ABSTRACT
Jaws are an amazing system as they can contribute to many aspects of life such as eating, defence, locomotion, etc. Each species has specific needs and thus each set of jaws tends to be adapted to fit these demands. In the animal kingdom, some jaws show unique characteristics, which not only terrify us, but also provide the species with amazing abilities for hunting and feeding performance. Snakes’ jaws evolved to match their nutritional needs, conferring them hunting abilities. Evolutionary pressures have forced their jaws to evolve to match their nutritional needs. In fact, their jaw’s gape can stretch to an incredible extent to allow for the ingestion of large prey. This is enabled by the chemical properties present in their jaw, such as the proteins elastin and collagen, as well as the structure of the joints of their jaw, such as the quadrate bone. Spider jaws are tiny yet extremely powerful tools. Also called chelicerae, these are mainly made of chitin. Chitin in some spiders’ chelicerae is reinforced with metal ions to give it the ability to bite through the chitin cuticle of their prey. Spider fangs, which form a part of the jaw, can secrete effective venom that varies from species to species. This essay will analyze the unusual jaws of aquatic invertebrates. For example, chiton, leeches and sea urchins each have very fascinating teeth which have evolved for the mechanical purpose of better obtaining food.
INTRODUCTION
Throughout all levels of biology, from a nanoscale, to a molecular level, and even to organs, the concept of structure fitting function is ubiquitous. Structure and function have an intimate connection that directly affects one-another; for instance, if the structure of an object changes, so will its function, and vice versa (Barrickman 2022). This concept can allow for a deeper understanding into the correlation between animal jaw’s chemistry and their functions.
The jaw is one of the most vital organs in animals as it is essential to their survival. It contributes to their eating, defense, locomotion etc. Therefore, by studying the differences in the molecular structure of several animal species’ jaws, one can better grasp their function.
I. THE SNAKE JAWS AND THE CHEMICAL COMPOSITION OF THE JOINT BETWEEN THEM
A. HOW SNAKES SWALLOW LARGE PREY
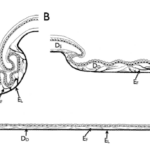
Predation of animals often depends on many varying external and internal factors, such as the accessibility of prey, the danger of capturing prey, hunger levels, reproductive status, and sex. These factors cause snakes to often catch one large prey and live off of that nutrition for several days, weeks or even months (Munsell, 2022). Often, snakes’ prey approximates 20-60% of their own body weight (Siers et al., 2018). Therefore, the size of their prey and their dependency on hunting large animals has consequently evolved their jaws to be able to open unusually wide, as can be observed in Figure 1.

Figure 1 : Demonstrating the immense jaw gape size that the snake has due to its structure enabling it to be flexible (Mclendon, 2022).
B. THE QUADRATE BONE AND THE SNAKE JAW GAPE SIZE
The jaws of snakes contain movable elements allowing them to open wide. Their upper and lower jaws are connected by a joint via the quadrate bone, shown in Figure 2(A). This incredibly fascinating joint allows the upper and lower jaws to move independently from one another. Functioning together with the upper and lower jaw as part of a closed kinematic chain, the quadrate bone is made of cartilage, which allows for an even broader range of movement in this joint (Freckelton, 2022).
In addition to the quadrate bone, the upper jaw in snakes is distinctly mobile. In most mammals, the upper jaw is fused to the skull; however, in snakes, the upper jaw is seen to be connected to muscles and ligaments, allowing for maximal movement independently from the rest of the skull. This is one element that enables the jaw to expand to up to 150 degrees, as seen in Figure 2(B) (Perry, 2004).
The lower jaw also contains elements that allow for increased gape size. This is mainly done by the separation of the left and right sides of their lower jaw, attached by elastic filaments. This allows the two sides of the jaw to move independently, as well as creating the ability for snakes to open their jaws even further laterally (Palci et al., 2016).

Figure 2 : (A): The skeleton of the snake skull demonstrating the jaw. The quadrate bone can be observed as well as the separate entities of the upper and lower jaw, enabling further flexibility (Freckelton, 2022). (B): An image of the snake jaw where the gape size is shown to be as wide as 150 degrees (King, 2002).
As observed, the quadrate bone plays a vital role in the flexibility and the wide gape size that the snake jaw has. In addition, the upper and lower jaws are split into two separate entities and this allows them to open horizontally to a greater width.
C. ELASTIN AND COLLAGEN IN THE SNAKE JAW
Another important element when looking at the flexibility of snake jaws and how their chemical components allow for a large range of opening is the connective tissue and skin around the jaws. The dermis is the main component of the skin that contains all the structural proteins, blood vessels, nerves and more. The dermis and the joint capsule of the snake jaw is composed of fibrous connective tissue, the key components of which are elastin and collagen, which allows the joint to stretch (Close et al., 2014).
In the dermis, elastin plays a major role in the range of motion of snakes’ jaw. Elastin can be compared to a spring in the sense that it can have both a stretched and relaxed state. During the stretched state, the length of the elastin increases. Elastin is an inherently disordered protein that participates in the hierarchical assembly of connective tissues, as can be seen in Figure 3 below. Elastin molecules are formed into fibers through cross-linking, which is the linking of two or more molecules together (Eyre, 2005). The enzyme involved in cross-linking elastin molecules into fibers is lysyl oxidase. Due to its disordered nature at the molecular scale, elastin must be able to also recoil back after being stretched, which is done through its cross-links.
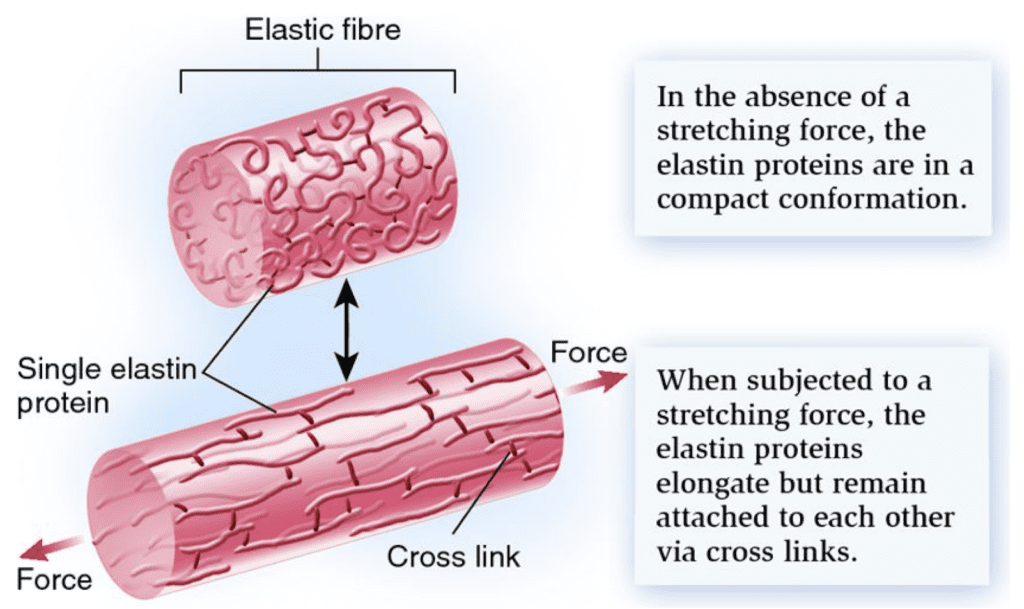
Figure 3 : Elastin in a compressed then stretched state showing its elastic abilities. Cross-links can also be observed demonstrating the highly organized protein (Biology Forums Gallery 2011).
Due to the ability of elastin to stretch and recoil back to its original state, it is appropriate that there is high elastin content in the dermis of the snake jaw, as shown in Figure 4. This allows snakes to widen their mouths to ingest large prey without irreversible consequences for the snake jaw (Close et al., 2014). In addition, the interfolded region of the epidermis allows for increased extensibility. The epidermis does this by unfolding itself to a linear structure, as can be seen in Figure 4.

Figure 4 : A: The skin in its resting state demonstrates the elastin (EF, EL) and the dermis (Ds) near each other and curved. B: The skin is in a slightly more stretched state. C: The skin is completely stretched out representing the elastin and the dermis far from one another (Close el al., 2014).
While elastin allows for opening the jaws, by contrast, collagen securely limits the jaws’ abnormal opening. Thus, it has different functions compared to elastin (Close et al., 2014). It is vital to the structural rigidity of an organ and does not have the ability to stretch as elastin does, as shown in Figure 5 (Osborn, 2020). While they have very different purposes in the cell (elasticity versus rigidity), collagen and elastin also exhibit many similarities. To name one, collagen also contains cross-links, and is cross-linked by the same enzyme as elastin: lysyl oxidase (Mayumi et al., 2021).

Figure 5 : (A) Collagen imaged in more detail demonstrating its rigid structure in comparison to (B) where elastin can be seen as more flexible with more cross-linkage sites (Osborn, 2020).
In a study comparing elastin to collagen, Mecham (2008) found that elastin contains 15-20 cross-linking sites, and collagen contains only 1-2 sites. As snake jaws require optimal opening range, it is consistent that they would contain more elastin than collagen and thus more cross-linking sites to enable reversible and fatigueless opening and closing.
II. SPIDER JAW
Spiders mainly prey on insects. They use their fangs to pierce through the insect cuticle, which is made of the same material as the spider fang: chitin polysaccharide. Chitin is assembled in a hierarchical fashion, and in the spider’s fangs it is reinforced in various ways to maximize its efficacy (Politi et al., 2012).
A. THE SPIDER FEEDING APPARATUS
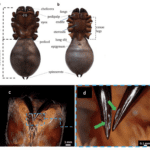
All spider species have one pair of chelicerae on the front side of the body (Figure 6) to seize, hold, or bite. Spiders use their chelicerae to move leaves and soil, to protect the cocoon containing eggs, or to crush dead prey (Machałowski et al., 2020).
The main biting apparatuses (jaws) of spiders are the chelicerae and cheliceral fangs in the cephalothorax. Arthropod chelicerae can be divided into three types: jacks-knife, scissor and three-segmented chelae [source?]. Most spiders also inject venom into prey via their fangs, which can be strong enough to pierce a human fingernail (Moon & Yu, 2007). Their venom glands are located in the fangs and in the anterior part of the cephalothorax (see Figure 6). In (a) and (b) we can see a general view (dorsal, ventral) of the cephalothorax and abdomen of a spider. We can see in (c) a photo of chelicerae of Theraphosidae spider and in (d) the fangs with openings to release venom (indicated by green arrows).
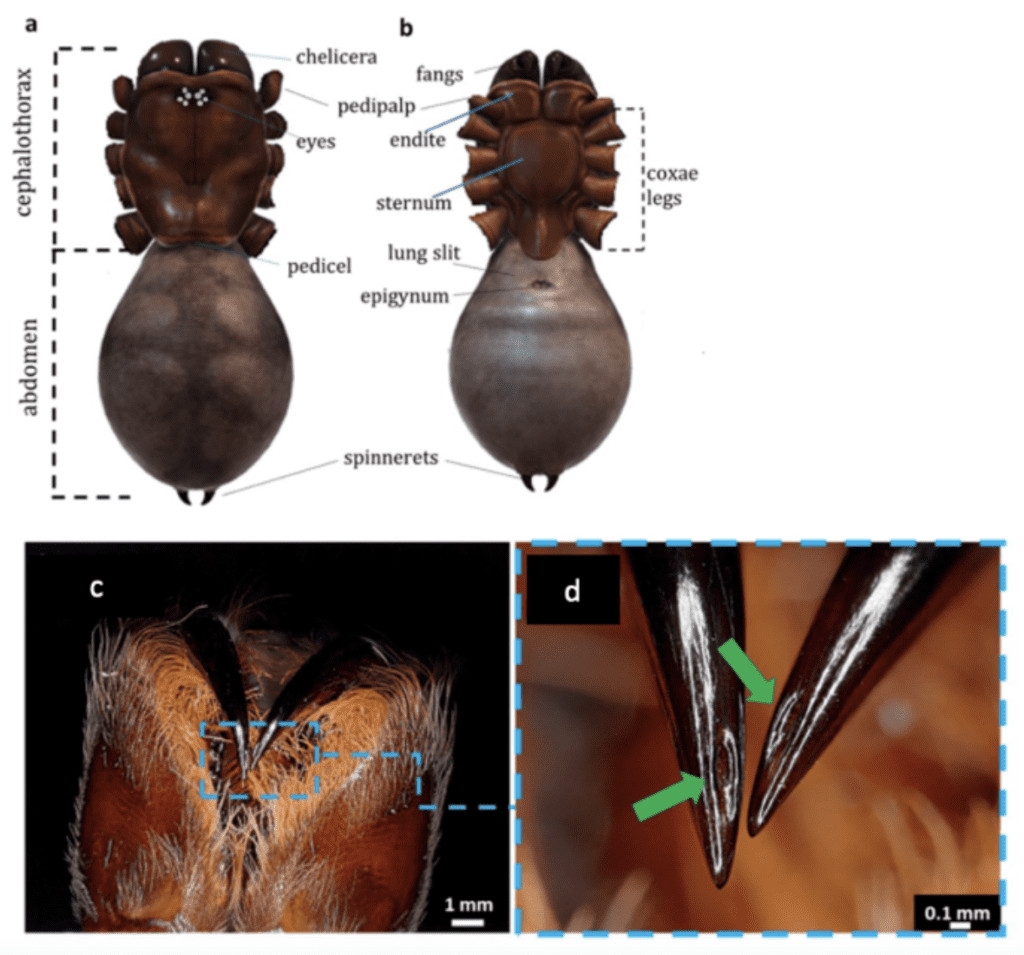
Figure 6 : Morphology of a spider cephalothorax, abdomen, leg and fangs (Machałowski et al., 2020).
The chelicera consists of two parts: a basal portion and a movable fang. Figure 7 illustrates an example of a chelicera in Trichonephila clavata and highlights how the tips of the hinged fangs are very sharp.

Figure 7 : The chelicerae in Trichonephila clavata located in the cephalothorax. BS stands for basal segment, FG stands for movable cheliceral fang, PT for promarginal teeth and RT for retromarginal teeth (Moon & Yu, 2007).
The position of the chelicerae divides the order of spiders into two suborders. The Orthognatha and the Labidognatha. Tarantulas are part of the Orthognatha and have a horizontally directed chelicera that works in a parallel sense whereas Labidognatha have vertical chelicerae that work in an opposition sense. These Labidognatha have a larger span and can therefore take on and defeat larger prey. The biting apparatus of most spiders can also penetrate large objects. In the case of T. Clavata spiders, their fangs are sharp, and they have a hinged fang in the chelicera that folds. They can penetrate flat surfaces such as a palm of a human hand because of the chelicerae hinged to the cephalothorax and fangs, by opening both joints fully (Machałowski et al., 2020).
To crunch and hold prey tightly, spiders have rows of teeth that are a very important part of their feeding apparatus. Most spiders develop cheliceral teeth on the inside of their chelicerae. They are considered a part of the spider’s jaws with the fangs hinged at their end. Each spider species has its own pattern of cheliceral teeth although some crab spiders do not even have cheliceral teeth. Thus, the number of teeth allows for the classification of spiders. Robusticoelotes spiders have more than five promarginal and retromarginal cheliceral teeth whereas Coelotinae spiders have only three promarginal and two retromarginal cheliceral teeth. The cheliceral fang of spiders, linked to the chelicera as already seen, consists of an organic hypodermic needle – or a “spider’s venom-injecting tooth”, present in almost all spiders (Moon & Yu, 2007). Spider fangs are composed of bio-composites, chitin, proteins and metal ion cross-linking, in the form of a light-weight structure (Machałowski et al., 2020). They are typically a few millimeters long, of conical shape, hollow and curved. (Bar-On et al., 2014). An interesting fact about chelicerae is that when rubbed together, they emit sounds used in defense mechanisms to scare away enemies.
B. CHITIN: A BIOLOGICAL POLYMER
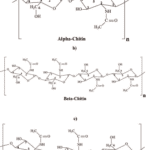
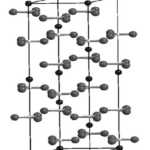
Chitin is a biological polysaccharide that contains nitrogen. It is chemically stable, with the following formula: (C8H13NO5)n\. Chitin is the second most abundant natural polysaccharide after cellulose. It is similar to cellulose but contains an acetamide group instead of a hydroxyl group. This polysaccharide exists as a rigid crystalline nanofiber or in its pure form as a linear homopolymer of N-acetylglucosamine linked by β- 1, 4 glycosidic bonds with deacetylated glucosamine residues. Chitin is a polymer but not a protein since it is not made of amino acids but of amino sugars. It is insoluble in most organic solvents like water. Natural chitin has three different crystalline allomorphic forms depending on the chitin strands’ arrangement: α-chitin, β-chitin, and γ-chitin (Figure 8). The α-form found in arthropod skeletons is the most thermodynamically stable due to its additional hydrogen bonds, which allow an anti-parallel fiber arrangement. β -chitin has chains arranged in a parallel direction, and γ-chitin has two chains that share the same polarity and one arranged in the opposite direction (Rouhani Shirvan et al., 2019)

Figure 8 : (a-c) Molecular structures of α-chitin (a), β-chitin (b), and γ-chitin C (Rufato et al., 2018).
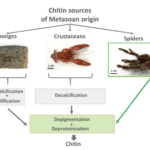
Chitin is usually extracted from fungal biomass or from seafood processing wastes (from shrimp, crab, and lobster exoskeletons), mostly from taxonomic groups (protists, sponges, insects). Naturally occurring chitin can also be collected from the moulting cuticles of spiders or other arthropods (see Figure 9 and Figure 10).

Figure 9 : Cuticle moults from diverse Theraphosidae species (Schofield et al., 2021).
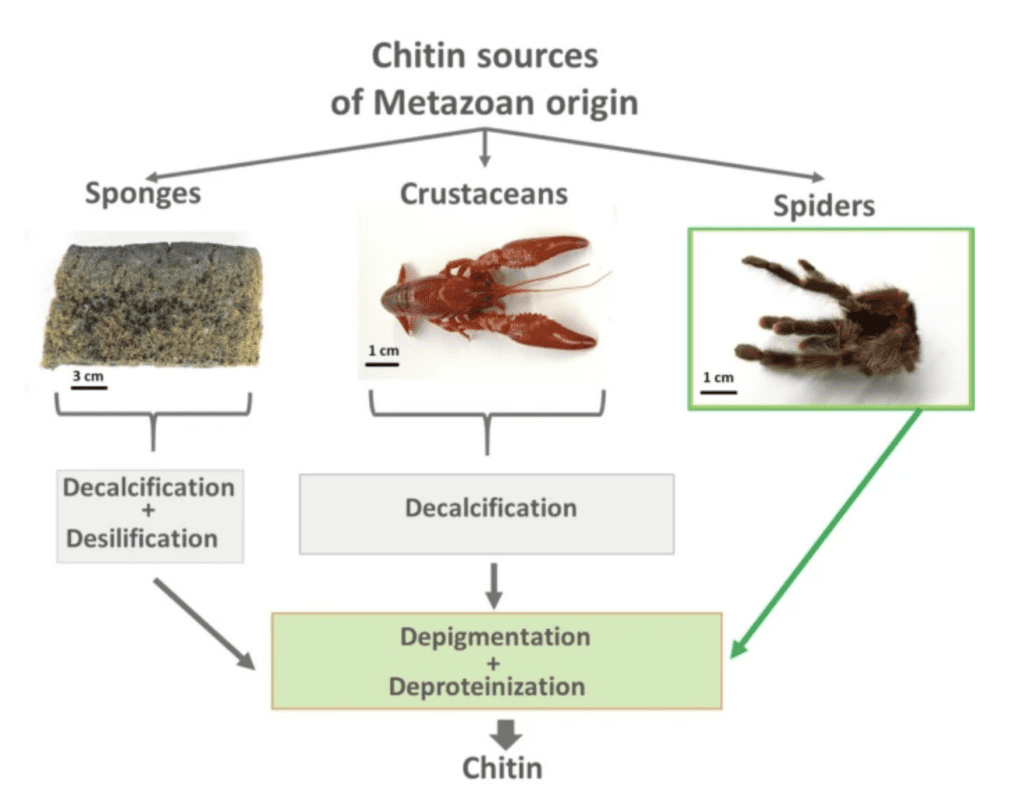
Figure 10 : Chitin isolation procedures from different species (Machałowski et al., 2020).
The body of an Araneae spider is covered by an exoskeletal cuticle. That cuticle is composed of an elongated microfiber coating with a chitin-protein complex. This complex of chitin with cross-linked protein makes up 90% of the organic exoskeleton. There are three different chitin-protein layers: the exoendocuticle, the intermediate mesocuticle, and a thin layer called the epicuticle. The exocuticle is the hardest layer and is not present in all exoskeleton regions but is present in the cephalothorax and legs. This chitin layer is used to protect the delicate animal tissues, since they don’t have internal skeletons (Machałowski et al., 2020).
Some Araneae have thick hair type cuticles, others have porous cuticle layers or waxy surfaces (Machałowski et al., 2020). An interesting thing about spider cuticle is that it is responsible for the different colors found in spiders due to its different ways of reflecting light, and different pigments (Machałowski et al., 2020). For example, for the Theridiidae spider Phoroncidia rubroargentea, the silvery apparent cuticle on their abdomen is due to regularly arranged guanine micro platelets as seen on Figure 11. Guanine crystals contain platelets with multiple layers that reflect and transmit light from layer to layer (Kariko et al., 2018).

Figure 11 : Structural characteristics of the guanine microplates in the silver regions (Kariko et al., 2018). (a) Optical image of the silver region, where individual guanocytes are visible. (b) A three-dimensional rendering of the microtomed block in the silver region based on confocal data. The yellow arrows indicate the grain boundary junctions among adjacent guanocytes. The white arrows indicate the immature circular-shaped guanocytes. (c) A scanning electron microscopy image of an individual guanocyte, showing multiple layers of guanine crystals. (d) A SEM image of guanine crystals. (e–h) Bright-field images of guanine crystals. The yellow and white arrows in (h) indicate the rough and smooth interfaces between the outer crystals and inner region. (i) High-magnification image showing the doublet structures (yellow arrows) (Kariko et al., 2018).
C. CHITIN REINFORCEMENT
Spiders mainly feed on insects using their fangs and chelicerae. In addition to cleaning and stretching the egg sac to release spiderlings, the fangs are mostly used to puncture insect cuticles and inject them with venom. When performing this action, these tiny “tools” experience compression, bending and twisting or deformation, since they are made of chitin fibres embedded in a protein-rich matrix. The reinforcement of this composite material by cross-links using metal ions, allows this stiff tip to penetrate insects’ cuticles and makes the tip resistant to damage over time. Even if it goes through a moulting process each year, it still must be intact enough for multiple prey captures throughout the year (Bar-On et al., 2014).
In Schofield’s study (Schofield et al., 2021), spider fangs have been analysed for their hardness, modulus of elasticity, loss tangent, abrasion resistance, energy of fracture and impact resistance. There are two groups of inorganic-enriched biological materials in fangs. The first is the biomineralized tissue that incorporates an organic matrix and calcium, iron and silicon-based mineral phase. The second group is one with enriched materials “Metal-Halogen” or “Heavy Element Biomaterials” called HEBs. The dominant inorganic elements in this material are zinc, manganese, bromine and copper and they take up around 20% of the fangs’ total weight. This HEB material group phase does not have two distinct phases, which differentiates it from the first biomineralized tissues phase. Figure 12 illustrates these different HEB in different animal “tools” (Schofield et al., 2021).
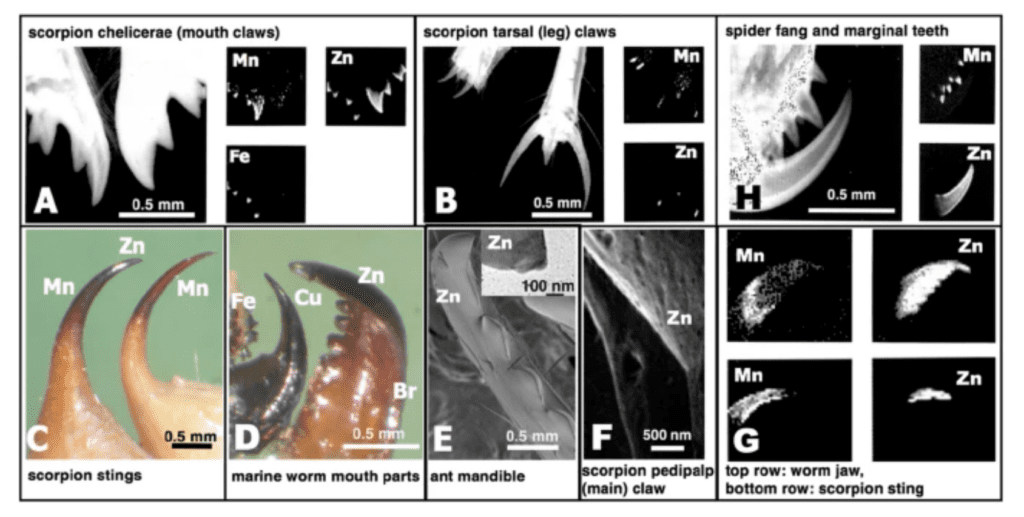
Figure 12: Images of HEB-containing “tools” with dominant inorganic elements indicated. On the top right: spider fang and marginal teeth (Schofield et al., 2021).
These HEB tissues located in “contact region tools” such as fangs, claws or teeth alter their mechanical properties. Spider fang is composed of 3% Mn and 16% Zn. We can see in the first study research diagram (Figure 13) the effects of the presence of these HEBs on different properties of the fang.

Figure 13: Results from mechanical testing. Zn-, Mn- and non-HEB (Schofield et al., 2021).
Figure 13 illustrates that for every property except abrasion resistance, Zn is stronger or equal to the non-HEB mineral-enriched material. This is also true for manganese except for the damping, and the energy fracture and impact resistance for which there is no manganese present. Figure 14 illustrates the contribution of HEB to various mechanical properties. It also compares these materials to engineering materials and biomineralized tissues.
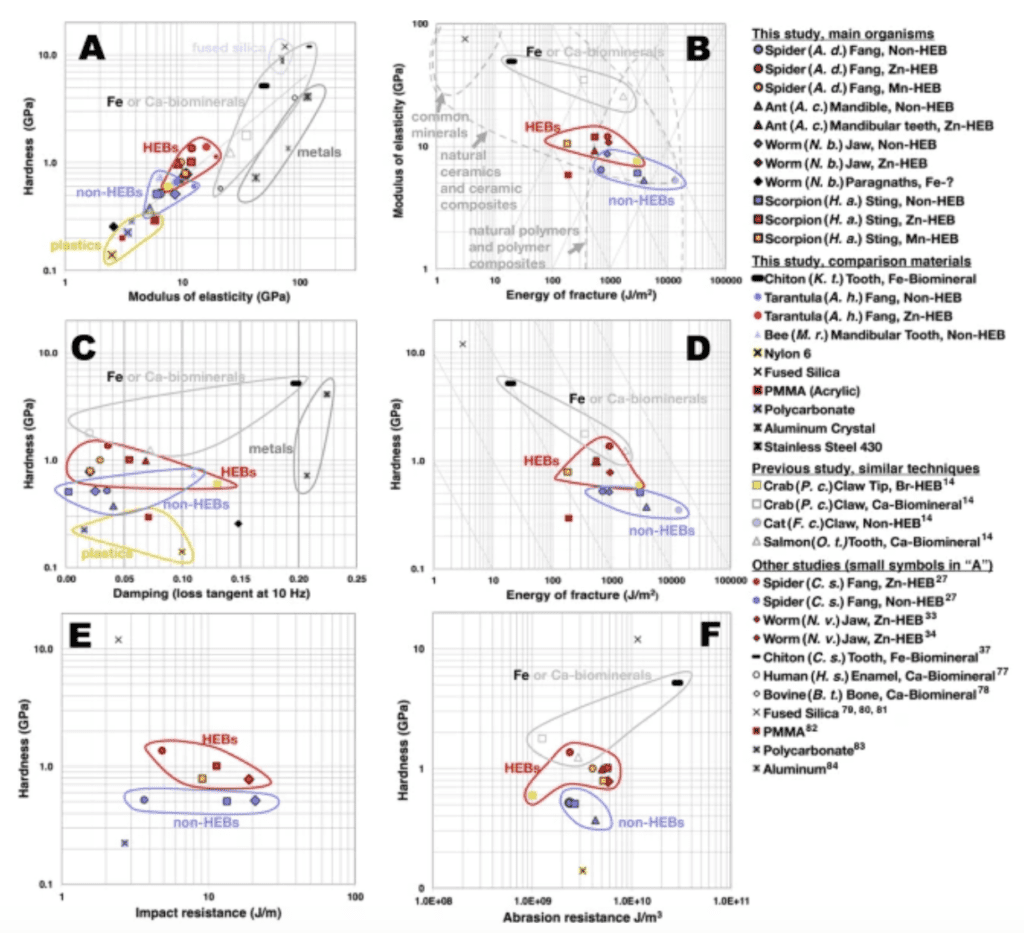
Figure 14: Graphs of pairs of material properties, showing the different mechanical characteristics for HEBs, non-HEBs, biomineralized tissues and engineering materials (Schofield et al., 2021).
We can find a link between hardness and modulus of elasticity. A high modulus of elasticity means high hardness because when a material has less tendency to deform under pressure, it suffers smaller lasting indentation (and is therefore harder). For the spider fang, the materials with HEBs have a higher modulus of elasticity and are also harder (Figure 14 A). Zn- and Mn-HEBs are 2 times harder than plastics, slightly harder than aluminum, stainless steel and fused silica but only 1/5 as hard as the Fe-biomineral in the chiton teeth (magnetite, see below). Thus, scratches in HEB-teeth would be the same as those made in mammal teeth made of crystalline calcium phosphate with a high percentage of mineral salts. Figure 14 B shows that the higher the modulus of elasticity, the lower the energy required to fracture the fang. Figure 14 C shows that the higher modulus of elasticity is associated with less energy dissipation (damping). Zn- and Mn-HEBs have a lower damping than the Fe-biomineral in chiton teeth. We can notice that the HEBs need more energy to fracture than the chiton Fe-biomineral but about the same range as the Ca-biominerals. Finally, the impact resistance property does not give a lot of information, but it is seen that the HEBs are more abrasion-resistant than all other materials. Zn-HEBs are harder and stiffer than Mn-HEBs but they both have the same resistance. This might explain why the Mn is located in areas between the zinc materials, since it reduces stress concentration and fracturing possibility.
In brief, the insects’ “tools,” such as the spider fang, are very precise and sharp, which is attributable to the basic polymer structure of chitin. This basic structure is further reinforced by many heavy metals that fill in the spaces between proteins (Schofield et al., 2021). Zn- and Mn- are bound to the protein matrix in the spider fang tip via histidine residues that are in the tip (chelation and cross-linking). They are incorporated after ecdysis (cuticle moulting) (Politi et al., 2017). This reinforcement of the chitin allows it to be a powerful “tiny tool.” Its viscous and tough properties are enhanced thanks to the heavy metals, which make it harder and more resistant to irreversible deformation during prey capture. It is crucial for the spider’s survival to have powerful fangs that can last until the next cuticle shedding.
D. SPIDER VENOM INJECTION SYSTEM
Numerous animals develop toxic secretions, including spiders. These can be harmful through direct contact, ingestions (poison) or through a wound (as injected by fangs, the toxins are called venoms). Spider venom is stored and produced in venom glands in the cephalothorax. It is then injected into the prey by the chelicerae and cheliceral fangs (Moon & Yu, 2007). These glands evolved over time and got bigger in size over eons. The location of the chelicerae changed with the evolution from orthognath (fangs facing down) to labidognath (fangs facing each other). Most spiders, whether they produce webs or not, use venom for hunting and defense. They produce not only venom but also a protein-based glue that they spit in order to immobilize their victim from a distance of 2 cm (Langenegger et al., 2019). Spider venom is divided into four groups: small molecular mass compounds, antimicrobial peptides, peptide neurotoxins, proteins and enzymes. Small molecular mass compounds include ions, organic acids, nucleotides, amino acids, amines, and polyamines. Antimicrobial peptides include hydrophobic amino acids.
Some spiders are euryphagous and generalist, others are stenophagous and specialist – they are specialized in a specific prey. For example, Zodariidae species are specialized in ants, Ammoxenidae in termites, and Arnaeidae in moths. Specialized spider venom is therefore less diversified. Over time, according to Langenegger et al., natural selection via the arms race between predators and prey has evolved and is one of the most important drivers of venom composition (Langenegger et al., 2019). This arms race has resulted in the evolution and adaptation of the predator for handling prey, therefore changing the selection pressure on the prey (Dawkins & Krebs, 1979). The composition of venom is associated with predator-prey interactions. It can be composed of new components targeting specific sites on the cell membranes of prey tissue. The main difference between prey-specific and non prey-specific venom is its toxic compounds (Pekár et al., 2018). These neurotoxin peptides weaken and alter a range of receptors on membranes of cells like nerves and muscles. The venom neurotoxins first spread into the cellular membrane and then diffuse to reach their target channel (Langenegger et al., 2019). Enzymes were for a long time assumed to have a minor role in spider venoms. They facilitate the diffusion of toxins in the prey organism. Others are used in the venom gland of the spider, and another group of enzymes is used to directly target important organs of the prey (Langenegger et al., 2019). In order for them not to poison themselves, spiders can either reshape the target protein in their body so they do not bind to the target within their own body, get rid of the toxin from the body right after ingesting it or finally, develop a system that can bind the toxin in order for it not to reach the target in the first place (“sequestration”).
Spiders biting chitin with chitin creates an interesting engineering conundrum. Their jaws evolved in a way that made it possible for them to be tiny but still very powerful. Chitin in the spider jaw is reinforced with heavy metals, which makes it stronger and more resistant to deformation occurring during a hunt. To defeat prey, spiders also use venom secreted in the glands and injected through their fangs. These venoms have a variety of compositions depending on the spider’s feeding specialization.
III. “BIZZARE” JAWS
A. CHITON TEETH
It may be hard to believe, but the sea animal with the hardest teeth is the chiton, an incredibly ancient, slow-crawling, and snail-like mollusk. This animal has magnificent teeth made of magnetite, the hardest material ever formed by a living organism. All of this occurs on a tongue-like structure called the radula, which supports more than 100 rows of teeth (Lindberg, 2001), Figure 16.
In fact, in chitons, only two of the teeth in each row are mineralized. The mineral of the working surface layer is the hard iron oxide called magnetite (Fe3O4), whose unit cell schematic is represented in Figure 15A. The teeth are actually magnetic. The remainder of the tooth is composed of softer and more pliable mineral phases carbonated apatite (Ca9.75[(PO4)5.5(CO3)0.5CO3) (Figure 15B). The two layers are separated by a thin third layer also containing an amorphous mineral, ferrihydrite. This third layer possibly functions as a gasket allowing the two major layers with distinctly different mechanical properties to “work” together (Wang et al., 2022).

Figure 15 : Unit cell schematic for magnetite (A) (Schmitz-Antoniak, 2015) and carbon apatite (B) (Corno et al., 2011).
The chiton teeth’s organic matrix contains proteins and carbohydrates. The protein is negatively charged, avidly binding the iron and the carbohydrates which are positively charged, as well as attracting magnesium and sodium. This network of proteins and carbohydrates forms the magnetite shield that covers the teeth – the magnetite. The hardness of the tip is about twice that of the other parts of the tooth. These properties are the key to the functioning of the tip as a self-sharpening grinding tool (Wang et al., 2022).
Chitons wear down their teeth at the rate of approximately a row a day, but also have the ability to produce new teeth rows at the same rate. They produce teeth for grinding the rocky substrate in order to extract the algae. An in-depth analysis of tooth structure and development can be seen in Figure 16 below.
Chitons’ ability to assemble such an incredible substance from easily available components makes the chiton an excellent model system to study biological mechanisms and design principles (Lindberg, 2001). This principle is similar to how spiders use metals to reinforce their chitin as stated above, and therefore bite chitin. Heavy metals integrated in their jaw makes it stronger and more resistant to damage, much like the way minerals are used in chiton teeth.
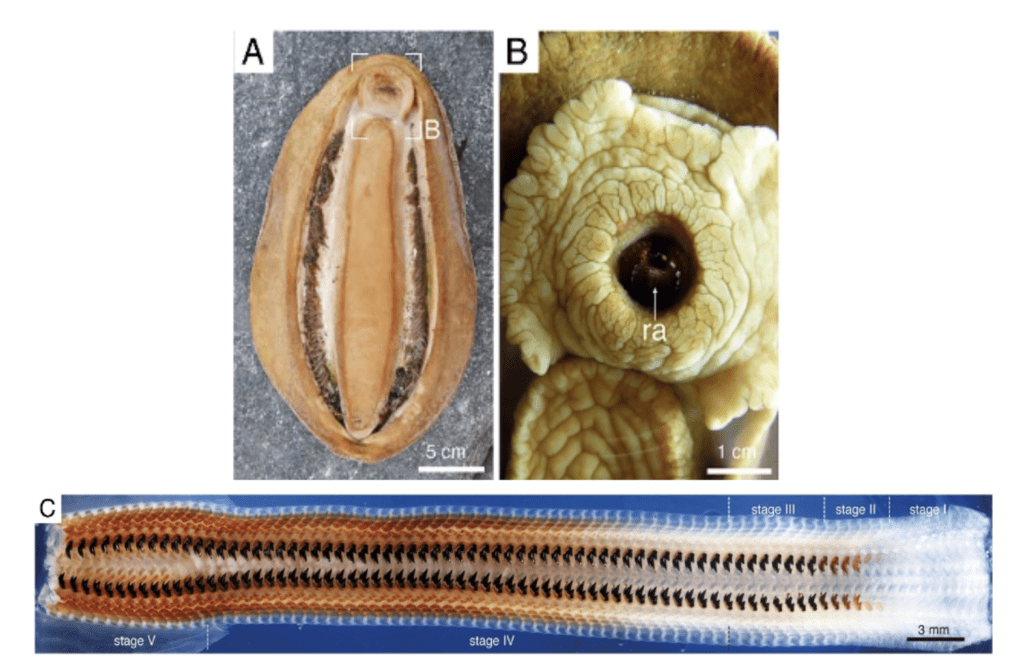
Figure 16: Radula teeth of C. stelleri. (A) Ventral aspect of C. stelleri. (B) Mouth and protruding anterior end of radula (ra) (8). (C) Mosaic image of the entire radula showing all stages of development, including deposition of the organic scaffold (stage I), infiltration of the cusp with ferrihydrite (stage II), conversion to magnetite (stage III), mineralization of the core (stage IV), and mature teeth (stage V) (Stegbauer et al., 2021).
B. LEECH TEETH
The teeth of leeches are also quite phenomenal. These animals have 3 jaws made up of 300 tiny teeth which can easily penetrate any skin. A study done in 2012 by the Technical University in Prague, Faculty of Mechanical Engineering, revealed the unique composition and properties of the leech teeth (Sepitka et al., 2012). Samples were taken from dissected leech jaws and ground using monocrystalline diamond suspension to a surface roughness of 40nm which is appropriate for micromechanical testing.
From the composition analysis, the significant elements of leech teeth were identified. The tooth is composed mainly of calcium (41.9 %), oxygen (41.2 %) and carbon (11.4 %), with other constituents present in small quantities (F 2.1 %, Na 1.0 %, P 0.9 %, S 0.6 % and Mg 0.6 %). In addition, hydroxyapatite (HA or HAP) (Ca10(PO4)6(OH)2), a substance of mineral component in bones, was also present in the leech teeth (Choi, 2010).
Figure 17 shows an oral sucker of a leech attached to a prewarmed glass plate as if it was the skin of a host. The pharynx is contracted and the leech ejects all the air between the sucker and the skin making for a tight latching. The pharyngeal muscles contract and expand in a rhythmic fashion which creates a suction used to draw blood from its host/prey. During this time the leech jaws and saw-like teeth also penetrate deeper and deeper into the host’s skin while emitting saliva which contains an anesthetic. In the middle of the throat, at the imaginary point of intersection of the jaw, the pharynx (throat) opens to let in the blood (Roth, 2016).
Leech saliva contains an enzyme called hirudin. Leeches produce hirudin so that no clotting is formed while they are drawing blood from their host during feeding. In fact, leeches can also be used as a treatment in patients with blood clots. Once the leech is applied to the area, it releases the hirudin from its saliva at the bite site and is said to remove some of the blood that has built up due to the clotting, hence relieving some of the pressure beneath the tissue. Due to the coagulation properties of the hirudin, blood can flow for up to 3 hours after the leech has finished feeding and has been removed (Markwardt, 2002).

Figure 17 : Oral sucker of leech attached to a prewarmed glass plate (Roth, 2016).
C. SEA URCHIN TEETH
Sea urchins also possess a very interesting feeding apparatus. They have evolved very dynamic teeth for the mechanical function of obtaining food more efficiently. The feeding apparatus of the sea urchin is referred to as “Aristotle’s Lantern” (Figure 18).
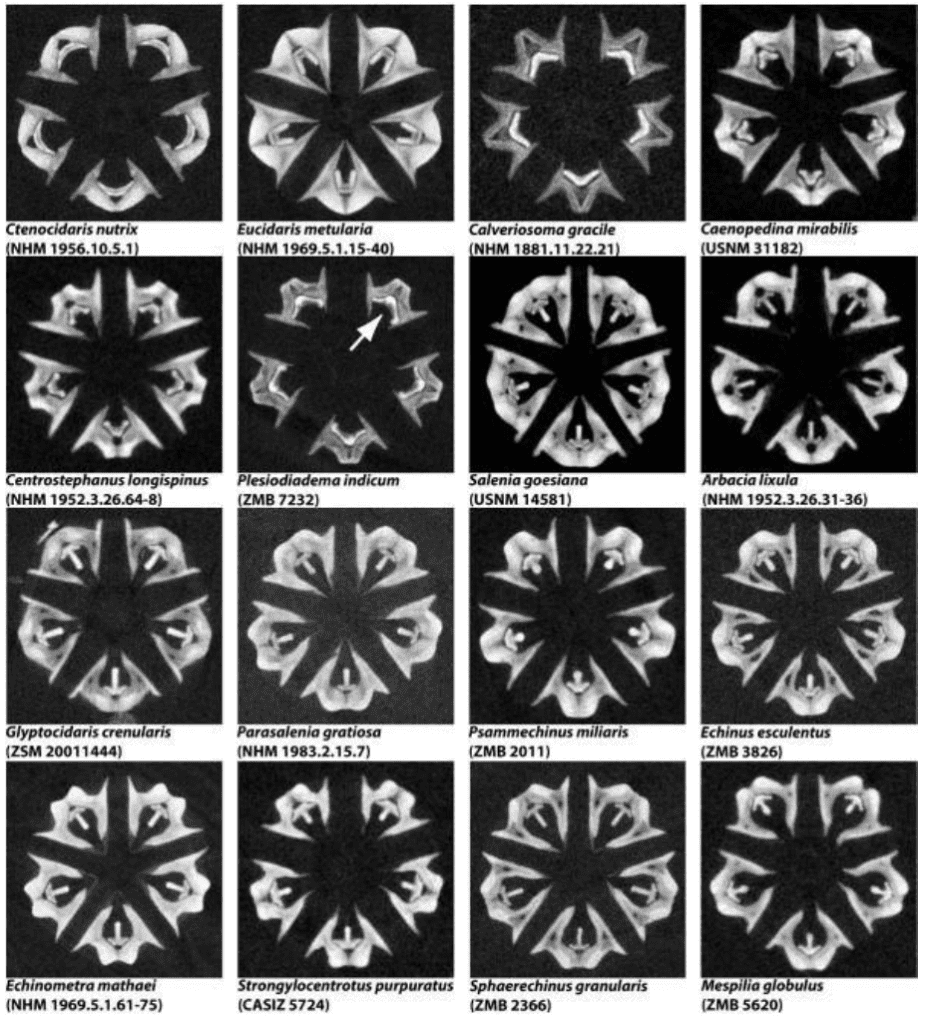
Figure 18 : Virtual horizontal μCT sections through Aristotle’s Lantern, the sea urchin feeding apparatus (Ziegler et al., 2010).
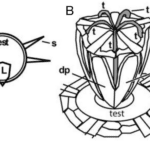
The jaw structure has five-fold symmetry, in the sense that there are five pyramid-like jaw sections, each containing a single tooth (Figure 19). The teeth attach with collagenous tissue (connective tissue) to the pyramid to a structure referred to as the dental slide, to which they are merely supported and extend beyond the dental slide as curved cantilevers (Figure 19 B). The teeth in each of these sections are fully capable of growing continuously as they can undergo all the stages of mineralization (Stock, 2014).

Figure 19: Geometry of Aristotle’s lantern of a regular sea urchin. (A) depicts the core lantern “L” with spines “S”. (B) displays the structure of the Camarodonta lantern along with the test opening through which the lantern protrudes during feeding. A pair of complementary demi-pyramids “dp” and “dp” are labeled on the face of the lantern closest to the viewer, and all five teeth “t” of the lantern are labeled (Stock, 2014).
As we can see, sea urchins have a set of five teeth. They only need these five teeth because they are self-sharpening and capable of replacing material that has been lost on a tooth via abrasion. This principle is similar to how you would sharpen a knife by selectively removing small bits of material from the edge. Sea urchin teeth are mineralized in two stages. The first forms the primary, secondary and carinar process plates and the lamella-needle-prism complexes, parts of the Aristotle Lantern. The second stage cements the first stage materials together. Both stages require magnesian calcite (Ca,Mg)CO3. This regeneration process means that the tooth has a range of developmental states which are different in composition. We find cellular syncytia at the beginning of the tooth shaft and a highly dense structure at the incisal end (tip). The mature portions of each tooth consist of single crystal calcite, whose unit cell schematic is represented in Figure 20 below. The second-stage mineral that cements the disparate plates and prisms together has a much higher Mg content than the first-stage prisms and needles and allows the tooth to be self-sharpening (Stock, 2014).
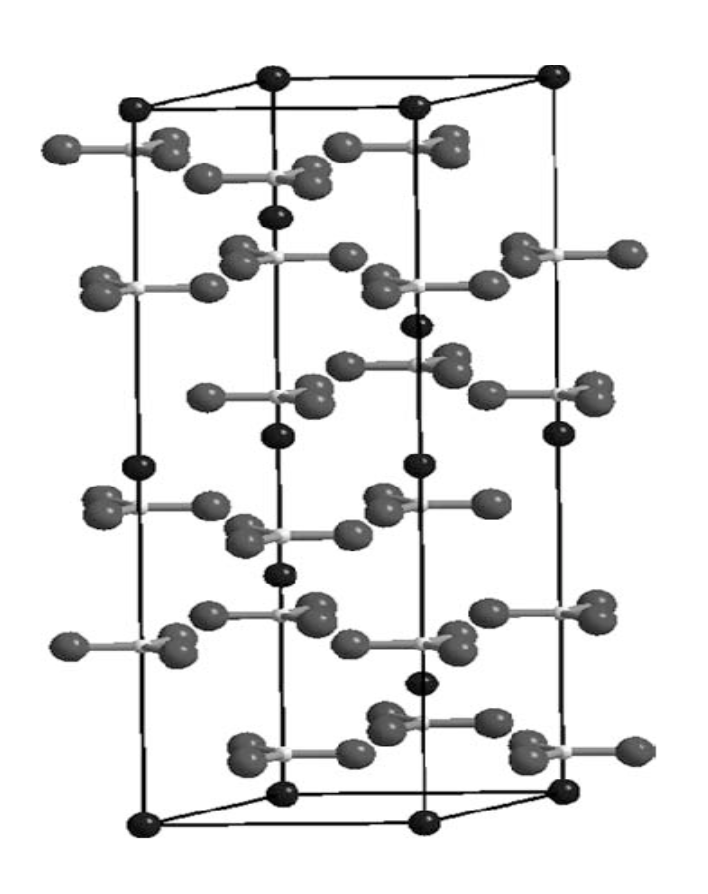
Figure 20: Unit cell schematic of crystal calcite (Dove et al., 2005).
The reinforcement strategy of using Mg for solution hardening and strengthening of the base material of the tooth is not the only method employed by sea urchins to obtain more effective teeth. They also strengthen the base material of the tooth through dispersion strengthening via macromolecules within the crystal lattice, and inclusion toughening via voids. The structure is also supported through crystallographic alignment. Overall, these evolved teeth prove very useful for sea urchins (Stock, 2014).
CONCLUSION:
Animal jaws’ functionalities dictate their survival as they are vital for eating, hunting, defense, locomotion, etc. Due to the importance of their functions, the jaws are formed by complex elements to allow these functions to be properly carried out. As seen in every level of biology, structure and function are intimately related and the structure of the jaw is what dictates its function. Through several different animals, one can study how these structures allow for the proper functionalities of the jaws, in order to gain a deeper understanding on how they may carry out their functions that are intimately vital to survival. Snakes have a complex jaw system which is structurally elastic, allowing it to open widely and ingest larger prey. This is attributable to the chemical composition of the joint capsule and the skin, including abundant elastin and fewer collagen proteins. Spiders, on the other hand, boast structurally hardened jaws as they need to hunt on tough shelled prey. Their jaws, made of chitin, are able to bite through prey chitin with heavy metals, structural reinforcement. Spider fangs also inject venom, which makes their whole biting apparatus even more powerful. That venom made of proteins, toxins and enzymes has different complex characteristics depending on the spider species. In addition, many animals possess extremely fascinating jaws that have evolved to be completely unique based on their specific needs. Leeches, chiton and sea urchins in particular have bizarre jaws that have evolved, and display characteristics unique to their species related to their functions and needs for survival. By studying these animals and the structure of their feeding apparatus, one of the most vital elements of an organism, one can gain a deeper understanding of how its structure is vital for its function.
References
Amisha, Malik, P., Pathania, M., & Rathaur, V. K. (2019). Overview of artificial intelligence in medicine. J Family Med Prim Care, 8(7), 2328-2331. https://doi.org/10.4103/jfmpc.jfmpc_440_19
Bar-On, B., Barth, F. G., Fratzl, P., & Politi, Y. (2014). Multiscale structural gradients enhance the biomechanical functionality of the spider fang. Nature Communications, 5(1), 3894. https://doi.org/10.1038/ncomms4894
Barrickman, N., Kathy Bell, D. V. M., & Chris Cowan, M. S. (n.d.). Chapter 10: Structure Determines Function. Human Biology. Retrieved October 27, 2022, from https://slcc.pressbooks.pub/humanbiology/chapter/chapter-12-organ-systems-of-the-human-body/
Biology Forums Gallery. Biology Forums. (2011). Retrieved November 15, 2022, from https://biology-forums.com/index.php?action=gallery%3Bsa
Choi, C. Q. (2010). T. Rex of Leeches Has Enormous Teeth. LiveScience. https://www.livescience.com/10984-rex-leeches-enormous-teeth.html
Close, M., & Cundall, D. (2014). Snake lower jaw skin: Extension and recovery of a hyperextensible keratinized integument. Journal of Experimental Zoology Part A: Ecological Genetics and Physiology, 321(2), 78-97. https://doi.org/https://doi.org/10.1002/jez.1839
Corno, M., Chiatti, F., Pedone, A., & Ugliengo, P. (2011). In Silico Study of Hydroxyapatite and Bioglass®: How Computational Science Sheds Light on Biomaterials. In (pp. 275-298). https://doi.org/10.5772/24956
Dalla Valle, L., Nardi, A., Belvedere, P., Toni, M., & Alibardi, L. (2007). β-keratins of differentiating epidermis of snake comprise glycine-proline-serine-rich proteins with an avian-like gene organization. Developmental Dynamics,236(7), 1939-1953. https://doi.org/https://doi.org/10.1002/dvdy.21202
Dawkins, R., & Krebs, J. R. (1979). Arms Races between and within Species. Proceedings of the Royal Society of London. Series B, Biological Sciences, 205(1161), 489-511. http://www.jstor.org/stable/77442
Dove, M., Swainson, I., Powell, B., & Tennant, D. (2005). Neutron powder diffraction study of the orientational order–disorder phase transition in calcite, CaCO3. Physics and Chemistry of Minerals, 32, 493-503. https://doi.org/10.1007/s00269-005-0026-1
Eyre, D. R., & Wu, J.-J. (2005). Collagen Cross-Links. Topics in Current Chemistry, 207–229. https://doi.org/10.1007/b103828
Freckelton, P. (2022, September 15). Snake myths: Snakes dislocate their jaws to eat large meals. Snake Myths: Snakes dislocate their jaws to eat large meals. Retrieved November 6, 2022, from https://www.forpetessnakes.ca/2021/06/01/snake-dislocate-their-jaws-to-eat/
Kariko, S., Timonen, J. V. I., Weaver, J. C., Gur, D., Marks, C., Leiserowitz, L., Kolle, M., & Li, L. (2018). Structural origins of coloration in the spider Phoroncidia rubroargentea Berland, 1913 (Araneae: Theridiidae) from Madagascar. J R Soc Interface, 15(139). https://doi.org/10.1098/rsif.2017.0930
King, R. B. (2002). Predicted and Observed Maximum Prey Size – Snake Size Allometry. Functional Ecology, 16(6), 766–772. http://www.jstor.org/stable/826607
Langenegger, N., Nentwig, W., & Kuhn-Nentwig, L. (2019). Spider Venom: Components, Modes of Action, and Novel Strategies in Transcriptomic and Proteomic Analyses. Toxins, 11(10), 611. https://www.mdpi.com/2072-6651/11/10/611
Lindberg, D. R. (2001). Molluscs. In S. A. Levin (Ed.), Encyclopedia of Biodiversity (Second Edition) (pp. 373-383). Academic Press. https://doi.org/https://doi.org/10.1016/B978-0-12-384719-5.00096-4
Machałowski, T., Amemiya, C., & Jesionowski, T. (2020). Chitin of Araneae origin: structural features and biomimetic applications: a review. Applied Physics A, 126(9), 678. https://doi.org/10.1007/s00339-020-03867-x
Markwardt, F. (2002). Hirudin as alternative anticoagulant–a historical review. Semin Thromb Hemost, 28(5), 405-414. https://doi.org/10.1055/s-2002-35292
Mayumi, K., Liu, C., Yasuda, Y., & Ito, K. (2021). Softness, Elasticity, and Toughness of Polymer Networks with Slide-Ring Cross-Links. Gels, 7(3), 91. https://www.mdpi.com/2310-2861/7/3/91
Mclendon, R. (2022, October 3). Pythons can swallow almost anything. A new study shows how. ScienceAlert. Retrieved November 6, 2022, from https://www.sciencealert.com/pythons-can-swallow-almost-anything-a-new-study-shows-how
Mecham, R. P. (2008). Methods in elastic tissue biology: elastin isolation and purification. Methods, 45(1), 32-41. https://doi.org/10.1016/j.ymeth.2008.01.007
Munsell, J. (2022, March 23). How long can a snake go without eating? the facts. How Long Can a Snake Go Without Eating. Retrieved November 6, 2022, from https://reptileslife.com/how-long-can-a-snake-go-without-eating/
Moon, M.-J., & Yu, M.-H. (2007). Fine structure of the chelicera in the spider Nephila clavata. Entomological Research, 37(3), 167-172. https://doi.org/https://doi.org/10.1111/j.1748-5967.2007.00108.x
Osborn, D. (2020, January 6). Difference Between Collagen and Elastin. Difference Between Similar Terms and Objects. http://www.differencebetween.net/science/difference-between-collagen-and-elastin/.
Pekár, S., Líznarová, E., Bočánek, O., & Zdráhal, Z. (2018). Venom of prey-specialized spiders is more toxic to their preferred prey: A result of prey-specific toxins. Journal of Animal Ecology, 87(6), 1639-1652. https://doi.org/https://doi.org/10.1111/1365-2656.12900
Politi, Y., Pippel, E., Licuco-Massouh, A. C. J., Bertinetti, L., Blumtritt, H., Barth, F. G., & Fratzl, P. (2017). Nano-channels in the spider fang for the transport of Zn ions to cross-link His-rich proteins pre-deposited in the cuticle matrix
Palci, A., Lee, M. S. Y., & Hutchinson, M. N. (2016). Patterns of postnatal ontogeny of the skull and lower jaw of snakes as revealed by micro-CT scan data and three-dimensional geometric morphometrics. Journal of Anatomy, 229(6), 723-754. https://doi.org/https://doi.org/10.1111/joa.12509
Perry, L. (2004, August 20). How snakes work. HowStuffWorks. Retrieved November 6, 2022, from https://animals.howstuffworks.com/snakes/snake.htm
Politi, Y., Priewasser, M., Pippel, E., Zaslansky, P., Hartmann, J., Siegel, S., Li, C., Barth, F. G., & Fratzl, P. (2012). A Spider’s Fang: How to Design an Injection Needle Using Chitin-Based Composite Material. Advanced Functional Materials, 22(12), 2519-2528. https://doi.org/https://doi.org/10.1002/adfm.201200063
Roth, M. (2016). The Biology of Leeches. Musculoskeletal Key. https://musculoskeletalkey.com/the-biology-of-leeches/
Rouhani Shirvan, A., Shakeri, M., & Bashari, A. (2019). 5 – Recent advances in application of chitosan and its derivatives in functional finishing of textiles. In I. Shahid ul & B. S. Butola (Eds.), The Impact and Prospects of Green Chemistry for Textile Technology (pp. 107-133). Woodhead Publishing. https://doi.org/https://doi.org/10.1016/B978-0-08-102491-1.00005-8
Rufato, K., Galdino, J., Ody, K., Pereira, A., Corradini, E., Martins, A., Paulino, A., Fajardo, A., Aouada, F., La Porta, F., Rubira, A., & Muniz, E. (2018). Hydrogels Based on Chitosan and Chitosan Derivatives for Biomedical Applications. In (pp. 1-40). https://doi.org/10.5772/intechopen.81811
Schmitz-Antoniak, C. (2015). X-ray absorption spectroscopy on magnetic nanoscale systems for modern applications. Reports on Progress in Physics, 78, 062501. https://doi.org/10.1088/0034-4885/78/6/062501
Schofield, R. M. S., Bailey, J., Coon, J. J., Devaraj, A., Garrett, R. W., Goggans, M. S., Hebner, M. G., Lee, B. S., Lee, D., Lovern, N., Ober-Singleton, S., Saephan, N., Seagal, V. R., Silver, D. M., Som, H. E., Twitchell, J., Wang, X., Zima, J. S., & Nesson, M. H. (2021). The homogenous alternative to biomineralization: Zn- and Mn-rich materials enable sharp organismal “tools” that reduce force requirements. Scientific Reports, 11(1), 17481. https://doi.org/10.1038/s41598-021-91795-y
Sepitka, J., Lukevs, J., Jiroušek, O., Kytyr, D., & Valach, J. (2012, 10/01).
Composition, Structural and Material Properties of Leech Teeth – Example of Bioinspiration in Materials Research. Chemické Listy, 106, 523-524.
Siers, S. R., Yackel Adams, A. A., & Reed, R. N. (2018). Behavioral differences following ingestion of large meals and consequences for management of a harmful invasive snake: A field experiment. Ecol Evol, 8(20), 10075-10093. https://doi.org/10.1002/ece3.4480
Stegbauer, L., Smeets, P. J. M., Free, R., Wallace, S. G., Hersam, M. C., Alp, E. E., & Joester, D. (2021). Persistent polyamorphism in the chiton tooth: From a new biomineral to inks for additive manufacturing. Proceedings of the National Academy of Sciences, 118(23), e2020160118. https://doi.org/doi:10.1073/pnas.2020160118
Stock, S. R. (2014). Sea urchins have teeth? A review of their microstructure, biomineralization, development and mechanical properties. Connect Tissue Res, 55(1), 41-51. https://doi.org/10.3109/03008207.2013.867338
Tolinski, M. (2009). Crosslinking. Crosslinking – an overview | ScienceDirect Topics. Retrieved October 27, 2022, from https://www.sciencedirect.com/topics/chemistry/crosslinking
Wang, T., Huang, W., Pham, C. H., Murata, S., Herrera, S., Kirchhofer, N. D., Arkook, B., Stekovic, D., Itkis, M. E., Goldman, N., Zepeda-Ruiz, L., Freychet, G., Zhernenkov, M., Nemoto, M., Arakaki, A., & Kisailus, D. (2022). Mesocrystalline Ordering and Phase Transformation of Iron Oxide Biominerals in the Ultrahard Teeth of Cryptochiton stelleri. Small Structures, 3(4), 2100202. https://doi.org/https://doi.org/10.1002/sstr.202100202
Wyneken, J. (2014). SKELETAL ANATOMY AND FUNCTION IN REPTILES. Retrieved November 6, 2022, from https://cdn.ymaws.com/members.arav.org/resource/resmgr/Files/Proceedings_2014/43.pdf
Zhu, X., Radovic-Moreno, A. F., Wu, J., Langer, R., & Shi, J. (2014). Nanomedicine in the management of microbial infection – Overview and perspectives. Nano Today, 9(4), 478-498. https://doi.org/https://doi.org/10.1016/j.nantod.2014.06.003Ziegler, A., Storm, M., Steinke, T., Beckmann, F., Prohaska, S., & Ziegler, A. (2010). Opportunities and challenges for digital morphology. Biology Direct, 5, 45. https://doi.org/10.1186/1745-6150-5-45