Abstract
Through this essay’s analysis, the chemistry of keratin unveils its versatility as the main building block for animal hair, horns, teeth, and the subject of the discussion, the claw. This research paper focuses on the chemistry of keratin in animal claws and a variety of claw phenomena that can be explained through chemical mechanisms, such as locomotive tracking, chemical communication, and venomous systems.
Introduction
At a glance, keratin seems like a simple protein. However, the deeper the analysis of this compound gets, the more is uncovered about its versatile and complex structures.
Claw chemistry is first analysed through four different magnifications. Each magnification provokes curiosity into a deeper exploration of the next magnification, as each layer contributes to making the claw structure even more durable and functional to assist in an animal’s survival.
Once basic claw chemistry is dissected and comprehended, niche examples of animals with unique claw chemistries can be analyzed, including cougars and other felines, duck-billed platypuses, and centipedes. Each example discussed possesses unique claw features that distinguish them from the rest of the animal domain.
Analysis of Claw Keratin on Macro and Micro Levels
Many birds and reptiles grow claws. Birds can use these claws to perch on surfaces and attack animals, while reptiles can use them to climb and dig. The claws of these animals must be durable to sustain their weight during climbing and preserve their pointy shape for catching prey. They are mainly made of the protein group of keratins (Alibardi, 2021), a very strong building block. Keratin is present in scales, feathers, and hair, for example (Alibardi et al., 2007). What differentiates claws from similar structures is that they are round keratin appendages at the digits, unlike nails which are flat (Alibardi, 2021). There are two main types of keratins found in claws. The alpha conformation is used in mammals and a tougher beta conformation is used in bird and reptile claws (Wang et al., 2016). The molecular weight of beta keratins is 22 kDa and that of alpha keratins is 50 kDa (Wang et al., 2016). Other conformations of keratin, such as phi keratin, are seen in scales and feathers, although they are rarer and will not be the focus of our analysis (Wyld, 1983).
Claw Overview
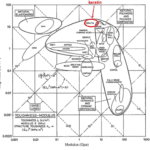
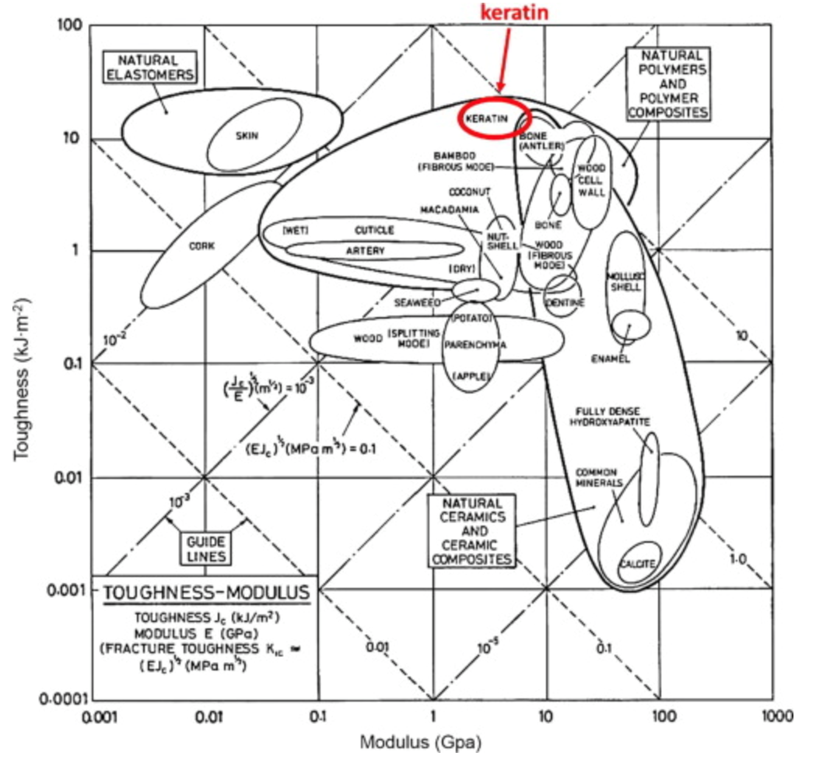
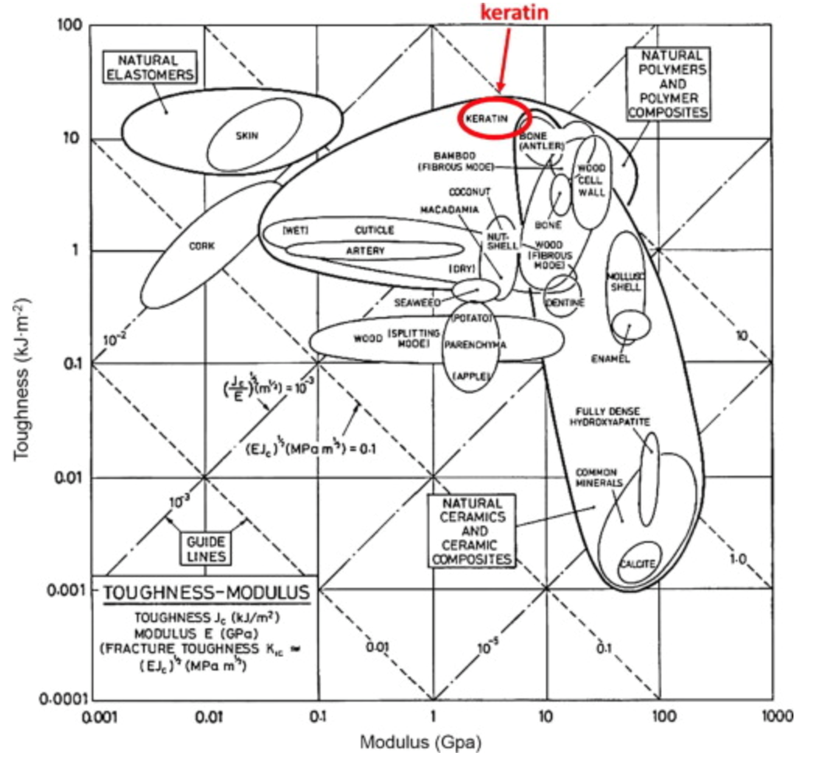
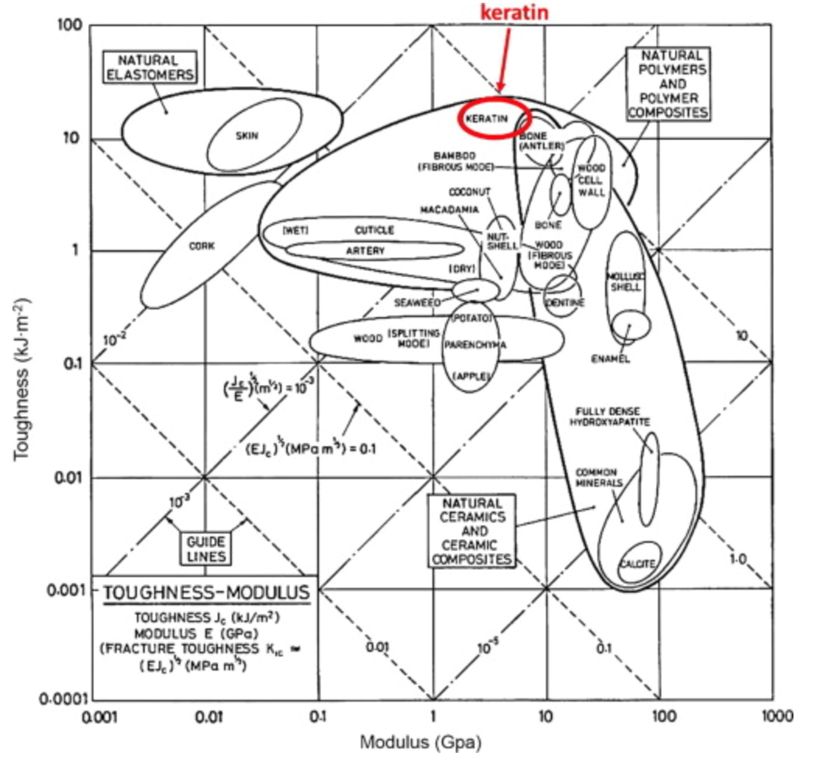
The keratin structure is very tough and has a high modulus compared to other biological materials (Wang et al., 2016). The toughness of a material is measured by the amount of energy it can absorb without fracturing (Shewmon P. G., 2022). The Young’s modulus of a material is defined by the ratio of tensile stress and tensile strain (Gregerson, 2022). The tensile stress is the stress that stretches or lengthens a material (Engineering ToolBox, 2005) and the tensile strain is defined as the deformation of the material in response to the application of tensile stress. As seen in Figure 1, the only other materials studied with a higher modulus would be antlers, indicative of their toughness. Furthermore, the structure of the claw keratin has a similar modulus lengthwise and perpendicular to the claw. Therefore, the claw has an isotropic construction. Isotropy is the property of having “homogeneity in all directions” (Irsay, 2010), so the physical property of the modulus would be similar if measured in different axes, such as collinear and perpendicular to the claw. Some animals with keratin appendages such as horses and their hooves have a more significant difference between the moduli. This is due to the need for the horse to stand on its hooves compared to the multipurpose use of the claw requiring it to be tough in many directions to ensure adaptability (Wang et al., 2016). In the case of horses, the keratin would be anisotropic as the fibers would be directionally dependent to keep its integrity (Irsay, 2010).
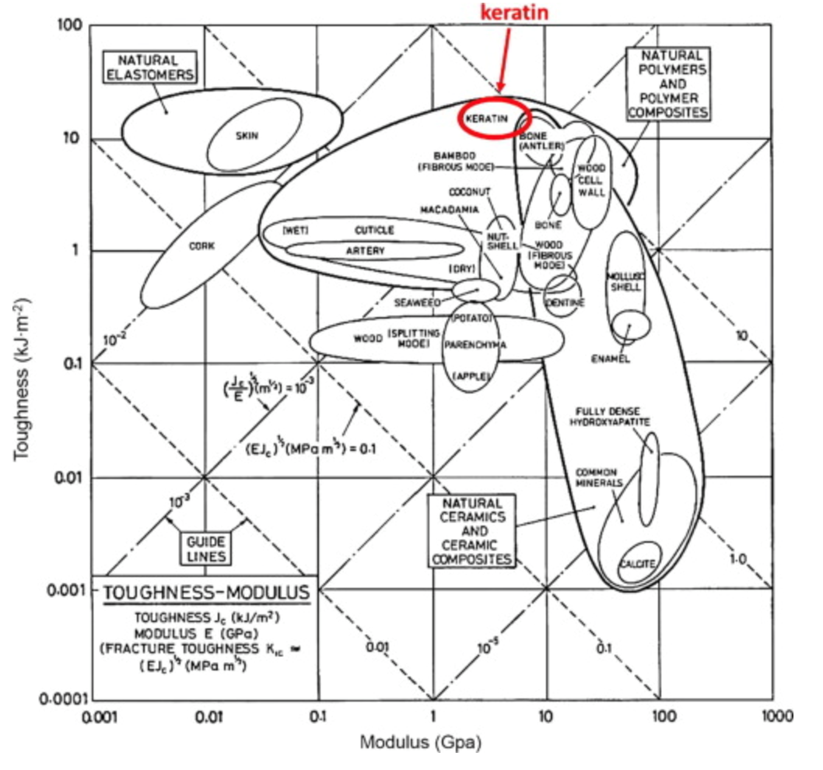
Fig. 1 Young’s modulus vs toughness graph of different biological materials (Wang et al, 2016).
For mammals, two complements to alpha keratin are seen: the intermediate filament keratin and the keratin associated proteins (Wang et al., 2016). The intermediate filaments are the fibrous part of the keratin structure, while the associated proteins merge with the intermediate filaments to create strong corneous appendages. The sole presence of alpha keratins in mammal claws may be due to the specific associated proteins that cause that conformation (Wang et al., 2016).
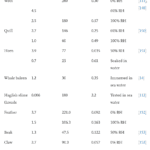
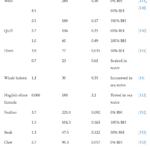
Keratin is denatured at 100°C, meaning normal weather temperatures will not affect the durable properties of the claws (Chilakamarry, C. R. et al., 2021). However, wetness affects the modulus and hardness of the claw negatively (Wang et al., 2016). The modulus, fracture strength, and fracture strain are considerably decreased when the relative humidity is 50% and even more when the relative humidity is 100%, as seen in Table 1. The fracture strength is associated with Young’s modulus, and the fracture strain refers to the ability of the claw not to fracture.
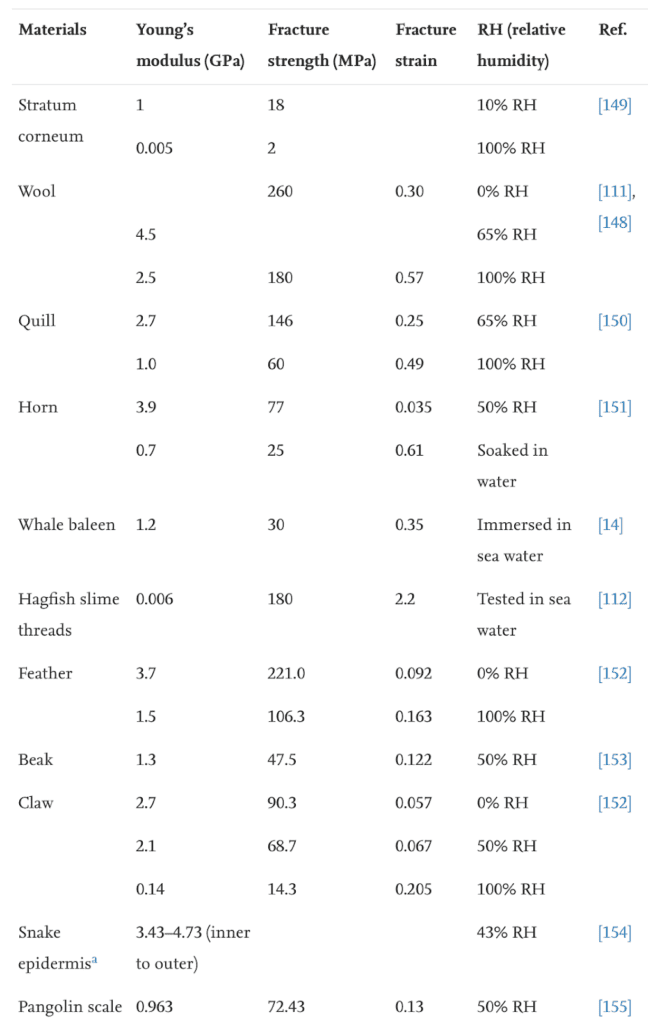
Table 1 Fracture strength of claws in water (Adapted from B. Wang et al., 2016).
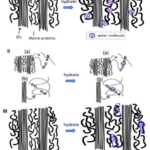
The water decreases the strength of the hydrogen bonds between keratin, distancing intermediate filaments, as seen in Figure 2. The water interacts with matrix proteins, creating a network that makes them more pliant, as seen in Figure 2. This segmentation decreases the stiffness of the claw.
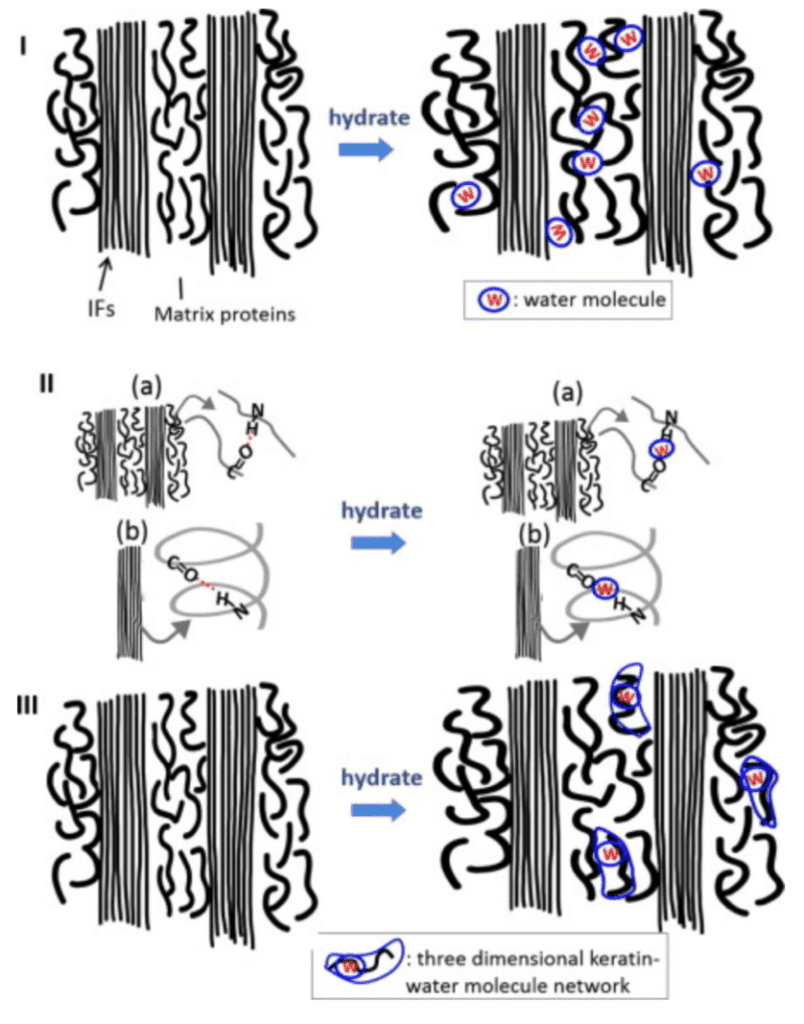
Fig. 2 The interaction of water with the keratin protein (Adapted from Adapted from B. Wang et al, 2016).
Claws Under the Microscope
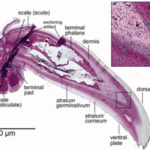
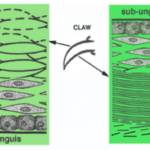
The general parts of the claw are the phalanx and the upper and inner portions, as seen in Figure 3. The phalanx refers to the bone part of the animal digit that the claw surrounds. For the passerines, a family of songbirds, there is fat tissue near the base of the claw to dampen the forces that the claw is subject to (Van Hemert, C, 2012). The upper part of the claw is referred to as the dorsal plate, and the underside is the ventral plate.
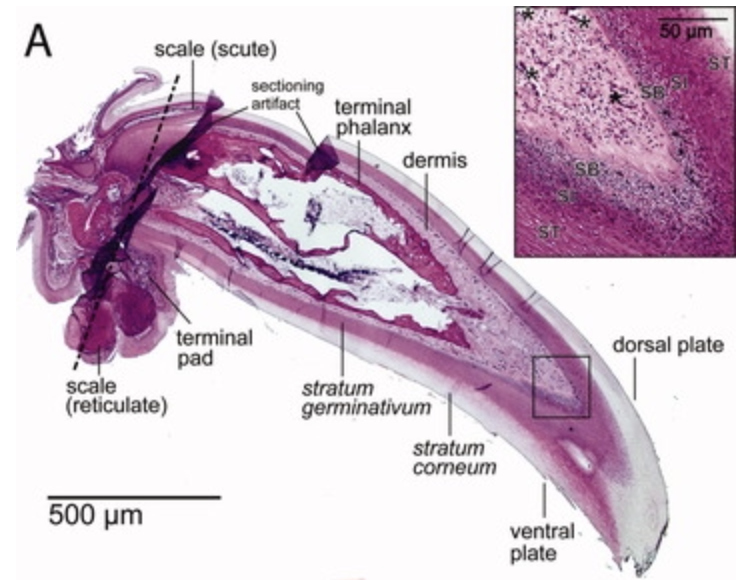
Fig. 3 The inside of a passerine claw (Adapted from Van Hemert, C, 2012).
In the case of reptiles, the top layer is called the unguis, and the layer that is pointing towards the ground is the subunguis, as seen in Figure 3. The scales cover the internal claw sections of the unguis and subunguis (Alibardi, 2021). From bone towards the surface of the claw, there are several differentiated layers. Going from the phalanx to the surface of the claw: there is the basal layer, the intermediate layer, the transitional layer, and the corneous layer, as seen in Figure 4. Sometimes there are more differentiated layers, depending on the animal.
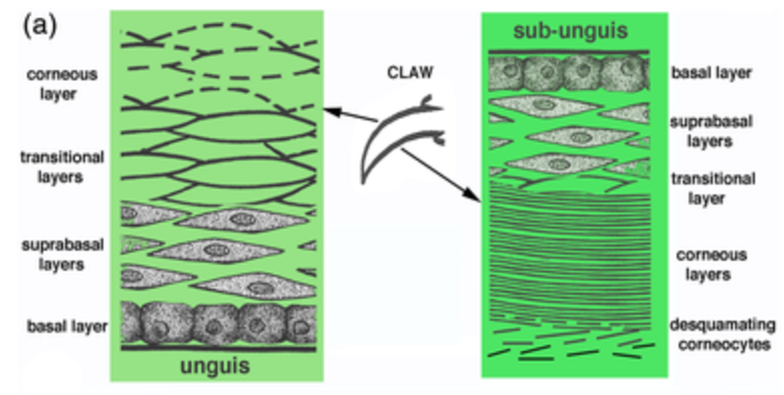
Fig. 4 Reptile claw and cell composition of outer claw portion (Adapted from Alibardi, 2021).
The growth of claws in mammals occurs at the germinal claw matrix, at the internal base of the claws. However, for reptiles, this is done not only at the base, but also along much of the upper and lower base plates (Alibardi, 2021). This can be seen in the subunguis with the weaker keratinocytes. The point of growth in places other than the germinal matrix allows for the replacement of the components lost in the desquamation of the claws by the environment, as seen in Figure 4.
On a smaller scale, some extra minerals can complement the claw. Phosphorus and magnesium are present in the claw keratin for structural purposes (Ethier, 2013). There are residues of zinc, copper, iron, and manganese indicative of the presence of enzymes involved in the growing of the claw (Ethier, 2013). There are also other minerals that can be observed on animal claws, such as the heavy metals lead and mercury, and non-essential elements, such as strontium. Residues of these many elements accumulate chronologically under the claw and can help to guess where the animal has been in its habitat (Ethier, 2013). This is done by linking trace elements in the claw with a particular geographic location. However, the scarceness of the minerals that accumulate in the claw does not allow to plot a comprehensive path of the animal, nor the specific location where the animal resides (Ethier, 2013).
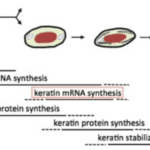
At the cellular level, specialized cells called keratinocytes build up the claws. The -cyte suffix means that the cell is mature. The keratins are translated from mRNA and accumulate in great numbers in these cells (B. Wang et al., 2016). They slowly start to replace the cytoplasm. Simultaneously, the cells move from the germinal layer into more external layers, becoming corneocytes and compacting into a dense corneous layer (Alibardi, 2021). As these cells are being compacted to produce this very tough structure, they are considered dead in the exterior part of the claws, as seen in Figure 5. All that remains are the large amounts of keratin that were contained in these cells (B. Wang et al, 2016). The nucleus and other organelles are also removed in this programmed death of the cells. The keratin filaments end up being parallel to the growing claw portion (B. Wang et al, 2016). As stated earlier, even if the fibers are in the same direction, the keratin in claws still has isotropic properties.
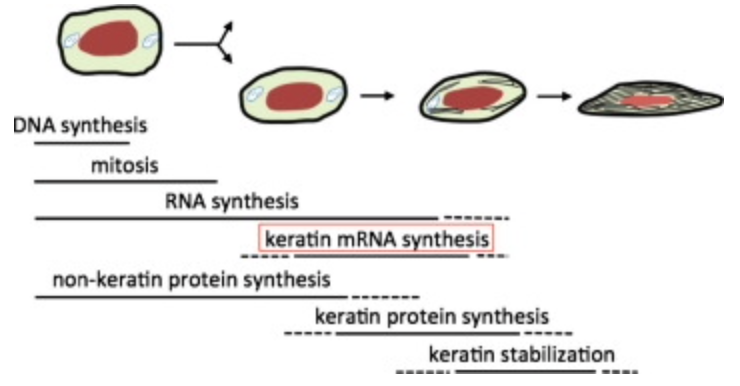
Fig. 5 The life of a keratinocyte (B. Wang et al, 2016).
For reptiles, the basal layer of the unguis has cuboidal cells that become squamous at the suprabasal layer, as seen in Figure 4. The epidermal tissue pointing outwards has different types of cell shapes: cuboidal are cube-shaped, squamous seem squashed and columnar look like longer cuboidal cells, among others. In the transitional layer, the cells start to die, being squashed even more. In the corneous layer, the keratin is tightly wound and is tough enough to withstand forces from many directions.
For passerines, the basal layer of the dorsal and ventral plate are cuboidal or columnar cells (Van Hemert, C., 2012). In the intermediate layer, cells of the dorsal portion are polyhedral and have a nucleus. In the ventral portion of the claw, the cells are condensed horizontally into spindle shapes with flattened nuclei (Van Hemert, C., 2012). Then there are the transitional layer and the cornified epidermal layer where both the dorsal and ventral portions join in a sharp ridge, exposing some transitional cells (Van Hemert, C., 2012). This section is then prone to more wear as it is not as strong as the corneous sections. In the chickadee, a small bird, this fault is fixed with the thickening of this junction to compensate for the accelerated degradation of that part of the claw (Van Hemert, C., 2012).
Analysis of Keratin on Nano Levels – 10-8-10-10 m
Keratin Filaments – 10-8-10-9 m
The common utilization of keratin across such a wide variety of species is proof of its immensely beneficial properties. Keratin possesses one of the highest toughness values among all biological materials, explaining its presence in many different animals. It is in fact the most abundant structural protein in the cytoplasm of most epithelial cells (Feng et al., 2013). To fully understand this powerful group of proteins, the core structures of keratin compositions will be discussed: the filament-matrix structure.
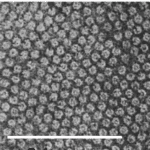
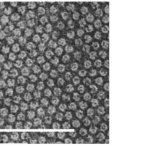
Mercer and Birbeck observed the densely packed filaments forming fibrous keratin through electron microscopy in 1957. These filaments differ significantly between α-keratins and β-keratins. β-keratin filaments measure about 30-40 Å in diameter (Filshie & Rogers, 1961), which is only around half of α-keratin filaments, which measure around 70-100 Å; a sample micrograph of α-keratin filaments can be seen in Figure 6 (Wang et al., 2016). In addition, the β-keratin filaments found within the claw of the Varanus varius, shown in Figure 7, measured 35 Å in diameter (Whitmore, 1971).
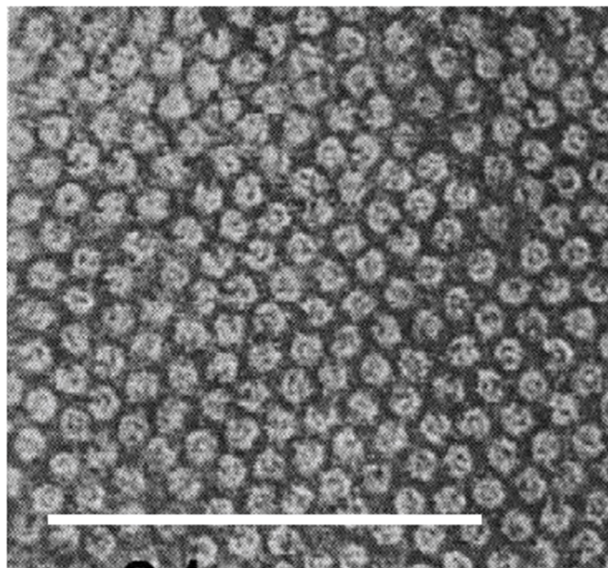
Fig. 6 Electron micrograph of a Merino wool cross section, showcasing α-keratin filaments as the rounded formations. Scale bar: 100 nm (Adapted from Parry, 2021).

Fig. 7 Specimen of Varanus varius, the lace monitor, with claws in clear display (Adapted from Monaco Nature Encyclopedia, Courtesy of Robert Ashdown, 2019).
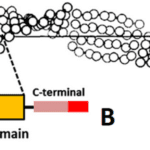
The formation of β-keratin filaments begins with the folding of the central region of the keratin’s polypeptide chain into four lateral β-strands, chains of three to ten amino acids connected by a backbone in an extended conformation (Wang et al., 2016). Hydrogen bonds link these β-strands into a β-sheet, and two β-sheets are stacked together and folded into a helix shape. (Fraser & Parry, 1996). Through this process, the β-pleated sheet structure, as seen in Figure 8, is formed.
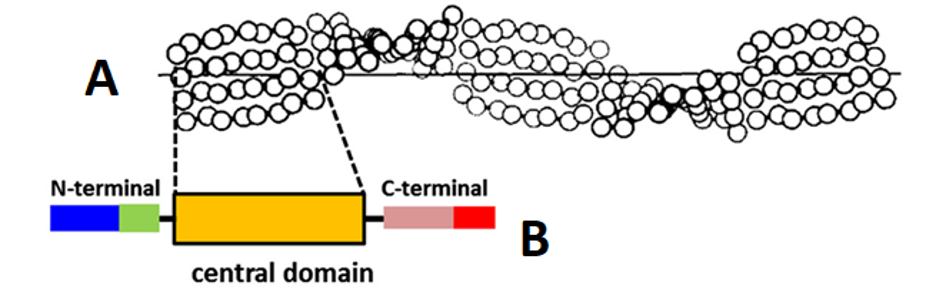
Fig. 8 Distorted β-sheet (A) along with a single molecular unit (B) of β-keratin filament (Adapted from Wang et al., 2016).
These β-pleated sheets form the β-keratin filaments that construct the claws of reptiles and birds. Furthermore, the terminal ends of the peptide chains wrap around the filaments to assist in forming a matrix of filaments by cross-linking the sulfur in cysteine residues through disulfide bonds and essentially act as a cement binding the filaments together (Fraser & Parry, 2011). An example of this filament-matrix structure can be seen in an electron micrograph of a fowl claw cross-section, shown in Figure 9.
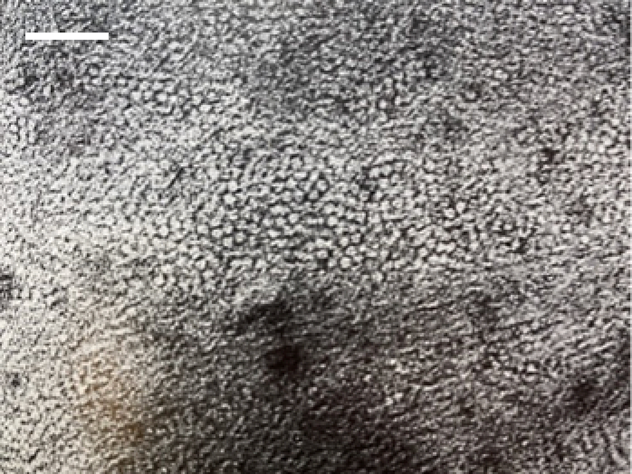
Fig. 9 Electron micrograph of a fowl claw cross-section stained with potassium permanganate – the rounded formations are the cross-sections of the filaments. Scale bar: 20 nm (Adapted from Fraser et al., 1972).
We can examine the filaments densely packed together into a matrix, with each filament measuring around 35 Å in diameter (Fraser et al., 1972). Chemical continuity is present within the filament-matrix structure of β-keratins since this leads to the filaments also forming the matrix. However, this is not the case for the construction of filament-matrix structures of α-keratins.
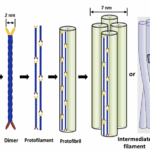
The formation of α-keratin filaments, or intermediate filaments, begins with two polypeptide chains forming right-hand α-helices (meaning these helices twist clockwise when pointed away from the viewer). These α-helices combine to form a coiled coil shape, the dimer, through disulfide bonds cross-linking. Further disulfide bonds connect multiple dimers into a structure named the protofilament. Two protofilaments associate laterally into a protofibril, and four protofibrils unite to form intermediate filaments (Herrmann & Aebi, 2004). These intermediate filaments serve as scaffolding for the cytoskeleton (Bragulla & Homberger, 2009). The process of the formation of α-keratin filaments, or intermediate filaments, is shown in Figure 10.
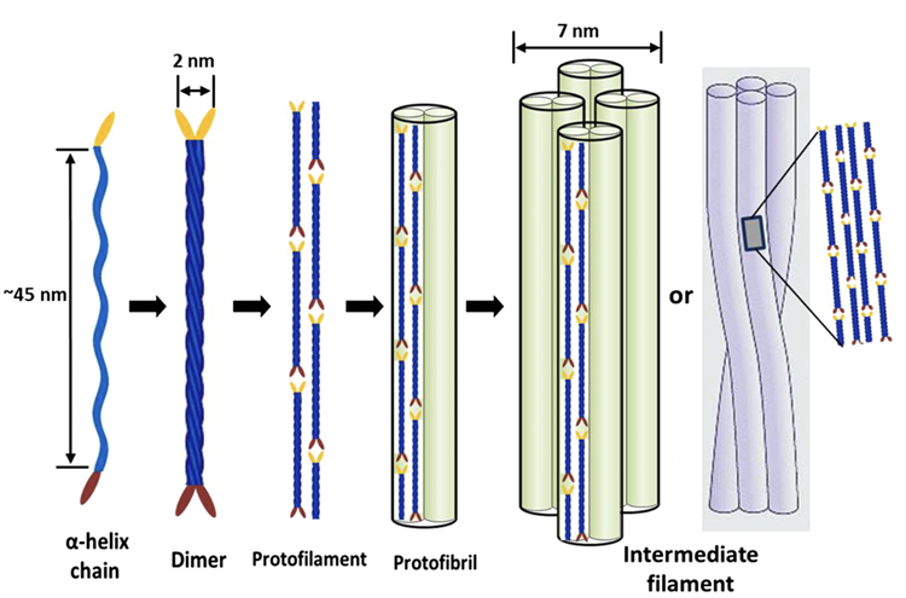
Fig. 10 The assembly process of an intermediate filament, beginning with α-helix polypeptide chains (Adapted from Wang et al., 2016).
However, these intermediate filaments are insufficient to form the filament-matrix structure, in contrast to our β-keratin filament-matrix. Instead, the low-sulfur intermediate filaments are bound together by high-sulfur protein groups, forming the matrix (Rogers et al., 1959). These high-sulfur protein groups differ significantly between different animals, but may consist of high-tyrosine and high-sulfur keratins (Gillespie & Inglis, 1965). In the filament-matrix structure of α-keratins, the intermediate filaments mainly determine the shape and are consistent throughout different keratins. In comparison, the high-sulfur protein groups in the matrix are numerous and highly variable, but do not contribute much to the filament-matrix structure’s shape (Fraser et al., 1972). These phenomena lead to what is essentially a one-dimensional crystal structure of low-sulfur keratin proteins (Fraser et al., 1972).
Both the matrices formed out of intermediate filaments and β-keratin filaments are amorphous (Wang et al., 2016). Furthermore, these keratin filament networks are highly dynamic to allow for constant remodeling and rejuvenation (Windoffer & Leube, 2004). These filament-matrix structures are the core of what gives keratin its incredible toughness, leading to its presence in many different animal species.
Keratin Cross-Linking by Transglutaminase – 10-9 m
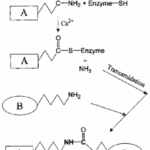
A crucial aspect of the strength of keratins comes from the many chemical bonds such as lysine bonds which form their structure. One of the catalysts for these bonds comes from a group of enzymes known as transglutaminases. Transglutaminases cross-link polypeptide chains through ε-(y-glutamyl) lysine bonds into branching networks, and for keratin specifically they act during the final stages of keratinization (Buxman & Wuepper, 1975). They do so by cleaving off terminal peptides and forming y-glutamyl bonds between lysine and glutamine, Figure 11 shows an example.
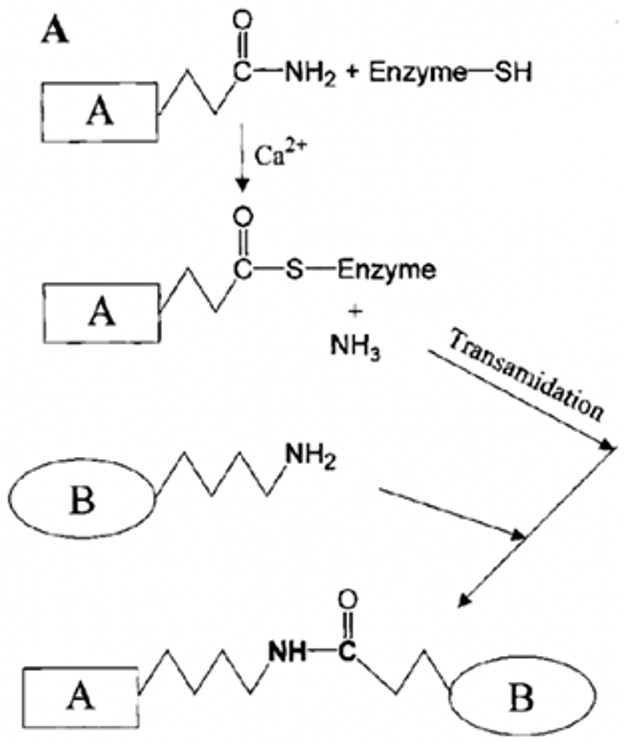
Fig. 11 Production of a glutamyl-lysine protein cross-link by a transglutaminase (Adapted from Griffin et al., 2003).
The keratinocyte-focused transglutaminase is type 1 transglutaminase. These enzymes are strictly specialized for glutamine but not for lysine, allowing for substitutes to replace lysine as a substrate; however, in the case of keratin, lysine is often used (Griffin et al., 2002). Only terminal domain lysines and glutamines are used for cross-linking, despite both mostly existing within the central domain of polypeptide chains; the central domain mainly serves to provide fillers or spacers to permit cross-linking (Steinert et al., 1999). This phenomenon results in only 2-3% of available lysine in keratin participating in cross-linking, which is surprising given the strength that cross-linking provides to keratins (Candi et al., 1998).
Transglutaminase cross-linking allows for the creation of keratin filament-matrix structures and is necessary for augmenting the toughness within keratin-based appendages such as claws, by forming a continuous and interconnected covalently crosslinked network. Specific lysine components of keratin are highly conserved to allow for cross-linking (Candi et al., 1998). Transglutaminases are a crucial component of the process of keratinization as their formation leads to the emergence of covalent bonds crucial to the properties of toughness and strength within keratin. They are a fascinating example of the chemistry within the keratin of claws.
Keratin Protein Folding – 10-10 m
Diving beyond the formation of filaments by keratins during their quaternary structure protein folding, α and β-keratin amino acid sequences are discussed, they can vary in length across different species but are similar in sequence throughout (Bragulla & Homberger, 2009). They form the tertiary structures of dimers and β-strands that create keratin filaments, which are themselves constructed out of secondary structures of α-helices and β-sheets, respectively.
Finally, the highest magnification we shall examine are primary structure amino acid sequences. As keratins are a group of proteins and thus do not have a single defined sequence, it is more appropriate to draw comparisons and find patterns within sequences of keratins across different species.
A defining characteristic of the keratin amino acid sequence is the commonality of cysteine (Cys), a sulfur-containing amino acid. This amino acid is crucial as it allows for strong disulfide bonds to take place between keratins, which greatly contribute to the strength that keratins provide by stabilizing and hardening the material. They are also necessary for the formation and maintenance of perinuclear networks of keratin filaments (Feng & Coulombe, 2015). As such, it is clear why cysteine is omnipresent within the many different types of keratins. Example keratin sequences with cysteines are shown in Figure 12 below.

Fig. 12 Keratin-14 sequences of several mammals, with cysteine residues highlighted in yellow (Adapted from Feng & Coulombe, 2015).
For β-keratins found specifically in avian and reptilian claws, cysteine is present in even higher amounts, suggesting that claw proteins are specialized to create a durable material suitable for the many mechanically demanding functions of the claw (Toni, 2008). The more cysteine present, the more disulfide bonds can form, leading to a more robust material. This pattern is consistent when comparing lizard claw proteins to soft keratin proteins such as those found in the skin’s epidermis.Lizard claws have a higher sulfur content, indicating a higher cysteine content (Wang et al., 2016).
It is the central region of the polypeptide chains in β-keratin that fold into β-pleated sheets – polypeptide chains of around eight amino acids each fold into the β-strands that later become β-sheets (Fraser, 1996). By way of comparison, only 30% of the polypeptide chains in seagull feather rachis, the upper portion of the feather shaft, contribute to the formation of β-sheets (Fraser et al., 1971). The N-terminal heads and C-terminal tails of the molecular units of β-keratin, shown in Figure 8, contribute to the formation of the matrix through disulfide bonds cross-linking, and thus contain much of the cysteine present within the polypeptide chain (Fraser & Parry, 2011).
α-keratins, while different in many ways, follow similar rules to β-keratins. For example, the sequence Cys-Cys was found to be characteristic of mammalian hard keratin (Lindley & Haylett, 1967). While α-keratins contain less sulfur than β-keratins, the high-sulfur protein groups that form the matrix are variable and homologous (Haylett & Swart, 1969).
Claw Chemistry in Felines
Chemical Isotopes in Claws used for Tracking Animal Movement
After analyzing the detailed structure of keratin, the substance from which claws are made, a unique application in the chemical profiling of feline claws is found – specifically the chemical profiles of keratin depending on the environment of the animal.
The general chemical structure of different claws has the same foundation; however, a slight change in environment can affect the overall chemical profile of the claw keratin.
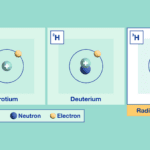
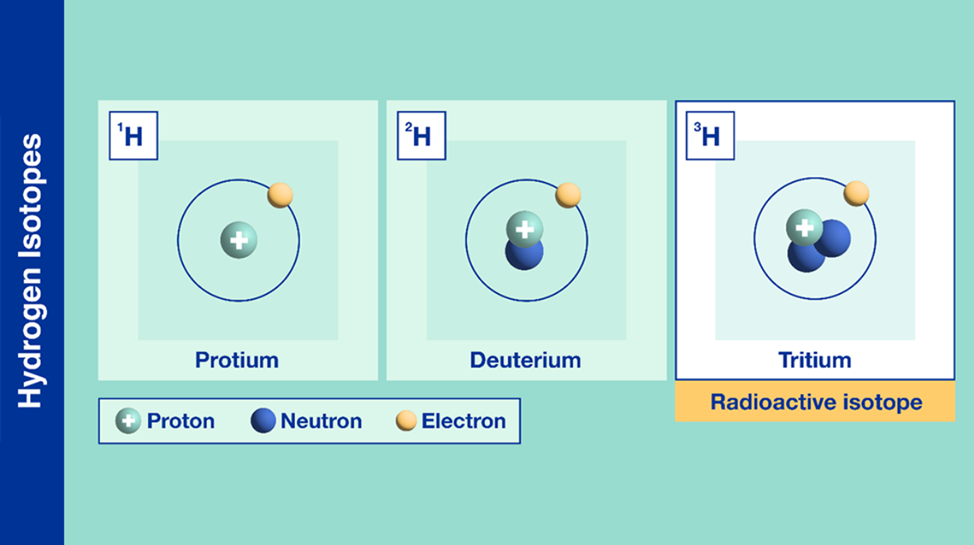
Isotopes are chemical elements with the same number of protons, but a different number of neutrons. An isotope has the same atomic number as the comparable chemical element, but it has a different atomic mass (Daya, 2022). An example of hydrogen isotopes is examined below in Figure 13.
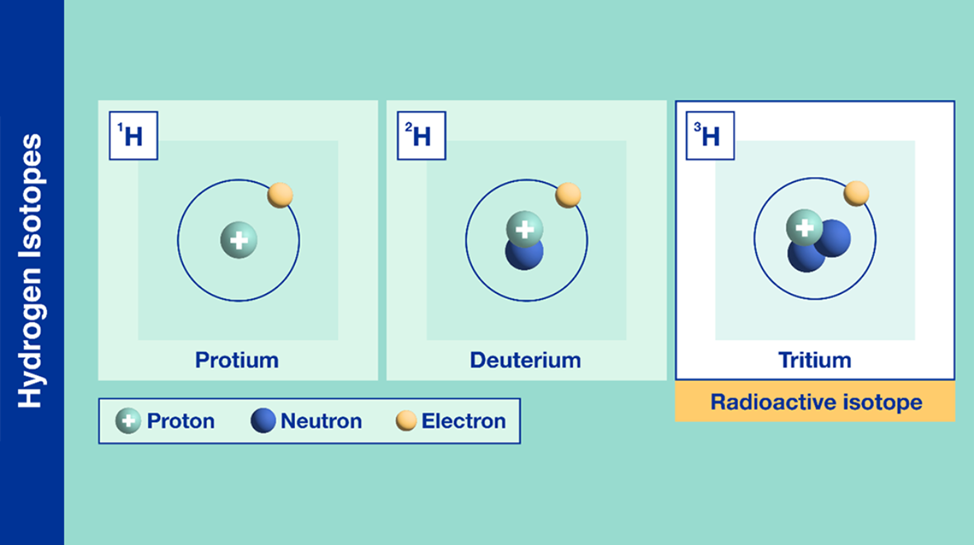
Fig. 13 Different types of isotopes for the chemical element hydrogen (Adapted from IAEA, Courtesy of Daya, 2022).
Knowing this, there are many different isotopes to consider when examining different environments. Isotopes can be present anywhere from the soil on the ground to the food consumed daily. With a consistent diet and habitat, an animal’s chemical composition and profile can vary slightly depending on what types of isotopes they are exposed to on a daily basis (Ethier et al., 2014).
The chemical profile in claw keratin contains the isotope of hydrogen, deuterium (shown in Figure 13). The claws of a cougar can be perceived from afar in Figure 14.
Fig. 14 Cougar (Adapted from Vincent, 2022).
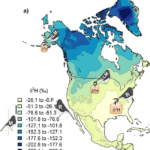
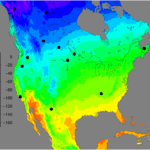
Deuterium has a larger atomic mass in comparison to the regular hydrogen atom described in the periodic table (Daya, 2022). Deuterium can be incorporated into the cougar’s environment through the local water supply which is distributed through the rain into the soil of the animal’s environment. This creates an overall heavier water molecule within this location (Marris, 2008). The difference in the chemical profiles of a variety of claw keratins can be determined by finding the differences in atomic mass (Marris, 2008). This is true for various animals such as flying animals, land animals, and insects. This slight change in the chemical profiles of animal claws provides an insight into the dynamics of wildlife. Through analyzing the isotopes’ effect on animal claws, researchers can estimate the locations the animals have migrated to in the past (Singh et al., 2019). This form of chemistry aids in animal tracing and provides evidence of animal movement. For example, the migration and movement of the juncos is analyzed through the stable isotope percentage, as shown in Figure 15. The isotope percentage in the figure below is calculated by taking the average atomic mass of the isotopes within a region and multiplying this value by the decimal equivalent of the species’ relative abundance. Relative abundance can be calculated by taking the number of species in the area and dividing this value by the total of all populations of species in the area. The legend in Figure 15 shows the colours on the map that correspond to a specific isotope percentage.
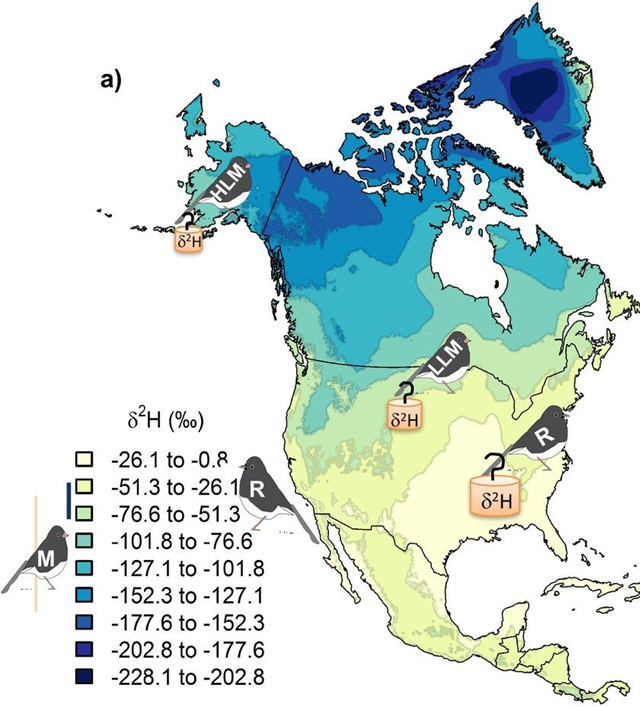
Fig. 15 The concentration of juncos within the latitudes of North America are analyzed through a diagram of hydrogen isotopes (Adapted from CSH, Courtesy of D. Singh et al., 2019).
For more clarification, another example of animal tracing can be analyzed: cat claw animal tracing. The isotope samples collected from feline claws can be compared with the hydrogen isotope composition within the claw. The hydrogen isotope compositions allow for estimating where the feline lives by comparing its claw chemistry with the map shown in Figure 16 below. Different isotope percentages of felines within the general area are colour coded on the legend corresponding to the map.Therefore, this graph can be used as a reference point to determine a feline’s original location based on claw profiling.
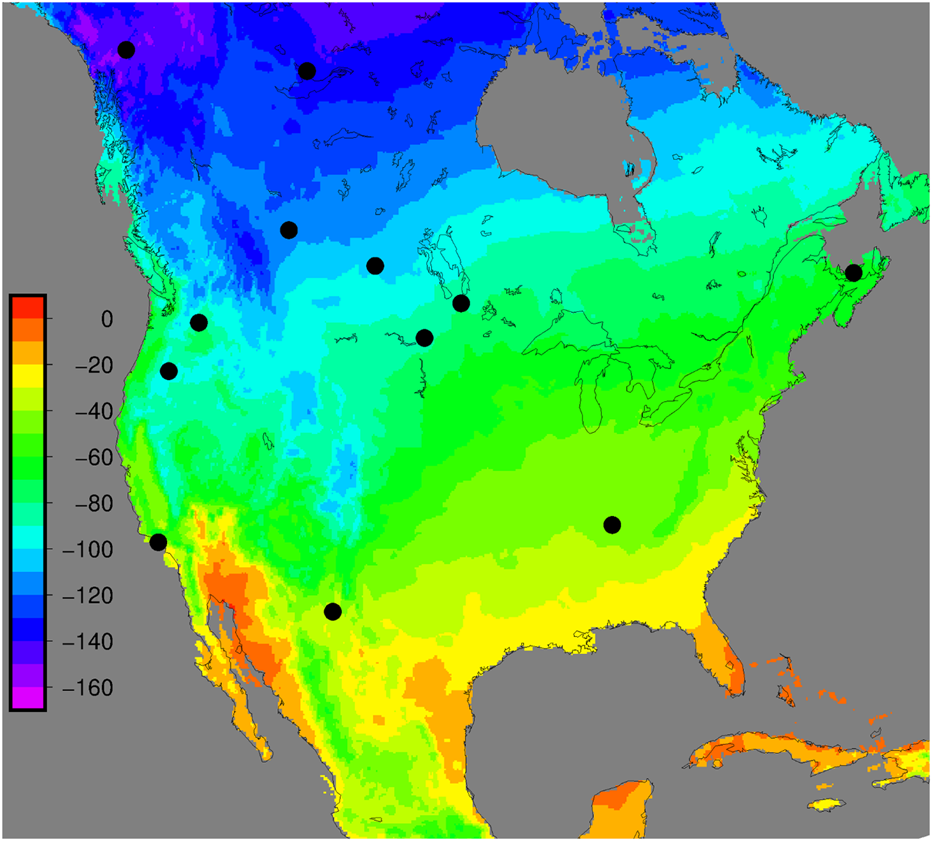
Fig. 16 Hydrogen isotope composition of felines. The legend on the left represents the levels of hydrogen isotopic composition (Adapted from PLOS, Courtesy of Hobson et al., 2019).
Chemical Communication through Clawing
Beyond enabling the tracking of feline migration due to their isotopic signatures, feline claws aid in enabling chemical communication between intraspecies members of the family Felidae. In typical feline behavior, members of the family, shown in Figure 17, maintain the habit of scratching surfaces with their claws. Although there are several reasons for this behavior, one of the main ones is that scratching surfaces allows felines to deposit their chemical signal, generally in the form of scents and pheromones. By leaving chemical signatures in their wake, felines are able to communicate chemically with other members of their species (DePorter, T. L. & Elzerman, A. L., 2019).

Fig. 17 Member species of the family Felidae (Adapted from Pop Chart, n.d.).
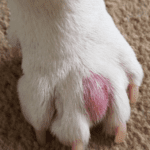
How exactly do felines execute this complicated form of communication? Initiated by the claws, these tools serve to carve a deep gash into the surface being scratched. Not only does the gash provide a secure place to deposit the feline’s chemical signature, but the clawing action itself stimulates the release of the cat’s scents and pheromones. These chemical signatures are released from the interdigital glands located between the individual digits of the cat’s paw, shown in Figure 18 (DePorter, T. L. & Elzerman, A. L., 2019).
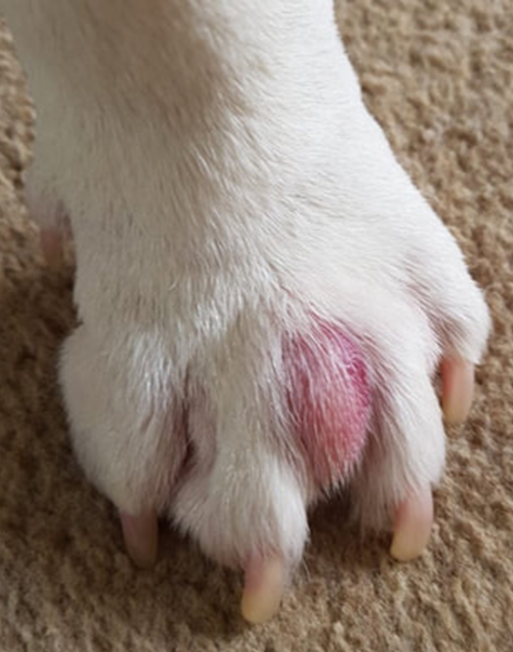
Fig. 18 Swollen interdigital gland for viewing and referencing its location on the feline paw (Adapted from Interdigital cysts, n.d.).
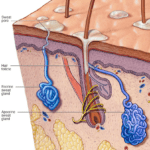
The interdigital glands of felines that release their communicative scents are the eccrine sweat glands, as shown in Figure 19. These glands release their secretions through pores which lead directly to the surface of the skin on their paw pads (Sergiel et al, 2017).
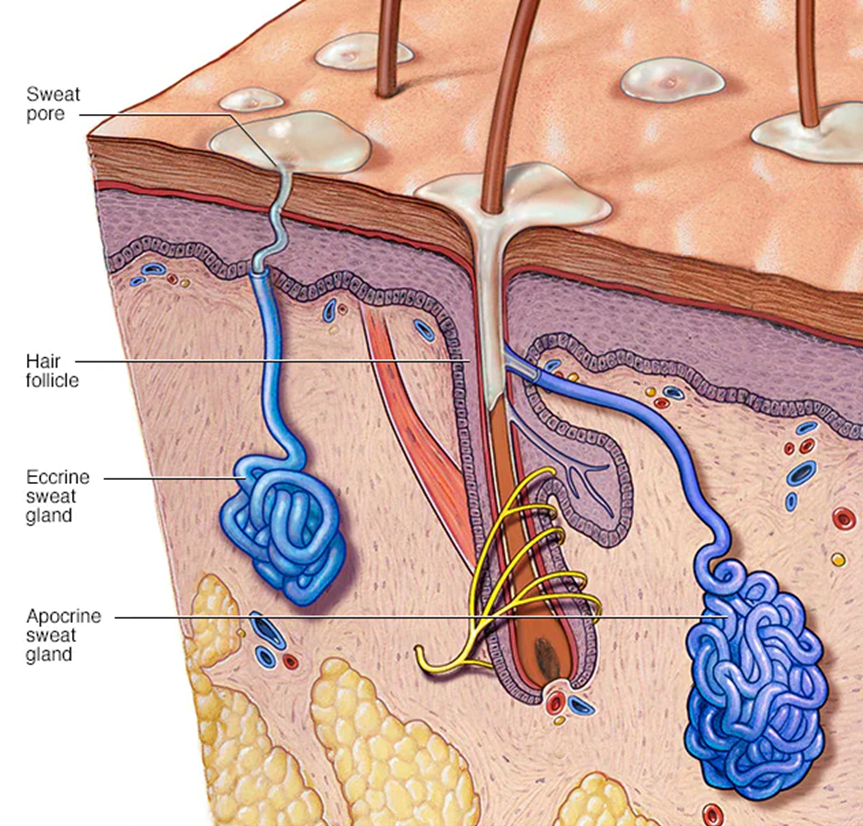
Fig. 19 Eccrine sweat glands: the feline interdigital glands responsible for secreting their marking scents left after scratching (Adapted from Mayo Clinic, 2020).
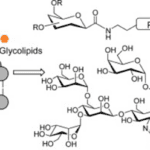
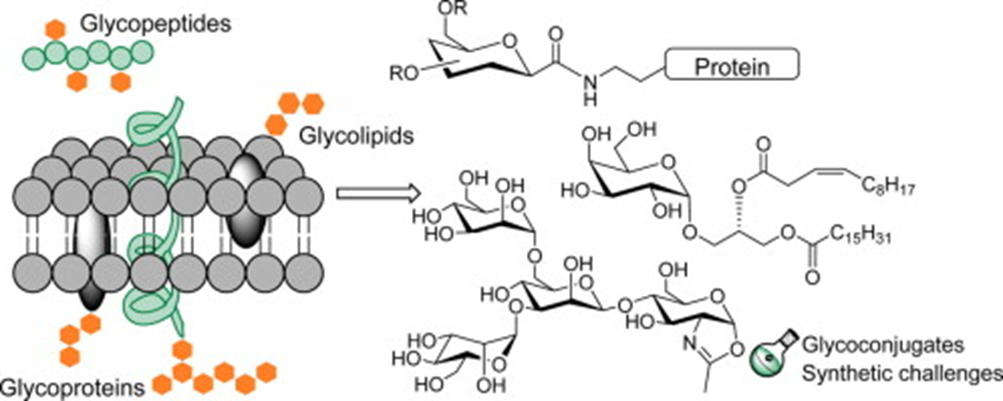
Researchers have found that the secretory products of the feline eccrine sweat glands include high amounts of glycoconjugates, examples of which are shown in Figure 20. Glycoconjugates represent a broad group of hybrid biochemicals, volatile in some cases, containing a carbohydrate chemically bonded to a secondary component. Some of these secondary components include peptides, proteins, and lipids (among others), which when combined with the carbohydrate, form glycopeptides, glycoproteins, and glycolipids, respectively (Lee, R. T. & Lee; Y. C., 1997). Although the exact glycoconjugates involved in scent communication and secreted by feline eccrine sweat glands remain unknown, researchers recognize the significance of glycoconjugates in feline territorial scent marking as well as tracking and orientation capabilities. This is because the eccrine sweat glands often release their secretions during a feline’s fear reactions, or more specifically, a feline’s sympathetic nervous system’s response to dangerous, stress-inducing situations, which include confrontations between unfamiliar felines (Cleveland Clinic, 2022). Thus, the scents from the sweat deposited through scratching serve as alarm marks with a chemical signal that prompts avoidance in other felines, the importance of which will be expanded upon later (Sergiel et al, 2017).
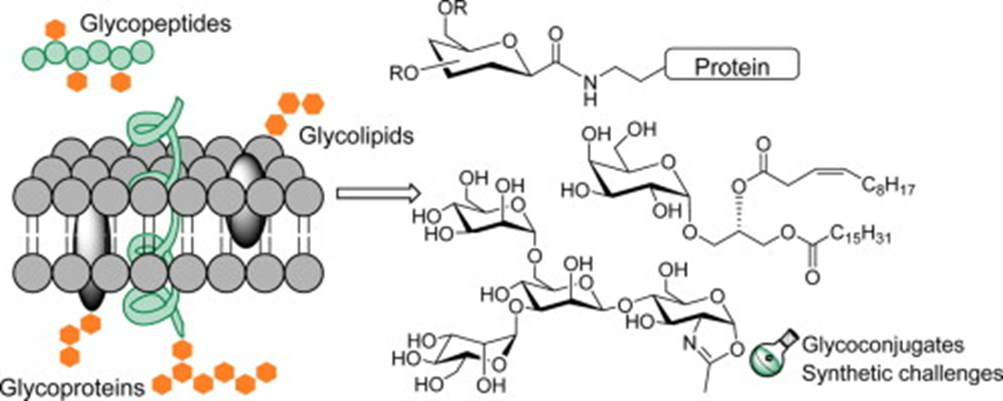
Fig. 20 Examples of various types of glycoconjugates (Crucho et al, 2015).
In addition to scent, the other chemical signaling compounds used in feline chemical communication are pheromones. They are composed of a distinct combination of different chemical compounds unique to each species, resulting from having evolved with intraspecies communication. Pheromones elicit a specific response from the individual detecting it (Vitale Shreve, K. R. & Udell, M. A., 2017). Although pheromones are also involved in mating and can evoke responses of attraction and arousal, similar semiochemicals can provoke contrasting responses such as avoidance. This is a reaction to detecting alarm pheromones excreted by felines marking their territory. These alarm pheromones, among many others, are also released through the feline interdigital glands, although not necessarily the eccrine sweat glands. The specific pheromones released through the interdigital glands are the feline interdigital semiochemicals, which are distinctly involved in territorial communication (DePorter, T. L. & Elzerman, A. L., 2019).
Defining semiochemicals
Semiochemicals are chemicals that convey a signal between different organisms in order to prompt a change in behavior of the recipient organism. A specific semiochemical is either considered a pheromone or an allelochemical. While pheromones mediate interactions between organisms of the same species (intraspecific interactions), allelochemicals mediate interactions between organisms of different species (interspecific interactions). Therefore, semiochemicals are used as a broad term to describe any chemical signaling between organisms, whether intra- or inter- species related (El-Shafie, H.A.F., and Faleiro, J.R., 2017).
The specific location of the interdigital glands serves to enhance the feline ability to chemically communicate with others of the same species. The paw pads between which the interdigital glands are located possess incredible sensitivity to sensory stimuli, which instigate a reaction in the feline. This ultimately leads to the production of glandular secretions containing the chemical signaling compounds as the feline scrapes the substrate (Sergiel et al, 2017). Moreover, the claws positioned at the forefront of the paw perform the scratching necessary to trigger the release of the feline interdigital semiochemicals as well as to deposit both those pheromones and the scents from the eccrine sweat glands into the scratched surface (Pageat, P. & Gaultier, E., 2003). Thanks to the configuration of their paws and their possession of claws, which are shown in Figure 21, felines can effectively trigger the release of their chemical signatures and leave a durable scent mark.
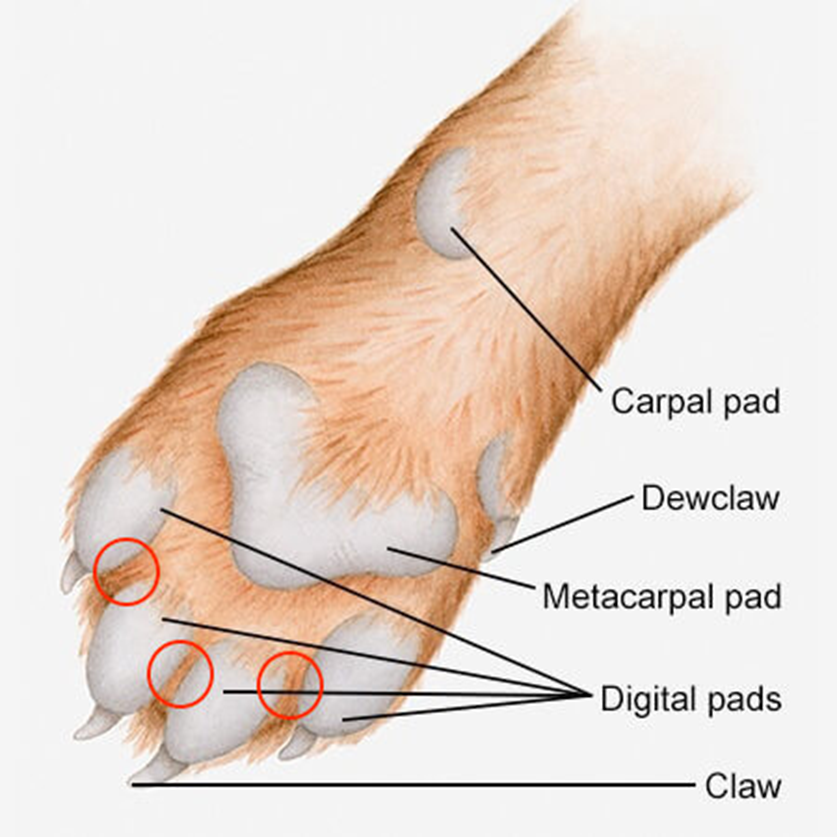
Fig. 21 Configuration of the feline paw (Adapted from Annex Cat Rescue, 2021). Location of feline interdigital glands circled in red.
Furthermore, considering that the primary function of the eccrine sweat glands is in fact to improve the feline paw’s frictional capacities, along with the scratching of the claws, felines can also provide significant visual signals, as shown in Figure 22 below, alerting other felines of their presence (Sergiel et al, 2017).

Fig. 22 Feline territorial claw marks left from scratching, serving as a visual signal of their presence (Kapama, 2012).
By indicating their presence in this way, felines can mark their territory. This becomes incredibly important for feline self-preservation considering serious conflict may occur during direct encounters between cats unfamiliar with each other, as shown in Figure 23 below. Thus, indirect communication through chemical signaling advertises one feline’s presence to another and warns the other to stay away (Sergiel et al., 2017).

Fig. 23 Territorial fight to the death between felines of different species resulting in the leopard killing the caracal (Africa Geographic Photographer of the Year, 2021).
Claw Chemistry of Venomous Animals
Duck-Billed Platypus Venom
Another unique animal claw to analyze is the male duck-billed platypus’, shown in Figure 24. These small, furry creatures are located in many different parts of Australia and are known for their duck-billed snout, which can help them detect prey using the multiple receptors on their bill (Brouw, 2020). However, the most intriguing – and unexpected – aspect of these animals is the male duck-billed platypus’s ability to secret venom when needed.
Defining the difference between Venom, Poison, and Toxins
The distinguishing feature between venom and poison is the mode of entry of the toxins into their victim. Venom refers to a toxin that is injected into its victim (actively executed by the venomous entity). Poison refers to a toxin that is ingested by the victim (passively executed by the poisonous entity). Toxins are substances composed of organic compounds that damage the enzymes of the organism intoxicated, thereby harming the organism’s health. A substantial dose of toxins, if not detoxified or expelled, may lead to the death of the afflicted organism (Rafferty, J. P., 2017).

Fig. 24 Male duck billed platypus (Adapted from Nature Picture Library, Courtesy of Doug Gimesy, 2020).
The male platypus starts developing venom in a gland called the crural gland during mating season in an attempt to assert dominance and compete with other platypuses for female attention (Brouw, 2020). Figure 25 demonstrates the path the venom travels in, from its creation in the crural gland to its movement through venom ducts and its ultimate ejection from a spur in the hind legs. The spurs – a type of claw usually located near the back of the paw – are roughly half an inch long (Whittington et al., 2010).
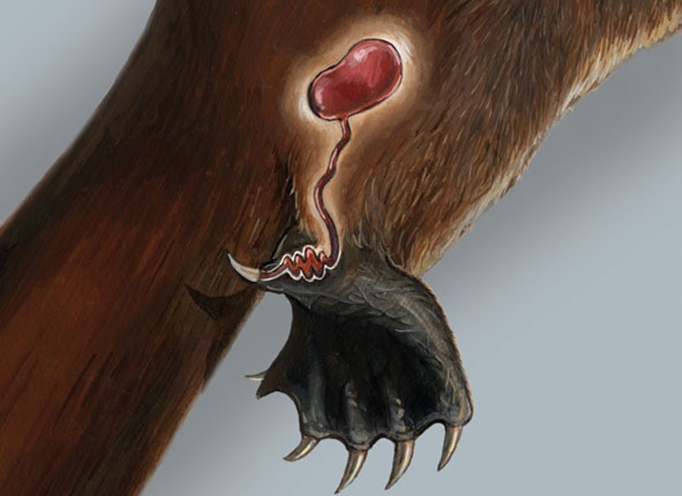
Fig. 25 Venom Secretion Mechanism (Adapted from Animal Diversity Web, courtesy of Sorin et al., 2004). This image shows the crural gland that connects to the venom ducts which are connected to the spur.
The venom excreted by the platypus claw is made of various proteins, which can be categorized into 13 different toxin groups (Whittington et al., 2010). These toxins include ornithorhynchus venom C-type natriuretic peptide (ovCNP-39), Ornithorhynchus nerve growth factors (NGF), and defensin-like peptides (Koh, 2009). However, the central toxin group that provides the most pain in the platypus’s victim is the NGF protein group.
Focusing on NGF protein and chemical structure, it is found that they have a tertiary structure made of cysteine and extended disulfides, as well as beta sheets (Bradshaw, 1977). To elaborate, beta sheets are secondary protein structures formed with hydrogen bonding between carbonyl O and hydrogen different amino acids, creating a pleated shape. NGF proteins are related to a protein called insulin-like protein (ILP), which is responsible for growth regulation and maintenance of sensory neurons. With this protein, NGF proteins can attach themselves to specific nuclear binding sites and create a cascade of different chemical reactions in the central nervous system (Petruska et al., 2009). To clarify, NGF proteins play a major role in nociception (pain caused by tissue damage) because they sensitize the reaction of the exposure of any nociceptive stimulus (pain stimulants) from the posttranscriptional mechanisms that can change gene expression within the area affected by the platypus’s venom (Petruska et al., 2009). The change in gene expression occurs within the dorsal root ganglia (central collection of nerves) which affects recovery of injury and prolongs the pain. NGF causes severe pain to its victims because it stimulates the activation of inflammatory cells to release inflammatory mediators, which can cause great pain as they directly affect neurons.
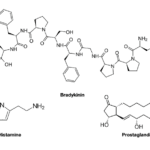
These toxin groups within the platypus venom cause chemical signals to be sent to the brain which convey a strong pain due to inflammatory response to injury (Jami, et al., 2017). The damaged cells that cause the inflammatory response release chemicals such as histamine, bradykinin, and prostaglandins which trigger swelling (Bronco et al., 2018). These chemicals are represented in Figure 26 below.
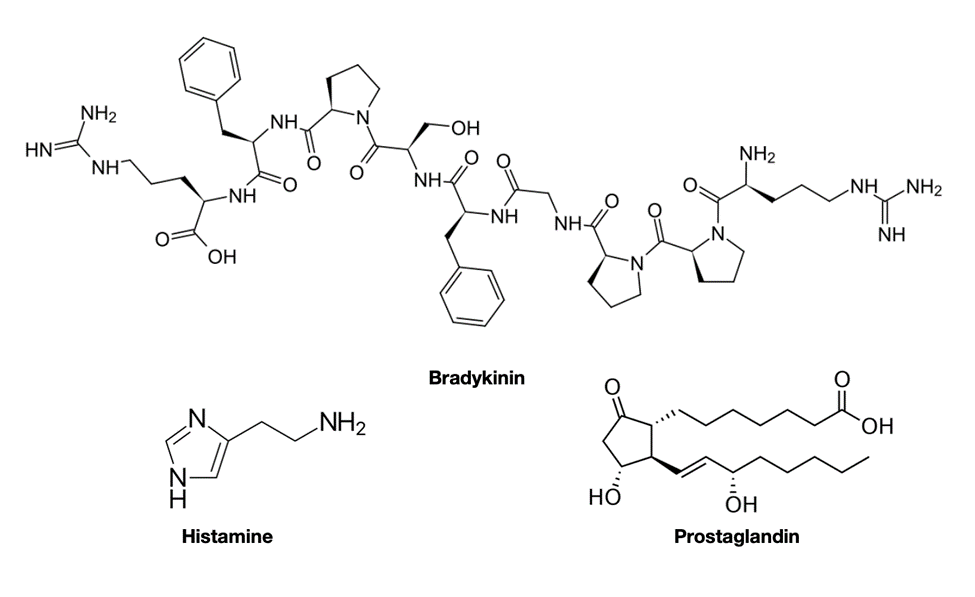
Fig. 26 Histamine, bradykinin, and prostaglandin chemical formula (Courtesy of Jung, 2022).
Centipede ‘Poison’ Claws
Another incredibly interesting animal that exemplifies the chemical applications of claws is the centipede, shown in Figure 27 below.

Fig. 27 Centipede (Johnny B’s Pest Control, 2019).
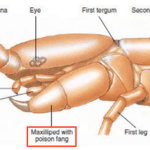
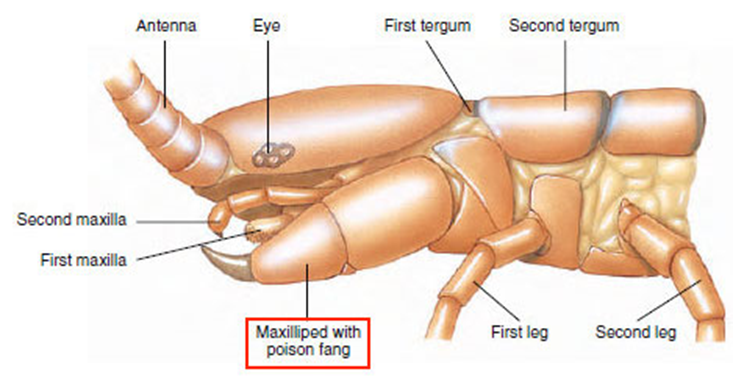
Constituting the class Chilopoda, centipedes have a long, segmented body, each consisting of a single pair of jointed appendages. The appendages on each body segment, besides the primary one consisting of the creature’s head, the appendages on each body segment are considered its legs and are used for moving. The appendages on the primary body segment include the centipede’s antennae, mandibles, and most notably its maxilliped, also known as its poison claws, shown in Figure 28 (Wildlife Journal Junior, 2022).
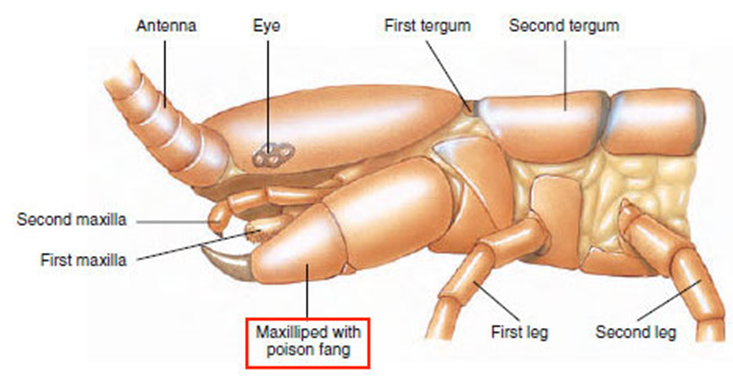
Fig. 28 Diagram of the primary body segment of a centipede (Adapted from Biocyclopedia, n.d.). Note the maxilliped, or poison claw, highlighted in red.
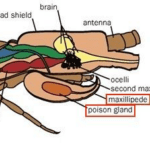
Considering their classification as carnivorous predatory arthropods, centipedes use their poison claws to capture and paralyze their prey, predominantly insects. With their strong poison claws, centipedes pierce the flesh of their prey to inject the venom that will paralyze, if not kill, their victim (Stankiewicz et al, 1999). The venom itself, different for each species of centipede, is excreted by the poison gland shown in Figure 29, located within the maxilliped posterior and attached via a duct to the sharp claw injecting the venom into the centipede’s victim.
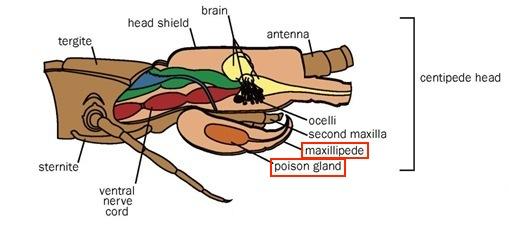
Fig. 29 Diagram of the location of the poison gland within the poison claw (maxilliped) (Adapted from Biocyclopedia, n.d.).
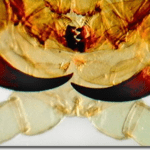
Although their appendages (shown in Figure 30) will be referred to as poison claws and poison glands (because that is their official designation), they are in fact considered venomous entities, given the technical definitions of the terms poisonous and venomous previously defined (Rafferty, J. P., 2017).
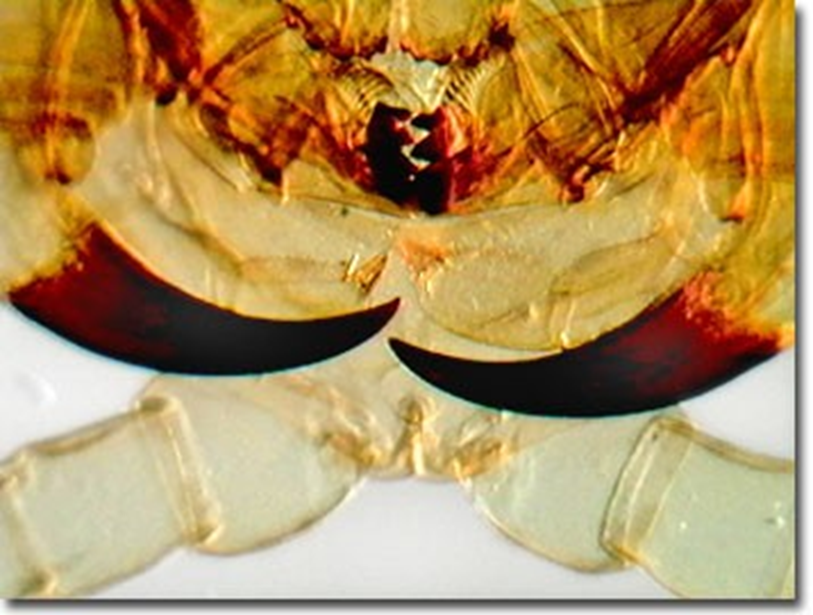
Fig. 30 Centipede poison claws at high magnification (Olympus, 2015).
Most centipede venom consists of a complex mixture of different chemical compounds that, combined, result in a distinctly toxic substance. Typically, centipede venom contains a mixture consisting of serotonin, histamine, lipids, lipoproteins, polysaccharides, and various enzymes, present in differing amounts (Erickson, T. B., 2007; Stankiewicz et al., 1999). The exact compounds and overall composition of the venom varies between species and depends on the prey it is meant to immobilize. For example, the species Scolopendra subspinipes produces a high molecular acidic cardiotoxic protein (Stankiewicz et al., 1999) in its venom, which invokes hypotension directly followed by hypertension (Erickson, T. B., 2007). The species Scolopendra morsitans produces acetylcholine in its venom, potent enough to induce cardiac arrest in an animal 2-4 times its size (Stankiewicz et al, 1999). In these cases, both venoms serve to affect the cardiovascular system of its prey, but because the prey themselves are different for the different specet al.the chemicals involved vary.
In a study published in the Journal of Chemical Ecology (Maschwitz et al, 1979), researchers explored the secretions of the centipede genus Asandada, a subset of the order Scolopendromorpha encompassing the most massive, aggressively predacious, and toxic Chilopoda. Researchers found that in the venom secreted by Asandada, the toxin hydrogen cyanide was present. Through an in-depth analysis paired with experimentation, the researchers deduced that the Asandada most likely produce their hydrogen cyanide in a cyanogenic reaction catalyzed by an enzyme not yet identified. The precursors of this reaction were found to be mandelonitrile and benzoyl cyanide, presumably produced in the sternal glands of the Asandada. The exact mechanism for the production of hydrogen cyanide in Asandada, however, is still yet to be discovered, although its presence is confirmed (Maschwitz et al, 1979).
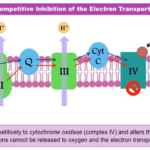
Hydrogen cyanide toxin functions by interfering with an organism’s use of oxygen. More specifically, it inhibits the enzyme cytochrome oxidase, which is required for the terminal step of electron transport in the process of cellular respiration. This prevents cells from utilizing oxygen, as shown in Figure 31. Depending on the cells inhibited by hydrogen cyanide, the affected organism can lose consciousness, experience respiratory arrest, suffer cardiac irregularities, or endure all of these effects (National Library of Medicine, 2002). Exposure to hydrogen cyanide in substantial concentrations can therefore be fatal (Centers for Disease Control and Prevention, 2021). Thus, the presence of hydrogen cyanide in the venom of the Asandada genus of centipede serves well to immobilize their prey for easier consumption.
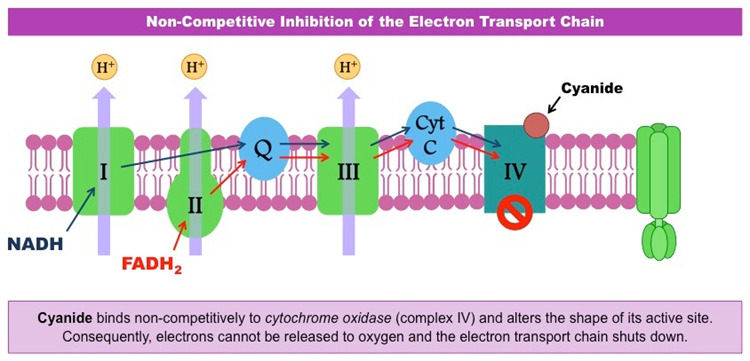
Fig. 31 Diagram of the electron transport step in cellular respiration and how hydrogen cyanide interferes with cytochrome oxidase to prevent oxygen utilization (BioNinja, n.d.).
Conclusion
The complex structure of claws in animals is necessary to accommodate their multivariate functions in different animals. The choice of the strong keratin protein group as a building block permits the claw to sustain large forces such as the body weight of the animals which use them while climbing. The keratin group’s strength is due to their intramolecular forces and how the keratin is woven together. The interweaving of keratin filaments into filament-matrix structures, as well as the strong presence of cysteine allowing for solid disulfide bonds; both contribute to the high toughness property of keratin claws. Claws possess useful physical properties provided by their structures. They can also be used to send signals to other animals through scent and pheromones. Isotopes present in the claws change depending on the animal’s environment, possibly causing changes within the properties of the claws, and enabling their tracking. Another case of more advanced use of chemistry within claws is the presence of venom in the platypus’ hind claws and the centipedes’ claws, which enable competition during mating and serve to dissuade predators, and paralyze or kill prey. The study of the chemistry of the claws furthers the understanding of the keratin protein group, while also inspiring biomimetic technology based on new proteins found in venomous animals. Furthermore, current research in the biomedical field is being done to apply insulin-like proteins from the duck-billed platypuses’ venom as a potential cure for type 1 diabetes. The further research of the pheromones and chemicals present on claws will help to localize species and track their movements and habits. Both animals in the ‘venomous claws’ section produce venom of interest with significant importance in medicine and bioengineering applications. Indeed, with protein engineering, one can make materials similar to keratin from scratch and use biomimicry to make carbon nanotubes, for example (Wang B. et al., 2016).
References
Africa Geographic Photographer of the Year. (2021). Cat fight: Leopard vs. caracal. Africa Geographic. https://africageographic.com/stories/cat-fight-leopard-vs-caracal/
Alibardi, L. (2021). Development, structure, and protein composition of reptilian claws and hypotheses of their evolution. Anat Rec, 304: 732– 757. https://doi.org/10.1002/ar.24515
Alibardi, L., Toni, M. and Dalla Valle, L. (2007). Hard cornification in reptilian epidermis in comparison to cornification in mammalian epidermis. Experimental Dermatology, 16: 961-976. https://doi-org.proxy3.library.mcgill.ca/10.1111/j.1600-0625.2007.00609.x
Annex Cat Rescue. (2021). Did you why a cat has dew claws on each foot? Facebook. https://www.facebook.com/AnnexCatRescue/photos/a.442658303648/10158309867818649/?epik=dj0yJnU9QlppQkJMQllUVERhUE44YkFnVjQ3aElieS1aSGt5amomcD0wJm49VUZSanJoLWUxS0pSajVrVTl6R1ZudyZ0PUFBQUFBR05WNnhZ
Biocyclopedia. (n.d.). Class chilopoda. Biocyclopedia | Study and Refer in subjects of Science (Biology and Chemistry) & Socialize. https://biocyclopedia.com/index/general_zoology/class_chilopoda.php
BioNinja. (n.d.). Enzyme inhibition. https://ib.bioninja.com.au/higher-level/topic-8-metabolism-cell/untitled-6/enzyme-inhibition.html
Brouw, B. (2020, June 17). Wide world of venom – the platypus. University of Melbourne. (Accessed October 24, 2022) https://biomedicalsciences.unimelb.edu.au/departments/department-of-biochemistry-and-pharmacology/engage/avru/blog/wide-world-of-venom-the-platypus
Bradshaw, R. A., Frazier, W. A., Pulliam, M. W., Szutowicz, A., Jeng, I., Hogue-Angeletti, R. A., Boyd, L. F., & Silverman, R. E. (1977). Structure-function relationships of nerve growth factor and insulin. Abstracts, 339. https://doi.org/10.1002/pro.5560031102
Bragulla, H. H., & Homberger, D. G. (2009). Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. Journal of Anatomy, 214(4), 516–559. https://doi.org/10.1111/j.1469-7580.2009.01066.x
Branco, A. C., Yoshikawa, F. S., Pietrobon, A. J., & Sato, M. N. (2018). Role of histamine in modulating the immune response and inflammation. Mediators of Inflammation, 2018, 1–10. https://doi.org/10.1155/2018/9524075
Branco, A. C., Yoshikawa, F. S., Pietrobon, A. J., & Sato, M. N. (2018). Role of histamine in modulating the immune response and inflammation. Mediators of Inflammation, 2018, 1–10. https://doi.org/10.1155/2018/9524075
Buxman, M. M., & Wuepper, K. D. (1975). Keratin cross-linking and epidermal transglutaminase. A review with observations on the histochemical and immunochemical localization of the enzyme. Journal of Investigative Dermatology, 65(1), 107–112. https://doi.org/10.1111/1523-1747.ep12598072
Candi, E., Tarcsa, E., Digiovanna, J. J., Compton, J. G., Elias, P. M., Marekov, L. N., & Steinert, P. M. (1998). A highly conserved lysine residue on the head domain of type II keratins is essential for the attachment of keratin intermediate filaments to the cornified cell envelope through isopeptide crosslinking by transglutaminases. Proceedings of the National Academy of Sciences, 95(5), 2067–2072. https://doi.org/10.1073/pnas.95.5.2067 Gregersen, E. (2022). Young’s modulus. Encyclopedia Britannica. https://www.britannica.com/science/Youngs-modulus
Centers for Disease Control and Prevention. (2021). Hydrogen cyanide. The National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/topics/hydrogen-cyanide/default.html#:~:text=Hydrogen%20cyanide%20(HCN)%20is%20a,Exposure%20can%20be%20fatal
Chilakamarry, C.R., Mahmood, S., Saffe, S.N.B.M. et al. (2021). Extraction and application of keratin from natural resources: a review. 3 Biotech 11: 220. https://doi.org/10.1007/s13205-021-02734-7
Cleveland Clinic. (2022, June 6). Sympathetic Nervous System (SNS). https://my.clevelandclinic.org/health/body/23262-sympathetic-nervous-system-sns-fight-or-flight#:~:text=Your%20sympathetic%20nervous%20system%20is%20best%20known%20for%20its%20role,your%20get%20out%20of%20danger
Crucho, C. I., Correia-da-Silva, P., Petrova, K. T., & Barros, T. (2015). Recent progress in the field of glycoconjugates. Carbohydrate Research, 402, 124–132. https://doi.org/https://doi.org/10.1016/j.carres.2014.10.005
Daya, P. (2022, August 19). What are isotopes? IAEA. (Accessed October 25, 2022) https://www.iaea.org/newscenter/news/what-are-isotopes
DePorter, T. L., & Elzerman, A. L. (2019). Common feline problem behaviors: Destructive scratching. Journal of Feline Medicine and Surgery, 21(3), 235-243. https://doi.org/10.1177/1098612×19831205
El-Shafie, H. A. F., & Faleiro, J. R. (2017). Semiochemicals and Their Potential Use in Pest Management. In (Ed.), Biological Control of Pest and Vector Insects. IntechOpen. https://doi.org/10.5772/66463
Engineering ToolBox, (2005). Stress, Strain and Young’s Modulus. https://www.engineeringtoolbox.com/stress-strain-d_950.html
Erickson, T. B. (2007). Arthropod Envenomation and parasitism. Wilderness Medicine, 947-982. https://doi.org/10.1016/b978-0-323-03228-5.50049-5
Ethier, D. M., Kyle, C. J., & Nocera, J. J. (2014). Tracking animal movement by comparing trace element signatures in claws to spatial variability of elements in soils. Science of The Total Environment, 468-469, 699–705. https://doi.org/10.1016/j.scitotenv.2013.08.091
Feng, X., & Coulombe, P. A. (2015). A role for disulfide bonding in keratin intermediate filament organization and dynamics in skin keratinocytes. Journal of Cell Biology, 209(1), 59–72. https://doi.org/10.1083/jcb.201408079
Feng, X., Zhang, H., Margolick, J. B., & Coulombe, P. A. (2013). Keratin intracellular concentration revisited: Implications for keratin function in surface epithelia. Journal of Investigative Dermatology, 133(3), 850–853. https://doi.org/10.1038/jid.2012.397
Fraser, R. D. B., MacRae, T. P., & Rogers, G. E.(1972). Keratins: Their composition, structure and biosynthesis. Bannerstone House.
Fraser, R. D. B., MACRAE, T., PARRY, D., & SUZUKI, E. (1971). The structure of feather keratin. Polymer, 12(1), 35–56. https://doi.org/10.1016/0032-3861(71)90011-5
Fraser, R. D. B., & Parry, D. A. D. (1996). The molecular structure of reptilian keratin. International Journal of Biological Macromolecules, 19(3), 207–211. https://doi.org/10.1016/0141-8130(96)01129-4
Fraser, R. D. B., & Parry, D. A. D. (2011). The structural basis of the filament-matrix texture in the avian/reptilian group of hard β-keratins. Journal of Structural Biology, 173(2), 391–405. https://doi.org/10.1016/j.jsb.2010.09.020
Gimesy, D. (2015). Platypus on the edge. Doug Gimesy Photography. Retrieved October 26, 2022, from http://gimesy.com/galleries/photo-documentary/platypus/
Gregersen, E. (2022). Young’s modulus. Encyclopedia Britannica. https://www.britannica.com/science/Youngs-modulus
Griffin, M., Casadio, R., & Bergamini, C. M. (2002). Transglutaminases: Nature’s biological glues. Biochemical Journal, 368(2), 377–396. https://doi.org/10.1042/bj20021234
Haylett, T., & Swart, L. S. (1969). Studies on the high-sulfur proteins of reduced merino wool. Textile Research Journal, 39(10), 917–929. https://doi.org/10.1177/004051756903901004
Herrmann, H., & Aebi, U. (2004). Intermediate filaments: Molecular structure, assembly mechanism, and integration into functionally distinct intracellular scaffolds. Annual Review of Biochemistry, 73(1), 749–789. https://doi.org/10.1146/annurev.biochem.73.011303.073823
Inglis, A. S., & Gillespie, J. M. (1965). High-sulphur proteins as a major cause of variation in sulphur content between α-keratins. Nature, 207(5003), 1293–1294. https://doi.org/10.1038/2071293a0
Interdigital cysts. (n.d.). HOOSIER BULLDOG RESCUE. https://www.hoosierbulldogrescue.com/interdigital-cysts.html
Irsay, L., Mandl, P., & Balint, P. V. (2010). Pitfalls of Gray-Scale Artifacts. R. J. Wakefield, & M. A. D’Agostino (Ed.), Essential Applications of Musculoskeletal Ultrasound in Rheumatology (pp. 35). Euler.
Jami, S., Erickson, A., Brierley, S., & Vetter, I. (2017). Pain-causing Venom peptides: Insights into sensory neuron pharmacology. Toxins, 10(1), 15. https://doi.org/10.3390/toxins10010015
Johnny B’s Pest Control. (2019). Johnny B’s Pest Control. https://www.johnnybpestcontrol.com/2019/06/18/the-huge-and-venomous-biting-centipede-species-that-you-do-not-want-to-find-within-your-home/
Kapama. (2012). Why do the big cats scratch the trees? Private Luxury Safari Game Lodge | Kapama Private Luxury Game Lodge. https://www.kapama.com/rangerblog/why-do-the-big-cats-scratch-the-trees/
Koehler, G., & Hobson, K. A. (2019). Tracking cats revisited: Placing terrestrial mammalian carnivores on Δ2H and Δ18o isoscapes. PLOS ONE, 14(9). https://doi.org/10.1371/journal.pone.0221876
Koh, J. M., Bansal, P. S., Torres, A. M., & Kuchel, P. W. (2009). Platypus Venom: Source of novel compounds. Australian Journal of Zoology, 57(4), 203. https://doi.org/10.1071/zo09040
Lee, R. T., & Lee, Y. C. (1997). Glycoproteins II. In New Comprehensive Biochemistry (pp. 1-652).
Lindley, H., & Haylett, T. (1967). Occurrence of the sequence in Keratins. Journal of Molecular Biology, 30(1), 63–67. https://doi.org/10.1016/0022-2836(67)90243-4
Marris, E. (2008). Cougar’s movements betrayed by Claw Analysis. Nature. https://doi.org/10.1038/news.2008.1021
Maschwitz, U., Lauschke, U., & Wurmli, M. (1979). Hydrogen cyanide-producing glands in a scolopender,Asanada n.sp. (Chilopoda, scolopendridae). Journal of Chemical Ecology, 5(6), 901-907. https://doi.org/10.1007/bf00990212
Mayo Clinic. (2020). Sweat glands. https://www.mayoclinic.org/diseases-conditions/hyperhidrosis/multimedia/sweat-glands/img-20007980
National Library of Medicine. (2002). Hydrogen cyanide: Acute exposure guideline levels – Acute exposure guideline levels for selected airborne chemicals. National Center for Biotechnology Information. https://www.ncbi.nlm.nih.gov/books/NBK207601/#:~:text=HCN%20is%20a%20systemic%20poison,arrest%2C%20and%20ultimately%2C%20death
Olympus. (2015). Molecular expressions: Science, optics & you – Olympus MIC-D: Oblique gallery – Centipede poison claws. Molecular Expressions: Images from the Microscope. https://micro.magnet.fsu.edu/optics/olympusmicd/galleries/oblique/centipedepoisonclaw.html
Pageat, P., & Gaultier, E. (2003). Current research in canine and feline pheromones. Veterinary Clinics of North America: Small Animal Practice, 33(2), 187-211. https://doi.org/10.1016/s0195-5616(02)00128-6
Parry, D. A. D. (2021). Structures of the ß-keratin filaments and keratin intermediate filaments in the epidermal appendages of birds and reptiles (sauropsids). Genes, 12(4), 591. https://doi.org/10.3390/genes12040591
Petruska J.C., Mendell L.M. (2009). Nerve Growth Factor. Academic Press. https://doi.org/10.1016/B978-008045046-9.00672-0
Pop Chart. (n.d.). The Entire Felidae Family. Facebook. https://www.facebook.com/popchart.co/
Rafferty, J. P. (2017). What’s the difference between venomous and poisonous? Encyclopedia Britannica. https://www.britannica.com/story/whats-the-difference-between-venomous-and-poisonous
Rogers, G. E. (1959). Electron microscope studies of hair and wool. Annals of the New York Academy of Sciences, 83(3), 378–399. https://doi.org/10.1111/j.1749-6632.1960.tb40914.x
Rogers, G. E., & Filshie, B. K. (1961). Some aspects of the ultrastructure of α-keratin, bacterial flagella, and feather keratin. Ultrastructure of Protein Fibers, 123–138. https://doi.org/10.1016/b978-1-4832-2838-9.50011-8
Sergiel, A., Naves, J., Kujawski, P., Maślak, R., Serwa, E., Ramos, D., Fernández-Gil, A., Revilla, E., Zwijacz-Kozica, T., Zięba, F., Painer, J., & Selva, N. (2017). Histological, chemical and behavioural evidence of pedal communication in Brown bears. Scientific Reports, 7(1). https://doi.org/10.1038/s41598-017-01136-1
Shewmon P. G. (2022). Testing mechanical properties. Encyclopedia Britannica. https://www.britannica.com/science/metallurgy/Testing-mechanical-properties#ref622779
Singh, D., Reed, S. R., Kimmitt, A. A., Alford, K. A., & Ketterson, E. D. (2019). Breeding at higher latitude as measured by stable isotope is associated with higher photoperiod threshold and delayed reproductive development in a songbird. BioRxiv. https://doi.org/10.1101/789008
Sorin, A., & Myers, P. (2001). Ornithorhynchidae (platypus). Animal Diversity Web. Retrieved October 26, 2022, from https://animaldiversity.org/accounts/Ornithorhynchidae/
Stankiewicz, M., Hamon, A., Benkhalifa, R., Kadziela, W., Hue, B., Lucas, S., Mebs, D., & Pelhate, M. (1999). Effects of a centipede venom fraction on insect nervous system, a native xenopus oocyte receptor and on an expressed drosophila muscarinic receptor. Toxicon, 37(10), 1431-1445. https://doi.org/10.1016/s0041-0101(99)00089-6
Steinert, P. M., Candi, E., Tarcsa, E., Marekov, L. N., Sette, M., Paci, M., Ciani, B., Guerrieri, P., & Melino, G. (1999). Transglutaminase crosslinking and structural studies of the human small proline rich 3 protein. Cell Death Differentiation, 6(9), 916–930. https://doi.org/10.1038/sj.cdd.4400568
Toni, M. (2008). Cytochemical and molecular characteristics of the process of cornification during feather morphogenesis. Progress in Histochemistry and Cytochemistry, 43(1), 1–69. https://doi.org/10.1016/j.proghi.2008.01.001
Van Hemert, C., Handel, C.M., Blake, J.E., Swor, R.M., & O’Hara, T.M. (2012). Microanatomy of passerine hard-cornified tissues: Beak and claw structure of the black-capped chickadee (Poecile atricapillus). J. Morphol., 273: 226-240. https://doi.org/10.1002/jmor.11023
Vincent, M. (2022, June 10). Reports of cougar near michaelis boulevard and 84 avenue. City of Grande Prairie. Retrieved October 27, 2022, from https://cityofgp.com/city-services/service-updates/reports-cougar-near-michaelis-boulevard-and-84-avenue
Vitale Shreve, K. R., & Udell, M. A. (2017). Stress, security, and scent: The influence of chemical signals on the social lives of domestic cats and implications for applied settings. Applied Animal Behaviour Science, 187, 69-76. https://doi.org/10.1016/j.applanim.2016.11.011
Wang, B., Yang, W., McKittrick, J., & Meyers, M. A. (2016). Keratin: Structure, mechanical properties, occurrence in biological organisms, and efforts at bioinspiration. Progress in Materials Science, 76, 229–318. https://doi.org/10.1016/j.pmatsci.2015.06.001
Whittington, C. M., Papenfuss, A. T., Locke, D. P., Mardis, E. R., Wilson, R. K., Abubucker, S., Mitreva, M., Wong, E. S. W., Hsu, A. L., Kuchel, P. W., Belov, K., & Warren, W. C. (2010). Novel Venom Gene Discovery in the platypus. Genome Biology, 11(9). https://doi.org/10.1186/gb-2010-11-9-r95
Wildlife Journal Junior. (2022). Chilopoda – Centipedes. New Hampshire’s PBS Station. https://nhpbs.org/wild/chilopoda.asp#:~:text=They%20can%20have%2012-100,capture%20and%20paralyze%20its%20prey
Windoffer, R., & Leube, R. E. (1999). Detection of cytokeratin dynamics by time-lapse fluorescence microscopy in living cells. Journal of Cell Science, 112(24), 4521–4534. https://doi.org/10.1242/jcs.112.24.4521
Windoffer, R., & Leube, R. E. (2004). Imaging of keratin dynamics during the cell cycle and in response to phosphatase inhibition. Intermediate Filament Cytoskeleton, 321–352. https://doi.org/10.1016/s0091-679x(04)78012-7
Wyld, J. A., & Brush, A. H. (1983). Keratin Diversity in the Reptilian Epidermis. The journal of experimental zoology, 225: 387-396. https://doi.org/10.1002/jez.1402250306