Abstract
While coral polyps have been extensively studied across various scientific perspectives, this paper will specifically explore them from a chemical standpoint. The coral polyp’s biomineralization process is investigated, highlighting its critical role in creating a suitable habitat. The chemical reaction between bicarbonate and calcium produces aragonite crystals which form the polyp’s exoskeleton. This intricate process connects closely with the chemical mechanisms of polyp’s symbiotic algae, the zooxanthellae. Pulsations of coral polyps can enhance this mutualistic and photosynthetic relationship between coral polyps and zooxanthellae. Additionally, the long-term maintenance of this symbiotic relationship is very important as environmental stress acts on corals. This environmental stress causes photoinhibition, as well as an increase in reactive oxygen species, which are two factors involved in coral bleaching.
Introduction
Coral polyps are intricate tentacle-bearing organisms that belong to the phylum Cnidaria and are the building blocks of coral colonies. Through a process known as biomineralization, coral polyps form the solid structure of coral reefs. Coral reefs are some of nature’s most fascinating structures. They are essential for marine biodiversity and play a vital role in the intricate web of life beneath the waves. Although they make up less than 1 % of the ocean’s floor, they are home to a quarter of all marine life (National Oceanic and Atmospheric Administration, 2019). Through satellite imagery, coral reefs are easily visible from space (Fig. 1) due to the iridescent blues of shallow lagoons that contrast sharply with the dark blues of deep water (NASA, 2016).

Fig. 1 An astronaut aboard the International Space Station used a powerful lens to photograph these three reefs in Australia’s Great Barrier Reef on Oct. 12, 2015. (NASA, 2016)
Furthermore, coral reefs get their bright colors from the symbiotic relationship between coral polyps and colorful photosynthetic algae called zooxanthellae. These algae live within the tissues of the coral polyps and provide them with essential nutrients, including oxygen and sugars produced through photosynthesis. In return, polyps provide zooxanthellae with protection and nutrients. The vibrant hues seen in coral reefs are a result of the pigments in the zooxanthellae, which can range from greens and browns to various shades of blue, purple, and even fluorescent colors. The health and color of a coral reef depend on the well-being of this symbiotic relationship. Stressors like increased water temperatures can lead to coral bleaching, which causes corals to lose their colorful appearance and die (National Oceanic and Atmospheric Administration, 2019). This paper will go over and explain the key aspects relating to biomineralization, symbiosis, coral bleaching, and coral polyp pulsations that improve photosynthesis.
Coral Polyp Biomineralization
Overview
Coral polyps, living at the ocean’s depths, face an array of physical hazards, including waves, currents, and other hydraulic forces (Chamberlain, 1978). These forces pose a risk to their delicate soft tissues. Thus, coral polyps need a suitable habitat that offers protection and stability, allowing them to thrive despite challenges. Through a process called biomineralization, coral polyps can create an exoskeleton, made primarily out of calcium carbonate. Over time, as individual polyps within the colony continue to grow and reproduce, they collectively contribute to the growth of the colony’s exoskeleton. This layer-by-layer growth process forms the sturdy and protective skeletal structure of the coral colony, which serves as the foundation for coral reefs (Drake et al., 2019). Coral polyps benefit from the reefs in a variety of ways. For starters, coral reefs offer protection to the polyps and serve as stable substrates for them to attach and grow upon. Furthermore, coral reefs foster diverse ecosystems, providing essential food sources and resources crucial for the survival of the polyps (National Oceanic and Atmospheric Administration, 2019).
The exoskeleton is formed by a process called biomineralization. Living organisms can precipitate minerals within their tissues. In the case of coral polyps, biomineralization is responsible for creating the calcium carbonate crystals that compose their exoskeletons. Specifically, the calcium carbonate crystals (CaCO₃) are a product of a chemical reaction involving calcium ions (Ca²⁺) and bicarbonate ions (HCO₃⁻) as shown by the following equations (Tambutté et al., 2011):
HCO₃⁻ ⇌ CO₃²⁻ + H⁺ (1)
Ca²⁺ + CO₃²⁻ ⇌ CaCO₃ (2)
Net equation : Ca²⁺ + HCO₃⁻ ⇌ CaCO₃ + H⁺ (3)
While seawater contains an excess of calcium (Ca²⁺) and bicarbonate ions (HCO₃⁻), calcium carbonate crystals (CaCO3) are more likely to naturally precipitate in the coral tissue (Tambutté et al., 2011). Calcium carbonate exists in different polymorphs such as calcite, aragonite, vaterite, amorphous calcium and carbonate. Calcite belongs to the hexagonal crystal system. Its unit cell (smallest repeating structure in a crystal lattice) is rhombohedral, meaning it has three pairs of equal sides with angles of 60 and 120 degrees (Al Omari et al., 2016). Aragonite also belongs to the hexagonal crystal system. However, its unit cell is orthorhombic, meaning it has three pairs of unequal sides with angles of 90 degrees (Jellison et al., 2019). The arrangement of calcium and carbonate ions in aragonite differs from that in calcite, leading to distinct crystal structures.
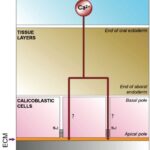
The aragonite crystals form at the interface between coral tissue and the substrate. The exact cellular and molecular processes involved in coral biomineralization are complex and the subject of ongoing research, but certain aspects are understood (Tambutté et al., 2011). Before explaining this process, it is crucial to understand the polyp’s composition (Fig. 2).
Calicodermis: Calicodermis is a delicate and intricate layer enveloping the polyp. Within the calicodermis, numerous specialized cells called calicoblasts perform essential functions. They primarily secrete an organic matrix that contributes to the formation of aragonite crystals. Calicoblasts also actively facilitate the transport of calcium and carbonate ions to the mineralization site within the polyp’s tissues (Drake et al., 2019; CDHC, n.d.).
Extracellular Calcifying Medium (ECM): The extracellular calcifying medium is a semi-enclosed extracellular compartment of calicoblasts. It is located between the skeleton and the calicodermis. The ECM offers an ideal environment for the formation of aragonite crystals (Drake et al., 2019).
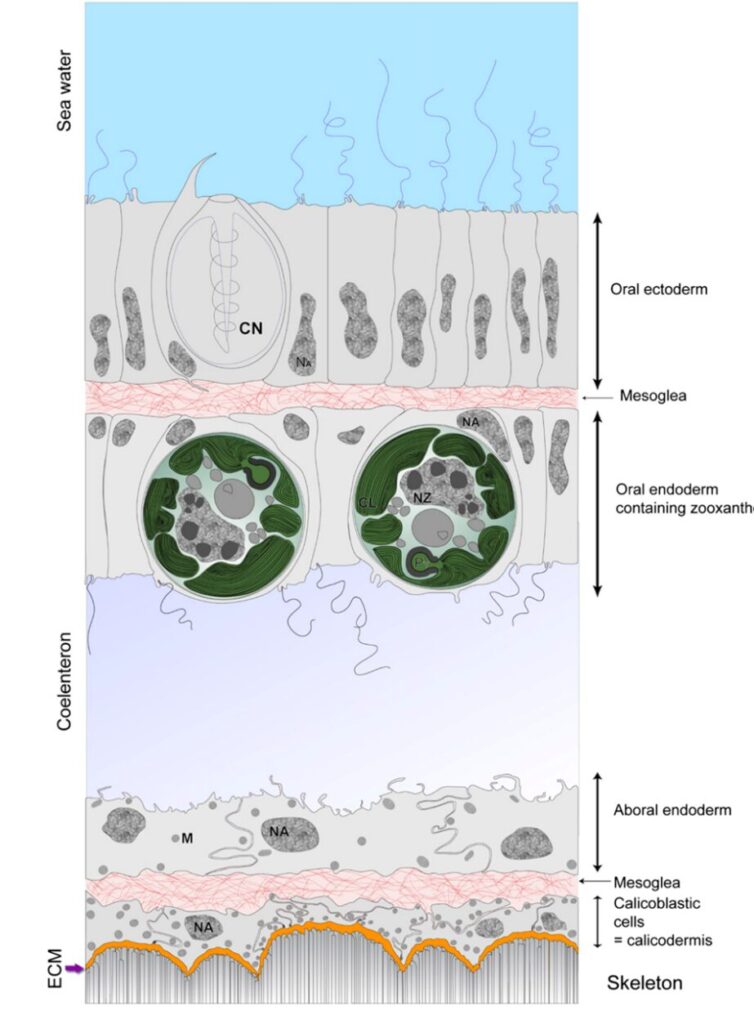
Fig. 2 Schematic representation of the histology of the coenosarc (drawn from a transmission electron microscopy image of the coral S. pistillata). (Tambutté et al., 2011)
Ion Uptake
Coral polyps take in dissolved calcium (Ca²⁺), carbonate (CO₃²⁻) and bicarbonate (HCO₃⁻) ions from the surrounding seawater (Fig. 3). Ions are moved across the epithelial layers (thin tissues that make up the outer body) through two main pathways: the paracellular pathway, which relies on diffusion or the flow of seawater, and the transcellular pathway, which involves active or facilitated transport. These pathways can operate independently or in combination (Tambutté et al., 2011).
The paracellular pathway entails ion movement between cells through intercellular spaces or junctions. The permeability of intercellular junctions to ions and molecules determines the percentage that a paracellular route can represent (Tambutté et al., 2011).
The transcellular pathway involves the active or facilitated transport of ions through individual cells. Specifically, calicoblastic cells can help with the transport of these ions. Calcium channels, within their cell membrane, facilitate the entry of calcium ions by diffusion into the cells. Calicoblastic cells contain a plasma membrane calcium ATPase (PMCA), which is a calcium pump also located in the cell membrane. Its role is to actively transport calcium ions (Ca²⁺) out of the calicoblastic cytosol and into the ECM (Tambutté et al., 2011).
Calicoblastic cells may also utilize bicarbonate transporters, such as those from the solute carrier 4 gamma (SLC4γ) family. These transporters introduce bicarbonate ions (HCO₃⁻) from the cytosol of the calicoblastic cells into the extracellular calcifying medium (Drake et al., 2019).
Carbon dioxide (CO₂) can enter the calicoblastic cells via diffusion. Once in the cell, it can undergo hydration which is a chemical reaction catalyzed by the enzyme carbonic anhydrase. This reaction converts CO₂ into bicarbonate ions (HCO₃⁻), making bicarbonate available for use in calcification (Drake et al., 2019).
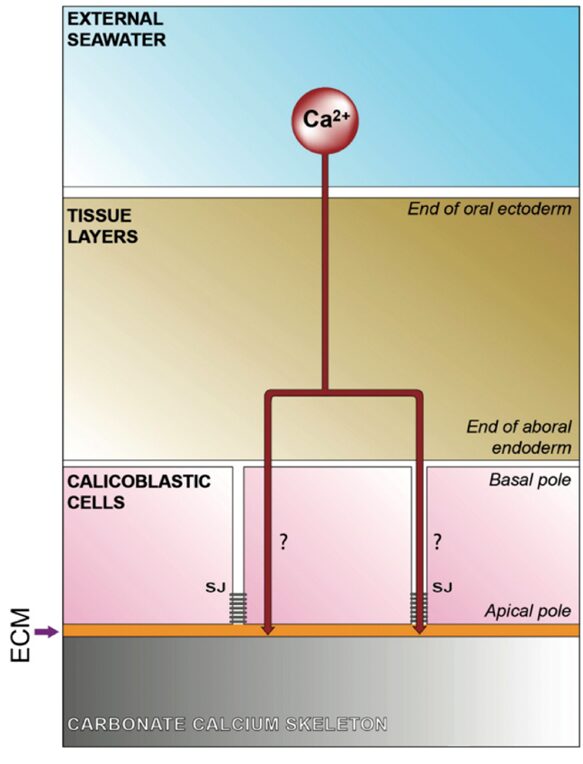
Fig. 3 Schematic representation of the transfer of calcium at the level of the calicoblastic cell layer (calicodermis). The proportion of calcium transferred via the paracellular vs transcellular pathway remains to be determined. (Tambutté et al., 2011)
Formation of Aragonite crystals
Once the necessary ions are present and conditions are favorable, aragonite crystals begin to form in the extracellular calcifying medium (ECM). Coral biomineralization is influenced by the aragonite saturation state, denoted as Ωarag. It is a measure of how favorable the conditions are for the formation of aragonite. This parameter links the concentrations of dissolved calcium ions (Ca²⁺) and carbonate ions (CO₃²) to their equilibrium as follows (Tambutté et al., 2011):
Ωarag = [Ca²⁺] × [CO₃²⁻] / Ksp. (4)
Ksp is the solubility of aragonite. When the aragonite saturation state is greater than 1, it indicates that the conditions are favorable for precipitation and calcium carbonate can form. In general, aragonite saturation values above 3 are considered optimal. Values of less than 1 indicate that dissolution is favored (Anderson et al., 2022). Dissolution is the process by which calcium carbonate minerals break down and dissolve in the surrounding environment.
As calcification proceeds, a buildup of H⁺ forms in the ECM because hydrogen ions are a product, as seen in Equation (3). Consequently, the pH of the ECM falls. Since hydrogen ions exhibit a stronger affinity for carbonate ions (CO₃²⁻) than calcium ions (Ca²⁺), hydrogen ions bond with carbonate ions (CO₃²⁻) and form bicarbonate ions (HCO₃⁻) (Bennett, 2018). Subsequently, there is a lower concentration of carbonate ions (CO₃²⁻) in the ECM. Since the concentration of carbonate ions (CO₃²⁻) falls, the aragonite saturation state decreases (Equation 2).
The pH of the extracellular calcifying medium can be manipulated to maintain a high aragonite saturation state. Raising the pH of the ECM can be achieved by the plasma membrane calcium ATPase (PMCA). The PMCA exchanges hydrogen ions (H⁺) and calcium ions (Ca²⁺) across the calicoblastic membrane (Drake et al., 2019). The expulsion of H⁺ raises the pH of the ECM. Thus, the increase in pH ensures a higher concentration of carbonate ions (CO₃²⁻). In turn, the aragonite saturation state increases, and the calcification process continues.
Control of Crystal Polymorphism
Calcium carbonate possesses several polymorphs. It can crystallize into different structures, with aragonite and calcite being the most common in coral skeletons. The organic matrix within the coral skeleton plays a crucial role in controlling this choice. The organic matrix is a complex mixture of organic compounds, including proteins, polysaccharides, lipids, and other biomolecules. It promotes the formation of aragonite over calcite, even in conditions that would otherwise favor calcite.
Aragonite is 16% more soluble than calcite. Aragonite’s higher solubility may provide an advantage for corals (Curl, 1962). While increased solubility might seem counterintuitive, it may allow corals to control the growth and maintenance of their skeletons more effectively in response to changing environmental conditions. Aragonite has a refractive index of 1.53, whereas calcite’s refractive index is 1.49 (MineralRocks, n.d.). The higher refractive index might also benefit the coral polyp by potentially increasing light scattering. However, it is important to note that this hypothesis requires further testing for validation. Furthermore, aragonite also has a higher density and hardness than calcite. Aragonite has a score of 3.5-4 on Mohs scale (MineralRocks, n.d.) which measures the hardness of minerals. On the other hand, calcite has a hardness of 3 on Mohs scale (MineralRocks, n.d.). Aragonite also has a density of 2.93 g/cm³ (WebMineral, n.d.-a), while calcite has a density of 2.71 g/cm³ (WebMineral, n.d.-b). The higher density and hardness of aragonite may contribute to the structural strength and resilience of coral skeletons. This can be important for withstanding physical stress and maintaining the integrity of the coral reef structure.
The mechanism by which the organic matrix enhances the production of aragonite crystal is not completely understood yet. However, it is believed that certain proteins which make up the organic matrix have properties that encourage the nucleation of aragonite crystals over calcite. (Yuyama & Higuchi, 2019)
Effects of Ocean Acidification on Coral Calcification
The Earth’s oceans actively exchange carbon dioxide (CO₂) with the atmosphere. The amount of CO₂ dissolved in water depends on its partial pressure and chemical reactions with other solutes. Partial pressure of carbon dioxide (pCO₂) refers to the equilibrium gas phase pressure (found in the air above a waterway) corresponding to the dissolved carbon dioxide. Over the past few decades, human activities, primarily the burning of fossil fuels, have significantly raised atmospheric pCO₂ levels (Australian Online Coastal Information, 2015). This increase in atmospheric pCO₂ has led to a rise in seawater CO₂ concentration and a decrease in pH, a phenomenon known as ocean acidification. In just the last two centuries, ocean water has experienced a 30% increase in acidity, marking a rapid transformation in ocean chemistry, unmatched in the past 50 million years (Bennett, 2018). Ocean acidification affects coral calcification by making it more challenging for corals to build and maintain their calcium carbonate skeletons. With lower pH levels, there are fewer carbonate ions (CO₃²⁻) available for corals to use in the calcification process. In consequence, the aragonite saturation stature is also lower and thus, the calcification process becomes slower. This reduced availability of carbonate ions makes it more challenging for corals to precipitate calcium carbonate and build their skeletons. Additionally, in more acidic conditions, existing calcium carbonate structures, such as coral skeletons, become more vulnerable to dissolution (Fig. 4). This can lead to the erosion and weakening of the reef structure. Over time, prolonged exposure to ocean acidification can have significant consequences for coral reef health and the entire ecosystem they support. It is projected that by around 2080, the increasing acidity of ocean conditions will lead to a situation where even healthy coral reefs will erode more rapidly than they can naturally recover (Bennett, 2018).
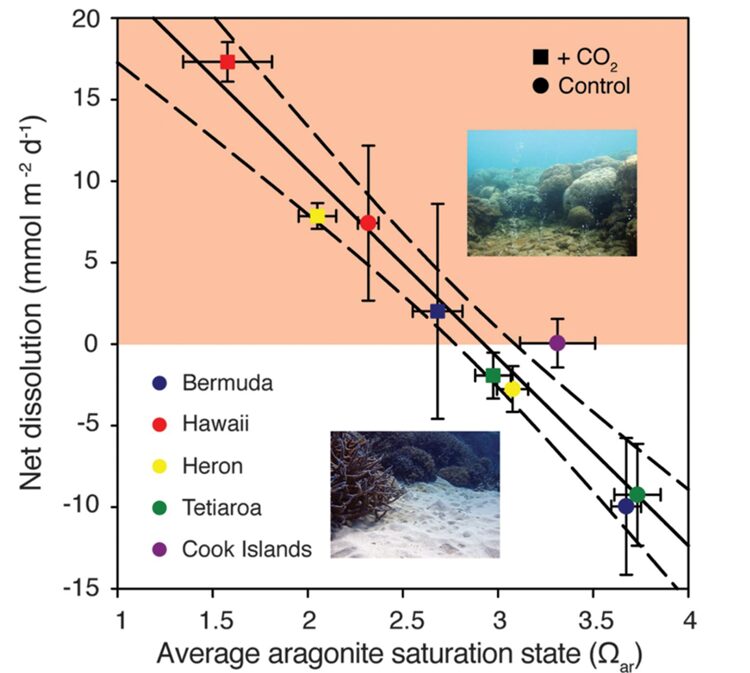
Fig. 4 Average CaCO₃ permeable sediment dissolution rates for each set of control (circles) and high pCO₂ (squares) treatments for each of the five reefs as a function of seawater average aragonite saturation state (Ωarag). There is a strong inverse relationship between the aragonite saturation state (Ωarag) of seawater and the average dissolution rates of calcium carbonate permeable sediment. As Ωarag decreases, the dissolution rates tend to increase, as evidenced by the negative slope in the regression equation. As previously mentioned, a decrease in pH results in a lower aragonite saturation state (Ωarag). Thus, a decrease in pH will lead to an increase in the dissolution rate. (Eyre et al., 2018)
Symbiotic relationship with the Zooxanthellae
Overview
Single celled, photosynthetic algae live on coral polyps and are key to the coral reef colonies’ success. These single celled algae are called zooxanthellae, and they engage in a mutually beneficial relationship with the polyps. The zooxanthellae are photosynthetic and thus provide the coral polyp with energy converted from light while the polyp supplies the zooxanthellae with chemicals essential for photosynthesis, as well as a safe habitat. While the zooxanthellae undergo photosynthesis, the polyp cells undergo cellular respiration. These two processes are the basis for this symbiotic relationship (Muller-Parker et al., 2015). In fact, coral polyps obtain about 10-40 % of their food by predation while the rest is supplied by the zooxanthellae (Titlyanov & Titlyanova, 2020).
Formation of the symbiosis
The formation of the symbiosis between the single celled algae and the polyp is not random and occurs in two processes. Vertical transmission, that is when the symbiodinium, which is a genus of dinoflagellates that is open to symbiosis, is acquired maternally, either in asexual reproduction where a part of the coral splits off with the existing zooxanthellae, or in sexual reproduction where the zooxanthellae is embedded in the coral larvae eggs and thus present since the beginning. However, the most prevalent method is horizontal transmission which occurs when the algae symbiont is acquired from the environment (Muller-Parker et al., 2015). This process occurs in the larval stage while the polyps are still motile and was once thought random. However, it has been shown that it is rather selective. In an experiment conducted by Yamashita and his coworkers (Yamashita et al., 2014), they showed that the symbiotic relationship formed was highly selective. They conducted an experiment where they exposed coral larvae to different concentrations of different types of zooxanthellae. Their results showed that the favored zooxanthellae were not related to their abundance but rather to their type.
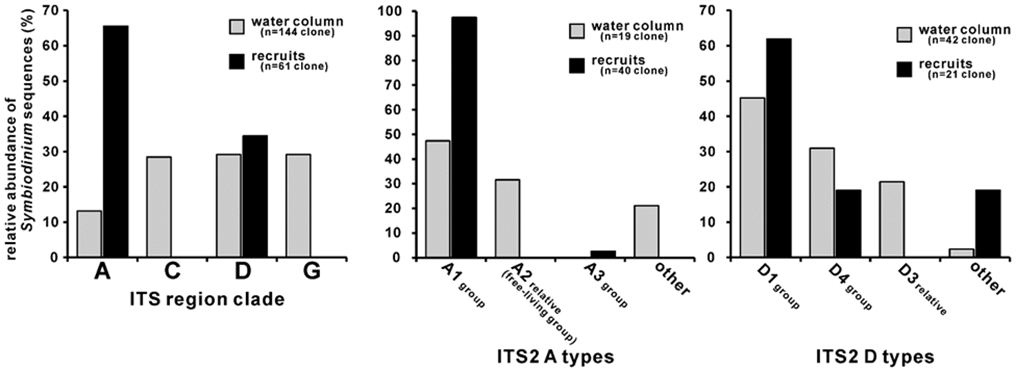
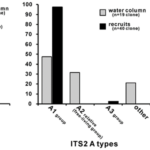
Fig. 5 Symbiodinium compositions of water column and naturally settled Acropora coral recruits. The water samples were collected during the coral mass spawning period in 2011 (gray bars), and the recruits were collected approximately 2 weeks after spawning (black bars). Left histograms show that the clade composition of the recruits did not reflect that of the water column (p<0.05). Clades A and D were detected in both the water and recruit samples, and these Symbiodinium clones were further sorted into ITS2 types. ITS2 are internal transcribed spaces, a region of the nuclear ribosomal DNA used as a DNA barcode. These sequences were used because of their conserved regio for designing universal primers. Type A1 was the dominant clade A type within the recruits (middle histogram). Right histogram showing clade D ITS2 type compositions; type D1 and D4 group sequences were predominant in both water and recruit samples. Type D3 relative sequences were only detected from water samples. In clade A and D, Symbiodinium type compositions were different between recruits and environments (p<0.05). (Yamashita et al., 2014)
Furthermore, the disaccharide Trehalose was found to play a part as a chemical messenger in the establishment of symbiosis. The establishment of symbiosis involved two steps: attraction and uptake. In the attraction step it was discovered that the symbiodinium secreted trehalose. However, to check if this played any role, Hagedorn and his coworkers placed F.scutaria larvae without symbionts in a Y maze with trehalose and glycerol at the ends (Hagedorn et al., 2015),. They found that the larvae were attracted by chemotaxis towards the end of the maze containing trehalose and glycerol. Therefore, the trehalose secreted attracts the larvae accentuating the selectivity of the attraction process.
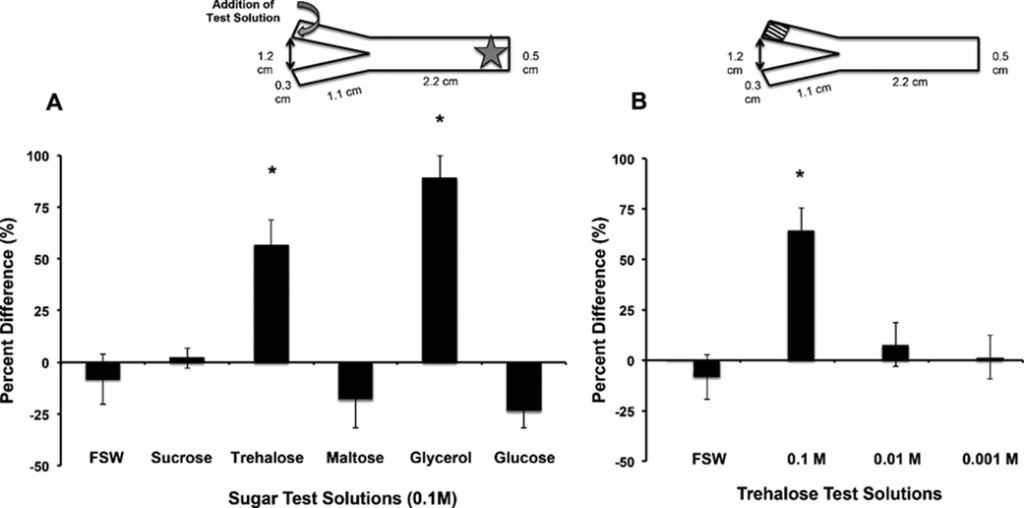
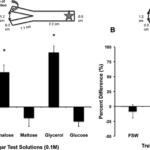
Fig. 6 Trehalose and glycerol are chemo-attractants for F. scutaria larvae. F. scutaria larvae were placed in a Y-maze, and their behavior monitored. The test solutions were introduced to the maze at the ends of each arm, indicated by the curved gray arrow, while the larvae were added at the base, as indicated by the star (inset A). A) Only glycerol and trehalose solutions demonstrated clear chemo-orienting behavior different from the controls (*P < 0.05, ANOVA, Tukey’s Multiple Comparison test) while the other solutions remained neutral (P > 0.05, ANOVA, Tukey’s Multiple Comparison test). B) Only the 0.1 M trehalose demonstrated clear chemo-orienting behavior different from the 0.01 and 0.001M trehalose solutions and controls (*P < 0.05, ANOVA, Tukey’s Multiple Comparison test). (Hagedorn et al., 2015)
Photosynthesis (Satpati & Pal, 2020)
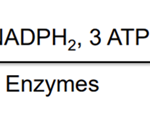
Photosynthesis is the process of carbon fixation from inorganic (CO2) to organic (sugars), producing carbohydrates with the help of sunlight, water, and chlorophyll. It is divided into two stages: the light dependent stage and the light independent stage. The light dependent stage depends on the electron transport chain which occurs in two distinct light harvesting complexes Photosystem I &II (PSI and PSII). Upon illumination, the incident light, made up of photons, excites the cells of PSII which break down water molecules O2, H+ and electrons. The electrons reduce NADP+ into NADPH2 while the protons (H+) are transported across the thylakoid membrane. This creates a pH gradient which drives the formation of ATP. The PSII then transfers the electrons to the PSI reaction center, which is excited with the aid of incident photons. The electron is then used to reduce NADP+ to NADP.
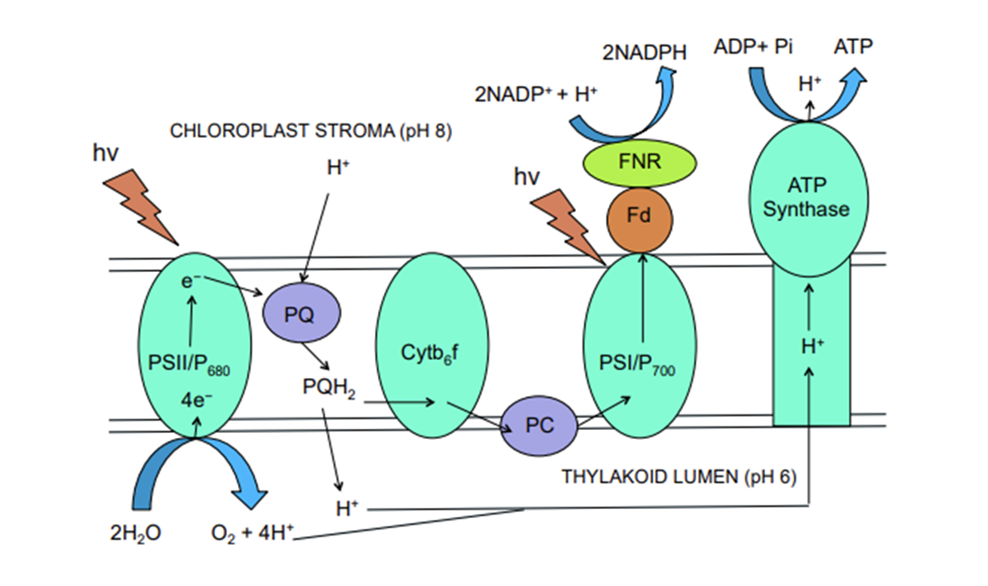
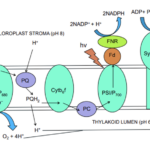
Fig. 7 Diagram illustrating the electron transport chain in the light dependent part of photosynthesis. Light is absorbed by the PSII which breaks down the water into oxygen and H+. The proton is then transported through the thylakoid lumen across the thylakoid membrane moving against its concentration gradient leading to the synthesis of ATP. In the meantime, the electron moves through the chloroplast stroma to the Plastoquinone (PQ) which transports the electron to cytochrome b6f complex, another electron carrier, which transports the electron to the PSI system. In the PSI system, the electron along with incident light reduce the NADP+ to NADP. (Satpati & Pal, 2020)
The light independent phase entails the fixation of organic carbon. This process comprises 4 main steps and is denoted as Calvin Cycle, a series of enzyme catalyzed reactions leading to organic carbon formation. The first step is carboxylation where CO₂ binds to rubisco converting RuBP into 3 PGA. The second step is reduction which includes two complementary steps. The first turns phosphoglycerate into diphosphoglycerate, then into phosophoglyceraldehyde using NADPH2. Regeneration involves a series of chemical reactions combining 4C, 5C, 6C and 7C sugar phosphates. Transketolase and Aldolase are the two major enzymes that help in the regeneration of RuBP (5C sugar phosphate) from 6C and 3C sugars. Then the overall reaction becomes (5):
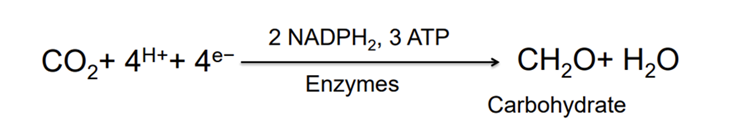
Then, the resulting chemical reaction for the 2 phases of photosynthesis is:
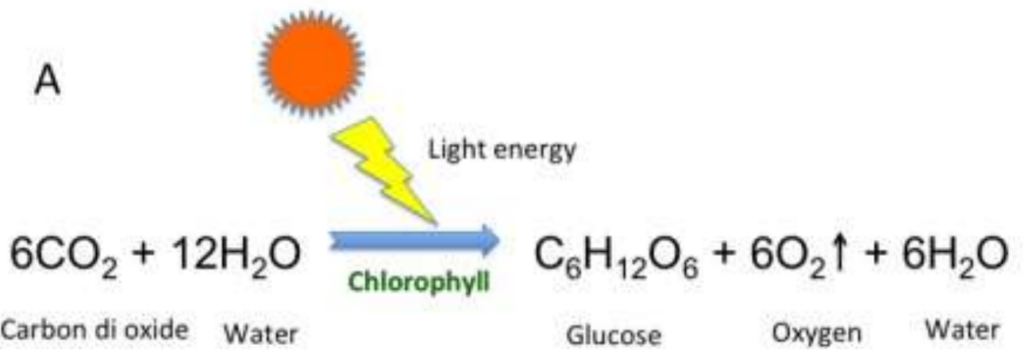
Fig. 8 The overall chemical reaction for the photosynthesis of the zooxanthellae algae. (Satpati & Pal, 2020)
Therefore, photosynthesis is a crucial mechanism that occurs in the zooxanthellae. This process utilizes carbon dioxide and water to create sugars oxygen and water. These are essential to the polyp, as they are required for cellular respiration.
Cellular respiration
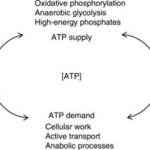
Coral polyp cells contain several genes coding for mitochondrial functions and thus synthesize their own ATP for energy (Levy et al., 2021). Cellular respiration is the process of consuming oxygen and nutrients to produce energy stored in the bonds of the ATP molecule. The whole point of cellular respiration is to provide the cell with enough ATP to fulfill its function (McClelland, 2011).
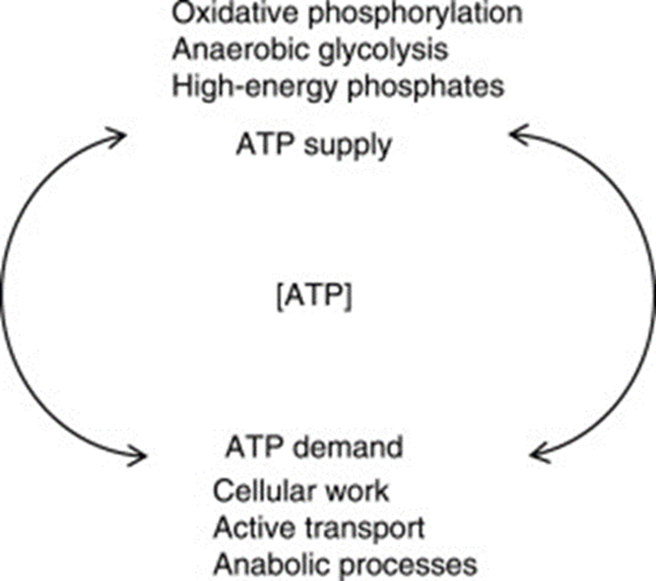
Fig. 9 ATP homeostasis is maintained through the balance between ATP-supply pathways and ATP-demand pathways. (McClelland, 2011)
Coral polyps undergo aerobic cellular respiration where organic compounds react with oxygen to produce carbon dioxide, and water and energy in the form of ATP. Here is the chemical reaction for this process when the organic compound is glucose:
C_6 H_{12} O_6 + 6O_2 → 6CO_2 + 6H_2 O+ATP \ \ \ \ \ \ (6) In cellular respiration, the polyp uses sugars and oxygen to produce energy for basic cell functions.
The exchange between the two
Within the coral symbiosis, zooxanthellae’s photosynthesis yields oxygen and sugars, which, in turn, provide essential resources for polyp cellular respiration, where oxygen and sugars are consumed to produce carbon dioxide and water. These byproducts are subsequently utilized by the zooxanthellae in a continuous cycle of interdependence.
To further understand the balance between photosynthesis and respiration, gross photosynthetic rate and respiration rate are calculated. The photosynthetic rate relies on the concentration of HCO3–, the flux density and the maximum photosynthetic rate.
P_g=P_{max} tanh(E/E_k ) [HCO_3^- ]_{coe}/(K_{HCO_3} +[HCO_3^- ]_{coe} ) \ \ \ \ \ \ \ \ \ (7)Where E is the photon flux density, Pmax is the maximum photosynthetic rate and Ek is a constant. [HCO3]coe is the concentration inside coelenteron (the cavity of the cnidaria) and KHCO3 is a half-saturation constant. [HCO3–]coe can be calculated from the total alkalinity and the dissolved organic carbon from equilibrium calculations (Nakamura et al., 2013).
The respiration rate depends on the level of dissolved oxygen and the maximum respiration rate.
R=R_{max} (CH_2 O)/(K_{CH_2 O} +CH_2 O)×(DO_{coe})/(K_{DO}+DO_{coe} ) \ \ \ \ \ \ \ \ (8)Where Rmax is the maximum respiration rate, and KCH2O and KDO are half-saturation constants for CH2O and DO, respectively (Nakamura et al., 2013).
Using Pg and R, the efficiency of this relationship can be tested, as analyzing the ratio of Pg to R reveals the efficiency of photosynthesis. Two cases can arise: a ratio greater than one and a ratio less than one. If the ratio is greater than one, then Pg > R. Thus, all energy requirements of the polyp are satisfied by photosynthesis. If the ratio is less than one, then Pg < R. Thus, the energy supplied by photosynthesis is insufficient to meet the polyp’s requirements for carrying out its cellular metabolic processes. This defines the polyps’ energy budget, where the excess or deficit can be calculated (Leletkin, 2000).
Exchange of nitrogen and carbon
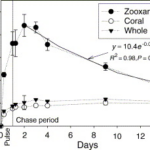
NO3− is a major inorganic nutrient found in seawater which is increased in the presence of river estuaries. The coral polyp lacks the NO3− and NO2− reductases that are used to synthesize organic N from these inorganic molecules. It must have another mechanism to uptake these ions. Research by Tanaka and coworkers (Tanaka et al., 2006) revealed that the zooxanthellae possessed NO3− and NO2− reductases, and that they featured the initial site to take up NO3− and synthesize it into organic N. In fact, the organic processed N is translocated to the host coral with the photosynthetic products, as the N content in the coral host contains no NO3−. As can be witnessed in the figure below, the quantity of organic N increases in the zooxanthellae and then decreases gradually accompanied by a gradual increase in the quantity of N in the coral.
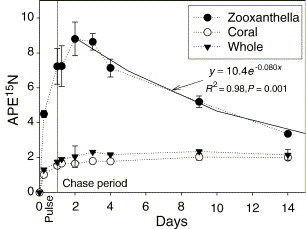
Fig. 10 Temporal changes in APE15N of the zooxanthella, the host coral, and the whole coral–algal tissue (average ± range of duplicates) during the pulse-chase experiment. The exponential curves are regression for the data between 2 and 14 days. During the pulse period, it was nighttime from 0.38 to 0.83 days. (Tanaka et al., 2006)
During algal photosynthesis, more than 70% of organic N synthesized from NO3− by zooxanthellae were translocated to their host coral as soon as they were produced (Tanaka et al., 2006).
The Problem: Increased Coral Bleaching
Overview
As explained in the previous section, corals and zooxanthellae have a mutualistic relationship and thus depend on each other to survive. Coral tissues provide the algae with a stable environment and the resources necessary for photosynthesis, while zooxanthellae supply the coral with the products from the photosynthetic process. Healthy corals are also bright and colorful because of the pigments produced by the algae. When this mutualistic relationship is put under stress by the environment, coral bleaching occurs. Bleaching is the major mechanism responsible for scleractinian (stony corals) mortality because they lose the zooxanthellae as part of their response to stress. Consequently, they also lose their color and appear white or very pale (Figure 12), while also giving up on their major source of food. This causes the corals to be vulnerable, more susceptible to disease, and unable to grow and reproduce at their usual rates. Prolonged bleaching can therefore lead to coral mortality. If the stressors are temporary and the conditions improve, the corals can recover by taking up new zooxanthellae (Lesser, 2010). Coral bleaching has recently become a bigger concern due to elevated seawater temperatures caused by climate change and is now a global issue affecting reefs around the world. At the current pace of climate change, Scleractinia corals may not be able to adapt physiologically since evolution occurs over long timescales.
Over many years, studies have revealed that the most common response to higher temperatures in corals is oxidative stress (Lesser, 2010). This stress can be further enhanced when corals are exposed to high irradiances of solar radiation, coupled with this increase in temperature. Oxidative stress refers to the production and accumulation of reactive oxygen species (ROS) such as superoxide radicals, singlet oxygen, hydrogen peroxide and hydroxyl radicals (Lesser, 2010). This stress occurs within an organism when the reactive oxygen species exceed the organism’s capacity to control their levels (Plass-Johnson et al., 2014). Excess levels of reactive oxygen species can cause damage to various cellular components including lipids, proteins, DNA, membranes, and organelles, which can lead to the activation of cell death processes. Depending on the severity and length of exposure to environmental stressors, damage in cell processes can significantly influence coral bleaching (Lesser, 2010).
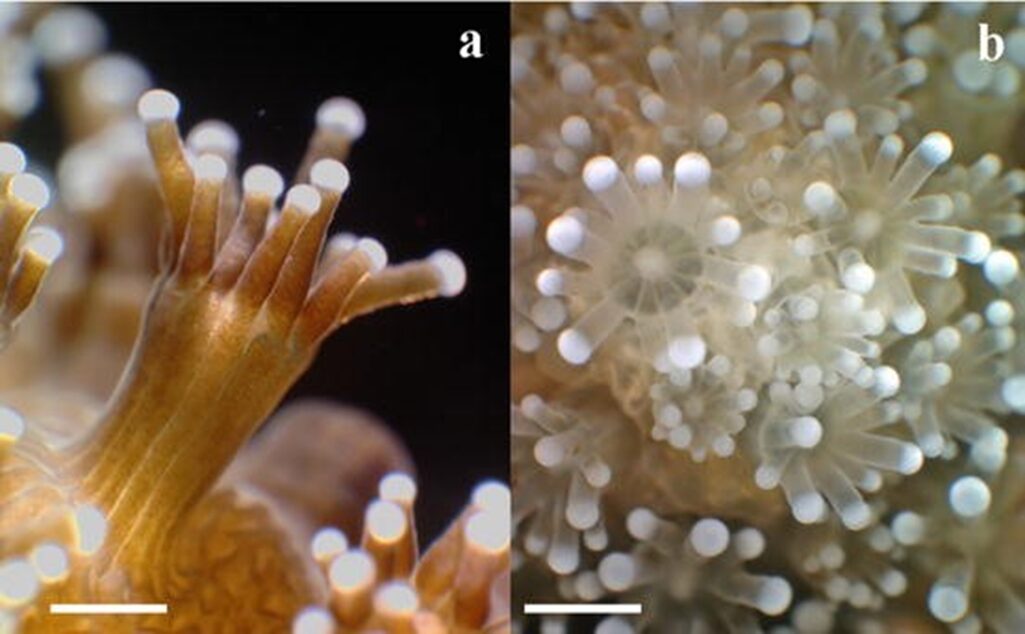
Fig. 11 Macro photographs of scleractinian coral Stylophora pistillata showing bleached and unbleached polyp tissue: (a) side view showing unbleached polyps with visible zooxanthellae population; (b) top view of bleached polyps with transparent tissue. (Plass-Johnson et al., 2014)
Mechanisms of Coral Bleaching
A study was carried out by Lesser (1996) to shed light on the mechanisms by which environmental stressors impact photosynthetic organisms, particularly zooxanthellae in coral symbiosis (Lesser, 2010). Many more recent experiments on zooxanthellae have been conducted but have only supported the results obtained in this study (Lesser, 2010). This experiment tests cultures of zooxanthellae isolated from the sea anemone Aiptasia pallide. The cultures of zooxanthellae are semicontinuous, which means that they are maintained under controlled conditions, with periodic changes being made to them. The conditions experienced by the zooxanthellae in this experiment attempt to simulate and replicate the nutrient and light regimes that they encounter within corals (Lesser, 2010). This is done to ensure that the experiment mimics the natural conditions in which these organisms live. The cultures are then exposed to elevated temperature stress and ultraviolet radiation (UVR) to simulate the increase in seawater temperatures and high irradiances of solar radiation occurring in nature. Throughout the experiment, various parameters related to the light and dark reactions in photosynthesis were monitored to assess the effects of these conditions. The production of reactive oxygen species and antioxidant enzymes are also monitored. The results indicate that when the zooxanthellae are exposed to an increased temperature (from 25°C to 31°C), photosynthesis, PSII function, growth rates, and rubisco are significantly affected. These components further decline when the cultures are exposed to UVR in addition to elevated temperatures. This shows that the two stressors have a detrimental effect on the photosynthetic process of zooxanthellae, a process that coral populations are dependent on. The observed negative impacts are always accompanied by an influx of superoxide radicals, hydrogen peroxide, and antioxidant enzymes. This suggests that ROS production is a crucial factor in the damage caused by environmental stress. The antioxidant defenses in biological systems are used to prevent or limit the cascade of ROS reactions (shown in Figure 13), and therefore reduce damage caused by oxidative stress. The goal of these systems is to eliminate singlet oxygens at their site of production, as well as eliminate or lower the amounts of reduced oxygen intermediates such as superoxide radicals and hydrogen peroxide. This is essential to prevent the formation of hydroxyl radicals, which is the most harmful type of reactive oxygen species. In the experiment, the zooxanthellae cultures are also exposed to exogenous antioxidants named ascorbate and catalase. Ascorbate acts as a nonenzymatic quencher of hydroxyl radicals, while catalase produces water and oxygen after decomposing hydrogen peroxide. After one-hour, photosynthetic performance improves by 24% in the cultures affected by temperature alone, and 37% in the cultures affected by both temperature and UVR. This demonstrates that antioxidants can minimize the effects of ROS and enhance photosynthesis under stress conditions (Lesser, 2010).
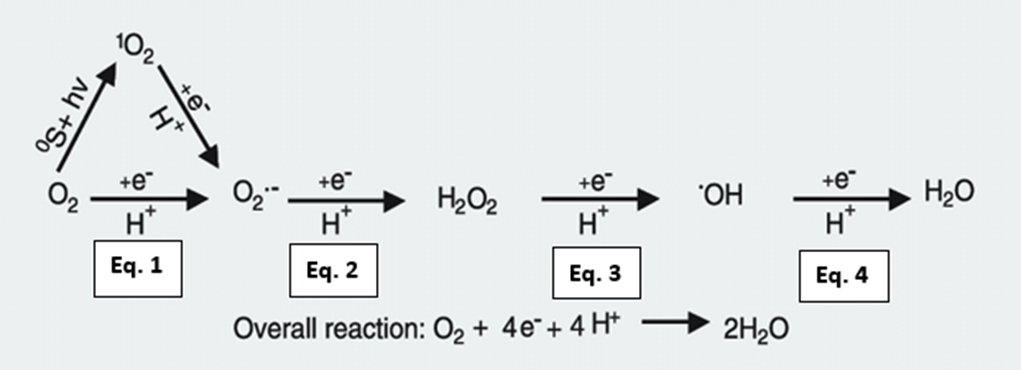
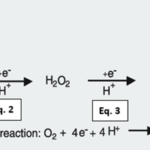
Fig. 12 All cells engaged in photosynthesis and respiration, including zooxanthellae within coral tissues, produce ROS. They are created as natural byproducts of these metabolic processes. The primary ROS generated in these cellular processes are superoxide radicals (O2•-) through the univalent pathway (Eq. 1). Superoxide radicals can further react and be converted into hydrogen peroxide (H2O2) because of the continued reduction of O2•- (Eq. 2). Hydrogen peroxide can give rise to hydroxyl radials, which is the most damaging ROS (HO•) (Eq. 3). Hydroxyl radicals can be further reduced to form the hydroxyl ion (OH–) and then water (H2O) because of exposure, and the utilization of oxygen molecules within the cellular environment (Eq. 4). (Scandalios, 2005)
Although coral has photoprotective mechanisms to minimize oxidative damage, the damage that is still occurring needs to be resolved. The corals invest energy to repair damaged components, while also synthesizing antioxidant enzymes to counteract the effects of the ROS. This energetic cost leads to a decrease in the efficiency of photosynthesis, while also resulting in further oxidative damage to components (Lesser, 2010). As the host cells experience exposure to, and damage from accumulated ROS, several mechanisms can lead to the uncoupling of the symbiotic relationship between zooxanthellae and coral polyps (Gates et al., 1992). Firstly, host cells can initiate a process called apoptosis, which is caused by ROS exposure or extensive damage to cellular components like DNA. Apoptosis is a regulated cellular process, where cells that have suffered irreparable damage go through a systematic form of self-deconstruction. If the level of damage is severe, the cell can undergo necrosis. Necrosis is a more chaotic form of cell death that lacks a highly regulated and controlled pathway seen in apoptosis. In addition, zooxanthellae can be released from the host coral cell into the gastrovascular cavity by exocytosis. Exocytosis involves no loss of the host cell and uses secretory vesicles to expel the algae out of the cell membrane (Yellowlees et al., n.d.). The coral cell containing the zooxanthellae can also detach from the endoderm entirely. This further contributes to the uncoupling of the mutualistic relationship since the cell disconnects the zooxanthellae from the host tissue (Plass-Johnson et al., 2014).
Did you know? Apoptosis is a highly controlled process involved in patterning and development. It is characterized by cell shrinkage which involves a decrease is cell volume as well as the disassembly and recycling of cellular components. This allows for the efficient and non-inflammatory removal of cells that are no longer needed or that are potentially harmful to the organism.
Necrosis is caused by the failure of regulatory mechanisms due to severe damage or energy depletion, which leads to cell swelling and bursting. Unlike the controlled process of apoptosis, necrosis is a non-specific, unregulated response to cellular stress and injury (Fink & Cookson, 2005).
These processes can occur when the presence of zooxanthella is more detrimental than beneficial for the host. As explained previously, when the coral endures environmental stressors for extended periods of time, photosynthesis is inhibited, and the production of harmful reactive oxygen species increases. This damage can be energy intensive to repair and can threaten the coral’s survival. To cope with the stress and reduce the energy burden, corals eject their zooxanthellae. While this expulsion can result in the loss of the colorful pigments in the zooxanthellae, and the coral’s main source of food, it conserves energy because the corals no longer need to invest in maintaining the symbiotic relationship or repairing any damage caused. This is only a short-term solution because in the long-term, the absence of the mutualistic relationship is more detrimental to the coral (Lesser, 2010).
Pulsations and Photosynthetic Efficiency
Overview
Corals have a significant and strong reliance on photosynthesis to grow and thrive in low-nutrient tropical environments. They are reef-building organisms and therefore, this reliance on sunlight poses many potential problems for coral colonies composed of hundreds to thousands of corals at one time. Firstly, corals are exposed to many different wavelengths and intensities of light depending on the time of day and year and are subject to lower light intensity levels than land plants as they live underwater. In addition, water pollution poses a risk to the growth of coral colonies as it inhibits the light from reaching the corals in the water. Corals therefore must find ways to counteract these problems (Coral Basics | Flower Garden Banks National Marine Sanctuary, n.d.). The polyps of certain coral families can pulsate, which has a positive effect on carbon acquisition which in turn enhances the photosynthetic efficiency of coral’s symbiotic algae. The positive effect of these pulsations on the photosynthetic chemical reaction of coral’s symbiotic algae is explained through the analysis of a study of four different Heteroxenia fuscescens (Xeniidae family soft corals) colonies at 5 to 10-meter depth (Kremien et al., 2013). To begin, from the information of these 4 colonies, it is observed that the polyps pulsate more than 95% of the time and that there is short (15-20 minutes) intervals without pulsation, which can be considered rest. It is also recorded that resting intervals occurr when the intensity of solar radiation is less than 50 % of the daily maximum, which highlights the connection between these pulsations and photosynthesis. The choice of rest periods during low light intervals (which are intervals of low photosynthetic efficiency) shows that there is an obvious connection between these pulsations and the efficiency of the photosynthetic chemical reactions and cycles (Kremien et al., 2013).
Regarding the mechanism of pulsations, coral polyp tentacles exhibit a rhythmic alteration in orientation, transitioning from a fully extended configuration in the open state to a tightly compacted configuration in the closed state. from the following section will delve into this mechanism and focus on how exactly it enhances photosynthesis.
The chemical effects of pulsations on photosynthesis
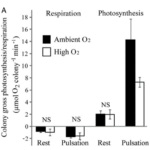
The pulsations have a very strong effect on the respiratory and photosynthetic efficiency of the symbiotic zooxanthellae present in corals’ tissues. These effects are measured through a comparison of respiration and photosynthesis rates between different pulsating and resting Heteroxenia fuscescens colonies. Based on measured changes in oxygen concentration, it is shown that when submerged in ambient levels of oxygen (∼200 μM) the colony’s gross respiration rate during coral polyp pulsation is approximately two times higher than during rest periods and that the gross photosynthesis rate of a colony is over seven times higher during pulsations than when the polyps are at rest (Fig. 14 & Fig. 15) (Kremien et al., 2013).
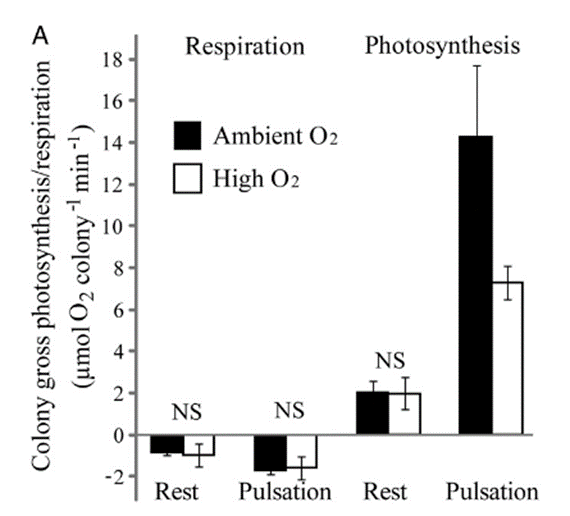
Fig. 13 Average rates of gross photosynthesis and dark respiration in pulsating and resting states measured under both ambient (represented by filled bars) and high (represented by open bars) oxygen concentrations. (Kremien et al., 2013)
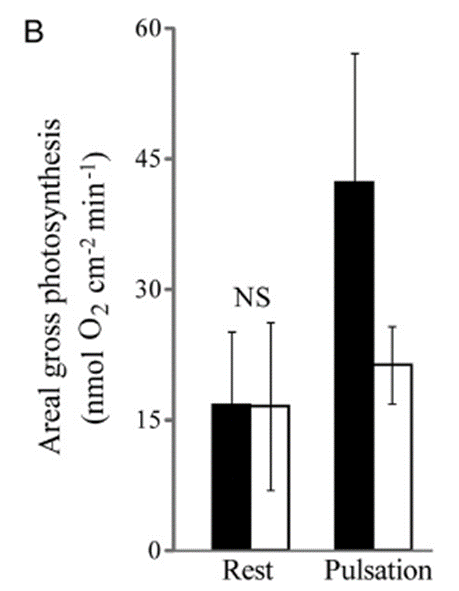
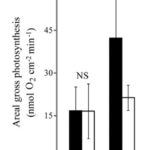
Fig. 14 Average rates of gross photosynthesis, adjusted for the surface area of the colony that exposed to downward light. (Kremien et al., 2013)
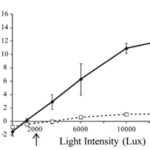
A very similar pattern is found when analyzing the change of net photosynthesis in response to varying levels of light intensity in the form of a Photosynthesis-Irradiance curve (P-I curve) (Fig 16). The findings indicate that in the pulsating corals, the P-I curve exhibits a significantly steeper slope, resulting in approximately ten times higher maximum photosynthesis compared to the resting corals. Notably, this enhancement of photosynthesis is evident even under low light conditions, such as 3500 Lux, which is equivalent to the light intensity experienced at a depth of 10 meters approximately one hour after sunrise or before sunset (Kremien et al., 2013).
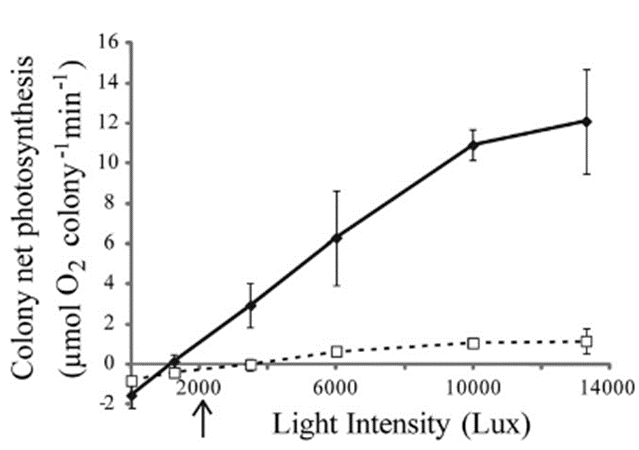
Fig. 15 P-I curves for H. fuscescens, with solid lines representing pulsating conditions and dotted lines resting conditions. Each data point represents the average net photosynthesis (based on data from three colonies) at various levels of irradiance. The arrow located below the horizontal axis points to the in-situ light intensity approximately one hour before darkness at a depth of around 10 meters. (Kremien et al., 2013)
As explained, there is an obvious beneficial connection between coral polyp pulsations and photosynthetic efficiency. First, as a result of coral polyp’s pulsations, there is an increased carbon acquisition experienced by the symbiotic zooxanthellae present in corals’ tissues. This occurs due to a pulsation-driven increase in oxygen efflux from the organism’s tissues. When water flows around the coral, it carries away the oxygen produced during photosynthesis. As a result, high oxygen levels do not build up within the tissues, which is crucial for the efficient functioning of the photosynthetic process. This constant efflux of oxygen associated with the pulsations allows for the maintenance of a low concentration of oxygen in, and around the coral, and therefore enhances the binding capability of CO2 by ribulose-1,5-bisphosphate carboxylase oxygenase (RuBisCO) enzyme in the carbon fixation stage of the Calvin cycle. On the other hand, when there is no flow (coral polyps at rest), there is a buildup of oxygen produced through photosynthesis within the tissue which diminishes the capacity of RuBisCO to bind with CO2, resulting in a reduction in the photosynthesis process and redirecting carbon toward the wasteful photorespiration pathway (Mass et al., 2010). Furthermore, the occurrence of rest periods during the late afternoon to early evening, when the light intensity consistently remains below 50% of the daily maximum, suggests that interruptions in pulsations happen when the reliance on oxygen removal from the tissues to regulate photosynthesis rate diminishes (Kremien et al., 2013). This pulsation induced oxygen efflux accounts for 56% of the total photosynthesis enhancement caused by pulsations. The remaining enhancement results from surface area change.
The pulsations of coral polyps also enhance photosynthesis through an increase in surface area exposed to light. This increase leads to an increased absorption of light as a result of a greater area available to absorb light. The surface area of a coral polyp that is exposed to light during pulsations is 2.69 times larger than the surface area exposed when the polyps are resting. Very similarly, photosynthesis by zooxanthellae is also 2.68 times higher during pulsating periods than when the polyp is at rest. Despite this, the enhancement of the chemical process of carbon acquisition by pulsations is proven by increasing the oxygen concentration around the corals to an abnormally high level (600–1,000 μM). As seen by the open bars in Figure 14 and 15, while increased oxygen concentration has no effect on photosynthesis during rest, it does have an impact in the presence of pulsation. In contrast, during pulsation, the mixing and removal of oxygen-rich water from the coral interface become more critical for enhancing photosynthesis (Kremien et al., 2013).
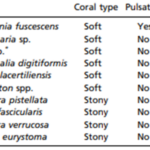
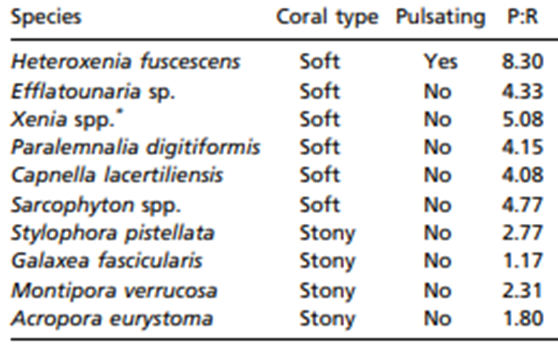
In terms of absolute gain, the increase in the colony’s photosynthesis rate by pulsation (+12.2 μmol O2 per min) greatly surpasses the added metabolic cost of pulsations (−0.9 μmol O2 per minute) (Kremien et al., 2013). Overall, this is very important because living in low-nutrient environments and relying on photosynthesis, which is driven by inconsistent, external factors such as sunlight to survive and grow poses several problems for these reef-building marine organisms. As a result, certain families of corals (Xeniidae) have gone through evolutionary processes that allow the coral polyps to pulsate which leads to an optimization of photosynthesis through oxygen efflux and increasing surface area exposed to light. The adaptive benefit of these polyp pulsations can be seen through a comparison of photosynthesis to respiration ratios of nine non-pulsating corals to the pulsating Heteroxenia fuscescens coral (Table 1). The photosynthesis to respiration ratio for the nine non-pulsating corals ranges from 1.17 to 5.08 whereas the photosynthesis to respiration ratio of the pulsating Heteroxenia fuscescens coral is considerably higher at a ratio of 8.3. Pulsations enable H. fuscescens to achieve an exceptionally high energy output and show how certain species of corals have undergone special evolutionary processes that create specific design solutions to allow them to counteract many issues such as sunlight inconsistency water pollution (Kremien et al., 2013).
Table 1
Table showing photosynthesis to respiration ratio of ten different coral species (Kremien et al., 2013).
Conclusion
In conclusion, coral polyps make use of biomineralization, symbiosis, bleaching and pulsations to overcome problems that impede their survival and to enhance their multitude of functions in the marine ecosystem. Firstly, coral polyps face threats from waves, currents, and hydraulic forces that can harm their delicate tissues. They require a suitable and secure habitat where they can find protection and stability, enabling them to grow and thrive. Their solution is to create an exoskeleton through a process called biomineralization. Over time, as individual polyps grow and reproduce, the exoskeletons will accumulate and collectively form the foundations of coral reefs. Coral reefs provide protection and stability for polyps to grow and prosper.
Moreover, they rely on zooplankton for feeding, however, they are not abundant enough to satisfy all the polyp needs and thus they engage in a mutualistic relationship with zooxanthellae providing them with most of the energy they require for survival. The complementarity of coral respiration and zooxanthellae photosynthesis ensure the continuation of this relationship as the products of one are the reactants of the other. In this way, the polyp maximizes the use of its resources rather than ejecting its waste into the environment. Additionally, the polyp lacks the enzymes responsible for synthesizing organic N from inorganic ones needed for cellular functions. Therefore, it benefits from a symbiotic relationship where the zooxanthellae can synthesize organic N and transport it to its host coral.
Furthermore, one of the most prevalent issues today is the increase in seawater temperatures due to climate change. This minimizes the efficiency of photosynthesis while also producing reactive oxygen species, which are harmful to the zooxanthellae-coral mutualistic relationship. The corals have developed a design solution that is geared towards self-preservation. Although they rely on these algae for key life processes such as eating and calcification, corals expel the zooxanthellae to preserve energy because the relationship can be more detrimental than beneficial under these environmental conditions.
Finally, as reef building marine organisms that live in low nutrient tropical waters, corals must also rely on photosynthesis to feed and grow into large colonies of thousands of corals. However, relying on sunlight as a means of feeding poses several problems such as the exposure to different intensities of light depending on the time of day, time of year, their depth and even the level of water pollution. To overcome these problems, certain species of corals have gone through evolutionary processes that allow the coral polyps to pulsate. This pulsation allows corals to counteract these problems as it allows for the enhancement of photosynthesis through the efflux of oxygen, increased carbon acquisition and an increased surface area exposed to sunlight.
The complexity and ingenuity of such a small natural system highlights the need to preserve these biodiverse beings in the face of ongoing environmental threats. Moving forward, hopefully there exists an innovative solution that can preserve the beauty and vitality of coral polyps.
References
Al Omari, M. M. H., Rashid, I. S., Qinna, N. A., Jaber, A. M., & Badwan, A. A. (2016). Calcium Carbonate. Profiles of Drug Substances, Excipients and Related Methodology, 31–132. https://doi.org/10.1016/bs.podrm.2015.11.003
Anderson, R. J., Hines, E., Mazzini, P. L. F., Elliott, M., Largier, J. L., & Jahncke, J. (2022). Spatial patterns in aragonite saturation horizon over the northern California shelf. Regional Studies in Marine Science, 52, 102286. https://doi.org/10.1016/j.rsma.2022.102286
Australian Online Coastal Information. (2015). Partial pressure of carbon dioxide. OzCoasts. https://ozcoasts.org.au/indicators/biophysical-indicators/water_column_partial_pressure/#:~:text=The%20partial%20pressure%20of%20carbon%20dioxide%20(PCO2)%20is%20the%20gas
Bennett, J. (2018, December 18). Ocean Acidification. Smithsonian Ocean. https://ocean.si.edu/ocean-life/invertebrates/ocean-acidification
Brittain, H. G. (2016). Profiles of drug substances, excipients, and related methodology. Elsevier Academic Press.
CDHC. (n.d.). Coral Anatomy and Histopathology Terms. Coral Disease & Health Consortium. Retrieved November 8, 2023, from https://cdhc.noaa.gov/coral-disease/glossary-hist/
Coral Basics | Flower Garden Banks National Marine Sanctuary. (n.d.). Flowergarden.noaa.gov. Retrieved November 8, 2023, from https://flowergarden.noaa.gov/education/coralbasics.html#:~:text=Hundreds%20to%20thousands%20of%20coral
Curl, R. (1962). The Aragonite-Calcite Problem.
Drake, J. L., Mass, T., Stolarski, J., Von Euw, S., van de Schootbrugge, B., & Falkowski, P. G. (2019). How corals made rocks through the ages. Global Change Biology, 26(1), 31–53. https://doi.org/10.1111/gcb.14912
Eyre, B. D., Cyronak, T., Drupp, P., De Carlo, E. H., Sachs, J. P., & Andersson, A. J. (2018). Coral reefs will transition to net dissolving before end of century. Science, 359(6378), 908–911. https://doi.org/10.1126/science.aao1118
GAS EXCHANGE Tissue Respiration Contents Cellular Respiration Mitochondrial Respiration Specializations in Mitochondrial Respiration of Fish. (2011).
Gates, R. D., Baghdasarian, G., & Muscatine, L. (1992). Temperature Stress Causes Host Cell Detachment in Symbiotic Cnidarians: Implications for Coral Bleaching. The Biological Bulletin, 182(3), 324–332. https://doi.org/10.2307/1542252
Hagedorn, M., Carter, V., Zuchowicz, N., Phillips, M., Penfield, C., Shamenek, B., Vallen, E. A., Kleinhans, F. W., Peterson, K., White, M., & Yancey, P. H. (2015). Trehalose Is a Chemical Attractant in the Establishment of Coral Symbiosis. PLOS ONE, 10(1), e0117087. https://doi.org/10.1371/journal.pone.0117087
Jellison, G. E., Leonard, D. N., Anovitz, L. M., Cheshire, M. C., Specht, E. D., & Rosseel, T. M. (2019). Crystallographic orientation of orthorhombic aragonite using reflection generalized ellipsometry. Journal of Applied Physics, 126(4). https://doi.org/10.1063/1.5109093
Kremien, M., Shavit, U., Mass, T., & Genin, A. (2013). Benefit of pulsation in soft corals. Proceedings of the National Academy of Sciences, 110(22), 8978–8983. https://doi.org/10.1073/pnas.1301826110
Leletkin, V. (2000). The Energy Budget of Coral Polyps. Russian Journal of Marine Biology, 26(6), 389–398.
Lesser, M. P. (2010). Coral Bleaching: Causes and Mechanisms. Coral Reefs: An Ecosystem in Transition, 405–419. https://doi.org/10.1007/978-94-007-0114-4_23
Mass, T., Genin, A., Shavit, U., Grinstein, M., & Tchernov, D. (2010). Flow enhances photosynthesis in marine benthic autotrophs by increasing the efflux of oxygen from the organism to the water. Proceedings of the National Academy of Sciences, 107(6), 2527–2531. https://doi.org/10.1073/pnas.0912348107
McClelland, Grant. (2011). Tissue respiration | Cellular Respiration. Encyclopedia of Fish Physiology. 2. 951-958. 10.1016/B978-0-12-374553-8.00120-9.
MineralRocks. (n.d.). Aragonite Vs. Calcite – Differences and Similarities. Allmineralsrock. Retrieved November 15, 2023, from https://www.allmineralsrock.com/compare/aragonite-vs-calcite
Muller-Parker, G., D’Elia, C. F., & Cook, C. B. (2015). Interactions Between Corals and Their Symbiotic Algae. Coral Reefs in the Anthropocene, 99–116. https://doi.org/10.1007/978-94-017-7249-5_5
Nakamura, T., Nadaoka, K., & Watanabe, A. (2013). A coral polyp model of photosynthesis, respiration and calcification incorporating a transcellular ion transport mechanism. Coral Reefs, 32(3), 779–794. https://doi.org/10.1007/s00338-013-1032-2
NASA. (2016). Great Barrier Reef Near Whitsunday Islands – NASA. https://www.nasa.gov/image-article/great-barrier-reef-near-whitsunday-islands/#:~:text=Reefs%20are%20easy%20to%20spot
National Oceanic and Atmospheric Administration. (2019a). Zooxanthellae…What’s That – Corals: NOAA’s National Ocean Service Education. Noaa.gov. https://oceanservice.noaa.gov/education/tutorial_corals/coral02_zooxanthellae.html
National Oceanic and Atmospheric Administration. (2019b, February 1). Coral Reef Ecosystems. Www.noaa.gov; National Oceanic and Atmospheric Administration. https://www.noaa.gov/education/resource-collections/marine-life/coral-reef-ecosystems
Plass-Johnson, J. G., Cardini, U., van Hoytema, N., Bayraktarov, E., Burghardt, I., Naumann, M. S., & Wild, C. (2014). Coral Bleaching. Environmental Indicators, 117–146. https://doi.org/10.1007/978-94-017-9499-2_9
Refractive Index Database. (2023). Refractive index of CaCO3 (Calcium carbonate, Calcite) – Ghosh-o. Refractiveindex.info. https://refractiveindex.info/?shelf=main&book=CaCO3&page=Ghosh-o
Satpati, G. G., & Pal, R. (2020). Photosynthesis in algae. Applied Algal Biotechnology, Nova Science Publishers, Inc, 49-68.
Scandalios, J. G. (2005). Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Brazilian Journal of Medical and Biological Research, 38(7), 995–1014. https://doi.org/10.1590/s0100-879×2005000700003
Tambutté, S., Holcomb, M., Ferrier-Pagès, C., Reynaud, S., Tambutté, É., Zoccola, D., & Allemand, D. (2011). Coral biomineralization: From the gene to the environment. Journal of Experimental Marine Biology and Ecology, 408(1-2), 58–78. https://doi.org/10.1016/j.jembe.2011.07.026
Tanaka, Y., Miyajima, T., Koike, I., Hayashibara, T., & Ogawa, H. (2006). Translocation and conservation of organic nitrogen within the coral-zooxanthella symbiotic system of Acropora pulchra, as demonstrated by dual isotope-labeling techniques. Journal of Experimental Marine Biology and Ecology, 336(1), 110–119. https://doi.org/10.1016/j.jembe.2006.04.011
Titlyanov, E. A., & Titlyanova, T. V. (2020). Symbiotic Relationships between Microalgal Zooxanthellae and Reef-Building Coral Polyps in the Process of Autotrophic and Heterotrophic Nutrition. Russian Journal of Marine Biology, 46(5), 307–318. https://doi.org/10.1134/s1063074020050107
WebMineral. (n.d.-a). Aragonite Mineral Data. Webmineral.com. Retrieved November 15, 2023, from https://webmineral.com/data/Aragonite.shtml
WebMineral. (n.d.-b). Calcite Mineral Data. Webmineral.com. https://webmineral.com/data/Calcite.shtml
Yamashita, H., Suzuki, G., Kai, S., Hayashibara, T., & Koike, K. (2014). Establishment of Coral–Algal Symbiosis Requires Attraction and Selection. PLoS ONE, 9(5), e97003. https://doi.org/10.1371/journal.pone.0097003
Yellowlees, D., Hoegh-Guldberg, O., & Oliver, J. (n.d.). Coral bleaching: implications for the Great Barrier Reef Marine Park.
Yuyama, I., & Higuchi, T. (2019). Differential gene expression in skeletal organic matrix proteins of scleractinian corals associated with mixed aragonite/calcite skeletons under low mMg/Ca conditions. PeerJ, 7, e7241. https://doi.org/10.7717/peerj.7241