Abstract
The narwhal’s tusk is a fascinating organ as it has the composition of a tooth but very different properties, in particular its ability to grow in a straight line. To understand the design mechanism allowing this particular feature, we will first present the composition, function and properties of the tusk. We will then be able to tackle the mechanism behind the tusk’s capacity to grow in a straight line. A comparative study with the elephant’s tusk will then be made to deepen our understanding of those design mechanisms.
Introduction
Tusks are a particular form of elongated, continuously growing protruding teeth. This specificity is present in several animals, such as pigs, hippos, elephants, and narwhals. In these tusked animals, usually both males and females have tusks, but some singularities may be observed from one species to another, such as a larger size in the male, or no tusks at all for the female narwhal. The design of the tusk is specific to the species; usually, they are curved, have a smooth continuous surface, and are located on both sides of the animal. However, the situation of narwhal is quite different as only males have a single straight helical tusk. The function of the tusk is varied: it can act as a secondary sexual character, a means for the male to assert his dominance, but it is not always very clear. The goal of this paper is to explain the design principle that allows narwhal tusks to grow perfectly linear. However, for us to understand the mechanism behind it, let us first take a look at the composition and properties of narwhal tusks.
Composition and Properties of the Tusk
The narwhal, monodon Monoceros, is known and easily recognizable thanks to the presence of its tusk, reaching three meters long on some animals (Nweeia et al., “Considerations of Anatomy, Morphology, Evolution, and Function for Narwhal Dentition”). Tusks are horizontally embedded teeth in the upper jaw of the narwhal and erupt through the left side of the maxillary bone by piercing through the lip in males (Nweeia et al., “Considerations of Anatomy, Morphology, Evolution, and Function for Narwhal Dentition”). The tusk on the right side usually does not erupt and remains embedded in the bone and is no longer than 30 cm. The eruption of a tusk through the left side is rare but occurs sometimes. When two tusks are present, an extreme form of dental asymmetry can be observed, the helical shape rotates in the left-handed side, independently of the tooth position. Females usually have two embedded tusks, neither erupting (Nweeia et al., “Vestigial Tooth Anatomy and Tusk Nomenclature”). A narwhal has other pairs of upper teeth qualified as vestigial as they perform no function (Nweeia et al., “Considerations of Anatomy, Morphology, Evolution, and Function for Narwhal Dentition”). The nature of this tooth has been much discussed in the past to determine if narwhal tusks are incisors or canines. The incisor or canine teeth are determined “by their embryologic origins within identified areas of the dental lamina, the developing osseous premaxilla, maxilla and mandible and morphologic characteristics (Nweeia et al., “Vestigial Tooth Anatomy and Tusk Nomenclature”). Some observations that teeth were implanted in the intermaxillary bone, or an alveolus common to the maxilla and intermaxillary led some researchers to define this tooth as a peculiarly modified incisor tooth (Turner, 1872). However, the erupted tusk is located within the alveolar sockets in the maxillary bone, which led to defining them as canine. Thus, the tusk has the composition of a canine tooth but inverted. Normally, there would be a hard coating of enamel, dentin, cementum around the sensitive nerves, whereas in the narwhal tusk the nerves are on the outside and the dense material is on the inside. The enamel has been replaced by cementum as intermediary tissue to attach to the bone (Nweeia et al., “Vestigial Tooth Anatomy and Tusk Nomenclature”). The cementum is more flexible and contains less mineral content than in other animal’s tusks. The dentin contains millions of tubules traversing the mixture of protein and minerals, as stated in Fig. 1.
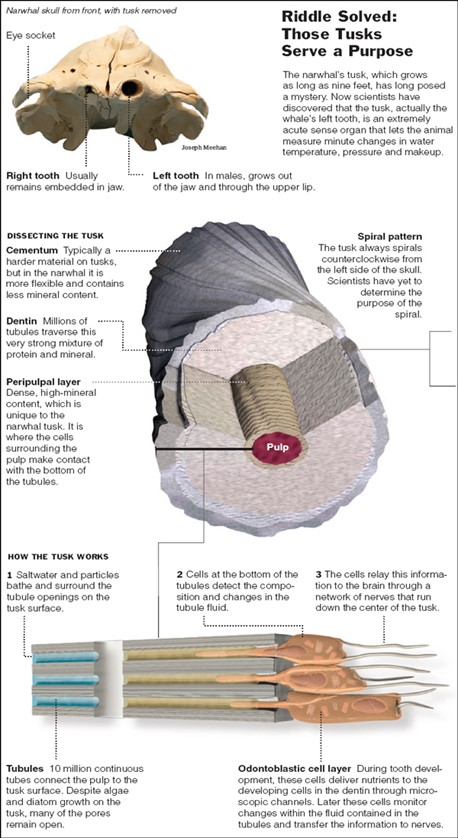
The tusk is made of ivory, which has a special composition. The dentine is composed of a matrix of a small particle in a ground substance containing dentinal tubules. Those tubules might be straight and aligned in sheets to form microlaminae in the length of the tusk; however, in the narwhal, the tubules are helical. The ivory matrix is the major component of the narwhal tusk (Locke, 2008). The cementum sheath around the dentine emerges from the skull into the sea, such that all connection with the periodontal fibers is lost and gives specific mechanical properties such as stiffness and toughness to the tusk (Brear et al., 1993). The narwhal tusk is continuously growing by constant deposition of material at the root.
Hypotheses for the Tusk’s Function
Discussion of Some of the Many Speculations
The function of the narwhal’s tusk is one intriguing, yet not fully answered, question in the scientific community. Of course, researchers have speculated for years on this subject but the fact that narwhals spend most of their lives hidden under the arctic ice complicates the research opportunities. Over the years, here are a few hypotheses that have come up for the function of the narwhal’s tusk. It has been speculated that the tusk serves to defend the narwhal against predators of the deep sea, or disturb them (Freuchen and Salomonsen, 1958). Some also thought the tusk was used as an ice drill, piercing through thin layers of ice for the narwhal’s breathing needs (Tomilin, 1967; Scoresby, 1820). Moreover, it was also theorized that the tusk was an organ involved in the narwhal’s cooling mechanism (Dow and Hollenberg, 1977). It was found with further research that a lot of these theories were outdated and highly improbable. However, there are still a few conjectures that remain plausible, namely the environmental sensor and sexual selection ones.
Environmental Sensor Theory
Let us start by analyzing the environmental sensor theory. As the narwhal’s tusk has a structure similar to a tooth’s, and since we know that teeth are partially made of a soft tissue called dentin that is filled with nerves, we can then conclude that a tusk contains many nerve endings. As discussed above, we also know the tusk has the particularity of being structured like an “inside out” tooth, due to its soft exterior and hard interior. Therefore, there are many nerve endings near the surface of the tusk. This leads to the environmental sensor theory, in which it is speculated that the tusk is used to detect parameters of the waters surrounding it, namely salt concentration, water pressure (and thus depth) and temperature (“Narwhals” | Cool Antarctica, 2001). In an experiment led by Dr. Martin Nweeia and colleagues, narwhals were placed in waters of various salinity. An increased heart rate was detected when in high salt concentration (high water concentration suggests danger and entrapment, as it means waters are freezing) and contrarily, a decreased heart rate was noticed when in fresh waters (Nweeia et al., “Considerations of Anatomy, Morphology, Evolution, and Function for Narwhal Dentition”). This thus suggests that narwhals may detect salinity variations. Even though Dr. Nweeia and colleagues encourage further research on the other potential parameters the tusk could detect, there are still a few flaws with this theory. For instance, if tusks are really those indispensable organs used in important water variable detection, it begs the question: “Why are females not equipped with this biological structure?”
Sexual Selection Theory
Although the tusk as an environmental sensor is not a completely rejected theory, the “scientific consensus” (Laidre, n.d.) of the answer to this question is that the tusk is an organ that evolved from sexual selection. According to Kristin Laidre — “University of Washington scientist who studies the ecology and population dynamics of Arctic marine mammals, including the impacts of climate change” (Laidre, n.d.), — “When the males are provided with weapons which females do not possess, there can hardly be a doubt that they… have been acquired through sexual selection” (Laidre, n.d.). Even though it is possible, although rare, for females to grow tusks, it may be speculated, when seeing two narwhals tusking, that these two combatant are males fighting for the female’s attention (Montemurno, 2019). One could ask: “Why such a colossal organ, requiring complicated growing mechanisms, simply to have a female’s cognizance?”
Indeed, it seems a little extravagant. However, sexual selection mechanisms are ubiquitous amongst living species, and take a greater role than one would expect. The peacock’s feathers, the deer’s antlers or the song and dances of countless bird species are only a few examples that spring quickly into our minds. Yet, many species, including humans, are subject to sexual selection and play an important role in the species development, as they can “help maintain dominance hierarchies” (Laidre, n.d.) or “help young males develop skills necessary for performance in adult sexual roles” (Laidre, n.d.). For those reasons, a lot of scientists believe the tusk is a trait that evolved strictly for the purpose of impressing female narwhals and thus ensuring reproduction.
Mechanisms Allowing the Tusks to Grow Straight
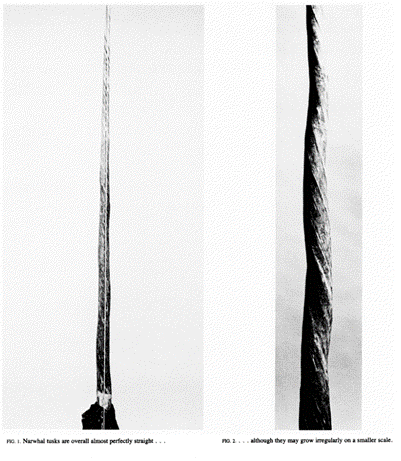
The narwhal tusk, unlike the tusks of the mammoth, boar or elephant, grows perfectly straight. It is also the only species of whale with a tusk. Most narwhals have only one tusk and it is found on the left-side of their heads; however, some narwhals do grow two. These cases are especially peculiar since both tusks grow in a left-handed spiral versus spiraling opposite one another (Kingsley and Ramsay, 1988). The narwhal tusk is a tooth and would grow curved at such lengths if not for perfect balancing of new material around the tooth root. A curved tusk would significantly hamper the swimming abilities of the narwhal. The evolutionary solution the narwhal developed to solve this issue of curvature is to grow its tooth in a spiral. The tusk twists as it grows, every part of its base spends even amounts of time in regions of the socket where growth is slightly faster or slower, evening out the effects (Kingsley and Ramsay, 1988).
Here it is seen how the tusk does curve as it grows, but the curves ‘even out’ almost perfectly, because the unevenness in the root tissue that causes curvature is evenly distributed by the continual rotation of the tooth as it grows (Kingsley and Ramsay, 1988).
Comparison between Elephants’ and Narwhals’ Tusks
During the process of evolution, tusks of different animals have evolved into different shapes and internal structures to align with their required functions and meet their different needs for strength and toughness.

As mentioned previously, tusks consist of a peripheral component, the cementum, a soft enamel layer, and a main core of dentine (Purk, 2017). The dentine is composed of peritubular dentin, a collagen matrix of intertubular dentin, and dentinal tubules that are about 5 µm in diameter (Locke, 2007). The structure of the tusk can be represented by the structure of reinforced concrete: “In the matrix of collagen fibers and particles, dentinal tubules are like the metal rods in the pebbles and cement of concrete” (Locke, 2007). Each dentinal tubule is encircled by peritubular dentin, and in between them, there is the collagen matrix of intertubular dentin (Purk, 2017). Within the dentin, dentinal tubules are aligned into sheets. These axial sheets of aligned dentinal tubules are called microlaminae (Locke, 2007).
Different kinds of tusks are characterized by the shape, orientation, and position of the dentinal tubules (Locke, 2007). Therefore, by examining the shape, orientation, and position of the dentinal tubules within the dentin of the elephant and narwhal tusks, we would like to provide a suggestion that might explain why elephant tusks are normally curved whereas narwhal tusks are straight.
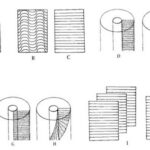
To observe a general pattern of the dentinal tubules arrangement in three dimensions, four different variables need to be considered (Locke, 2007). The first variable is the shape of the dentinal tubules. They are commonly straight but can also be curled into wave-like shapes which can be found in pigs’ dentin (Fig. 5b and c). The second variable is the orientation of the dentinal tubules within the microlaminae. They can be transverse or angled to the tusk vertical axis (Fig. 5d and e). Then, the orientation of the microlaminae may also be different. For example, as shown in Fig. 5f and g, the microlaminae can be longitudinal and normal or longitudinal but angled to the bottom face of the tusk. Besides, they can also be helical in the tusk vertical axis (Fig. 5h). Finally, the orientation of the dentinal tubules in one microlaminae and its adjacent microlaminae can be the same or different (Fig. 5i and j). If the orientation is different in the adjacent microlaminae sheet, an overall helicoidal pattern of the arrangement of dentinal tubules can be observed. Narwhal tusks are characterized by this helicoidal pattern (Locke, 2007).
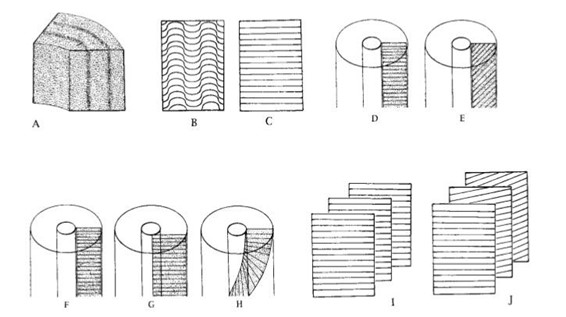
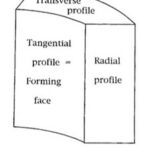
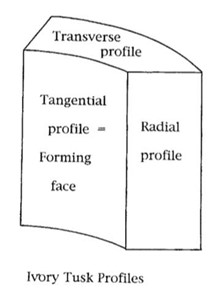
To observe and study the pattern of dentinal tubules arrangement in elephants and narwhals’ tusks, in the research from Locke (2007), the tusks were cut in a specific way which is shown in Fig. 5 so that radial, transverse, and tangential faces were exposed. After the patterns in the three different surfaces were inspected with epi-illumination, a three-dimensional tusk model was built (Locke, 2007). Then the dentinal tubules arrangement of narwhal and elephant’s tusks were examined in transverse, tangential, and radial profiles respectively.
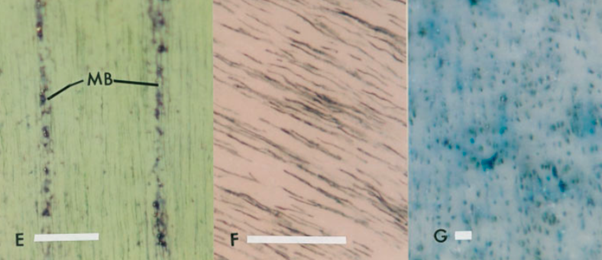
In the transverse profiles of the narwhal tusk, the area within and in between the black shaded region is where the dentinal tubules are located (Fig. 7e). The transverse profiles show that the dentinal tubules are radially oriented. In addition, in the transverse profiles, dentinal tubules are in short sections which indicates that they are not exactly oriented transversely but at an angle to the tusk vertical axis since, otherwise, they would be shown in a complete form with very long length (Locke, 2007). In the radial profiles (Fig. 7f), the black lines are the dentinal tubules. It also indicates that the dentinal tubules orient at an angle to the tusk vertical axis (Locke, 2007). Besides, the black dots in the tangential profiles (Fig. 7g) show that the dentinal tubules are twisted and form a helical structure through the thickness of narwhal’s dentine (Locke, 2007). As mentioned previously, the peripheral cementum of the narwhal tusks is twisted into a counterclockwise spiral from the base to the tip. These three different profiles have shown that within the dentine, the dentinal tubules are arranged in a helical structure and the microlaminae are arranged in a clockwise helix (Locke, 2007). It is interesting to note that the peripheral cementum is in a counterclockwise spiral whereas the inner dentine is in a clockwise helix. This opposing double spiral structure is one of the striking features of narwhals.
However, compared to narwhals’ tusks, the tusks of elephants are in different shapes and have different internal structures. In the transverse profiles (Fig. 8a) of stained elephant and mammoth ivory, there is a unique checkered pattern which is also known as the Schreger pattern (Locke, 2007). The tangential profiles (Fig. 8b) show that the arrangement of the dentinal tubules is in a feather-like pattern. Each row of the feather pattern corresponds to a Schreger column shown in Fig. 8a. It is important to note that this feather-like pattern indicates that the dentinal tubules are arranged in truncated helicoids instead of complete helicoids (Locke, 2007). This truncated-helicoidal model is also supported by the radial profiles (Fig. 8c) since in the radial profiles, there is a long wave pattern instead of complete helicoids (Locke, 2007). This truncated-helicoidal arrangement of the dentinal tubules is the one of the key differences between the structures of elephants and narwhals’ tusks.
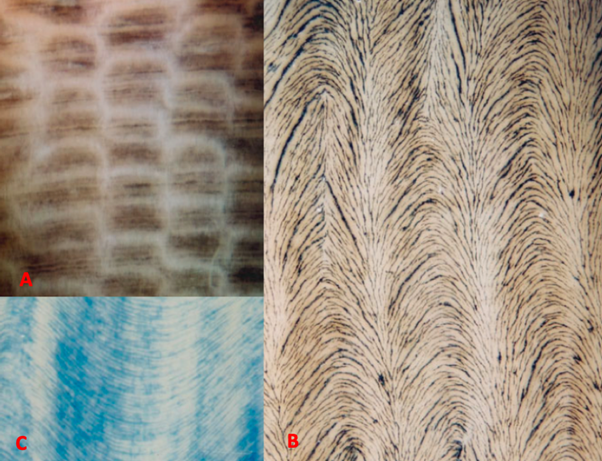
According to Currey et al. (1994) and Kingsley and Ramsay (1988), the opposing double spiral structure might be the mechanism that allows narwhals to have straight tusks. By comparing the differences between the structures of elephants’ tusks and narwhals’ tusks, we can conclude that the presence of the truncated helicoidal structure and the lack of this opposing double spiral structure might be one of the reasons why elephants’ tusks are curved.
Conclusion
The design and method of growth of the Narwhal tusk is very unique, being in essence an inside-out tooth, growing on a straight axis and always in a left-hand spiral. Today, the reasons for the spiraled growth are known to be for the straight growth of the tooth, although the use of the tusk remains unresolved. Indeed, there are multiple plausible competing theories this paper has explored, with the tusk likely being for sexual character and/or acting as some sort of sensory organ, but no theory has been confirmed. The rotational method for straight-axis growth is remarkable and rare, with the helicoidal pattern of dentin differentiating the narwhal tusk from the tusks of other animals. The singular nature of the narwhal tusk, isolated to such a unique creature, is precisely what makes the study of it so important and fascinating. Further research into its function and evolution is a necessity, as such special cases are those where there stands the most to be learned, and they can say the most about the environment that shaped them.
References
Antarctica, C. (2001). Narwhals – Facts and Adaptations. Retrieved from https://www.coolantarctica.com/Antarctica%20fact%20file/wildlife/Arctic_animals/narwhal-arctic-whale.php
Brear, K., Currey, J., Kingsley, M., & Ramsay, M. (2009). The mechanical design of the tusk of the narwhal (Monodon nonoceros: Cetacea). Journal of Zoology, 230, 411-423. doi:10.1111/j.1469-7998.1993.tb02693.x
Constantine, D., & Gröndahl, M. (2005). Riddle Solved In: The New York Times. Retrieved from https://archive.nytimes.com/www.nytimes.com/imagepages/2005/12/12/science/20051213_NARWHAL_GRAPHIC1.html
Currey, J. D., Brear, K., & Zioupos, P. (1994). Dependence of mechanical properties on fibre angle in narwhal tusk, a highly oriented biological composite. Journal of Biomechanics, 27(7), 885-897. doi:10.1016/0021-9290(94)90261-5
Dow, P. R., & Hollenberg, M. J. (1977). The tuskal pulp of the narwhal, Monodon monoceros. Oral Surgery, Oral Medicine, Oral Pathology, 44(1), 135-146. doi:10.1016/0030-4220(77)90255-9
Freuchen, P., & Salomonsen, F. (1958). The Artic Year (1 ed.): Putnam.
Graham, Z. A., Garde, E., Heide-Jørgensen, M. P., & Palaoro, A. V. (2020). The longer the better: evidence that narwhal tusks are sexually selected. Biology Letters, 16(3), 20190950. doi:doi:10.1098/rsbl.2019.0950
Kingsley, M., & Ramsay, M. (1988). The Spiral in the Tusk of the Narwhal. Arctic, 41. doi:10.14430/arctic1723
Laidre, K. (n.d.). Narwhal FAQ. Retrieved from http://staff.washington.edu/klaidre/narwhal-faq/
Locke, M. (2008). Structure of ivory. Journal of Morphology, 269(4), 423-450. doi:https://doi.org/10.1002/jmor.10585
Montemurno, M. (2019). What Exactly IS a Narwhal Tusk? Retrieved from https://oceanconservancy.org/blog/2019/03/08/exactly-narwhal-tusk/
Nweeia, M., Eichmiller, F., Nutarak, C., Eidelman, N., Giuseppetti, A., Quinn, J., . . . Angnatsiak, D. (2008). Considerations Of Anatomy, Morphology, Evolution, and Function for Narwhal Dentition.
Nweeia, M. T., Eichmiller, F. C., Hauschka, P. V., Tyler, E., Mead, J. G., Potter, C. W., . . . Black, S. R. (2012). Vestigial Tooth Anatomy and Tusk Nomenclature for Monodon Monoceros. The Anatomical Record, 295(6), 1006-1016. doi:10.1002/ar.22449
Purk, J. H. (2017). 8 – Morphologic and structural analysis of material-tissue interfaces relevant to dental reconstruction. In P. Spencer & A. Misra (Eds.), Material-Tissue Interfacial Phenomena (pp. 205-229): Woodhead Publishing. Retrieved from https://www.sciencedirect.com/science/article/pii/B978008100330500008X
Scoresby, W. (2011). An Account of the Arctic Regions: With a History and Description of the Northern Whale-Fishery (Vol. 1). Cambridge: Cambridge University Press. Retrieved from https://www.cambridge.org/core/books/an-account-of-the-arctic-regions/FDDD133256BB26AEF96F5F01B1F97FFF
Tomilin, A. G. (1967). Cetacea. Jerusalem: Israel Program for Scientific Translations.
Turner. (1872). Some Observations on the Dentition of the Narwhal (Monodon Monoceros). Journal of Anatomy and Physiology, 7(Pt 1), 75-79.