Abstract
Daphnia, referred to as water fleas due to their distinctive hop-and-sink swimming pattern, are commonly found navigating freshwater lakes and ponds. Their phenotype, finely tuned to their environment and way of life, is dynamically adaptive throughout their lifecycle, showcasing variations in eye size, carapace shape, and swimming behaviors. Their compound eye, a singular structure comprising numerous independently functioning units, further emphasizes their evolutionary adaptations. Intriguingly, external factors influence their eye size and entire body structure, reflecting the profound influence of their environment and trophic interactions. This paper delves into a comprehensive exploration of these aspects, explicitly analyzing their implications on Daphnia‘s fitness and subsequent evolutionary trajectory shaped by natural selection. Overall, this paper provides an overall understanding of their locomotion, mechanical structures, optimized visual system, and defense mechanisms and their importance in Daphnia‘s survival.
Introduction
Daphnia, commonly known as water fleas, are small, freshwater crustaceans belonging to the family Daphniidae and order Cladocera(Daphnia, 2023). The genus Daphnia is subdivided into several subgenera, including Daphnia, Australodaphnia, and Ctenodaphnia (Daphnia, 2023). Daphnia magna is amongst the largest species in size within the genus, with females reaching up to 5 mm and males about 2 mm (Daphnia magna, 2023). Daphnia is a widely studied zooplankton in relation to food webs, predator-prey interactions, and other biological and ecological considerations. These water fleas utilize a hop-and-sink mechanism that makes quick, impulsive jumps by beating their antennae to propel themselves forward. They are found in any freshwater system; however, their movement is limited in vertical displacement in water columns due to their small sizes. Daphnia are known for their distinct morphology, rapid reproductive capabilities, and sensitivity to environmental changes, making them invaluable subjects for ecological, toxicological, and physiological studies. Their widespread distribution in freshwater habitats, ease of cultivation, and transparent exoskeleton, allowing for clear visualization of internal processes, have contributed to their prominence in scientific investigations.

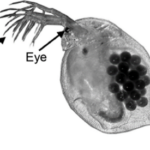
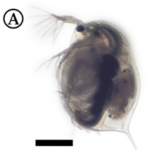
Fig.1: Micrography of a Daphnia with eggs (Algar, 2018).
Moreover, Daphnia’s trophic relations significantly impact their behaviour and changes through their evolutionary biology. As herbivorous zooplankton, Daphnia primarily consume phytoplankton, exerting top-down control on algal populations within aquatic habitats. As filter feeders, meaning that they are able to intake a certain volume of water and use their mouths as a water filter to only keep in food, their feeding behaviour regulates algal biomass. Therefore, it affects the water quality and nutrient dynamics. Daphnia serves as a significant food source for various predators including phantom midge, fish, insects, amphibians, and other crustaceans (Stollewerk, 2010). This predation pressure functions as a mechanism of top-down regulation in the trophic pyramid, and food resource availability significantly impacts population dynamics and growth rates. The interplay of Daphnia within the trophic web elucidates their pivotal position, influencing the structure and function of freshwater ecosystems.
The environment of Daphnia primarily consists of freshwater ecosystems, such as lakes, ponds, and rivers. Thus, water chemistry, temperature, nutrient availability, and the presence of predators significantly impact them. As a result, Daphnia’s survival is directly linked to their ability to adapt to various conditions within their habitat. For instance, they exhibit behavioral and morphological adaptations to cope with changing temperatures, fluctuating nutrient levels, and predation pressures. As a result, they develop inducible defenses, altering their morphology to enhance survival rates. The dynamic nature of freshwater environments constantly challenges Daphnia, necessitating continuous adaptation and contributing to the diversification and evolution of this essential aquatic organism.
This paper elaborates on the hop-and-sink mechanism of Daphnia‘s locomotion and the mechanical adaptions crucial for their defined motion in the water, as well as the high flexibility of their antennae. The unique structure of Daphnia‘s carapace, a lightweight structure capable of high mechanical impacts, and its role in their swimming behaviour are also explored better to understand Daphnia’s various phenotypic adaptations throughout evolution. Further, the paper discusses their apposition-compound eye and the optimization of their visual system in response to changes in their environmental conditions and their inducible morphological defenses that thwart predators. Finally, these various mechanical structures and developments are linked together through the notion of “survival of the fittest” and how these adaptations have allowed Daphnia to optimize its survival.
Hop-and-Sink Locomotion
Daphnia’sswimming mechanism is referred to as the “hop-and-sink” method, as shown in Figure 2, and is a biomechanical process that allows for efficient navigation through their aquatic world. Daphnia uses rapid, synchronized movements of their antennae and thoracic limbs to generate propulsive forces. The water flea extends its antennae forward, creating a water current that propels itself in the opposite direction. This action, followed by a rapid flexion of the antennae and thoracic limbs, creates an upward thrust, and the downward motion resembles a “sink,” preparing them for the next “hop.” This cyclic “hop-and-sink” motion allows Daphnia to move through the water swiftly and effectively (Skipper et al., 2019).
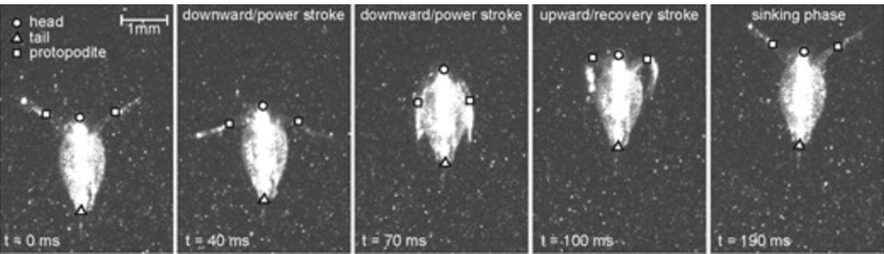
Fig. 2: Sequence of particle image velocimetry (PIV) images showing the typical stroke cycle for Daphnia magna (specimen 4; 2.57 mm) (Skipper et al., 2019).
Mechanical Adaptations
Daphnia have developed various mechanical adaptations essential for their defined motion in the water and high flexibility of their antennae, enabling precise control of their movements. For instance, the “hop-and-sink” motion depends on the periodical beating of the second set of antennae. More specifically, the second set of antennae is divided into two branches: exopodite and endopodite. As can be seen from Figure 2, a typical motion cycle includes a downward-directed power stroke followed by a recovery stroke, a sinking stage, which enables a flexible asymmetric antennae motion, and an overall breaststroke style of swimming. During the cycle, the branched antennae spread out in the downward stroke to increase the drag force on appendages and, thus, increase thrust. Through the recovery stroke, the antennae move upwards, and the distal branches fold downwards to reduce drag. The distal branches then extend above the head to initiate another stroke cycle. This movement is examined by the flow field around the body, as shown in Figure 3. During the power stroke, two viscous vortex rings — antennal vorticity and body vorticity form on the two sides of the body, become strengthened during the recovery stroke, and decay slowly in the sinking phase. Using time-resolved tomographic particle image velocimetry (tomo-PIV), Skipper et al. found that Daphnia’s velocity experiences a dramatic increase during the power stroke, reaching up to 1000 body lengths/s2, and decelerates to a steady sinking speed during recovery stroke (Skipper et al., 2019). Using the breaststroke style of swimming, the interruption of the flow structure around Daphnia is minimized due to its vertical swimming direction, and the antennae are curled close to the body during the recovery stroke. Hence, the flexibility of the antennae enables Daphnia to swim with minimized interference and high control (Skipper et al., 2019). More specifically, the second antennae form a particular muscle system to expand energy in environmental adaptation. As the main swimming organ, the second antennae are powered by three muscles, longer than those in closely related species (Hebert, 1978). First, the longer muscles shorten faster than the short fibers, increasing the distance the antennae move on each stroke and contributing to Daphnia’s rapid swimming. Secondly, the nerve supply to the antennae originates primarily from a significant branch of the ventral nerve cord on its respective side. The antennal protopod/head joint structure comprises chitinous rings with interposing thin arthrodial membranes, enabling one ring to contract against adjacent ones. This system provides the antennae with enough flexibility and sensitivity during swimming and allows Daphnia to use their proximal muscles, in addition to their antennae and distal muscles, to make sweeping motions for guidance of direction (Fryer, 1991). This precise coordination allows Daphnia to detect environmental changes and carry out multiple sensory functions such as food capture, predator avoidance, and navigation.

Fig.3: Flow field created by Daphnia magna (specimen 8; 2.45 mm) (Skipper et al., 2019).

Fig. 4: Image of a swimming gravid female daphniid (Daphnia magna), approximately 4 mm long, with important anatomical features labeled: the branched second antennae, the eye, and the posterior spine (Skipper et al., 2019).
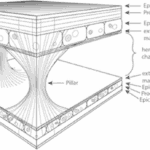
Moreover, Daphnia’s carapace is a lightweight structure capable of withstanding high mechanical impacts. It comprises an integumental fold with two integumental layers piled in a reverse complement manner. The space between the layers is filled with hemolymph, interconnected by irregularly dispersed structures known as the pillars, which are solid columns with broad bases primarily composed of chitin fibers. The slim waists of these pillars are made of intermediate filaments that extend to the extracellular matrix and are anchored vertically in the procuticle, which provides an optimal load angle to withstand tensile forces generated from hemolymph between the two layers of the carapace when encountering force from the environment. On the other hand, the whole integumental covering of Daphnia has been found to be permeable to oxygen to a certain degree. As can be seen from Figure 5, the proximal integument is thinner than the distal integument. While the thicker outer layer does not seem to contribute to gas exchange, the thinner inner layer is covered by a delicate cuticle, which is several times thinner than the cuticle on other parts of the body, contributing to its low diffusive resistance that facilitates Daphnia’s oxygen intake from the water environment (Pirow et al., 1999) (Kruppert et al., 2016). This results in the high hemoglobin oxygen saturation near the posterior margin of the carapace and further supports their swimming by providing a sufficient oxygen supply. At the same time, the carapace and antennae can form an aerofoil profile that assists in lifting and the control of swimming direction (Watling & Thiel, 2013). Moreover, the streamlined shape of the carapace can help reduce water resistance and thus contribute to more efficient and energy-saving swimming. The carapace can also increase the speed of vertical migration when avoiding predators (Horstmann et al., 2022). Hence, Daphnia’s carapace contributes to protective mechanical support, increasing respiration rate and direction control, and reducing water resistance, which results in more efficient swimming.
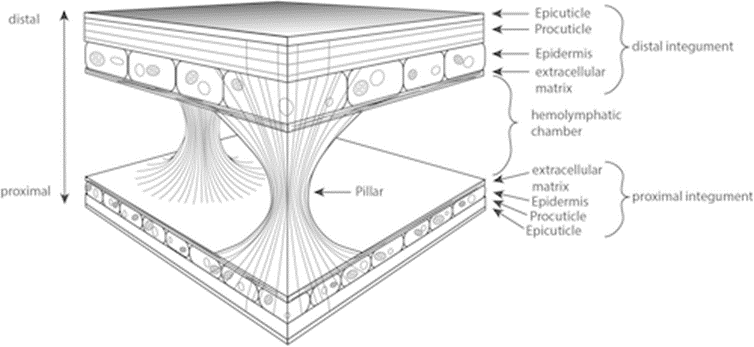
Fig. 5: Schematic drawing of the Daphnia carapace structure, showing the two integumental layers in reverse complement manner and interconnected by pillars (Kruppert et al., 2016).
Stoke’s Law
One unique characteristic of Daphnia’s hop-and-sink is that their swimming is able to counter the impact of Stoke’s law. This law is applied to situations where a spherical object with a low Reynolds number experiences a drag force in a Newtonian fluid. The force of viscosity on a small sphere moving through a viscous fluid is the following, composed of units below (SI units):
![]()
Equation 1: Frictional force according to Stoke’s law
- Fd: frictional force (Stokes’ drag) that is acting on the interface between the fluid and the particle (N)
- μ: dynamic viscosity (Pa*s)
- R: radius of the spherical object (m)
- v: flow velocity relative to the object (m/s).
Daphnia can avoid following Stokes’ law, allowing them to swim more efficiently and rapidly as they experience less resistance and thus, require less energy. While Stoke’s law predicts the sinking rate of particles in a fluid, free-swimming Daphnia have been observed to sink slower than predicted (Gorski & Dodson, 1996). This is due to their “hop and sink” behaviour where they make small jumps and push lighter water down, creating a water body below (Gries et al., 1999). This movement allows them to maintain their position in the water column without expending as much energy as continuous swimming does. The density gradient of the surrounding water environment influences the size of the water body created through this movement (Gries et al., 1999). Additionally, their swimming velocity in quiescent flow increases with their body length; however, turbulence affects their speed which is largely characterized by the dissipation of turbulent kinetic energy (Serra et al., 2019). That is, under calm, undisturbed flow conditions, the swimming velocity of Daphnia has a positive correlation with its body length. However, in the presence of turbulent conditions, their swimming dynamics undergo alterations, dictated by the specific level of turbulence marked by the dissipation of turbulent kinetic energy. In fact, such turbulence exerts a profound influence on their survival, swimming behavior, and filtering efficacy. When subjected to turbulent environments, Daphnia’s repertoire of swimming patterns, encompassing cruising, hopping, sinking, and looping, is notably affected by the intensity of turbulence. For dissipation rates below a critical threshold, the swimming velocity is contingent upon both the water flea’s length and the prevailing turbulence level. This adaptive response to varied turbulence levels enhances Daphnia’s swimming efficiency and speed across diverse environmental settings. Thus, the ability for Daphnia to adeptly adjust to varying turbulence levels significantly augments their efficiency and speed in swimming. Specifically, while their swimming velocity amplifies with body length in tranquil flow conditions, the introduction of turbulence prompts nuanced alterations in swimming speeds and behaviors, intricately tied to the degree of turbulence (Serra et al., 2019).
External Factors
External factors, particularly environmental stressors, significantly impact Daphnia’s swimming behaviour. Research by Egan et al. revealed that exposure to pesticides like neonicotinoids and anthranilic diamides can disrupt Daphnia’s navigation efficiency in water. These findings highlight that pesticides like CHL and IMI, along with contaminated surface waters, lead to abnormal swimming behaviour in Daphnia, impacting their overall fitness, as demonstrated by (Egan et al., 2023). Additionally, Langer et al. showed that Daphnia’s defense strategies help them evade predators from different species. For instance, they decrease their swimming speed as well as their vertical position or increase their nearest neighbor distance. It is important to note that Daphnia are known for their ability to show plastic response to environmental challenges. For example, by adapting their behaviour, morphology, and life-history in the presence of predators, some morphological adaptations are in the form of neckteeth, spines, or even thorns. Concerning changes in life history, this study goes through parameters such as the fact that Daphnia trade somatic growth with reproduction in the presence of fish. Further, in the presence of predators, Daphnia adapt their swimming in various ways, which include inducible migration patterns, including diel vertical and horizontal migrations. As such, Daphnia remain in deeper nutrient-poor regions during the day to avoid fish, while they move towards euphotic zones during the night.
Optimization of The Eyes
Daphnia have apposition compound eyes that become fused during the embryonic stage (Brandon, 2015). A compound eye is a composite structure that includes numerous optical elements, each with its own photoreceptors, and where the individual units, known as ommatidia, function collaboratively to form a cohesive image (Land, 1999). Daphnia‘s eyes consist of 22 ommatidia and exhibit a relatively spherical shape, resulting in diminished visual acuity due to the substantial inter-ommatidial angle of 38 to 54 degrees, and difficulties for Daphnia to see other organisms at certain distances. Nevertheless, they exhibit enlarged photoreceptor cells, enhancing their ability to capture photons effectively and detect potential predators (Hathaway & Dudycha, 2018).

Fig. 6: The Compound Eye (black) of Daphnia (Company, 2010).
Optics of the Compound Eye
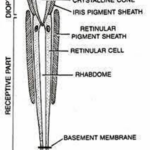
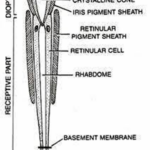
Within the compound eye, incident light traverses the cornea and is received by the rhabdom, subsequently transmitted to retinular cell axons. These axons then convey the acquired information to the retina for image formation. There are two types of compound eye: superposition and apposition, and as previously stated, Daphnia have apposition compound eyes. Superposition compound eyes have ommatidia that each interact with each other, creating images that are superimposed. Conversely, within an apposition eye, each optical element functions as an isolated radiometer, resulting in the creation of images formed through the received photons from the region the eye is facing. More specifically, apposition-type ommatidia allow light to converge once passing through the cornea, where it then forms an inverted image on the rhabdom’s tip. The rhabdom, a cylindrical bundle of photoreceptor cells, has a refractive index that is radially gradient. That is, the rhabdom will converge light to allow for the image to be sent to the retinular cell axons. Comparatively, apposition compound eyes are typically found within species active in well-illuminated environments as they permit a lower photon flux due to each ommatidium’s independence. Superposition compound eyes, on the other hand, are typically found in species that are nocturnal or crepuscular (Cronin, 1986).
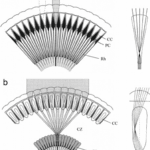
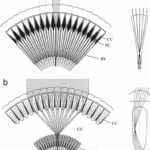
As shown in Figure 7, in apposition compound eye, the ray passes through the cornea and goes down the rhabdom because of its gradient refractive index, then converging to the bottom of the ommatidium after which the electric signal is transformed into an electrical one through phototransduction. The main difference between apposition and superposition compound eye is clear when looking at Figure 8. At the top of that figure, the light is only received by one singular ommatidium and every ommatidium is independent from the others. This means that a certain ommatidium could receive a light signal and another one could receive a completely different one. At the bottom of Figure 8, there is a visual description of light interaction in a superposition compound eye. The light is received by different ommatidia, and the rays are converged by all of them. The waves are superposed and converge to one cellular termination.
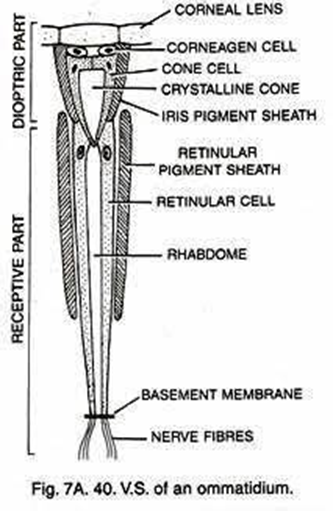
Fig. 7: Scheme of an apposition-type ommatidium with its parts identified. Daphnia possesses 22 of those in their compound eye (Uday, 2017).

Fig. 8: Scheme of image formation in the two different compound eye types (apposition type in ‘a’ and superposition type in ‘b’) (Arikawa et al., 2014).
Snell’s Law
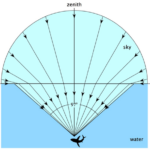
Light is absorbed by Daphnia’s typical habitats, such as ponds and lakes, and in the presence of pollutants or particles, such water bodies can also absorb or scatter light, resulting in a lower light intensity in the water than outside. Since Daphnia have a very low visual acuity, their eyes aid them to reorient themselves according to the contrast created by Snell’s window. Snell’s window is the circle through which there is transmission of light when looking at an environment with a lower refractive index from an environment with a higher refractive index as it can be seen in Figure 9 (Meester, 2009). In this circle, it is possible to see the sky and the normal angle between the incident light and the top of the water is smaller than the critical angle for total internal refraction (Brandon, 2015). As a result, Daphnia are able to change their location in the water depending on the time of the day, a phenomenon known as diel vertical migration. Diel vertical migration happens when Daphnia change their depth in the water depending on the time of the day (Meester, 2009). The contrast, which is what Daphnia detect, changes during the day because of light intensity.
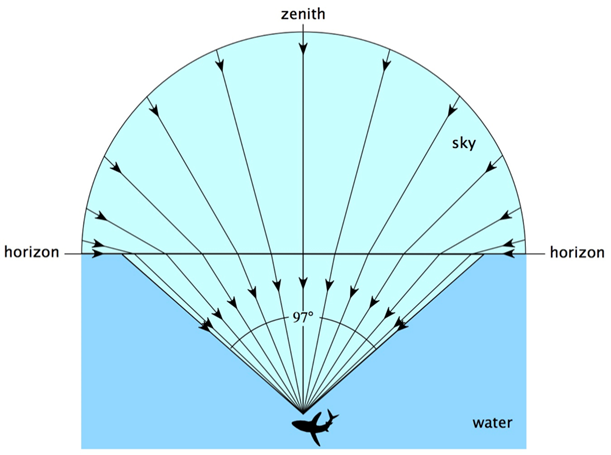
Fig. 9: Representation of Snell’s window. Outside of Snell’s window, all the light coming from under the surface is reflected (Sarika, 2020).
![]()
Equation 2: Snell’s law
- n: refractive index
- Θ: angle of the light
- λ: wavelength of the light
- v: speed of the light in the medium
With Snell’s law, it is possible to find Snell’s window. In fact, when going from water to air, light has a larger refracted angle than incident angle. Therefore, a certain incident angle will give a 90° refracted angle. At that point, referred to as the critical angle, there will be no light transmission from the incident medium to the next. All incident angles that are bigger than the critical angle will cause total internal reflection meaning that all the light will be reflected.
In doing so, they are able to retrieve information that dictates whether they should go up or down in the water to escape visual predators and avoid damaging ultraviolet light. Additionally, the compound eye can support Daphnia in moving away from the shore as a protection mechanism known as “shore flight”. That is, they are able to detect the degrees of polarization caused by the shore and notably, locate it without being able to see it, allowing them to flee and hide in plants (Brandon, 2015). Furthermore, some researchers have realized that Daphnia can detect and follow light sources (Hathaway & Dudycha, 2018). Overall, it is still uncertain what Daphnia need to see and why this would explain them having a compound eye. However, one thing that is now clear is that it is not used for seeing because of how poor their visual acuity is. What is however possible to see is that Daphnia respond to light stimuli.
Compound Eye Behaviour
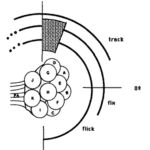
There are three types of behavioural characteristics of the compound eye: flick, an ephemeral rotation elicited by a brief flash of light; fixation, a maintained eye orientation in response to a stationary light source over an extended period; and tracking, the smooth pursuit of a moving stimulus. In fact, a study found that certain eye regions experience specialization for different behavioural responses (Consi et al., 1990). Data has shown that a small light stimulus elicits the three responses which overlap with one another within the medial region but to different extents. For instance, a flick is inducible if placed anywhere between 120 and 90 degrees on the eye with the exception of the null region that has neither response (Consi et al., 1990). While the initiation of the eye flick and fixation within the dorsal-most boundaries are inconclusive, tracking occurs in response to moving stimulus found within the region from 120 to 20 degrees on the eye (Consi et al., 1990). Indeed, the interpretation of the stimulus position in terms of ommatidia is supported by the analysis of the size and shape characteristics of the receptive fields within each ommatidium, as well as their relative placement in the eye (Consi et al., 1990). A receptive field is the region of the retina where light alters the firing of the neuron and Consi et al. found a yield of receptive fields to be around 40 degrees in diameter with central zones of 100% sensitivity about ten degrees in diameter and steep sides. That is, the receptive fields of neighboring ommatidia overlap just slightly, allowing for small or no blind gaps between them. This leads to the conclusion that single bilateral pairs of medial ommatidia are commonly stimulated throughout the cases of the experiment (Consi et al., 1990).
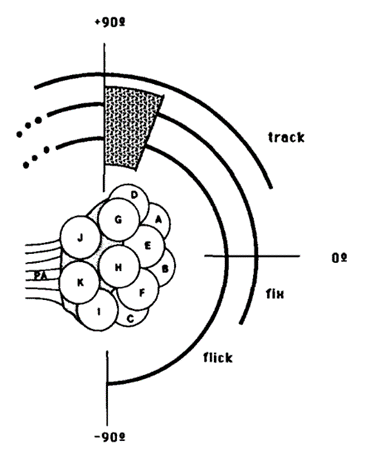
Fig. 10: Behavioral regions on the Daphnia compound eye. Each arc represents the region of the eye in which a stimulus can elicit the indicated response. Null region is shaded (Consi et al., 1990).
Further, behavioural assignments may be approximated regarding the medial ommatidia. In reference to Figure 10, the eye flick is elicited by a flash of light that individually stimulates any of the ommatidia D, A, B, or C, and stable fixation occurs in the receptive fields of ommatidia, D, A, or B (Consi et al., 1990). Upon long durations of stimuli, partial fixation along the large rotations and oscillations are observed in the ventral half of the receptive field of ommatidium B and dorsal half of the receptive field of C lie in the area (Consi et al., 1990). The null region coincides with the dorsal half of the receptive field of ommatidium D, and the tracking zone includes the receptive fields of ommatidia D and A (Consi et al., 1990). All three behaviors may be elicited by stimuli dorsal to the receptive field of ommatidium D. Finally, the receptive field of ommatidium G may extend medially to provide input signal (Consi et al., 1990).
Additionally, Daphnia photoreceptors have a sigmoid dependence of the peak depolarization with stimulus intensity ranging from threshold to saturation at around three to four log units (Consi et al., 1990). At a higher intensity, eye movements do not reflect the response characteristics of the photoreceptors and the peak of the photoreceptor potential saturates and remains constant during the brief light flashes (Consi et al., 1990). The response of the eye flick diminishes progressively as light intensity surpasses a defined ‘peak response’ threshold. Evidently, if the Daphnia nervous system is modulating the output to the eye muscles to decrease, then the decline in behavioural response must take place at light intensities lower than saturation levels (Consi et al., 1990). In cases of prolonged stimuli, the amplitude of the receptor potential diminishes as the photoreceptors adapt after the initial surge (Consi et al., 1990). This adaptation could potentially account for the reduced fixation responses observed at elevated intensities. Another explanation for the reduction in eye excursion at high light intensities is the scattering of the light entering other ommatidia and therefore, producing an antagonistic response (Consi et al., 1990). This phenomenon is observed when two stimuli that induce opposite eye movements are presented simultaneously.
Overall, the oculomotor system of Daphnia appears to be an integral component of its body orientation control system, where any contrast detected by the movable eye allows the body to reposition in alignment with the eye in a favorable manner. This concept suggests that Daphnia‘s oculomotor system responds to stationary and moving stimuli within the tracking zone through eye flicks, fixations, or tracking. Beyond the tracking zone, flicks and fixations likely serve to initiate an eye movement towards the stimulus. A combination of eye and body movements may be necessary to bring the stimulus into the tracking zone, where the eye movement alone can effectively follow it. In fact, researchers proposed that the prominent contrast boundary provided by Snell’s window serves as a major orientational landmark for Daphnia (Ringelberg, 2007). As a result, two potential visual goals for the oculomotor system emerge. Firstly, the eye and body system may strive to align the null region with a strong contrast boundary. Alternatively, the two contrast boundaries on either side of Snell’s window may offer a dual stimulus, resulting in stationary eye position when their effects on the oculomotor system balance.
Eye Plasticity
Plasticity is an evolutionary advantage. In nature, only the fittest individuals survive to changes in the environment through natural selection, and as a result, a species must adapt to its environment through various mechanical adaptations. Daphnia exhibit eyes that have plasticity, allowing them to change to a certain extent in response to external changes. More specifically, the sizes of their eyes can adjust accordingly to environmental conditions. Increasing the size of their eyes allow them to capture more photons and become more responsive to light. According to a study measuring the effects of light intensity on Daphnia, the eye size of two species (D. pulicaria and D. pulex) increased when they were in environments with a high light intensity; whereas other species (D. parvula and D. obtusa) experienced no notable augmentation in eye size when exposed to the same intensity of light. In fact, an important correlation was found between eye size and the availability of resources. That is, Daphnia can afford to enlarge their eyes at the expense of energy when they have access to abundant resources. However, for those that struggle to survive with fewer resources, their priority is finding food instead of enhancing their sight. While having a larger eye allows them to be more sensitive to light, Daphnia inevitably must focus on preserving their energy for swimming, seeking food, and escaping predators (Brandon & Dudycha, 2014). Another study found that Daphnia living in environments with many predators have a greater tendency to have enlarged eyes than others to allow for improved detection. Additionally, the presence of predators causes a vertical migration within Daphnia populations, forcing them to swim deeper into lakes and ponds with weaker light and thus, fewer photons to capture for imaging. That is, if a Daphnia were to be exposed to an increased count of predators for most of their life, their eye size will likely be transmitted down to their offspring. However, changes would occur again if the predator count decreased for an extended period of time. Overall, Daphnia have an optimized visual system that continuously improves throughout their lifetime, with large changes occurring typically during their maximal growth periods. Such plasticity-based changes are also transgenerational (Beston et al., 2019).
Inducible Morphological Defenses that Thwart Predators
One peculiar property observed amongst all species of Daphnia is phenotypic plasticity, which refers to the ability of Daphnia to express different physical, physiological, or behavioural patterns depending on their environmental conditions. This can entail the temperature, the photoperiod, diurnal and nocturnal routines, population density or scarcity of food. As such, many Daphnia possess various inducible defense mechanisms ranging from physical to behavioural changes, which include crown of thorns, elongation of their tail and spine, and fortification of their exoskeleton.
Crown of Thorns
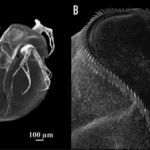
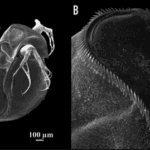
According to a study on the morphological features of subgenus Daphnia atkinsoni that were previously thought to be exclusive to the genes of the population of the subgenus, tests showed that such morphological features were inducible. The results were obtained by comparing molecular data and barcodes collected from different kinds of Daphnia. In particular, a feature named “crown of thorns” was found not to be at all DNA-based but instead to depend on the environmental conditions of the organism. It consists of “a dorsal extension of the carapace into the head shield that forms a heart-shaped lobe, [in some species, covering] a large portion of the head and […] armed by long spines” (Petrusek et al., 2009).
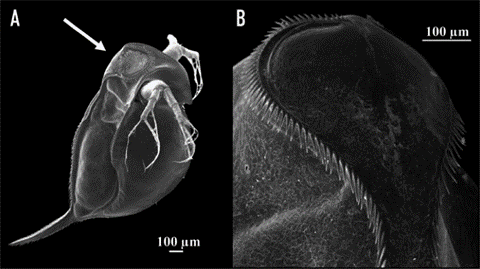
Fig. 11: The induced crown of thorns on Daphnia atkinsoni. A shows the Daphnia with its induced changes. The crown is made up of the spines circling around on the top of its head as shown closer up in B (Laforsch et al., 2009).
Due to Daphnia’s ability to produce a crown of thorns in an environment inhabited by predators, an insightful examination was conducted to observe the resulting induced mechanism and its impact on species survival. Notably, the crown of thorns’ induction was primarily triggered by kairomones, specific chemical cues emitted by predators—specifically, the kairomones of tadpole shrimp. The presence of these chemical cues led to a substantial allocation of resources by the organism towards fortifying its carapace, resulting in increased thickness and width of the supporting pillars within both layers of the carapace (Laforsch et al., 2004). While the physical alterations may not manifest overtly, they bear significant implications for the survival rates of the species. Numerically evaluating these alterations within a population originating from Hungary revealed a noteworthy escalation in relative tail length, rising from approximately 25% to 45% when comparing the population exposed to kairomones with the unexposed population. Furthermore, other physical attributes, such as relative lobe and shield widths, displayed notable increases of 10% and 5%, respectively (Petrusek et al., 2009). To evaluate the survivability of Daphnia from the same origin, a controlled experiment was conducted involving ten organisms with the induced trait exposed to a single predator for a duration of 30 minutes. This experiment was repeated ten times, and the resultant average survival rate demonstrated nearly completely preserving the introduced Daphnia population. Although a few casualties were observed, the magnitude of change was considerable compared to the control group, which lacked the induced feature when introduced to the predator. The average survival rate of the control group remained substantially low at approximately three individuals remaining, a significant disparity compared to the induced group.
Elongation of the tail, the spine, and the fortified exoskeleton
Depending on the genome of the Daphnia, pillar reinforcement is done in different areas, meaning that the induced changes on the carapace can vary. Through the same process by which the D. atkinsoni induces its crown of thorns, other species of Daphnia demonstrate different forms when in the presence of predators. For example, the Daphnia magna, when exposed to predators, will reinforce the top of its head and elongate its tail. Studies have been made to illustrate the elongation areas of the subgenus graphically (Horstmann et al., 2021).
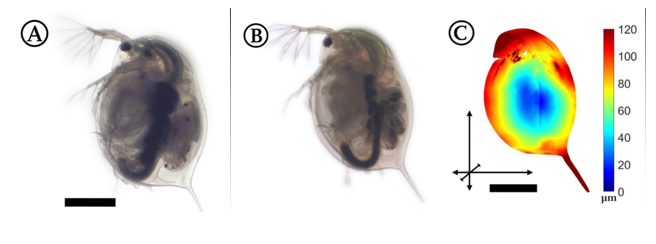
Fig. 12: Part A shows D. magna in its regular state, Part B shows D. magna with its induced morphological change and Part C, shows the zones on D. magna where reinforcement is present. The scale bar represents the length of the deformation in the different areas (Horstmann et al., 2021).
In this case, specifically, the Daphnia induces these changes when it detects the kairomones of the Triops tadpole shrimp. Another example would be the Daphnia barbata, which shows another way the animal can transform. When in the presence of its predator’s kairomones, the organism makes its head much pointier and elongates its tail (Horstmann et al., 2021).

Fig. 13: Part A shows D. barbata in its regular state, Part B shows D. barbata with its induced morphological change and Part C, shows the zones on D. barbata where reinforcement is present. The scale bar represents the length of the deformation in the different areas (Horstmann et al., 2021).
As with the previous species, Figure 13 (A) shows D. barbata in its regular state, Figure 13 (B) shows the inducible morphological changes, and Figure 13 (C) shows the zones where the changes are most important. It is important to note that the changes in this image are induced by the kairomones of the Triops tadpole shrimp, as D. barbata does not present identical changes with all predators.

Fig. 14: Part A shows Daphnia longicephala in its regular state, Part B shows D. longicephala with its induced morphological change and Part C, shows the zones on D. longicephala where reinforcement is present. The scale bar represents the length of the deformation in the different areas (Horstmann et al., 2021).
Another interesting example would be D. longicephala, which shows notable changes compared to the previous two examples. D. longicephala, when in contact with Notonecta shrimp kairomones, forms a large lobe on its head and elongates its tail. While there are some similarities in the changes induced within the different subgenus, fundamental differences exist with the different forms induced. The main purpose of the changes is to increase the survivability against predation, which raises the question: How does the difference in these changes help increase Daphnia’s survival?
These morphological changes are very effective in increasing the predation survival rate within Daphnia because of their specificity against certain predators. In fact, “These inducible morphological defenses are discussed to operate as anti-lock-and-key mechanisms, where the morphological trait interferes with the predator’s mouthparts or handling organs and hampers prey consumption” (Kruppert et al., 2017). As such, the different subgenus of Daphnia have adapted to defend themselves against the predators in their environment, which allows the demonstration of many morphological changes, even within a single subgenus of Daphnia. For example, D. barbata has different inducible forms depending on the kairomones it is subjected to (Horstmann et al., 2021). In fact, when presented to the tadpole shrimp Triops or the Notonecta, the changes observed are slightly different. When comparing the two, it is possible to observe a difference in the lower abdominal area’s reinforcement (see Figure 13 for the Triops-induced changes). This morphological change is absent when the organism is in the presence of the Notonecta. This observation further reinforces the idea that the possibilities of inducible morphological changes are genetically inherited and present themselves when the organism is in certain conditions. It also supports the theory that the induced changes work in an anti-lock and key mechanism since they are not the same within one subgenus and vary depending on the predators’ kairomones detected.
Effects of Induced Changes
Upon examination of the crown of thorns, it becomes evident that induced changes significantly enhance the survival rate of Daphnia. However, the magnitude of this impact on survivability raises the question of how such drastic changes are achieved. By analyzing Young’s modulus distinction between a standard and an induced carapace, the implications of these alterations become more apparent. Young’s modulus, a fundamental material property, provides insights into its resistance to pressure and deformability. It denotes the ratio of tensile stress to strain, and it is mathematically represented as:
![]()
Equation 3: Young’s modulus
- E: Young’s modulus (Pa)
- σ: stress (Pa)
- ε: strain
This constant parameter proves valuable in observing variations in strain as stress is applied to an object (Physics A level revision resource: Introduction to Young’s Modulus). Consequently, when comparing two objects subjected to identical stress, their respective Young’s modulus values offer insights into the strain level each material is enduring. Specifically, a lower modulus indicates greater strain for the material under the same stress, elucidating the underlying mechanics of these induced adaptations.
An experiment involving a distinct subgenus of Daphnia determined Young’s modulus for the carapace exhibiting induced carapace changes and those without such alterations (Laforsch et al., 2004). The study focused on two variants of Daphnia: D. pulex and D. longicephala. The modulus measurements revealed approximately 1.66 MPa and 2.93 MPa values for undefended and defended D. pulex, respectively. Similarly, for undefended and defended D. longicephala, the modulus readings were 5.12 MPa and 15.59 MPa, respectively. Significant disparities exist between the modulus values for the undefended and defended states. Further, induced forms of Daphnia are more resistant to strain than their non-induced counterparts when subjected to identical tensile stress levels.
Conclusion
Daphnia are characterized by their distinctive anatomical features, including their swimming mechanism, compound eye, and carapace structure; they exhibit remarkable resilience and flourishing capabilities within their ecological niche. Beyond their physical attributes, Daphnia showcase evolutionary responses to counteract several relevant issues. As explained, Daphnia’s carapace is lightweight for optimized swimming but can also withstand high mechanical impacts to increase the survival rate of the genus and make the structure more resistant overall. Therefore, Daphnia developed two integumental layers connected by pillars between which flows hemolymph. Thanks to this fluid, the carapace can withstand high tensile forces while keeping its structure less heavy than if it had been made of a continuous block of chitin. To allow Daphnia to maintain a fixed position in a given water column without spending too much energy, the “hop and sink” behaviour was developed, enabling Daphnia to create a water body below them through the repetitive motion and then sink slower than what fluid mechanics laws would expect, overcoming effects of the Stoke’s Law. Furthermore, Daphnia inhabit a low-lit environment, and like most moving organisms, Daphnia must be able to orient themselves. Resultantly, Daphnia do so by developing large photoreceptor cells in their ommatidia, allowing them to capture a large number of photons of which they can distinguish the contrast at the border of Snell’s window. Daphnia are able to use that marker as a reference to orient their body in the water. On top of that, Daphnia also have very low visual acuity because of their sizeable ommatidial angle, making their oriental perception of their environment more challenging, and as a result, Daphnia developed the ability to detect the degree of polarization caused by the shore.
Nevertheless, many species have a common challenge: staying fit within a changing environment. The attributes used to define a species’ environment encompass the other species present, the available resources, and its physical characteristics. Thanks to natural selection, species have adapted to the average conditions of their respective environments. However, this environment undergoes continuous changes. Daphnia found a solution to that: plasticity. As developed in the paper, due to such environmental variations, Daphnia’s phenotype may undergo various changes, whether that be eye plasticity, with larger eyes with species living deeper to escape predators to catch more photons, or smaller eyes in populations that live in water bodies with low resources to save energy. Their bodies are able to change depending on predator presence to increase survival rate with the development of the crown of thorns, the elongation of the tail, the spine, or the fortification of the exoskeleton. Daphnia found the balance between energy saving and optimized fitness, adapted to a changing environment. Overall, through their body, swimming mechanism, eye, and phenotypic plasticity, Daphnia triumphed in finding solutions to nature’s challenges. Although this paper primarily focused on Daphnia‘s mechanical structures and physical interactions within their environment, a fraction of its chemical interactions, specifically the impact of neonicotinoids and anthranilic diamides on its navigational behavior, were also discussed. Future exploration into Daphnia‘s chemical interactions with its surroundings presents an enticing avenue for comprehensive investigation.
References
Algar, A. (2018). Daphnia (water flea) with eggs. Nikon Small World. Retrieved October 8 from https://www.nikonsmallworld.com/galleries/2018-photomicrography-competition/daphnia-water-flea-with-eggs
Arikawa, K., Wakakuwa, M., & Kinoshita, M. (2014). Spectral Sensitivity of Insect Photoreceptors. Japanese journal of applied entomology and zoology, 58, 5-11. https://doi.org/10.1303/jjaez.2014.5
Beston, S. M., Dudycha, J. L., Post, D. M., & Walsh, M. R. (2019). The evolution of eye size in response to increased fish predation in Daphnia. Evolution, 73(4), 792-802. https://doi.org/https://doi.org/10.1111/evo.13717
Brandon, C. S. (2015). The Evolutionary Biology of Vision in Daphnia.
Brandon, C. S., & Dudycha, J. L. (2014). Ecological constraints on sensory systems: compound eye size in Daphnia is reduced by resource limitation. Journal of Comparative Physiology A, 200(8), 749-758. https://doi.org/10.1007/s00359-014-0918-y
Company, C. B. S. (2010). Daphnia pulex. In. flickr.
Consi, T. R., Passani, M. B., & Macagno, E. R. (1990). Eye movements in Daphnia magna. Journal of Comparative Physiology A, 166(3), 411-420. https://doi.org/10.1007/BF00204815
Cronin, T. W. (1986). Optical Design and Evolutionary Adaptation in Crustacean Compound Eyes. Journal of Crustacean Biology, 6(1), 1-23. https://doi.org/10.2307/1547926
Daphnia. (2023, August 21). Wikipedia. Retrieved October 2 from https://en.wikipedia.org/wiki/Daphnia
Daphnia magna. (2023, February 23). Wikipedia. Retrieved October 2 from https://en.wikipedia.org/wiki/Daphnia_magna
Egan, N., Stinson, S. A., Deng, X., Lawler, S. P., & Connon, R. E. (2023). Swimming Behavior of Daphnia magna Is Altered by Pesticides of Concern, as Components of Agricultural Surface Water and in Acute Exposures. Biology, 12(3), 425. https://www.mdpi.com/2079-7737/12/3/425
Fryer, G. (1991). Functional morphology and the adaptive radiation of the Daphniidae (Branchiopoda: Anomopoda). Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 331(1259), 1-99. https://doi.org/doi:10.1098/rstb.1991.0001
Gorski, P. R., & Dodson, S. I. (1996). Free-swimming Daphnia pulex can avoid following Stokes’ law. Limnology and Oceanography, 41(8), 1815-1821. https://doi.org/https://doi.org/10.4319/lo.1996.41.8.1815
Gries, T., Jöhnk, K., Fields, D., & Rudi Strickler, J. (1999). Size and structure of ‘footprints’ produced by Daphnia: impact of animal size and density gradients. Journal of Plankton Research, 21(3), 509-523. https://doi.org/10.1093/plankt/21.3.509
Hathaway, C. R., & Dudycha, J. L. (2018). Quantitative measurement of the optomotor response in free-swimming Daphnia. Journal of Plankton Research, 40(3), 222-229. https://doi.org/10.1093/plankt/fby014
Hebert, P. D. N. (1978). The adaptive significance of cyclomorphosis in Daphnia: more possibilities. Freshwater Biology, 8(4), 313-320. https://doi.org/https://doi.org/10.1111/j.1365-2427.1978.tb01452.x
Horstmann, M., Tollrian, R., & Weiss, L. C. (2021). Thwarting predators? A three-dimensional perspective of morphological alterations in the freshwater crustacean Daphnia. PLoS One, 16(7), e0254263. https://doi.org/10.1371/journal.pone.0254263
Horstmann, M., Weiss, L. C., & Tollrian, R. (2022). Specific Turbulence- and Chaoborus-Induced Morphotypes Affect the Streamlining Properties of Daphnia cucullata [Original Research]. Frontiers in Ecology and Evolution, 9. https://doi.org/10.3389/fevo.2021.825765
Kruppert, S., Horstmann, M., Weiss, L. C., Schaber, C. F., Gorb, S. N., & Tollrian, R. (2016). Push or Pull? The light-weight architecture of the Daphnia pulex carapace is adapted to withstand tension, not compression. Journal of Morphology, 277(10), 1320-1328.
Kruppert, S., Horstmann, M., Weiss, L. C., Witzel, U., Schaber, C. F., Gorb, S. N., & Tollrian, R. (2017). Biomechanical properties of predator-induced body armour in the freshwater crustacean Daphnia. Scientific Reports, 7(1), 9750. https://doi.org/10.1038/s41598-017-09649-5
Laforsch, C., Ngwa, W., Grill, W., & Tollrian, R. (2004). An acoustic microscopy technique reveals hidden morphological defenses in <i>Daphnia</i>. Proceedings of the National Academy of Sciences, 101(45), 15911-15914. https://doi.org/doi:10.1073/pnas.0404860101
Land, M. (1999, June 1, 2020). Compound eyes. Britannica. Retrieved September 20 from https://www.britannica.com/science/photoreception/Compound-eyes
Langer, S. M., Weiss, L. C., Ekvall, M. T., Bianco, G., Hansson, L.-A., & Tollrian, R. (2019). A three-dimensional perspective of Daphnia’s swimming behavior with and without predator cues. Limnology and Oceanography, 64(4), 1515-1525. https://doi.org/https://doi.org/10.1002/lno.11132
Meester, L. D. (2009). Diel Vertical Migration. In G. E. Likens (Ed.), Encyclopedia of Inland Waters (pp. 651-658). Academic Press. https://doi.org/https://doi.org/10.1016/B978-012370626-3.00151-4
Petrusek, A., Tollrian, R., Schwenk, K., Haas, A., Laforsch, C., & Berenbaum, M. R. (2009). A “Crown of Thorns” Is an Inducible Defense That Protects Daphnia against an Ancient Predator. Proceedings of the National Academy of Sciences of the United States of America, 106(7), 2248-2252. http://www.jstor.org/stable/40272657
Physics A level revision resource: Introduction to Young’s Modulus. University of Birmingham.
Pirow, R., Wollinger, F., & Paul, R. J. (1999). The sites of respiratory gas exchange in the planktonic crustacean daphnia magna: an in vivo study employing blood haemoglobin as an internal oxygen probe. Journal of Experimental Biology, 202(22), 3089-3099. https://doi.org/10.1242/jeb.202.22.3089
Ringelberg, J. (2007). The photobehaviour of Daphnia spp. as a model to explain diel vertical migration in zooplankton. Biological Reviews, 74, 397-423. https://doi.org/10.1111/j.1469-185X.1999.tb00036.x
Sarika, A. (2020). What is Snell’s window and how does it depend on the observer’s depth? 9TO5Science. Retrieved September 20 from https://9to5science.com/what-is-snell-39-s-window-and-how-does-it-depend-on-the-observer-39-s-depth
Serra, T., Müller, M. F., & Colomer, J. (2019). Functional responses of Daphnia magna to zero-mean flow turbulence. Sci Rep, 9(1), 3844. https://doi.org/10.1038/s41598-019-40777-2
Skipper, A. N., Murphy, D. W., & Webster, D. R. (2019). Characterization of hop-and-sink daphniid locomotion. Journal of Plankton Research, 41(2), 142-153. https://doi.org/10.1093/plankt/fbz003
Stollewerk, A. (2010). The water flea Daphnia – a ‘new’ model system for ecology and evolution? Journal of Biology, 9(2), 21. https://doi.org/10.1186/jbiol212
Uday, L. (2017). Why are arthropod eyes called “compound”? Socratic Q&A. Retrieved September 20 from https://socratic.org/questions/5440fe58581e2a5bfaf7fe58
Watling, L., & Thiel, M. (2013). Functional morphology and diversity. Oxford University Press. https://academic.oup.com/book/4028