Abstract
The beauty of shells hides an elaborate interrelationship of structures that provides a protective layer against environmental and predatorial threats. The constant need to defend their soft body has driven mollusks to develop complex shell structures that maximize their chances of survival. This essay reviews the understanding of the process of biomineralization and the roles of inorganic and organic molecules in mollusk shell formation and structure. This includes the uptake, movement and organization of precursor ions into various crystal lattices. It also details the involvement of organic macromolecules as calcification inhibitors, and structural supports for the architectural organization of the shell. Interestingly, two phyla have separately developed and utilized similar processes. The chemical, mineral and structural machinery described in mollusks closely resemble that of brachiopods. A comparative analysis is conducted to describe the parallel, yet independent, evolution of these shell formation mechanisms. This concurrent evolution of shell features furthers our understanding of how adaptive the shell mineralization process of mollusks must be to respond to their survival needs.
Introduction
About 550 to 580 million years ago, the first mollusks crawled on the seabed without a shell.In doing so, their ventral and dorsal sides were exposed to environmental constraints. The internal organs of mollusks were protected by a thick layer of tissue covered with a rigid cuticle. Over the centuries, there has been a union between the calcium carbonate ingested by the mollusk and the cuticle. This process has increased the resistance of this protective coating. This evolutionary transformation is thought to have given rise to the premise for the formation of a single shell.
Mollusks, invertebrate organisms, are one of the largest branches of the animal kingdom, with more than 100,000 species, such as gastropods, abalones, or mussels. These living organisms show great diversity and can possess various shell types. Many mollusks possess shells that are essential for their survival and evolution as it helps in protecting their soft body from environmental stresses. For example, predation represents one of these environmental constraints. The shell also helps to give optimal support to the soft body of the mollusk.
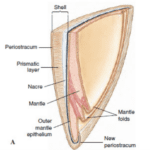
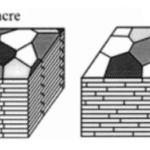
The exoskeleton shell is composed of an inorganic matrix, i.e., calcium carbonate, which is secreted by the mantle of the mollusk. The mantle corresponds to a tissue that adheres to the inner wall of the opening of the shell. It is important to note that organic matrices are also present in the form of proteins and affect the shell’s creation. Thus, it is possible to separate the shell of a mollusk into three distinct parts, namely the periostracum, the middle prismatic layer and the nacreous layer. The periostracum represents a thin envelope that surrounds the shell’s outer layer. This envelope is composed of conchiolin, which corresponds to a complex of polysaccharides and chitinous proteins. The primary function of the periostracum is to limit the effects of erosion on the underlying limestone layers. The middle prismatic layer is made up of calcium carbonate crystals in the form of calcite. Then, the inner nacreous layer is located on the inner face of the shell, the side of the mantle. This last layer is an association of aragonite lamellae (Figure 1).
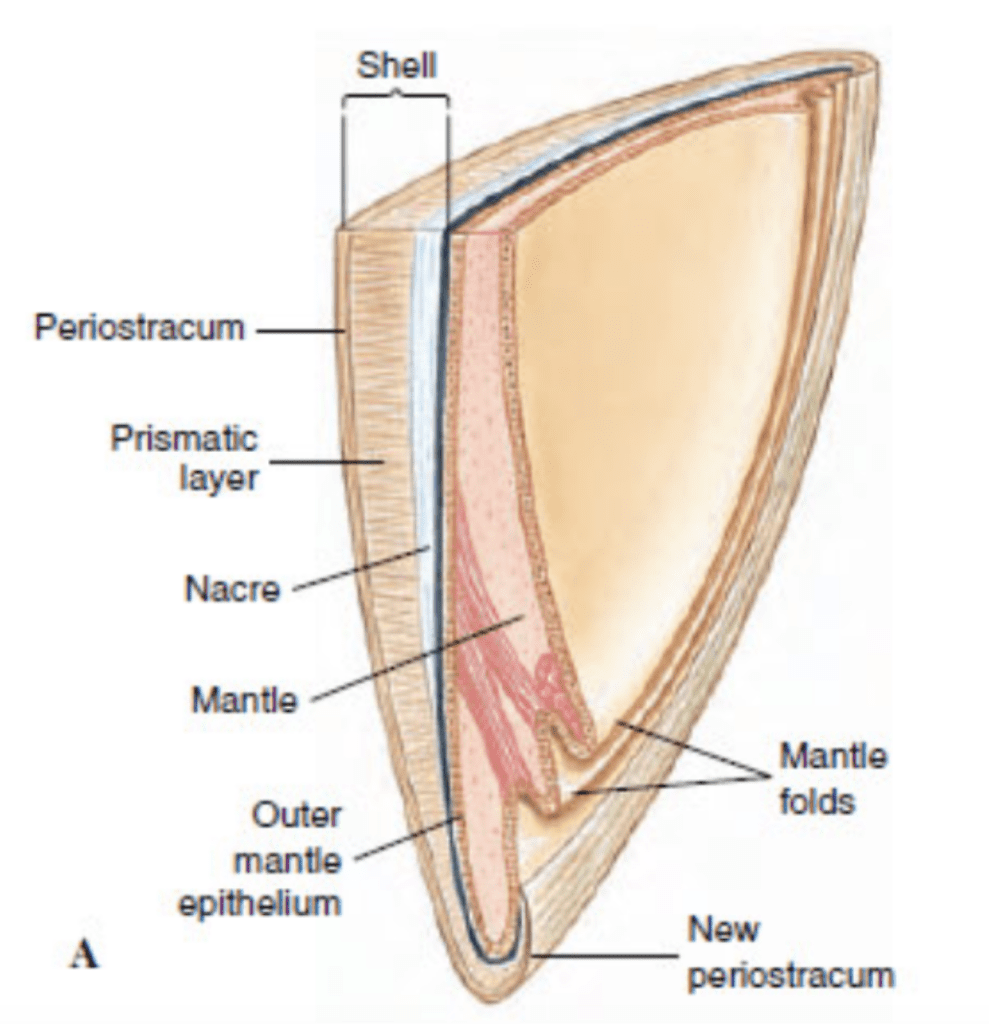
Fig. 1. Vertical cross-section of a bivalve’s shell and mantle (Adapted from Patrick De Deckker et al., 2016).
What is so incredible about the molluscan shell, is that it is primarily made from mineralized calcium carbonate. These minerals are quite brittle, easily fractured and cannot withstand the mechanical stresses to which mollusks are subjected to in their environment. To solve this, a complex shell-forming process that utilizes both calcium carbonate minerals, as well as a strengthening organic matrix, is employed.
This paper will develop an in-depth analysis of exactly how these shells come to be. Calcium carbonate, as the material base of the shell, will be examined, along with its mineralized forms and the differences between them. The machinery used to convert solubilized calcium carbonate found in sea water into a protective coat will also be analyzed.
Elemental concepts of biomineralization
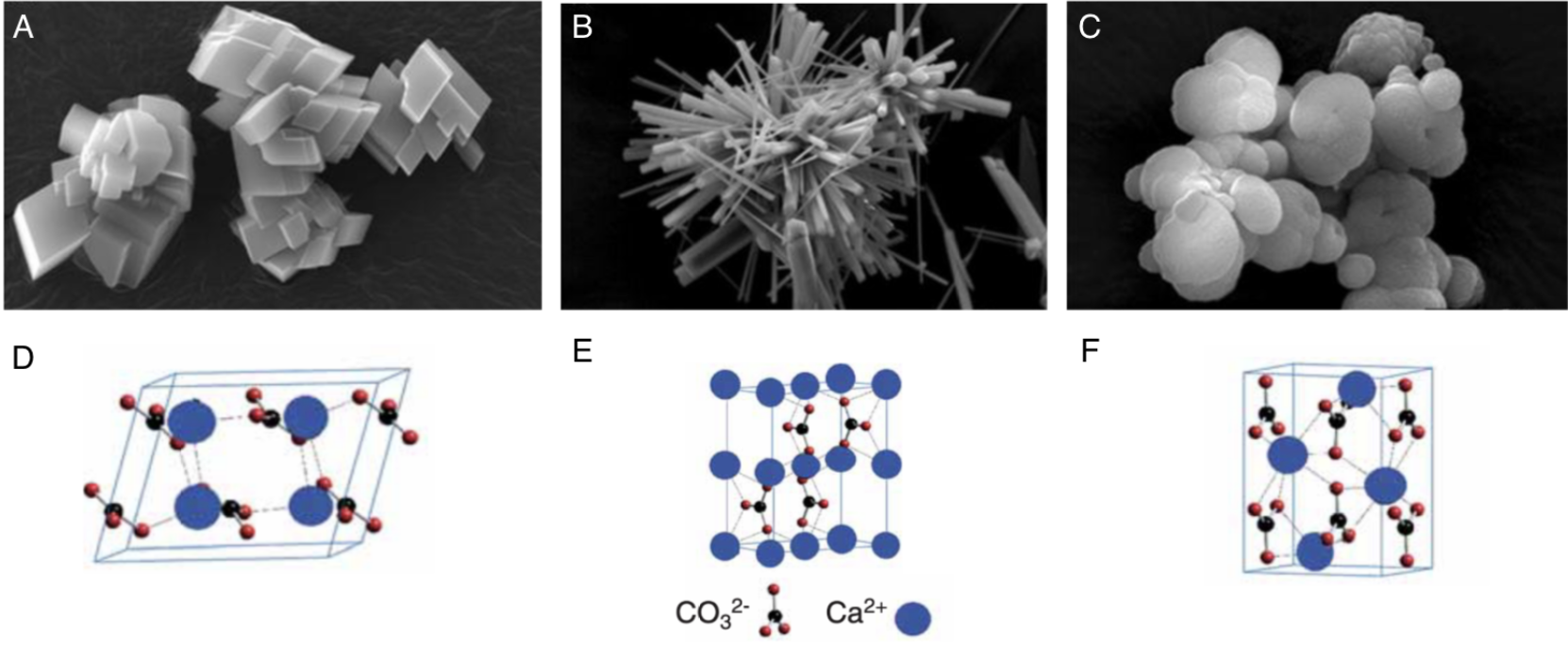
The process of biomineralization consists in the precipitation of minerals to hard tissues composed of organic (proteins, lipids, polysaccharides) and inorganic (Calcium carbonate CaCO3) compounds, such as shells and bones. (Estroff, 2008; Louis et al., 2022) It is important to note that the term “bio” refers to the fact that the process is driven in a living organism. Then, the term “mineralization” refers to the fact that the product of this process is composed of calcite or aragonite minerals. The range of inorganic compounds produced through biomineralization includes calcium phosphate, silicon dioxide, iron dioxides, calcium carbonate and calcium oxalate. The main inorganic constituent of the shell is calcium carbonate, commonly known as limestone. This section will focus on the calcification in seashells, which produces three types of crystal arrangements (polymorphs): calcite, aragonite, and occasionally, vaterite (Figure 2).

Fig. 2. Scanning electron microscope (SEM) images of synthesized calcium carbonate polymorphs (A. calcite, B aragonite, C vaterite) and their associated schematic crystal structures (D. calcite, E. aragonite, F. vaterite). Adapted from (Maleki Dizaj et al., 2015; Ševčík et al., 2018)
The production of calcium carbonate is described by the following chemical formula:
Ca2+ + HCO3– ⇌ CaCO3 (s) + H+ (1)
where calcium and bicarbonate ions react to form calcium carbonate and an H+ proton. The chemical equation (1) is reversible because it is possible for the reaction to occur in both directions, either from left to right or from right to left (De Deckker et al., 2016). If the chemical reaction occurs from left to right, it corresponds to the precipitation of calcium carbonate. The precipitation that occurs in shell formation will form an ordered 3-dimensional pattern, thus a crystal. The crystals that are formed are aragonites. However, when the reaction occurs from right to left, it corresponds to the dissolution of aragonite. The dissolution phenomenon forms a homogeneous mixture containing a solute and a solvent (Larsen, 2015).
The biomineralization of a shell also includes the interaction of the resultant calcium carbonate with macromolecules to form biominerals. (Marin et al., 2012) Indeed, the mollusk shell is composed of 95-99.9% calcite and aragonite, while the remaining 0.1-5% includes proteins, glycoproteins, polysaccharides and vaterite (Nudelman, 2015). This combination of inorganic calcium carbonate and organic components enhances the shell structure’s mechanical properties and plays an important role in the mineralization process.
The movement of precursor ions in the calcium carbonate formation process
The biomineralization process begins with the uptake of precursor ions, calcium and bicarbonate, which can be extracted from the external environment or from the mollusk’s own metabolism. The relative concentration of ions in a mollusk is comparable to that of seawater, except with a larger proportion of calcium and bicarbonate. This indicates that most of the calcium and bicarbonate ions originate from seawater, whereas the surplus is provided through its metabolism (Marin et al., 2007). Calcium ions are from seawater absorbed in the gills, whereas the remaining portion is produced through digestion. These two factors dictate that shell growth is closely related to temperature, salinity, and food availability (Larsen, 2015).
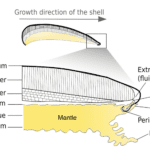
These ions are then transported toward the mineralization site in two ways. The ions can move in soluble form in the hemolymph, a fluid analogous to blood that is found in organisms such as arthropods, insects and mollusks.(Machałowski & Jesionowski, 2020; Marin et al., 2012). Or, they can alternatively move in clustered form, granules, which can be intra- or extracellular. This form offers additional advantages such as being compact in volume and trapping heavy metals by binding to silver, aluminum, cadmium, cobalt, chromium, copper, iron, manganese, nickel, lead, and zinc, and being readily available for repairs. (Marin et al., 2012; Simkiss, 1977) The calcification process takes place in the extrapallial space (EPS) (Figure 3), a space filled with the extrapallial fluid (EPF), a supersaturated fluid containing calcium and carbonate ions (Furuhashi et al., 2009; Louis et al., 2022). The calcium ions can reach the EPS by diffusion, through Ca2+-ATPase (a form of P-ATPase that transfers calcium) or via intercellular spaces. In contrast, the bicarbonate ions are transported to the EPS through HCO3–/Cl– exchangers and HCO3–/Na+ transporters (Figure 3) (Xiang et al., 2013). The resulting H+ from the calcification process can overly acidify the medium, which is processed either by reabsorption in the mantle or extrusion via H+-ATPases transmembrane pumps (Figure 3). Alternatively, because the excess reagent is bicarbonate, it can chemically react with the hydron (H+) to produce carbon dioxide (CO2) and water (H2O) that will be gradually dispersed in the mollusk environment (Larsen, 2015).

Fig. 3. Schematic calcification model of bivalve shells. Calcium ions reach the EPF via diffusion (purple), Ca2+-ATPase (red) or intercellular spaces. Bicarbonate ions reach the EPF via HCO3–/Cl– exchangers (dark blue) and HCO3–/Na+ transporters (light blue). H+ can be absorbed or released by the mantle via H+-ATPases transmembrane pumps (dark red). Adapted from (Louis et al., 2022).
All of these pathways reside within the mantle, which needs to be crossed by all ions coming from the mollusk’s body in order to reach the EPS (Figure 4). The mantle comprises the inner epithelium, the outer epithelium, and internal tissue such as pallial muscle or nerve fibers, and has the role of gradually secreting extrapallial fluid into the extrapallial cavity (Benzerara, 2022). The edge of the mantle consists of multiple folds, three in bivalves and two in gastropods, one of which forms the periostracal groove. The periostracum (Figure 4) contributes to provide support for the mineralization process and acts as a barrier between the mineralization area and the surrounding environments (Marin et al., 2007). The concept of a confined space where minerals can be synthesized is an essential criterion of biologically controlled mineralization (Talham, 2002), as it allows for inorganic ions and macromolecules to interact in a controlled manner to form specific structures (Marin et al., 2012).

Fig. 4. View of M. galloprovincialis demonstrating various components involved in mineralization. Adapted from (Louis et al., 2022).
The mechanism of crystallization during biomineralization
The transition from aqueous precursor ions to crystalline biomineral occurs at the distal border of the EPS. This process is controlled, as the EPF contains organic mineralization inhibitors, which prevent the uncontrolled precipitation of calcium carbonate. Because the EPF is supersaturated with calcium and bicarbonate, these organic molecules (particularly acidic proteins) transiently maintain calcium in solution by inhibiting or allowing precipitation when needed.
As mentioned earlier, some organic compounds that are either produced from the mollusk’s cells or ingested, including proteins, glycoproteins, acidic polysaccharides, chitin and lipids, combine with calcium carbonate to form the shell crystal structure. The various hierarchical crystal shapes, or microstructures (Figure 5), are influenced by three main factors: macromolecules, crystal competition and self-organization of the matrix structure (Louis et al., 2022).

Fig. 5. SEM images illustrating examples of bivalve microstructures: cross-foliated calcite (A), fibro-prismatic calcite (B), chalky calcite (C), nacre of a deep-sea mussel (D), prismatic aragonite (E) and contact between prismatic and nacreous layer (F) (Louis et al., 2022).
The organic macromolecules, polysaccharides and proteins, guide the crystallization process by varying their charge densities (Giuffre et al., 2013). Crystal competition allows for the creation of a well-defined growth front. The growth front will be sub-perpendicular or perpendicular to the growth surface, as the fastest growing crystals of the aggregate follow this direction. These crystals will outcompete crystals that are growing obliquely, forcing them to progressively follow a co-oriented growth direction (Checa, 2018). This phenomenon occurs abundantly in the shell’s prisms, which are calcitic and aragonitic needles secreted from the inner surface of the periostracum at the growing shell edge (Figure 6).

Fig. 6. SEM images of examples of crystal competition in prismatic microstructures of bivalve shells, where the growth front is perpendicular or perpendicular to the growth surface. In A (Unio pictorum) and B (Anodonta), the aragonitic prisms are in contact with the nacreous layer. In C (Mytilus edulis), are thin oblique calcitic prisms. In D (Atrina rigida), they are calcitic prisms (Marin et al., 2007).
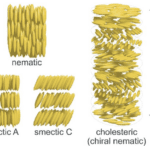
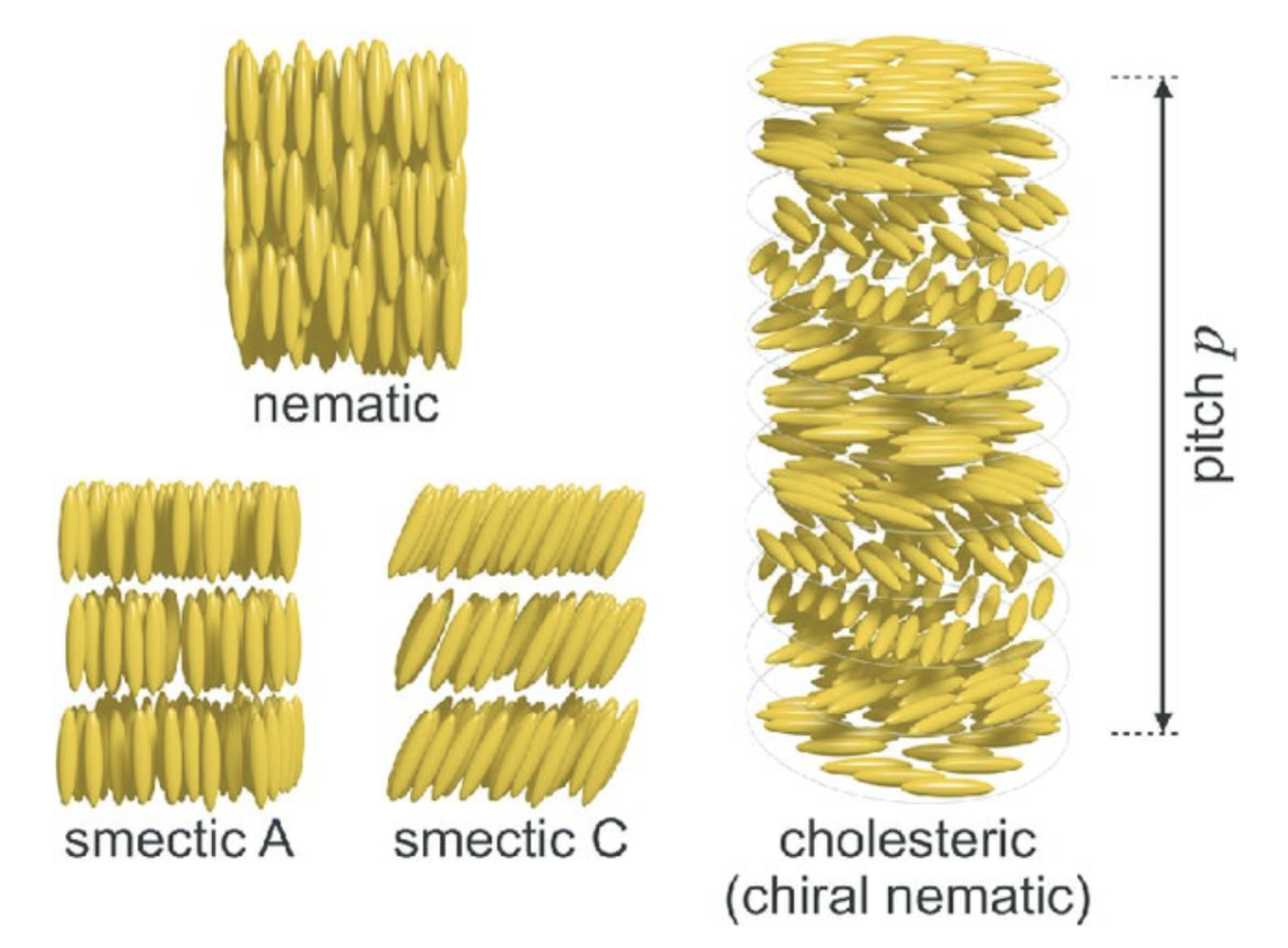
Finally, the self-organization of the matrix structure allows for precise control of the distribution, orientation and distances between nanolayers within the shell. This can be accomplished through liquid crystals, a state of matter that has properties of both liquids and crystals. A common configuration for liquid crystal is the cholesteric phase, where fibers align in organized parallel planes, with each plane rotating slightly with respect to the preceding plane. The orientation will be the exact same once a certain distance is reached (Figure 7). These patterns have been recognized in the periostracum of gastropods (Checa, 2018).

Fig. 7. Schematic of liquid crystal mesophases. The nematic phase only oriented on the long axis aligned with the elongated molecules. The smectic phases (smectic A and smectic C), which exhibit two types of layered orientations and the chiral nematic (cholesteric) phase, where the individual nematic layers stack together with a twist leading to helicoidal order with a pitch p. (Parry & Savin, 2016).
The cholesteric phase is one of three possible chiral mesophases of liquid crystals, which also include the smectic and nematic phases (Figure 7) (Bouligand, 1972). Chiral mesophases are a state of matter between a liquid and a solid, which self-assemble to form organized patterns due to the chirality of the particles (characteristic of an object that cannot be superimposed to its mirror image) (Calus et al., 2016). The cholesteric phase, specifically, has been observed in a variety of biological materials such as DNA, chromatin, collagen and viruses, and are better known under the name Bouligand structures (Mitov, 2017).
The stages of aragonite crystallization
As highlighted by the chemical reaction (1), the phenomenon of calcium carbonate crystallization is necessary for the biomineralization process (Boon et al., 2020). The formation of a crystal separates into two distinct stages, nucleation and growth (Figure 8).

Fig. 8. The crystallization phenomenon requires two steps, nucleation and growth. Adapted from (Sauter et al., 2015).
The nucleation process also separates into two parts, the mechanisms of primary nucleation and secondary nucleation. Thus, during the mechanism of primary nucleation, the concentration of ions (Ca2+ and HCO3+) exceeds the solubility constant of aragonite (Ka). Precipitation is therefore possible when [Ca2+]*[HCO3+]>Ka. By agglomerating in the form of an aggregate, the solute constituents present in this supersaturated solution induces the formation of a crystalline germ (Benzerara, n.d.). Curie’s law governs crystalline germs. This law highlights a principle of energy minimization. Indeed, the crystal’s growth promotes a position of equilibrium that maintains optimal cohesion within the crystal network. Then, the aggregates will form many nuclei, which are fine crystals. The secondary nucleation mechanism forms other crystals from previously existing crystals through primary nucleation. Then, the second stage of crystallization, growth, increases the size of pre-existing crystal structures formed during nucleation (Larsen, 2015).
It is possible to establish a relationship between the nucleation rate and the surface energy. Surface energy is required to break intermolecular bonds in a solution (Tran et al., 2016). Assuming that the value of supersaturation is constant, it highlights that the smaller the surface energy, the higher the nucleation rate (Boon et al., 2020).
\begin{equation}\tag{2}
J=A\exp\left[\frac{-B\gamma^3}{\sigma^2}\right]
\end{equation}Thus, the variable J represents the nucleation rate. Variable A corresponds to a pre-exponential factor. Then, the term B is Boltzmann’s constant (1.38 × 10-23 m2· kg/s2· K-1). This variable B establishes a relationship between the temperature of a system and its energy on a microscopic scale. The variable γ corresponds to surface free energy, and the variable σ corresponds to supersaturation (Boon et al., 2020).
Growth units, entities composed of ions existing in the mother solution, settle on places where the crystal lattice has minimal energy. This step ensures crystal growth. The contact between the crystal lattice surface and the growth units forms integration sites (Sun & Bhushan, 2012). This step increases the layers on the surface of the crystal. Also, impurities occupying an integration site can slow the growth process.
The chemical relationship between aragonite and calcite.
Calcium carbonate, the product of the chemical reaction (1), comes in two distinct forms: calcite or aragonite. Thus, these two compounds have a crystal lattice characterized as crystals. A crystal lattice is a periodic and symmetric arrangement of atoms within a three-dimensional space. The rigid atomic structure of aragonite is an orthorhombic Bravais lattice (Larsen, 2015). From a microscopic point of view, the lengths of an orthorhombic unit cell (a, b and c) are unequal. While the angles α, β and γ have a common value, i.e. 90° (Figure 9).
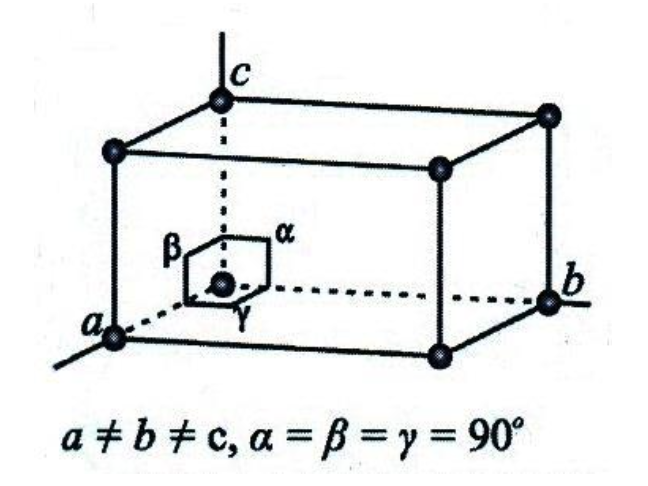
Fig. 9. An orthorhombic unit cell that has distinct lengths (a, b and c) and angles between the axes (α, β and γ) (Adapted from Larsen, 2015).
The structure of calcite is a network of rhombohedral Bravais (Larsen, 2015). From a microscopic point of view, only two of the three lengths of an orthorhombic unit cell (a, b and c) are equal. None of the angles α, β and γ are equal to 90° (Figure 10).

Fig. 10. A rhombohedral unit cell with distinct lengths (a, b and c) and angles between the axes (α, β and γ) (Adapted from Larsen, 2015).
The significant difference between these two crystal lattices lies in the arrangement of atoms that generate different physical and chemical properties (Larsen, 2015). Indeed, the thermodynamics of aragonite are more stable than calcite when temperatures and pressures are high. In contrast, calcite is much more stable within ambient conditions on the earth’s surface (Benzerara, n.d.).
By heating aragonite, its crystal lattice, which is stable when in contact with high temperatures, reconfigures itself to match the atomic arrangement of calcite. During this transformation, the heavy isotopes of carbon and oxygen present in the aragonite crystal lattice will also change so that they conform to the atomic structure of calcite. Therefore, the isotopes of these atoms will become lighter because there are fewer neutrons in the atom’s nucleus. Thus, this chemical conversion of aragonite to calcite is called epitaxial nucleation; it occurs due to a change in pressure or temperature (Sauter et al., 2015). This transformation results from the absence of thermodynamic equilibrium. In addition, the kinetics of this transformation, which occur under ambient conditions, are very slow. Therefore, this transformation of aragonite into calcite lasts several million years, i.e. on average, 10 million years. Since calcite and aragonite are closely related, these two compounds can coexist harmoniously (Larsen, 2015). Indeed, specific conditions favour the formation of aragonite on the earth’s surface to the detriment of calcite.
The conditions that favour the crystallization of aragonite are primarily the differences in the atomic structure of calcite and aragonite (Figure 11). It is important to note that the carbonate molecules in calcite are harmoniously arranged. While for aragonite, the carbonate molecules are dispersed within the compound.

Fig. 11. Aragonite and calcite are shown through a projection of crystal structures vertical to the CO3 layers (top row) and a horizontal projection of the CO3 layers (second row) (Bayarjargal et al., 2018).
By replacing calcium, the magnesium atom (Mg) can enter the calcite structure. This phenomenon destabilizes calcite’s structure because magnesium’s atomic radius is less than that of calcium. The calcite precipitation rate can decrease sharply due to the increase in magnesium concentration relative to calcium concentration (Boon et al., 2020). While for aragonite, the magnesium atom cannot enter the atomic structure of this compound. Thus, the increase in magnesium concentration does not impact the aragonite precipitation rate. Therefore, from a kinetic point of view, the crystallization of aragonite is favoured within the ambient conditions of the earth’s surface (Benzerara, n.d.).
The presence of aragonite in mother-of-pearl
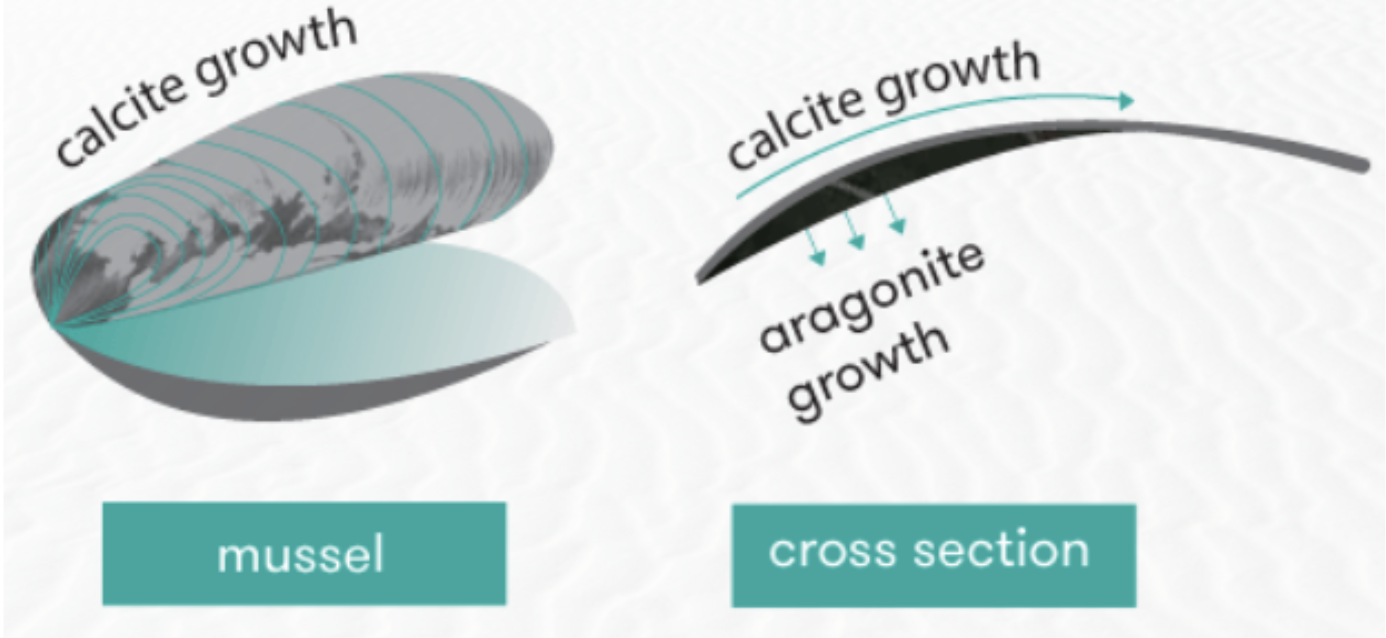
It is, therefore, essential to emphasize the relationship between the calcite layer and that which is composed of aragonite. Indeed, during the elongation of the shell, a layer of calcite accumulates on the outer edge of the shell (Patrick De Deckker et al., 2016). In comparison, the aragonite layer forms inside the shell wall. This characteristic portion of the shell is called the pearly layer (Figure 12).

Fig. 12. The illustration of a mussel shows calcite and aragonite growth regions. Adapted from (Patrick De Deckker et al., 2016).
Mother-of-pearl is a white material that lines the inner wall of the shell (Figure 13). The iridescent appearance characteristic of this material is due to the particular structure of the aragonite crystal lattice (Patrick De Deckker et al., 2016). Crystals can form a series of uniform plates that diffract light.

Fig. 13 An abalone shell (Haliotis tuberculata) is seen from an inside and outside point of view. The pearly layer occurs on the inner layer of the shell. Adapted from (Benzerara, n.d.).
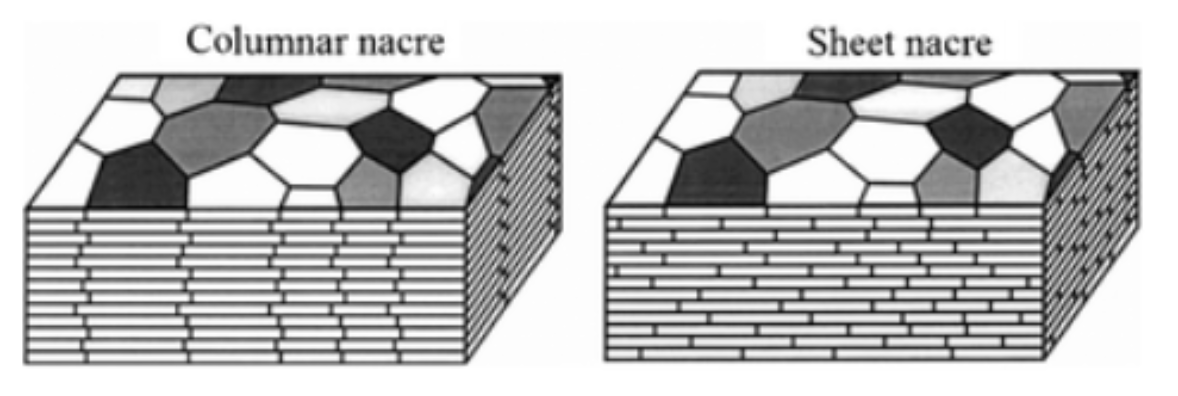
It is possible to give a distinctive appearance in the pearly microstructure with parallel filling of laminar tablets on the deposition surface (Larsen, 2015). It is important to note that the arrangement of laminar tablets, which are presented as rounded polygons, is divided into two classes, either column or sheet. The significant difference is that for the column layout, the centers of the laminar tablets are aligned with those below (Sun & Bhushan, 2012). While arranged in a sheet, laminar tablets are stacked like a brick wall (Figure14).
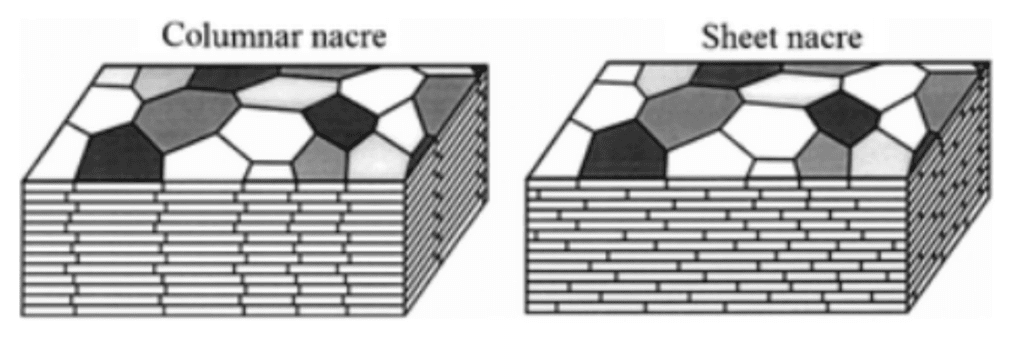
Fig. 14 A schematic illustration that shows the stacking of laminar tablets in a column or sheet arrangement (Adapted from Sun & Bhushan, 2012).
Functions of Organic Molecules within the Shell
As mentioned previously, while most of the shells’ weight is composed of inorganic mineral material (Marin et al., 2012), its distinguishing characteristic is its organic portion. On its own, calcium carbonate is brittle, but organic material allows the mollusk to organize and control the calcification of calcium carbonate to form sturdier structures, granting the shell much more structural stability and resistance. These organic compounds lie in crystalline matrices, molecular structures which guide the growth of the shell, arranging the minerals in sturdier orientations. In the micro-architecture of the shell’s layers, one could say that while calcium carbonate is the raw material, the organic portion is the architect of the microarchitecture of the shell.
The diversity of organic molecules present in shells’ matrices is immense, counting over 40 proteins, a variety of polysaccharides and even trace amounts of lipids (Marin et al., 2012).
In line with the paper’s objective of presenting the principal chemical processes occurring within mollusk shells, this subsection will only present the principal functions of proteins and chitin (a polysaccharide), the two most abundant organic molecule groups in mollusk shells.
Chitin functions
Chitin (Figure 15) is the second most abundant polysaccharide on the planet after cellulose and is known for its structural roles in the exoskeletons of insects and crustaceans as well as the cell walls of fungi and bacteria (Muzzarelli, 1977). Chitin’s structural strength comes from its beta glucose linkage. It is observed in three different forms: α, β, and γ.
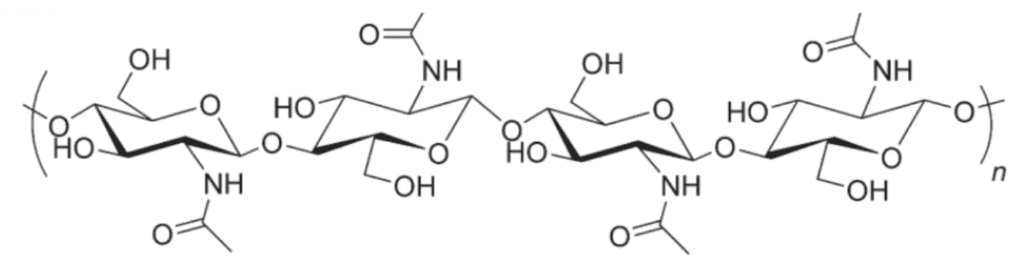
Fig. 15 Chitin molecule. As can be seen here, it is made from a chain of glucose subunits (Song et al. 2012).
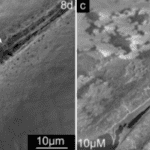
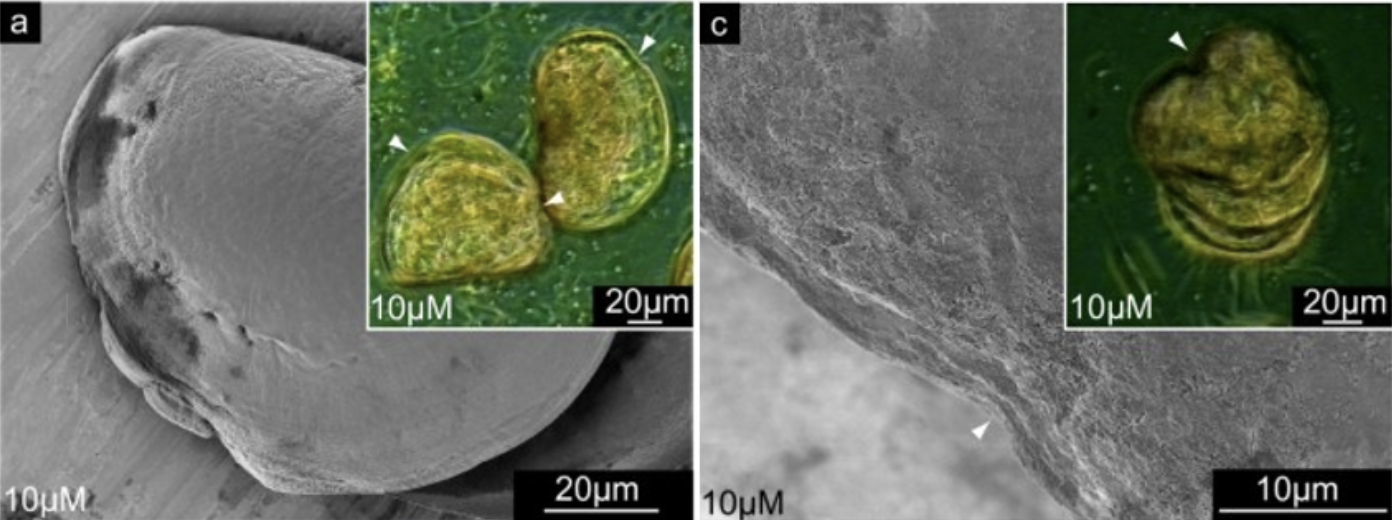
In mollusks, chitin is of the β type and is frequently documented (Marin et al., 2012). It is greatly suspected to play a role in shell formation since its inhibition in mollusk larvae using the drug NikkomycinZ results in significant deformations of shell structure, as seen in Figures 16 and 17 (Schönitzer & Weiss, 2007).

Fig. 16 Scanning electron microscopy (SEM) images of mollusk larvae grown in NikkomycinZ (left and right) exhibit abnormal curved deformations along their shell edge, not observed in larvae not grown in NikkomycinZ (not pictured) (Schönitzer and Weiss, 2007).

Fig. 17 Scanning electron microscopy (SEM) images of mollusk larvae after 8 days of growing in 0 µM (left) and 10 µM (right) of NikkomycinZ. Larvae grown in the absence of the inhibitor have teeth-like structures on their hinge. Larvae grown with the inhibitor sometimes do not develop teeth-like structures, affecting the functionality of their hinge (Schönitzer & Weiss, 2007).
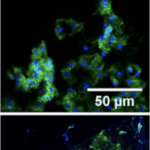
While the function of chitin is well-known in fungi, bacteria and other organisms, chitin’s role in mollusks is still being discovered. One of the proposed functions of chitin in mollusks is shell healing (Chan et al., 2018). To determine the chitin molecules’ position relative to mollusk cells during regeneration, researchers labelled the chitin of a damaged mollusk shell (in the process of regeneration) in a fluorescent green colour. As observed in Figure 18, the chitin molecules and mollusk cells were indeed closely positioned during the early hours of shell repair before spreading across the rest of the shell surface, suggesting that chitin plays a role in shell regeneration.

Fig. 18 Distribution of chitin (green) and cell nuclei (blue) after 5h, 18h, and 27h from top to bottom (Chan et al., 2018).
Proteins Functions
Ideally, experts would have liked to conjure a table of all proteins in mollusks along with each of their functions. However, proteomic studies have revealed that nacre composition is not uniform across species (Marin et al, 2012).
Indeed, different species harbour different proteins in their nacreous layer, leading to the conclusion that while nacre is a common feature across several mollusks, it has mutated multiple times since its original apparition in a common ancestor or was never present in a common ancestor at all but rather evolved convergently (Marin et al, 2012). Because of this lack of uniformity in nacre composition, most shell proteins discovered may be specific to the bivalve pearl oyster “Pinctada” and the edible gastropod “Haliotis”, the most studied mollusks due to their importance in the jewelry and food industries (Marin et al., 2012).
Prismalin and Nacrein: Calcification inhibition
Mollusks can regulate calcium carbonate calcification using inhibitors. Like a chisel chipping away at a rock, inhibitors can hinder calcification, altering the neat geometrical shapes of calcium carbonate crystals into the required shape for functional shell layers.
One such inhibitor is prismalin, which has been documented to alter the shape of calcium carbonate crystals in the prismatic layer. Indeed, as seen in Figure 19, the higher the concentration of prismalin, the greater the calcification inhibition.

Fig. 19 Spectrophotometer data from four different solutions. ○:10 mM Tris/HCl •: 0.2 μM prismalin ▵: 0.4 μM prismalin ▴: 2.0 μM prismalin. The filled triangle data points, representing the solution with the highest prismalin concentration, allowed the most light to pass through, indicating the greatest calcification inhibition.
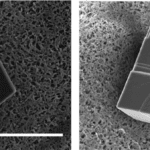
Furthermore, when the mineral was allowed to calcify without the presence of prismalin, it formed crystals with smooth hexahedral (six-sided) shapes. However, as seen in Figure 20, the crystals remained hexahedral in the presence of prismalin, but their surface was now “rough” and “steps were formed” (Suzuki et al., 2004). This result showed that prismalin alters calcium carbonate crystal geometry by “inhibiting the layer-by-layer growth of calcium carbonate crystals” (Suzuki et al., 2004).
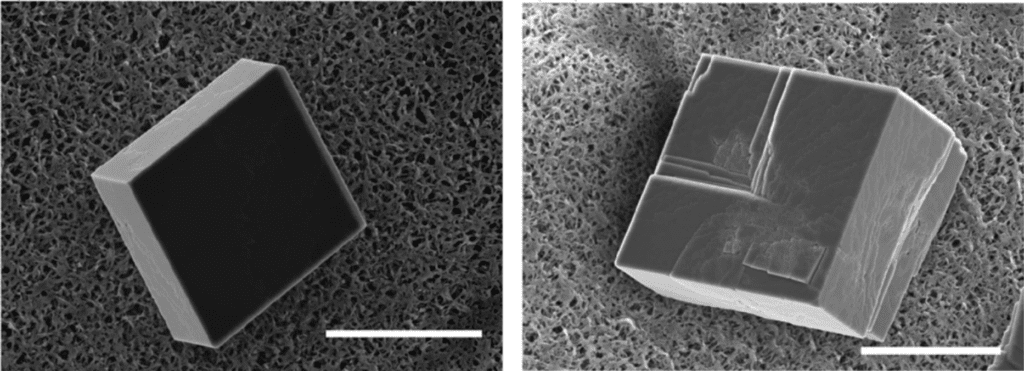
Fig. 20 Scanning electron microscope (SEM) images of two calcium carbonate crystals, one smooth and grown without prismalin (left) and one rough with “steps” on its surface grown with prismalin (right) (Suzuki et al., 2014). Scale bar: 15 µm.
Nacrein resides in both the nacreous and prismatic layers, and as seen in Figure 21, inhibits calcium carbonate calcification like prismalin (Song et al., 2019; Miyamoto et al., 2005).

Fig. 21 The higher the concentration of nacrein, the less the pH drops, indicating a reduction in release of H+ ions. As per Equation (1), this indicates an inhibition of calcium carbonate calcification (Miyamoto et al., 2005).
Pif and N16: Calcification Template and Chitin Binding
Many proteins serve as molds and templates for calcium carbonate calcification. Proteins Pif and N16 from the nacreous layer are prime examples. These molecules bind to themselves to form hydrogels to regulate calcium carbonate calcification and crystallization. As seen in Figure 22, once calcium carbonate has calcified in the regulating hydrogel, it makes use of a second property: chitin binding. Pif and N16’s chitin-binding sites allow them to bind to chitin and be added to the new nacreous layer being formed (Chang and Evans, 2015) (Perovic et al., 2014) (Song et al., 2019).

Fig. 22 Proposed mechanism of the role of the N16 protein in the formation of the nacreous layer framework. Blue circles are calcium carbonate minerals, while blue rectangles are calcified minerals. A represents the self-binding phase, B the hydrogel phase, C the crystallized phase, and D the chitin-binding phase (Adapted from Perovic et al., 2014).
See the Appendix for more proteins and protein functions which are beyond the scope of this paper.
Brachiopods and Brachiopod Shell Synthesis
We will now turn our attention briefly away from the Mollusca phylum and discuss brachiopods. Brachiopods are shell-calcifying marine invertebrates with a rich and well documented geological history tracing all the way back to the Cambrian era (Harper et al., 2017). They are similar to and are grouped with Mollusca under the lophotrochozoan umbrella, as can be seen in Figure 23 (Wernström, 2022).

Fig. 23 A section of the phylogenetic tree, including the lophotrochozoan classification, Brachiopoda, and Mollusca (Harper et al., 2017).
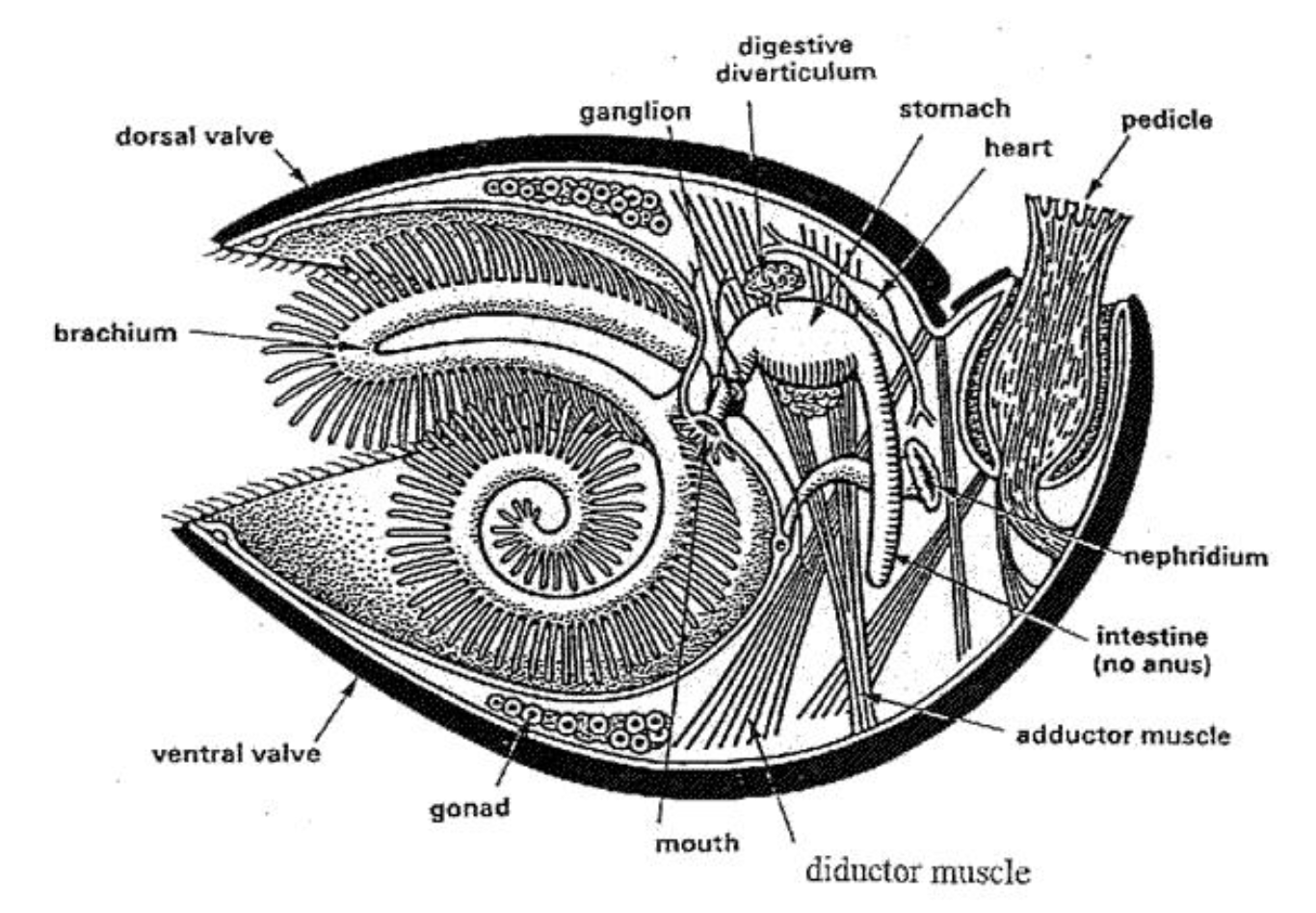
Their general macrostructure tends to include a shell containing 2 valves, which are held together with skeletonized brachial supports (Figure 24)(Harper et al., 2017).
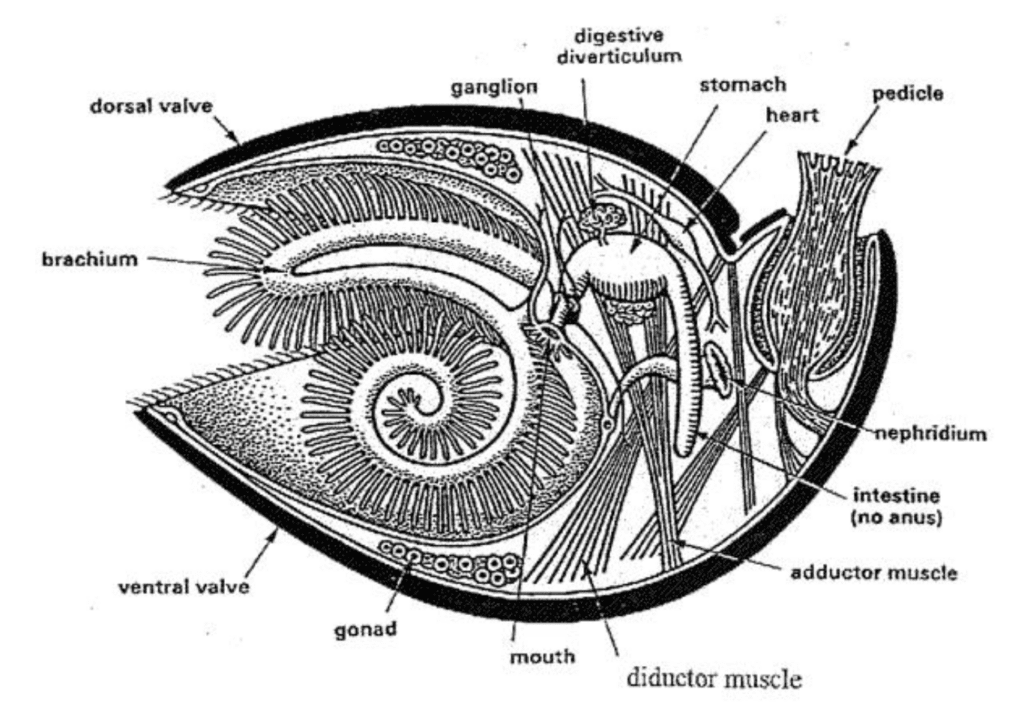
Fig. 24: Basic macrostructure of the Brachiopod shell. Organs are shown, though the key shell feature is the presence of two separate valves (dorsal and ventral). Note that the cut made to obtain this cross-section was made on the plane of symmetry of the brachiopod shell (Alex Trekeisen, n.d.).
On the inside, their shells contain impressions where their muscle system, mantle canals, and nervous system fit (Harper et al., 2017). The phylum itself contains three subphyla, Linguliformea, Craniiformea, and Rhynchonelliformea, each differing in their shell morphologies and soft-body anatomies (Harper et al., 2017). The relevant feature, which is important to this paper and conserved throughout the phylum, is the presence of a calcified protective shell, which is similar to the mollusk in many ways.
Chemically, brachiopod and molluscan shell compositions are very similar, though their mineralogies tend to differ slightly. Both are composed primarily of calcium carbonate, crystalized as either calcite or aragonite with a low concentration protein biopolymer matrix around the crystalline structures (Checa, 2018). These differences do result in morphologically different microstructures, as illustrated in Figure 25.
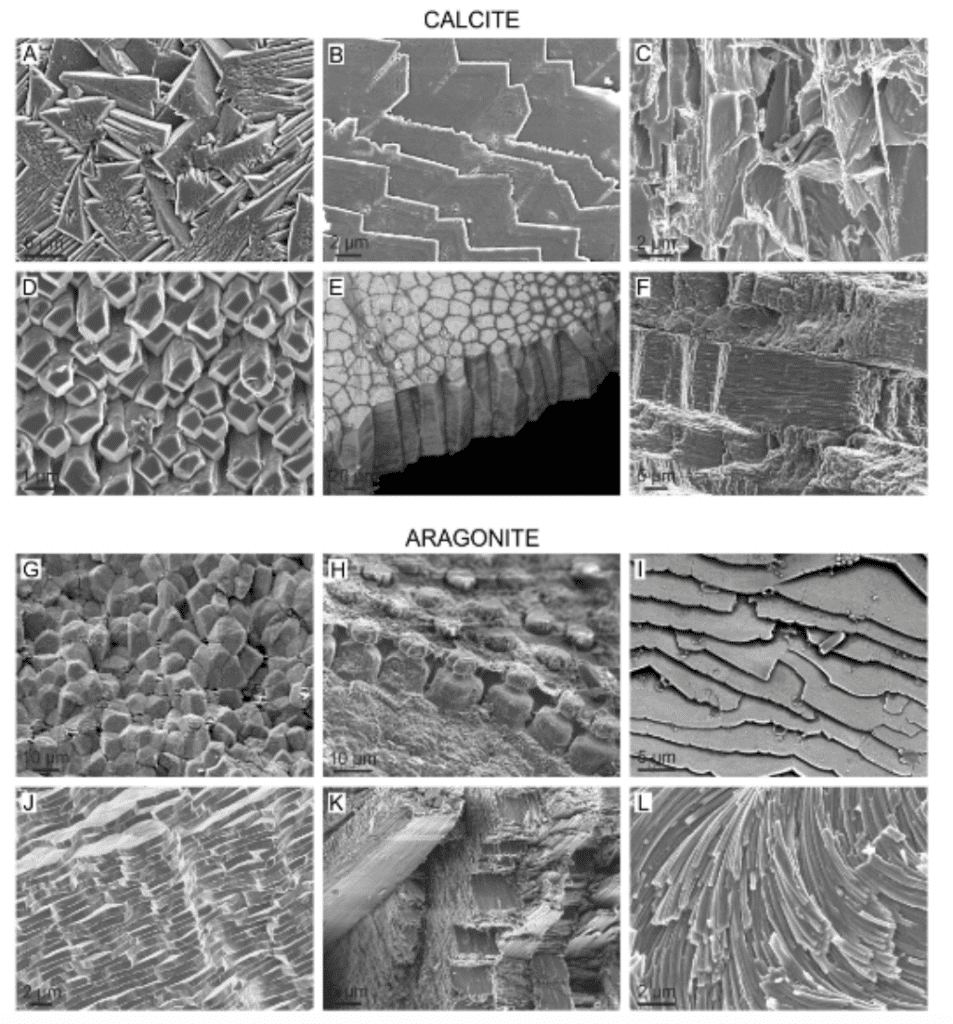
Fig. 25: Scanning electron microscope images of calcite and aragonite microstructures from the following mollusc species (from A to L): Cellana toreuma, Anomia ephippium, Crassostrea angulata, Mytilus chilensis, Pinctada margaritifera, Scutellastra tabularis, Entodesma navicula, Neotrigonia lamarckii, Rokopella euglypta, Neotrigonia bednalli, Semicassis granulata, Cuvierina columnella (Checa, 2018).
While the Mollusca phylum contains species exhibiting both aragonitic and calcitic mineralization, no brachiopods are known to exhibit aragonitic mineralization (Hall & Kennedy, 1967). This is a potential contributor to the variety of shell microstructures observed in mollusks.
The main difference between the shell structure of the two phyla lies in its formation process. The order and mechanism of mineral deposition and biopolymer formation, which informs on the microstructure, differs between the two, thus resulting in different shell microstructures (Simonet Roda et al., 2019). To illustrate, we will compare the formation process of calcitic brachiopod (so called) “semi-nacre” (Figure 26) (which is also sometimes present in mollusc shells), and molluscan aragonitic nacre.
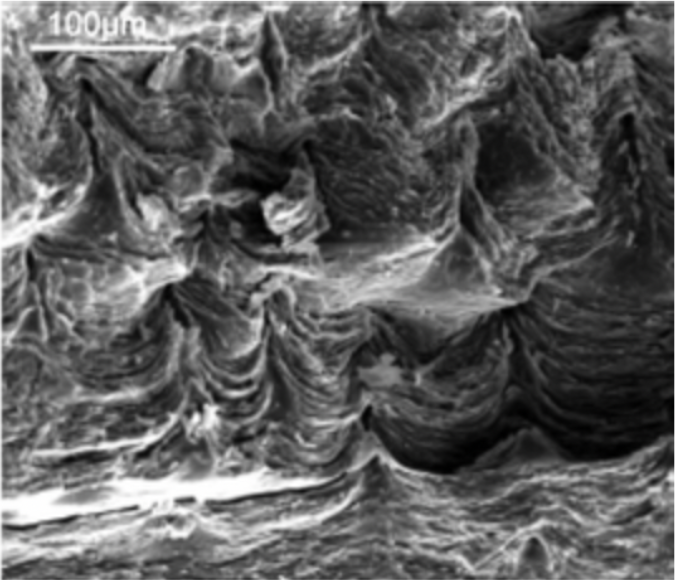
Fig. 26 Scanning electron microscope image (detecting secondary electrons) of the semi-nacre layer found in the mollusk Novocrania huttoni (England et al., 2007).
The first major contributor between these two structures revolves around the mantle’s positioning relative to the shell. In brachiopods semi-nacre, the mineral-secreting epithelial cell interface is always in contact with the mineral layer, whereas in mollusks, these two layers are always separated by an interlamellar membrane (Simonet Roda et al., 2019), which itself is composed of fibrils of β- chitin, and surrounded by acidic proteins (Checa et al., 2009). These membranes facilitate the growth of nacre tablets on top of one another, allowing them to form stacked towers instead of just growing upwards (Checa et al., 2009). The second major contributing factor here revolves around the order in which the mineral and biopolymer sections are added. In brachiopods, calcified fibers are covered with the biopolymeric layer as the final step, once the fiber has already grown (Simonet Roda et al., 2019). In the case of aragonitic nacre, the intermembrane surface forms first as a scaffold, into which calcium carbonate crystallizes into aragonite (Simonet Roda et al., 2019).
Thus, while the chemical, and even sometimes mineral, identity of shells may be identical, the mechanism of assembly is ultimately what will inform on the final observed microstructure. In fact, electron backscatter diffraction has been used to compare the ultrastructure, substructure, and crystallography of both nacre and semi-nacre (Figure 27) (England et al., 2007).
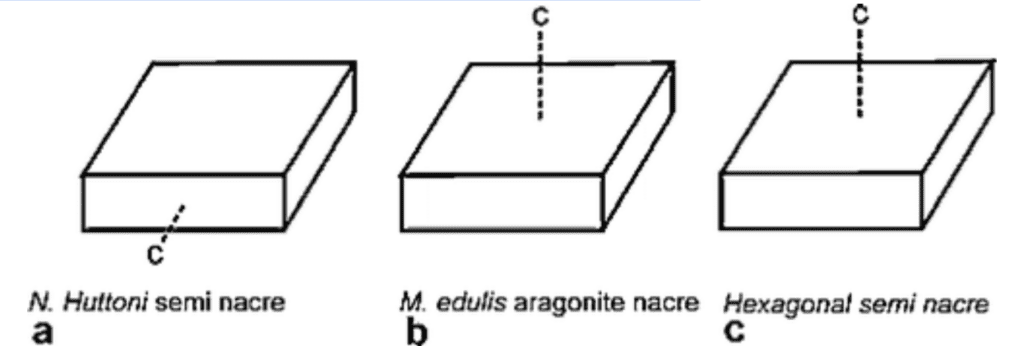
Fig. 27: An illustration depicting the crystallographic c-axis in three different calcium carbonate structures. Note that the c-axis is a crystallographically determined property of crystals and is used for characterization. The c-axis differs in a. (brachiopod semi-nacre) compared to b. (mollusk aragonite nacre) and c. (semi-nacre from an entirely different calcifying phylum, Bryozoa) (England et al., 2007).
This effort confirmed that while the two structures are somewhat similar in their ultrastructure and substructure, their respective crystallographies differ significantly (England et al., 2007). The two structures are similar in many ways but possess key differences that techniques like crystallography can pinpoint, and can be used as means of classification.
This comparative analysis aims to highlight the chemical similarities between mollusks and brachiopods, but also that chemical identity is not solely responsible for the microstructure of nacre and semi-nacre. Secondly, the differences between both shell material secretion and assembly are intended to provoke the following question: How closely related are mollusks and brachiopods, and what separates them as different phyla?
The Evolution of Mollusk and Brachiopod Shells
As previously mentioned, brachiopods and mollusks are both classified as Lophotrochozoans, and their shell structures are very similar: chemically, minerally (in some instances), and to a certain extent microstructurally. The particular differences which set them apart were also highlighted. Another difference which will be discussed here is the genetic machinery used by both phyla for shell formation.
While brachiopods and mollusks are in distinct phyla, they clearly have some evolutionary relationship to each other, and indeed, are both descendants of biomineralizing Cambrian ancestors (Wernström et al., 2022). In fact, transcriptome and genome searches have revealed that both brachiopods and mollusks share a conserved set of orthologous genes which are involved in biomineralization (Wernström et al., 2022). Some of these genes were detected to be expressed in various biomineralizing cell types of the juvenile brachiopod, Terebratalia transversa (Figure 28)(Wernström et al., 2022).

Fig. 28: The brachiopod Terebratalia transversa (Digimorph, n.d.).
Furthermore, an extension of this analysis leads to the finding that many of these key genes were not detected and in fact lost in both Bryozoan and Phoronid phyla, which indicates that these phyla possess a different biomineralizing strategy (Wernström et al., 2022). This finding supports the idea that these key proteins are involved in biomineralization.
While much of the biomineralizing genetic machinery may be conserved between brachiopods and mollusks, further studies using techniques such as proteome analysis and bioinformatic tools like the Basic Local Alignment Search Tool (BLAST) have been used to identify a set of shell matrix proteins in a specific brachiopod, Laqueus rubellus (Figure 29), which are not expressed in mollusks (Isowa et al., 2015).

Fig. 29: The Brachiopod Laqueus rubellus (ViaNet Conchology, n.d.).
The same group of researchers determined that some of those proteins contain structures, such as NAD(P) binding domains, which hint at the existence of completely unknown shell-forming ion control systems in brachiopods, which are not characterized in mollusks (Isowa et al., 2015). This is particularly interesting, because it suggests the existence of a completely different shell mineralizing system in brachiopods as compared to mollusks (Isowa et al., 2015). Despite their shell structures being so similar, the mechanism for shell formation differs significantly between the two phyla (Isowa et al., 2015).
The important conclusion of these genetic analyses is that it is very likely that brachiopods and mollusk shells evolved in a convergent fashion. Despite their similarities, the mechanisms for shell formation are incredibly different, both from physiological (mantle interactions) and proteomic (genetic machinery involved) perspectives.
The Message of the Shells’ Evolutionary Relationship
This paper has thoroughly investigated the chemical process which drives the calcification of molluscan shells. However, the question remains: why is this a good solution to protect the mollusk? Clearly, the shell is strong and can withstand massive amounts of mechanical stress, making shell calcification an excellent solution to protect the mollusk. However, the chemical, structural, and genetic comparison outlined above suggest that mineralized shells are not merely a good, but rather an absolutely exceptional solution. These structures have evolved separately two different times, which provides a tangible piece of evolutionary evidence that the chemical process which drives shell calcification, and the use of these calcified minerals to build shells is one of the best protective mechanisms available to the mollusk and other soft bodied marine organisms.
Conclusion
The biomineralized shell is a wildly successful defense mechanism in the animal kingdom as evidenced by its effectiveness in mollusks. A feature so widespread must be a practical evolutionary solution, a practicality which is not concealed in mollusks.
Mollusks absorb readily available calcium carbonate from seawater via diffusion, membrane proteins and intercellular spaces. Once saturation concentrations are surpassed, crystals can nucleate and grow. But mineral precipitation only occurs with precise architectural regulation. Crystal competition, self-organization of the matrix structure, as well as organic molecules control the crystallization of calcium carbonate into calcite and aragonite crystals, two molecules with different properties and growth areas in the shell.
The organic molecules responsible for crystallization are an arsenal of proteins, polysaccharides and lipids consisting of dozens of compounds. By inhibiting nucleation and serving as scaffolds, they micromanage and dictate the crystallization of calcium carbonate, elevating the shell’s structural integrity to a higher level.
Furthermore, shells are such practical defense mechanisms that they have evolved independently multiple times. While having differences in their nacre structure, calcite and aragonite shells of both phyla have evolved separately in various species of mollusks and brachiopods.
The elaborate structural composition of shells allows them to survive and enables humans to utilize their material in various industries. Ceramics, concrete production, and wastewater treatment are some of the many ways that this intricate biomineralization process has been applied to our own needs.
Appendix
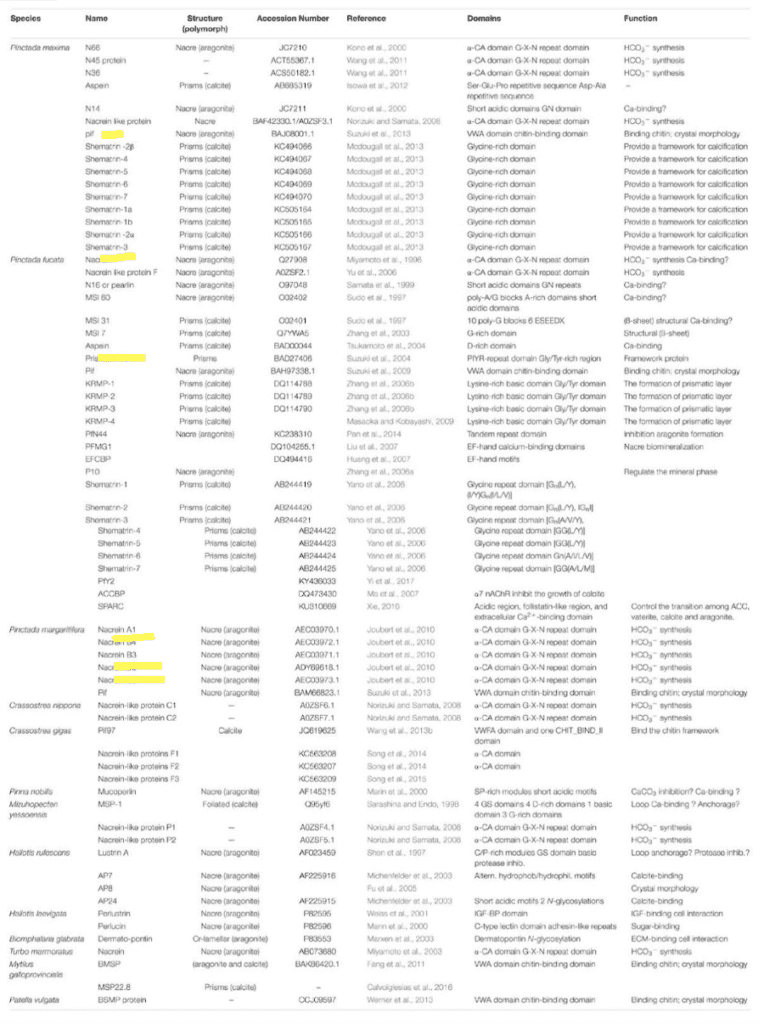
Fig. 30 Table of proteins and their functions in 13 species of mollusk. Proteins covered in this paper are highlighted in yellow. “HCO3- synthesis” refers to calcium carbonate calcification inhibition since it is the product of calcification’s reverse reaction (see Equation (1) ). (Song et al., 2019).
Sources
6. Biominerals and Biomineralization—Knovel. (n.d.). Retrieved November 4, 2022, from
Addadi, L., Joester, D., Nudelman, F., & Weiner, S. (2006). Mollusk Shell Formation: A Source of New
Concepts for Understanding Biomineralization Processes. Chemistry – A European Journal, 12(4), 980–987. https://doi.org/10.1002/chem.200500980
Alex Trekeisen – Brachiopods. (n.d.). Retrieved November 18, 2022, from
https://www.alexstrekeisen.it/english/sedi/brachiopods.php
Ball, J. (2017). Liquid crystals and their defects.
Bayarjargal, L., Fruhner, C.-J., Schrodt, N., & Winkler, B. (2018). CaCO 3 phase diagram studied with Raman spectroscopy at pressures up to 50 GPa and high temperatures and DFT modeling. Physics of the Earth and Planetary Interiors, 281. https://doi.org/10.1016/j.pepi.2018.05.002
Benzerara, K. (n.d.). Des structures cristallines dans les organismes biologiques. Planet-Vie. Retrieved
November 6, 2022, from https://planet-vie.ens.fr/thematiques/cellules-et- molecules/molecules/des-structures-cristallines-dans-les-organismes
Biological inorganic chemistry: Structure and reactivity – University of Toronto. (n.d.). Retrieved
November 4, 2022, from https://librarysearch.library.utoronto.ca/discovery/fulldisplay/alma991106247373206196/01UTORONTO_INST:UTORONTO
Boon, M., Rickard, W. D. A., Rohl, A. L., & Jones, F. (2020). Stabilization of Aragonite: Role of Mg2+
and Other Impurity Ions. Crystal Growth & Design, 20(8), 5006–5017. https://doi.org/10.1021/acs.cgd.0c00152
Bouligand, Y. (1972). Twisted fibrous arrangements in biological materials and cholesteric mesophases. Tissue and Cell, 4(2), 189–217. https://doi.org/10.1016/S0040-8166(72)80042-9
Całus, S., Busch, M., Kityk, A. V., Piecek, W., & Huber, P. (2016). Chiral Phases of a Confined Cholesteric Liquid Crystal. The Journal of Physical Chemistry C, 120(21), 11727–11738.https://doi.org/10.1021/acs.jpcc.6b03553
Chan, V. B. S., Johnstone, M. B., Wheeler, A. P., & Mount, A. S. (2018). Chitin Facilitated
Mineralization in the Eastern Oyster. Frontiers in Marine Science, 5. https://www.frontiersin.org/articles/10.3389/fmars.2018.00347
Chang, E. P., & Evans, J. S. (2015). Pif97, a von Willebrand and Peritrophin Biomineralization Protein,
Organizes Mineral Nanoparticles and Creates Intracrystalline Nanochambers. Biochemistry, 54(34), 5348–5355. https://doi.org/10.1021/acs.biochem.5b00842
Checa, A. G. (2018). Physical and Biological Determinants of the Fabrication of Molluscan Shell
Microstructures. Frontiers in Marine Science, 5. https://www.frontiersin.org/articles/10.3389/fmars.2018.00353
Checa, A. G., Cartwright, J. H. E., & Willinger, M.-G. (2009). The key role of the surface membrane in
why gastropod nacre grows in towers. Proceedings of the National Academy of Sciences, 106(1),
38–43. https://doi.org/10.1073/pnas.0808796106
Clark, M., Peck, L., Arivalagan Immanuel, J., Backeljau, T., Berland, S., Cardoso, J., Caurcel, C.,
Chapelle, G., De Noia, M., Dupont, S., Gharbi, K., Hoffman, J., Last, K., Marie, A., Melzner, F.,
Michalek, K., Morris, J., Power, D., Ramesh, K., & Harper, E. (2020). Deciphering mollusc shell
production: The roles of genetic mechanisms through to ecology, aquaculture and biomimetics.
Biological Reviews, 95. https://doi.org/10.1111/brv.12640
Digimorph—Terebratalia transversa. (n.d.). Retrieved November 6, 2022, from
http://digimorph.org/specimens/Terebratalia_transversa/whole/
England, J., Cusack, M., Dalbeck, P., & Pérez-Huerta, A. (2007). Comparison of the Crystallographic
Structure of Semi Nacre and Nacre by Electron Backscatter Diffraction. Crystal Growth &
Design, 7(2), 307–310. https://doi.org/10.1021/cg060374p
Estroff, L. A. (2008). Introduction: Biomineralization. Chemical Reviews, 108(11), 4329–4331.
https://doi.org/10.1021/cr8004789
Falini, G., & Fermani, S. (2004). Chitin mineralization. Tissue Engineering, 10(1–2), 1–6.
https://doi.org/10.1089/107632704322791646
Furuhashi, T., Schwarzinger, C., Miksik, I., Smrz, M., & Beran, A. (2009). Molluscan shell evolution
with review of shell calcification hypothesis. Comparative Biochemistry and Physiology Part B:
Biochemistry and Molecular Biology, 154(3), 351–371.
https://doi.org/10.1016/j.cbpb.2009.07.011
Giuffre, A. J., Hamm, L. M., Han, N., De Yoreo, J. J., & Dove, P. M. (2013). Polysaccharide chemistry
regulates kinetics of calcite nucleation through competition of interfacial energies. Proceedings
of the National Academy of Sciences, 110(23), 9261–9266.
https://doi.org/10.1073/pnas.1222162110
Hall, A., & Kennedy, W. J. (1967). Aragonite in Fossils. Proceedings of the Royal Society of London.
Series B, Biological Sciences, 168(1013), 377–412.
Isowa, Y., Sarashina, I., Oshima, K., Kito, K., Hattori, M., & Endo, K. (2015). Proteome analysis of shell
matrix proteins in the brachiopod Laqueus rubellus. Proteome Science, 13(1), 21.
https://doi.org/10.1186/s12953-015-0077-2
Jackson, A., Vincent, J., & Turner, R. M. (1988). The Mechanical Design of Nacre. Proceedings of The
Royal Society of London. Series B, Biological Sciences (1934-1990), 234, 415–440.
https://doi.org/10.1098/rspb.1988.0056
Kong, J., Liu, C., Yang, D., Yan, Y., Chen, Y., Huang, J., Liu, Y., Zheng, G., Xie, L., & Zhang, R.
(2018). Alv Protein Plays Opposite Roles in the Transition of Amorphous Calcium Carbonate to
Calcite and Aragonite during Shell Formation. Crystal Growth & Design, 18(7), 3794–3804.
https://doi.org/10.1021/acs.cgd.8b00025
Larsen, S. (2015). Recrystallization of Biogenic Aragonite Shells from Archaeological Contexts and
Implications for Paleoenvironmental Reconstruction. WWU Graduate School Collection.
https://doi.org/10.25710/xnkp-9e39
Louis, V., Besseau, L., & Lartaud, F. (2022). Step in Time: Biomineralisation of Bivalve’s Shell.
Frontiers in Marine Science, 9. https://www.frontiersin.org/articles/10.3389/fmars.2022.906085
Machałowski, T., & Jesionowski, T. (2020). Hemolymph of molluscan origin: From biochemistry to
modern biomaterials science. Applied Physics A, 127(1), 3.
https://doi.org/10.1007/s00339-020-04166-1
Maleki Dizaj, S., Barzegar-Jalali, M., Zarrintan, M., Adibkia, K., & Lotfipour, F. (2015). Calcium
carbonate nanoparticles as cancer drug delivery system. Expert Opinion on Drug Delivery, 12, 1-
12. https://doi.org/10.1517/17425247.2015.1049530
Marin, F., Le Roy, N., & Marie, B. (2012). The formation and mineralization of mollusk shell. Frontiers
in Bioscience – Scholar, 4 S(3), 1099–1125. Scopus. https://doi.org/10.2741/s321
Marin, F., Luquet, G., Marie, B., & Medakovic, D. (2007). Molluscan Shell Proteins: Primary Structure,
Origin, and Evolution. In Current Topics in Developmental Biology (Vol. 80, pp. 209–276).
Academic Press. https://doi.org/10.1016/S0070-2153(07)80006-8
McConnaughey, T. A., & Gillikin, D. P. (2008). Carbon isotopes in mollusk shell carbonates. Geo-
Marine Letters, 28(5), 287–299. https://doi.org/10.1007/s00367-008-0116-4
Mitov, M. (2017). Cholesteric liquid crystals in living matter. Soft Matter, 13(23), 4176–4209. h t tps://doi.org/10.1039/c7sm00384f
Miyamoto, H., Miyoshi, F., & Kohno, J. (2005). The Carbonic Anhydrase Domain Protein Nacrein is
Expressed in the Epithelial Cells of the Mantle and Acts as a Negative Regulator in Calcification
in the Mollusc Pinctada fucata. Zoological Science, 22(3), 311–315.
https://doi.org/10.2108/zsj.22.311
Muzzarelli, R. A. A. (2013). Chitin. Elsevier.
Nudelman, F. (2015). Nacre biomineralisation: A review on the mechanisms of crystal nucleation.
Seminars in Cell & Developmental Biology, 46, 2–10. https://doi.org/10.1016/j.semcdb.2015.07.004
Parry, A., & Savin, T. (2016). Recent advances in the biomimicry of structural colours. Chem. Soc. Rev., 45. https://doi.org/10.1039/C6CS00129G
Patrick De Deckker, P., Reeves, J., & Prendergast, A. (2016, December 5). She sells sea shells….
Curious. https://www.science.org.au/curious/earth-environment/sea-shells
Perovic, I., Chang, E. P., Lui, M., Rao, A., Cölfen, H., & Evans, J. S. (2014). A Nacre Protein, n16.3,
Self-Assembles To Form Protein Oligomers That Dimensionally Limit and Organize Mineral
Deposits. Biochemistry, 53(16), 2739–2748. https://doi.org/10.1021/bi401721z
Sauter, A., Roosen-Runge, F., Zhang, F., Lotze, G., Feoktystov, A., J. Jacobs, R. M., & Schreiber, F.
(2015). On the question of two-step nucleation in protein crystallization. Faraday Discussions,
179(0), 41–58. https://doi.org/10.1039/C4FD00225C
Schönitzer, V., & Weiss, I. M. (2007). The structure of mollusc larval shells formed in the presence of
the chitin synthase inhibitor Nikkomycin Z. BMC Structural Biology, 7(1), 71.
https://doi.org/10.1186/1472-6807-7-71
Ševčík, R., Šašek, P., & Viani, A. (2018). Physical and nanomechanical properties of the synthetic
anhydrous crystalline CaCO3 polymorphs: Vaterite, aragonite and calcite. Journal of Materials
Science, 53. https://doi.org/10.1007/s10853-017-1884-x
Simkiss, K. (1977). Biomineralization and detoxification. Calcified Tissue Research, 24(1), 199–200. h ttps://doi.org/10.1007/BF02223316
Simonet Roda, M., Griesshaber, E., Ziegler, A., Rupp, U., Yin, X., Henkel, D., Häussermann, V.,
Laudien, J., Brand, U., Eisenhauer, A., Checa, A. G., & Schmahl, W. W. (2019). Calcite fibre
formation in modern brachiopod shells. Scientific Reports, 9(1), Article 1.
https://doi.org/10.1038/s41598-018-36959-z
Song, X., Liu, Z., Wang, L., & Song, L. (2019). Recent Advances of Shell Matrix Proteins and Cellular
Orchestration in Marine Molluscan Shell Biomineralization. Frontiers in Marine Science, 6.
https://www.frontiersin.org/articles/10.3389/fmars.2019.00041
Song, E.-H., Shang, J., & Ratner, D. M. (2012). 9.08—Polysaccharides. In K. Matyjaszewski & M. Möller (Eds.), Polymer Science: A Comprehensive Reference (pp. 137–155). Elsevier. https://doi.org/10.1016/B978-0-444-53349-4.00246-6
Sun, J., & Bhushan, B. (2012). Hierarchical structure and mechanical properties of nacre: A review. RSC
Advances, 2(20), 7617–7632. https://doi.org/10.1039/C2RA20218B
Suzuki, M., Murayama, E., Inoue, H., Ozaki, N., Tohse, H., Kogure, T., & Nagasawa, H. (2004).
Characterization of Prismalin-14, a novel matrix protein from the prismatic layer of the Japanese
pearl oyster (Pinctada fucata). Biochemical Journal, 382(Pt 1), 205–213.
https://doi.org/10.1042/BJ20040319
Talham, D. R. (2002). Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry
Stephen Mann. Oxford University Press, New York, 2001. Crystal Growth & Design, 2(6), 675–
675. https://doi.org/10.1021/cg020033l
Tran, R., Xu, Z., Radhakrishnan, B., Winston, D., Sun, W., Persson, K. A., & Ong, S. P. (2016). Surface
energies of elemental crystals. Scientific Data, 3(1), Article 1. https://doi.org/10.1038/sdata.2016.80
ViaNet Conchology. (n.d.). Retrieved November 6, 2022, from
Wernström, J. V., Gąsiorowski, L., & Hejnol, A. (2022). Brachiopod and mollusc biomineralisation is a
conserved process that was lost in the phoronid–bryozoan stem lineage. EvoDevo, 13(1), 17.
https://doi.org/10.1186/s13227-022-00202-8
Xiang, L., Su, J., Zheng, G., Liang, J., Zhang, G., Wang, H., Xie, L., & Zhang, R. (2013). Patterns of
Expression in the Matrix Proteins Responsible for Nucleation and Growth of Aragonite Crystals
in Flat Pearls of Pinctada fucata. PLOS ONE, 8(6), e66564.