Abstract
Lactation constitutes an important aspect of mammalian physiology and reproduction and is a key trait that defines mammals. The evolution of lactation is a story of great controversy, however, and the main theories behind the apparition of mammary glands will be discussed in the following paragraphs. In addition, the origin as well as the general anatomy of mammary glands serves as a unifying factor among the mammalian class and will be examined to provide the basis on which diversity in mammalian lactation evolved. Furthermore, diverse lactation strategies and/or structures will be explored through a plethora of different mammals, examining how those unique strategies are an evolutionary response to specific environments and selective pressures. The biomechanics of mammalian lactation will be investigated as well, including the differential pressures involved in the suckling mechanism with respect to Boyle’s Law and mechanical principles. Details regarding the biomechanics of the lactation process will also be explained including lactogenesis, teat stimulation, pressure receptors, the release of oxytocin, and fluid dynamics. Finally, a case study of the bioengineering principles displayed in the dairy industry will be examined and related to the biomechanics of the lactation process.
Introduction
Lactation is a complex form of adaptation that has evolved over the course of many centuries and can be considered an essential biomechanism to the mammalian class. Milk isn’t only a source of nutrients, but also contains antimicrobial proteins and components related to the immune system. The ancestor of the mammals first used milk to protect the egg from drying out (Oftedal, 2012).
However, after 200 million years of evolution, the reproductive strategies of mammals have changed. Some continue to lay eggs whereas others give birth, consequently affecting their use of milk. Nowadays, most mammals use milk as a source of nutrients for the young. However, different environments and selective pressures have led to different lactation strategies (Oftedal, 2012). Nevertheless, there is a common pattern to all mammals in the mechanism of the suction of the milk: utilizing a partial vacuum mechanism where pressure differentials lead the milk towards the nipple where the young can then drink it (German et al., 1992). Mammalian milk is a good source of nutrients that have been utilized by human activities for centuries. Nowadays, the dairy industry uses a plethora of different strategies to harness natural biomechanics and efficiently acquire the important resource that is milk, notably through the use of the milk machine.
Origin of Lactation
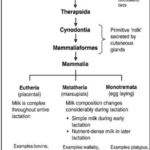
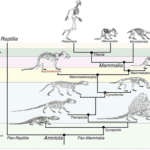
In the history of evolution, lactation played a big role for mammals in differentiating them from other species. Nowadays, all mammals can lactate. This means that before the existence of mammals, a common ancestor could lactate. Indeed, 310 million years ago, it was the synapsids that diverged from another branch in the phylogenetic tree (Figure 1) leading to egg-laying creatures like birds and reptiles (Sauropsids). The synapsids laid eggs like reptiles, but unlike the sauropsids, they didn’t lay eggs with a calcified eggshell. A calcified eggshell is resistant to vapor loss and has small pores for gas exchange, but the synapsids evolved in another path. They instead laid eggshells of diverse crystalline form that gain a lot of water but could also lose water rapidly. So, the synapsids eggs were relying on the water environment, burying their eggs in a moisture-laden soil. Then, during the mid-late Permian period (274 million years ago), the synapsids were going extinct because of the appearance of their successor, the therapsids.
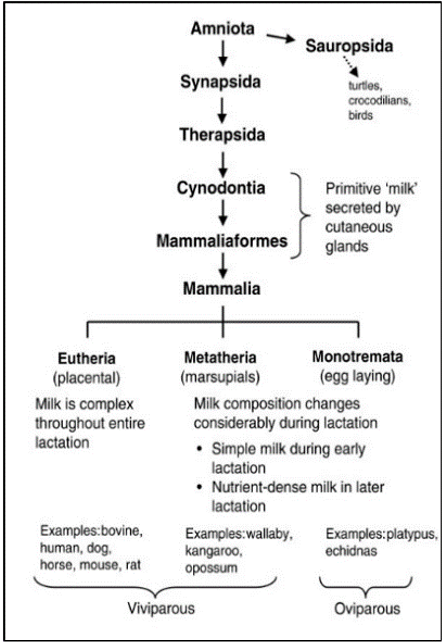
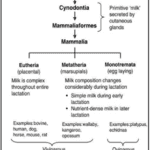
However, at the end of the Permian period (252 million years ago), a massive extinction had eliminated the therapsids, but their descendants, the cynodonts survived. The cynodonts had increased their body temperature, and the eggs had to be incubated to increase thermal stability. The soil had the moisture needed for the egg, but the temperature was too low. It seems impossible to incubate the eggs without facing dehydration, so it needed a source of external moisture. That’s why the skins of the cynodonts evolved to eject milk that had all the nutrients for protecting the eggs from drying out. Then, in the late Triassic period (201 million years ago), the mammaliaforms had succeeded the cynodonts (Oftedal, 2012). The cynodonts and the mammaliaforms were becoming smaller in size with each generation because the reptiles were ruling the Triassic period and mammaliaforms survived by evading the attention of big reptiles by being small (Black, 2020). That meant that the eggs were also smaller, and the newborn would also be smaller. These undeveloped young didn’t have teeth that could continuously be replaced and didn’t have strong jaws to eat adult food. Once born, the young were dependent on milk as a source of the nutrients needed to develop their bodies. After the mammaliaforms, in the late Jurassic period (160 million years ago), mammals appeared (Figure 2). There were three types of mammals that had evolved: the monotremes, the marsupials and the eutherians. The monotremes kept laying eggs, but the marsupials, and the eutherians changed that with the placenta, giving birth instead. However, all mammals lactate to give their young the nutrients and resources they need to develop (Oftedal, 2012).

Origin of the Mammary Glands
The mammary glands are what produce milk and there was no fossilized proof to see an intermediate form of the mammary glands. The theory of evolution was even criticized because of this lack of intermediate form. However, in the second edition of On the Origin of the Species (1872), Darwin thought that the mammary glands evolved from glands that were inside the brood pouches in which aquatic species keep their eggs and nourished the young. It is now clear that the mammary glands had evolved from skin glands (Capuca & Akers, 2009).
In monotremes like the platypus (Figure 1), they have no nipples and thus the mammary ducts open directly in the areola, where there is a hair follicle. The association between the hair follicle and the mammary glands is evident. For the marsupials, the same association can be made. For example, the koala has hair remaining on their nipples during their development. For the eutherians, there is an inhibition of the hair follicle in the mammary glands (Lefèvre et al., 2010). This association between the hair follicles and mammary glands proves that the mammary glands came from the sweat glands.
General Anatomy of the Mammary Glands
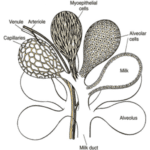
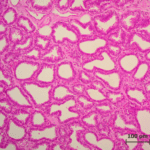
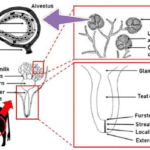
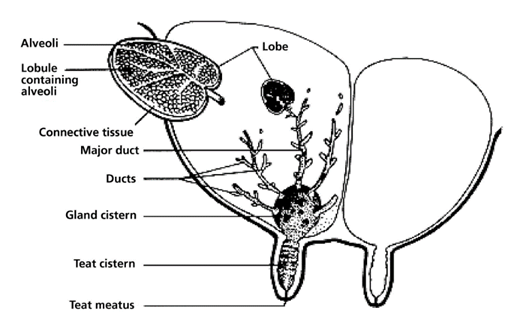
The mammary glands are positioned in the breasts in primates like chimpanzees, the udder in ruminants like cows and the dug in canines. The mammary glands are composed of mammary alveoli (Figure 3). The design of the alveoli is a small cavity that can produce milk and where milk is stored. These groups of alveoli form lobules, which are densely surrounded by a complex web of capillaries. These capillaries provide a pathway for hormone transport and are involved in the contraction of myoepithelial cells. All the alveoli in the same lobule are connected to one milk duct where the milk can go towards the nipple. The mammary gland could contain only one lobule (simple mammary gland) or it could contain many lobules (complex mammary gland). The number of mammary glands is different for each species (“Mammary gland,” 2021).
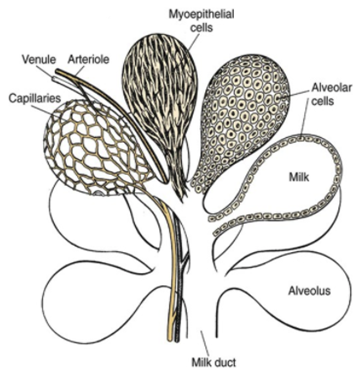
Diversity of Lactation Strategies
There exists a diversity of lactation strategies among mammals. For example, the monotremes, which can hatch eggs, have no nipples. For the marsupials like the opossum, once the young are born, the mother produces milk with nutrients that are constantly changing with respect to the growth of the young. Furthermore, the eutherians produce milk from mammary glands through a complex mechanism that leads the milk towards the nipple. Thus, each species has a unique strategy for lactation depending on their environment and the selective pressures they face (Oftedal, 2012).
Diversity Among Lactation Strategies
Among terrestrial mammals, the most important milk-producing animals for agriculture are ruminants like cows, sheep, and goats. The ruminants began to evolve about 50 million years ago and they can live in different places with various climates (Hackmann & Spain, 2010). They are unique because they have a stronger digestive system and more mammary glands than other mammals. A study by Boutinaud et al. (2003) found out that in ruminants, two factors can affect the evolution of mammary glands. First and foremost, frequent milking develops mammary glands. Furthermore, the more growth hormone, the more milk they make because growth hormones stimulate secretory cells that produce milk. This can explain why ruminants can lactate a considerable amount of milk and have several mammary glands.
Cow’s milk is an important source of dairy products. Cows were evolved from the aurochs, which are a wild bovine species and became domestic about 10,000 years ago (McTavish et al., 2013). They live in grasslands, pastures, forests, and they eat grass which contains dietary fiber. By increasing the amount of rumen acetate in fiber, cows can transfer fiber nutrition into milk fat (Esmail, 2021). Cows have four mammary glands (udders) that take nutrients from blood and synthesize it into milk. Milk is produced by the alveoli within every mammary gland, then the calf sucks the teat to get the milk (Holstein Foundation, 2017).
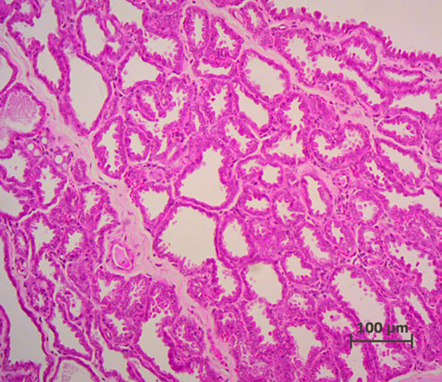
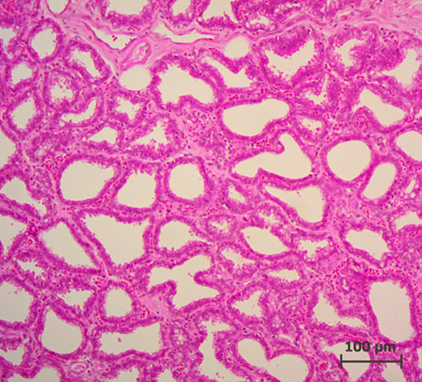
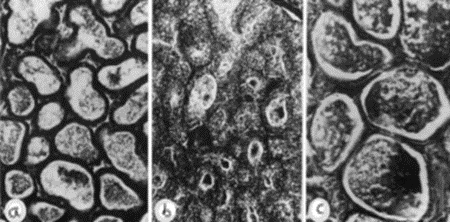
Lactation of sheep and goats shows a similar pattern as with the cow. They are both artiodactyls, which is the same subclass of mammals as cows. Sheep and goats live in mountainous or hilly areas, and they eat grass and plants. As seen from Figure 4 & 5, the mammary glands pattern, size, and shape of alveoli tissue of sheep and goats are very similar, which means that their lactation strategies are almost the same (Lérias et al., 2014). Like sheep and goats, ruminants’ mammary glands have many branches and when sucking the teats, milk can travel from alveoli tissues to the teats through many mammary ducts.
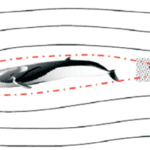
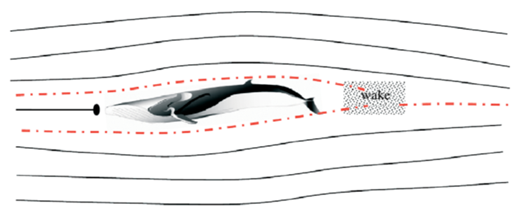
The Blue Whales are Cetaceans which is a subclass of marine mammals, and they can be found in all the major oceans. Their lactating method is similar to other mammals, but they have a unique characteristic that their nipples are not protruding. Calves put pressure on nipples and use their tongues to suck the milk into their mouth. The two nipples in the whale are recessed under the belly with an overlying skin fold, to not disturb the streamline shape of the whale and its hydrodynamics (Whales online, n.d.). This streamline shape minimizes drag and thus reduces pressure on both sides of the body (Figure 6). If two nipples are protruding, they would act as two blocks separating the water flow so that they need more energy and strength to swim (Vogel, 1996). From the evolutionary point of view, this lactating feature of Cetaceans such as whales can be explained; cetaceans originated about 50 million years ago from terrestrial mammals and they got adapted to living underwater during the evolution process (Thewissen et al., 2009). Now they can swim a long distance and at high speed among all the marine mammals which is determined by their ability to control the flow in the water (Fish et al., 2008).
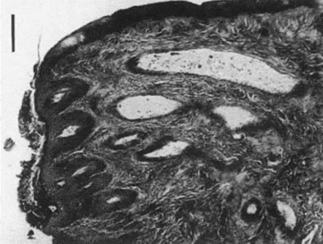
Lactation is not something only female animals can do; males can do too. Some male lactations are functional (need-based) like infant feeding, some are role-based, like being a paternal caregiver, and some are fortuitus (Cohen & Otomo, 2019). But only in a few mammals is lactation a normal male function. The Dayak fruit bat is an example. Dayak fruit bats can be found exclusively in Southeast Asia and their habitat can be found in tropical rainforests (Francis, 1994). Lactating male Dayak fruit bats’ nipples are smaller and alveoli tissues are larger than females (Figure 7 and 8). This means that milk is concentrated in alveoli and the sucking behavior of baby bats is rare (Francis et al., 1994). Besides, they have large mammary gland beneath the wings. The reason why male Dayak fruit bats have evolved the ability to secret small amounts of milk from mammary glands needs to be further investigated, but an article by Kunz & Hosken (2009) offers a possible reason: this male lactation serves as a paternal care function and male Dayak fruit bats may face selective pressure. Dayak fruit bats are listed as “nearly threatened” on the IUCN list and their number is decreasing (Csorba et al., 2020).
Platypuses (Figure 9) belong to a unique subclass of mammals called monotremes that lactate like other mammals, but unlike mammals, they lay eggs (Grant, 2007). Platypuses are only found in Australia and their habitat can be found along stream banks and they seek shelter in burrows. A female platypus will lay her eggs in a burrow and incubate the eggs until they hatch. The lactation mechanisms of platypuses are unique in comparison to other mammals as the milk is ejected through the mother’s skin due to the absence of a teat or nipple structure (Sharp et al., 2011). The location of the mammary glands is under the skin of the female’s abdomen and the mammary glands divide into two patches of skin, also known as areolae. The areolae are the structures where milk is ejected through the skin and onto the fur on the female’s abdomen (Grant, 2007). The lactation process is initiated by the stimulation of the skin just above the areolae which is done by the infant platypuses either suckling or prodding this region (Grant, 2007; Sharp et al., 2011). Furthermore, the action of suckling or prodding the skin above the areolae forces the milk through the female’s skin once the milk reaches the areolae from the mammary alveoli and ducts. The unique lactation mechanisms of monotremes such as platypuses are explained by evolution, as monotremes are thought to have split from other mammals at least 166 million years ago (Langley, 2016). This explains why monotremes, like the platypus, still lactate through their skin compared to mammals that over time evolved divergent lactation strategies and structures such as teats (Sharp et al., 2011). Differences in the evolutionary pathways between monotremes and mammals provide insight into why differences in lactation anatomy and mechanisms exist for the female platypus.

The Mechanics of Milk Ejection
As a response to their different environments and lifestyles, all mammals supply milk to their offspring in a unique fashion. However, a distinct trait that unites all mammals is the evolution of a secondary palate (Johnson et al., 2010). This palate allows for simultaneous nursing and breathing and is a key element in the act of suckling; an essential feeding activity adopted by all mammals. In fact, even aquatic mammals such as sperm whales suckle by making use of their secondary palate known as the baleen (The Editors of Encyclopaedia Britannica, 2010; Sivacolundhu, 2014). By making use of this functional adaptation, young infant mammals are able to create a negative pressure system within the oral cavity, forcing milk into their mouths.
A study by German et al. (1992) sought to understand this mechanism, and figure 10 illustrates an infant miniature pig suckling to demonstrate the efficacy of the strategy.
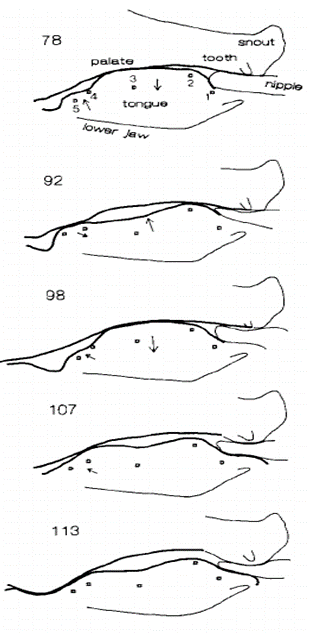
The mechanism works as follows: the distinctly prolonged mouth of the infant pig allows the nipple to enter the oral cavity, creating an airtight seal with the snout and the tongue. The tongue of the infant is initially making contact with the palate in the posterior region of the oral cavity. This allows for as small of an air pocket as possible within the system. However, the tongue then moves back and the point of contact with the palate moves to the anterior region of the mouth, increasing the volume of the system in which the nipple resides (German et al., 1992).
As the infant moves their tongue towards the front of the oral cavity, thereby increasing the volume of the air pocket, the pressure ultimately drops. This adheres to Boyle’s law, which states that pressure and volume are inversely proportional (P1V1=P2V2) (“Boyle’s Law,” 2005). The consequent pressure differential allows for the higher-pressure system of the greater mammary duct of the nursing mother (or father in certain cases) to eject the milk into the mouth of the nursing infant, assuming the infant provides enough light suction to overcome the resistance of the nipple sphincter. Once the mouth is filled with milk, the point of contact between the tongue and the palate of the infant is reverted back to the posterior region of the mouth, allowing the infant to relax the tongue and swallow the newly acquired milk. This process then repeats itself in a cyclic manner (German et al., 1992).
This suckling mechanism is common to all mammals in the animal kingdom, as a direct result of the evolution of the secondary palate. However, in order for lactation to occur, other biomechanical processes must first take place.
Milk first appears in the alveoli of the mammary glands through lactogenesis, a biochemical process that occurs following parturition and the subsequent drop in progesterone (Cowie et al., 1980; Sivacolundhu, 2014). When it comes time for lactation, the stimulus of the teat/nipple by the mouth of the infant triggers a signal through the nervous system which travels up the spinal cord to the brain of the nursing parent. In short, this stimulation causes the release of the hormone oxytocin into circulation (Bisset & Clark, 1968).
The circulation of this hormone causes the contraction of the myoepithelial cells and muscle cells surrounding the alveoli and upper mammary ducts. In terms of fluid dynamics, the decreased volume of the alveoli causes the milk to be pushed out of the upper alveoli and mammary ducts due to the fact that milk is incompressible. As a result, the milk is ejected into the larger lower mammary ducts, or the cisterns/sinuses in some mammalian species. As the contraction continues and the milk descends into the extremity of the nipple/teat, the negative pressure and light suction induced by the mouth of the infant is sufficient to transfer the milk to the young, as the liquid will tend to go to the lower pressure system (Bisset & Clark, 1968).
In some species, a significant amount of milk is present in the lower mammary duct/cisterns prior to the release of oxytocin, allowing for a process known as passive withdrawal. This allows for the transfer of milk without the need for epithelial cell contraction, and only the surface tension of the milk and resistance of the nipple/teat sphincter must be overcome by the infant (Bisset & Clark, 1968).
Bioengineering and Lactation
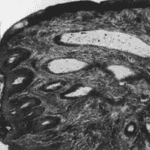
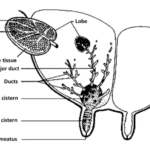
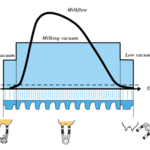
In the dairy industry, the mechanics of dairy cow lactation are extensively studied to both maximize the yield of milk and improve the efficiency of milk production. Bioengineering principles led to the creation of the milking machine that uses the biological demands and mechanisms of cow lactation to efficiently extract milk from the teats of a cow via a vacuum system (Holstein Foundation, 2017). The process of lactation for dairy cows varies slightly from other mammals due to a unique structure called udders (Figure 11, Figure 12) which overall increases the efficiency of the lactation process in comparison to other mammals.
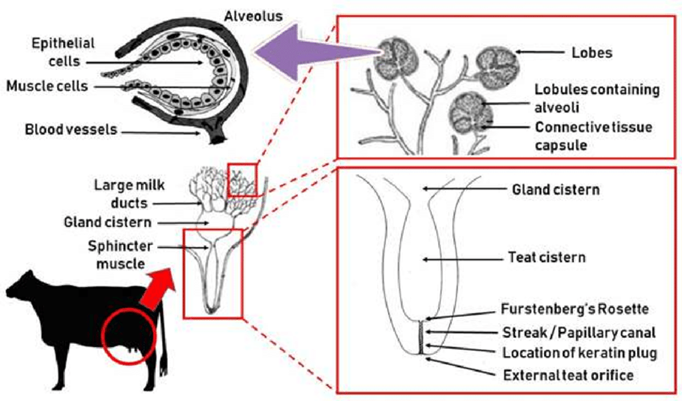
The milking process begins with the pre-stimulation of the teats and this stage usually occurs before the milking machine is even secured to the cow’s teats. Pre-stimulation can be achieved by the act of cleaning and drying the teats and/or hand massaging the teats (Svennersten-Sjaunja, 2001). Next, a structure of the milking machine called the teat cups are then properly secured to the teats of the cow and the stimulation stage begins (Svennersten-Sjaunja, 2001). Stimulation of the teats can be attained by the attachment of the teat cups or even the sounds or sight of the milking machine or a calf (Holstein Foundation, 2017; Svennersten-Sjaunja, 2001). In addition, some dairy farmers will place feeding concentrate in the milking parlor which can also act as a teat stimulate in preparation for lactation (Svennersten-Sjaunja, 2001). In response to stimulation, pressure sensitive receptors in the cow’s teats are activated and consequently nerve impulses travel to the pituitary gland initiating the release of oxytocin into the bloodstream (Fish et al., 2008). The increased levels of oxytocin in the bloodstream cause the myoepithelial cells that line both the mammary ducts and alveoli to contract. It is the myoepithelial cells contracting rhythmically that are responsible for milk being forced into the gland cistern (Svennersten-Sjaunja, 2001). The gland cistern (Figure 12) is a storage structure unique to ruminants and fills continuously and quickly during the milk “let-down” from the mammary alveoli and ducts (Holstein Foundation, 2017). This results in an increase in the efficiency of the milking process as there is always a large and readily available supply of milk in the lower ducts. Once the milk reaches the gland cistern, the milking machine can then remove the milk easily (Holstein Foundation, 2017; Neville, 1995). The “let-down” reflex is vital as without the contraction of the myoepithelial cells, the milk stored in the alveoli would not be accessible to be removed by the milking machine (Svennersten-Sjaunja, 2001).
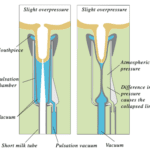
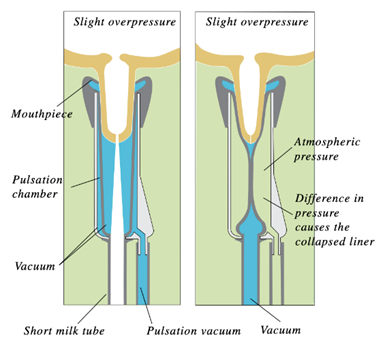
The milking machine implements a vacuum system that decreases the external pressure at the end of the teat and therefore produces a pressure differential across the teat orifice (Holstein Foundation, 2017). This difference in pressure allows milk to flow out of the cow’s teat into the milk tube of the milking machine (Figure 13) (Holstein Foundation, 2017). Additionally, the milking machine successfully extracts milk from the teats due to a difference in pressure between a structure of the milking machine called the liner and the inner wall of the udder (Svennersten-Sjaunja, 2001). This is due to the liner forming a secure seal around the teat during the milking process (Svennersten-Sjaunja, 2001). The machine ultimately extracts milk due to constant pressure from the vacuum system that is disturbed by the opening and closing motions of the liner (Svennersten-Sjaunja, 2001). This manmade mechanism was inspired by observations of a calf suckling on a teat as constant suckling had resulted in both lymph and blood accumulating in the teat (Svennersten-Sjaunja, 2001).
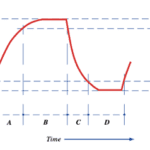
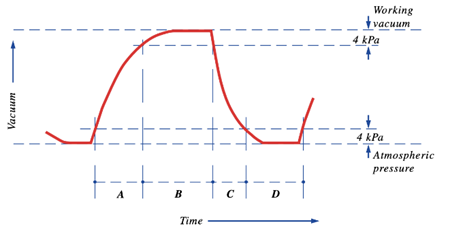
The pulsation cycle (Figure 14) illustrates the opening and closing of the liner and is divided into 4 phases (Svennersten-Sjaunja, 2001). The cycle begins with Phase A where the liner of the machine opening, therefore, allowing milk to exit the end of the teat. Furthermore, Phase B is referred to as the milking phase and is when milk continuously flows from the teat. Following this, Phase C represents the liner beginning to close and ultimately stops the flow of milk observed in Phase B. Finally, Phase D is called the resting phase, and this is when the liner remains closed before the pulsation cycle restarts (Svennersten-Sjaunja, 2001). The pulsation cycle that the machine undergoes is an engineering design based on the observations of calf suckling which is an interesting example of the influence of natural processes on engineering creations.
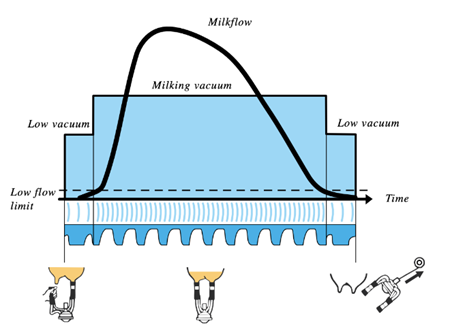
On a larger spectrum, the milking machine performs three cycles: the pre-milking phase, milking phase and post-milking phase (Figure 15) (Svennersten-Sjaunja, 2001). The milking cycle is composed of differing pressures and pulsations being applied to the teat from the milking machine depending on the rate of milk flow from the teat. The pre-milking phase occurs prior to the flow of milk surpassing the “low flow limit” (Svennersten-Sjaunja, 2001) depicted in Figure 15. Once the flow rate exceeds this minimum, the milking phase begins and consequently the vacuum level increases gradually and milk flows from the teat due to the pressure differential created between the teat and the machine (Holstein Foundation, 2017; Svennersten-Sjaunja, 2001). The milking phase continues until the flow rate of milk falls below the low flow limit where the machine then automatically decreases the vacuum level, and the cycle transitions into the post-milking phase (Svennersten-Sjaunja, 2001).
The mechanisms of the lactation process of dairy cows have been thoroughly observed and examined and this natural process has led to the creation of the milking machine that has resulted in the increase of both output and efficiency for the dairy industry.
Conclusion
Lactation is a unique feature of mammalian physiology which differentiates mammals from other species. The product of lactation (milk) provides key nutritional value, immune support, and hydration to the infant offspring of the nursing parent. In terms of evolution, lactation was first used to protect soft-shelled eggs from drying out. However, after the evolution, the young of the mammals were dependent on milk as a source of nutrients and lactation was used to feed offspring directly.
The main theory in terms of the evolution of mammary glands is that they evolved directly from skin glands. This theory is supported by the association of the hair follicle and the mammary gland, made clear in particular by the case of the platypus (which lactates through hair follicles). The common anatomy of all mammary glands consists of the lobules, made up of alveoli, the milk ducts, and the epithelial cells which are responsible for the movement of milk. However, each species has unique anatomical elements within its mammary glands; this causes each species to have a unique lactation strategy.
The unique strategy of lactation of each species is based on the environment of their habitat and the selective pressures they face. Among terrestrial animals, ruminants are the main lactating species. They all produce a considerable amount of milk and have several mammary glands that are similar in construction. The blue whale, on the other hand, is more distinct from the plethora of terrestrials as it is aquatic. This environment changes the way in which their physiology is constructed so as to not disturb their hydrodynamics, and it changes the rate of nursing as well.
Selective pressures may also affect lactation in terms of parental roles, as some rare species are shown to have male nursing parents. This is the case in particular for the Dayak fruit bat. Furthermore, some species’ physiology took a different evolutionary pathway, as is explained by the lack of nipples for the platypus.
Although each species has a different mechanism for lactation, there are some unifying biomechanisms that each species utilizes when it comes to feeding. Lactogenesis, for example, is the production of milk in the alveoli which occurs shortly after parturition. In all lactating mammals, it is the release of the hormone oxytocin which causes the contraction of the epithelial cells and the subsequent transport of the milk from the site of lactogenesis to the lower ducts of the mammary glands.
Another unifying biomechanism that unites mammals revolves around the utilization of the secondary palate, a unique and distinct evolutionary adaptation that defines mammals. This palate allows for suckling, a biomechanical process that takes place in the oral cavity of the feeding infant. By moving the tongue and changing the volume of the air cavity surrounding the nipple, the infant can create negative pressure. This forces milk into the mouth of the infant, ready to be digested.
Finally, the dairy industry has improved the efficiency and yield of the cow lactation process by the invention of the milking machine. Through the application of bioengineering principles, the milking machine implements a vacuum system to improve the natural biomechanical processes of lactation. The milking machine reflects the biomechanics of lactation as the vacuum creates a pressure differential across the teat orifice and the liner which replicates the suckling mechanism of a calf but in a much more efficient manner. The milking machine not only improves the efficiency of the lactation process but also maximizes milk yield, therefore, improving the productivity of the dairy industry.
Thus, lactation not only constitutes a defining trait of all mammals, but it has been proven to be a crucial element to the survival of mammalian ancestors. Nursing is an essential element in the bond of infant and mother, and as engineers, we can take inspiration from this zero-learning-curve design.
References
Bisset, G. W., & Clark, B. J. (1968). Synthetic 1-N-carbamylhemicystine-2-O-methyltyrosine-oxytocin (N-carbamyl-O-methyl-oxytocin): a specific antagonist to the actions of oxytocin and vasopressin on the uterus and mammary gland. Nature, 218(5137), 197-199. https://doi.org/10.1038/218197b0
Black, R. (2020, September 30). Why Did Our Mammal Ancestors Stop Laying Eggs? Discover. https://www.discovermagazine.com/planet-earth/why-did-our-mammal-ancestors-stop-laying-eggs
Boutinaud, M., Rousseau, C., Keisler, D. H., & Jammes, H. (2003). Growth hormone and milking frequency act differently on goat mammary gland in late lactation. J Dairy Sci, 86(2), 509-520. https://doi.org/10.3168/jds.S0022-0302(03)73629-7
Boyle’s Law. (2005). In D. M. Considine (Ed.), Van Nostrand’s Scientific Encyclopedia (9 ed.). John Wiley & Sons, Inc. https://doi.org/https://doi.org/10.1002/0471743984.vse1242
Cohen, M., & Otomo, Y. (2019). Making Milk: The Past, Present and Future of Our Primary Food. Bloomsbury Publishing.
Cowie, A. T., Forsyth, I. A., & Hart, I. C. (1980). Lactation. In A. T. Cowie, I. A. Forsyth, & I. C. Hart (Eds.), Hormonal Control of Lactation (pp. 146-229). Springer Berlin Heidelberg. https://doi.org/10.1007/978-3-642-81389-4_4
Csorba, G., Bumrungsri, S., Francis, C., Bates, P., Gumal, M., & Kingston, T. (2020, March 16). Dayak Fruit Bat. IUCN Red List of Threatened Species. Retrieved November 5, 2021, from https://dx.doi.org/10.2305/IUCN.UK.2020-3.RLTS.T6931A22029918.en
Esmail, S. H. (2021, May 27). Factors affecting fibre utilisation in ruminant feeds. Dairy Global. https://www.dairyglobal.net/Nutrition/Articles/2021/5/Factors-affecting-fibre-utilisation-in-ruminant-feeds-751088E/
Fish, F. E., Howle, L. E., & Murray, M. M. (2008). Hydrodynamic flow control in marine mammals. Integrative and Comparative Biology, 48(6), 788-800. https://doi.org/10.1093/icb/icn029
Francis, C. M. (1994). Vertical stratification of fruit bats (Pteropodidae) in lowland dipterocarp rainforest in Malaysia. Journal of Tropical Ecology, 10(4), 523-530. https://doi.org/10.1017/S0266467400008191
Francis, C. M., Anthony, E. L. P., Brunton, J. A., & Kunz, T. H. (1994). Lactation in male fruit bats. Nature, 367(6465), 691-692. https://doi.org/10.1038/367691a0
German, R. Z., Crompton, A. W., Levitch, L. C., & Thexton, A. J. (1992). The mechanism of suckling in two species of infant mammal: Miniature pigs and long-tailed macaques. Journal of Experimental Zoology, 261(3), 322-330. https://doi.org/https://doi.org/10.1002/jez.1402610311
Grant, T. (2007). Platypus. CSIRO Publishing. https://books.google.ca/books?id=xIUaGpG6CIIC
Hackmann, T. J., & Spain, J. N. (2010). Invited review: Ruminant ecology and evolution: Perspectives useful to ruminant livestock research and production. Journal of Dairy Science, 93(4), 1320-1334. https://doi.org/10.3168/jds.2009-2071
Holstein Foundation. (2017). Milking and Cactation. Holsteinfoundation.Org. http://www.holsteinfoundation.org/pdf_doc/workbooks/Milking_Lactation_Workbook.pdf
Johnson, G., Frantzis, A., Johnson, C., Alexiadou, V., Ridgway, S., & Madsen, P. T. (2010). Evidence that sperm whale (Physeter macrocephalus) calves suckle through their mouth [https://doi.org/10.1111/j.1748-7692.2010.00385.x]. Marine Mammal Science, 26(4), 990-996. https://doi.org/https://doi.org/10.1111/j.1748-7692.2010.00385.x
Khan, S., Manjusha, K. M., & Banu S, A. (2020). Surgical affections of Udder and Teat in Large Animals.
Kunz, T. H., & Hosken, D. J. (2009). Male lactation: why, why not and is it care? Trends in Ecology & Evolution, 24(2), 80-85. https://doi.org/https://doi.org/10.1016/j.tree.2008.09.009
Langley, L. (2016, July 30). How the Venomous, Egg-Laying Platypus Evolved. National Geographic. https://www.nationalgeographic.com/animals/article/animals-platypus-evolution-science
Lefèvre, C. M., Sharp, J. A., & Nicholas, K. R. (2010). Evolution of Lactation: Ancient Origin and Extreme Adaptations of the Lactation System. Annual Review of Genomics and Human Genetics, 11(1), 219-238. https://doi.org/10.1146/annurev-genom-082509-141806
Lérias, J. R., Hernández-Castellano, L. E., Suárez-Trujillo, A., Castro, N., Pourlis, A., & Almeida, A. M. (2014). The mammary gland in small ruminants: major morphological and functional events underlying milk production – a review. Journal of Dairy Research, 81(3), 304-318. https://doi.org/10.1017/S0022029914000235
Mammary gland. (2021, October 12). Wikipedia. Retrieved November 4, 2021, from https://en.wikipedia.org/w/index.php?title=Mammary_gland&oldid=1049514745
McTavish, E. J., Decker, J. E., Schnabel, R. D., Taylor, J. F., & Hillis, D. M. (2013). New World cattle show ancestry from multiple independent domestication events. Proceedings of the National Academy of Sciences, 110(15), E1398-E1406. https://doi.org/10.1073/pnas.1303367110
Monotremata: Life History & Ecology. (1994). University of California Museum of Paleontology. Retrieved November 5, 2021, from https://ucmp.berkeley.edu/mammal/monotremelh.html
Neville, M. C. (1995). C – Sampling and Storage of Human Milk. In R. G. Jensen (Ed.), Handbook of Milk Composition (pp. 63-79). Academic Press. https://doi.org/https://doi.org/10.1016/B978-012384430-9/50006-8
Oftedal, O. T. (2012). The evolution of milk secretion and its ancient origins. Animal, 6(3), 355-368. https://doi.org/https://doi.org/10.1017/S1751731111001935
Potvin, J., Goldbogen, J. A., & Shadwick, R. E. (2009). Passive versus active engulfment: verdict from trajectory simulations of lunge-feeding fin whales <i>Balaenoptera physalus</i>. Journal of The Royal Society Interface, 6(40), 1005-1025. https://doi.org/doi:10.1098/rsif.2008.0492
Sharp, J. A., Menzies, K., Lefevre, C., & Nicholas, K. R. (2011). Milk | Milk of Monotremes and Marsupials. In J. W. Fuquay (Ed.), Encyclopedia of Dairy Sciences (Second Edition) (pp. 553-562). Academic Press. https://doi.org/https://doi.org/10.1016/B978-0-12-374407-4.00516-1
Sivacolundhu, R. K. (2014). Chapter 56 – Palate. In S. J. Langley-Hobbs, J. L. Demetriou, & J. F. Ladlow (Eds.), Feline Soft Tissue and General Surgery (pp. 683-690). W.B. Saunders. https://doi.org/https://doi.org/10.1016/B978-0-7020-4336-9.00056-1
Svennersten-Sjaunja, K. (2001). Efficient Milking. DeLaval. http://www3.delaval.com/ImageVaultFiles/id_27591/cf_5/Efficient-milking.PDF
The Editors of Encyclopaedia Britannica. (2010, November 18). palate. Encyclopedia Britannica. Retrieved November 5, 2021, from https://www.britannica.com/science/palate
The Mammary Gland. (2016, July 18). Veterian Key. https://veteriankey.com/the-mammary-gland-2/
Thewissen, J. G. M., Cooper, L. N., George, J. C., & Bajpai, S. (2009). From Land to Water: the Origin of Whales, Dolphins, and Porpoises. Evolution: Education and Outreach, 2(2), 272-288. https://doi.org/10.1007/s12052-009-0135-2
Vogel, S. (1996). Life in Moving Fluids: The Physical Biology of Flow – Revised and Expanded Second Edition. Princeton University Press.
Whales online. (n.d.). Mother-Calf Relationships. GREMM. Retrieved November 5, 2021, from https://baleinesendirect.org/en/discover/life-of-whales/behaviour/mother-calf-relationships/
Bibliography
Capuco, A. V., & Akers, R. M. (2009). The origin and evolution of lactation. J Biol, 8(4), 37. https://doi.org/10.1186/jbiol139
Farmer, C., Maes, D., & Peltoniemi, O. (2019). Mammary System. In Diseases of Swine (pp. 313-338). https://doi.org/https://doi.org/10.1002/9781119350927.ch18
Jackson School of Geosciences. (2018). Mammal Forerunner that Reproduced Like a Reptile Sheds Light on Brain Evolution. The University of Texas at Austin. https://news.utexas.edu/2018/08/29/mammal-forerunner-sheds-light-on-brain-evolution/
Neville, M. C., & Morton, J. (2001). Physiology and Endocrine Changes Underlying Human Lactogenesis II. The Journal of Nutrition, 131(11), 3005S-3008S. https://doi.org/10.1093/jn/131.11.3005S
Nickerson, S. C., & Akers, R. M. (2022). Mammary Gland: Anatomy. In P. L. H. McSweeney & J. P. McNamara (Eds.), Encyclopedia of Dairy Sciences (Third Edition) (pp. 157-166). Academic Press. https://doi.org/https://doi.org/10.1016/B978-0-08-100596-5.23019-9