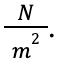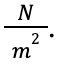Abstract
This essay dives into animal horns and the physical composition, mechanical properties, and ingenious designs that make them incredibly effective at everything they do. It features a selection of horn structures from different organisms in the animal kingdom, which demonstrates the diversity of horn structures while also showing the uniform characteristic of resilience and strength. Throughout this paper, it becomes evident that every aspect of an animal’s horns serves a particular purpose; from the molecular structure to intricate shapes, every feature contributes to the physical strength, efficacy, and mechanisms that make horns such a useful tool in an animal’s life.
Introduction
Fossil records of approximately 15 million years ago show the first signs of cranial appendages, including horns (“What’s the difference between horns and antlers?,” 2016). Standing the test of time and natural selection, it is evident that horns play a vital role in many organisms’ lives in terms of survival and reproductive success. Over the past 15 million years, organisms have evolved a wide range of horn shapes and sizes to better fit the needs of individual species, which will be explored throughout this paper.
These cranial appendages are extremely valuable to the species that possess them, as they depend on this headgear for defense against predators, interspecies territorial combat, dominance, or mating priority (Geist and Walther, 1974; Wilson, 1980). Horns are also responsible for digging soil, stripping bark from trees, and are used for mating displays.
As a result, permanent damage to the animal’s horns puts them at a devastating disadvantage, almost guaranteeing failure in survival and reproduction. Furthermore, unlike other cranial appendages such as antlers, horns are unable to grow or regenerate. Thus, the horn must be durable, strong, and able to withstand large loads and stresses to fulfill its functions (Tombolato et Al., 2010). This paper explores the impressive structure, mechanical properties, and different architectural designs found in horns that provide strength and resilience and are essential for the animal’s survival.
Fundamental Physics Concepts
This paper relies on basic yet fundamental physical and mechanical principles. It is important to understand what they mean in order to comprehend their application to the biological world such as animal horns. Below is a review of said principles.
Tensile Stress
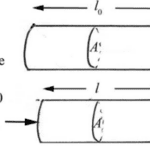
Tensile stress represents the force applied perpendicular to the area and can be calculated using their quotient (Dourmeashkin, 2022).

Fig. 1 Visual representation of tensile force on a cylindrical object (Dourmeashkin, 2022)
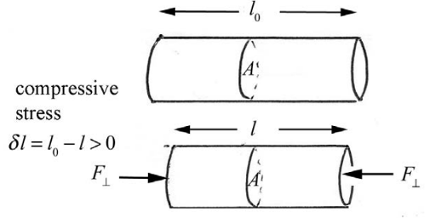
Compressive Strength
When two or more forces are directed towards each other with an object in between. Compressive stress results in compressive strain.
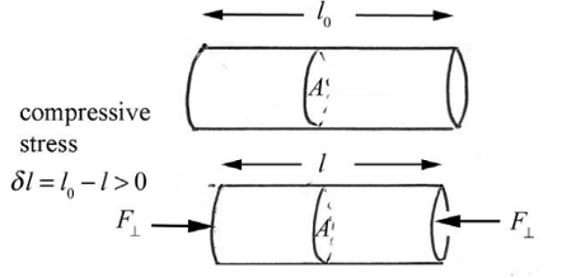
Fig. 2 Visual representation of compressive force on a cylindrical object (Dourmeashkin, 2022)
Young’s Modulus
Young’s modulus is a constant which expresses the elasticity of a substance. It can be calculated by determining the ratio of stress acting on an object over its strain. The units are N/(m^2).
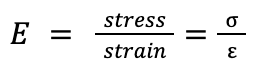
Equation 1: Formula for calculating Young’s Modulus
It is assumed that for a given material, their Young’s modulus constant is the same when it is subjected to tension or compression. The well know material steel has a large young’s modulus of E = 200 x 109 Nm2 , implying that it experiences little strain. A small E value describes a material with high elasticity which is more vulnerable to changes in shape when subjected to external forces (Hearn, 1997).
Normally, Young’s modulus is the same for an object under compression and tension. However, when this is not the case it is referred to as stress state asymmetry. For example, concrete can sustain significant compressive stress, but not tensile stress (Dourmeashkin, 2022).
Beam Theory
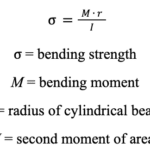
Without taking into account how an animal fights, it is possible to study the mechanical properties of horns by applying the engineering beam theory. This requires the assumption that any horn is a short, linear cylinder beam which is fixed at one end and free at another. Beam theory quantifies the bending stress of a cylindrical beam by multiplying the bending moment, M, with the radius, r, and dividing their product by the second moment of area, I (Kitchener, 1985).
Equation 2: Formula for calculating the bending strength of a cylindrical beam.
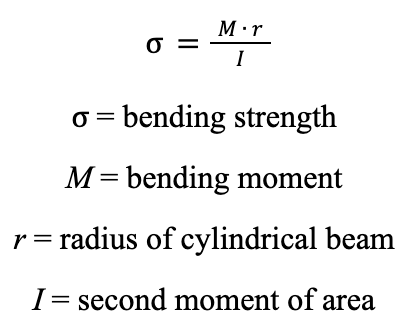
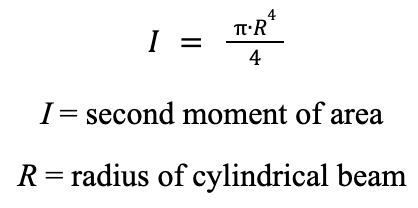
The bending moment represents the product of the force acting on the bean and the distance between the two points. The second moment of area represents how effective the beam is at resisting force.
Equation 3: Formula for calculating the second moment of area of a cylindrical beam.
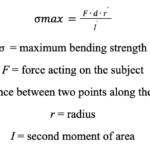
The maximum bending strength can be calculated by multiplying the force acting on the horn with the distance between the two points at the base of the horn and its radius. This is then divided by the second moment of area at the base of the horn (Kitchener, 1985).
Equation 4: Formula for calculating the maximum bending strength of the cylindrical beam.

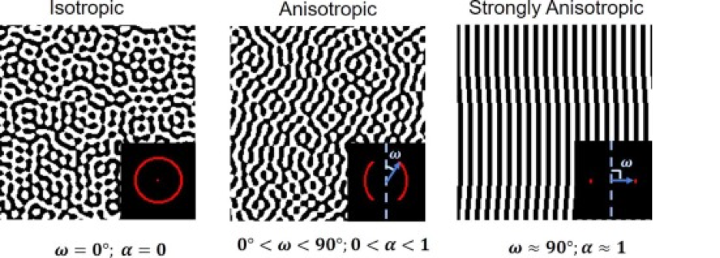
Anisotropy
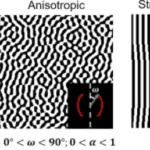
A material is anisotropic if its properties vary depending on the direction and orientation that they are measured in. When the composition of a material follows a particular pattern in a singular direction or plane, it is often anisotropic, such as fibrous materials. As a result, horn structures are anisotropic as they are primarily made up of keratin. In contrast, an isotropic material is an object with uniform strength in all directions (Intron, 2022).
Quantifying anisotropy is not the goal of this paper; however, Figure 3 introduces a visual depiction of isotropic and anisotropic behavior and how it is determined by angles created by the compositions of a material (Iyer, et al., 2020).

Fig. 3 Qualitative and quantifying representation of anisotropy (Iyer, et al., 2020).
Mechanical Properties of Horns
Now that there is a solid understanding of the basic physics principles used to quantify and describe horns, one can dive into the fascinating mechanical properties behind these cranial appendages.
Hierarchical Structure of the Bovid Horn
Horns are mostly found in animals that fall into the category of Bovidae: cloven-hoofed, ruminant animals, such as goats, antelopes, buffalos, etc. Consequently, while this paper touches on horn applications outside of this biological family, it mainly focuses and goes more into depth on topics surrounding the structure and mechanisms behind bovid horns. In this section, the physical composition and mechanics of bovid horns will be discussed; that being said, other possible structures do exist in other biological groups and specific organisms, such as in invertebrates and reptiles.

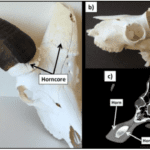
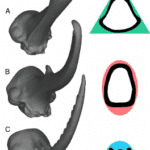
Fig. 4 a) Horn (Sheath) and horn core (Bone), b) Cross-section of the inside of the horn core, c) Axial view of the spatial arrangement of the horn sheath, horn core, and brain of the bovid (Wheatley, 2016).
Macroscopically, the horns of Bovidae are composed of two parts; they consist of a bony core covered by a keratinous sheath (Fig. 4). In particular, the horn’s sheath poses very interesting mechanical properties that are essential to the organism’s survival and reproductive success. In order to understand the mechanics behind the sheath, one needs a deeper understanding of its structure (Zhang et al., 2013).
At a molecular level, the amino acids of keratin come together to form an elongated unit of amino acids, which are referred to as protofibrils. These fibrils then bind to one another to produce microfibrils, called intermediate filaments (IFs). Moreover, these IFs then intertwine and orient themselves randomly into an amorphous protein matrix to produce lamellae, a very fine layer of α-keratin. Within the sheath, these lamellae are layered and bounded together by other proteins, which creates tubules (Zhang et al., 2013).
To summarize, the tubules captured by 3D imagery in Fig. 5 are aligned in the growth direction, longitudinally, while the laminae are angled 30 degrees to the growth direction. Within the keratin cells there are also intermediate filaments organized in random orientations that do not follow a particular geometric pattern. The structures described above are highlighted in Figure 5.

Fig. 5 Hierarchical structure of a bovine horn (Huang et al., 2017).
These keratin tubules are aligned with the direction of growth of the horns. Furthermore, due to the parallel nature of the tubules in the longitudinal direction, the composition of the horn’s sheath is considered a composite material with a fiber-reinforced body. Bovidae horns are more resilient along their direction of growth, which is ideal for an organism with tendencies to charge, ram, and stab in combat. Specifically, the α -keratinous structure forms a nanoscale composition with fiber-reinforcement, which increases durability, provides higher fracture toughness, and prevents cracks from extending further (Zhang et al., 2013) (Zhao et al., 2010). This composite material bears many similarities to mechanisms and structures engineered by humans today.

Fig. 6 Reinforced concrete with embedded steel rods (Ryan, 2010).
To illustrate, consider a more familiar, man-made material with the same principles, such as reinforced concrete (Fig. 6). Reinforced concrete consists of steel rods embedded into the concrete so that the two materials act together in resisting forces. Similarly to the bovid horn’s tubules, the steel framework absorbs the tensile and shear stresses, and the concrete resists compressive stresses. Regular concrete is incapable of doing this, especially when it comes to withstanding wind, earthquakes, changing temperatures, etc. Through this example, it is evident that nature has designed an optimal material for a biological structure that undergoes such a great deal of stress throughout an animal’s lifetime (Britannica, 2020).
One demonstration of the durability of this material is in comparing the specific work of fracture, a material constant and its ability to withstand stress. In horns the specific work of fracture varies with the length of the horns, so in waterbuck horns it ranges between 10-80 kJ m−2, while mouflon horns range between 12-60 kJ m −2 from the base to the end of their horns. In comparison to other materials, it is apparent how resilient Bovidae horns really are. They are more resilient to stress than most other biological and even synthetic materials. For example, horns have a higher specific work of fracture than antlers (6.2 kJ m−2), glass (5 J m −2), mild steel (26 kJ m −2), and even bulletproof glass (33 kJ m −2) (Zhang et al., 2013).
Mechanical Properties of Horns
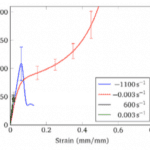
The mechanical nature of keratin makes it an excellent material for animal parts, such as horns. Such properties vary according to water concentrations, structure, and amino acid composition. Keratinous sheaths, found in bovid horns, provide strength, durability, and energy absorption and vary in shape and mechanical properties from species to species. Traditionally, keratin load-deformation profile is divided into three sections: an elastic region, yield region and post-yield region. A plastic region is a region where a material changes form permanently (Shanmukha, 2017). In contrast, the elastic region on a stress and strain curve is a region where a material is deformable, although it will return to its original shape once the force is removed (elastic region). As seen in Figure 6, stress initially increases linearly within the linear elastic region. At 2% the protein reaches critical stress and begins to uncoil into β-pleated sheets which is demonstrated by a plateau region. During the yield region the material begins to transition from elastic behavior to plastic behavior. Within the post-yield region, the strain and stress of the fibers and intermediate fibers are positively correlated while the matrix is in a plateau (Lazarus et al., 2021).
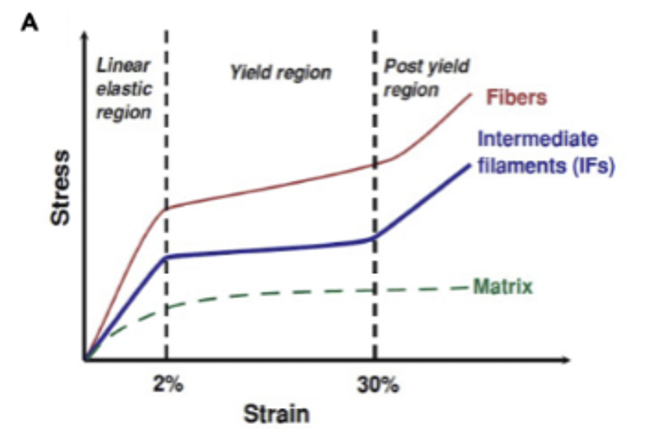
Fig. 7 Stress and strain curve of α-keratin material divided into three regions: linear elastic, yield and post-yield regions. This curve is limited and excludes additional factors such as structural deformation mechanics (Lazarus et al., 2021).
In the bighorn sheep horn, the α-keratin sheath is anisotropic, favoring the radial direction. The radial direction can be seen as the normal to the longitudinal direction. The keratin tubules are extended in the longitudinal or growth directions between lamellae, which provide the rigid structure with some strength and support. Moreover, what makes the composition of the horn unique is that despite requiring strength for survival, it lacks any mineral composites which typically add stiffness to other protein-based substances (Johnson et al., 2017).
Mechanical Properties of Tubules
Unlike hooves, horns are more vulnerable to impact due to their tubules being perpendicular to a load at the point of impact. A research paper by Lazarus et al. (2021), supports that when the loading compression is parallel to the lamellae, the strength of the horn decreases. This is due to delamination between the hard and soft phases. When compressive force is applied perpendicular to the tubules, their strength decreases leading to them collapsing (Lazarus et al., 2021). Moreover, keratin tubules are essential in absorbing impact energy. For instance, bighorn sheep ram into each other at speeds of up to 32 km/h; the reason they can withstand greater impact than other horned animals, such as domestic sheep, is due to the differences between their tubular structures (Huang et al., 2017).
In a study presented by Results in Physics, researchers found that horns with higher tubular density, such as the bighorn sheep, can absorb more energy due to plastic deformation. Furthermore, the failure impact data concludes that bighorn sheep horns have better impact resistance when impact speed is elevated. While domestic sheep share a similar fighting style, they ram into one another at slower speeds, since they tend to stand closer to their opponents than the bighorn sheep, and their failure impact data reflects this behavior. The impact test results infer that horn properties are engineered by evolution and designed to fit different combat behaviors (Zhang et al., 2018).
Applying Force to Horns
Types of Force Damages Endured by Keratinous Horns
Horns are often a primary weapon in animal conflict and as such endure great forces. To better understand horns, and their functional strengths, we can analyze their mechanical structure and reaction to external forces. The difference in mechanical properties such as stiffness and loading direction vary across species with respect to their fighting behavior; high stiffness and compressive strength in mountain goat horns support forces endured during stabbing. Flexibility and high tensile strength in pronghorn horns support interlocked pulling. Impact absorption properties in sheep distribute and limit cranial damage during butting. Given the inability of most horns to regrow, they must meet certain mechanical specifications to avoid fracture from damage both externally and internally.
External Damages on Keratinous Horns
External damage or any kind of external energy applied to horns without severely impacting the horn originates from multiple types of forces. The most common impact is dimple or depression, which results from an impact that creates a small dimple. The stronger the dimple force, the larger the impact energy and the more likely it is to have a dimple of larger diameter and depth (Zhang et al., 2018). The resistance of the horn material to fracture by a blow can be calculated using the impact strength equation:

Where: En is the normalized impact strength, E is the impact energy, ds are the cover plate aperture diameter, and t is the thickness of the specimen.
Another result of external damage experienced by horns are splits and cracks. The ability to resist cracks is directly correlated to load direction (how the horn distributes impact based on direction of impact) and horn hydration (Zhang et al., 2018). Load direction is greatly dependent on horn geometry and tubule structure. Tubules can redirect crack propagation, increasing the fracture toughness of horns. Because of this, the internal structure of most bovid horns is a fine tuned and unique arrangement of tubules of varying shape and geometry, to best accommodate their combative behavior (Li et al., 2011).
It has also been shown that hydration in horns greatly decreases their Young’s modulus and compressive strength in loading states, leading to a brittle horn. The Young’s modulus is a measure in pressure units derived from uniaxial stress and strain modeled by the aforementioned equation:
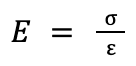
Significance of Hydration on Mechanical Properties
According to a study presented by Royal Society Interface, the water concentration of animal horns greatly impacts their mechanical properties. The study focused on the following animals: the mountain goat, domestic sheep, bighorn sheep and the pronghorn. In wet conditions, the mountain goat horns retain the most stiffness and strength, while the pronghorn horn performs at the lowest strength and stiffness (Zhang et al., 2018). There are several possible quantitative and qualitative explanations for these findings which include, but are not limited to, water absorption and evolutionary effects. The pronghorn horn has the highest water absorption efficiency. Hydration may decrease a horn’s likeliness of cracking or splitting in several ways. Water molecules can act as a swelling agent, increasing space between molecular chains and thus decreasing forceful chain interactions. Water molecules may also replace hydrogen bonding between chains, resulting in increased chain mobility.
Furthermore, when a horn is hydrated, it dissipates energy when a stress load is applied through absorption. Another reason for this is the softening of keratin protein leaving the structure more pliant (Zhang et al., 2018).
With an increase in water content, the Young’s modulus, tensile strength and microhardness of the horns in the longitudinal direction decrease, while the fracture strain increases. At 0% water content, the stiffness and strength of the material increase, but the horn becomes brittle. Dry horns are more susceptible to breakage at a lower strain level. However, a surplus of water is not ideal either, due to differences in mechanical behavior between the longitudinal and transverse directions (Zhao et al., 2010).
Anisotropy: Significance of Direction of Force
Normally, loads are applied to the distal end of the horn, rather than the proximal or base of the appendage. In this case, the horn experiences impact at the portion that is most vulnerable to damage, which results in maximal bending causing the proximal end to break. Without elaborating extensively on the geometry of the horn, it should be noted that due to its conical geometry it is designed for what is referred to as ‘equal strength’ (Li et al., 2011).
This implies the following:
- The transverse area increases towards the base which provides it with load bearing capability.
- The inner bony core contributes to the stable structure while preventing the outer sheath from buckling.
- The tough keratinous sheath protects against rupturing during combat.
Stress state asymmetry
Stress state asymmetry is an important material property of keratin. It is due to the material undergoing many deformation events involving both tension and compression.
The paper put forth by Material Science and Engineering, A recent study reveals the mechanical behavior of horns in the radial direction, under different conditions (Li et al., 2011). Based on the results in the article presented above, the mechanical behavior in the radial dry condition showed significant asymmetry. This implies that the horn performs differently when it is impacted by a force along a single axis (Johnson et al., 2017).
In the case of the horn, stress asymmetry reveals that the stress and strain relationship varies significantly depending on the type of force and the rate of loading applied to the horn. In particular, the radial direction experiences the largest disparity between compression and tension as demonstrated in Figure 8.
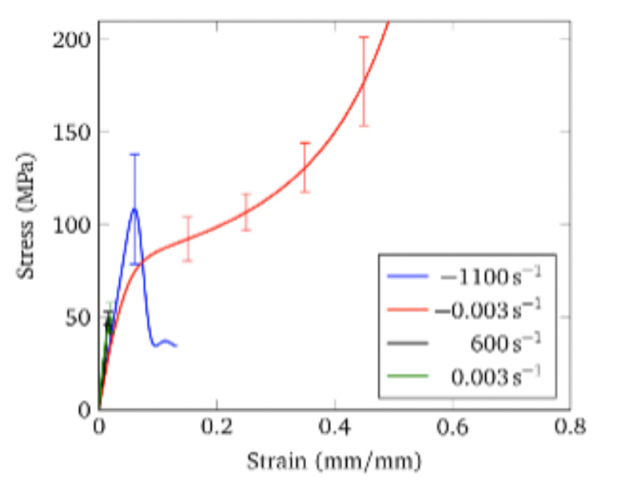
Fig. 8 Stress and strain results in the radial direction, under dry conditions. Positive and negative strain rates represent tension and compression, respectively (Johnson et al., 2017).
Results of Compression, Tension and Tensile Tests
Compressive Energy Absorption Depending on Direction
It is suggested by one study that compressive forces directed in the radial direction results in the dry horn being able to absorb more energy, as opposed to the transverse and longitudinal directions (Gatesy and McKittrick, 2018). The arrangement of lamellae is significant in explaining these findings. Typically, the lamellae are arranged along the radial direction which enables them to endure stress without significant deformation. In particular, sheep fight by exerting forces on their peers in a radial direction (Zhang et al., 2018).
Static Compression Tests Carried out on Bovid Animals
The study recorded in Static and dynamic mechanical properties of cattle horns finds that, after being subjected to a compressive force at the distal end, the horn only displays approximately one third of its total height. Figure 9 presents a qualitative and quantitative depiction of a horn under compression. Furthermore, from the naked eye there are no significant cracks or indents on the exterior keratinous sheath layer as well as the lower half of the horn. In a real scenario, such as two bulls wrestling, the horns of the animals would have been subjected to damage long before the maximum load that the sheath can withstand. This suggests that horn failure is mainly caused by the breakage of the bony core interior, which is known to have a relatively lower ability to withstand tensile stresses. It should be noted that typical force is not exerted in the optimal orientation for resistance (Li et al., 2011).
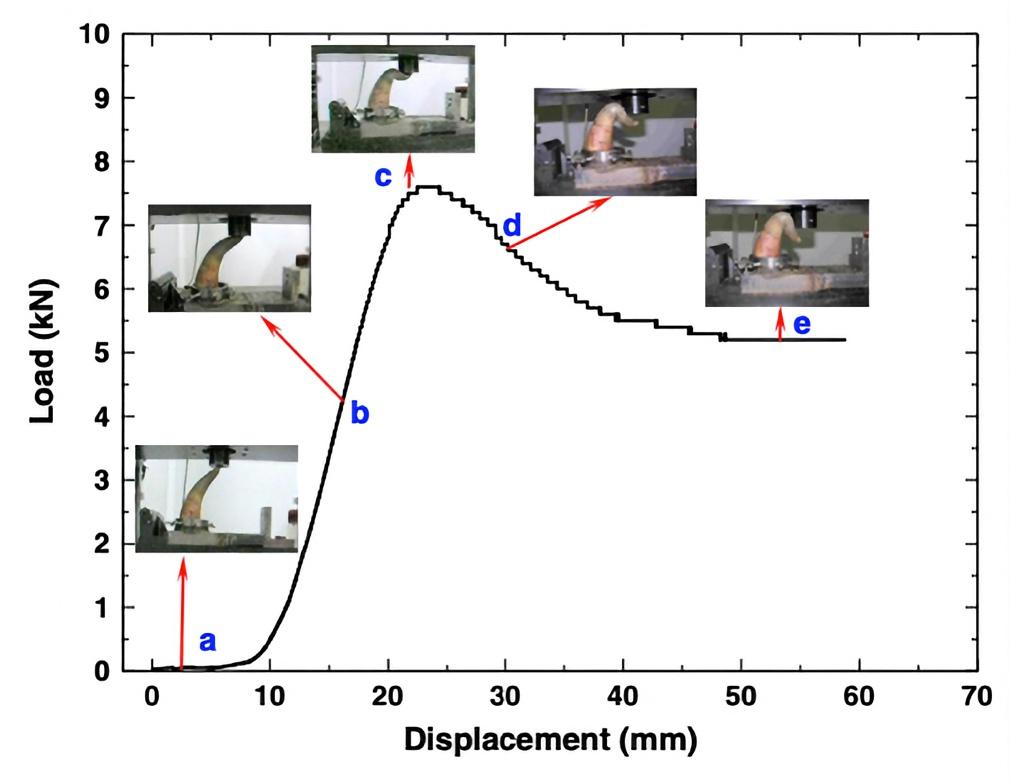
Fig. 9 Load versus displacement curve of a horn compression test (Li et al., 2011).
Comparison of Mechanical Properties Among Different Animal Horns
The difference between Young’s modulus values for different horns in different directions is not significant enough to conclude that stiffness is due to tubule density, shape and size of a horn. For example, the compressive yield strength of a mountain goat horn is much higher than that of other animal species in the radial and transverse direction, but not for the longitudinal direction. An explanation for this is because mountain goat horns tend to have a higher tubular density compared to other species, so their horns are more reinforced and fit for impact. The longitudinal direction is not affected by the presence of tubules, which could be due to the fighting method of the individual species. For example, mountain goats fight by stabbing and thrusting their horns, thus the strength properties of their horns must be strong enough to maintain the structure and integrity of the body part by absorbing the impact (Zhang et al., 2018).
In another study, it was found that the mechanical properties of horns vary depending on the type of species and where the sample was retrieved. For example, the stiffness and strength of buffalo and cattle horns increased from the proximal to the distal segment (Gatesy and McKittrick, 2018). However, in sheep horns, the Young’s modulus and ultimate strength in the middle region were more effective than the proximal and distal regions; the proximal and distal regions showed signs of delamination, while the middle was uniform. It is thought that the distal/thinner parts of the horns need to be stiff, strong, and tough to attack and defend. On the other hand, the sheep horn is not as susceptible to attack, since it is the middle region that is the most resilient and its horns are curled. With this said, it is understood that the spatial variation of mechanical properties in horns are dependent on the mechanical functions, material quality, and microstructures of the species (Zhang et al., 2018).
The Relationship Between Body Weight and Second Moment of Area
To study the mechanical properties without considering how an animal fights, engineering beam theory must be applied. This requires the assumption that any horn is a short, linear cylinder beam which is fixed at one end and free at another (Kitchener, 1985).
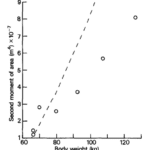
The article The effect of behaviour and body weight on the mechanical design of horns suggests that as the animal grows, the horns develop gradually and exponentially in tandem with its force bearing properties and weight. The result of their study reveals a positive linear relationship between the second moment of area of the base of the horn and the weight of the animal, as seen in Figure 10. Moreover, when researchers compared geometric growth of the horn, rather than its moment of area, the results revealed an exponential curve (Kitchener, 1985).
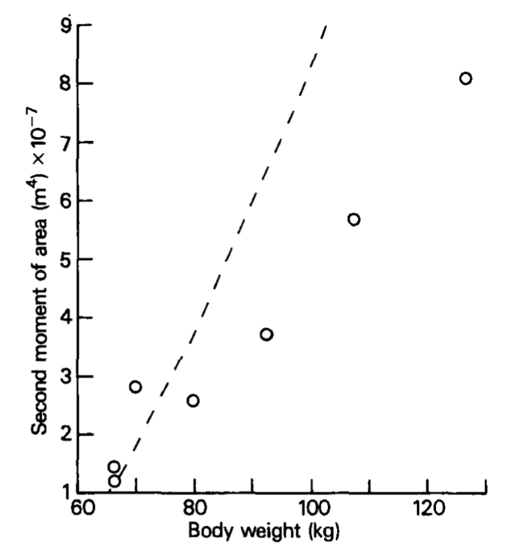
Fig. 10 Graph of the relationship between the second moment of area and body weight of an animal (Kitchener, 1985).
This trend is relatively constant among species with horns; however, each follows a different trajectory, making it nearly impossible to combine data from different animals. This may be due to the different fighting tactics of species and how their specific geometric orientation allows them to resist stress.
Horn Applications in the Animal Kingdom
Horns are mostly observed in Bovidae (e.g. goats, antelope, bison…); however, they can also be found amongst certain organisms outside of this family, like in invertebrates and reptiles. The majority of organisms who possess horns share the same composition and molecular structure in their headpieces, which was described above in Hierarchical Structure of the horn. Yet, the diversity amongst horn shapes and sizes is significant from species to species. These variations are due to evolutionary change to fit the specific roles of each species. To highlight some of the impressive designs and mechanics behind these evolutionarily engineered tools, in the following section, certain distinguished organisms are compared to tools designed by humans with similar mechanisms. These comparisons aim to aid in the understanding of the functionality, design, and principles behind the different types of horns.
Sword-like Structure of the Horned Beetle
Horns are expressed primarily in male beetles and are used for combat and especially to attract female beetles. Normally, horn-like structures increase an animal’s capital, however, this is not always the case for horned beetles. A study presented in the article Integrating Development with Evolution: A Case Study with Beetle Horns: Results from studies of the mechanisms of horn development shed new light on our understanding of beetle horn evolution reveals that “Beetle horns comprising more than 10% of the animal’s total body mass are not uncommon, and such large structures are energetically expensive to produce” (Emlen et al., 2000). Thus, the growth process depletes the beetles’ available resources which negatively impacts its development. Furthermore, having such a large structure greatly impacts the invertebrate’s mobility. Nonetheless, there are still considerable sexual and reproductive advantages of this evolutionary trait which is likely why it has become increasingly more common in the course of evolution. In particular, the Rhino beetle’s horn shape is dependent on its fighting style (Emlen et al., 2000).

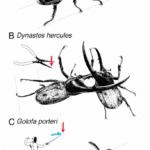
Fig. 11 Visual comparison between fighting tactics of beetle species Trypoxylus dichotomus, Dynastes hercules and Golofa porteri (McCullough et al., 2014).
The Hercules beetle (Dynastes Hercules)
The Hercules beetle is one of the largest subspecies of rhino beetles. It has a large curved upper horn which extends from its back and a smaller curved horn, curving up from its head. Together the horns form a pincher-like structure, which is ideal for its fighting style which involves grabbing a predator or prey’s entire body before throwing it to the ground. National Geographic reveals that “it can lift 850 times its own weight and, until recently, held the title of world’s strongest animal”. In short, this horn simulates the force like motion of scissors, as depicted in Figure 11 (Yong, 2022).
The Calliper beetle (Goloka porteri)
The combat style of the calliper beetle is to lift and shove its opponent. As a result, its horn is narrow and serrated at the edges. One can imagine the horn to mimic the motion of a fencer’s sword as depicted in Figure 11 (Yong, 2022).
Force Resistance of Beetle Horns
The study by McCullough et al. (2014) examines the effects of force on three different beetle species: Trypoxylus dichotomus, Golofa porteri and Dynastes hercules. The stress and strain energies were significantly low for all three beetles. The results of the study support the hypothesis that, “horns are structurally adapted to meet the functional demands of species-specific fighting” (McCullough et al., 2014). Differences in cross-sectional areas of horns are significant in a beetle’s ability to resist force. When the horn is bent in a predictable unidirectional way, such as in the Hercules beetle, there is a greater degree of resistance. This is because the mass is distributed further from the bending axis. To compensate for this nature of bending, the Hercules beetle has an elliptical cross section, as seen in Figure 12B. When a horn bends in an unpredictable way from many different angles, having a sword-like structure fairs best. The calliper beets have a circular cross-sectional area which allows them to distribute their mass equally along all possible bending axes, as shown in 12C. If a structure is repeatedly subjected to both bending and twisting forces, a non-circular cross-sectional area is most resistant to damage. In addition, force applied in a unidirectional manner results in the lowest degree of stress. The figure below demonstrates the different cross-sectional area shapes of the studied beetles (McCullough et al., 2014).
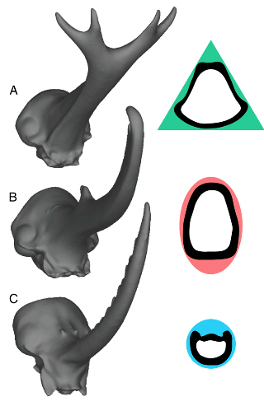
Figure 12: 3D and flat illustrations of different cross sectional area morphologies of beetle horns: (A) represents the triangle cross section in Trypocylus; (B) represents the elliptical area in Dynastes; and (C) is the circular cross section in Golofa (McCullough et al., 2014).
Corkscrew-Like Framework of the Common Eland Horns
The invention of the corkscrew is universally loved by all alcohol consumers since 1795. Its effective design allows one to pierce and uncork wine bottles by intertwining the screw with the cork (Scriber, 2015). Before the corkscrew was invented, the mechanics behind this tool were already being put into use by spiral horned animals, such as elands (Taurotragus oryx) (Fig. 14), using the same principles, not for uncorking spirits bur for fulfilling important roles – survival and reproductive success.
Elands are one of the largest antelopes in Africa. While the males are larger than the females, both sexes possess spiraled horns, similar to the structure of the basic corkscrew (Fig. 13) (Rowe, 1983). The males tend to have shorter, thicker and more pronounced spirals which are well designed through evolution to attack predators and for interspecific combat (Rowe, 1983). On the other hand, the females have longer horns which are effective at stabbing predators from afar to protect themselves and their offspring (Worthington, 1978). Animals using their horns for combat is not unusual, but what differentiates the eland horns in combat is their curved shape. This spiral structure proves to have a mechanical purpose, especially in male fights. They push their opponent with the distal regions of their horns with intertwined, twisting movements, while the spiral provides an increased grip in wrestling during interspecific combat between males (Worthington, 1978). The physics behind the shape and combat style are almost identical to that of a corkscrew; with pressure applied to the helix, it can dig and grip into a cork, or a rival.

Fig. 13 The common corkscrew (Horst, 2004)

Fig. 14 Common Eland (Taurotragus oryx) (Junek, 2010).
The advantages of the spiral shape are clear; however, it might be puzzling how the eland’s horns can withstand these loads without breakages. In a study on the composition and mechanical properties of the bony core and sheath of elands, it reveals that the density of the bone, ash content, bending strength, and impact work (energy it can absorb before breaking) decrease in value along the length of the horns from the proximal to the distal segment (from position 1-4, Fig. 15) (Cappelli, 2017). As mentioned in the previous sections, these spatial variations in mechanical properties depend mostly on the mechanical function of the horns. In another study, the researchers concluded similar results where these values decreased in the distal direction in cattle horns (cattle are also known to wrestle with each other) (Li, 2011). With all of this in mind, it was concluded that the variations along the eland horns are due to the fighting function within the horn. The region of the horn that experiences the greatest degree of stress is the proximal region which is the portion of the horn that is closest to the cranium. These regions undergo the most bending, which explains why the density, ash content, bending strength, and impact work are much higher in this area. It is significantly better suited to absorb energy during fights.

Fig. 15 Sampling technique in Eland bone and spiral structural composition study: (a) sampling positions ranging from 1-4; (b) in each sampling position, two bone samples were taken; (c) samples from spiral sheath (Cappelli, 2017).

Fig. 16 Elands locking horns (“Antelopes,” n.d.).
The spiral-shaped horns are associated with combat, since many organisms with this structure tend to wrestle and fight. With this in mind, the corkscrew shaped horns give the impression of having higher mechanical properties to withstand these stresses; however, the opposite is true. In the same study, the spiral showed smaller mechanical properties than a regular-straight horn, so what makes spiral-horns ideal for combat? In reality, it is a base for a lower impact fighting system (Cappelli, 2017). As mentioned before, the corkscrew architecture allows males to better grip during combat; however, another adaptation of this mechanism is that the spiral allows locking of horns, which prevents high impact to the skull by only allowing pushing in fights (Geist, 1966a). Imagine “sword fighting” with two corkscrews as a parallel; it is not hard to imagine how much easier it would be to grip the other’s spiral-sword as compared to a regular sword. It is also clear how often they would get caught and forced to wrestle rather than bashing each other repeatedly, thus reducing brute impact.
In addition to its beauty, the eland and other spiral horns are extremely effective in combat, not due to its stronger-than-average structural composition, but rather its perfectly engineered spiral design.
Mountain Sheep Horns – A Mechanical and Social Significance

Fig. 17 “The horn display” – a show of force by a larger ram through this “horn-threat,” a pattern constant between smaller and larger rams (Geist, 1966b).
Mechanics Meets Social Settings
A closer look into the North American wild sheep, also known as the Mountain sheep or rams, reflects a significant ramification of having a specific horn that dictates social hierarchy in finding mates, reproduction, and access to essential food resources. Recent studies on the exact evolutionary significance of horns classify various horns as weapons, shields, or shock absorbers but have shown all the former to explain little this evolution of horns in Mountain sheep.
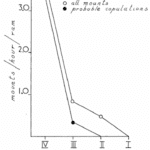
Instead, the hypothesis of horns as display organs was studied; indeed, horns’ size and shape affect social dynamics, reproductive advantage, and their bearers’ behaviour. Larger-horned rams in general dominate smaller-horned rams. Through this visual dominance rank, Mountain sheep with large horns breed with almost all female sheep, ewes, and exclude small-horned sheep (Geist, 1966b).
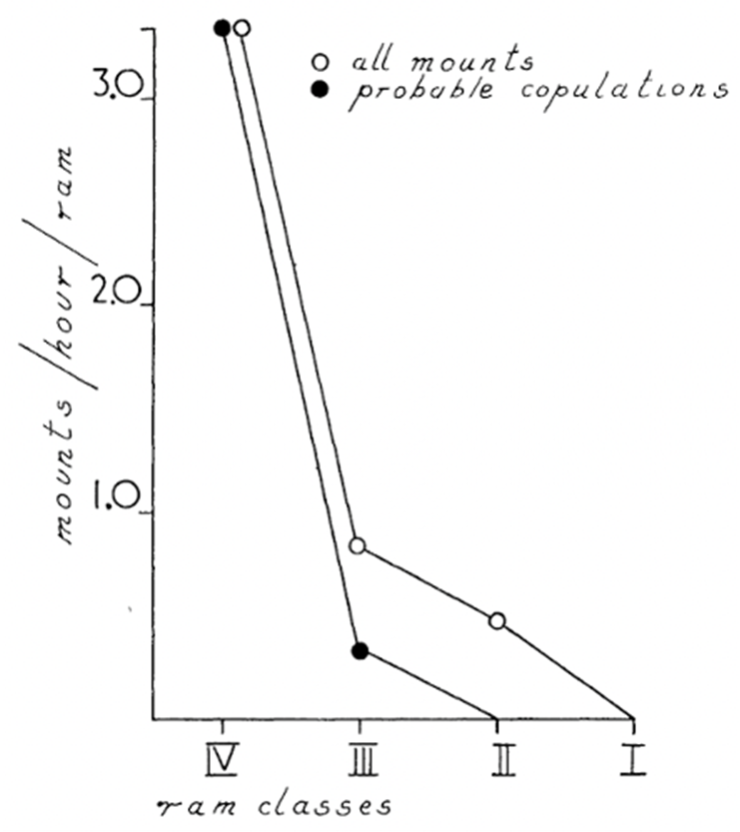
Fig.18 “The Variation of Number of All Mounts and Probable Copulations as a Function of Decreasing Order of Horn Size” (Geist, 1966b). This reveals how the largest class of Rams by far are the most likely to do mounting and almost all copulations with females.
Behavioral Impact of Horns on Rams’ Interactions
Rams responding to the largest horn size (Class IV), upon closer observation, are more frequently approached and acted upon by their peers in Class IV rather than the other smaller-horn classes. This shows that the Class IV rams’ social status heavily impacts the everyday behavior of rams centered around symbolic horn domination (Geist, 1966b).
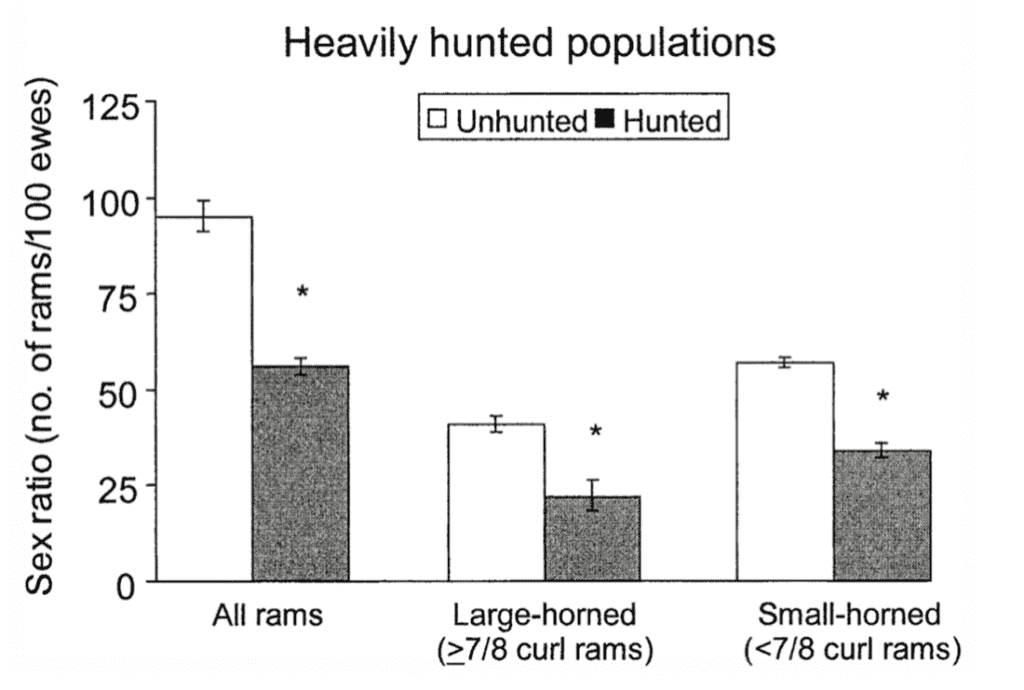
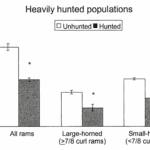
Fig.19 “The Variation of Sex Ratios (Number of rams/100 ewes) in Heavily Hunted and Unhunted Populations as a Function of Horn Size” (Singer & Zeigenfuss, 2002). This reveals how the largest class of rams decreased substantially, leaving way for small-horned rams to tend to more ewes.
Researchers have attributed this intrinsic behavior to how the Mountain Sheep invest large amounts of resources into the growth of their horns as well as their shapes to attain the symbolic curl, the representative of social status; those horns, in turn, represent about 12% of total body weight especially in older rams whose larger horns are more than ¾ of a full curl (Singer & Zeigenfuss, 2002).
Reproduction, Mortality, and Rate of Horn Growth
Interestingly, the larger-horned rams’ rate has been observed to reproduce more. However, as a result of their greater horn growth, they show higher mortality than non-breeding rams; this younger death of rams with good horn growth versus rams with poor horn growth is attributed to the greater participation in breeding activities (Geist, 1966b; Singer & Zeigenfuss, 2002).
Case Study: A Look into Hunted Large-Horned Rams
Given all of this, a significant social impact occurred as an indirect result of trophy hunting of the largest-horned rams, a multimillion-dollar activity in the inland United States, Canada, and Alaska where up to 2,000 such rams are hunted. This hunting gives smaller-horned rams more confidence in aggressively competing for ewes by interacting with them more, leading to greater rates of harassment and more courtship displays, which culminates in obtaining more copulations. Nonetheless, this leaves the ewes who are usually defended by their larger-horned rams more vulnerable, inciting them to distance themselves from the aggressive small-horned rams unlike in the unhunted herd (Singer & Zeigenfuss, 2002).
In short, with their intrinsic various rates of growth, horns in Mountain Sheep represent a significant symbol that drives the social and natural cycles in their communities, without which one cannot explain their hierarchical dynamics.
Club-like Structure of Giraffe Ossicones
Similar in function to horns, although distinguishable by their odd development and covering, giraffe ossicones are marvelous biological features. Found only in giraffes, okapi, and extinct relatives of the Giraffidae family, ossicones are formed from cartilage which hardens into bone. In early development, giraffe ossicones are not bony. The cartilage continues to ossify and harden until sexual maturity when the ossicones eventually fuse with the skull and appear structurally similar to other animals’ horns (Solounias, 1990).
Combat and Necking
Like other horned animals, giraffes primarily use their ossicones as weapons in combat. Male giraffes engage in combat behavior called ‘necking’. In giraffe herds, males will commonly fight each other for dominance, or territory. Dominant males will have more power in the herd, leading to greater opportunities for mating. However, giraffes may fight simply to establish dominance. Even in the absence of female giraffes, males will engage in combat. It is common to see necking in all male herds.

Fig. 20 Male giraffes engaged in necking (“Antelopes,” n.d.).
During necking, males will fight using their necks and heads. Swinging their heads like clubs, they bash each other using their bony heads and ossicones. The ossicones add bony mass to the giraffe skull, increasing the impact with which it can deliver a blow. Additionally, it concentrates the force of the blow onto the two points of the ossicones. The concentration of force generated by the momentum of the skull, focused onto two apexed horns, allows the strike to deliver a more focused and severe impact. Additionally, ossicones allow a giraffe to cut into an opponent’s flesh – something it could not accomplish simply with its skull. Giraffe necking is extremely violent and can leave unlucky opponents wounded or even dead. Because of the severity with which male giraffes club their skulls together, the outer layers of mature male giraffes ossicones and skulls are quite different from those of female or juvenile giraffes (Solounias, 1990).
Sexual Dimorphism
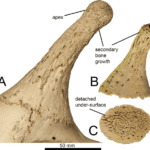
Both male and female giraffes have a pair of ossicones that fuse to the parietal bones of the skull. These parietal horns are composed of three structures: the frontoparietal base, the ossicone, and outer layers of secondary bone growth. The frontoparietal base and ossicone form similarly in male and female giraffes. The secondary bone growth, however, is linked to giraffe necking, the aggressive combative behaviour displayed only by males. Males club their heads with such force that it wears off the skin and fur tufts displayed by females and juvenile males. Furthermore, the tremendous force and cranial impact agitates the periosteum, the outer membrane of the bone, which stimulates secondary bone growth onto the ossicone, strengthening and reinforcing its structure (Solounias, 1990).

Fig. 21 (A) Adult giraffe ossicones, significant secondary bone growth. Irregular structure and apex formed from secondary growth. (B) Juvenile ossicones, not fully developed or fused (Danowitz 2017).
In Figure 21 the horns of an adult giraffe (A) and that of a juvenile (right) are examined. Due to secondary growth, the adult giraffe’s ossicones are larger and slightly more irregular than those of the juveniles. Because of the random nature with which secondary bone growth is stimulated, there is much variation between male giraffe ossicones. As such, secondary bone growth and overall horn structure is unique to every giraffe. Secondary bone growth however is not exclusively an epigenetic process derived from necking. Small amounts of secondary growth can be formed genetically as well. Genetic growth allows female giraffes to grow small amounts of secondary bone as well, however, due to lack of cranial impact resulting in less periosteum stimulation, female giraffes develop much less secondary growth than males (Mitchell et al., 2013; Solounias, 1990).
A very characteristic and interesting structure formed by secondary growth in male giraffe ossicones is the apex. Irregular secondary growth forms a rounded structure at the end of the horn which helps protect the vascularized and more vulnerable inside.
Okapi and Okapi Ossicones
The okapi is the only other non-extinct member of the Giraffidae family and as such is the only other species with ossicones. Although they share the same developmental and some structural similarities, giraffe and okapi ossicones are significantly different in function.
While giraffe ossicones add mass and a blunt clublike end to fight with, okapi ossicones are much more pointed. This can be attributed to the difference in combative behaviour and difference in stature. Because okapi do not possess the same long necks as giraffes they are unable to swing their necks to generate as much force when using their ossicones to fight. Because of this they do not benefit equally from the club-like structure of giraffe ossicones. The pointed ends of okapi ossicones allow them to focus the force of blows onto an even smaller area of impact than giraffes when fighting. Additionally, the sharp end may increase the chance of piercing the flesh of an opponent (Solounias, 1990) (Nasoori, 2020).
Only male okapi have ossicones. The core is formed on the frontal bone and similarly to the giraffe it develops into a network of vascularized and connective tissue. The frontal sinus in okapi also enlarges under the frontal bone and advances beneath the base of the ossicones. The ossicones grow with age and when the animal’s body reaches its mature size, the tip of the bony core is visible, protruding through the integument and remains skinless (Nasoori, 2020).
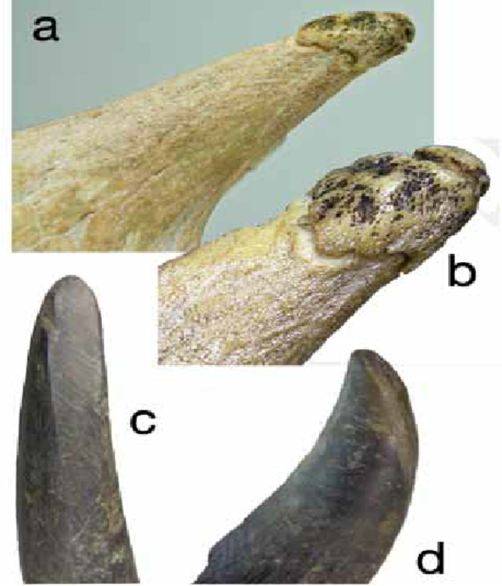
Fig. 23 Okapi Ossicones (Danowitz, 2017). A and B show clear illustrations of the ossicone tip. C and D show the shorter curved geometry of the ossicone compared to giraffe ossicones.
The tip of the Okapi ossicone is a very dense ossified structure containing crevices. Due to bone resorption the ossicone tip is considered ‘dead’. This concept of a dead yet still functional bone is similar to antlers, which can be seen as dead tissue due to the absence of a blood supply when shed (Nasoori, 2020).

Fig. 24 Male Okapi in Zoo (The Maryland Zoo, 2011).
Okapi ossicones are used in male–male combat involving neck clashing, similar to that of giraffes. They are also used in guarding females. Despite not having ossicones, in female okapi the frontal bone is bulged at what would be the ossicone site (Nasoori, 2020).
Conclusion
Throughout this paper, it becomes clear that horns have a unique structure and design that allows organisms to protect themselves against predators and fight for dominance, mating rights, and territory. These behaviors are physically taxing and require immense support and efficient engineering in order to withstand such forces and attacks. From the molecules that make up the horns to the impressive and intricate architectural designs, every aspect of the horns is carefully selected by the leading engineer – nature.
Starting with the physics seen at a molecular level, keratin was introduced as the main composition of animal horns. While keratin molecules are not particularly known for creating strong materials, like hair, wool, feathers (Numata & Kaplan, 2011), the manner in which the molecules create microfilaments and microstructures to further reinforce the structure results in a material less prone to fracture than bulletproof glass (Zhang et al., 2013). The methodical way that horns are pieced together molecularly is nothing short of ingenious mechanics, so much so that people have adopted such principles in reinforcing their own human-made structures.
Other mechanisms of bovid horns were examined. In particular, how the strength, density, and absorbance varied in different conditions/situations, such as water content; how they vary with the direction of the applied forces; how different segments and orientations of the horns vary, etc. Through compression, tension, and tensile tests, it became evident that while there are a few similarities amongst different species, ultimately, the mechanical properties of different types of organisms vary, mostly due to the functions that the animals need to fulfill.
This functional dependence is highlighted in the final section of this essay, where examples of different horn shapes and their functions are discussed in comparison to common tools in society. It is evident that the diverse shapes and mechanics of horns serve to fulfill its purpose, like a tool would. The sword-like structure of the horned beetle, the corkscrew-like shape of the common eland, and the club-like configuration of the giraffe, all have different fighting functions, and the animals, different uses for their horns, in different circumstances. As a result, their structures are considerably different in shape, but their foundations remain similar.
References:
Antelopes. Kid Cyber (n.d.). Retrieved from https://www.kidcyber.com.au/antelopes
Cappelli, J., García, A. J., Kotrba, R., Gambín Pozo, P., Landete-Castillejos, T., Gallego, L.,& Ceacero, F. (2017). The bony horncore of the common eland (taurotragus oryx): Composition and mechanical properties of a spiral fighting structure. Journal of Anatomy, 232(1), 72–79. https://doi.org/10.1111/joa.12708
Dourmashkin, P. (2022, July 20). 26.2: Stress and Strain in Tension and Compression. Physics LibreTexts. Retrieved September 11, 2022, from https://phys.libretexts.org/Bookshelves/Classical_Mechanics/Classical_Mechanics_(Dourmashkin)/26%3A_Elastic_Properties_of_Materials/26.02%3A_Stress_and_Strain_in_Tension_and_Compression
The Editors of Encyclopaedia Britannica. (2020). Reinforced concrete. Encyclopædia Britannica. Retrieved October 2, 2022, from https://www.britannica.com/technology/reinforced-concrete
Elastic region. Elastic Region | MATSE 81: Materials In Today’s World. (n.d.). Retrieved October 3, 2022, from https://www.e-education.psu.edu/matse81/node/2103
Emlen, D. J. (2000). Integrating development with evolution:a case study with Beetle Horns. BioScience, 50(5), 403. https://doi.org/10.1641/0006-3568(2000)050[0403:idweac]2.0.co;2
Geist, V. (1966). The evolution of horn-like organs. Behaviour, 27(1-2), 175–214. https://doi.org/10.1163/156853966×00155
Geist, V. (1966). The Evolutionary Significance of Mountain Sheep Horns. Evolution, 20(4), 558–566. https://doi.org/10.2307/2406590
Geist, V., & Walther, F. R. (1974). The behaviour of ungulates and its relation to management: The papers of an international symposium held at the University of Calgary, Alberta, Canada, 2-5 November 1971. International Union for Conservation of Nature and Natural Resources.
Hearn, E. J. (1997). Simple stress and strain. Mechanics of Materials 1, 1–26. https://doi.org/10.1016/b978-075063265-2/50002-5
Horst, F. (2004). A Simple Corkscrew [image]. Wikipedia. https://simple.wikipedia.org/wiki/Corkscrew#/media/File:Korkenzieher.jpg
Huang, W., Zaheri, A., Jung, J.-Y., Espinosa, H. D., & Mckittrick, J. (2017). Hierarchical structure and compressive deformation mechanisms of bighorn sheep (ovis canadensis) horn. Acta Biomaterialia, 64, 1–14. https://doi.org/10.1016/j.actbio.2017.09.043
Iyer, A., Dulal, R., Zhang, Y., Ghumman, U. F., Chien, T. Y., Balasubramanian, G., & Chen, W. (2020). Designing anisotropic microstructures with spectral density function. Computational Materials Science, 179, 109559. https://doi.org/10.1016/j.commatsci.2020.109559
Johnson, K. L., Trim, M. W., Francis, D. K., Whittington, W. R., Miller, J. A., Bennett, C. E., & Horstemeyer, M. F. (2017). Moisture, anisotropy, stress state, and strain rate effects on bighorn sheep horn keratin mechanical properties. Acta Biomaterialia, 48, 300–308. https://doi.org/10.1016/j.actbio.2016.10.033
Junek, T. (2010). Giant Eland, with Detail of the Horns [photograph]. Wikipedia. https://en.wikipedia.org/wiki/Tragelaphini#/media/File:Western_Derby_Eland_(Taurotragus_derbianus_derbianus)_1.jpg
Kiley-Worthington, M. (1978). The causation, evolution and function of the visual displays of the eland (taurotragus oryx). Behaviour, 66(3-4), 179–222.
Kitchener, A. (1985). The effect of behaviour and body weight on the mechanical design of horns. Journal of Zoology, 205(2), 191–203. https://doi.org/10.1111/j.1469-7998.1985.tb03528.x
Kitchener, A. (1988). An analysis of the forces of fighting of the Blackbuck ( antilope cervicapra ) and the Bighorn Sheep ( ovis canadensis ) and the mechanical design of the Horn of Bovids. Journal of Zoology, 214(1), 1–20. https://doi.org/10.1111/j.1469-7998.1988.tb04983.x
Kitchener, A. (1987). Fracture toughness of horns and a reinterpretation of the horning behaviour of bovids. Journal of Zoology, 213(4), 621–639. https://doi.org/10.1111/j.1469-7998.1987.tb03730.x
Lazarus, B. S., Chadha, C., Velasco-Hogan, A., Barbosa, J. D. V., Jasiuk, I., & Meyers, M. A. (2021). Engineering with keratin: A functional material and a source of bioinspiration. IScience, 24(8), 102798. https://doi.org/10.1016/j.isci.2021.102798
Li, B.-W., Zhao, H.-P., & Feng, X.-Q. (2011). Static and dynamic mechanical properties of cattle horns. Materials Science and Engineering: C, 31(2), 179–183. https://doi.org/10.1016/j.msec.2010.08.016
Li, B. W., Zhao, H. P., Feng, X. Q., Guo, W. W., & Shan, S. C. (2010). Experimental study on the mechanical properties of the horn sheaths from cattle. Journal of Experimental Biology, 213(3), 479–486. https://doi.org/10.1242/jeb.035428
McCullough, E. L., Tobalske, B. W., & Emlen, D. J. (2014). Structural adaptations to diverse fighting styles in sexually selected weapons. Proceedings of the National Academy of Sciences, 111(40), 14484–14488. https://doi.org/10.1073/pnas.1409585111
Mitchell, G., Roberts, D., Sittert, S., & Skinner, J. D. (2013). Growth patterns and masses of the heads and necks of male and female giraffes. Journal of Zoology, 290(1), 49–57. https://doi.org/10.1111/jzo.12013
Nasoori, A. (2020). Formation, structure, and function of extra‐skeletal bones in mammals. Biological Reviews, 95(4), 986–1019. https://doi.org/10.1111/brv.12597
Numata, K., & Kaplan, D. L. (2011). Biologically derived scaffolds. Advanced Wound Repair Therapies, 524–551. https://doi.org/10.1533/9780857093301.4.524
Rowe-Rowe, D. T., & Meester, J. (1982). Habitat preferences and abundance relations of small mammals in the natal Drakensberg. South African Journal of Zoology, 17(4), 202–209. https://doi.org/10.1080/02541858.1982.11447804
Ryan, V. (2010). Composite Materials – Reinforced Concrete [image]. Technology Student. https://www.technologystudent.com/joints/reinforc1.html
Scriber, B. (2021, May 3). Tools: Seven twists in Corkscrew history. Culture. Retrieved October 2, 2022, from https://www.nationalgeographic.com/culture/article/tools-seven-twists-in-corkscrew-history#:~:text=The%20earliest%20dated%20device%20is,firmer%20fit%20with%20the%20cork
Shanmukha. (2017, February 5). Elastic region, plastic region, Yield Point. CIVIL ENGINEERING. Retrieved October 3, 2022, from https://knowledge4civil.wordpress.com/2017/02/05/elastic-region-plastic-region-yield-point/#:~:text=The%20region%20of%20the%20stress,is%20called%20the%20plastic%20region
Singer, F. J., & Zeigenfuss, L. C. (2002). Influence of trophy hunting and horn size on mating behavior and survivorship of mountain sheep. Journal of Mammalogy, 83(3), 682-698.
Solounias, N. & Tang, N. “The Two Types of Cranial Appendages in Giraffa Camelopardalis (Mammalia, Artiodactyla).” Journal of Zoology, vol. 222, no. 2, 1990, pp. 293–302., https://doi.org/10.1111/j.1469-7998.1990.tb05678.x.
Shi, X., Wang, Y., Jia, R., Liu, F., & Zhang, J. (2019). Experimental investigations on microstructures and mechanical properties of white yak horns sheath. Results in Physics, 13, 102174. https://doi.org/10.1016/j.rinp.2019.102174 Tombolato, L., Novitskaya, E. E., Chen, P.-Y., Sheppard, F. A., & McKittrick, J. (2010). Microstructure, elastic properties and deformation mechanisms of horn keratin. Acta Biomaterialia, 6(2), 319–330. https://doi.org/10.1016/j.actbio.2009.06.033
Trim, M. W., Horstemeyer, M. F., Rhee, H., El Kadiri, H., Williams, L. N., Liao, J., Walters, K. B., McKittrick, J., & Park, S.-J. (2011). The effects of water and microstructure on the mechanical properties of bighorn sheep (ovis canadensis) horn keratin. Acta Biomaterialia, 7(3), 1228–1240. https://doi.org/10.1016/j.actbio.2010.11.024
What’s the difference between horns and antlers? (2016). American Museum of Natural History. Retrieved October 2, 2022, from https://www.amnh.org/explore/news-blogs/news-posts/horns-and-antlers-what-s-the-difference#:~:text=In%20the%20fossil%20record%2C%20antlers,re%20not%20just%20for%20looks
What is Anisotropic or Anisotropy (n.d.). Instron. Retrieved October 1, 2022, from https://www.instron.com/en/resources/glossary/a/anisotropic-or-anisotropy
Wheatly, B. (2016). Horn and Horn Core [image]. Research Gate. https://www.researchgate.net/publication/306373431_Horn_and_horn_core_trabecular_bone_of_bighorn_sheep_rams_absorbs_impact_energy_and_reduces_brain_cavity_accelerations_during_high_impact_ramming_of_the_skull/figures?lo=1
Wilson, E. O. (1998). Sociobiology. The Belknap Press.
Yong, E. (2021, May 4). Rhino beetle weapons match their fighting styles. Science. Retrieved October 1, 2022, from https://www.nationalgeographic.com/science/article/rhino-beetle-weapons-match-their-fighting-styles
Yu, Y.-J., Zou, M., Xu, S.-C., Zhang, R.-R., Wang, H.-X., & Liu, J.-T. (2014). Structure and mechanical characteristic of cattle horns. Journal of Mechanics in Medicine and Biology, 14(06). https://doi.org/10.1142/s0219519414400119
Zhang, Y., Huang, W., Hayashi, C., Gatesy, J., & McKittrick, J. (2018). Microstructure and mechanical properties of different keratinous horns. Journal of The Royal Society Interface, 15(143), 20180093. https://doi.org/10.1098/rsif.2018.0093
Zhang, Q.-bin, Li, C., Pan, Y.-ting, Shan, G.-hua, Cao, P., He, J., Lin, Z.-shi, Ao, N.-jian, & Huang, Y.-xiong. (2013). Microstructure and mechanical properties of horns derived from three domestic bovines. Materials Science and Engineering: C, 33(8), 5036–5043. https://doi.org/10.1016/j.msec.2013.08.034
Zhu, B., Zhang, M., & Zhao, J. (2016). Microstructure and mechanical properties of sheep horn. Microscopy Research and Technique, 79(7), 664–674. https://doi.org/10.1002/jemt.22681