Abstract
Biological information storage and processing form the foundation for understanding organisms’ operation in nature. In this report, we examine the signaling and mathematical components of the skin from a portfolio of animals to illustrate their significance in governing the biomolecular and structural components in which they are intertwined. Particularly, we look at how mathematics dictates the pigment design of the integument via pattern formation, and how the design of the skin’s sensory mechanisms enables the functions of photoreception, thermoreception, and mechanoreception. Furthermore, we touch on how these designs enable natural solutions for mate selection, camouflage, niche construction, and predation. To conclude, we tie in the importance of these designs for organisms’ survival and suggest paths for further exploration or application of the mechanisms discussed.
Pattern Formation
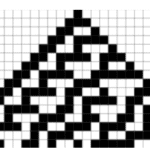
Turing’s paper The Chemical Basis of Morphogenesis proposed one of the best-known models to describe pattern formation — the reaction-diffusion (RD) model — which researchers have been exploring for over half a century. In this report, we will look at the RD model for skin pigment patterning in zebrafish Danio rerio (Fig. 1a). This organism is ideal to examine the biological implementation and mechanism of the model since:
(i) they have yellow and black stripes comprising of melanophores and xanthophores that after ablation — selectively destroying cells. In this case, xanthophores and melanophores are destroyed with a laser, — regenerate and self-organize into labyrinthine patterns suggestive of the Turing model;
(ii) there are a variety of known genetic manipulation techniques and mutant lines of the fish available to explore the biomolecular mechanism of pattern formation (Watanabe and Kondo, 2015).
Recently, another model for pattern formation has for the first time been observed in the ocellated — having eye-like markings of its skin — lizard Timon lepidus (Fig. 1b and c). Pattern formation in the ocellated lizard acts as a living cellular automaton (CA) which consists of discrete units (in this case scales) where the color of each scale depends on the color of the adjacent (neighboring) scales rather than a continuous concentration field of interacting and diffusing pigment cells or signals as suggested by the RD model (Manukyan et al., 2017). Furthermore, the connection of these two models of pattern formation from two scientific giants of the twentieth century can inform the discovery of biological mechanisms previously unknown in many organisms, leading to advances in evolutionary and morphological biology among others.

The self-emerging patterns described by Turing’s model rely on a pre-pattern or gradient of chemicals, deemed morphogens, enabling cell differentiation in a threshold dependent way and directing morphogenesis (Maini et al., 2012). To illustrate a mechanism for this idea, Turing proposed a reaction between two chemicals in which interaction and diffusion occur to cause an initially uniform mixture of chemicals to become non-uniform. In this case differences in the rate of diffusion of the two chemicals are necessary but not sufficient conditions for diffusion-driven instability — Turing’s counterintuitive idea that two stabilizing processes could result in instability leading to spatial self-organization of morphogens and ultimately pattern formation (Lacitignola, 2016). — The simplest model of diffusion driven instability can occur in the following system of partial differential equations:
{∂ω\over ∂t}=DΔ ⃗ω+F(ω)(1)
Turing’s analysis of this proposed model shows that with the right system kinetics the morphogens assume a steady state in the absence of diffusion and become unstable in its presence. In the above equation ω is a vector of concentrations of the two morphogens u(r,t) and v(r,t) at the spatial position r = (x,y) at time t. The diagonal matrix D contains constant diffusion coefficients, and F accounts for the reaction kinetics through the source terms f(u,v) and g(u,v). Delta stands for the Laplacian operator: (∂2/ ∂x2)+ (∂2/ ∂y2). Following Turing’s analysis, the homogeneous equilibrium Pe = ωe = (ue, ve) ensures that the reaction kinetics F(ωe) = 0 and the steady state’s behavior can be studied with small perturbations (δω) near ωe. Following this linear stability analysis, the Turing space where instability arises is found to be bounded by two inequalities (Lacitignola, 2016):
J ^e_{11} + J^e_{22} < 0(2)
J ^e_{11}J^e_{22} − J^e_{12}J^e_{21} > 0(3)
dJ^e_{11} + J^e_{22} > 0(4)
{(dJ^e_{11} + J^e_{22})^2\over 4d}>det(J(u_e, v_e))(5)
where J is the Jacobian matrix evaluated at the steady state Pe, and d is the diffusion coefficient chosen as a bifurcation parameter. Using linear stability analysis by studying small perturbations (δω) on the steady state is useful for:
(i) determining the bifurcation thresholds for diffusion driven instability;
(ii) identify the Turing space where Turing instabilities can occur;
(iii) approximating the characteristic length of the resulting patterns.
However, it does not tell us about the morphology of the patterns formed by diffusion driven instability (Lacitignola, 2016). To determine the generated pattern by the Turing instability, nonlinear bifurcation theory is applied to detect the bifurcation thresholds and relate the diffusion driven instability to morphological patterns such as spots, stripes, etc.
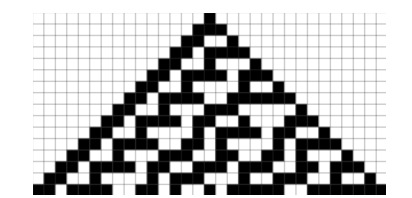
The second model of pattern formation, a Neumann CA (Fig. 2), is a mathematical idealization of a physical system. It is composed of a collection of elements called cells which each have states and are connected to other cells to form an n-dimensional lattice. Through discrete time steps and a set of local rules that specifies the state of a cell based on the states of neighboring cells in the previous time step, the CA evolves as the states of the cells are updated simultaneously over an arbitrary number of time steps (Wolfram, 1983). In this way, a CA is not only useful in modelling biological self-reproduction as Neumann first proposed, but also any physical system containing discrete elements that interact locally.
Connecting these mathematical models to the biological mechanisms of pattern formation requires answering the following question: what constitutes a pattern? As Turing suggested, a pattern can be thought of as a loss of homogeneity due to perturbations leading to spatial self-organization of morphogens that direct the formations of stripes, spots, labyrinths, etc. in the case of skin pigment patterning. Because of this, understanding the mathematics of the self-organizing systems that set up these nonhomogeneous pre-patterns of morphogens can provide insight into the biological mechanisms behind how macroscopic patterns form solely through the interactions of microscopic components (Widelitz et al., 2006).
In the Turing RD model, patterning begins with a homogenous distribution of cells, activators, and inhibitors in the absence of diffusion until random perturbations initiate their distribution into a spatial pattern that governs pattern formation in a deterministic way (Widelitz et al., 2006). Likewise, the CA model is also deterministic since the state of every cell depends on local rules; however, despite these mathematical models, there are still large variations between patterning in individuals of the same species or genus that possess very similar DNA. The reason for this can be inferred as follows: DNA is transcribed to RNA and proteins that build cells with unique characteristics. The interactions of these cells are therefore not identical because of their individual chemical and micro-environmental properties, although the cells and signaling molecules are similar. This means that the results of patterning are both deterministic as per the models and stochastic due to genetic and epigenetic factors (Widelitz et al., 2006). However, it is thought that combinations of the CA and RD patterning mechanisms likely results in the formation of more complex patterns that are relatively robust to genetic and epigenetic variation.
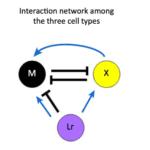
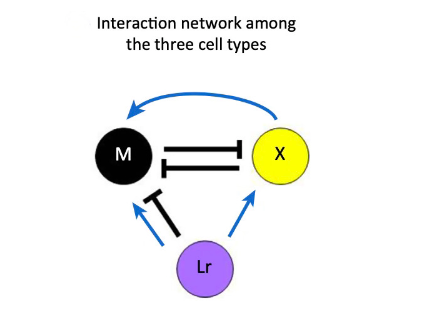
One interesting case of pigment patterning that follows the RD model, despite having a different biochemical mechanism for pattern formation than proposed, is found in zebrafish. Zebrafish have stripes on their bodies and fins composed of melanophores and xanthophores. Additionally, in the body trunk of the zebrafish, iridophores are found to colocalize with the previously mentioned pigment-containing cells (Watanabe and Kondo, 2015). In mutant lines that lack one of the three types of chromatophores, patterning is lost, suggesting that the pattern results from interactions between these pigment cells. In a study by Parichy and Turner (2003), they found that once xanthophores in the zebrafish were ablated, melanophores died shortly after, indicating that melanophores require continuous signaling for their survival. Nakamasu et al. (2009) similarly ablated various pigment cells in zebrafish to determine the effects of their loss on neighboring cells. They found that xanthophores eliminate neighboring melanophores in a competition for survival while they also enhance survival and development through long-range signaling. The results of this study were used to derive distance-dependent feedback loops between pigment cells by Wantanabe and Kondo. The competition between melanophores and xanthophores is double inhibitory, acting as a positive feedback loop while a second long-range feedback loop results from melanophores inhibiting local xanthophores as xanthophores have a long-range positive effect on melanophores (Watanabe and Kondo, 2015).
These two feedback loops (Fig. 3) are consistent with the Turing model’s necessary condition for long-range inhibition and short-range activation despite the absence of diffusion. Instead of diffusion, xanthophores are found to extend dendrites toward melanophores, causing them to move away angularly while the xanthophores follow them in a spiraling chasing mechanism, distinct from the mechanism postulated by Turing. Despite not involving diffusion, the mechanism is mathematically equivalent to the RD model and has prompted the generation of another model (Bullara and Decker, 2015) to account for immobile agents that can generate Turing patterns and undergo Turing instabilities to reproduce patterns. Additionally, the lack of this chasing mechanism in zebrafish mutants without patterns further suggests the mechanism’s role in pattern formation where the combination of dendrites and long-range projections from melanophores to xanthophores mimics the effect of fast and slow diffusing molecules respectively.
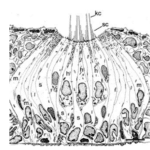
In the case of the ocellated lizard Timon Lepidus, its scales change from brown to a labyrinth of green and black during development from juvenile to adult. During its lifetime, each scale probabilistically flips between the two colors, granting its pattern recognition as a living CA (Edelstein- Keshet, 2017). The CA, in the case of the ocellated lizard, comprises scales as mesoscopic elements whose color is determined by microscopic interactions of pigment cells. To explain how a discrete CA arises from the continuous Turing RD model, the researchers looked at the morphology of the lizard’s scales. Since the superficial Oberhautchen along with the other structural components of the lizard’s integument thins as the scale forms the hinge joint, researchers assumed that the three-dimensional structure creates a partial barrier between the scales, allowing each scale to be considered a discrete unit that assumes a homogeneous color (black or green on the dorsal scales) (Manukyan et al., 2017). Furthermore, simulations show that to reproduce the observed pattern, the color of a scale must depend on the color of its neighbors and can probabilistically shift from black to green or vice versa depending on the state of neighboring scales. In this way, the patterning mechanism is analogous to a CA where over time the development of the pattern follows a set of local rules to determine the state of every scale (Edelstein-Keshet, 2017). Additionally, the authors confirmed that the discrete CA mechanism arises from the continuous RD model by superposing it on a lattice where at the borders, diffusion is drastically reduced (by about 80 %) and running a computer simulation to generate pattern formations that correlate well with the observed pattern in the ocellated lizard.
Photoreception
Animals detect light using sensors known as photoreceptor cells. These cells are found not only in the retinas of animal eyes, but also outside the eyes, where they are often called the extraocular photoreceptor cells (Ramirez et al., 2011). This section of the report explores the receptor mechanisms of these cells within animal skin, where they confer a particular form of photoreception known as the dermal light sense (Millott, 1968). The skin is a particularly important site of photoreception as it is the first part of the body to receive light (Kelley and Davies, 2016).
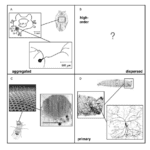
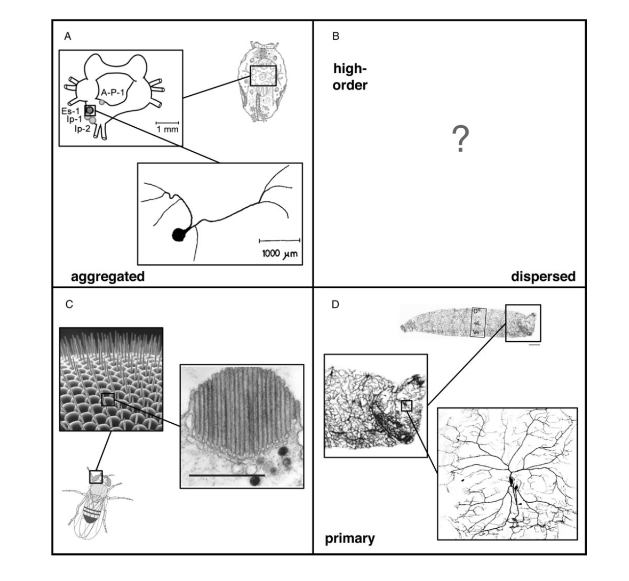
Structurally, all photoreceptor cells can be classified using two axes, the first axis describing the extent to which photoreceptor cells are distributed (aggregated versus dispersed), and the second axis identifying receptors as either first or higher-order neurons (Ramirez et al., 2011). Taken together, these two axes for receptor classification produce four separate quadrants (Fig. 4). The receptors that confer the dermal light sense belong in the fourth quadrant, which contains cells that are dispersed and are first-order neurons. In essence, photoreceptor cells of the skin can be referred more specifically as dispersed photoreceptor cells (Ramirez et al., 2011).
In understanding the operations involved in dispersed photoreception, one can refer to the molecular basis of light sensitivity in animals. Although molecular synthesis is forged primarily from data on retinal photoreceptor cells, it is safe to assume that all photoreceptor cells, including the dispersed photoreceptor cells, are considered in the same framework (Ramirez et al., 2011). Photoreceptor cells can be categorized by the degree of similarity between the molecular components that make up their phototransduction cascades — process by which the absorbed light triggers an electrical response (the neural signal) in rods and cones. (Luo et al., 2009). — Phototransduction begins as a photon of light being absorbed by a visual pigment that consists of a chromophore — molecule in a given material that absorbs particular wavelengths of visible light, and in doing so confers color on the material (Millington, 2009). — This chromophore is bound to a seven transmembrane domain G-protein — proteins that are involved during signal transduction and signal amplifications through their interaction with protein coupled receptors, thus modulating the events of the downstream effectors (Jones and Assmann, 2004) — coupled receptor known as an opsin (Ramirez et al., 2011) Opsins can be categorized into four separate clades defined by the G-protein with which they interact. The resulting categories include Gt-opsins, Gq-opsins, Gs-opsins, and Go-opsins. The four opsin clades are each associated with distinct sets of downstream secondary messengers —small intracellular molecules that mediate the effects of first messengers, i.e., neurotransmitters and hormones (Meyer, 1997) — and ion channels. For example, Gt-opsins activate transducin, which signals through a cyclic nucleotide second messenger that closes cyclic nucleotide gated (CNG) ion channels (Fu and Yau, 2007). In contrast, Gq-opsins involve the secondary messenger inositol triphosphate that leads to intracellular calcium release and the opening of transient receptor potential cation (TRPC) channels (Hardie, 2001). The distinct phototransduction cascades contribute to an understanding of variation in photoreceptor cell physiology in different animals and their body parts. Specifically, the state change of the ion channel following phototransduction changes the membrane potential of the cell while the direction of the voltage change depends on the type of phototransduction pathway involved (Ramirez et al., 2011). Hence, every photoreceptor cell utilizes its distinct phototransduction cascade, providing its unique molecular solution for detecting light.
The underlying mechanisms of light detection by the dispersed photoreceptor cells are critical to animal behavior and survival. In poikilothermic animals — animals whose internal temperature varies considerably, — non-visual photoreceptions can occur in chromatophores (Kelley and Davies, 2016). Chromatophores contain the pigments responsible for both generating and mediating the body coloration of amphibians, reptiles, insects, cephalopods, and other poikilothermic animals (Parker, 2012; Bagnara and Hadley, 1973; Umbers et al., 2014, all as cited in Kelley and Davies, 2016) with the general exception of mammalian vertebrates. We examine the diversity and sensitivity of dermal photoreception in different animals and their functional roles in mediating animal behavior.
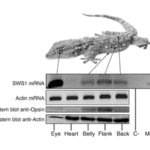
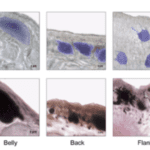
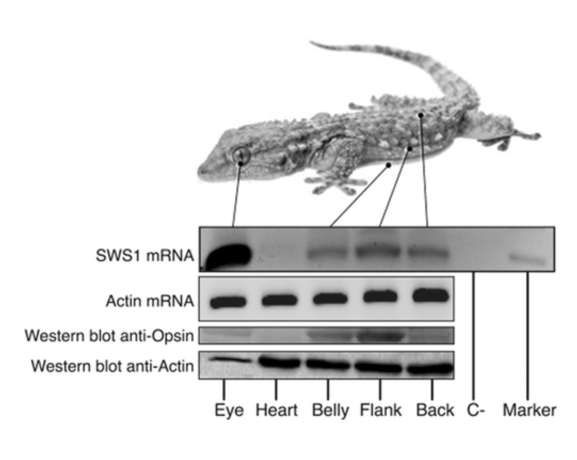
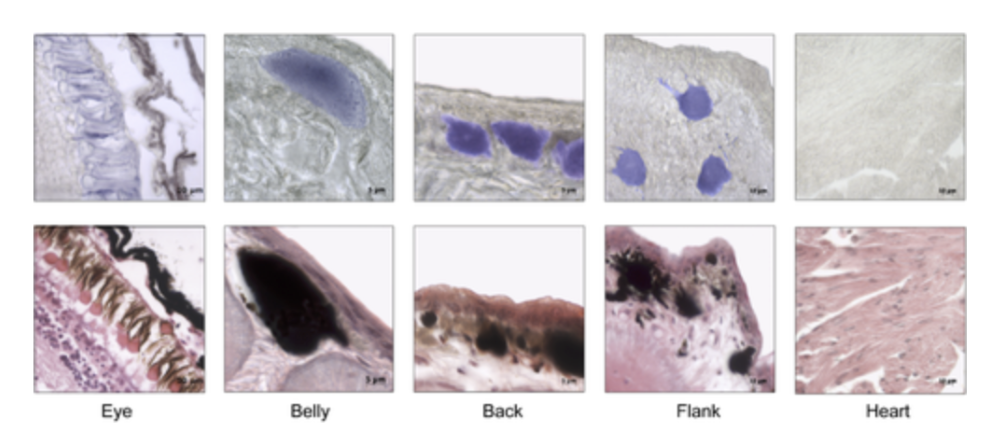
The cutaneous photosensitivity known for the class Reptilia is the direct response of melanophores to light that supplements neural and hormonal control of color change in the skin (Parker, 2012). In the case of the moorish gecko Tarentola mauritanica, its skin melanophores may activate a process of color change autonomously with light acting on the skin melanophores (Fulgione et al., 2014). This yields evidence of crypsis mediated by dermal light sensitivity in reptiles. For T.mauritanica, the darkening in its skin color can occur as a response that involves the activation of melanophores either via the alpha-melanophore– stimulating hormone (a-MSH) or under nervous control. On the other hand, it can also be a local response exerted directly by melanophores (Fulgione et al., 2014). The study by Fulgione and his team in 2014 explores how the moorish gecko T.mauritanica obtains and processes information on background shading to modify its body color. According to the study, the darkening in the T.mauritanica skin does not involve the activation of melanin production in melanophores by a-MSH, which eliminates the role of hormonal control in the animal’s coloration. Furthermore, the study tested for gene expression of photosensitive protein SWS1 (short wavelength-sensitive opsin) in the gecko through semi-quantitative reverse transcriptase polymerase chain reaction (RT-PCR) on SWS1 mRNA (Kojima et al., 1992; Loew, 1994). SWS1 mRNA was observed in skin tissues taken from T.mauritanica belly, back and flanks. In particular, the team observed distinctively higher levels of opsin-protein in the flanks than elsewhere (Fig. 5). The result was further borne out by immunohistochemistry analysis which showed melanophores in the skin are rich in melanin (Fig. 6), suggesting that the moorish gecko’s melanophores are light sensitive and can therefore be held responsible for skin darkening (Fulgione et al., 2014). Likewise, the study indicates the surrounding light is perceived by the animal’s skin receptors, irrespective of circulating levels of a-MSH. It also provides evidence for opsin expression in the skin, both at mRNA and protein levels, strongly linked to melanophores in the dermis. T.mauritanica skin acts as both a receptor and effector of skin darkening, independently from visual inputs. In other words, the gecko’s skin is photosensitive and melanophores may activate skin darkening autonomously, which produces rapid modification of body color for camouflage or communication.
In comparison, cephalopod skin is also capable of processing light through the skin through their extraocular photoreceptors to produce dramatic color and pattern changes. There are several photo-transduction components in cephalopod skin, where they serve a distributed light sensing system. More specifically, the presence of rhodopsin, Gqα and sTRP channels (Kingston et al., 2015) suggest the presence of dermal light sensing in cephalopod skin because all are components that can serve extraocular photoreception, duplicating their function in retinal photoreceptors. Most significantly, antibody labeling of rhodopsin, retinochrome and Gqα in the highly folded membranes, radial muscle fibers and sheath cells of chromatophore organs suggests a photoreceptive function (Kingston et al., 2015). In cephalopod skin, the chromatophore photoreceptors act as a local system affecting individual cells, within a broader system of cells immediately adjacent to the photoreceptive cell or in coordination with the central nervous system. Thus, sensing by chromatophores could alter a single chromatophore component (pigment cell membrane, radial muscles, sheath cell), or the entire organ, to make it more or less likely to change its state of expansion or retraction (Kingston et al., 2015). In this case, individual chromatophore organs would respond to light locally. Alternatively, local receptors could communicate with one another among the chromatophores via the gap junctions that exist between adjacent muscle cells and allow electrical interactions (Cloney and Florey, 1968), so that small regional areas of chromatophores would respond to light stimuli as a unit. Finally, phototransduction induced signals produced by chromatophores may travel by afferent nerve fibers to the central nervous system to provide additional information about the environment in which the cephalopod exists. This information itself could serve ultimately to affect chromatophore behavior (Kingston et al., 2015). Likewise, visual opsins and other components of visual phototransduction exist in the cephalopod skin, and specifically in their chromatophore organs, indicating its potential of dermal photoreception.
Thermoreception
The skin or the surface of organisms acts as an interface between the organism and its surroundings, governing the interaction between these two internal and external environments. Therefore, the thermosensory ability of the skin is also a crucial function in addition to photoreception. It plays a key role in processes like thermoregulation where homeostasis regulates and maintains a constant body temperature for endotherms like mammals; it also modulates behavioral output responses to different thermal stimuli of ectotherms like nematodes. The neuronal thermoresponse in animals can be categorized into two major types: innocuous temperature detection — harmless temperature detection, usually for finding food, thermoregulation and locating habitable environments (McKemy, 2007) — and nociception — dangerous and potentially damaging temperature detection, pain receptors use to avoid dangerous and harmful environmental conditions — (McKemy, 2007). This section of the report aims to explore innocuous thermal detection seen in nematodes (i.e., roundworms), specifically the free-living nematode Caenorhabditis elegans and parasitic nematodes, and investigate the neural circuitry behind these responses.
Thermoreception in nematodes is evidence of nature’s successful design for an effective thermosensory model. Nematodes have been around for millennia, having gone through numerous evolutionary stages; they are abundant and can be found everywhere in all sorts of habitats like in soil and in humans. The size of these organisms is such that their surface area to volume ratio is large, and thus nematode interactions with the environment is done primarily through the surface of the entire worm, as a result we see little distinction between their skin and body. This gives rise to unique and interesting thermoreception capabilities, hence the reason for exploring the “skin” of these roundworms (McKemy, 2007).
Furthermore, these ectotherms, lacking both eyes and a homeostatic internal temperature regulatory system, rely on their sensory receptors and neurons to navigate towards regions with favorable conditions ensuring their survival. In addition, thermal detection is also used to avoid dangerous and damaging temperature-extreme environments. For parasitic nematodes in particular, it is vital to be able to sense their host and navigate to facilitate host-attachment. This is to ensure that they acquire a host during their infective larval stage (McKemy, 2007), as not all parasitic nematodes may survive as free-living organisms in the absence of a host (Perry et al., 2011). Therefore, thermoreception seen in these animals is a great place to study effective thermosensory models in nature.
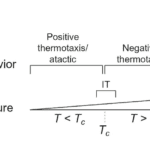
C. elegans, in addition to their use of a single sensory neuron AFD to regulate navigation, also utilizes a complex learning and memory model where prior exposure to ideal environmental conditions drives these nematodes to gravitate towards temperate zones (Kimura et al., 2004). Their behavioral responses are generated from minor flux in external temperature; as demonstrated in the study by Goodman and Sengupta (2018) the sensitivity of the worms’ receptors can detect change as little as 0.05 °C over a 10 degrees temperature range. Thermal input through nerve endings at the surface results in one of the four behavioral outputs: Positive thermotaxis (PT), negative thermotaxis (NT), isothermal tracking (IT) and atactic (i.e., insensitive to thermal gradient) behavior (Goodman and Sengupta, 2018). Shown in the study, an NT response is triggered when C. elegans are placed in a thermal gradient at a temperature greater than the temperature the worms were previously exposed to (TC). At temperatures lower than TC, the worms exhibit a PT navigational behavior or are atactic, and at T=TC ± ∼2°C they exhibit IT (Fig. 7).
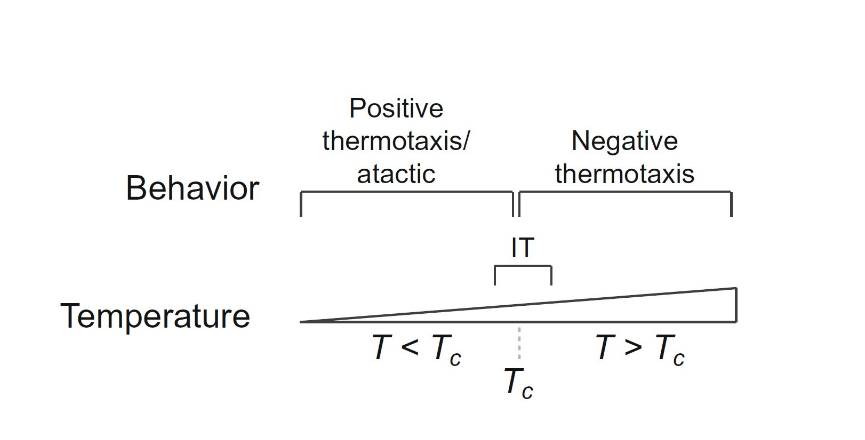
Interestingly, the threshold TC is dynamic and changes according to previous exposure of C. elegans to this environment (Clark et al., 2006) and correlates it to how well-fed they were in that condition (Goodman and Sengupta, 2018), i.e., if these animals were in a well-fed condition under a certain ambient temperature then this would become their new threshold TC and behavioral responses (PT, NT, IT) will all be shifted to correspond with this new TC temperature. This feedback mechanism resets TC after 3 -5 hours and updates the system’s threshold to constantly react to external changes (Bryant et al., 2018). Thus, the combination of the feedback mechanism and navigational behavioral response is a great strategy that worms implement to survive in a versatile and constantly fluctuating soil environment to seek ideal regions of soil (where there is access to food source and allows for growth). To further reinforce this, Goodman and Sengupta (2018) showed that when C. elegans were starved in a certain ambient temperature they displayed atactic behaviors and showed no changes in TC. Therefore, C. elegans seem to “remember” a certain temperature range when they are well-fed and use this as a guide for navigational behaviors to seek out similar regions with the help of their AFD sensory neuron and its feedback mechanism that constantly updates TC. AFD neurons are activated by fluctuations in thermal input as opposed to input of a constant and prolonged thermal environment (Goodman and Sengupta, 2018). A warming temperature change will cause the intracellular calcium concentration to increase and vice versa for cooling, a bidirectional response (Clark et al., 2006).
Parasitic nematodes also rely on thermoreception to similarly seek ideal conditions. However, unlike C. elegans these nematodes rely on a host and thus thermoreception found in these worms are instead modified to facilitate host-seeking behaviors. The life cycle of parasitic nematodes involves a developmental period called the third-larval stage (iL3) where these worms are infective and are effectively seeking a host (Bryant et al., 2018). iL3 nematodes are found to also exhibit NT and PT behaviors and utilizes learning and memory to reset a threshold temperature TC in addition to a preferred temperature TH. Seen in the two human-parasitic nematodes A. ceylanicum and S. stercoralis, A. ceylanicum has TH closer to the human body temperature of around 38 °C whilst S. stercoralis preferred higher temperatures nearing 40 °C (Bryant et al., 2018). Most mammalian-parasites have an observed TH higher than that of mammalian skin temperature to trigger a stronger drive towards the heat source when detection of temperatures is below TH and closer to host temperature (Bryant and Hallem, 2018). This maximizes the chance of seeking a host and triggering favorable behaviors to invade a host before iL3s pass their infective stage.
Observed in the study was the effect of the external temperature gradient on the crawling speed of the worms in addition to the change in direction differing from C. elegans; a steeper thermal gradient resulted in a faster speed and a shallow or isothermal environment displayed slower crawling speeds. Another noticeable difference lies in the fact that a thermal gradient in a cooler temperature range will result in faster crawling speeds than in a warmer range (Bryant et al., 2018). Furthermore, as these iL3s approach regions with temperature nearing TH they change gear and switch from using long-range navigation to local search possibly to increase the chance of attaching onto the host (Gang and Hallem, 2016). The switch between PT to NT and long-range to local range is a lot more complicated for parasitic nematodes as it is extremely important for them to be able to differentiate environmental heat sources from host thermal cues. When mammalian-parasitic iL3s approach regions with temperature near TH, host invasion behaviors are also triggered, an example is nictation where the worm erects itself upright on its tail and waves its head to increase the chance of attaching to the host and skin penetration (Bryant and Hallem, 2018). Therefore, due to the need to seek a host, parasitic nematodes have a more specialized thermosensory response than C. elegans as they are not free-living and rely heavily on being able to occupy a host to ensure survival.
Mechanoreception
While photoreception and thermoreception are useful means for the detection of electromagnetic and thermal stimuli, detection of mechanical stimuli is also useful to many organisms. The lateral line system of fish is a skin-based system of mechanoreception which responds to hydrodynamic stimuli (water movements). Such stimuli can include large-scale ambient water motions, or local flow perturbations created by animate or inanimate objects. The lateral line system is useful to fish in many respects. It is used for detection of prey, avoidance of predators and obstacles, and movement. For example, in the blind Mexican cavefish (Astyanax mexicanus) the lateral line system replaces sight. Approaching obstacles create predictable changes in the self-generated field of turbulence around the body of a fish. A. mexicanus takes advantage of this to avoid obstacles (Montgomery et al., “Sensory Ecology and Neuroethology of the Lateral Line”). The lateral line, like any sensory system, is exposed to noise, and it must be able to differentiate useful stimuli from this noise. It is of particular interest to see how this is accomplished, but the structure and basic operation of the lateral line must first be understood.
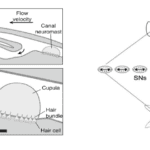
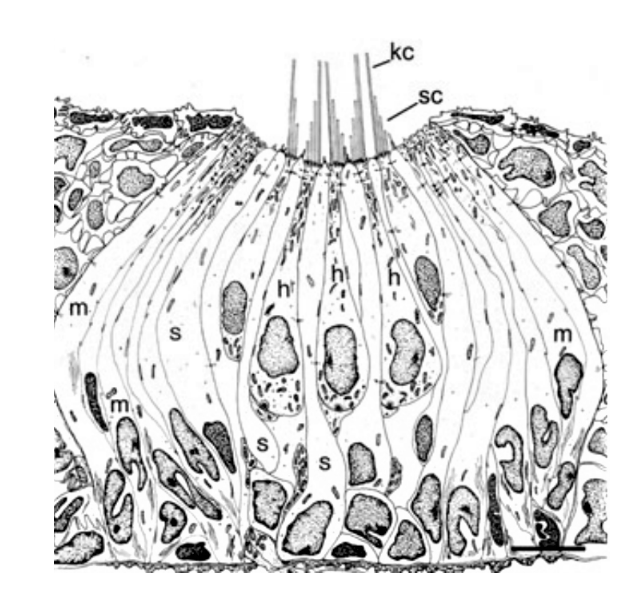
Sensory receptors called neuromasts are the fundamental components of the lateral line. They are found in the skin of the fish, or integrated into canals underlying the skin, and are dispersed throughout the body. The neuromasts themselves contain sensory hair cells which detect hydrodynamic stimuli. Each neuromast contains hair bundles, and each individual bundle has a specific orientation. Bundles with opposing orientation are found within a neuromast and define the neuromast’s axis of best sensitivity. A given hair bundle is composed of a single kinocillium and multiple shorter stereocilia located to one side of the kinocilium. The neuromast’s hair bundles are not in direct contact with water but are covered with a sphere or cylinder of gelatinous material called cupula. This structure is the biomechanical interface between neuromasts and the surrounding environment, its base covers the entire neuromast. Two types of non-sensory cells can be found in the neuromast; mantle and support cells (Fig. 8).
Neuromasts are innervated by afferent and efferent neurons. Within a neuromast, an afferent fiber innervates multiple hair bundles of the same orientation while bundles of the opposite orientation are innervated by a different neuron. Efferent neurons innervate multiple hair bundles regardless of their orientation. Two types of neuromasts are found in the lateral line: superficial neuromasts (SNs) and canal neuromasts (CNs). Superficial neuromasts are embedded in the exterior surface of the skin. Canal neuromasts are found in a network of canals which are part of the lateral line system and are found under the skin (Fig. 9a). The canals of the lateral line go from head-to-tail and from dorsal to ventral regions, and the linear arrays of superficial neuromasts generally follow the canals (Fig. 9b). The CNs are much larger than the SNs, and typically contain hundreds or thousands of hair cells while SNs usually contain around 10 hair cells. Innervation varies between CNs and SNs; an afferent fiber can innervate multiple SNs but rarely innervates more than one CN. There is also no evidence of afferent neurons innervating SNs and CNs simultaneously. A given efferent neuron can, however, be found innervating multiple SNs and CNs (Webb, 2014; Chagnaud and Coombs, 2014). The differences between CNs and SNs are of great importance to the operation of the lateral line system and to noise reduction.
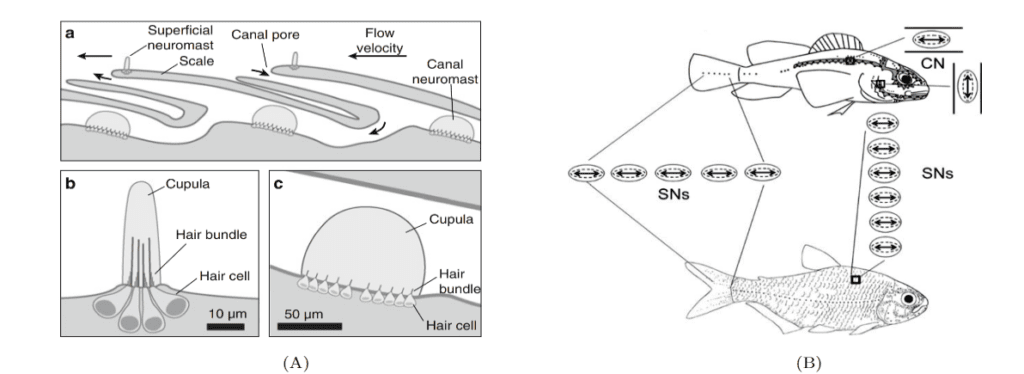
As water flows over a neuromast, it creates drag on the cupula. The neuromast’s hair bundles will bend in response to this drag force. This bending causes a change in membrane potential of the hair cells and a subsequent release of neurotransmitters. As a result, there is an increase in the firing rate of afferent neurons innervating the hair cells. A deflection of the kinocilium away from stereocilia causes excitatory responses, whereas a deflection of the kinocilium towards the stereocilia causes an inhibitory response. While the fish’s surface is always exposed to water flow, the lateral line canals only experience water flows when there is a pressure difference between two pores of a canal. For a fluid, changes in the pressure gradient will typically result in changes in acceleration, thus CNs are classified as acceleration detectors. SNs are not selectively sensitive to pressure gradients and are classified as velocity detectors. Using their lateral line, fish can determine the position of an object relative to them, the size and shape of the object, and its direction and speed if it is moving. However, for this to be possible, noise must be eliminated (McHenry and Liao, 2014; Bleckmann and Zelick, 2009).
Two principal sources of hydrodynamic noise include self-generated turbulence (caused by the fish’s own movements), or ambient water currents (flow of a river, waves, etc.). The simplest way in which the fish can deal with noise is to sit still, or glide instead of swimming. Some fish exhibit this behavior when hunting, though it is only of use for self-generated noise. Another mechanism of noise reduction is inherent to the differences between SNs and CNs. SNs are sensitive to unidirectional flows as well as low-frequency oscillating water flows up to tens of Hertz. CNs are sensitive to oscillating flows from 10- 500Hz. This difference is likely due to structural distinctions between CNs and SNs, as well as differing properties of their sensory hair cells. Large-scale ambient water flows, or self-generated flows usually occur at lower frequencies. CNs can thus “ignore” these lower frequencies and may detect higher frequency stimuli generated by the movement of other animals. This variation in receptor sensitivity for different stimulus frequencies is defined as the frequency response. For a given receptor, this parameter can be modelled mathematically using a transfer function. These functions are useful tools for describing input- output relationships (Hambley, 2011); in this case the function describes the relationship between stimulus and receptor response. A Fourier transform of a signal in the time domain (in this case the signal is a hydrodynamic stimulus) will yield a result in the frequency domain. By evaluating the transfer function for the frequencies present in the signal, one can get an idea of a sensor’s response to various frequencies. The canal system itself acts as a noise filter, reducing sensitivity to low frequencies. This is because viscous forces predominate at low frequencies. The velocity of water close to the canal wall is thus reduced by drag and has a reduced effect on CNs. Noise can also be reduced during processing of lateral line information by the nervous system. For example, two receptors may be exposed to a common noise, while only one receptor is exposed to a stimulus. If the responses generated by these receptors are compared, it is possible to “extract” only the useful stimulus. The fish may also have an idea of the afferent input resulting from its own movements and could use this knowledge to filter out said input. Finally, efferent innervation of neuromasts is also involved in noise reduction. The efferent system may modulate the sensitivity of hair cells in neuromasts and is proposed to function in two distinct modes: feedback and feed forward mode. In feedback mode, the system reacts to overstimulating inputs and reduces hair cell sensitivity. In feed forward mode, the system responds proactively; if another sense detects a potential stimulus, hair cell sensitivity is reduced (Montgomery et al., “Behavior and physiology of mechanoreception”; van Netten and McHenry, 2014; Chagnaud and Coombs, 2014).
The stimuli to which the lateral line system is subjected can be very complex. This has resulted in the evolution of an elaborate mechanosensory system which can deal with such levels of complexity. It is clear that noise reduction mechanisms are crucial for making sense of the hydrodynamic stimuli experienced by the fish, and that such mechanisms are empowered by the complex design of the lateral line system.
Conclusion
In nature, pattern formation serves to enable several critical functions for an organism’s survival including camouflage, thermoregulation, and sexual selection. By exploring two mathematical models and how they govern pigment pattern formation in zebrafish and the ocellated lizard, we have presented a basis with the power to inform the discovery of new molecular mechanisms, which in turn can inform mathematical biology. As such, molecular biology and mathematics operate in a feedback loop to aid discovery into the incredible process of pattern formation with significant implications in the area of developmental biology. Furthermore, better models of pattern formation could be usefully applied and tuned to biomimetic applications such as medical imaging software that better predicts the growth of melanomas using a cellular automaton.
Animals detect light using sensors known as photoreceptor cells. Best known from the retinas of animal eyes, photoreceptor cells also provide a significant function on the skin of some animals, where they detect light to mediate behaviors like change in skin color that are important for survival. Regulatory independence from ocular photoreception is advantageous for rapid dermal changes in color, due to the lack of requirement of complex neural or endocrine signaling pathways. In addition, direct dermal photosensitivity can supplement any photic input received by the retina and provide luminance information in regions outside the field of view or outside the spectral range of the visual photoreceptors. This is particularly important when the head of the animal is hidden or buried, but other parts of the body are exposed to environmental light (Kelley et al., 2016). The ability to detect changes in environmental light also provides animals with important temporal information about their surroundings that can be used to regulate activities such as foraging and reproductive behaviors, as well as regulating the cyclic changes in body coloration (Caswell, 1950). In complex organisms such as cephalopods, dermal photosensitivity allows rapid adjustment in coloration for both social signaling and camouflage, thereby providing optimal patterning despite not possessing classical ocular color vision (Mäthger et al., 2010). Light detection by photoreceptors present in particularly vulnerable body parts, such as the tails of sea snakes, aids their ability to detect dark crevices to remain concealed from predators (Zimmerman and Heatwole, 1990). Whichever behaviors are exhibited, individual chromatophores may function and sense light independently, or operate in conjunction with nearby receptors to provide regionalized light detection, thereby providing supplementary information to that received by the retina.
Demonstrated above is the importance of thermoreception for organisms. Although not discussed in this report is the role of thermoreception in thermoregulation to maintain a constant body temperature for endotherms, this is another vital mechanism that these animals rely on to protect their internal metabolic activities. This negative feedback loop is analogous to our very familiar example of the thermostat regulating the temperature of our house during the winter. Therefore, the mechanism and modelling of thermoreception in endotherms is shown to have very useful applications, thus suggesting that thermoreception in ectotherms as discussed above could also be of great potential for applicational use. Nematodes are fascinating creatures being able to navigate without eyes and sense their soil environment for zones of adequate food and nutrients. Being one of the oldest creatures on Earth and still thriving in nature these roundworms have developed a thermoreception sensory model that is worth investigating. Free-living nematodes like C. elegans have been greatly studied and these incredible findings have shed light into the “learning” and “memory” capability of these worms. Although parasitic nematodes have been found to display similar mechanisms, being a specialist species, these nematodes have a more complex model to facilitate their host-seeking needs. Therefore, further investigation into thermoreception and its regulatory responses of parasitic nematodes may bring about innovative discoveries and possible biomimetic applications.
The structural and functional difference between CNs and SNs is one of the intricacies of the lateral line system which makes noise reduction possible. This is a good example of how seemingly minor design elements can have important repercussions for the operation of a system. A complementary relationship exists between SNs and CNs which allows for the filtering out of noise. This relationship is a direct result of the structural characteristics of CNs and SNs, and of their placement within the lateral line system. This relationship is also crucial for the general function of the lateral line system. For example, SNs has an important role in the mediation of rheotactic behaviors in slow currents. This is caused by the innervation of multiple SNs by individual afferent fibers. Because of this, irregularities in a flow field are averaged out (input from multiple receptors is averaged), and the SNs can better detect unidirectional stimuli (which occur in water currents). In contrast, a single afferent fiber will not innervate more than one CN. Thus, irregularities in the flow field stand out when detected by CNs. Such irregularities can occur in the form of abrupt movements of prey, indicating that CNs are more suited for prey localization. For a more profound insight into the relationship between SNs and CNs, and how it affects general lateral line operation, The Lateral Line System is a highly recommended volume. It also explores other interesting subjects, such as the organization of the lateral line in the central nervous system.
Our investigation of signaling and pattern formation in the skin has given us a deeper understanding of how such a complex organ can be viewed from an engineering and design perspective. The systems explored above are made up of many moving parts which interact in complex ways to allow the system to fulfil its functions. We explored how the interactions of pigment cells produce skin patterns and how these interactions can be modelled mathematically. We gained an understanding of how various molecular components interact to allow for photoreception. We also learned how the thermoreception system of nematodes is designed to be adaptive and respond differently to changing temperatures. Finally, we saw how the lateral line system of fish is designed to reduce hydrodynamic noise. Research into these various topics has shown us that the designs of nature are highly complex. Understanding them can provide valuable insight for engineers looking to create designs for biological applications of surfaces, or to mimic surface interactions of life through their designs.
References
BLECKMANN, H., & ZELICK, R. (2009). Lateral line system of fish. Integrative Zoology, 4(1), 13-25. doi:https://doi.org/10.1111/j.1749-4877.2008.00131.x
Bryant, A. S., & Hallem, E. A. (2018). Temperature-dependent behaviors of parasitic helminths. Neuroscience Letters, 687, 290-303. doi:10.1016/j.neulet.2018.10.023
Bryant, A. S., Ruiz, F., Gang, S. S., Castelletto, M. L., Lopez, J. B., & Hallem, E. A. (2018). A Critical Role for Thermosensation in Host Seeking by Skin-Penetrating Nematodes. Current Biology, 28(14), 2338-2347.e2336. doi:10.1016/j.cub.2018.05.063
Bullara, D., & De Decker, Y. (2015). Pigment cell movement is not required for generation of Turing patterns in zebrafish skin. Nature Communications, 6(1), 6971. doi:10.1038/ncomms7971
Caswell, H. (1950). Rhythmic Color Change in the Lizard Xantusia vigilis. Copeia, 1950, 87. doi:10.2307/1438950
Chagnaud, B. P., & Coombs, S. (2014). Information Encoding and Processing by the Peripheral Lateral Line System. In S. Coombs, H. Bleckmann, R. R. Fay, & A. N. Popper (Eds.), The Lateral Line System (pp. 151-194). New York, NY: Springer New York. Retrieved from https://doi.org/10.1007/2506_2013_15
Clark, D. A., Biron, D., Sengupta, P., & Samuel, A. D. T. (2006). The AFD Sensory Neurons Encode Multiple Functions Underlying Thermotactic Behavior in <em>Caenorhabditis elegans</em>. The Journal of Neuroscience, 26(28), 7444-7451. Retrieved from https://www.jneurosci.org/content/jneuro/26/28/7444.full.pdf
Cloney, R. A., & Florey, E. (1968). Ultrastructure of cephalopod chromatophore organs. Zeitschrift für Zellforschung und Mikroskopische Anatomie, 89(2), 250-280. doi:10.1007/bf00347297
Commons, W. (2005). Zebrafish. In (Vol. 820 × 392, pp. A female specimen of a zebrafish (Danio rerio) breed with fantails). Wikimedia Commons. Retrieved from https://commons.wikimedia.org/wiki/File:Zebrafisch.jpg
Edelstein-Keshet, L. (2017). How the lizard gets its speckled scales. Nature, 544(7649), 170-171. doi:10.1038/544170a
Fu, Y., & Yau, K.-W. (2007). Phototransduction in mouse rods and cones. Pflugers Archiv : European journal of physiology, 454(5), 805-819. doi:10.1007/s00424-006-0194-y
Fulgione, D., Trapanese, M., Maselli, V., Rippa, D., Itri, F., Avallone, B., . . . Raia, P. (2014). Seeing through the skin: dermal light sensitivity provides cryptism in moorish gecko. Journal of Zoology, 294(2), 122-128. doi:https://doi.org/10.1111/jzo.12159
Gang, S. S., & Hallem, E. A. (2016). Mechanisms of host seeking by parasitic nematodes. Sensing and signaling in parasitism, 208(1), 23-32. doi:10.1016/j.molbiopara.2016.05.007
Goodman, M. B., & Sengupta, P. (2018). The extraordinary AFD thermosensor of C. elegans. Pflügers Archiv – European Journal of Physiology, 470(5), 839-849. doi:10.1007/s00424-017-2089-5
Hambley, A. R. (2011). Electrical Engineering: Principles and Applications (5 ed.): Pearson
Hardie, R. C. (2001). Phototransduction in Drosophila melanogaster. Journal of Experimental Biology, 204(Pt 20), 3403-3409.
Jones, A. M., & Assmann, S. M. (2004). Plants: the latest model system for G-protein research. EMBO reports, 5(6), 572-578. doi:10.1038/sj.embor.7400174
Kelley, J. L., & Davies, W. I. L. (2016). The Biological Mechanisms and Behavioral Functions of Opsin-Based Light Detection by the Skin. Frontiers in Ecology and Evolution, 4(106). doi:10.3389/fevo.2016.00106
Kimura, K. D., Miyawaki, A., Matsumoto, K., & Mori, I. (2004). The C. elegans Thermosensory Neuron AFD Responds to Warming. Current Biology, 14(14), 1291-1295. doi:10.1016/j.cub.2004.06.060
Kingston, A. C., Kuzirian, A. M., Hanlon, R. T., & Cronin, T. W. (2015). Visual phototransduction components in cephalopod chromatophores suggest dermal photoreception. Journal of Experimental Biology, 218(Pt 10), 1596-1602. doi:10.1242/jeb.117945
Kojima, D., Okano, T., Fukada, Y., Shichida, Y., Yoshizawa, T., & Ebrey, T. (1992). Cone visual pigments are present in gecko rods. Proceedings of the National Academy of Sciences of the United States of America, 89, 6841-6845. doi:10.1073/pnas.89.15.6841
Lacitignola, D. (2016). The Mathematical Beauty of Nature and Turing Pattern Formation. Matematica nella Societa e nella Cultura, 1.
Loew, E. R. (1994). A third, ultraviolet-sensitive, visual pigment in the Tokay gecko (Gekko gekko). Vision Research, 34(11), 1427-1431. doi:10.1016/0042-6989(94)90143-0
Luo, D. G., Su, C. Y., & Yau, K. W. (2009). Photoreceptors: Physiology. In Encyclopedia of Neuroscience (pp. 677-686): Elsevier Ltd. Retrieved from http://www.scopus.com/inward/record.url?scp=84882909735&partnerID=8YFLogxK
Maini, P. K., Woolley, T. E., Baker, R. E., Gaffney, E. A., & Lee, S. S. (2012). Turing’s model for biological pattern formation and the robustness problem. Interface Focus, 2(4), 487-496. doi:doi:10.1098/rsfs.2011.0113
Manukyan, L., Montandon, S. A., Fofonjka, A., Smirnov, S., & Milinkovitch, M. C. (2017). A living mesoscopic cellular automaton made of skin scales. Nature, 544(7649), 173-179. doi:10.1038/nature22031
Mäthger, L. M., Roberts, S. B., & Hanlon, R. T. (2010). Evidence for distributed light sensing in the skin of cuttlefish, <i>Sepia officinalis</i>. Biology Letters, 6(5), 600-603. doi:doi:10.1098/rsbl.2010.0223
McHenry, M. J., & Liao, J. C. (2014). The Hydrodynamics of Flow Stimuli. In S. Coombs, H. Bleckmann, R. R. Fay, & A. N. Popper (Eds.), The Lateral Line System (pp. 73-98). New York, NY: Springer New York.
McKemy, D. D. (2007). Temperature sensing across species. Pflügers Archiv – European Journal of Physiology, 454(5), 777-791. doi:10.1007/s00424-006-0199-6
Meyer, J. S. (1997). Chapter 5 – Principles of Neurotransmission and Implications for Network Modeling. In J. W. Donahoe & V. Packard Dorsel (Eds.), Advances in Psychology (Vol. 121, pp. 82-104): North-Holland. Retrieved from https://www.sciencedirect.com/science/article/pii/S016641159780091X
Millington, K. R. (2009). 9 – Improving the whiteness and photostability of wool. In N. A. G. Johnson & I. M. Russell (Eds.), Advances in Wool Technology (pp. 217-247): Woodhead Publishing. Retrieved from https://www.sciencedirect.com/science/article/pii/B9781845693329500095
Millot, N. (1968). The dermal light sense. In Symposia of the Zoological Society of London (pp. 36): The Society. Retrieved from https://books.google.ca/books?id=TPJJAQAAIAAJ
Montgomery, J., Bleckmann, H., & Coombs, S. (2014). Sensory Ecology and Neuroethology of the Lateral Line. In S. Coombs, H. Bleckmann, R. R. Fay, & A. N. Popper (Eds.), The Lateral Line System (pp. 121-150). New York, NY: Springer New York. doi:https://doi.org/10.1007/2506_2013_17
Montgomery, J. C., Windsor, S., & Bassett, D. (2009). Behavior and physiology of mechanoreception: separating signal and noise. Integr Zool, 4(1), 3-12. doi:10.1111/j.1749-4877.2008.00130.x
Nakamasu, A., Takahashi, G., Kanbe, A., & Kondo, S. (2009). Interactions between zebrafish pigment cells responsible for the generation of Turing patterns. Proceedings of the National Academy of Sciences of the United States of America, 106(21), 8429-8434. doi:10.1073/pnas.0808622106
Parichy, D. M., & Turner, J. M. (2003). Temporal and cellular requirements for Fms signaling during zebrafish adult pigment pattern development. Development, 130(5), 817-833. doi:10.1242/dev.00307
Parker, G. H. (2012). Animal Colour Changes and Their Neurohumours: A Survey of Investigations 1910-1943: Cambridge University Press. Retrieved from https://books.google.ca/books?id=8OKAEmARDIoC
Perry, R. N., & Moens, M. (2011). Survival of parasitic nematodes outside the host. Molecular and Physiological Basis of Nematode Survival, 1-27. doi:10.1079/9781845936877.0001
Ramirez, M. D., Speiser, D. I., Pankey, M. S., & Oakley, T. H. (2011). Understanding the dermal light sense in the context of integrative photoreceptor cell biology. Visual Neuroscience, 28(4), 265-279. doi:10.1017/s0952523811000150
van Netten, S. M., & McHenry, M. J. (2014). The Biophysics of the Fish Lateral Line. In S. Coombs, H. Bleckmann, R. R. Fay, & A. N. Popper (Eds.), The Lateral Line System (pp. 99-119). New York, NY: Springer New York. doi: https://doi.org/10.1007/2506_2013_14
Watanabe, M., & Kondo, S. (2015). Is pigment patterning in fish skin determined by the Turing mechanism? Trends in Genetics, 31(2), 88-96. doi:10.1016/j.tig.2014.11.005
Webb, J. F. (2014). Morphological Diversity, Development, and Evolution of the Mechanosensory Lateral Line System. In S. Coombs, H. Bleckmann, R. R. Fay, & A. N. Popper (Eds.), The Lateral Line System (pp. 17-72). New York, NY: Springer New York. doi: https://doi.org/10.1007/2506_2013_12
Weisstein, E. W. (n.d.). Cellular Automaton. Retrieved from https://mathworld.wolfram.com/CellularAutomaton.html
Widelitz, R. B., Baker, R. E., Plikus, M., Lin, C. M., Maini, P. K., Paus, R., & Chuong, C. M. (2006). Distinct mechanisms underlie pattern formation in the skin and skin appendages. Birth Defects Res C Embryo Today, 78(3), 280-291. doi:10.1002/bdrc.20075
Wolfram, S. (1983). Statistical mechanics of cellular automata. Reviews of Modern Physics, 55(3), 601-644. doi:10.1103/RevModPhys.55.601
Yu, Yanxun V., Bell, Harold W., Glauser, Dominique A., Van Hooser, Stephen D., Goodman, Miriam B., & Sengupta, P. (2014). CaMKI-Dependent Regulation of Sensory Gene Expression Mediates Experience-Dependent Plasticity in the Operating Range of a Thermosensory Neuron. Neuron, 84(5), 919-926. doi:10.1016/j.neuron.2014.10.046
Zimmerman, K., & Heatwole, H. (1990). Cutaneous Photoreception: A New Sensory Mechanism for Reptiles. Copeia, 1990, 860. doi:10.2307/1446454