Abstract
Armed with their network of pseudopodia and armoured by their distinctive shells, foraminifera are single-celled protists known for being ecologically successful marine microorganisms. Rare are the eukaryotes capable of surviving in oxygen-depleted environments, and rarer still are those that have evolved to prefer anoxic or hypoxic conditions, and yet that is precisely the case for some species of benthic foraminifera. Much of their success can be attributed to the plethora of intracellular chemical processes they undergo, which have allowed them to thrive in a variety of chemical environments. This paper namely discusses calcification, symbiosis, kleptoplasty, bacterial farming, denitrification, and lipid metabolism. It is assumed that the reader has adequate knowledge on universal natural processes such as diffusion, respiration, and photosynthesis, but links to educational sources have been provided where appropriate.
Introduction
Foraminifera are most often identified by their test, or shell, whose chemical composition is predominantlyCaCO3 (Hansen, 1999). The complex chemical process that creates this material is known as calcification, and the challenge for foraminifera is to collect the necessary reactants to perform calcification and to structure the CaCO3 into a usable, crystalline shell.
Symbiosis is very prevalent in foraminifera, specifically endosymbiosis of other protists, including dinoflagellates, diatoms, cyanobacteria, unicellular Chlorophyta, and Rhodophyta (Lee 2006). Most studies on foraminifera symbiosis focus on photosymbiotic relationships, which enable foraminifera to survive in low-oxygen environments. That is not to say that all symbiosis in foraminifera is photosymbiosis – as will be covered in later sections, denitrification is also a symbiotic role. Denitrification is typically performed by foraminifera that live in anoxic conditions below the photic zone. Some foraminifera show a preference for denitrification-based respiration over aerobic respiration. As a rule, foraminifera symbiosis is species specific, and foraminifera can identify their symbiotic targets by interacting with the target’s surface glycoproteins via their pseudopodia (Chai & Lee, 2000). Once ingested, their glycoproteins prevent symbiont digestion.
Some foraminifera are able to steal functional plastids from their prey for their own use. This is not classified as a symbiotic relationship as the original host of the plastids is dead. This is termed kleptoplasty, and so far, it is restricted to foraminifera from the Rotaliid and Miliolid families taking chloroplasts from diatoms (Glock, 2023). A definitive role of kleptoplasty in foraminifera is the acquisition of photosynthetic ability (LeKieffre, 2018) – but kleptoplasty is also seen in foraminifera that live below the photic level, suggesting that these stolen chloroplasts are capable of more than just photosynthesis. On the other hand, bacterial (and fungal) farming is observed in non-symbiont bearing foraminifera. These foraminifera secrete a sulphated glycosaminoglycan, upon which bacterial and fungal colonies feed. The foraminifera farmers then use chemotactic processes to systematically harvest the bacteria and fungi.
1. Calcification
One of the defining features of Foraminifera, discussed extensively in the previous physics essay, is the test — the shell that forms the outermost surface of the organism. Most foraminifera have tests that fall into one of two categories: agglutinated or calcareous. Agglutinated tests consist of foreign particles that are cemented together using the organism’s organic material (Goldstein, 1999). On the other hand, calcareous tests comprise calcium carbonate (CaCO3), which is secreted following a complex chemical process, shown in equation (3) — most foraminifera tests are formed this way (Hansen, 1999).
Throughout this section, the primary focus will be on species that have calcareous tests, and the process by which they precipitate: calcification.
![]()
![]()
![]()
Equations: [1] The net ionic equation of calcium carbonate. [2] Dissolved inorganic carbon and the bicarbonate buffer system (Raymond & Hamilton, 2018). [3] An example chemical reaction for calcification in aquatic organisms (Raymond & Hamilton, 2018).
As shown in equation (1), the general process of calcification requires calcium ions and carbonate ions to precipitate into the solid calcium carbonate. The ions needed for this process can be found in the ocean; Ca2+ can be found in abundance in the ocean because it is also found in earth’s crust (Calcium (Ca) and water) and carbonate is also found in the ocean as a part of a system of dissolved inorganic carbon (or DIC), equation (2). Seawater is foraminifera’s primary source for the calcification reactants (de Nooijer et al., 2014). In addition to collecting enough reactants for calcification, Foraminifera must form the collected calcium carbonate into multiple chambers of lamellar shells (crystalline calcite and aragonite) as discussed in the previous physics paper. The mechanisms by which Foraminifera can collect enough Ca2+ and DIC from the ocean and the way it can form CaCO3 into a usable test are the main chemical design solutions to the Foraminifera’s calcification challenge.
Ion collection
Ion concentration challenge
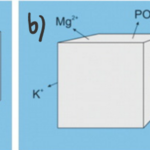
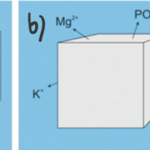
One major challenge for calcareous foraminifera is the need to concentrate enough calcium ions and dissolved inorganic carbon from seawater. As an example, to produce one new test chamber, younger foraminifera of some species need to process 50-100 times their own volume of seawater to collect enough calcium ions and approximately 3000 times their volume to collect enough carbonate ions (de Nooijer, Langer, et al., 2009). However, the amount of carbonate ions needed for calcification is significantly reduced by Foraminifera’s ability to take advantage of other forms of DIC for calcification—forms shown in Figure 1a. This has been proven by observations of high pH where foraminifera calcification is occurring, which suggests that the basic, and more abundant (based on pKa values) bicarbonate and carbon dioxide are being converted into carbonate for calcification (Spero et al., 1997). Even considering that foraminifera can broadly use all DIC as a source for carbonate, DIC concentrations are still one fifth of calcium ion concentrations. To successfully concentrate enough calcium ions and carbonate, there are two general mechanisms that Foraminifera uses: The first is to extract the needed ions from seawater (Fig. 1a below), and the second is to store seawater in intercellular reservoirs and expel all other ions (Fig. 1b below) (de Nooijer et al., 2014).
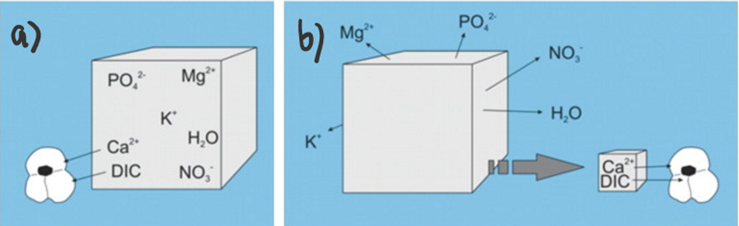
Figure 1. a) Diagram representing foraminifera’s specific selection of ions from seawater. b) Diagram representing foraminifera uptake of seawater and reduction of undesired ions. Adapted from de Nooijer et al. (2014).
Calcium ions
In some species of Foraminifera, there is evidence that calcium ions are stored in the reservoirs. For example, Toyofuku et al. (2008) showed that the species of foraminifera Ammonia beccarii was able to perform calcification even when placed in seawater without Ca2+. Even though the A. beccarii was still able to perform calcification in the experiment, the test was malformed, demonstrating that calcium directly from seawater is still important even in the case of reservoirs. Intracellular reservoirs are created using endocytosis and exocytosis of seawater (de Nooijer et al., 2014) — presumably increasing the desired ion concentration in these reservoirs.
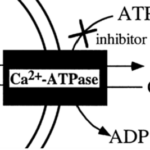
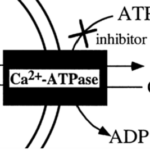
As opposed to using internal Ca2+ reservoirs, foraminifera can take calcium ions directly from surrounding sea water — de Nooijer et al. (2014) state that this is the most common source that foraminifera use for calcification. Interestingly, passive diffusion of Ca2+ cannot describe this mechanism since calcite (a specific structure of CaCO3) made by most foraminifera species has a much lower magnesium ion concentration compared to calcite precipitated inorganically (Kitano et al., 1975) — indicating that the foraminifera mechanism has some sort of specificity towards calcium. Therefore, it has been proposed that transmembrane proteins in foraminifera such as active ion pumps and ion channels facilitate specific transport of Ca2+ into the cytoplasm. One proposed mechanism is the use of ATP-driven active ion pumps to either pump calcium ions into the cytoplasm or pump magnesium outside the cell (de Nooijer et al., 2014)—as some transporters, such as some Ca-ATPases have been shown to be specific enough when it comes to magnesium and calcium (Drake et al., 1996). Specifically, the active ion pump Ca2+-ATPase is thought to be used in foraminifera as it would expel H+ while intaking Ca2+—shown in Figure 2 below. Zeebe and Sanyal (2002) states that H+ removal is a more efficient way to specifically intake Ca2+ as opposed to expulsing Mg2+ and corals have also been shown to use H+ expulsion for calcium intake (McConnaughey & Whelan, 1997).
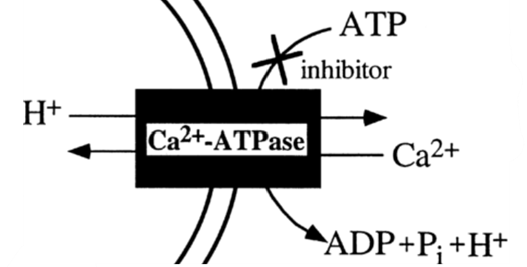
Figure 2. Simplified diagram representing Ca2+-ATPase pump, the double curved lines represent the cell membrane but directionality of the curve is ignored in this case. Adapted from Waldeck et al. (1998).
Dissolved inorganic carbon
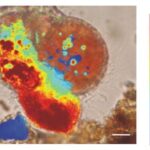
Similar to calcium reservoirs, some species of foraminifera have DIC reservoirs; proven by an experiment performed by ten Kuile and Erez (1987) where Amphistegina lobifera was first exposed to labelled 14C and the labelled carbon was found in later calcite formations even without environmental 14C. As with calcium ions, dissolved inorganic carbon comes from seawater. One of the ways by which foraminifera achieve a high concentration of inorganic carbon is by using a pH gradient. Bentov et al. (2009) found that pH is high inside of the foraminifera cell and low extracellularly. They proposed that this pH gradient causes CO2 to enter the cytoplasm. Erez (2003) measured pH in foraminifera vacuoles using “ratio imaging of the pH indicator Snarf-Dextran to Ca green-Dextran” and found that a foraminifer is able to increase the pH of its vacuoles to approximately 8.5 – 9. This technique used fluorescent Dextran probes that emit light when exposed to different pHs, and the relative proportion of the two probes (seen through fluorescence) is used to calculate pH (Bright et al., 1989). They also showed that foraminifera would increase the vacuole pH as it approaches the calcification site; this would indicate that the organism is concentrating DIC using CO2 to provide enough DIC to perform calcification. This relationship between vacuoles with high pH and calcification can be seen in Figure 3 below. It is also important to note that high pH levels in vacuoles cause the bicarbonate—which is common in seawater—to be converted into the carbonate needed for calcification; this conversion is part of the bicarbonate buffer system shown in equation (2).
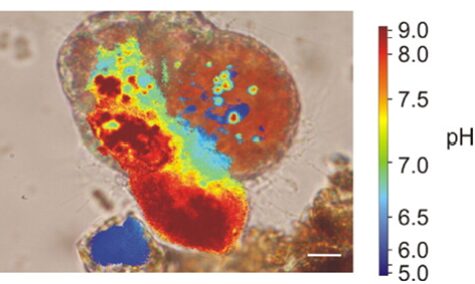
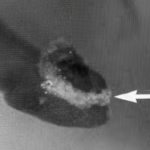
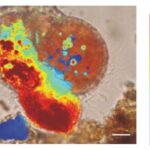
Figure 3. A microscopic image of foraminifera Cibicides lobatus with pH values coloured. The most red area is also the site where a new test chamber is being formed (calcification). The scale bar is 10 μm. Adapted from (de Nooijer, Toyofuku, & Kitazato, 2009).
Symbiosis and calcification
Although the majority of calcification is associated with seawater, symbionts such as algae can also play a role in foraminifera calcification. There are two proposed ways in which algae may help foraminifera calcification. The first equation describes bicarbonate splitting (Ter Kuile & Erez, 1991)—the use of bicarbonate for photosynthesis as opposed to CO2. This describes equation (3); the product carbon dioxide is used by the symbiont for photosynthesis and the product CaCO3 is used by foraminifera for its test. However, an alternate benefit is the production of ATP by the algal symbiont for use in active transport. This was proposed by Erez and Luz (1983) since symbionts treated with herbicide that blocks carbon fixation (and not the light-dependent reactions) did not decrease the calcification of the foraminifera. As mentioned earlier, active transport/ion pumps play a role in the concentration of calcium ions and, therefore, an increase in ATP from symbionts would improve the foraminifera’s calcification.
Formation of CaCO3
Nucleation challenge
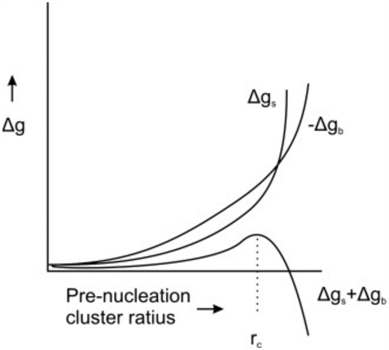
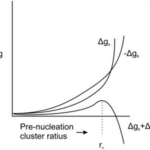
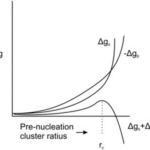
Figure 4. Free energy, Δg, as a function of radius of a pre-nucleation sphere of ions (de Nooijer et al., 2014). Δgs represents free energy at the surface, Δgb represents free energy of the ion bulk, and rc represents the critical radius at which nucleation occurs.
When CaCO3 forms as a result of calcification, there must be a point where the crystalline structure nucleates—usually a solid surface (De Yoreo & Vekilov, 2003). This is caused by the need to stabilize unbound ions—or reduce interfacial free energy; which can be achieved by either bonding to a solid surface or by the clustering of crystals (De Yoreo & Vekilov, 2003). In Figure 4, there is clearly an energy barrier that CaCO3 nucleation must overcome to start forming a crystalline structure, using binding with a surface. Interestingly, this nucleation energy is the reason that foraminifera can form two types of CaCO3 shell: calcite and aragonite. Calcite is the stable form of crystalline calcium carbonate whereas aragonite is more soluble but metastable—it may transform to the more stable calcite over time (Chandler, 2015). The two types of structure differentiate at the nucleation phase, and the only reason that the less stable aragonite forms is because of high Mg2+ concentrations, which increases the free energy barrier to forming calcite (Chandler, 2015): perhaps this is a reason foraminifer specifically create lower intracellular magnesium concentrations.
Organic matrix
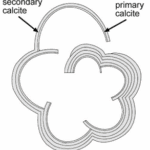
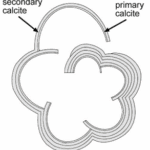
The nucleation of CaCO3 in foraminifera occurs on the primary organic sheet (or POS) which forms in the cytoplasm in preparation for calcification along with vacuoles, mitochondria, and other organic particles (Banner et al., 1973). The POS is made up of unbranched polysaccharides (Hottinger & Dreher, 1974) and proteins (Robbins & Brew, 1990). The polysaccharides are what determine that shape of the new growth of the foraminifera test (de Nooijer et al., 2014) while proteins connected to the polysaccharides may be charged and bind to CaCO3(Towe & Cifelli, 1967), reducing the nucleation free energy barrier. Once CaCO3 is nucleated somewhere on the POS, it spreads to cover both sides of the primary sheet (de Nooijer et al., 2014). The rest of test is grown in secondary calcium carbonate layers on top of the primary layer (Reiss, 1957) since the existing crystalline structure removes the nucleation energy barrier. This double layer is known as lamellar, and is shown in Figure 5 below.
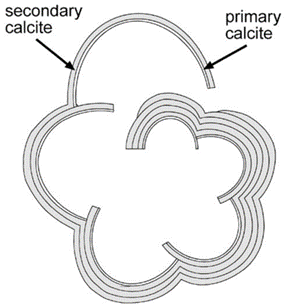
Figure 5. Diagram depicting the calcite test of foraminifera (Erez, 2003). The thinner, inner layer is labelled as “primary calcite” and the thicker, outer layers are labelled as “secondary calcite.”
2. Symbiosis
Symbiosis in foraminifera occurs mainly as photosymbiosis – this section will explore the diversity of applications it has within foraminifera.
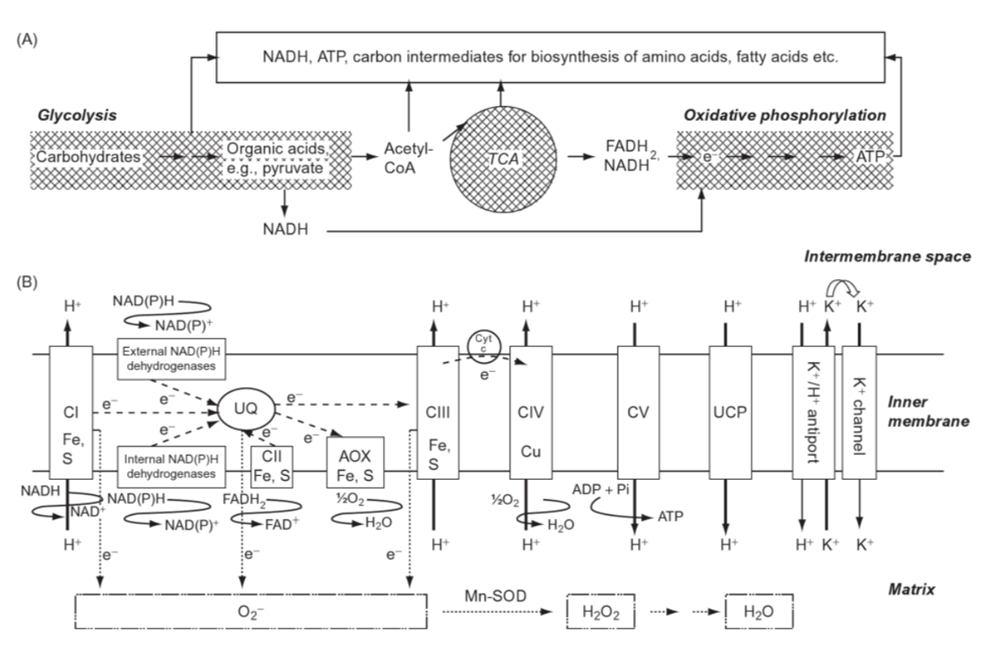
Figure 6. Scheme of respiration in the mitochondria. (A) Main steps of respiration: glycolysis in the cytosol and plastids, tricarboxylic acid cycle (TCA) and oxidative phosphorylation in mitochondria; (B) organization of the electron transport process on the inner membrane of mitochondria; CI complex I (NADH dehydrogenase), CII complex II (succinate dehydrogenase), CIII complex III (cytochrome bc1 complex), CIV complex IV (cytochrome c oxidase), CV complex V (ATP synthase); UQ: ubiquinone; cyt c: cytochrome c; UPC: uncoupling protein; Mn-SOD: Mn superoxide dismutase. Note the many instances of Fe(III) in the process (Marschner, 2011).
In the species Virgulinella fragilis, which lives in O2 depleted zones, researchers observed that the host’s mitochondria surround the symbiotic δ-proteobacteria, which supports the idea that the foraminifera host is sequestering oxygen produced by their endosymbionts for the creation of its own ATP (see Fig. 6) (Tsuchiya et al., 2015). Interestingly, V. fragilis shows a low diversity of endobionts, suggesting that there is an additional motive in selecting δ-proteobacteria specifically. Some δ-proteobacteria, like Anaeromyxobacter dehalogenans, can reduce oxidizing metals like Fe(III) in a mechanism that is independent of redox-conditions (Olson et al., 2008). Reduced Fe(III) is important for the chloroplast’s photosynthetic electron-transport (see Fig. 6), which makes A. dehalogenans the perfect photosymbiont for V. fragilis in its O2 depleted environment (Tsuchiya et al., 2015).
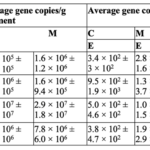
It has been suggested that V. fragilis’s endosymbiotic relationship enables them to oxidize sulfide, but this has yet to be confirmed (Tsuchiya et al., 2015). The ability to oxidize reduced sulfur compounds into sulfuric acid provides microorganisms with an oxygen-alternative for respiration. This has been observed in other benthic foraminifera, such as the Ammonia, Haynesina, and Elphidium families, which are known to host sulfur-cycle bacteria (in addition to kleptoplasts!) (Salonen et al., 2019). In fact, the genomes of foraminifera in these families contain some of their symbiotic bacteria’s genes (refer to Table 1), which allow them to actively participate in the sulfur and nitrogen cycle. For example, it has been hypothesized that they are able to create sulfolipids through the sulfate activation pathway (Nomaki et al., 2016). Sulfolipids can protect photosynthetic machinery under stressful conditions, which theoretically allows these foraminifera to protect their kleptoplasts. As will be seen in the next section, there are additional instances of horizontal gene transfer strengthening the link between kleptoplasty and symbiosis.
Table 1. Average functional genes copy numbers of Nitrogen and Sulfur cycle genes (± standard deviation) in sediment samples and inside the foraminiferal cells.
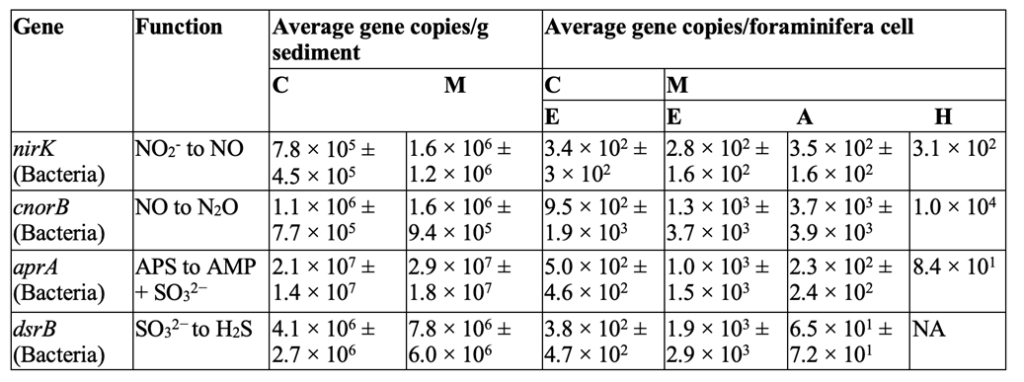
C: de Cocksdorp, M: Mokbaai, E: Elphidium sp., A: Ammonia sp., H: Haynesina sp., NA: Below the detection limit of the qPCR assay. This table displays the non-foraminifera genes found inside various foraminifera genomes Note the corresponding functions in the second column for each gene (Salonen et al., 2019).
Symbiosis in V. fragilis is considered robust, in the sense that the presence (or absence) of symbionts does not seem to be determined by environmental factors. This is not the case for foraminifera in the family Ammonia, where specimens collected in June-July showed no endosymbionts, while specimens collected in March (when temperatures and oxygen levels are low) did contain endobionts (Nomaki et al., 2016). This suggests that the Ammonia family use endosymbiosis to cope with the seasonal environmental variability that occurs within their lifetime.
Another mechanism that foraminifera use to adapt to ongoing environmental changes is symbiont shuffling, as seen in the species Pararotalia calcariformata of the Calcarindae family (Schmidt et al., 2018). This species can host a diverse community of diatom endosymbionts, each performing overlapping roles to different degrees of efficiency. Some diatoms are more thermally tolerant than others, such as Amphora coffeaeformis. This means that when external temperatures rise, P. calcariformata regulates the reproductive success of its endosymbionts to favor A. coffeaeformis. The mechanisms by which symbiont shuffling occurs have not yet been determined, as it is a relatively new discovery due to past technological limitations. However, a recent paper identifies symbiont shuffling in the genus Amphistegina, and the authors suggest that it is in fact a widespread adaptation (Prazeres et al, 2021).
Not all foraminifera use-and-abuse their symbionts – some foraminifera, like Heterosteginales depressa and Marginopora vertebralis protect their endosymbionts during stressful conditions (Lintner et al., 2023; Petrou et al. 2017). It has been experimentally observed that H. depressa enters a dormancy-like state when in darkness for extended periods of time (15 days) and relies on heterotrophic feeding (glucose uptake) to maintain its metabolism (Lintner et al., 2023). These foraminifera hosts do not consume their endosymbionts – in fact, the endosymbiont population appears to continue growing (albeit minimally) during this stressful time. Once photic conditions are re-established, the endosymbionts reward their hosts by performing unknown metabolic functions that enable carbonate, nitrate, and ammonium uptake to resume. This provides H. depressa with all the tools it needs to survive periods of darkness.
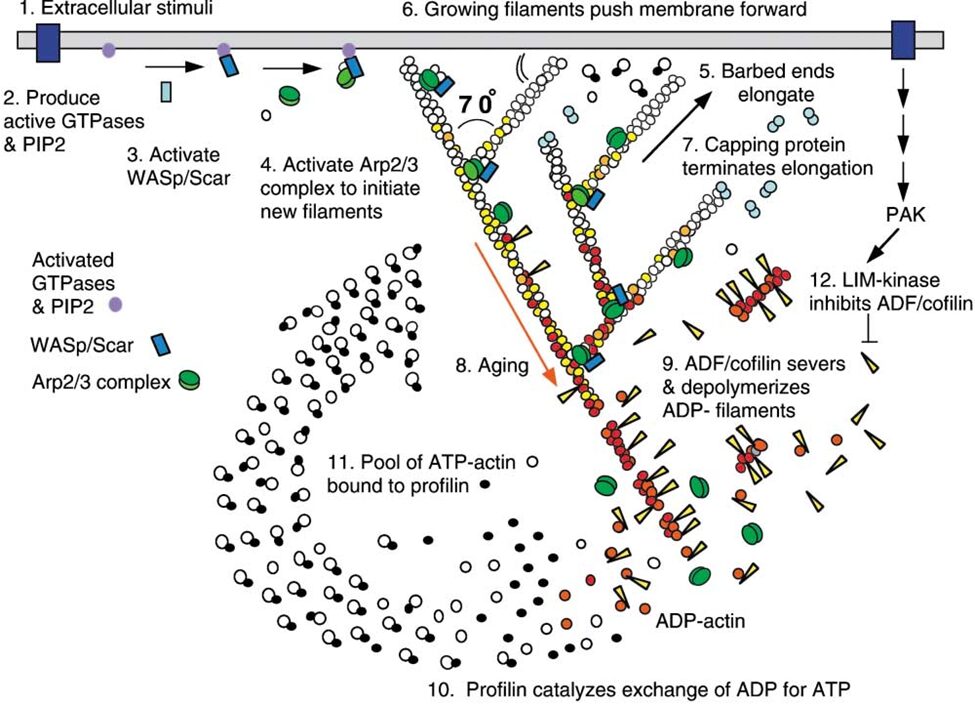
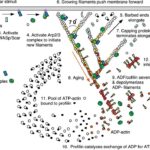
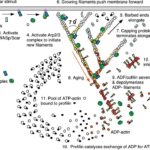
Figure 7 Actin Polymerization and Depolymerization. Figure shows actin regulation at the leading edge of a motile cell – these are universal concepts. Actin polymerization and depolymerization occurs at both ends of the filament, but the filament is polar and shows preference for polymerizing at the (+) end and depolymerization at the (-) end. Actin monomers are added to the filament in the ATP state, and spontaneous hydrolysis of ATP to ADP recruits the protein cofilin, which accumulates and eventually severs the actin filaments to increase the rate of depolymerization. Profilin recycles the actin monomers (catalyzing the exchange of ADP for ATP). Initiation proteins such as WASp and the Arp2/3 complex act as nucleation sites for new actin filaments. Capping proteins associate with the (+) ends of polymerizing filaments to stop polymerization (but prevent depolymerization), which creates short (and thus sturdy) filaments. Various kinases and phosphatases regulate the entire process by phosphorylating and dephosphorylating both the actin proteins and the polymerization/depolymerization proteins (Pollard & Borisy, 2003)

Figure 8. Heterostegina Depressa. (2.4 mm across) in a rock pool.
Both H. depressa (Fig. 8) and M. vertebralis maintain their endosymbionts close to their test surface, which supposedly optimizes photosynthesis (Lintner et al., 2023; Petrou et al. 2017). In high irradiance conditions, M. vertebralis can retract their photosymbionts away from the shell surface via rapid cytoskeletal restructuring (Petrou et al. 2017). Specifically, the host receives an unidentified infochemical signal from its photosymbiont, triggering actin filament restructuring that moves the symbionts. Refer to Figure 7 for an overview of actin polymerization and depolymerization. There are other ways that foraminifera use to protect their cellular components from light damage, such as physically moving away from the light, however, M. vertebralis’s mechanism allows it to remain in photic conditions so that other light-dependent reactions can proceed unimpeded.
3. Kleptoplasty

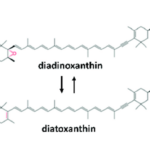
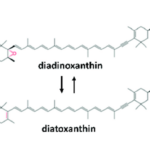
For a long time, kleptoplasty was thought to have evolved as an alternative to symbiosis, but recent discoveries of common ancestry between the kleptoplasts within the Hauerina diversaspecies and the symbionts in the Alveolinidae family suggests that kleptoplasty evolved in parallel to endosymbiosis (Pinko et al, 2023). Genomic analysis of foraminifera that host both symbionts and kleptoplasts frequently shows how foraminifera can take their symbiont’s genes to support and protect their kleptoplasts. As mentioned in the previous section, genes taken from sulfur-oxidizing symbionts potentially allows foraminifera in the Ammonia, Haynesina, and Elphidium genus to synthesize protective sulfolipids (Nomaki et al., 2016). An additional example is seen in Haynesina germanica, whose genome encodes the diatom-specific proteins diadinoxanthin de-epoxidase (DDE) and diatoxanthin epoxidase (DEP) (Jauffrais et al., 2017). Normally, foraminifera must replenish their chloroplasts daily (due to their inability to fix damaged chloroplasts), but in H. germanica the presence of DDE and DEP extends the survival of chloroplasts for up to a week. These molecules catalyze the conversion between diadinoxanthin to diatoxanthin as part of the xanthophyll cycle (see Fig. 9). The activity of DDE and DEP is regulated by pH rather than intracellular signalling, which exempts H. germanica from having to play an active role in photoprotection (beyond expressing the DDE and DEP genes, of course) (Latowski et al., 2011).
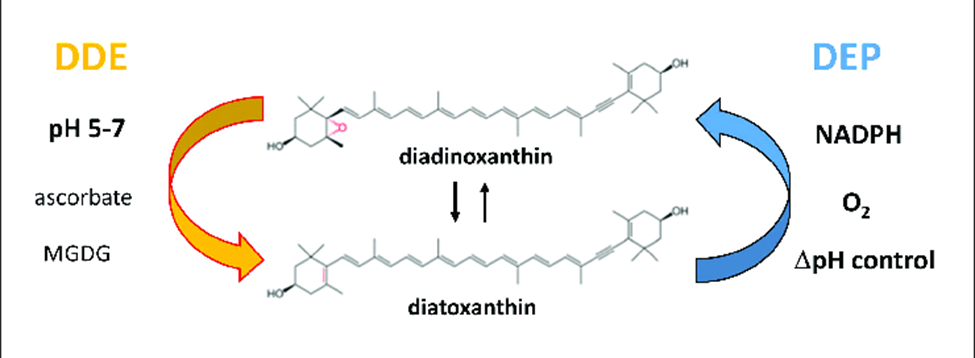
Figure 9 Diatoxanthin Cycling in Chloroplasts. The de-epoxidation reaction occurs in the thylakoid lumen, using DDE (diatoxanthin de-epoxidase). The epoxidation reaction occurs in the stroma, using DEP (diatoxithin epoxidase). Light decreases the pH in the thykaloid lumen (via the electron transport chain). Excessive light favors DDE activity over DEP, increasing the diatoxanthin/diadinoxanthin ratio. Diatoxanthin providing a photoprotective role via nonphotochemical quenching of energy.

The mechanism by which the xanthophyll cycle protects chloroplasts from photo-oxidative damage is as follows (refer to Fig. 9): diatoxanthin in the thylakoid membrane dissipates energy via non-photochemical quenching, which prevents the formation of reactive oxygen species (Lavaud et al., 2002). This is only needed when there is excessive light exposure, so diatoxanthin is typically converted into diadinoxanthin by DEP (located in the stroma). The first step of photosynthesis is the transport of electrons across the thylakoid membrane, decreasing the thylakoid lumen pH. Excessive light leads to excessive pH decrease, which favors DDE activity over DEP, increasing the presence of diatoxanthin in the thylakoid membrane.
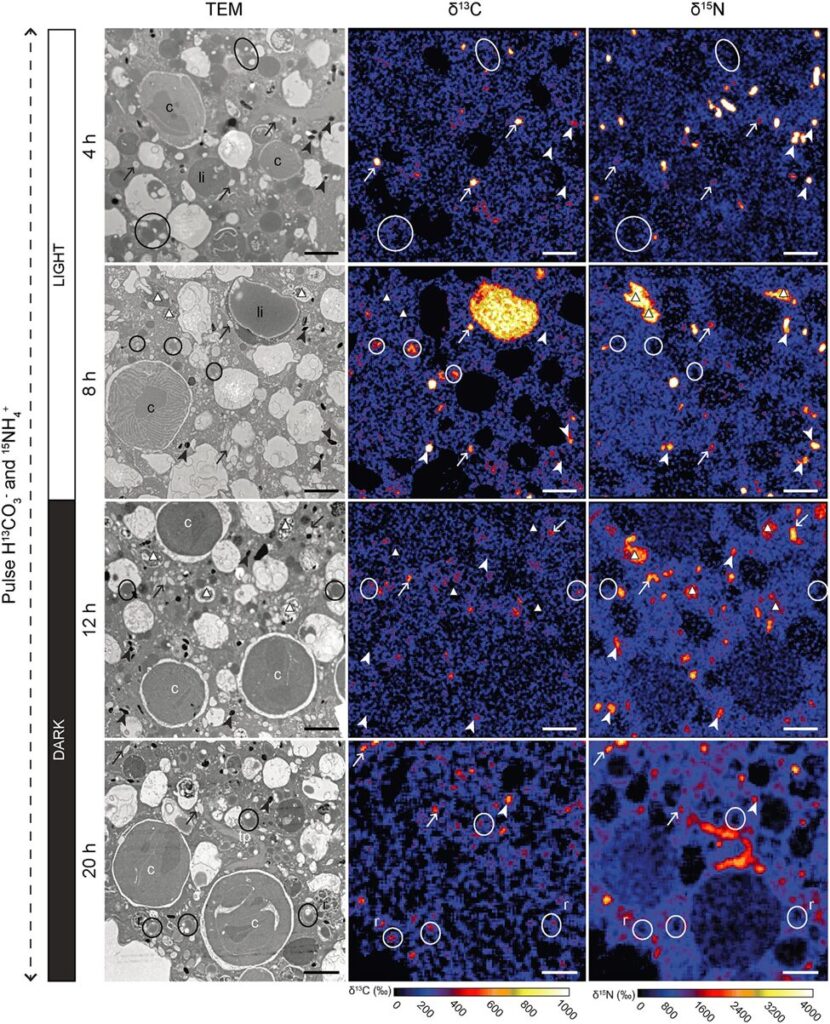
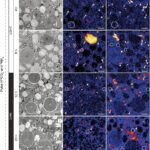
Figure 10. Transmission Electron Microscopy Showing Inorganic Carbon and Nitrogen Assimilation by Electron-Opaque Bodies. The electron opaque-bodies are indicated by the arrowheads, while the chloroplasts are indicated by “c”. Note how inorganic carbon and nitrogen is localized to the electron opaque-bodies but not the nearby chloroplasts. This indicates that foraminifera are able to assimilate inorganic carbon and nitrogen themselves without the help of chloroplasts. Scale bars: 2um (LeKieffre et al., 2018).
The question remains as to why foraminifera developed kleptoplasty when symbiosis can provide them photosynthetic abilities as well. When researchers measured the photosynthetic activity of chloroplasts in Hauerina diversa (a foraminifera)and compared it to that of photosymbionts in the closely related Amphistegina lobifera (also a foraminifera), they found that kleptoplasty was less efficient (in terms of nanograms of carbon uptake, per hour, per specimen) (Pinko et al, 2023). Logically, there must be some advantage to kleptoplasty, otherwise it would not be competitive enough to be found in foraminifera. Researchers suggest that despite a decrease in photosynthetic ability, there are still enough photosynthates produced for host consumption, probably because the host doesn’t need to share with symbionts. Other researchers have probed into the idea that chloroplasts perform some non-photosynthetic function, because kleptoplasty has been observed in foraminifera that live in aphotic environments (Gomaa et al., 2021). For a few years it was thought that kleptoplasty allows foraminifera to fix inorganic nitrogen via the glutamine synthetase and glutamate 2-oxoglutarate aminotransferase (GOGAT) pathway (Grzymski et al., 2002), but transmission electron microscopy experiments show that assimilated nitrogen doesn’t localize to chloroplasts (refer to Fig. 10), indicating that the foraminifera are able to fix inorganic nitrogen themselves (via the GOGAT pathway) (Jauffrais et al., 2019; Gomaa et al., 2021). Interestingly, the proteins that Nonionella stella uses to fix nitrogen were acquired from its kleptoplasts (not symbionts) via horizontal gene transfer at some point in evolution (Gomaa et al., 2021).
4. Bacterial and Fungal Farming
Bacterial and fungal farming by foraminifera refers to a feeding strategy where certain smaller, non-symbiont bearing, epiphytic foraminifera graze on seagrass leaves and create embankment-like organic traces. These traces serve as a fertile substrate for the growth of bacteria and fungi. The foraminifera then graze on the bacteria and fungi, suggesting a farming strategy where they cultivate and harvest these microorganisms as a food source (Langer & Gehring, 1993).
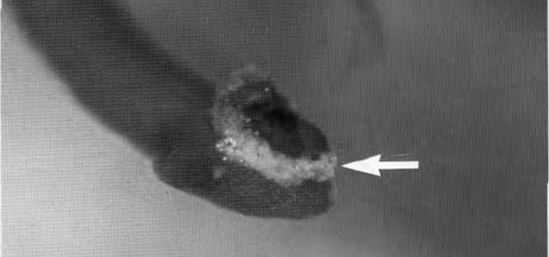
Figure 11: Adhesive, whitish glycosaminoglycans secreted by Rosalina globularis on a dasycladacean algae for temporary attachment (arrow) (Langer, M. R., & Gehring, C. A. 1993, P6).
The substrate secreted by foraminifera is a type of sulfated glycosaminoglycans (GAGs) (refer to Fig. 11). Historically referred to as mucopolysaccharides, the current scientific discourse favors glycosaminoglycan, since each molecule is a derivative of either galactosamine or glucosamine (Langer & Gehring, 1993). These GAGs are intricate chains of complex sugars, distinguished by their sulfation, a process unique to foraminifera among their microfaunal peers. It begins with the formation of a protein linkage region on which chains of heparin/heparan sulfate or chondroitin/dermatan sulfate are polymerized. Synthesis is facilitated by a group of glycosyltransferases – enzymes that are encoded by the EXT family genes for heparin/heparan sulfate and by chondroitin synthases for chondroitin/dermatan sulfate (Kitagawa H. 2002).
Foraminifera exhibit what seems to be repeated grazing activity on previously produced and colonized traces (refer to Fig. 12). This implies a chemotaxis mechanism, but the question remains on whether it is directed by an attraction to their own GAGs or perhaps to the bacteria and fungi themselves (Langer & Gehring, 1993).
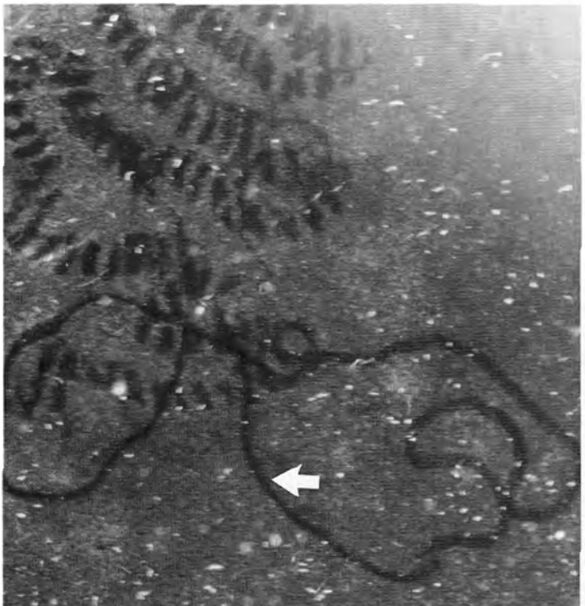
Figure 12: Light microscope observations of foraminifera in culture vessels. Irregular grazing traces of a smaller textulariid foraminifera on algal-covered glass surfaces in a culture vessel (arrow, note difference in secretion traces). (Langer, M. R., & Gehring, C. A. 1993, P6)
5. Denitrification
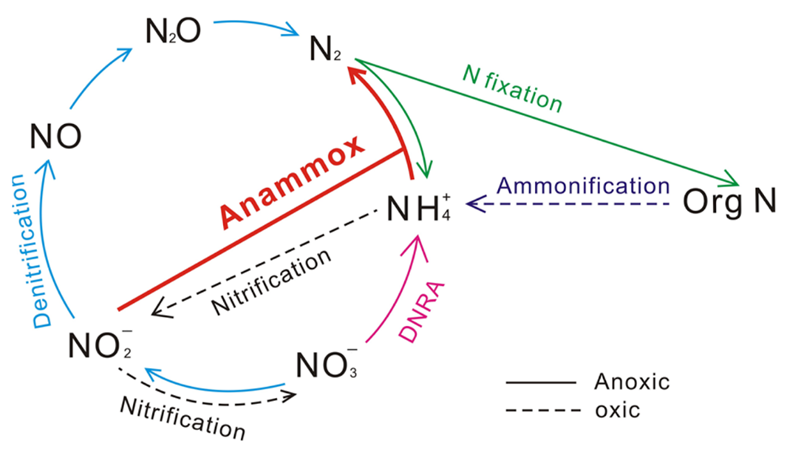
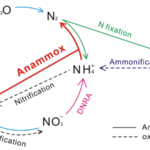
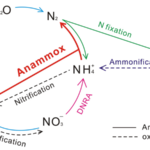
Figure 13 Nitrogen Cycle. Blue arrows denote the denitrification process, which reduces oxidized forms of nitrogen. Pink arrow denotes the net DNRA process, which uses nitrate to form ammonium – figure is misleading in that it ignores the nitrite intermediate. Red arrow denotes the anammox process which creates nitrogen gas using ammonia and nitrite (Wei & Zhang, 2023).
Denitrification involves reducing oxidized forms of nitrogen for energy production. It can be the preferred respiration pathway in some foraminifera, where intracellular nitrate stores lead to more efficient nitrate metabolism during denitrification compared to dioxygen metabolism during aerobic respiration (Glock et al., 2019). Foraminifera perform anaerobic respiration by oxidizing organic matter and then using oxidized forms of nitrogen as electron acceptors, eventually producing nitrogen gas (see eq. [4] to [7], and Fig.13). An alternative respiratory mechanism that also uses oxidized nitrogen as an electron acceptor is the DNRA (Dissimilatory Nitrate Reduction to Ammonium) pathway (see eq. [8] and Fig.13) (Lam & Kuypers, 2011). When both the foraminifera and their endosymbiont use the DNRA pathway, they compete for nitrate, which may explain why denitrification is more common than DNRA (Sommer et al., 2016). Endosymbionts can remove the ammonium via anaerobic ammonium oxidation – referred to as anammox – using nitrite as a reactant (see Fig. 13) (Thamdrup & Dalsgaard, 2002).
Denitrification Equations
![]()
![]()
![]()
![]()
DNRA (Dissimilatory Nitrate Reduction to Ammonium) pathway
![]()
Foraminifera are the only eukaryotes known to perform complete denitrification to nitrogen gas (Risgaard-Petersen, 2005), but they probably acquired the genes needed to perform denitrification via horizontal gene transfer from a prokaryote donor (Woehle et al., 2022). Cases where foraminifera’s endosymbionts support their denitrification have been observed – specifically, in the rotaliid genus Globobulimina, foraminifera in the Peruvian oxygen minimum zone were observed to rely on their Desulfobacteraceae endosymbionts (Woehle et al., 2022). These endosymbionts perform the first step of DNRA to produce nitrite, which can be used by the foraminifera host for denitrification (see Fig. 13). In this specific case, the foraminifera rely on its endosymbionts because it lacks the gene encoding nitrate reductase, meaning that it cannot perform the first step of denitrification on its own (see equations (4) – (7)).
6. Peroxisomes and lipid metabolism
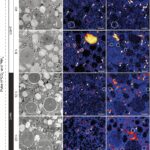
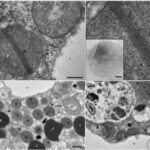
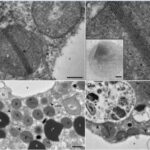
Robust foraminifera populations occur across oxygen-gradients within chemoclines, and certain of those foraminifera contain masses of peroxisomes, which are often associated to the presence of endoplasmic reticulum (Bernard and Bowser, 2008). Peroxisomes are spherical vacuoles of approximately 500nm in size (Fig. 14A), which contain a crystalline structure (Fig. 14B).
Furthermore, peroxisomes are hypothesized to mediate aerobic respiration through the catalyzation of O2 production–this requires further investigation (B&B, 2008).
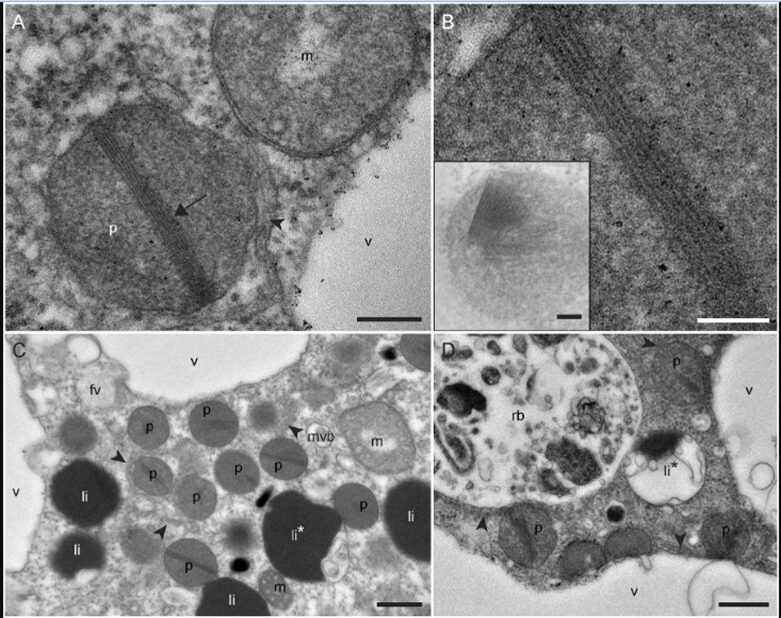
Figure 14. Peroxisomes of benthic foraminifer from transmission electron micrographs in N. labradorica. A) Peroxisome surrounded by endoplasmic reticulum (ER). B) Magnified image of peroxisome’s crystalline structure, seen in A. C) and D) are peroxisomes in the cytoplasm, where rb denotes residual bodies, v denotes vacuoles, and li are liquid droplets whereas li* are liquid droplets in lysis (LeKieffre et al, 2018).
To thrive in oxygen-depleted conditions (i.e. hypoxic or anoxic environments), protists have been shown to modify the functions of their peroxisomes & mitochondria. In particular, two benthic foraminifera species–N. Stella and B. argentea–within the Santa Barbara Bassin (SBB) utilize adaptable mitochondrial and peroxisomal metabolism; interestingly, a (meta)transcriptomic analysis by Power et al. revealed contrasting utilizations of these organelles.
Let’s first highlight some key differences between the two species outlined in the study by Power et al. For one, N. stella are most abundant in anoxic conditions, and they are a calcareous species characterized by an apparent lack of food vacuoles; instead, they have an abundance of intracellular lipid droplets. Contrastingly, B. argentea are most abundant in hypoxia, that is in aquatic environments where O2 is present, but the O2 saturation is less than 30 %. They contain food vacuoles and were predicted to utilize the putative peroxisomal gluconeogenesis and a full TCA cycle (which is the second stage of cellular respiration) but lacks the expression of key beta oxidation and glyoxylate cycle genes.
Furthermore, B. argentea exhibited potential capabilities for substrate-level phosphorylation through the interplay of succinate: acetate CoA transferase (SCoAT) and succinyl-CoA synthetase (SCS). Significantly, while genes encoding SCS and SCoAT have been recognized in related species previously, this study marks the first documentation of the expression of the SCoAT gene in foraminifera. The employment of substrate-level phosphorylation and the consistent expression of denitrification pathways by B. argentea in hypoxic conditions suggest a potential lifestyle as a facultative anaerobe.
In contrast, N. stella exhibited more metabolic responses to the chemical amendments tested in the study, whether it be under hypoxic or anoxic conditions, for it was found that they employed a combination of peroxisomal and mitochondrial pathways. For example, the introduction of H2O2 resulted in the up-regulation of genes (i.e. increased activity/expression of genes) associated with beta-oxidation and the glyoxylate cycle. Note that since beta-oxidation is the process of the breakdown of molecules of fatty acids (which occurs in the mitochondria for eukaryotes) (Houten et al, 2010), this response is likely connected to the usage of intracellular lipid droplets. Remarkably, the metabolic profile of N. stella under H2O2 amendment appears to be consistent to its native metabolic state in the sediments of the SBB: no differential gene expression was detected between H2O2 amendments under anoxia or hypoxia compared to their respective conditions, without those amendments (i.e. the control samples).
Ultimately, the predictions obtained from Power et al.’s study indicates that combinations of metabolic pathways found in mitochondria related organelles (MROs) (refer to Fig.14) are likely to be localized in the mitochondria of N. stella and B. argentea. Despite this, the mitochondria of both species still maintain the oxygen-dependent Electron Transport Chain (ETC), plus they have the potential for anaerobic respiration through denitrification. This marks the first time that these distinctive metabolic capacities have been identified in protists, and the current hypothesis is that they may contribute to the adaptability of the benthic foraminifera studied, thereby enabling their survival in both oxygen-depleted and aerated conditions.
Overall, both species show distinct metabolic adaptations to anoxic and hypoxic conditions, and they were found to have different capabilities when it came to using the compounds of the SBB, which indicates that metabolism of benthic foraminifera is not uniform across species. On one hand, N. stella are professionals when it comes to surviving the hypoxic conditions of the SBB: this study found that their mitochondrial metabolism is flexible, for their function coordinates with that of the predicted metabolism of their peroxisomes. On the other hand, the metabolism that was predicted for B. argentea the implies that they digest the contents of their food vacuoles, as facultative anaerobes would.
Ultimately, this study sets benthic foraminifera’s mitochondrial metabolisms apart from those observed in conventional MROs present in anaerobic protists. This suggests that their mitochondria have evolved specialized metabolic tools, allowing them to support their ability to respond to varying oxygen concentrations characteristic of the SBB chemocline. Additionally, the plasticity observed in the mitochondria is further influenced by the peroxisomes in both species. What is particularly noteworthy is the fact that these peroxisomes exhibit metabolic distinctiveness not only from each other but also from known anaerobic peroxisomes. N. stella upregulate peroxisomes because they use their lipid droplets as carbon sources in anoxia, while B. argentea downregulate the activity of their peroxisomes.
Conclusion
Foraminifera exhibit many interesting and complex adaptations that allow them to survive in harsh environments. Staggeringly, these adaptations to anoxic and hypoxic conditions–and their relative rarity amongst other eukaryotes–could mean that the foraminifer species that possess them could benefit from the expansion of oxygen-depleted zones. When they lack the necessary organelles to live in an environment, they form symbiotic relationships with micro-organisms that have already developed the appropriate adaptations. For example, foraminifera that reside in low-oxygen environments form symbiotic relationships with photosynthesizing micro-organisms. In an alternative adaptation, foraminifera can also simply steal the organelles they need via kleptoplasty. Horizontal gene transfer from their symbionts and kleptoplasts equips foraminifera with the additional tools that they need to adapt to challenging environmental conditions.
Foraminifera provide protection and nutrients to their endosymbionts, and in return, the symbionts aid in respiration (via photosynthesis and/or sulfur oxidation). Foraminifera have numerous ways of protecting their symbionts (and kleptoplasts) from photo-oxidative damage; Marginopora vertebralis rapidly remodel their actin cytoskeleton move their photosymbionts away from the shell surface in excessive light conditions, while Haynesina germanica passively protect their kleptoplasts by constitutionally expressing diatom diatoxanthin and diadinoxanthin proteins (part of the xanthophyll cycle). Some foraminifera are more selfish in their symbiotic relationships, only ingesting (but not digesting!) other micro-organisms when environmental conditions are tough (e.g., the genus Ammonia). In between these two extremes are the foraminifera that play host to many different symbionts simultaneously, controlling their symbiont’s reproductive success to favor those which are best suited to the current environmental conditions (Pararotalia calcariformata).
Bacterial farming is a clever mechanism that foraminifera have developed which saves them the trouble of hunting for food by simply excreting nutrients that promote bacterial and fungal colony growth in the foraminifera’s local environment. Foraminifera then use chemotaxis to systematically harvest these micro-organisms.
All foraminifera have shells, and although the net ionic equation for calcification is simple (Ca2+(aq) + CO32-(aq) = CaCO3), foraminifera need to overcome the challenge low ion concentration in seawater. They do this by active transport and deliberately created pH gradients and overcome the nucleation free energy of CaCO3 crystallization by using ordered and polar organic polymers.
References
Bentov, S., Brownlee, C., & Erez, J. (2009). The role of seawater endocytosis in the biomineralization process in calcareous foraminifera. Proceedings of the National Academy of Sciences, 106(51), 21500-21504.
Bright, G. R., Fisher, G. W., Rogowska, J., & Taylor, D. L. (1989). Chapter 6 Fluorescence Ratio Imaging Microscopy. In D. L. Taylor & Y.-L. Wang (Eds.), Methods in Cell Biology (Vol. 30, pp. 157-192). Academic Press. https://doi.org/https://doi.org/10.1016/S0091-679X(08)60979-6
Chai, J., & Lee, J. J. (2000). Recognition, Establishment and Maintenance of Diatom Endosymbiosis in Foraminifera. Micropaleontology, 46, 182–195. http://www.jstor.org/stable/1486189
Chandler, D. L. (2015). Mystery solved: Why seashells’ mineral forms differently in seawater. MIT News. Retrieved 11/07 from https://news.mit.edu/2015/why-seashell-mineral-forms-differently-in-seawater-0302
Correia MJ, Lee JJ. Chloroplast retention by Elphidium excavatum (Terquem). Is it a selective process? Symbiosis. 2000; 29: 343–55.
de Nooijer, L. J., Langer, G., Nehrke, G., & Bijma, J. (2009). Physiological controls on seawater uptake and calcification in the benthic foraminifer Ammonia tepida. Biogeosciences, 6(11), 2669-2675.
de Nooijer, L. J., Spero, H. J., Erez, J., Bijma, J., & Reichart, G. J. (2014). Biomineralization in perforate foraminifera. Earth-Science Reviews, 135, 48-58. https://doi.org/https://doi.org/10.1016/j.earscirev.2014.03.013
de Nooijer, L. J., Toyofuku, T., & Kitazato, H. (2009). Foraminifera promote calcification by elevating their intracellular pH. Proceedings of the National Academy of Sciences, 106(36), 15374-15378. https://doi.org/doi:10.1073/pnas.0904306106
De Yoreo, J. J., & Vekilov, P. G. (2003). Principles of crystal nucleation and growth. Reviews in mineralogy and geochemistry, 54(1), 57-93.
Drake, S. K., Lee, K. L., & Falke, J. J. (1996). Tuning the equilibrium ion affinity and selectivity of the EF-hand calcium binding motif: substitutions at the gateway position. Biochemistry, 35(21), 6697-6705.
Erez, J. (2003). The source of ions for biomineralization in foraminifera and their implications for paleoceanographic proxies. Reviews in mineralogy and geochemistry, 54(1), 115-149.
Erez, J., & Luz, B. (1983). Experimental paleotemperature equation for planktonic foraminifera. Geochimica et Cosmochimica Acta, 47(6), 1025-1031. https://doi.org/https://doi.org/10.1016/0016-7037(83)90232-6
Glock, N. (2023). Benthic foraminifera and gromiids from oxygen-depleted environments – survival strategies, biogeochemistry and trophic interactions. Biogeosciences, 20(16), 3423–3447. https://doi.org/10.5194/bg-20-3423-2023
Goldstein, S. T. (1999). Foraminifera: A Biological Overview. In Modern Foraminifera. Springer Netherlands. https://books.google.ca/books?id=K-3tUmXW-IgC
Gomaa, F., Utter, D. R., Powers, C., Beaudoin, D. J., Edgcomb, V. P., Filipsson, H. L., Hansel, C. M., Wankel, S. D., Zhang, Y., and Bernhard, J. M. (2021) Multiple integrated metabolic strategies allow foraminiferan protists to thrive in anoxic marine sediments, Sci. Adv., 7, eabf1586, https://doi.org/10.1126/sciadv.abf1586,
Goss, R., & Latowski, D. (2020). Lipid Dependence of Xanthophyll Cycling in Higher Plants and Algae. Frontiers in plant science, 11, 455. https://doi.org/10.3389/fpls.2020.00455
Grzymski, J., Schofield, O. M., Falkowski, P. G., and Bernhard, J. M. (2002) The function of plastids in the deep-sea benthic foraminifer, Nonionella stella, Limnol. Oceanogr., 47, 1569–1580, https://doi.org/10.4319/LO.2002.47.6.1569, 2002
Hansen, H. J. (1999). Shell Construction in Modern Calcareous Foraminifera. In Modern Foraminifera. Springer Netherlands. https://books.google.ca/books?id=K-3tUmXW-IgC
Hottinger, L., & Dreher, D. (1974). Differentiation of protoplasm in Nummulitidae (foraminifera) from Elat, Red Sea. Marine Biology, 25, 41-61.
Houten, S. M., & Wanders, R. J. (2010). A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation. Journal of inherited metabolic disease, 33(5), 469–477. https://doi.org/10.1007/s10545-010-9061-2
Jauffrais, T., Jesus, B., Metzger, E., Mouget, J.-L., Jorissen, F., and Geslin, E. (2016) Effect of light on photosynthetic efficiency of sequestered chloroplasts in intertidal benthic foraminifera (Haynesina germanica and Ammonia tepida), Biogeosciences, 13, 2715–2726, https://doi.org/10.5194/bg-13-2715-2016
Jauffrais, T., Jesus, B., Méléder, V., & Geslin, E. (2017). Functional xanthophyll cycle and pigment content of a kleptoplastic benthic foraminifer: Haynesina germanica. PloS one, 12(2), e0172678. https://doi.org/10.1371/journal.pone.0172678
Jauffrais T., LeKieffre C., Schweizer M., Geslin E., Metzger E., Bernhard J. M., Jesus B., Filipsson H. L., Maire O., Meibom A. (2019) Kleptoplastidic benthic foraminifera from aphotic habitats: Insights into assimilation of inorganic C, N, and S studied with subcellular resolution. Environ. Microbiol. 21, 125–141
Jesus, B., Jauffrais, T., Trampe, E.C.L. et al. (2022) Kleptoplast distribution, photosynthetic efficiency and sequestration mechanisms in intertidal benthic foraminifera. ISME J 16, 822–832 https://doi.org/10.1038/s41396-021-01128-0
Kitagawa H. (2002). Yakugaku zasshi : Journal of the Pharmaceutical Society of Japan, 122(7), 435–450. https://doi.org/10.1248/yakushi.122.435
Kitano, Y., Okumura, M., & Idogaki, M. (1975). Incorporation of sodium, chloride and sulfate with calcium carbonate. Geochemical Journal, 9(2), 75-84.
Lam, P. & Kuypers, M. M. M. (2011). “Microbial Nitrogen Processes in Oxygen Minimum Zones”. Annual Review of Marine Science. 3: 317–345. Bibcode:2011ARMS….3..317L. doi:10.1146/annurev-marine-120709-142814. hdl:21.11116/0000-0001-CA25-2. PMID 21329208.
Langer, M. R., & Gehring, C. A. (1993). Bacteria farming; a possible feeding strategy of some smaller motile foraminifera. The Journal of Foraminiferal Research, 23(1), 40–46.
Latowski, D., Kuczyńska, P., & Strzałka, K. (2011). Xanthophyll cycle–a mechanism protecting plants against oxidative stress. Redox report: communications in free radical research, 16(2), 78–90. https://doi.org/10.1179/174329211X130209517399
Lavaud, J., Rousseau, B., van Gorkom, H. J., & Etienne, A. L. (2002). Influence of the diadinoxanthin pool size on photoprotection in the marine planktonic diatom Phaeodactylum tricornutum. Plant physiology, 129(3), 1398–1406. https://doi.org/10.1104/pp.002014
Lee, J.J. (2006) Algal symbiosis in larger foraminifera. Symbiosis 42, 63–75
LeKieffre, C., Jauffrais, T, Geslin, E., Jesus, B., Bernhard, J.M., Giovani, M.E., Meibom, A. Inorganic carbon and nitrogen assimilation in cellular compartments of a benthic kleptoplastic foraminifer. Sci. Rep. 8, 10140 (2018).
LeKieffre, C., Bernhard, J. M., Mabilleau, G., Filipsson, H. L., Meibom, A., & Geslin, E. (2018). An overview of cellular ultrastructure in benthic foraminifera: New observations of rotalid species in the context of existing literature. Marine Micropaleontology, 138, 12-32. https://doi.org/https://doi.org/10.1016/j.marmicro.2017.10.005
Lenntech B.V(2007). Calcium (Ca) and water.https://www.lenntech.com/periodic/water/calcium/calcium-and-water.htm
Lintner, M., Lintner, B., Schagerl, M. et al. The change in metabolic activity of a large benthic foraminifera as a function of light supply. Sci Rep 13, 8240 (2023). https://doi.org/10.1038/s41598-023-35342-x
Marschner, H. (2011). Marschner’s Mineral Nutrition of Higher Plants. Academic Press.
McConnaughey, T., & Whelan, J. (1997). Calcification generates protons for nutrient and bicarbonate uptake. Earth-Science Reviews, 42(1-2), 95-117.
Olson, J., Spiro, T., Zumft, W., Ann Walker, F., Kitagawa, T., Scheidt, W. R., Farmer, P., Gilles-Gonzalez, M.A. (2008) The Smallest Biomolecules: Diatomics and their Interactions with Heme Proteins. Elsevier B.V.
Petrou, K., Ralph, P. & Nielsen, D. (2017). A novel mechanism for host-mediated photoprotection in endosymbiotic foraminifera. ISME J 11, 453–462 https://doi.org/10.1038/ismej.2016.128
Pinko, D., Abramovich, S., Rahav, E., Belkin, N., Rubin-Blum, M., Kucera, M., Morard, R., Holzmann, M., Arbu, U. (2023) Shared ancestry of algal symbiosis and chloroplast sequestration in foraminifera.Sci. Adv.9, eadi3401 DOI:10.1126/sciadv.adi3401
Pollard, T.D., & Borisy, G.G. (2003). Cellular Motility Driven by Assembly and Disassembly of Actin Filaments. Cell, 112, 453-465
Powers, C., Gomaa, F., Billings, E. B., Utter, D. R., Beaudoin, D. J., Edgcomb, V. P., Hansel, C. M., Wankel, S. D., Filipsson, H. L., Zhang, Y., & Bernhard, J. M. (2022). Two canonically aerobic foraminifera express distinct peroxisomal and mitochondrial metabolisms [Original Research]. Frontiers in Marine Science, 9. https://doi.org/10.3389/fmars.2022.1010319
Prazeres, M., Roberts, T. E., Ramadhani, S. F., Doo, S. S., Schmidt, C., Stuhr, M., & Renema, W. (2021). Diversity and flexibility of algal symbiont community in globally distributed larger benthic foraminifera of the genus Amphistegina. BMC microbiology, 21(1), 243. https://doi.org/10.1186/s12866-021-02299-8
Raymond, P. A., & Hamilton, S. K. (2018). Anthropogenic influences on riverine fluxes of dissolved inorganic carbon to the oceans. Limnology and Oceanography Letters, 3(3), 143-155. https://doi.org/https://doi.org/10.1002/lol2.10069
Reiss, Z. (1957). The Bilamellidea, nov. superfam., and remarks on Cretaceous globorotaliids. Contributions from the Cushman Foundation for Foraminiferal Research, 8, 127-145.
Risgaard-Petersen, N. et al., (2006) Evidence for complete denitrification in a benthic foraminifer. Nature 443, 93–96
Robbins, L., & Brew, K. (1990). Proteins from the organic matrix of core-top and fossil planktonic foraminifera. Geochimica et Cosmochimica Acta, 54(8), 2285-2292.
Salonen, I. S., Chronopoulou, P., Bird, C., Reichart, G., & Koho, K. A. (2019). Enrichment of intracellular sulphur cycle -associated bacteria in intertidal benthic foraminifera revealed by 16S and aprA gene analysis. Scientific reports, 9(1), 11692. https://doi.org/10.1038/s41598-019-48166-5
Schmidt, C., Morard, R., Romero, O., & Kucera, M. (2018). Diverse Internal Symbiont Community in the Endosymbiotic Foraminifera Pararotalia calcariformata: Implications for Symbiont Shuffling Under Thermal Stress. Frontiers in microbiology, 9, 2018. https://doi.org/10.3389/fmicb.2018.02018
Shimojima, M. (2011) Biosynthesis and functions of the plant sulfolipid. Progress in Lipid Research, 5(3) https://doi.org/10.1016/j.plipres.2011.02.003
Spero, H. J., Bijma, J., Lea, D. W., & Bemis, B. E. (1997). Effect of seawater carbonate concentration on foraminiferal carbon and oxygen isotopes. Nature, 390(6659), 497-500.
Sommer, S., et al., (2016).Depletion of oxygen, nitrate and nitrite in the Peruvian oxygen minimum zone cause an imbalance of benthic nitrogen fluxes. Deep Sea Res Part I 112, 113–122
ten Kuile, B., & Erez, J. (1987). Uptake of inorganic carbon and internal carbon cycling in symbiont-bearing benthonic foraminifera. Marine Biology, 94, 499-509.
Ter Kuile, B. H., & Erez, J. (1991). Carbon Budgets for Two Species of Benthonic Symbiont-Bearing Foraminifera. Biol Bull, 180(3), 489-495. https://doi.org/10.2307/1542350
Thamdrup, B., Dalsgaard, T. (2002) Production of N(2) through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments. Appl Environ Microbiol 68, 1312–1318
Towe, K. M., & Cifelli, R. (1967). Wall ultrastructure in the calcareous foraminifera: crystallographic aspects and a model for calcification. Journal of Paleontology, 742-762.
Toyofuku, T., Jan de Nooijer, L., Yamamoto, H., & Kitazato, H. (2008). Real‐time visualization of calcium ion activity in shallow benthic foraminiferal cells using the fluorescent indicator Fluo‐3 AM. Geochemistry, Geophysics, Geosystems, 9(5).
Tsuchiya, M., Toyofuku, T., Uematsu, K., Brüchert, V., Collen, J., Yamamoto, H., & Kitazato, H. (2015). Cytologic and Genetic Characteristics of Endobiotic Bacteria and Kleptoplasts of Virgulinella fragilis (Foraminifera). The Journal of eukaryotic microbiology, 62(4), 454–469. https://doi.org/10.1111/jeu.12200
Nomaki, H., Bernhard, J.M., Ishida,A., Tsuchiya, M., Uematsu, K.,Tame, A., Kitahashi, T., Takahata, N., Sano, Y. and Toyofuku, T. (2016) Intracellular Isotope Localization in Ammonia sp. (Foraminifera) of Oxygen-Depleted Environments: Results of Nitrate and Sulfate Labeling Experiments. Front. Microbiol.7:163.doi:10.3389/fmicb.2016.00163
Wei, C., & Zhang, W. (2023). Nitrogen Contribution Rate of Anammox in Different Systems and Its Relationship with Environmental Factors. Water, 15(11), 2101. MDPI AG. Retrieved from http://dx.doi.org/10.3390/w15112101
Zeebe, R. E., & Sanyal, A. (2002). Comparison of two potential strategies of planktonic foraminifera for house building: Mg2+ or H+ removal? Geochimica et Cosmochimica Acta, 66(7), 1159-1169.
Zumft, W G (1997). “Cell biology and molecular basis of denitrification”. Microbiology and Molecular Biology Reviews. 61 (4): 533–616. doi:10.1128/mmbr.61.4.533-616.1997. PMC 232623. PMID 9409151.
Banner, F., Sheehan, R., & Williams, E. (1973). The organic skeletons of rotaline foraminifera; a review. The Journal of Foraminiferal Research, 3(1), 30-42.
Bentov, S., Brownlee, C., & Erez, J. (2009). The role of seawater endocytosis in the biomineralization process in calcareous foraminifera. Proceedings of the National Academy of Sciences, 106(51), 21500-21504.
Bright, G. R., Fisher, G. W., Rogowska, J., & Taylor, D. L. (1989). Chapter 6 Fluorescence Ratio Imaging Microscopy. In D. L. Taylor & Y.-L. Wang (Eds.), Methods in Cell Biology (Vol. 30, pp. 157-192). Academic Press. https://doi.org/https://doi.org/10.1016/S0091-679X(08)60979-6
Calcium (Ca) and water. Lenntech B.V. Retrieved 11/07 from https://www.lenntech.com/periodic/water/calcium/calcium-and-water.htm
Chandler, D. L. (2015). Mystery solved: Why seashells’ mineral forms differently in seawater. MIT News. Retrieved 11/07 from https://news.mit.edu/2015/why-seashell-mineral-forms-differently-in-seawater-0302
de Nooijer, L. J., Langer, G., Nehrke, G., & Bijma, J. (2009). Physiological controls on seawater uptake and calcification in the benthic foraminifer Ammonia tepida. Biogeosciences, 6(11), 2669-2675.
de Nooijer, L. J., Spero, H. J., Erez, J., Bijma, J., & Reichart, G. J. (2014). Biomineralization in perforate foraminifera. Earth-Science Reviews, 135, 48-58. https://doi.org/https://doi.org/10.1016/j.earscirev.2014.03.013
de Nooijer, L. J., Toyofuku, T., & Kitazato, H. (2009). Foraminifera promote calcification by elevating their intracellular pH. Proceedings of the National Academy of Sciences, 106(36), 15374-15378. https://doi.org/doi:10.1073/pnas.0904306106
De Yoreo, J. J., & Vekilov, P. G. (2003). Principles of crystal nucleation and growth. Reviews in mineralogy and geochemistry, 54(1), 57-93.
Drake, S. K., Lee, K. L., & Falke, J. J. (1996). Tuning the equilibrium ion affinity and selectivity of the EF-hand calcium binding motif: substitutions at the gateway position. Biochemistry, 35(21), 6697-6705.
Erez, J. (2003). The source of ions for biomineralization in foraminifera and their implications for paleoceanographic proxies. Reviews in mineralogy and geochemistry, 54(1), 115-149.
Erez, J., & Luz, B. (1983). Experimental paleotemperature equation for planktonic foraminifera. Geochimica et Cosmochimica Acta, 47(6), 1025-1031. https://doi.org/https://doi.org/10.1016/0016-7037(83)90232-6
Goldstein, S. T. (1999). Foraminifera: A Biological Overview. In Modern Foraminifera. Springer Netherlands. https://books.google.ca/books?id=K-3tUmXW-IgC
Hansen, H. J. (1999). Shell Construction in Modern Calcareous Foraminifera. In Modern Foraminifera. Springer Netherlands. https://books.google.ca/books?id=K-3tUmXW-IgC
Hottinger, L., & Dreher, D. (1974). Differentiation of protoplasm in Nummulitidae (foraminifera) from Elat, Red Sea. Marine Biology, 25, 41-61.
Kitano, Y., Okumura, M., & Idogaki, M. (1975). Incorporation of sodium, chloride and sulfate with calcium carbonate. Geochemical Journal, 9(2), 75-84.
McConnaughey, T., & Whelan, J. (1997). Calcification generates protons for nutrient and bicarbonate uptake. Earth-Science Reviews, 42(1-2), 95-117.
Raymond, P. A., & Hamilton, S. K. (2018). Anthropogenic influences on riverine fluxes of dissolved inorganic carbon to the oceans. Limnology and Oceanography Letters, 3(3), 143-155. https://doi.org/https://doi.org/10.1002/lol2.10069
Reiss, Z. (1957). The Bilamellidea, nov. superfam., and remarks on Cretaceous globorotaliids. Contributions from the Cushman Foundation for Foraminiferal Research, 8, 127-145.
Robbins, L., & Brew, K. (1990). Proteins from the organic matrix of core-top and fossil planktonic foraminifera. Geochimica et Cosmochimica Acta, 54(8), 2285-2292.
Spero, H. J., Bijma, J., Lea, D. W., & Bemis, B. E. (1997). Effect of seawater carbonate concentration on foraminiferal carbon and oxygen isotopes. Nature, 390(6659), 497-500.
ten Kuile, B., & Erez, J. (1987). Uptake of inorganic carbon and internal carbon cycling in symbiont-bearing benthonic foraminifera. Marine Biology, 94, 499-509.
Ter Kuile, B. H., & Erez, J. (1991). Carbon Budgets for Two Species of Benthonic Symbiont-Bearing Foraminifera. Biol Bull, 180(3), 489-495. https://doi.org/10.2307/1542350
Towe, K. M., & Cifelli, R. (1967). Wall ultrastructure in the calcareous foraminifera: crystallographic aspects and a model for calcification. Journal of Paleontology, 742-762.
Toyofuku, T., Jan de Nooijer, L., Yamamoto, H., & Kitazato, H. (2008). Real‐time visualization of calcium ion activity in shallow benthic foraminiferal cells using the fluorescent indicator Fluo‐3 AM. Geochemistry, Geophysics, Geosystems, 9(5).
Thamdrup, B., Dalsgaard, T. (2002) Production of N(2) through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments. Appl Environ Microbiol 68, 1312–1318
Waldeck, A. R., Dam, K., Berden, J., & Kuchel, P. (1998). A non equilibrium thermodynamics model of reconstituted Ca2+-ATPase. European biophysics journal : EBJ, 27, 255-262. https://doi.org/10.1007/s002490050132
Zeebe, R. E., & Sanyal, A. (2002). Comparison of two potential strategies of planktonic foraminifera for house building: Mg2+ or H+ removal? Geochimica et Cosmochimica Acta, 66(7), 1159-1169.