (convergent evolution). It is energetically favourable for both to have evolved extended lifespans as both experience low rates of extrinsic mortality due to their agility when escaping predators. Bird tissues are resistant to ROS when compared to flightless mammals (Holmes et al., 2001). Liver tissue in pigeons was found to be more resistant to lipid peroxidation than rat tissue, caused by lower fatty acid unsaturation in the pigeons mitochondrial membrane. These same oxidation-resistant membranes were also observed in the heart tissues of canaries and budgerigars, two long-living bird species.
Hydrogen peroxide (H2O2) is an example of a very reactive ROS produced in the mitochondria that causes damage to the cell and organelles. H2O2 forms as a natural byproduct of mitochondrial metabolism, and some bat species have evolved characteristics to reduce its production. The little brown bat, with a remarkable maximum longevity of 34 years, has been observed to produce less than half the amount of H2O2 per mole of oxygen consumed by another mammalian species of comparable size, the short-tailed shrew. One suggested explanation to this efficiency is that of bats having a larger surface area in their inner mitochondrial membrane, or an extended electron transport chain to reduce waste electrons. Little brown bat endothelial cells also produce less ROS and show a resistance to cell autophagy induced by ROS (Munshi-South & Wilkinson, 2010). The little brown bat exhibits an incredibly long lifespan for its size of about 8 grams, as well as a strong resistance to hydrogen peroxide, one of the most contributing ROS to oxidative stress. This suggests that, at least for this species and possibly for other bats, there is a significant correlation between resistance to ROS and maximum lifespan.
Anti-Inflammatory Mechanisms
Inflammation is a natural process that the body undergoes in response to different external or internal elements. These can be viruses, microbes, damaged cells, or anything that should not be present in the system. However, as animals grow older and approach the end of their lifespans, many functions become diminished in the body, processes aren’t as efficient, and there is a general decline in the immune system. Many of these “age-related processes promote inflammatory activity, causing long term tissue damage and systemic chronic inflammation” (Masternak & Bartke, 2012), which is a constant inflammation of differing pain that can last up to years. This “state of low-grade nonresolving activation of inflammatory pathways” (Neves & Sousa-Victor, 2020) that often follows the ageing process has come to be known as ‘inflammaging’. Chronic inflammation is bad for the body as the excessive production of proinflammatory cytokines, the signalling molecules that promote inflammation, or a ‘cytokine storm,’ can lead to tissue damage and many age-related diseases such as atherosclerosis or Alzheimer’s disease.
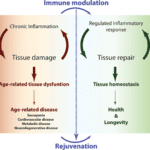
Chronic inflammation creates a positive feedback loop that accelerates tissue damage, leading to age-related tissue dysfunction and diseases, while a well-regulated inflammatory response does not damage the tissues and leads to a healthier and longer life (Fig. 6). Although the relation between chronic inflammation and ageing can’t be said for certain, it is obvious that “inflammation is a common denominator between several age-related pathologies and, independently of its role as the initial trigger driving the ageing process, contributes to the progression of tissue dysfunction” (Neves & Sousa-Victor, 2020).

As for the case with bats, one of the many aspects that contribute to their extended lifespan is their natural ability to suppress chronic inflammation as they approach old age. This is done through a multitude of genetic adaptations. It is hypothesized that bats have evolved these unique immune system adaptations as a means of countering the great strain that powered flight, which induces high amounts of metabolic stress, and viral infections. It would be false to believe that bats do not exhibit pro-inflammatory responses when such a reaction would be needed. Rather, bats’ pro-inflammatory responses are countered “by a sustained high-level transcription of anti-inflammatory cytokine Il-10” (Kacprzyk et al., 2017). A study done on the anti-inflammatory response in bat macrophages which studied the response that Myotis, the largest and longest living bat in the world, and the house mouse’s macrophages had against external stimuli. Although the bat had a similar pro-inflammatory response to the mouse’s, the big difference came when observing the anti-inflammatory response later on, with the greater mouse-eared bat (M. myotis) showing higher proportions of anti-inflammatory cytokines. Another difference in their immune response is the dampening of many proinflammatory mechanisms such as NLRP3 inflammasome activity and TNF-alpha expression. Both of these mechanisms have been linked to the “initiation and progression of atherosclerosis” (Gorbunova et al., 2020). Oftentimes, free DNA can wind up in a cell’s cytoplasm, which signals an alarm leading to inflammation. These pieces of DNA can be viral in nature, however, in senescent cells, there is often an accumulation of dead or damaged organelles and “the self-debris and self-molecules that result from unhealthy or dead cells are produced at a higher rate in aged tissues, while the mechanisms responsible for the disposal of harmful products of cellular damage progressively decline” (Neves & Sousa-Victor, 2020). Thus, scientists believe that the many inflammation-suppressing adaptations bats have evolved in order to accommodate a greater metabolic stress or to prevent viral infections has led them to limit inflammation and inflammation driven ageing, resulting in extraordinary longevity (Kacprzyk et al., 2017).
Tolerance and Coexistence with Viruses
The immune system protects organisms from potentially harmful substances. Primary defenses of many mammals include production of mucus and saliva, stomach acid and skin. Despite this, many viruses manage to penetrate these defenses and enter the body. Viruses will then seek to attach to a host cell and either replicate or go dormant. If the virus replicates, upon reaching a threshold of virus concentration, symptoms will be produced in the host. If the virus goes dormant, it will latently reside within the cell, not eradicated, but not replicating. A latent virus can reactivate and produce progeny. Most multicellular organisms have latent viruses within them. This poses a great threat to mammals. Bats, however, are unique among mammals in their excellent tolerance to viruses. Thus far, only a single RNA virus studied and found in the bat population, the Tacaribe virus in the Jamaican fruit bat, has been shown to consistently cause significant bat morbidity and mortality (Malmlov et al., 2017). This resilience allows them to be reservoir hosts to a multitude of viruses, especially RNA viruses. A reservoir host carries pathogens and can spread them to others but suffers no ill effects itself. In summary, bats carry a plethora of viruses, whilst neither becoming sick nor expending energy upon them. So where exactly does this exceptional tolerance come from? Mammals express innate immune receptors and inflammation-associated proteins. However, these characteristics are abnormally modified in bats, leading to an efficient combination of antiviral mechanisms and reduced inflammation.
Interferons (IFN) are a form of antiviral cytokine and represent an important component of antiviral defense. There exist 3 types of IFN, types I, II, and III. Type I and III IFNs are induced in vertebrates to combat viral infections (Clayton & Munir, 2020). Type I IFNs consist most notably of IFN-alpha and IFN-beta, which respond directly to viral infection, and IFN-delta, IFN-kappa, IFN-omega, and IFN-epsilon, the purposes of which are less clear (Randall & Goodbourn, 2008). Type III IFNs are composed of three main members, IFN-lambda-1, IFN-lambda-2, and IFN-lambda 3.
Both of these types of IFNs are activated through the same signalling pathway. They are stimulated in mammalian cells by pattern recognition receptors (PRRs) that recognize pathogen-associated molecular patterns (PAMPS), in infected cells (Banerjee et al., 2020). Once PAMPS are signalled, the immune system induces the expression of proinflammatory and antiviral cytokines such as IFN. These IFNs are then secreted within viral-infected cells (Onoguchi et al., 2007) to activate IFN-stimulated genes (ISGs) which protect against pathogens in various ways. ISGs can inhibit translation, inhibit viral entry, inhibit viral transcription, and sequester viral mRNA translation. Thus, an early IFN response is crucial to limiting virus propagation (Banerjee et al., 2020).
We now know that IFN are the first cytokines to respond to viral infection in bats (Clayton & Munir, 2020). Unlike other mammals, bats appear to express constitutively type I IFNs. That is to say that bats are always producing type I IFNs, whether an infection has been signalled or not.
The existence of IFN signalling pathways in bats was first proven in 1969 (Stewart, 1969). The first transcriptome analysis of IFNs in bats was conducted upon the black flying fox in 2016. In this analysis, it was found that the black flying fox possess a restricted type I IFN locus, smaller than that of other mammals (Zhou et al., 2016). This could highlight an optimization of natural selection of functional genes in accordance with the “less is more” theory. That is to say, the favoring of certain genes with advantageous consequences for the host while discarding the production of those less important (Olson, 1999). Notably, the black flying fox also has an expansion of IFN-delta and IFN-epsilon, uncommon in mammals (Clayton & Munir, 2020). This presence of lesser-known type I IFNs may somehow contribute to bats’ viral tolerance. Considered in accordance with the “less is more” theory, this could be indicative of unknown contributions of IFN-delta and IFN-epsilon to viral protection.
As for type III IFNs, bats appear to be relatively homologous to other mammals (Virtue, E.R., et al., 2011). Despite this, type III IFNs have been suggested to play an important role in bat antiviral immunity, because they are upregulated in response to viral infection whilst type I IFNs are simultaneously downregulated (Clayton & Munir, 2020). Given that both type I and III IFNs activate the same signalling pathway, it can be deduced that somehow the expression of type III IFN during a viral infection is more beneficial to bats than the expression of type I IFN. This may be related to the wide distribution of type III IFN in bats (Clayton & Munir, 2020). The more IFN receptors are present, the faster ISGs can be expressed and, consequently, the faster a virus can be contained. IRF7, a common interferon regulatory factor, is also distributed unusually broadly through the cells of the flying black fox (Zhou et al., 2014). IRF7 is generally thought of as a master regulator of the production of type I IFN. However, it also possesses the ability to coexpress type III IFNs with its type I IFNs (Wack et al., 2015). This further magnifies the flying black fox’s ability to produce IFN quickly in response to signals of infection. Interestingly, at least five species of fish have also been identified to have broadly distributed IRF7 (Zhou et al., 2014). This could be indicative of similarities in antiviral immunity between bats and fish. It is also noteworthy that, of the fish species identified, only the snakehead fish appears to have potential geographical overlap with the black flying fox.
Stimulator of interferon genes (STING) are transmembrane proteins that controls transcription of various host defence genes in the endoplasmic reticulum (Barber, 2015). Bats have a reduced STING response due to a mutation at the S358 phosphorylation site of STING (Nagaraja et al., 2022). This leads to a lessened release of IFN in response to DNA viruses. This means that while bats show a strong resilience to RNA viruses, they are somewhat more vulnerable to DNA viruses.
The excessive release of proinflammatory cytokines in response to infection can lead to tissue damage (Tisoncik et al., 2012). Bats have evolved mechanisms to prevent such unnecessary self-inflicted harm. For example, bat immune cells differ greatly in NLRP3 inflammasome activation in comparison to mouse cells, as those of bats show reduced activation when stimulated with NLRP3 ligands (Nagaraja et al., 2022). This shows an adapted repression of inflammatory response. As a result this repression of NLRP3 activation, there is also a reduced release of IL-1-beta cytokines, proinflammatory cytokines, in response to infection (Nagaraja et al., 2022).
A recent study showed that variations in bats’ CASP1, a protein coding gene, allows reduction of enzymatic activity afforded for IL-1-beta processing and pyroptosis (Goh et al., 2020). This same study also identified that some bat species show intact CASP1 function, instead have abolished IL-1-beta cleavage as an alternate strategy to reduce inflammation. In both cases, mutations have enabled bats to dampen inflammatory responses to RNA viruses (Goh et al., 2020).
These adaptations enable bats to avoid both needless expenditure of energy and damage from inflammation. This allows them to preserve their homeostasis at a lower cost, thus increasing longevity.
Conclusion
Senescence is inevitable. Eventually, all organisms must deteriorate and die. Nonetheless, the longevity of bats is exceptional. It can be attributed to various attributes, among which telomere maintenance mechanisms, decreased ROS production and increased telomere lengthening due to hibernation, sustained transcription of anti-inflammatory cytokines, constitutively expressed interferons, coexpression of interferons and dampened inflammatory responses to infection, stand out as key effects. Notice how the combination of dampened inflammatory responses to infection in tandem with the sustained transcription of anti-inflammatory cytokines support each other, both pre-empting the chronic feedback loop of chronic inflammation. Combinations of key effects not only impact senescence on an individual level; they also overlap creating a powerful compounding effect. Thus, the unique combination of characteristics present in bats allows them to defy senescence for an unusually long period of time. This combination of traits has not been discovered in other species as of yet. However, certain other species share in some of the traits highlighted in this paper. This poses two major questions for future study. First, how, and why did bats and these other species develop these traits in spite of inhabiting different environments? Secondly, what traits possessed by other species could further optimize the longevity of bats?
References
Banerjee, A., Baker, M. L., Kulcsar, K., Misra, V., Plowright, R., & Mossman, K. (2020). Novel Insights Into Immune Systems of Bats. Front Immunol, 11, 26. https://doi.org/10.3389/fimmu.2020.00026
Barber, G. N. (2015). STING: infection, inflammation and cancer. Nat Rev Immunol, 15(12), 760-770. https://doi.org/10.1038/nri3921
Buffenstein, R., Edrey, Y. H., Yang, T., & Mele, J. (2008). The oxidative stress theory of aging: embattled or invincible? Insights from non-traditional model organisms. AGE, 30(2), 99-109.
Clayton, E., & Munir, M. (2020). Fundamental Characteristics of Bat Interferon Systems. Front Cell Infect Microbiol, 10, 527921. https://doi.org/10.3389/fcimb.2020.527921
Foley, N. M., Hughes, G. M., Huang, Z., Clarke, M., Jebb, D., Whelan, C. V., Petit, E. J., Touzalin, F., Farcy, O., Jones, G., Ransome, R. D., Kacprzyk, J., O’Connell, M. J., Kerth, G., Rebelo, H., Rodrigues, L., Puechmaille, S. J., & Teeling, E. C. (2018). Growing old, yet staying young: The role of telomeres in bats’ exceptional longevity. Sci Adv, 4(2), eaao0926. https://doi.org/10.1126/sciadv.aao0926
Foley, N. M., Petit, E. J., Brazier, T., Finarelli, J. A., Hughes, G. M., Touzalin, F., Puechmaille, S. J., & Teeling, E. C. (2020). Drivers of longitudinal telomere dynamics in a long-lived bat species, Myotis myotis. Mol Ecol, 29(16), 2963-2977. https://doi.org/10.1111/mec.15395
Goh, G., Ahn, M., Zhu, F., Lee, L. B., Luo, D., Irving, A. T., & Wang, L. F. (2020). Complementary regulation of caspase-1 and IL-1beta reveals additional mechanisms of dampened inflammation in bats. Proc Natl Acad Sci U S A, 117(46), 28939-28949. https://doi.org/10.1073/pnas.2003352117
Gorbunova, V., Seluanov, A., & Kennedy, B. K. (2020). The World Goes Bats: Living Longer and Tolerating Viruses. Cell Metab, 32(1), 31-43. https://doi.org/10.1016/j.cmet.2020.06.013
Holmes, D. J., Fluckiger, R., & Austad, S. N. (2001). Comparative biology of aging in birds: an update. Exp Gerontol, 36(4-6), 869-883. https://doi.org/10.1016/s0531-5565(00)00247-3
Ineson, K. M., O’Shea, T. J., Kilpatrick, C. W., Parise, K. L., & Foster, J. T. (2020). Ambiguities in using telomere length for age determination in two North American bat species. Journal of Mammalogy, 101(4), 958-969. https://doi.org/10.1093/jmammal/gyaa064
Kacprzyk, J., Hughes, G. M., Palsson-Mcdermott, E. M., Quinn, S. R., Puechmaille, S. J., O’Neill, L. A. J., & Teeling, E. C. (2017). A Potent Anti-Inflammatory Response in Bat Macrophages May Be Linked to Extended Longevity and Viral Tolerance. Acta Chiropterologica, 19(2), 219-228. https://doi.org/10.3161/15081109acc2017.19.2.001
Lagunas-Rangel, F. A. (2020). Why do bats live so long?-Possible molecular mechanisms. Biogerontology, 21(1), 1-11. https://doi.org/10.1007/s10522-019-09840-3
Malmlov, A., Seetahal, J., Carrington, C., Ramkisson, V., Foster, J., Miazgowicz, K. L., Quackenbush, S., Rovnak, J., Negrete, O., Munster, V., & Schountz, T. (2017). Serological evidence of arenavirus circulation among fruit bats in Trinidad. PLOS ONE, 12(9), e0185308. https://doi.org/10.1371/journal.pone.0185308
Masternak, M. M., & Bartke, A. (2012). Growth hormone, inflammation and aging. Pathobiol Aging Age Relat Dis, 2. https://doi.org/10.3402/pba.v2i0.17293
Munshi-South, J., & Wilkinson, G. S. (2010). Bats and birds: Exceptional longevity despite high metabolic rates. Ageing Res Rev, 9(1), 12-19. https://doi.org/10.1016/j.arr.2009.07.006
Nagaraja, S., Jain, D., & Kesavardhana, S. (2022). Inflammasome regulation in driving COVID-19 severity in humans and immune tolerance in bats. J Leukoc Biol, 111(2), 497-508. https://doi.org/10.1002/JLB.4COVHR0221-093RR
Neves, J., & Sousa-Victor, P. (2020). Regulation of inflammation as an anti-aging intervention. Febs j, 287(1), 43-52. https://doi.org/10.1111/febs.15061
Olson, M. V. (1999). When less is more: gene loss as an engine of evolutionary change. Am J Hum Genet, 64(1), 18-23. https://doi.org/10.1086/302219
Onoguchi, K., Yoneyama, M., Takemura, A., Akira, S., Taniguchi, T., Namiki, H., & Fujita, T. (2007). Viral infections activate types I and III interferon genes through a common mechanism. J Biol Chem, 282(10), 7576-7581. https://doi.org/10.1074/jbc.M608618200
Pole, A., Dimri, M., & Dimri, G. P. (2016). Oxidative stress, cellular senescence and ageing. AIMS Molecular Science, 3(3).
Power, M. L., Foley, N. M., Jones, G., & Teeling, E. C. (2021). Taking flight: An ecological, evolutionary and genomic perspective on bat telomeres. Mol Ecol. https://doi.org/10.1111/mec.16117
Power, M. L., Power, S., Bertelsen, M. F., Jones, G., & Teeling, E. C. (2021). Wing: A suitable nonlethal tissue type for repeatable and rapid telomere length estimates in bats. Mol Ecol Resour, 21(2), 421-432. https://doi.org/10.1111/1755-0998.13276
Randall, R. E., & Goodbourn, S. (2008). Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol, 89(Pt 1), 1-47. https://doi.org/10.1099/vir.0.83391-0
Stewart, W., Allen, R., & Sulkin, S. (1969). Persistent infection in bats and bat cell cultures with Japanese encephalitis virus. Bacteriol. Proc,
Tisoncik, J. R., Korth, M. J., Simmons, C. P., Farrar, J., Martin, T. R., & Katze, M. G. (2012). Into the eye of the cytokine storm. Microbiol Mol Biol Rev, 76(1), 16-32. https://doi.org/10.1128/MMBR.05015-11
Virtue, E. R., Marsh, G. A., Baker, M. L., & Wang, L. F. (2011). Interferon production and signaling pathways are antagonized during henipavirus infection of fruit bat cell lines. PLOS ONE, 6(7), e22488. https://doi.org/10.1371/journal.pone.0022488
Wack, A., Terczynska-Dyla, E., & Hartmann, R. (2015). Guarding the frontiers: the biology of type III interferons. Nat Immunol, 16(8), 802-809. https://doi.org/10.1038/ni.3212
Wang, L., McAllan, B. M., & He, G. (2011). Telomerase activity in the bats Hipposideros armiger and Rousettus leschenaultia. Biochemistry (Mosc), 76(9), 1017-1021. https://doi.org/10.1134/S0006297911090057
Zhou, P., Cowled, C., Mansell, A., Monaghan, P., Green, D., Wu, L., Shi, Z., Wang, L. F., & Baker, M. L. (2014). IRF7 in the Australian black flying fox, Pteropus alecto: evidence for a unique expression pattern and functional conservation. PLOS ONE, 9(8), e103875. https://doi.org/10.1371/journal.pone.0103875
Zhou, P., Tachedjian, M., Wynne, J. W., Boyd, V., Cui, J., Smith, I., Cowled, C., Ng, J. H., Mok, L., Michalski, W. P., Mendenhall, I. H., Tachedjian, G., Wang, L. F., & Baker, M. L. (2016). Contraction of the type I IFN locus and unusual constitutive expression of IFN-alpha in bats. Proc Natl Acad Sci U S A, 113(10), 2696-2701. https://doi.org/10.1073/pnas.1518240113